Imeglimin Alleviates High-Glucose-Induced Bioenergetic and Oxidative Stress Thereby Enhancing Intercellular Adhesion in H9c2 Cardiomyoblasts
Abstract
1. Introduction
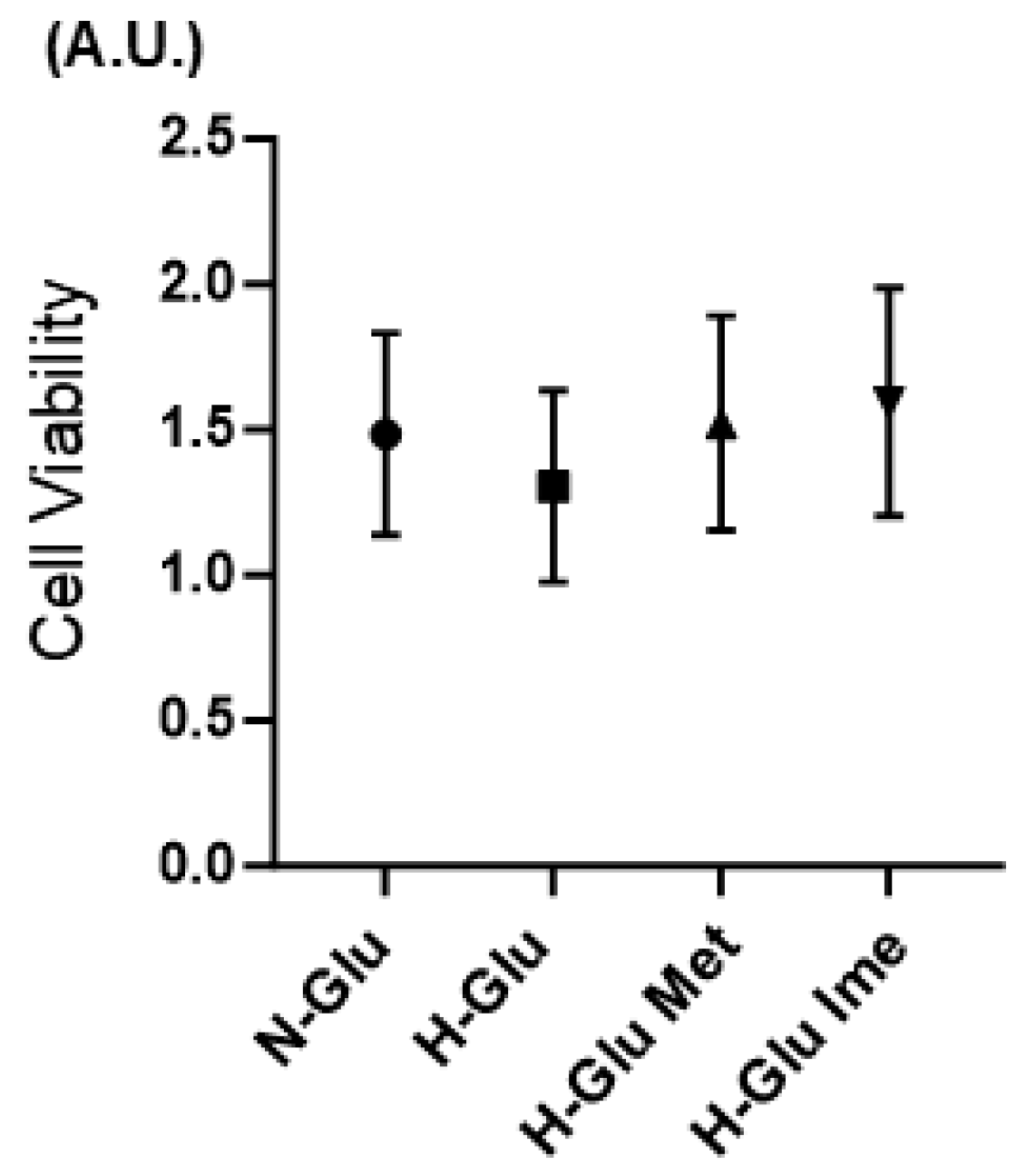
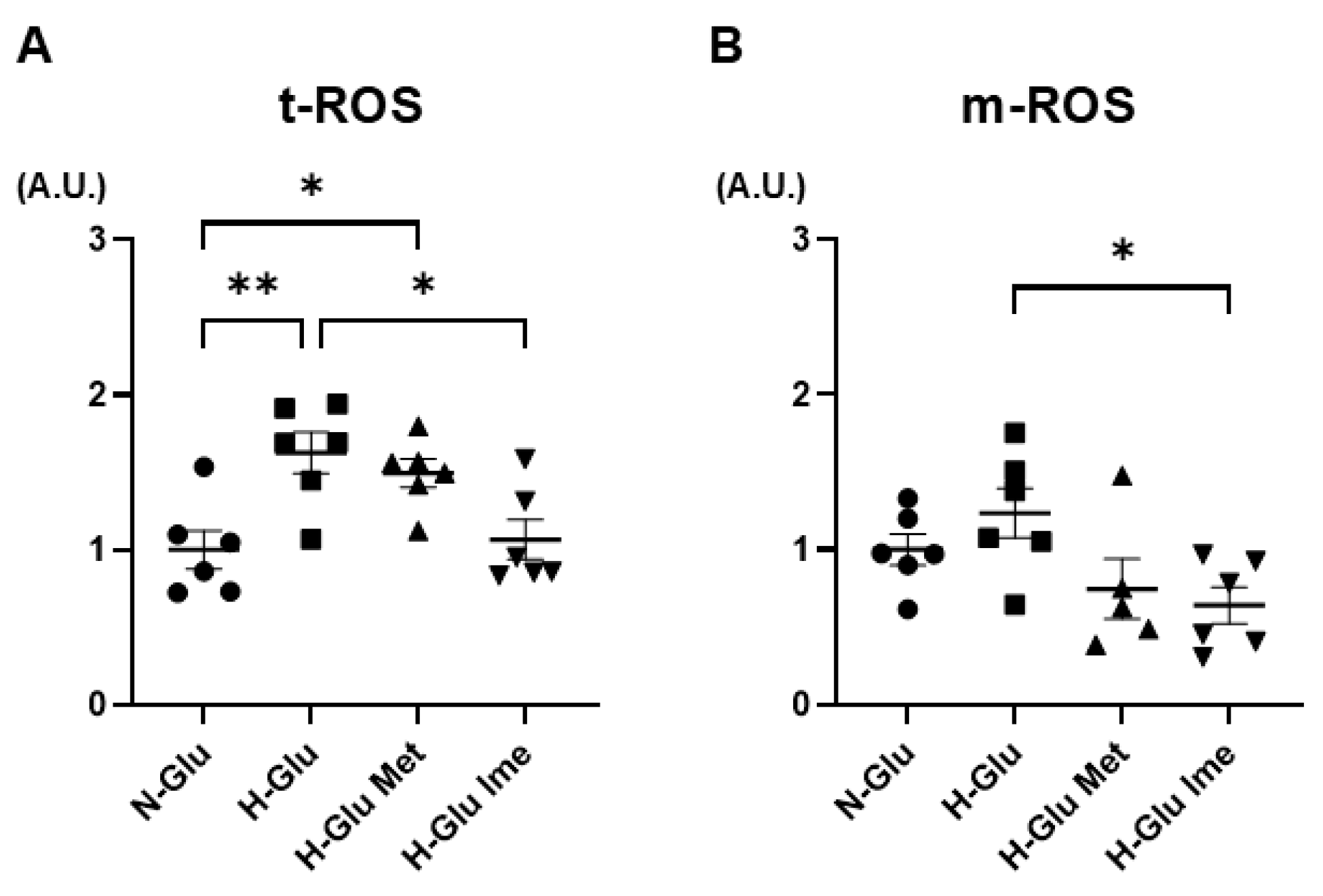
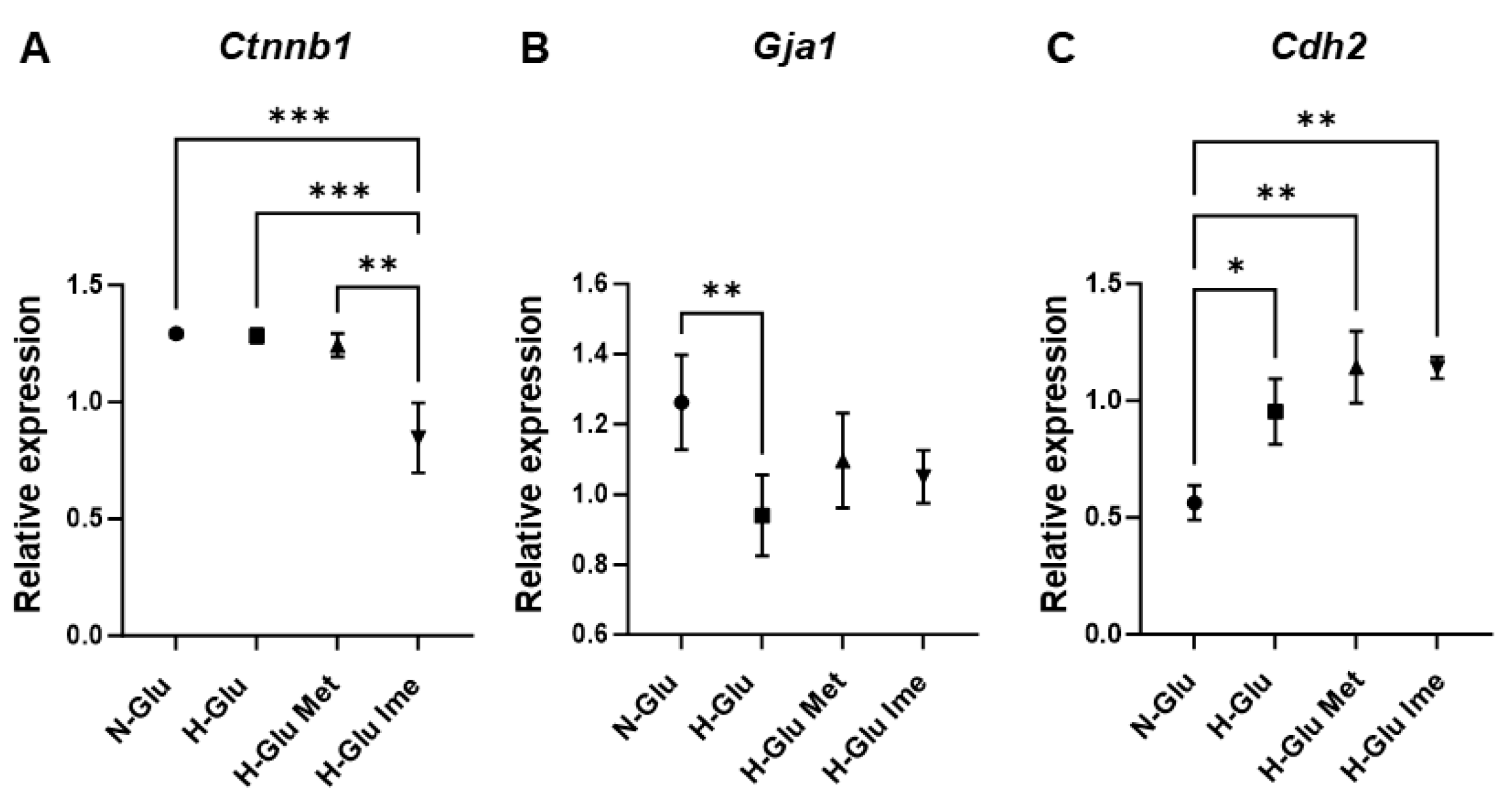
2. Results
3. Discussion
4. Materials and Methods
4.1. Planar Culture of H9c2 Cells
4.2. Cell Viability Assay
4.3. Seahorse Extracellular Flux Analysis
4.4. Measurement of Levels of Reactive Oxygen Species (ROS)
4.5. Measurement of TEER
4.6. Western Blotting
4.7. Quantitative Real-Time PCR
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010, 11, 31–39. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., III.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Hue, L.; Taegtmeyer, H. The Randle cycle revisited: A new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Iadicicco, I.; Donnarumma, M.; Galiero, R.; Acierno, C.; et al. Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications. Antioxidants 2025, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.R.; Chang, K.; Frangos, M.; Hasan, K.S.; Ido, Y.; Kawamura, T.; Nyengaard, J.R.; van den Enden, M.; Kilo, C.; Tilton, R.G. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes 1993, 42, 801–813. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Pandey, K.B.; Abidi, A.B.; Rizvi, S.I. Markers of Oxidative Stress during Diabetes Mellitus. J. Biomark. 2013, 2013, 378790. [Google Scholar] [CrossRef]
- Davargaon, R.S.; Sambe, A.D.; Muthangi, V.V.S. Toxic effect of high glucose on cardiomyocytes, H9c2 cells: Induction of oxidative stress and ameliorative effect of trolox. J. Biochem. Mol. Toxicol. 2019, 33, e22272. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Jiang, B.; Li, M.; Zhu, M.; Peng, Y.; Zhang, Y.L.; Wu, Y.Q.; Li, T.Y.; Liang, Y.; Lu, Z.; et al. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 2014, 20, 526–540. [Google Scholar] [CrossRef]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front. Public Health 2017, 5, 335. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Shah, R.B.; Singhal, S.; Dutta, S.B.; Bansal, S.; Sinha, S.; Haque, M. Metformin: A Review of Potential Mechanism and Therapeutic Utility Beyond Diabetes. Drug Des. Dev. Ther. 2023, 17, 1907–1932. [Google Scholar] [CrossRef]
- Hsu, S.K.; Cheng, K.C.; Mgbeahuruike, M.O.; Lin, Y.H.; Wu, C.Y.; Wang, H.D.; Yen, C.H.; Chiu, C.C.; Sheu, S.J. New Insight into the Effects of Metformin on Diabetic Retinopathy, Aging and Cancer: Nonapoptotic Cell Death, Immunosuppression, and Effects beyond the AMPK Pathway. Int. J. Mol. Sci. 2021, 22, 9453. [Google Scholar] [CrossRef]
- Johnson, R.; Sangweni, N.F.; Mabhida, S.E.; Dludla, P.V.; Mabasa, L.; Riedel, S.; Chapman, C.; Mosa, R.A.; Kappo, A.P.; Louw, J.; et al. An In Vitro Study on the Combination Effect of Metformin and N-Acetyl Cysteine against Hyperglycaemia-Induced Cardiac Damage. Nutrients 2019, 11, 2850. [Google Scholar] [CrossRef]
- Sanit, J.; Prompunt, E.; Adulyaritthikul, P.; Nokkaew, N.; Mongkolpathumrat, P.; Kongpol, K.; Kijtawornrat, A.; Petchdee, S.; Barrère-Lemaire, S.; Kumphune, S. Combination of metformin and p38 MAPK inhibitor, SB203580, reduced myocardial ischemia/reperfusion injury in non-obese type 2 diabetic Goto-Kakizaki rats. Exp. Ther. Med. 2019, 18, 1701–1714. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Shi, X.; Yang, L.; Hua, F.; Ma, J.; Zhu, W.; Liu, X.; Xuan, R.; Shen, Y.; et al. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging 2020, 12, 24270–24287. [Google Scholar] [CrossRef]
- Li, W.; Jin, S.; Hao, J.; Shi, Y.; Li, W.; Jiang, L. Metformin attenuates ischemia/reperfusion-induced apoptosis of cardiac cells by downregulation of p53/microRNA-34a via activation of SIRT1. Can. J. Physiol. Pharmacol. 2021, 99, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bai, Y.; Qi, Y.; Chang, C.; Jiao, Y.; Bai, Y.; Guo, Z. Metformin ameliorates ferroptosis in cardiac ischemia and reperfusion by reducing NOX4 expression via promoting AMPKα. Pharm. Biol. 2023, 61, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018, 9, 753. [Google Scholar] [CrossRef]
- Vuylsteke, V.; Chastain, L.M.; Maggu, G.A.; Brown, C. Imeglimin: A Potential New Multi-Target Drug for Type 2 Diabetes. Drugs RD 2015, 15, 227–232. [Google Scholar] [CrossRef]
- Konkwo, C.; Perry, R.J. Imeglimin: Current Development and Future Potential in Type 2 Diabetes. Drugs 2021, 81, 185–190. [Google Scholar] [CrossRef]
- Pirags, V.; Lebovitz, H.; Fouqueray, P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes. Metab. 2012, 14, 852–858. [Google Scholar] [CrossRef]
- Fouqueray, P.; Pirags, V.; Diamant, M.; Schernthaner, G.; Lebovitz, H.E.; Inzucchi, S.E.; Bailey, C.J. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapy. Diabetes Care 2014, 37, 1924–1930. [Google Scholar] [CrossRef]
- Fouqueray, P.; Pirags, V.; Inzucchi, S.E.; Bailey, C.J.; Schernthaner, G.; Diamant, M.; Lebovitz, H.E. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care 2013, 36, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Theurey, P.; Vial, G.; Fontaine, E.; Monternier, P.A.; Fouqueray, P.; Bolze, S.; Moller, D.E.; Hallakou-Bozec, S. Reduced lactic acidosis risk with Imeglimin: Comparison with Metformin. Physiol. Rep. 2022, 10, e15151. [Google Scholar] [CrossRef] [PubMed]
- Lachaux, M.; Soulié, M.; Hamzaoui, M.; Bailly, A.; Nicol, L.; Rémy-Jouet, I.; Renet, S.; Vendeville, C.; Gluais-Dagorn, P.; Hallakou-Bozec, S.; et al. Short-and long-term administration of imeglimin counters cardiorenal dysfunction in a rat model of metabolic syndrome. Endocrinol. Diabetes Metab. 2020, 3, e00128. [Google Scholar] [CrossRef]
- Aoyagi, K.; Nishiwaki, C.; Nakamichi, Y.; Yamashita, S.I.; Kanki, T.; Ohara-Imaizumi, M. Imeglimin mitigates the accumulation of dysfunctional mitochondria to restore insulin secretion and suppress apoptosis of pancreatic β-cells from db/db mice. Sci. Rep. 2024, 14, 6178. [Google Scholar] [CrossRef]
- Narukawa, Y.; Sugiyama, N.; Miura, J.; Yamashita, R.; Tominaga, S.; Izumi, Y.; Bamba, T.; Ishihama, Y.; Kashiwagi, Y.; Murakami, S. Chronic hyperglycemia reduces the expression of intercellular adhesion molecules and increases intercellular hyperpermeability in the periodontal epithelium. J. Periodontal Res. 2023, 58, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Tien, T.; Barrette, K.F.; Chronopoulos, A.; Roy, S. Effects of high glucose-induced Cx43 downregulation on occludin and ZO-1 expression and tight junction barrier function in retinal endothelial cells. Investig. Ophthalmol Vis. Sci. 2013, 54, 6518–6525. [Google Scholar] [CrossRef] [PubMed]
- Isaksen, J.L.; Sivertsen, C.B.; Jensen, C.Z.; Graff, C.; Linz, D.; Ellervik, C.; Jensen, M.T.; Jørgensen, P.G.; Kanters, J.K. Electrocardiographic markers in patients with type 2 diabetes and the role of diabetes duration. J. Electrocardiol. 2024, 84, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kobayashi, T.; Kuno, A.; Miki, T.; Tanno, M.; Kouzu, H.; Itoh, T.; Ishikawa, S.; Kojima, T.; Miura, T.; et al. Type 2 diabetes induces subendocardium-predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1054–H1065. [Google Scholar] [CrossRef]
- Gallego, M.; Zayas-Arrabal, J.; Alquiza, A.; Apellaniz, B.; Casis, O. Electrical Features of the Diabetic Myocardium. Arrhythmic and Cardiovascular Safety Considerations in Diabetes. Front. Pharmacol. 2021, 12, 687256. [Google Scholar] [CrossRef]
- Jin, X.; Jiang, Y.; Xue, G.; Yuan, Y.; Zhu, H.; Zhan, L.; Zhuang, Y.; Huang, Q.; Shi, L.; Zhao, Y.; et al. Increase of late sodium current contributes to enhanced susceptibility to atrial fibrillation in diabetic mice. Eur. J. Pharmacol. 2019, 857, 172444. [Google Scholar] [CrossRef]
- Dziubak, A.; Wójcicka, G.; Wojtak, A.; Bełtowski, J. Metabolic Effects of Metformin in the Failing Heart. Int. J. Mol. Sci. 2018, 19, 2869. [Google Scholar] [CrossRef]
- Schernthaner, G.; Brand, K.; Bailey, C.J. Metformin and the heart: Update on mechanisms of cardiovascular protection with special reference to comorbid type 2 diabetes and heart failure. Metab. Clin. Exp. 2022, 130, 155160. [Google Scholar] [CrossRef]
- Li, J.Z.; Li, Y.R. Cardiovascular Protection by Metformin: Latest Advances in Basic and Clinical Research. Cardiology 2023, 148, 374–384. [Google Scholar] [CrossRef]
- Nishikiori, N.; Watanabe, M.; Higashide, M.; Umetsu, A.; Ogawa, T.; Furuhashi, M.; Ohguro, H.; Sato, T. The Combination of PPARα Agonist GW7647 and Imeglimin Has Potent Effects on High-Glucose-Induced Cellular Biological Responses in Human Retinal Pigment Epithelium Cells. Bioengineering 2025, 12, 265. [Google Scholar] [CrossRef]
- Nishikiori, N.; Ohguro, H.; Watanabe, M.; Higashide, M.; Ogawa, T.; Furuhashi, M.; Sato, T. High-Glucose-Induced Metabolic and Redox Alterations Are Distinctly Modulated by Various Antidiabetic Agents and Interventions Against FABP5/7, MITF and ANGPTL4 in Melanoma A375 Cells. Int. J. Mol. Sci. 2025, 26, 1014. [Google Scholar] [CrossRef]
- Ohguro, H.; Higashide, M.; Nishikiori, N.; Ogawa, T.; Furuhashi, M.; Sato, T.; Watanabe, M. The distinct effects of metformin and imeglimin on high glucose-induced alterations in metabolic function and reactive oxygen species production in mouse Schwann cells are modulated by pemafibrate and/or fatty acid-binding proteins. Front. Cell. Neurosci. 2025, 19, 1634262. [Google Scholar] [CrossRef]
- Crowley, M.J.; Diamantidis, C.J.; McDuffie, J.R.; Cameron, C.B.; Stanifer, J.W.; Mock, C.K.; Wang, X.; Tang, S.; Nagi, A.; Kosinski, A.S.; et al. Clinical Outcomes of Metformin Use in Populations With Chronic Kidney Disease, Congestive Heart Failure, or Chronic Liver Disease: A Systematic Review. Ann. Intern. Med. 2017, 166, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, L. High-glucose-induced apoptosis, ROS production and pro-inflammatory response in cardiomyocytes is attenuated by metformin treatment via PP2A activation. J. Biosci. 2020, 45, 126. [Google Scholar] [CrossRef]
- Yang, F.; Qin, Y.; Wang, Y.; Meng, S.; Xian, H.; Che, H.; Lv, J.; Li, Y.; Yu, Y.; Bai, Y.; et al. Metformin Inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent Effects in Diabetic Cardiomyopathy. Int. J. Biol. Sci. 2019, 15, 1010–1019. [Google Scholar] [CrossRef]
- Yendapally, R.; Sikazwe, D.; Kim, S.S.; Ramsinghani, S.; Fraser-Spears, R.; Witte, A.P.; La-Viola, B. A review of phenformin, metformin, and imeglimin. Drug Dev. Res. 2020, 81, 390–401. [Google Scholar] [CrossRef]
- Kitakata, H.; Endo, J.; Hashimoto, S.; Mizuno, E.; Moriyama, H.; Shirakawa, K.; Goto, S.; Katsumata, Y.; Fukuda, K.; Sano, M. Imeglimin prevents heart failure with preserved ejection fraction by recovering the impaired unfolded protein response in mice subjected to cardiometabolic stress. Biochem. Biophys. Res. Commun. 2021, 572, 185–190. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, G.; Chen, W.; Zhang, B.; Zhang, J.; Chang, J.; Tang, C.; Qi, Y.; Yin, X. Metformin protects the myocardium against isoproterenol-induced injury in rats through alleviating endoplasmic reticulum stress. Die Pharm. 2014, 69, 64–69. [Google Scholar]
- Ren, J.; Dominguez, L.J.; Sowers, J.R.; Davidoff, A.J. Metformin but not glyburide prevents high glucose-induced abnormalities in relaxation and intracellular Ca2+ transients in adult rat ventricular myocytes. Diabetes 1999, 48, 2059–2065. [Google Scholar] [CrossRef]
- Wang, G.Y.; Bi, Y.G.; Liu, X.D.; Zhao, Y.; Han, J.F.; Wei, M.; Zhang, Q.Y. Autophagy was involved in the protective effect of metformin on hyperglycemia-induced cardiomyocyte apoptosis and Connexin43 downregulation in H9c2 cells. Int. J. Med. Sci. 2017, 14, 698–704. [Google Scholar] [CrossRef]
- Kajbaf, F.; De Broe, M.E.; Lalau, J.D. Therapeutic Concentrations of Metformin: A Systematic Review. Clin. Pharmacokinet. 2016, 55, 439–459. [Google Scholar] [CrossRef]
- Liu, X.D.; Li, Y.G.; Wang, G.Y.; Bi, Y.G.; Zhao, Y.; Yan, M.L.; Liu, X.; Wei, M.; Wan, L.L.; Zhang, Q.Y. Metformin protects high glucose-cultured cardiomyocytes from oxidative stress by promoting NDUFA13 expression and mitochondrial biogenesis via the AMPK signaling pathway. Mol. Med. Rep. 2020, 22, 5262–5270. [Google Scholar] [CrossRef]
- Hu, M.; Ye, P.; Liao, H.; Chen, M.; Yang, F. Metformin Protects H9C2 Cardiomyocytes from High-Glucose and Hypoxia/Reoxygenation Injury via Inhibition of Reactive Oxygen Species Generation and Inflammatory Responses: Role of AMPK and JNK. J. Diabetes Res. 2016, 2016, 2961954. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Iwashita, K.; Iwasa, M.; Kato, S.; Yamakage, H.; Suganami, T.; Tanaka, M.; Satoh-Asahara, N. Imeglimin Exhibits Novel Anti-Inflammatory Effects on High-Glucose-Stimulated Mouse Microglia through ULK1-Mediated Suppression of the TXNIP-NLRP3 Axis. Cells 2024, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Vial, G.; Lamarche, F.; Cottet-Rousselle, C.; Hallakou-Bozec, S.; Borel, A.L.; Fontaine, E. The mechanism by which imeglimin inhibits gluconeogenesis in rat liver cells. Endocrinol. Diabetes Metab. 2021, 4, e00211. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Z.; Shi, H. HIF-1 is involved in high glucose-induced paracellular permeability of brain endothelial cells. Cell. Mol. Life Sci. CMLS 2012, 69, 115–128. [Google Scholar] [CrossRef]
- Sajja, R.K.; Prasad, S.; Cucullo, L. Impact of altered glycaemia on blood-brain barrier endothelium: An in vitro study using the hCMEC/D3 cell line. Fluids Barriers CNS 2014, 11, 8. [Google Scholar] [CrossRef]
- Brown, R.C.; Morris, A.P.; O’Neil, R.G. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007, 1130, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Petersen, A.; Adelöf, J.; Hernebring, M.; Zetterberg, M. A Novel Primary Porcine Retinal Pigment Epithelium Cell Model with Preserved Properties. Curr. Eye Res. 2024, 49, 97–107. [Google Scholar] [CrossRef]
- Dubourg, J.; Perrimond-Dauchy, S.; Felices, M.; Bolze, S.; Voiriot, P.; Fouqueray, P. Absence of QTc prolongation in a thorough QT study with imeglimin, a first in class oral agent for type 2 diabetes mellitus. Eur. J. Clin. Pharmacol. 2020, 76, 1393–1400. [Google Scholar] [CrossRef]
- Abe, K.; Yano, T.; Tanno, M.; Miki, T.; Kuno, A.; Sato, T.; Kouzu, H.; Nakata, K.; Ohwada, W.; Kimura, Y.; et al. mTORC1 inhibition attenuates necroptosis through RIP1 inhibition-mediated TFEB activation. Biochim. Biophys. Acta.—Mol. Basis Dis. 2019, 1865, 165552. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yano, T.; Sato, T.; Umetsu, A.; Higashide, M.; Furuhashi, M.; Ohguro, H. mTOR Inhibitors Modulate the Physical Properties of 3D Spheroids Derived from H9c2 Cells. Int. J. Mol. Sci. 2023, 24, 11459. [Google Scholar] [CrossRef] [PubMed]
- Oouchi, Y.; Watanabe, M.; Ida, Y.; Ohguro, H.; Hikage, F. Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-β2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners. Int. J. Mol. Sci. 2021, 22, 7335. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Ohta, M.; Inoue, T.; Mizuno, K.; Isobe, T.; Tanabe, S.; Tanihara, H. Effects of K-115 (Ripasudil), a novel ROCK inhibitor, on trabecular meshwork and Schlemm’s canal endothelial cells. Sci. Rep. 2016, 6, 19640. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2α agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef]
- Itoh, K.; Hikage, F.; Ida, Y.; Ohguro, H. Prostaglandin F2α Agonists Negatively Modulate the Size of 3D Organoids from Primary Human Orbital Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohguro, H.; Watanabe, M.; Suzuki, M.; Ohara, N.; Ogawa, T.; Sato, T.; Yano, T. Imeglimin Alleviates High-Glucose-Induced Bioenergetic and Oxidative Stress Thereby Enhancing Intercellular Adhesion in H9c2 Cardiomyoblasts. Int. J. Mol. Sci. 2025, 26, 8913. https://doi.org/10.3390/ijms26188913
Ohguro H, Watanabe M, Suzuki M, Ohara N, Ogawa T, Sato T, Yano T. Imeglimin Alleviates High-Glucose-Induced Bioenergetic and Oxidative Stress Thereby Enhancing Intercellular Adhesion in H9c2 Cardiomyoblasts. International Journal of Molecular Sciences. 2025; 26(18):8913. https://doi.org/10.3390/ijms26188913
Chicago/Turabian StyleOhguro, Hiroshi, Megumi Watanabe, Megumi Suzuki, Naruki Ohara, Toshifumi Ogawa, Tatsuya Sato, and Toshiyuki Yano. 2025. "Imeglimin Alleviates High-Glucose-Induced Bioenergetic and Oxidative Stress Thereby Enhancing Intercellular Adhesion in H9c2 Cardiomyoblasts" International Journal of Molecular Sciences 26, no. 18: 8913. https://doi.org/10.3390/ijms26188913
APA StyleOhguro, H., Watanabe, M., Suzuki, M., Ohara, N., Ogawa, T., Sato, T., & Yano, T. (2025). Imeglimin Alleviates High-Glucose-Induced Bioenergetic and Oxidative Stress Thereby Enhancing Intercellular Adhesion in H9c2 Cardiomyoblasts. International Journal of Molecular Sciences, 26(18), 8913. https://doi.org/10.3390/ijms26188913







