Adipocyte-Derived Stem Cells in the Treatment of Spinal Cord Injuries in Animal Models: A Systematic Review
Abstract
1. Introduction
1.1. Spinal Cord Injury [SCI]
1.2. Phases of SCI
- -
- Acute phase (2 h–2 days): Characterised by hallmarks of acute injury, namely inflammation, haemorrhage and oedema. Other features of note are vascular disruption, cellular necrosis and axonal injury. There is also evidence that free radical generation further contributes to cellular damage in the acute phase [7].
- -
- Subacute phase (2 days–2 weeks): Phagocytosis of debris begins to occur; axonal growth is initiated. At this stage, astrocytes proliferate and migrate to the site of injury [8]. However, phenotypic changes lead to glial scarring which may later impede neural regeneration in the chronic phase.
- -
- Intermediate phase (2 weeks–6 months): Maturation of scarring and axonal sprouting begins.
- -
- Chronic phase (6 months onwards): Formation of chronic scar tissue which may impede axonal growth [9]. Wallerian degeneration also occurs at this stage, and long-term sequela such as chronic pain and motor dysfunction begin to develop.
1.3. Mesenchymal Stem Cells (MSCs)
1.3.1. ADSCs
1.3.2. Animal Models Relevant to SCI Research
2. Materials and Methods
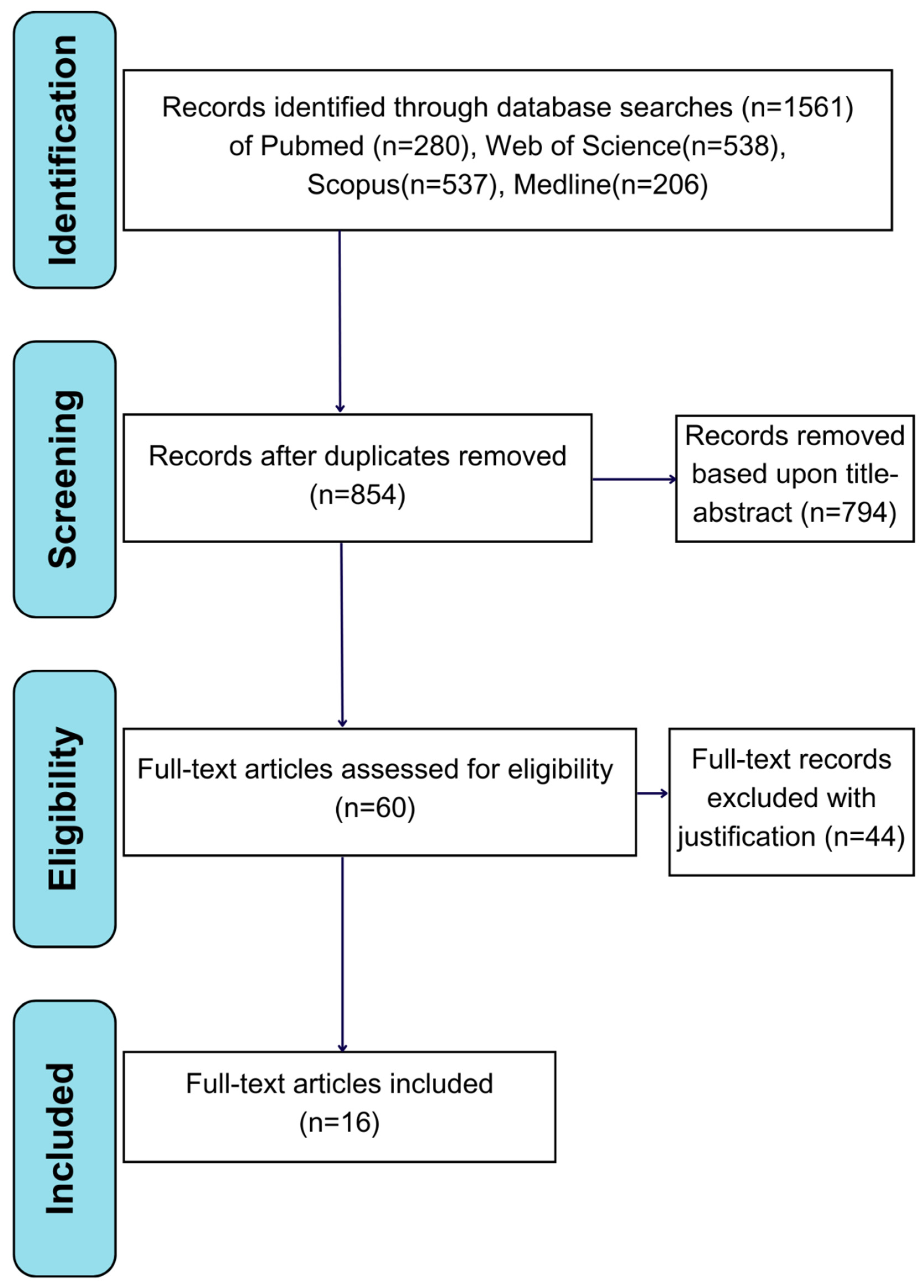
2.1. Search Strategy
2.2. Inclusion Criteria
- Studies carried out in animal models;
- Studies where the primary intervention is the administration of ADSCs;
- Studies displaying stem cell characterisation results;
- Studies that utilise the [BBB] scale to measure locomotor outcomes;
- English language.
2.3. Exclusion Criteria
- Studies with a total n value less than 10;
- Studies investigating conditions other than SCI;
- Studies not carried out in animal models;
- Studies lacking adequate control groups;
- Systematic reviews, the literature reviews and letters to editors;
- Studies not available in English.
2.4. Data Extraction
2.5. Quality Assessment
2.6. BBB Scoring System
3. Results
3.1. General Study Characteristics
3.2. Characterisation of Adipocyte-Derived Stem Cells
3.3. SCI Injury Models
3.4. BBB Scores
3.5. Secondary Bladder Functional Outcomes
3.6. Quality of Included Studies
4. Discussion
4.1. Stem Cells in Curing SCI
4.2. ADSC Characterisation
4.3. Quality of Experimental Models
4.4. ADSC Therapy and Its Effects on Locomotor Recovery
4.5. Other Effect Measures
4.6. Adjuvant Therapies
4.7. Limitations of Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.; Das, J.M.; Emmady, P.D. Spinal Cord Injuries. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ding, W.; Hu, S.; Wang, P.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal Cord Injury: The Global Incidence, Prevalence, and Disability from the Global Burden of Disease Study 2019. Spine 2022, 47, 1532–1540. [Google Scholar] [CrossRef]
- van Den Hauwe, L.; Sundgren, P.C.; Flanders, A.E. Spinal Trauma and Spinal Cord Injury [SCI]. In Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging; IDKD Springer Series; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Fyffe, D.C.; Botticello, A.L.; Myaskovsky, L. Vulnerable Groups Living with Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2011, 17, 1–9. [Google Scholar] [CrossRef]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Visavadiya, N.P.; Patel, S.P.; VanRooyen, J.L.; Sullivan, P.G.; Rabchevsky, A.G. Cellular and Subcellular Oxidative Stress Parameters Following Severe Spinal Cord Injury. Redox Biol. 2016, 8, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2018, 126, 39–43. [Google Scholar] [CrossRef]
- Li, X.; Yang, B.; Xiao, Z.; Zhao, Y.; Han, S.; Yin, Y.; Chen, B.; Dai, J. Comparison of subacute and chronic scar tissues after complete spinal cord transection. Exp. Neurol. 2018, 306, 132–137. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Camilleri, E.T.; Gustafson, M.P.; Dudakovic, A.; Riester, S.M.; Garces, C.G.; Paradise, C.R.; Takai, H.; Karperien, M.; Cool, S.; Sampen, H.-J.I.; et al. Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res. Ther. 2016, 7, 107. [Google Scholar] [CrossRef]
- Lin, C.S.; Ning, H.; Lin, G.; Lue, T.F. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy 2012, 14, 1159–1163. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy [ISCT®] Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Retinal Ischemia-Reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.L.B.; Hoa, N.D.; Thanh, V.V.; Van Thach, N.; Ngoc, V.T.N.; Dinh, T.C.; Phuong, T.N.T.; Toi, P.L.; Chu, D.T. Autologous Transplantation of Adipose-Derived Stem Cells to Treat Acute Spinal Cord Injury: Evaluation of Clinical Signs, Mental Signs, and Quality of Life. Maced. J. Med. Sci. 2019, 7, 4399–4405. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Safford, K.M.; Hicok, K.C.; Safford, S.D.; Halvorsen, Y.D.C.; Wilkison, W.O.; Gimble, J.M.; Rice, H.E. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2002, 294, 371–379. [Google Scholar] [CrossRef]
- Horie, T.; Hirata, H.; Sakamoto, T.; Kitajima, H.; Fuku, A.; Nakamura, Y.; Sunatani, Y.; Tanida, I.; Sunami, H.; Tachi, Y.; et al. Multiomics Analyses Reveal Adipose-Derived Stem Cells Inhibit the Inflammatory Response of M1-Like Macrophages Through Secreting Lactate. Stem Cell Res. Ther. 2024, 15, 485. [Google Scholar] [CrossRef]
- Bruun, K.; Schermer, E.; Sivendra, A.; Valaik, E.; Wise, R.B.; Said, R.; Bracht, J.R. Therapeutic applications of adipose-derived stem cells in cardiovascular disease. Am. J. Stem Cells 2018, 7, 94–103. [Google Scholar]
- Przekora, A.; Juszkiewicz, L. The Effect of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells’ Therapy in the Treatment of Chronic Posttraumatic Spinal Cord Injury in a Domestic Ferret Patient. Cell Transplant. 2020, 29, 0963689720928982. [Google Scholar] [CrossRef]
- Zhang, N.; Fang, M.; Chen, H.; Gou, F.; Ding, M. Evaluation of spinal cord injury animal models. Neural Regen. Res. 2014, 9, 2008–2012. [Google Scholar] [CrossRef]
- Ruberte, J.; Schofield, P.N.; Sundberg, J.P.; Rodriguez-Baeza, A.; Carretero, A.; McKerlie, C. Bridging mouse and human anatomies; a knowledge-based approach to comparative anatomy for disease model phenotyping. Mamm. Genome 2023, 34, 389–407. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Luo, J.; Sui, L.S.; Ma, X.; Yan, Z.J.; Lin, J.H.; Wang, Y.-S.; Chen, Y.-Z.; Jiang, X.-D.; Xu, R.-X. Effects of differentiated versus undifferentiated adipose tissue-derived stromal cell grafts on functional recovery after spinal cord contusion. Cell. Mol. Neurobiol. 2009, 29, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Takahara, E.; Kamizato, K.; Kakinohana, M.; Sunami, H.; Kise, Y.; Furukawa, K.; Ntege, E.H.; Shimizu, Y. Subpial Transplantation of Adipose-Derived Stem Cells Alleviates Paraplegia in a Rat Model of Aortic Occlusion/Reperfusion-Induced Spinal Cord Infarction. Regen. Ther. 2024, 26, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.H.; Lim, J.H.; Byeon, Y.E.; Park, J.R.; Seo, M.S.; Lee, Y.W.; Kim, W.H.; Kang, K.-S.; Kweon, O.-K. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J. Vet. Sci. 2009, 10, 273–284. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Y.; Zhang, H.; Min, S.; Yu, B.; He, B.; Jin, A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 2013, 15, 434–448. [Google Scholar] [CrossRef]
- Yousefifard, M.; Sarveazad, A.; Janzadeh, A.; Behroozi, Z.; Nasirinezhad, F. Pain Alleviating Effect of Adipose-Derived Stem Cells Transplantation on the Injured Spinal Cord: A Behavioral and Electrophysiological Evaluation. J. Stem Cells Regen. Med. 2022, 18, 53–63. [Google Scholar] [CrossRef]
- Aras, Y.; Sabanci, P.A.; Kabatas, S.; Duruksu, G.; Subasi, C.; Erguven, M.; Karaoz, E. The Effects of Adipose Tissue-Derived Mesenchymal Stem Cell Transplantation During the Acute and Subacute Phases Following Spinal Cord Injury. Turk. Neurosurg. 2016, 26, 127–139. [Google Scholar] [CrossRef]
- Ohta, Y.; Hamaguchi, A.; Ootaki, M.; Watanabe, M.; Takeba, Y.; Iiri, T.; Matsumoto, N.; Takenaga, M. Intravenous infusion of adipose-derived stem/stromal cells improves functional recovery of rats with spinal cord injury. Cytotherapy 2017, 19, 839–848. [Google Scholar] [CrossRef]
- Sarveazad, A.; Babahajian, A.; Bakhtiari, M.; Soleimani, M.; Behnam, B.; Yari, A.; Akbari, A.; Yousefifard, M.; Janzadeh, A.; Amini, N.; et al. The combined application of human adipose derived stem cells and Chondroitinase ABC in treatment of a spinal cord injury model. Neuropeptides 2017, 61, 39–47. [Google Scholar] [CrossRef]
- Takahashi, A.; Nakajima, H.; Kubota, A.; Watanabe, S.; Matsumine, A. Adipose-Derived Mesenchymal Stromal Cell Transplantation for Severe Spinal Cord Injury: Functional Improvement Supported by Angiogenesis and Neuroprotection. Cells 2023, 12, 1470. [Google Scholar] [CrossRef]
- Min, J.; Kim, J.H.; Choi, K.H.; Yoon, H.H.; Jeon, S.R. Is There Additive Therapeutic Effect When GCSF Combined with Adipose-Derived Stem Cell in a Rat Model of Acute Spinal Cord Injury? J. Korean Neurosurg. Soc. 2017, 60, 404–416. Available online: https://pubmed.ncbi.nlm.nih.gov/28689389/ (accessed on 25 May 2025). [CrossRef]
- Sarveazad, A.; Bakhtiari, M.; Babahajian, A.; Janzade, A.; Fallah, A.; Moradi, F.; Soleimani, M.; Younesi, M.; Goudarzi, F.; Joghataei, M.T. Comparison of human adipose-derived stem cells and chondroitinase ABC transplantation on locomotor recovery in the contusion model of spinal cord injury in rats. Iran. J. Basic Med. Sci. 2014, 17, 685. [Google Scholar]
- Villanova Junior, J.A.; Fracaro, L.; Rebelatto, C.L.K.; da Silva, A.J.; Barchiki, F.; Senegaglia, A.C.; Dominguez, A.C.; de Moura, S.A.B.; Pimpão, C.T.; Brofman, P.R.S.; et al. Recovery of motricity and micturition after transplantation of mesenchymal stem cells in rats subjected to spinal cord injury. Neurosci. Lett. 2020, 734, 135134. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ji, Z.; Wang, Y.; Li, T.; Luo, J.; Li, J.; He, L.; Wu, W. Human Adipose-Derived Stem Cells Combined with Nano-Hydrogel Promote Functional Recovery after Spinal Cord Injury in Rats. Biology 2022, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, J.; Guo, D.; Ji, N.; Liu, W.; Li, X.; Liu, H.; Zhang, C.; Zhao, M.; Li, H.; et al. Adipose-derived stem cell transplantation enhances spinal cord regeneration by upregulating PGRN expression. Neuroreport 2024, 35, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, H.; Zhang, K.; Xiao, D.J.; Wang, C.; Wang, Y.S. Adipose-derived stromal cells improve functional recovery after spinal cord injury through TGF-β1/Smad3/PLOD2 pathway activation. Aging 2021, 13, 4370–4387. [Google Scholar] [CrossRef]
- Oh, J.S.; Park, I.S.; Kim, K.N.; Yoon, D.H.; Kim, S.H.; Ha, Y. Transplantation of an adipose stem cell cluster in a spinal cord injury. Neuroreport 2012, 23, 277–282. [Google Scholar] [CrossRef]
- Fonseca, L.N.; Bolívar-Moná, S.; Agudelo, T.; Beltrán, L.D.; Camargo, D.; Correa, N.; Del Castillo, M.A.; de Castro, S.F.; Fula, V.; García, G.; et al. Cell surface markers for mesenchymal stem cells related to the skeletal system: A scoping review. Heliyon 2023, 9, e13464. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Schirmer, L.; Pinho, T.S.; Atallah, P.; Cibrão, J.R.; Lima, R.; Afonso, J.; B-Antunes, S.; Marques, C.R.; Dourado, J.; et al. Sustained Release of Human Adipose Tissue Stem Cell Secretome from Star-Shaped Poly [ethylene glycol] Glycosaminoglycan Hydrogels Promotes Motor Improvements after Complete Transection in Spinal Cord Injury Rat Model. Adv. Healthc. Mater. 2023, 12, e2202803. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Huang, Y.C.; Chueh, L.L.; Yeh, L.S.; Lin, C.S. Intra-articular transplantation of porcine adipose-derived stem cells for the treatment of canine osteoarthritis: A pilot study. World J. Transplant. 2014, 4, 196–205. [Google Scholar] [CrossRef]
- Ao, Z.; Cai, H.; Wu, Z.; Krzesniak, J.; Tian, C.; Lai, Y.Y.; Mackie, K.; Guo, F. Human Spinal Organoid-on-a-Chip to Model Nociceptive Circuitry for Pain Therapeutics Discovery. Anal. Chem. 2022, 94, 1365–1372. [Google Scholar] [CrossRef]
- Martín-López, M.; González-Muñoz, E.; Gómez-González, E.; Sánchez-Pernaute, R.; Márquez-Rivas, J.; Fernández-Muñoz, B. Modeling chronic cervical spinal cord injury in aged rats for cell therapy studies. Correction J. Clin. Neurosci. 2022, 96, 232. [Google Scholar] [CrossRef]
- Allen, K.J.; Leslie, S.W. Autonomic Dysreflexia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
| Reference | Brief Summary | ADSC Source | Total Number of Subjects (n) | ADSC Culture Conditions |
|---|---|---|---|---|
| Aras et al. (2016) [32] | Rat-derived ADSCs were implanted into the site of SCI, and the effects on locomotor recovery measured in rats. | Rat-derived ADSCs | 42 + 6 sacrificed during study | Harvested adipose tissue digested in DMEM, filtered and then centrifuged at 1200 rpm for 10 min. Medium replaced with fresh culture after 7 days then biweekly thereafter. |
| Ohta et al. (2017) [33] | ADSCs were harvested from the dorsal fat pads of rats, infused into rats following SCI and the impact upon locomotor recovery was measured. | Rat-derived ADSCs Dorsal fat pad | 30 | Centrifuge products suspended in medium at 37 °C in atmosphere of 5% CO2 95% air. Non-adherent cells removed after 24 h. |
| Zhou et al. (2013) [30] | Compared the effects of ADSC and BMSC implantation on functional recovery in rat models following SCI. | Human-derived ADSCs (bone-marrow cells also included) | 108 | ADSCs obtained following centrifuging process were suspended in DMEM with 10% FBS. Non-adherent cells removed by PBS wash. |
| Sarveazad et al. (2017) [34] | ADSCs were harvested from human tissue and implanted into rats alongside chondroitinase ABC, subsequent impact on functional recovery following SCI was measured. | Human-derived ADSCs | 30 | Isolated ADSCs suspended in medium (DMEM, HAM’s F-12, 10% FBS, 1% P/S). Cells incubated for 4 passages. |
| Takahara et al. (2024) [28] | ADSCs derived from rats were implanted in the subpial region, the impact upon functional recovery following aortic occlusion/reperfusion-induced SCI was measured in rats. | Rat-derived ADSCs | 12 | ADSCs cultured in DMEM with 10% FBS. Passage 0 ADSCs washed with PBS after 24 h initial incubation. Maintained in DMEM, 10% FBS and 1% P/S. Medium changed every other day. |
| Zhang et al. (2009) [27] | Differentiated and undifferentiated ADSCs were implanted into rats following SCI, with the relative impacts on functional recovery compared. | Rat-derived ADSCs | 46 | ADSCs obtained following centrifuging process maintained in DMEM/F12, 10% FBS, 1% P/S. Cultures incubated at 37 °C with 5% CO2. |
| Yousefifard et al. (2022) [31] | Human-derived ADSCs were implanted into rats following SCI, the impacts on functional recovery and pain following SCI were measured. | Human-derived ADSCs | 42 Excluded animals with BBB > 3 following SCI | ADSCS obtained following centrifugation process cultured in DMEM, Ham SF-12, 10% FBS, 1% P/S. Cells incubated until third passage. |
| Ryu et al. (2009) [29] | Canine-derived ADSCs were transplanted into dogs following SCI, the impacts on functional recovery were measured. | Canine-derived ADSCs from 2-year-old dog | 11 | ADSC pellets obtained after centrifuging cultured in DMEM, 10% FBS. Unattached cells washed off with PBS. Medium supplemented with rEGF, bovine pituitary extract, 2 mM N-acetyl-L-cysteine, 0.2 mM L-ascorbic acid, 2-phosphate, insulin and hydrocortisone. Medium changed at 48 h intervals until cell confluence was achieved. |
| Takahashi et al. (2023) [35] | Rat-derived ADSCs were implanted into rats following severe SCI, the impacts on functional recovery and the mechanisms that subserve this were examined. | Rat-derived ADSCs | 80 | Cells washed with PBS, cultured in DMEM and 10% FBS. Cultures maintained at 80–90% confluency at 37.5 °C with 5% CO2. |
| Min et al. (2017) [36] | Rat-derived ADSCs were infused into SCI rats alongside GCSF, the impacts upon functional recovery were measured. | Rat-derived ADSCs | 28 | Pellet obtained following centrifuging incubated at 37 °C in 5% CO2. Non-adherent cells removed by replacing medium; cells fed every 3 days by feeding with DMEM solution. |
| Sarveazad et al. (2014) [37] | Human-derived ADSC therapy and chondroitinase ABC therapy were compared in terms of their impacts on functional recovery following SCI in rat models. | Human-derived ADSCs | 24 | Formed plate following centrifuging process cultured in DMEM/Ham’s F-12, 10% FBS and 1% P/S. Flasks incubated at 37 °C, 5% CO2 and 98% humidity. |
| Junior et al. (2020) [38] | Human-derived ADSCs were implanted into rats following SCI, the impacts on locomotor recovery and bladder function were measured. | Human-derived ADSCs | 63 | Details of culture conditions unclear. |
| Li et al. (2022) [39] | Human-derived ADSCs and nano hydrogels were implanted into rats following SCI, the impact upon functional recovery was then measured. | Human-derived ADSCs | 32 | Cells cultured in ADSC complete medium. Culture changed one day after seeding, then every 3 days thereafter, for a total culture time of 7 days. |
| Zhang et al. (2024) [40] | Rat-derived ADSCs were implanted into SCI rats, the impact on functional recovery and PGRN expression was monitored. | Rat-derived ADSCs | 30 | Primary ADSCs cultured in DMEM, 10% FBS and 1% P/S. |
| Li et al. (2021) [41] | Human-derived ADSCs were implanted into rats following SCI, the impacts upon locomotor recovery and relevant molecular pathways were investigated. | Human-derived ADSCs | 30 | ADSCs cultured in alpha-MEM, 37 °C and 5% CO2. |
| Oh et al. (2012) [42] | Human-derived ADSC clusters were implanted into rats following SCI, the impact on locomotor recovery was examined. | Human-derived ADSCs | 30 | Cells cultured in DMEM/F-12. Plated in tissue flasks in culture conditions of humidified air at 37 °C 5% CO2 and 95% air. Culture changed to remove non-adherent cells, and media changed every other day. |
| References | Characterisation Method | Results |
|---|---|---|
| Aras et al. (2016) [32] | Flow cytometry | CD29, CD54 and CD90 expressed in rat-derived ADSCs. |
| Ohta et al. (2017) [33] | Flow cytometry and mRNA characterisation (RT-PCR) | Beta-3 tubulin and GFAP expression not significantly altered with regard to ADSC passage number. Significant reduction in nestin expression with regards to increasing passage number. |
| Zhou et al. (2013) [30] | Flowcytometry, immunocytochemistry and RT-PCR | CD29, CD44, CD90 and CD105 expressed in ADSCs. Cells positive for vimentin and BDNF. Nestin expressed in ADSCs at 10.25% ± 4.5%; Ki67 expressed in ADSCs at 57.22% ± 5.76%. |
| Sarveazad et al. (2017) [34] | Flow cytometry | CD44, CD73 and CD90 expressed in ADSCs. |
| Takahara et al. (2024) [28] | Flow cytometry | CD29 and CD90 expressed in ADSCs. |
| Zhang et al. (2009) [27] | Immunocytochemistry | Nestin, vimentin, CD44 and beta-tubulin III expressed in undifferentiated ADSCs. |
| Yousefifard et al. (2022) [31] | Flow cytometry | CD44, CD73 and CD166 expressed in ADSCs. |
| Ryu et al. (2009) [29] | Flow cytometry | First passage ADSCs expressed CD44, CD90, CD105 and MHC class I; partially positive for CD34. Seventh passage of ASSCs expressed CD44, CD90 and CD105. |
| Takahashi et al. (2023) [35] | Flow cytometry | Third passage ADSCs positive CD29 and CD90. |
| Min et al. (2017) [36] | Flow cytometry | ADSCs expressed CD73 and CD105 at rates of 90.1% and 98.6%. |
| Sarveazad et al. (2014) [37] | Flow cytometry | CD44, CD73 and CD90 expressed in ADSCs. |
| Junior et al. (2020) [38] | Immunophenotyping | CD29, CD73, CD90 and CD105 expressed in ADSCs. |
| Li et al. (2022) [39] | Flow cytometry | Fifth generation ADSCs expressed CD73, CD90 and CD105 > 95%. |
| Zhang et al. (2024) [40] | Flow cytometry | CD44 and CD90 expressed by ADSCs. |
| Li et al. (2021) [41] | Flow cytometry | ADSCs expressed high levels of CD13, CD44, CD73, CD90 and CD105. |
| Oh et al. (2012) [42] | Immunofluorescence | ADSCs between passages 3–5 expressed human CD29, CD90 and CD105. |
| References | SCI Injury Level | SCI Injury Method |
|---|---|---|
| Aras et al. (2016) [32] | T10–T11 | Severe SCI trauma. |
| Ohta et al. (2017) [33] | T10 exposed by laminectomy | Weight-drop method: 10 g weight from 25 mm height onto exposed spinal cord. |
| Zhou et al. (2013) [30] | Bilateral T9 exposure by dorsal laminectomy | CST, RST and dorsal columns cut with microscissors to depth of central canal. |
| Sarveazad et al. (2017) [34] | T8–T9 exposure by laminectomy | Incision and piston method, causing moderate contusion SCI. 10 g piston released from 12.5 cm distance. |
| Takahara et al. (2024) [28] | SCI induced by aortic occlusion/reperfusion leading to infarction [multi-level injury] | TairaMarsala technique: 2F Fogarty catheter introduced through left femoral artery for aortic occlusion. 20G catheter in left common carotid artery for blood withdrawal. Blood pressure maintained at 40 mmHg during 10 min occlusion period. |
| Zhang et al. (2009) [27] | T9–T10 exposure by laminectomy | Weight-drop method: 10 g rod, 2.5 mm in diameter dropped from 12.5 mm height. |
| Yousefifard et al. (2022) [31] | T6–T8 exposure by laminectomy | Moderate clip compression method: 60 s aneurysm clip compression at pressure 20 g/cm2. |
| Ryu et al. (2009) [29] | L4 exposure by hemilaminectomy | Epidural balloon catheter method, 12 h injury period. |
| Takahashi et al. (2023) [35] | T9–T10 exposure by total laminectomy | Contusion SCI: ACI device at 250 kD leading to severe SCI. |
| Min et al. (2017) [36] | T7 [bilateral dorsal laminectomy] | Contusion SCI: infinite horizon impactor device with 200 kdyn force. |
| Sarveazad et al. (2014) [37] | T8–T9 exposure by laminectomy | Weight drop; 10 g 2 mm diameter cylinder released from 12.5 cm distance. |
| Junior et al. (2020) [38] | Thoracolumbar region | Balloon-induced compressive SCI; filled with 80 microlitres saline. |
| Li et al. (2022) [39] | T10 exposure by laminectomy | Piston-induced SCI; 2.2 mm diameter impactor at 1.4 mm impact depth and 0.6 s residence time. |
| Zhang et al. (2024) [40] | T10 exposure | Contusional SCI; 10 g weight drop from 2.5 cm height. |
| Li et al. (2021) [41] | T10 exposure by laminectomy | Bulldog clamp method; 30 s injury period. |
| Oh et al. (2012) [42] | T9 exposure by laminectomy | Clip compression SCI; 10 min injury period. |
| Reference | Experimental Groups | Results |
|---|---|---|
| Aras et al. (2016) [32] | L + T L + T + PSH/Hyperacute L + T + MSC/Hyperacute L + T and L + T + PSH/Acute L + T & L + T + MSC/Acute | MSC therapy consistently resulted in significant difference in BBB scores overall. Significant differences found between following groups: L + T//L [p = 0.004 **] L + T//L + T + MSC/Hyperacute [p = 0.007 **] L + T//& L + T + MSC/Acute [p = 0.017 *] L + T + PSH/Hyperacute//L + T + MSC/Hyperacute [p = 0.0010 ***] L + T + PSH/Acute//L + T + MSC/Acute [p = 0.0014 **] No significant differences between L + T and L + T + PSH [both acute/hyperacute]. |
| Ohta et al. (2017) [33] | Saline treatment only ADSC therapy | Significantly higher BBB scores in ADSC group [all timepoints beyond 8 days, point of intervention]. p < 0.01 [*] at all timepoints therein, except 11-, 42-, 50-day timepoints [p < 0.05 [*]]. |
| Zhou et al. (2013) [30] | PBS control hBMSC hADSC | Significantly higher BBB scores in hADSC score vs. Control [all timepoints beyond 2 weeks, [p < 0.05 [*]]. Average BBB score of 12.5 in ADSC group, significantly higher than other 2 groups. No other significant differences between groups at any timepoint [not hADSC vs. hBMSC nor hBMSC vs. control]. One-way analysis of variance followed by Bonferroni post hoc testing. |
| Sarveazad et al. (2017) [34] | Sham SCI only hADSC ChABC hADSC + ChABC | Single treatment groups had significantly higher BBB scores compared to SCI only [all timepoints beyond 28 d in hADSC group; 14,21 + 63 d timepoints in ChABC group; p < 0.001]. Dual therapy group had significantly higher BBB scores compared to SCI only [all timepoints beyond 14 d] + compared to single treatment groups [all timepoints beyond 21 d]. [p < 0.001]. Treatment is superior to control, and dual treatment is superior to single therapy with respect to locomotor recovery. |
| Takahara et al. (2024) [28] | Control [PBS] ADSC | Significantly higher BBB scores in ADSC group vs. Control days 7 + 14 post-treatment [p < 0.05]. |
| Zhang et al. (2009) [27] | Sham control Saline control dADSC-P1 dADSC-P2 uADSC | Significant improvement in BBB score between cell-treated vs. Control groups at end of 12 weeks: p < 0.01: uADSC, dADSC-P2 p < 0.05: dADSC-P1 Significant difference between uADSC//dADSC-P2, dADSC-P1 groups [p < 0.05 both cases]. No other significant differences [recovery timecourses of uADSC//dADSC-P2 groups]. |
| Yousefifard et al. (2022) [31] | Intact Sham SCI only Vehicle [cell culture media] ADSC | Significantly improved BBB scores [ADSC group vs. SCI group, all timepoints beyond 4 weeks, p < 0.0001]. |
| Ryu et al. (2009) [29] | Control [SCI only] Vehicle [PBS] ADSC | Significantly higher Obly [modified BBB scores [ADSC group vs. Other groups, 5 + 9-week timepoints; p < 0.05]. ADSC group Olby scores increased 1 [3 W], 3.6 [5 W] and 4.6 [9 W]. Other groups consistently displayed Olby scores < 1. |
| Takahashi et al. (2023) [35] | Sham PBS no exercise PBS exercise ADSC no exercise ADSC exercise | Significantly higher BBB scores [ADSC-ex vs. All other groups at 7, 8, 10 weeks post-transplant; p < 0.05]. No other significant differences between groups, including ADSC no exercise vs. PBS [± exercise]. |
| Min et al. (2017) [36] | Sham GCSF ADSC ADSC + GSCF | At 8 W: ADSC, ADSC + GCSF groups had mean BBB scores of 17.37 ± 0.7 and 17.57 ± 0.5; significantly higher than GCSF, Sham; 16 ± 0.5 and 15.6 ± 0.5, respectively [p < 0.01]. No significant difference [ADSC//ADSC + GCSF groups nor GCSF//Control]. |
| Sarveazad et al. (2014) [37] | Control Sham ChABC hADSC | Significant difference between hADSCs//Control] 28 d, p < 0.01]. Significant difference in BBB score between Ch ABC//control [14 d, p < 0.001]. Significant difference between Ch ABC, hADSCs//Control [63 d, p < 0.01]. Intervention point at 7 days post-surgery. |
| Junior et al. (2020) [38] | Control hADSC hADSC + MPSS | Motor recovery at 21 d [hADSC] + 17 d [hADSC + MPSS] following second transplant. Median BBB score following motor stabilisation: 5.5 [hADSC] and 5.0 [hADSC + MPSS]; no subsequent regression of motor assessment. Significantly improved motor function [hADSC ± MPSS//Control]. p < 0.01. |
| Li et al. (2022) [39] | PBS ADSC RADA16-RGD ADSC + RADA16-RGD | Significantly higher BBB scores [ADSC + RADA16-RGD//All other groups] between 3 and 10 weeks. p values: ADSCs + RADA16-RGD//PBS: p < 0.01 at 4, 5, and 7 w; p < 0.001 after 3, 6, 8, 9, and 10 w. ADSCs + RADA16-RGD//ADSCs: p < 0.01 after 4 and 7 w; p < 0.001 after 3, 5, 6, 8, 9, and 10 w. ADSCs + RADA16-RGD//RADA16-RGD: p < 0.05 after 3, 4, 6, and 7 w; p < 0.01 after 8, 9, and 10 w. |
| Zhang et al. (2024) [40] | Sham SCI alone SCI + PBS SCI + ADSCs | Significantly higher BBB score in SCI + ADSC//SCI [p < 0.05 at 8 w, p < 0.01 at 9, 10 W]. Significantly higher BBB score in SCI + ADSC//SCI + PBS [p < 0.05 at 8, 10 W; p < 0.001 at 9 W]. |
| Li et al. (2021) [41] | Sham PBS SB ADSC ADSC + SB | Significant difference in BBB score [ADSC vs. Baseline at 3 d; at 7, 14 d, p < 0.05]. Significant difference in BBB score [ADSC + SB//Baseline at 3 d; 14, 21 d, p < 0.05]. Significant difference in BBB score [ADSC//ADSC + SB; at 28 d, p < 0.05]. |
| Oh et al. (2012) [42] | PBS 3DCM hADSC | Significant difference in BBB scores between 3DCM//hADSC, 3DCM//PBS, hADSC//3DCM beyond 3 W [p < 0.05]. Significant difference between 3DCM//hADSC, not hADSC//PBS at 1, 2 W [p < 0.05]. |
| Reference | Summary of Results | Significance |
|---|---|---|
| Junior et al. (2020) [38] | Reddish urine occurred in 43/63 (68.7%) rats for three days following SCI. Two animals (one from group A, one from B) urinary retention not responsive to bladder massage; cystocentesis resulted in death. Urinary continence recovery not observed in animals group A (control). Groups B (ADSC) and C (ADSC + MPSS) urinary continence recovery rates of 14/21 (66.66%) and 13/21 (61.9%); average of 9 days from the second transplant. Significant difference between A and B regarding urinary continence [p < 0.01], no significant difference between groups B and C [p < 0.0657]. | Evidence that ADSC therapy promotes statistically significant increases recovery of bladder function following SCI, as compared to control. No evidence that adjuvant therapy with MPSS yields additive effects with respect to ADSC therapy in terms of bladder function. |
| Zhang et al. (2024) [40] | Bladder histology examined following SCI. ADSC group showed less significant histological damage to bladder tissue (HE + Masson staining). ADSC groups displayed lesser degree of following histological changes: epithelial layer of bladder sphincter mucosa thickening, mucosal epithelial shedding, inflammatory cell infiltrate (lamina propria), elastic fibre reduction, collagen deposition, enlarged smooth muscle cell nuclei, muscle cell disorder and bladder stone formation. | Indication ADSC therapy reduces extent of damage following SCI with respect to bladder histology. Likely translates to improved bladder function. Statistical significance not quantified. Histology less severe (ADSC group), not identical to sham. |
| Study ID | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Randomised Sequence generation | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Baseline characteristics | 2 | 1 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 3 | 2 |
| Allocation concealment | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Random housing | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 |
| Blinding of allocation | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| Random outcome assessment | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| Blinding of assessment | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| Attrition bias | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 3 | 2 | 3 | 2 | 3 | 2 |
| Reporting bias | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Other sources of bias | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, F.; Ahmed, T.; Iqbal, H.; Vogt, A.; Khan, W. Adipocyte-Derived Stem Cells in the Treatment of Spinal Cord Injuries in Animal Models: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 9330. https://doi.org/10.3390/ijms26199330
Jamil F, Ahmed T, Iqbal H, Vogt A, Khan W. Adipocyte-Derived Stem Cells in the Treatment of Spinal Cord Injuries in Animal Models: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(19):9330. https://doi.org/10.3390/ijms26199330
Chicago/Turabian StyleJamil, Faraz, Taha Ahmed, Hamzah Iqbal, Antonia Vogt, and Wasim Khan. 2025. "Adipocyte-Derived Stem Cells in the Treatment of Spinal Cord Injuries in Animal Models: A Systematic Review" International Journal of Molecular Sciences 26, no. 19: 9330. https://doi.org/10.3390/ijms26199330
APA StyleJamil, F., Ahmed, T., Iqbal, H., Vogt, A., & Khan, W. (2025). Adipocyte-Derived Stem Cells in the Treatment of Spinal Cord Injuries in Animal Models: A Systematic Review. International Journal of Molecular Sciences, 26(19), 9330. https://doi.org/10.3390/ijms26199330







