Safety and Regenerative Properties of Immortalized Human Mesenchymal Stromal Cell Secretome
Abstract
1. Introduction
2. Results
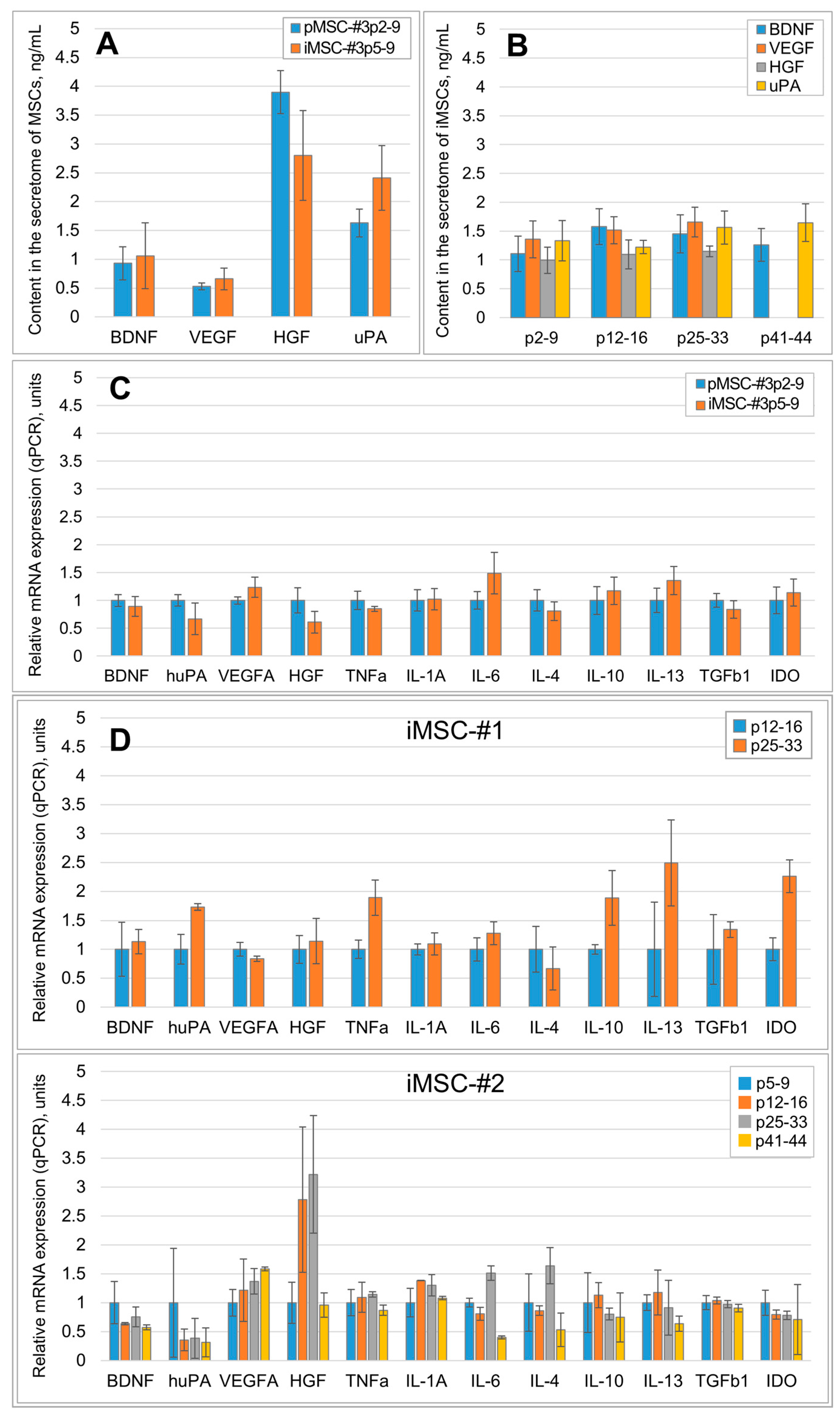
2.1. Immortalization Stabilizes the Qualitative and Quantitative Composition of the MSC Secretome
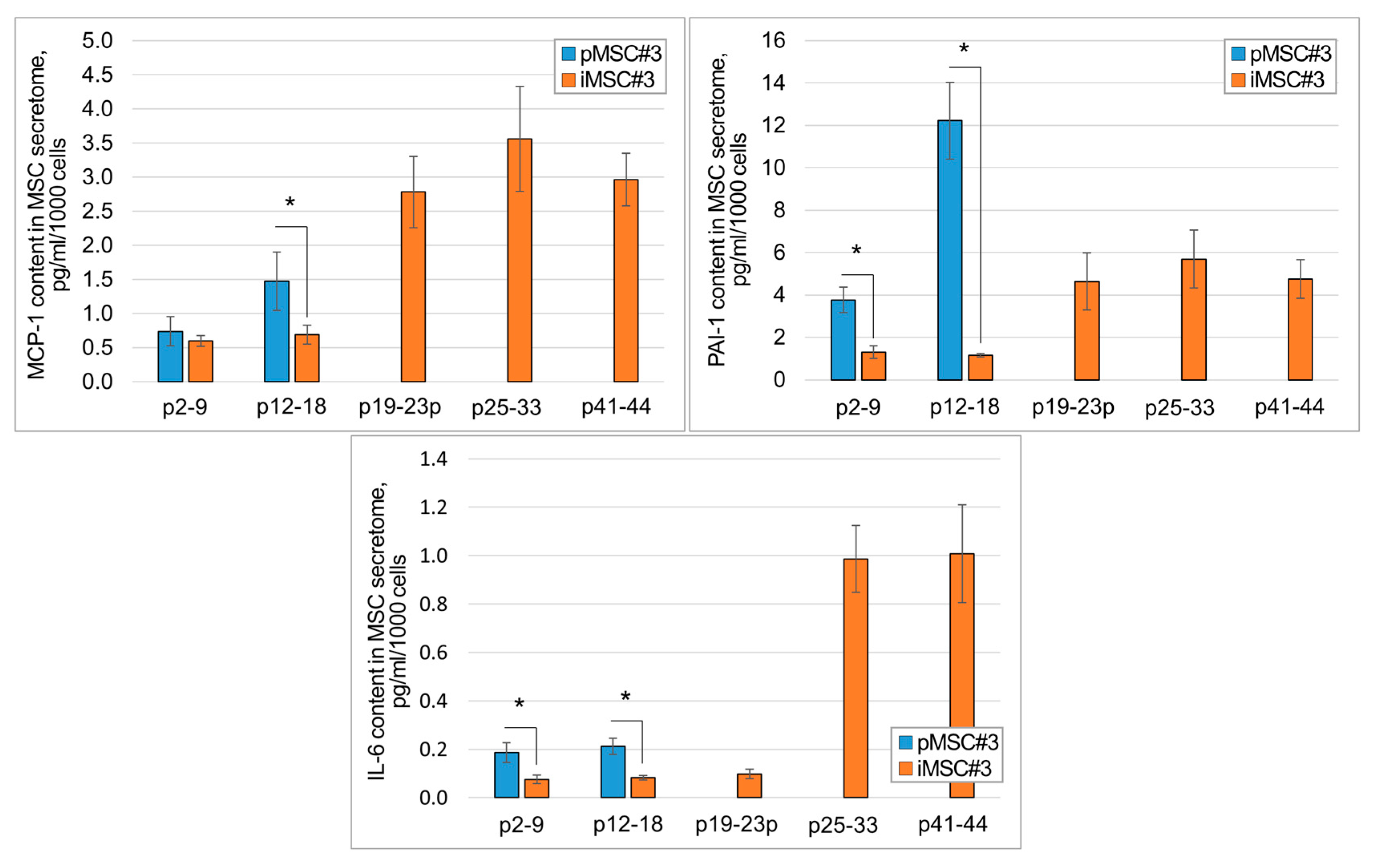
2.2. SASP Components Appear in the iMSC Secretome Later than in the Secretome of pMSCs
2.3. The iMSC Secretome Reveals the Same Pro-Regenerative Activity as the Secretome of pMSCs
2.3.1. iMSC Secretome Stimulates Secretory Activity of Leydig Cells
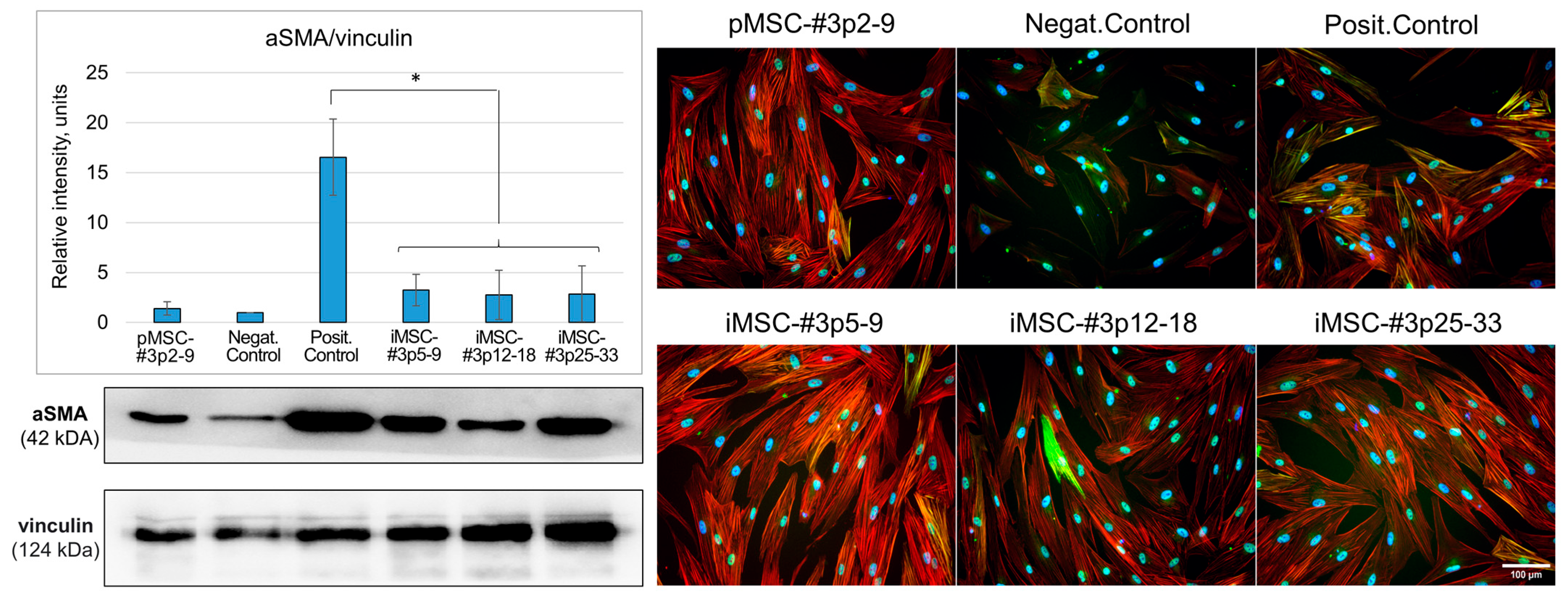
2.3.2. iMSC Extracellular Vesicles Prevent Fibrosis in the Model of Fibroblast to Myofibroblast Differentiation In Vitro
2.3.3. iMSC Secretome Stimulates Neurite Growth of Murine Sensory Ganglions
2.4. The Secretome of Immortalized MSCs Does Not Contain Detectable Amounts of Telomerase and Reveals No Transforming Activity
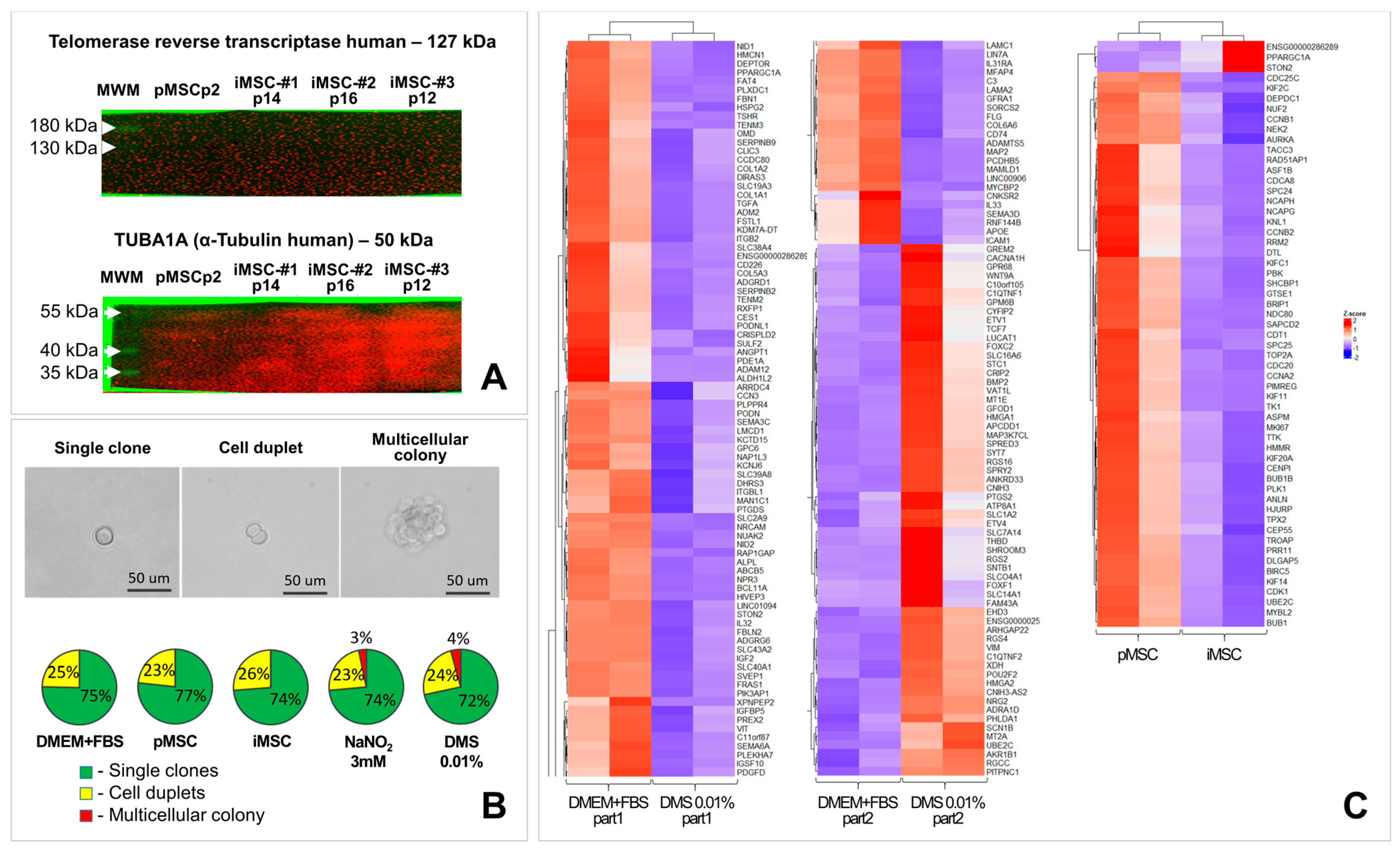
2.4.1. The iMSC Secretome Does Not Contain Detectable Amounts of Telomerase Protein or Telomerase Encoding Nucleic Acids
2.4.2. iMSC Secretome Does Not Induce Fibroblast Colony Formation in Soft Agar Colony Formation Assay
2.4.3. The iMSC Secretome Does Not Change the Expression of Pro- and Anti-Oncogenes in the Culture of Primary Human Dermal Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Animals
4.3. Immortalization of Human MSC Culture
4.4. Obtaining of Secretome of pMSCs and iMSCs
4.5. MSC-EV Isolation, Characterization, Nanoparticle Tracking Analysis
4.6. Analysis of Qualitative and Quantitative Composition of Secretome of iMSC
4.7. RNA Isolation, Reverse Transcription, qPCR
4.8. Assessing the Pro-Regenerative Activity of the Secretome of iMSC on Potency Assays In Vitro
4.8.1. Stimulation of Testosterone Production by Leydig Cell Culture (Leydig Cell Culture-Based Potency Assay)
4.8.2. Prevention of Fibroblast-to-Myofibroblast Differentiation (In Vitro Model of Fibrosis)
4.8.3. Stimulation of Neurite Growth of Murine Sensory Ganglions (In Vitro Model of Neuritogenesis)
4.9. Study of the Potential Transforming Activity of Secretome of iMSC
4.9.1. Detection of Telomerase Protein and Nucleic Acids Encoding It in the Secretome
4.9.2. Soft Agar Colony Formation Assay
4.9.3. Transcriptomic Analysis of Primary Human Fibroblasts Treated with the iMSC Secretome
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | ANalysis Of VAriance |
| a-SMA | alpha Smooth Muscle Actin |
| BDNF | Brain-Derived Neurotrophic Factor |
| bFGF | basic Fibroblast Growth Factor |
| BSA | Bovine Serum Albumin |
| cDNA | coding DeoxyriboNucleic Acid |
| CTGF | Connective Tissue Growth Factor |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| DMS | Dimethyl Sulfate |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| EGF | Epidermal Growth Factor |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| MSC-EV | Mesenchymal Stromal Cell-Derived Extracellular Vesicles |
| FBS | Fetal Bovine Serum |
| GDNF | Glial Cell-Derived Neurotrophic Factor |
| GDF15 | Growth Differentiation Factor 15 |
| GMFB | Glial Macrocytic Factor Beta |
| HDF | Human Dermal Fibroblasts |
| HDGF | Hepatoma-Derived Growth Factor |
| HGF | Hepatocyte Growth Factor |
| HNRNPU | Heterogeneous Nuclear Ribonucleoprotein U |
| HSP70/74 | Heat Shock Protein 70/74 |
| HSP90B1 | Heat Shock Protein 90 Beta Family Member 1 |
| IDO | Indoleamine 2,3-Dioxygenase |
| IGF-I | Insulin-Like Growth Factor I |
| IgG | Immunoglobulin G |
| IL-1A/4/6/10/13 | Interleukin-1a/4/6/10/13 |
| iMSC | immortalized Mesenchymal Stromal Cells |
| ISCT | International Society for Cell Therapy |
| KGF | Keratinocyte Growth Factor |
| LIF | Leukemia Inhibitory Factor |
| MANF | Mesencephalic-Astrocyte-Derived Neurotrophic Factor |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MEGF8 | Multiple Epidermal Growth Factor-like Domains 8 |
| MSC | Mesenchymal Stromal Cells |
| MMP-2/-9 | Matrix Metalloproteinase-2/-9 |
| MWCO | Molecular Weight Cutoff |
| MYDGF | Myeloid-derived growth factor |
| NDNF | Neuron-Derived Neurotrophic Factor |
| NEGR1 | Neuronal Growth Regulator 1 |
| NENF | Neuron-Enriched Neurotrophic Factor |
| OLFML1 | Olfactomedin-Like 1 |
| OLFML3 | Olfactomedin-Like 3 |
| PAAG | Polyacrylamide Gel |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| PDGF | Platelet-Derived Growth Factor |
| PEDF | Pigment Epithelium-Derived Factor |
| PBS | Phosphate-Buffered Saline |
| PlGF | Placental Growth Factor |
| pMSCs | primary Mesenchymal Stromal Cells |
| P-RAM Iss | Peroxidase-labeled rabbit anti-mouse isotype control antibodies |
| PVDF | Polyvinylidene Fluoride |
| qPCR | quantitative Polymerase Chain Reaction |
| RFTN1 | Raftlin1 |
| RT | Room Temperature |
| SASP | Senescence-Associated Secretory Phenotype |
| SDF-1 | Stromal-Derived Factor 1 |
| TCN2 | Transcobalamin II |
| TERT | Telomerase Reverse Transcriptase |
| TGFb | Transforming Growth Factor Beta |
| TIMP-1/-2 | Tissue Inhibitor of Metalloproteinases-1/-2 |
| TNFa | Tumor Necrosis Factor Alpha |
| tPA | tissue Plasminogen Activator |
| TUBA1A | Tubulin Alpha 1A |
| uPA | urokinase-type Plasminogen Activator |
| VASN | Vasorin |
| VEGF | Vascular Endothelial Growth Factor |
References
- Jin, Y.; Li, S.; Yu, Q.; Chen, T.; Liu, D. Application of stem cells in regeneration medicine. MedComm 2023, 4, e291. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Iismaa, S.E.; Kaidonis, X.; Nicks, A.M.; Bogush, N.; Kikuchi, K.; Naqvi, N.; Harvey, R.P.; Husain, A.; Graham, R.M. Comparative regenerative mechanisms across different mammalian tissues. npj Regen. Med. 2018, 3, 6. [Google Scholar] [CrossRef]
- Yanagida, H.; Kaibori, M.; Hijikawa, T.; Kwon, A.H.; Kamiyama, Y.; Okumura, T. Administration of rhHGF-activator via portal vein stimulates the regeneration of cirrhotic liver after partial hepatectomy in rats. J. Surg. Res. 2006, 130, 38–44. [Google Scholar] [CrossRef]
- Terenghi, G. Peripheral nerve regeneration and neurotrophic factors. J. Anat. 1999, 194, 1–14. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 21, 469. [Google Scholar] [CrossRef]
- Farajollahi, M.M.; Hamzehlou, S.; Mehdipour, A.; Samadikuchaksaraei, A. Recombinant proteins: Hopes for tissue engineering. Bioimpacts 2012, 2, 123–125. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Sorby-Adams, A.; Marcoionni, A.; Dempsey, E.; Woenig, J.; Turner, R. The Role of Neurogenic Inflammation in Blood-Brain Barrier Disruption and Development of Cerebral Oedema Following Acute Central Nervous System (CNS) Injury. Int. J. Mol. Sci. 2017, 18, 1788. [Google Scholar] [CrossRef]
- Sifat, A.; Vaidya, B.; Abbruscato, T. Blood-Brain Barrier Protection as a Therapeutic Strategy for Acute Ischemic Stroke. AAPS J. 2017, 19, 957–972. [Google Scholar] [CrossRef]
- Bernardo-Castro, S.; Sousa, J.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of Blood-Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Front. Neurol. 2020, 11, 594672. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Dabrowska, S.; Lukomska, B.; Janowski, M. Mesenchymal Stem Cells for Neurological Disorders. Adv. Sci. 2021, 8, 2002944. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yang, H.; Kim, M.W.; Cho, K.S.; Kim, D.S.; Yim, H.E.; Atala, Z.; Ko, I.K.; Yoo, J.J. The Delivery of the Recombinant Protein Cocktail Identified by Stem Cell-Derived Secretome Analysis Accelerates Kidney Repair After Renal Ischemia-Reperfusion Injury. Front. Bioeng. Biotechnol. 2022, 10, 848679. [Google Scholar] [CrossRef]
- Tögel, F.; Westenfelder, C. The role of multipotent marrow stromal cells (MSCs) in tissue regeneration. Organogenesis 2011, 7, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, A.; Kozlowska, U. Mesenchymal Stromal Cells and Tissue-Specific Progenitor Cells: Their Role in Tissue Homeostasis. Stem Cells Int. 2016, 2016, 2044285215. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Kalinina, N.; Kharlampieva, D.; Loguinova, M.; Butenko, I.; Pobeguts, O.; Efimenko, A.; Ageeva, L.; Sharonov, G.; Ischenko, D.; Alekseev, D.; et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. Ther. 2015, 6, 221. [Google Scholar] [CrossRef]
- Makridakis, M.; Roubelakis, M.G.; Vlahou, A. Stem cells: Insights into the secretome. Biochim. Biophys. Acta-Proteins Proteom. 2013, 1834, 2380–2384. [Google Scholar] [CrossRef]
- González-González, A.; García-Sánchez, D.; Dotta, M.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J. Stem Cells 2020, 12, 1529–1552. [Google Scholar] [CrossRef]
- Lopatina, T.; Kalinina, N.; Karagyaur, M.; Stambolsky, D.; Rubina, K.; Revischin, A.; Pavlova, G.; Parfyonova, Y.; Tkachuk, V. Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 2011, 6, e17899, Erratum in PLoS ONE 2019, 14, e0219946. https://doi.org/10.1371/journal.pone.0219946.. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.; Wu, P.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886, Correction in PLoS ONE 2024, 19, e0302417. https://doi.org/10.1371/journal.pone.0302417. [Google Scholar] [CrossRef] [PubMed]
- Onda, T.; Honmou, O.; Harada, K.; Houkin, K.; Hamada, H.; Kocsis, J. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J. Cerebr. Blood Flow Metab. 2008, 28, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.; Chopp, M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 2013, 31, 2737–2746. [Google Scholar] [CrossRef]
- Estrada, R.; Li, N.; Sarojini, H.; An, J.; Lee, M.J.; Wang, E. Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J. Cell Physiol. 2009, 219, 563–571. [Google Scholar] [CrossRef]
- Nazarie, I.S.R.; Gharbia, S.; Hermenean, A.; Dinescu, S.; Costache, M. Regenerative Potential of Mesenchymal Stem Cells’ (MSCs) Secretome for Liver Fibrosis Therapies. Int. J. Mol. Sci. 2021, 22, 13292. [Google Scholar] [CrossRef]
- Kondziolka, D.; Steinberg, G.; Wechsler, L.; Meltzer, C.; Elder, E.; Gebel, J.; Decesare, S.; Jovin, T.; Zafonte, R.; Lebowitz, J.; et al. Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. J. Neurosurg. 2005, 103, 38–45. [Google Scholar] [CrossRef]
- Lee, R.; Pulin, A.; Seo, M.; Kota, D.; Ylostalo, J.; Larson, B.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Arabi, T.Z.; Almasry, Y.; Xue, A.; Eirin, A.; Lerman, A.; Zhu, X.Y.; Lerman, L.O. Immune rejection of human mesenchymal stem cells compared to extracellular vesicles in mice with renal artery stenosis. Stem Cells Transl. Med. 2025, 14, szaf015. [Google Scholar] [CrossRef]
- Ghaderi, A.; Abtahi, S. Mesenchymal Stem Cells: Miraculous Healers or Dormant Killers? Stem Cell Rev. Rep. 2018, 14, 722–733. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Alvarez-Grech, J.; Vallejo-Cremades, M.; Expósito-Alcaide, M.; Merino, J.; Roda, J.; Díez-Tejedor, E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience 2011, 175, 394–405. [Google Scholar] [CrossRef]
- Ma, H.; Siu, W.S.; Leung, P.C. The Potential of MSC-Based Cell-Free Therapy in Wound Healing-A Thorough Literature Review. Int. J. Mol. Sci. 2023, 24, 9356. [Google Scholar] [CrossRef]
- Lalu, M.; Montroy, J.; Dowlatshahi, D.; Hutton, B.; Juneau, P.; Wesch, N.Y.; Zhang, S.; McGinn, R.; Corbett, D.; Stewart, D.A.; et al. from the Lab to Patients: A Systematic Review and Meta-Analysis of Mesenchymal Stem Cell Therapy for Stroke. Transl. Stroke Res. 2020, 11, 345–364. [Google Scholar] [CrossRef]
- Efimenko, A.; Starostina, E.; Kalinina, N.; Stolzing, A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J. Transl. Med. 2011, 9, 10. [Google Scholar] [CrossRef]
- Petrenko, Y.; Vackova, I.; Kekulova, K.; Chudickova, M.; Koci, Z.; Turnovcova, K.; Kupcova Skalnikova, H.; Vodicka, P.; Kubinova, S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci. Rep. 2020, 10, 4290. [Google Scholar] [CrossRef]
- Cui, L.; Golubczyk, D.; Tolppanen, A.; Boltze, J.; Jolkkonen, J. Cell therapy for ischemic stroke: Are differences in preclinical and clinical study design responsible for the translational loss of efficacy? Ann. Neurol. 2019, 86, 5–16. [Google Scholar] [CrossRef]
- Li, W.; Shi, L.; Hu, B.; Hong, Y.; Zhang, H.; Li, X.; Zhang, Y. Mesenchymal Stem Cell-Based Therapy for Stroke: Current Understanding and Challenges. Front. Cell. Neurosci. 2021, 15, 628940. [Google Scholar] [CrossRef] [PubMed]
- Alegre, M.; Bartman, C.; Chong, A. Microbes, and allogeneic transplantation. Transplantation 2014, 97, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Song, J.; Huang, X.; Pan, Z.; Goldbrunner, R.; Stavrin, L.; Lin, S.; Hu, W.; Zheng, F.; Stavrinou, P. Exosomes Derived from Mesenchymal Stem Cells: Novel Effects in the Treatment of Ischemic Stroke. Front. Neurosci. 2022, 16, 899887. [Google Scholar] [CrossRef] [PubMed]
- Chouaib, B.; Haack-Sørensen, M.; Chaubron, F.; Cuisinier, F.; Collart-Dutilleul, P.Y. Towards the Standardization of Mesenchymal Stem Cell Secretome-Derived Product Manufacturing for Tissue Regeneration. Int. J. Mol. Sci. 2023, 24, 12594. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, N.; Tyurin-Kuzmin, P.; Karagyaur, M.; Akopyan, Z.; Kulebyakin, K. Practical Use of Immortalized Cells in Medicine: Current Advances and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 12716. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.X.; Mao, X.M.; Lin, D.C.; Hong, Y.H.; Liang, G.S.; Chen, Q.X.; Chen, Q.H. Establishment and characterization of immortalized human eutopic endometrial stromal cells. Am. J. Reprod. Immunol. 2020, 83, e13213. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.M.; McDaniel, L.D.; Wright, W.E.; Shay, J.W.; Schultz, R.A. The establishment of telomerase-immortalized cell lines representing human chromosome instability syndromes. Hum. Mol. Genet. 2000, 9, 403–411. [Google Scholar] [CrossRef]
- Chalak, M.; Hesaraki, M.; Mirbahari, S.N.; Yeganeh, M.; Abdi, S.; Rajabi, S.; Hemmatzadeh, F. Cell Immortality: In Vitro Effective Techniques to Achieve and Investigate Its Applications and Challenges. Life 2024, 14, 417. [Google Scholar] [CrossRef]
- Chin, J.S.; Tan, M.L.L.; Lim, P.L.K.; Sharma, B.; Yeo, A.; Aw, Y.B.; Ng, Y.Z.; Bonnard, C.; Becker, D.L.; Mok, P. Secretome from Prolonged High-Density Human Wharton’s Jelly Stem Cell Culture Accelerates Wound Healing in Both In Vitro and In Vivo Models. Int. Wound J. 2025, 22, e70033, Erratum in Int. Wound J. 2025, 22, e70706. https://doi.org/10.1111/iwj.70706. [Google Scholar] [CrossRef]
- O’Sullivan, R.J.; Karlseder, J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef]
- Zietzer, A.; Hosen, M.; Goody, P.; Werner, N.; Nickenig, G.; Jansen, F. HnRNPU regulates intra- and intercellular microRNA trafficking in a sequence specific manner. Eur. Heart J. 2020, 41, 3611. [Google Scholar] [CrossRef]
- Zietzer, A.; Hosen, M.R.; Wang, H.; Goody, P.R.; Sylvester, M.; Latz, E.; Nickenig, G.; Werner, N.; Jansen, F. The RNA-binding protein hnRNPU regulates the sorting of microRNA-30c-5p into large extracellular vesicles. J. Extracell. Vesicles 2020, 9, 1786967. [Google Scholar] [CrossRef]
- Jin, H.J.; Lee, H.J.; Heo, J.; Lim, J.; Kim, M.; Kim, M.K.; Nam, H.Y.; Hong, G.H.; Cho, Y.S.; Choi, S.J.; et al. Senescence-Associated MCP-1 Secretion Is Dependent on a Decline in BMI1 in Human Mesenchymal Stromal Cells. Antioxid. Redox Signal. 2016, 24, 471–485. [Google Scholar] [CrossRef]
- O’Hagan-Wong, K.; Nadeau, S.; Carrier-Leclerc, A.; Apablaza, F.; Hamdy, R.; Shum-Tim, D.; Rodier, F.; Colmegna, I. Increased IL-6 secretion by aged human mesenchymal stromal cells disrupts hematopoietic stem and progenitor cells’ homeostasis. Oncotarget 2016, 7, 13285–13926. [Google Scholar] [CrossRef]
- Monakova, A.; Sagaradze, G.; Basalova, N.; Popov, V.; Balabanyan, V.; Efimenko, A. Novel Potency Assay for MSC Secretome-Based Treatment of Idiopathic Male Infertility Employed Leydig Cells and Revealed Vascular Endothelial Growth Factor as a Promising Potency Marker. Int. J. Mol. Sci. 2022, 23, 9414. [Google Scholar] [CrossRef]
- Sagaradze, G.; Basalova, N.; Kirpatovsky, V.; Ohobotov, D.; Nimiritsky, P.; Grigorieva, O.; Popov, V.; Kamalov, A.; Tkachuk, V.; Efimenko, A. A magic kick for regeneration: Role of mesenchymal stromal cell secretome in spermatogonial stem cell niche recovery. Stem Cell Res. Ther. 2019, 10, 342. [Google Scholar] [CrossRef]
- Grigorieva, O.; Basalova, N.; Dyachkova, U.; Novoseletskaya, E.; Vigovskii, M.; Arbatskiy, M.; Kulebyakina, M.; Efimenko, A. Modeling the profibrotic microenvironment in vitro: Model validation. Biochem. Biophys. Res. Commun. 2024, 733, 150574. [Google Scholar] [CrossRef] [PubMed]
- Basalova, N.; Sagaradze, G.; Arbatskiy, M.; Evtushenko, E.; Kulebyakin, K.; Grigorieva, O.; Akopyan, Z.; Kalinina, N.; Efimenko, A. Secretome of Mesenchymal Stromal Cells Prevents Myofibroblasts Differentiation by Transferring Fibrosis-Associated microRNAs within Extracellular Vesicles. Cells 2020, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Horie, H.; Hikawa, N.; Takenaka, T. Isolation and age-related characterization of mouse Schwann cells from dorsal root ganglion explants in type I collagen gels. J. Neurosci. Res. 1993, 35, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.R.; Meyer, A.; Loerch, S.; Campbell, Z.T. Protocol for the isolation and culture of mouse dorsal root ganglion neurons for imaging applications. STAR Protoc. 2023, 4, 102717. [Google Scholar] [CrossRef]
- Hindle, J.; Williams, A.; Kim, Y.; Kim, D.; Patil, K.; Khatkar, P.; Osgood, Q.; Nelson, C.; Routenberg, D.A.; Howard, M.; et al. hTERT-Immortalized Mesenchymal Stem Cell-Derived Extracellular Vesicles: Large-Scale Manufacturing, Cargo Profiling, and Functional Effects in Retinal Epithelial Cells. Cells 2024, 13, 861. [Google Scholar] [CrossRef]
- Krawczenko, A.; Bielawska-Poh, A.; Paprocka, M.; Kraskiewicz, H.; Szyposzynska, A.; Wojdat, E.; Klimczak, A. Microvesicles from Human Immortalized Cell Lines of Endothelial Progenitor Cells and Mesenchymal Stem/Stromal Cells of Adipose Tissue Origin as Carriers of Bioactive Factors Facilitating Angiogenesis. Stem Cells Int. 2020, 2020, 1289380. [Google Scholar] [CrossRef]
- Brancolini, A.; Bobbili, M.R.; Pultar, M.; Mazidi, Z.; Wieser, M.; Gamauf, J.; Roefs, M.T.; Corso, G.; Nivarthi, H.; Paredes, M.B.A.; et al. Immortalization of mesenchymal stromal cells by hTERT does not affect the functional properties of secreted extracellular vesicles. bioRXiv 2024. [Google Scholar] [CrossRef]
- Karagyaur, M.; Dzhauari, S.; Basalova, N.; Aleksandrushkina, N.; Sagaradze, G.; Danilova, N.; Malkov, P.; Popov, V.; Skryabina, M.; Efimenko, A.; et al. MSC Secretome as a Promising Tool for Neuroprotection and Neuroregeneration in a Model of Intracerebral Hemorrhage. Pharmaceutics 2021, 13, 2031. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Choi, S.C.; Kim, J.H.; Choi, J.H.; Joo, H.J.; Hong, S.J.; Lim, D.S. Cardiac Stem Cell Secretome Protects Cardiomyocytes from Hypoxic Injury Partly via Monocyte Chemotactic Protein-1-Dependent Mechanism. Int. J. Mol. Sci. 2016, 17, 800. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Park, C.Y.; Kim, J.H.; Joo, H.J.; Choi, S.C.; Choi, J.H.; Lim, I.R.; Park, J.H.; Hong, S.J.; Lim, D.S. Cardioprotective effects of genetically engineered cardiac stem cells by spheroid formation on ischemic cardiomyocytes. Mol. Med. 2020, 26, 15. [Google Scholar] [CrossRef] [PubMed]
- Kraskiewicz, H.; Paprocka, M.; Bielawska-Pohl, A.; Krawczenko, A.; Panek, K.; Kaczyńska, J.; Szyposzyńska, A.; Psurski, M.; Kuropka, P.; Klimczak, A. Can supernatant from immortalized adipose tissue MSC replace cell therapy? An in vitro study in chronic wounds model. Stem Cell Res. Ther. 2020, 11, 29. [Google Scholar] [CrossRef]
- Miura-Yura, E.; Tsunekawa, S.; Naruse, K.; Nakamura, N.; Motegi, M.; Nakai-Shimoda, H.; Asano, S.; Kato, M.; Yamada, Y.; Izumoto-Akita, T. Secreted factors from cultured dental pulp stem cells promoted neurite outgrowth of dorsal root ganglion neurons and ameliorated neural functions in streptozotocin-induced diabetic mice. J. Diabetes Investig. 2020, 11, 28–38. [Google Scholar] [CrossRef]
- Primak, A.; Kalinina, N.; Skryabina, M.; Usachev, V.; Chechekhin, V.; Vigovskiy, M.; Chechekhina, E.; Voloshin, N.; Kulebyakin, K.; Kulebyakina, M.; et al. Novel Immortalized Human Multipotent Mesenchymal Stromal Cell Line for Studying Hormonal Signaling. Int. J. Mol. Sci. 2024, 25, 2421. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Asgarpour, K.; Shojaei, Z.; Amiri, F.; Ai, J.; Mahjoubin-Tehran, M.; Ghasemi, F.; ArefNezhad, R.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs derived from mesenchymal stem cells: Cell-to-cell messages. Cell Commun. Signal. 2020, 18, 149. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, J.; Zhang, F.; Liu, L.; Hu, C. MSCs can be a double-edged sword in tumorigenesis. Front. Oncol. 2022, 12, 1047907. [Google Scholar] [CrossRef]
- Venugopal, C.; Wang, X.S.; Manoranjan, B.; McFarlane, N.; Nolte, S.; Li, M.; Murty, N.; Siu, K.W.; Singh, S.K. GBM secretome induces transient transformation of human neural precursor cells. J. Neurooncol. 2012, 109, 457–466. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Horio, E.; Sonoda, J.; Yamagishi, M.; Miyakawa, S.; Murakami, F.; Hasegawa, H.; Katahira, Y.; Mizoguchi, I.; Fujii, Y.; et al. Immortalization of Mesenchymal Stem Cells for Application in Regenerative Medicine and Their Potential Risks of Tumorigenesis. Int. J. Mol. Sci. 2024, 25, 13562. [Google Scholar] [CrossRef]
- Padgaonkar, M.; Shendre, S.; Chatterjee, P.; Banerjee, S. Cancer secretome: Finding out hidden messages in extracellular secretions. Clin. Transl. Oncol. 2023, 25, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- López de Andrés, J.; Griñán-Lisón, C.; Jiménez, G.; Marchal, J.A. Cancer stem cell secretome in the tumor microenvironment: A key point for an effective personalized cancer treatment. J. Hematol. Oncol. 2020, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, B.; Jahanafrooz, Z.; Allahdadi, S.; Daryani, S.; Dehghani, Z.; Sadeghi, M.; Pedram, M.S.; Dehghan, M.M. Transcriptomic and in vivo approaches introduced human iPSC-derived microvesicles for skin rejuvenation. Sci. Rep. 2023, 13, 9963. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Dzhauari, S.S.; Primak, A.L.; Basalova, N.A.; Kalinina, N.I.; Monakova, A.O.; Bozov, K.D.; Velichko, A.Y.; Illarionova, M.E.; Grigorieva, O.A.; Akopyan, Z.A.; et al. Overexpression of BDNF and uPA Combined with the Suppression of Von Hippel-Lindau Tumor Suppressor Enhances the Neuroprotective Activity of the Secretome of Human Mesenchymal Stromal Cells in the Model of Intracerebral Hemorrhage. Int. J. Mol. Sci. 2025, 26, 6697. [Google Scholar] [CrossRef]
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int. J. Mol. Sci. 2019, 20, 1656. [Google Scholar] [CrossRef]
- Basalova, N.; Arbatskiy, M.; Popov, V.; Grigorieva, O.; Vigovskiy, M.; Zaytsev, I.; Novoseletskaya, E.; Sagaradze, G.; Danilova, N.; Malkov, P.; et al. Mesenchymal stromal cells facilitate resolution of pulmonary fibrosis by miR-29c and miR-129 intercellular transfer. Exp. Mol. Med. 2023, 55, 1399–1412. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404, Correction in J. Extracell. Vesicles 2024, 13, e12451. https://doi.org/10.1002/jev2.12451. [Google Scholar] [CrossRef]
- ASTM E2834-12; Standard Guide for Measurement of Particle Size Distribution of Nanomaterials in Suspension by Nanoparticle Tracking Analysis (NTA). ASTM: West Conshohocken, PA, USA, 2018.
- Kulebyakina, M.A.; Basalova, N.; Butuzova, D.; Arbatsky, M.; Chechekhin, V.; Kalinina, N.; Tyurin-Kuzmin, P.; Kulebyakin, K.; Klychnikov, O.; Efimenko, A. Balance between Pro- and Antifibrotic Proteins in Mesenchymal Stromal Cell Secretome Fractions Revealed by Proteome and Cell Subpopulation Analysis. Int. J. Mol. Sci. 2023, 25, 290. [Google Scholar] [CrossRef] [PubMed]
- Avraham, O.; Feng, R.; Ewan, E.E.; Rustenhoven, J.; Zhao, G.; Cavalli, V. Profiling sensory neuron microenvironment after peripheral and central axon injury reveals key pathways for neural repair. eLife 2021, 10, e68457. [Google Scholar] [CrossRef] [PubMed]
- Korzhevskii, D.E.; Grigor’ev, I.P.; Gusel’nikova, V.V.; Kolos, E.A.; Petrova, E.S.; Kirik, O.V.; Sufieva, D.A.; Razenkova, V.A.; Antipova, M.V.; Chernysh, M.V. Immunohistochemical markers for neurobiology. Med. Acad. J. 2019, 19, 7–24. [Google Scholar] [CrossRef]
- Mender, I.; Shay, J.W. Telomerase Repeated Amplification Protocol (TRAP). Bio-Protocol 2015, 5, e1657. [Google Scholar] [CrossRef]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S.; Karuppusamy Rathinam, M.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The soft agar colony formation assay. J. Vis. Exp. 2014, e51998. [Google Scholar] [CrossRef]
- Mykhailenko, V.; Kyrychenko, V.; Bragin, A.; Chuiko, D. Generation, Evaluation, and Prospects of Further Use of Mutations Based on New Homozygous Self-Pollinated Sunflower Lines. In Genotoxicity and Mutagenicity-Mechanisms and Test Methods; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Kodama, F.; Umeda, M.; Tsutsui, T. Mutagenic effect of sodium nitrite on cultured mouse cells. Mutat. Res. 1976, 40, 119–124. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karagyaur, M.; Primak, A.; Basalova, N.; Monakova, A.; Tolstoluzhinskaya, A.; Kulebyakina, M.; Chechekhina, E.; Skryabina, M.; Grigorieva, O.; Chechekhin, V.; et al. Safety and Regenerative Properties of Immortalized Human Mesenchymal Stromal Cell Secretome. Int. J. Mol. Sci. 2025, 26, 9322. https://doi.org/10.3390/ijms26199322
Karagyaur M, Primak A, Basalova N, Monakova A, Tolstoluzhinskaya A, Kulebyakina M, Chechekhina E, Skryabina M, Grigorieva O, Chechekhin V, et al. Safety and Regenerative Properties of Immortalized Human Mesenchymal Stromal Cell Secretome. International Journal of Molecular Sciences. 2025; 26(19):9322. https://doi.org/10.3390/ijms26199322
Chicago/Turabian StyleKaragyaur, Maxim, Alexandra Primak, Nataliya Basalova, Anna Monakova, Anastasia Tolstoluzhinskaya, Maria Kulebyakina, Elizaveta Chechekhina, Mariya Skryabina, Olga Grigorieva, Vadim Chechekhin, and et al. 2025. "Safety and Regenerative Properties of Immortalized Human Mesenchymal Stromal Cell Secretome" International Journal of Molecular Sciences 26, no. 19: 9322. https://doi.org/10.3390/ijms26199322
APA StyleKaragyaur, M., Primak, A., Basalova, N., Monakova, A., Tolstoluzhinskaya, A., Kulebyakina, M., Chechekhina, E., Skryabina, M., Grigorieva, O., Chechekhin, V., Yakovleva, T., Turilova, V., Shagimardanova, E., Gazizova, G., Vigovskiy, M., Kulebyakin, K., Sysoeva, V., Dyachkova, U., Dzhauari, S., ... Tkachuk, V. (2025). Safety and Regenerative Properties of Immortalized Human Mesenchymal Stromal Cell Secretome. International Journal of Molecular Sciences, 26(19), 9322. https://doi.org/10.3390/ijms26199322






