Stefin A Regulation of Cathepsin B Expression and Localization in Cancerous and Non-Cancerous Cells
Abstract
1. Introduction
2. Results
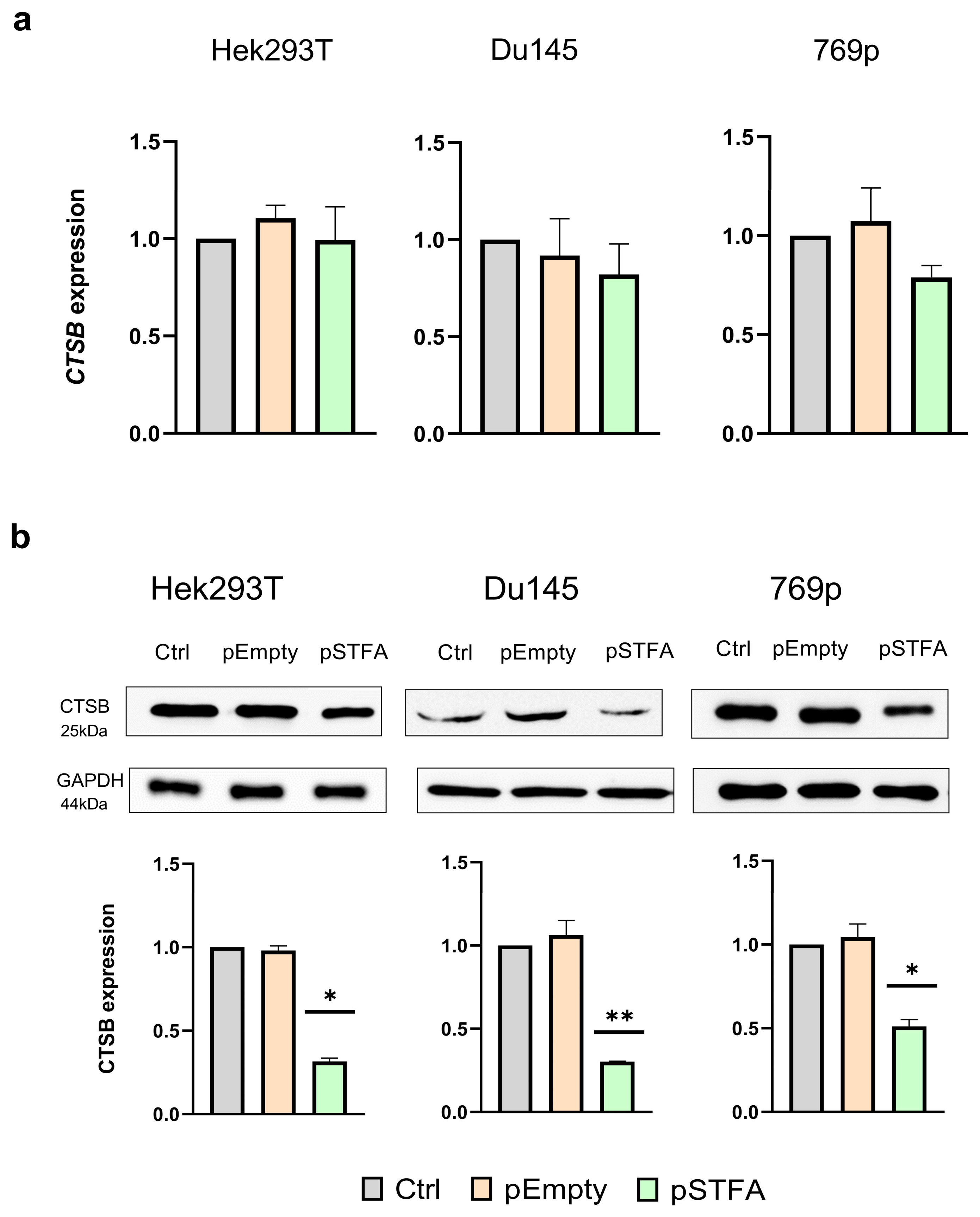
2.1. Effect of STFA on CTSB mRNA and Protein Expression
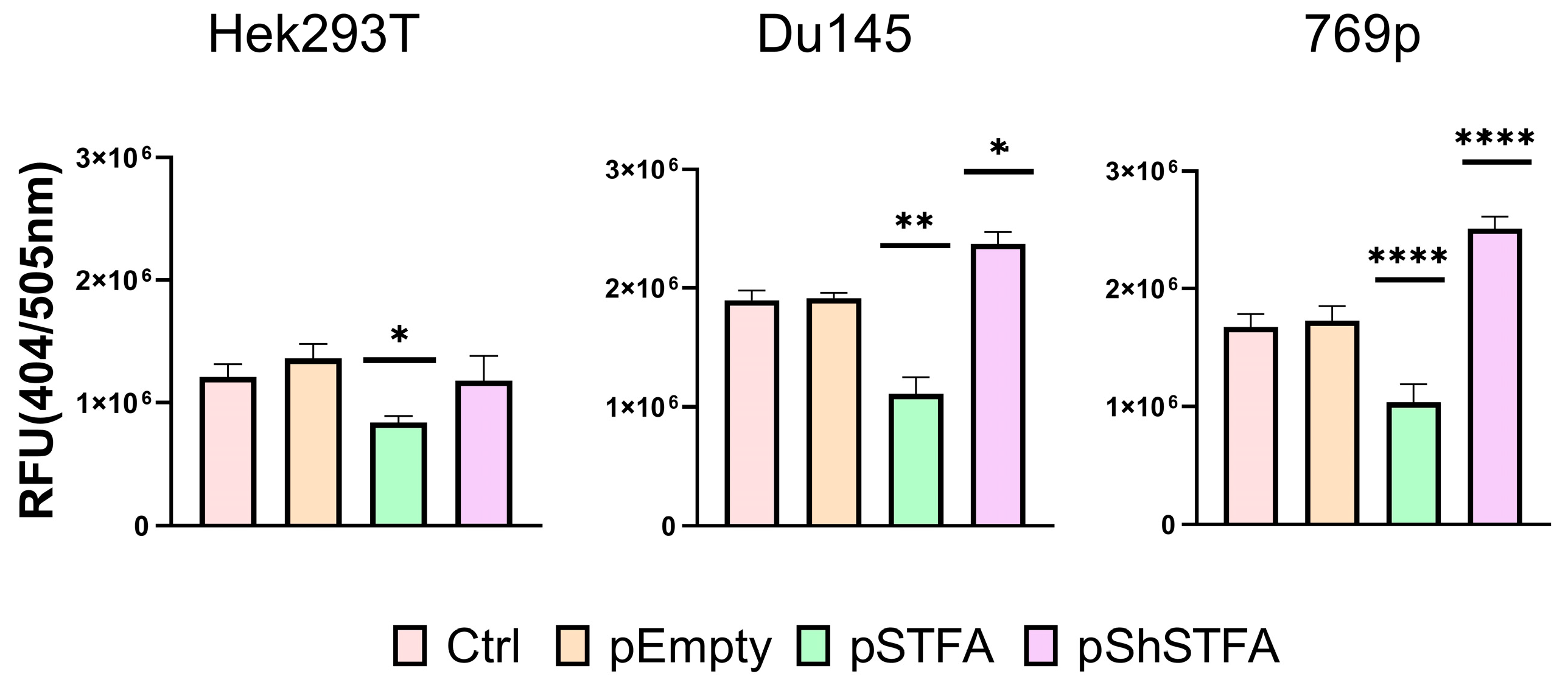
2.2. CTSB Activity Is Dependent on STFA Expression
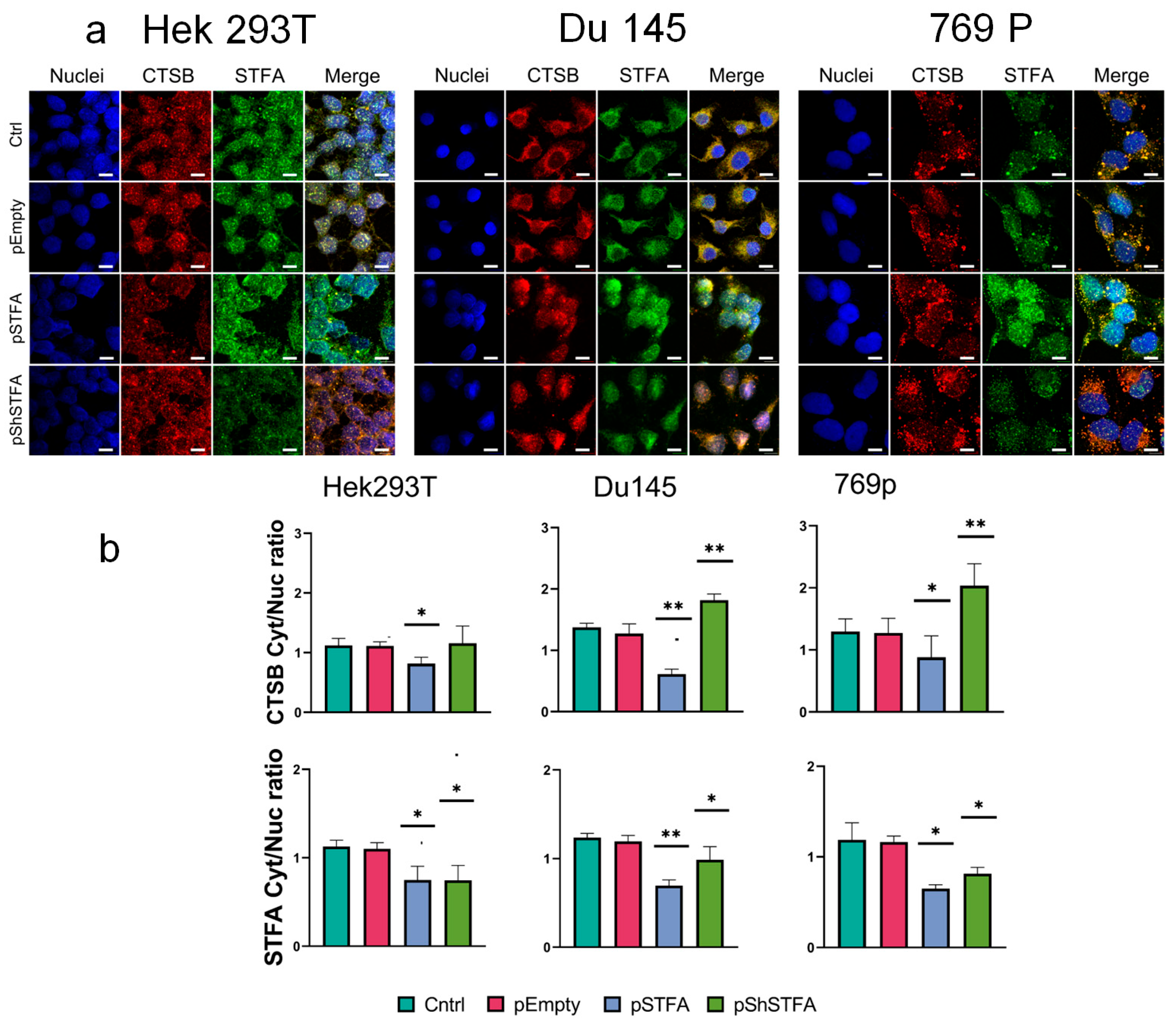
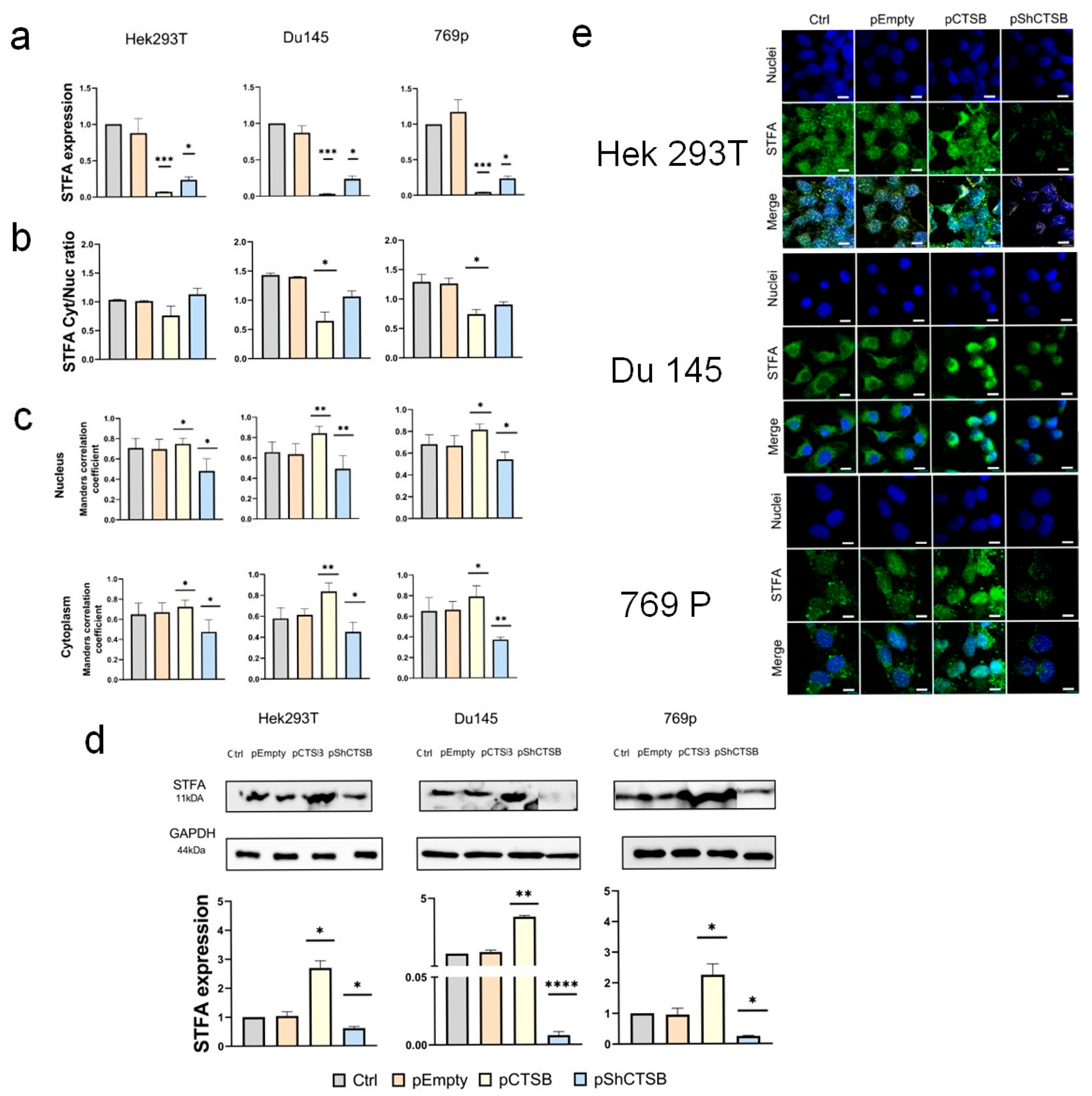
2.3. CTSB Biodistribution Is Dependent on STFA Expression
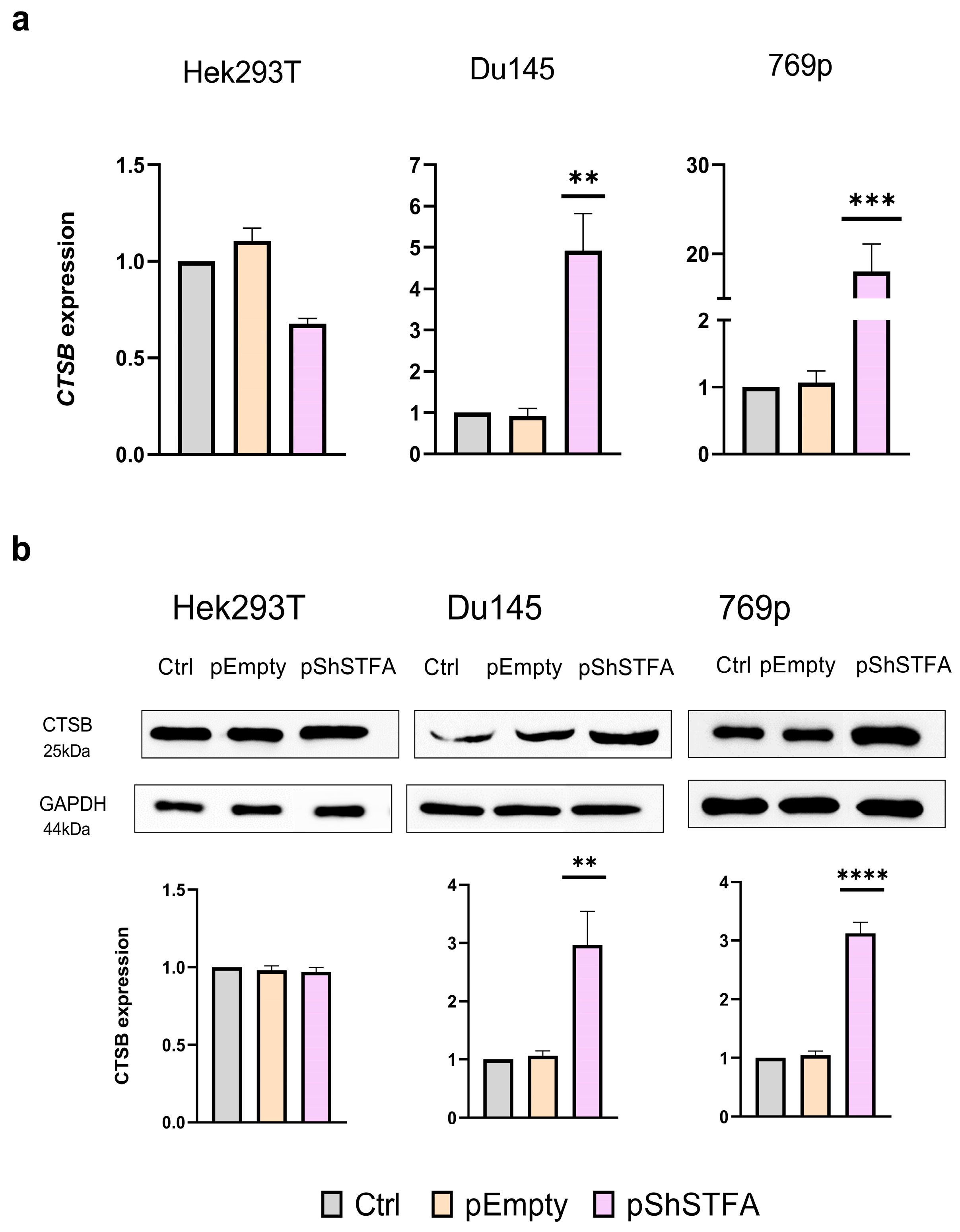
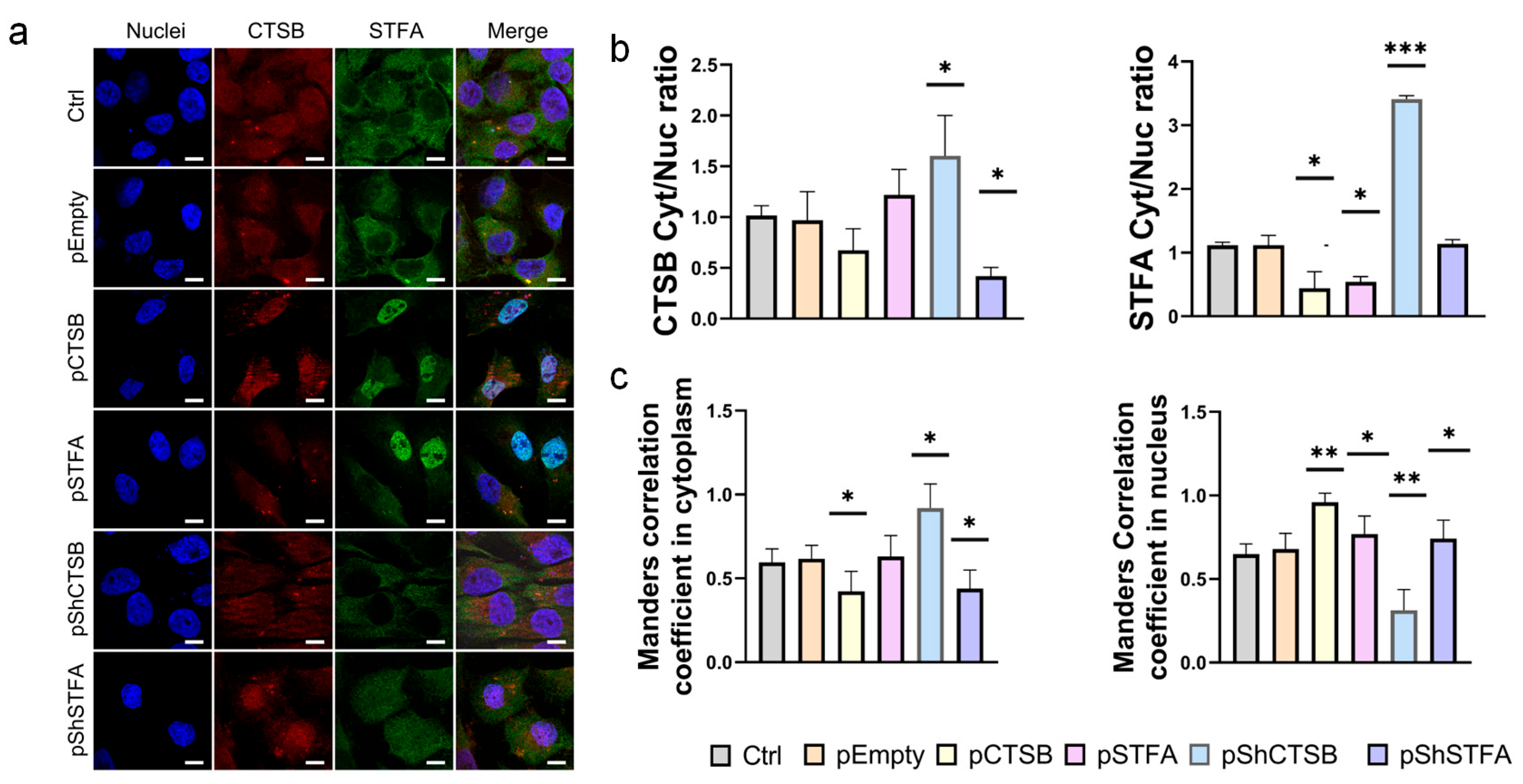
2.4. Effect of CTSB on STFA Expression and Distribution
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Transfection
4.3. Cathepsin B Activity Assay
4.4. RNA Isolation and Real-Time Polymerase Chain Reaction (RT-qPCR)
4.5. Western Blotting
4.6. Immunofluorescent Staining
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Z.; Xu, S.; Liu, C. Cathepsin B in cardiovascular disease: Underlying mechanisms and therapeutic strategies. J. Cell. Mol. Med. 2024, 28, e70064. [Google Scholar] [CrossRef] [PubMed]
- Zamyatnin, A.A., Jr.; Gregory, L.C.; Townsend, P.A.; Soond, S.M. Beyond basic research: The contribution of cathepsin B to cancer development, diagnosis and therapy. Expert Opin. Ther. Targets 2022, 26, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, M.; Yang, X.; Zhou, X.; Zhang, S. The role of cathepsin B in pathophysiologies of non-tumor and tumor tissues: A systematic review. J. Cancer 2023, 14, 2344. [Google Scholar] [CrossRef]
- Wang, H.; Inoue, A.; Lei, Y.; Wu, H.; Hong, L.; Cheng, X.W. Cathepsins in the extracellular space: Focusing on non-lysosomal proteolytic functions with clinical implications. Cell. Signal. 2023, 103, 110531. [Google Scholar] [CrossRef]
- Aggarwal, N.; Sloane, B.F. Cathepsin B: Multiple roles in cancer. Proteom.-Clin. Appl. 2014, 8, 427–437. [Google Scholar] [CrossRef]
- Cavallo-Medved, D.; Moin, K.; Sloane, B. Cathepsin B: Basis sequence: Mouse. AFCS-Nat. Mol. Pages 2011, 2011, A000508. [Google Scholar]
- Mendoza-Palomares, C.; Biteau, N.; Giroud, C.; Coustou, V.; Coetzer, T.; Authié, E.; Boulangé, A.; Baltz, T. Molecular and biochemical characterization of a cathepsin B-like protease family unique to Trypanosoma congolense. Eukaryot. Cell 2008, 7, 684–697. [Google Scholar] [CrossRef]
- Han, S.J.; Lee, H.T. Mechanisms and therapeutic targets of ischemic acute kidney injury. Kidney Res. Clin. Pract. 2019, 38, 427. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X. Cathepsin B as a target in cancer therapy and imaging. New J. Chem. 2022, 46, 19593–19611. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, T.; Zhang, S.; Li, Y.; Chen, Y.; Miao, Z.; Wang, X.; Yang, G.; Li, Q.; Zhang, L. Cathepsin B: The dawn of tumor therapy. Eur. J. Med. Chem. 2024, 269, 116329. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tang, Q.; Yan, X.; Xie, W.; Xu, Y.; Zhang, W. Cathepsins and neurological diseases: A Mendelian randomization study. Front. Neurosci. 2024, 18, 1454369. [Google Scholar] [CrossRef]
- Kryczka, J.; Papiewska-Pajak, I.; Kowalska, M.A.; Boncela, J. Cathepsin B is upregulated and mediates ECM degradation in colon adenocarcinoma HT29 cells overexpressing snail. Cells 2019, 8, 203. [Google Scholar] [CrossRef]
- Egorova, V.S.; Kolesova, E.P.; Lopus, M.; Yan, N.; Parodi, A.; Zamyatnin, A.A., Jr. Smart delivery systems responsive to cathepsin B activity for cancer treatment. Pharmaceutics 2023, 15, 1848. [Google Scholar] [CrossRef] [PubMed]
- Syrocheva, A.O.; Gorbacheva, V.I.; Egorova, V.S.; Zamyatnin, A.A.; Parodi, A.; Kolesova, E.P. Inorganic Silica Nanoparticles Increase Lysosomal Biology and Protease Activity. Int. J. Mol. Sci. 2025, 26, 8291. [Google Scholar] [CrossRef] [PubMed]
- Bien, S.; Ritter, C.A.; Gratz, M.; Sperker, B.; Sonnemann, J.; Beck, J.F.; Kroemer, H.K. Nuclear factor-κB mediates up-regulation of cathepsin B by doxorubicin in tumor cells. Mol. Pharmacol. 2004, 65, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Rudzinska-Radecka, M.; Frolova, A.S.; Balakireva, A.V.; Gorokhovets, N.V.; Pokrovsky, V.S.; Sokolova, D.V.; Korolev, D.O.; Potoldykova, N.V.; Vinarov, A.Z.; Parodi, A. In silico, in vitro, and clinical investigations of cathepsin B and stefin A mRNA expression and a correlation analysis in kidney cancer. Cells 2022, 11, 1455. [Google Scholar] [CrossRef]
- Peng, T.; Li, T.; Zhang, C. Correlation between cathepsins and the likelihood of renal cancer: A Mendelian randomization study. Postgrad. Med. J. 2025, 101, 730–736. [Google Scholar] [CrossRef]
- Podgorski, I.; Sloane, B.F. Cathepsin B and its role (s) in cancer progression. Biochem. Soc. Symp. 2003, 70, 263–276. [Google Scholar] [CrossRef]
- Gondi, C.S.; Rao, J.S. Cathepsin B as a cancer target. Expert Opin. Ther. Targets 2013, 17, 281–291. [Google Scholar] [CrossRef]
- Xiaofei, C.; Yanqing, L.; Dongkai, Z.; Dong, C.; Feng, Z.; Weilin, W. Identification of cathepsin B as a novel target of hypoxia-inducible factor-1-alpha in HepG2 cells. Biochem. Biophys. Res. Commun. 2018, 503, 1057–1062. [Google Scholar] [CrossRef]
- Illy, C.; Quraishi, O.; Wang, J.; Purisima, E.; Vernet, T.; Mort, J.S. Role of the occluding loop in cathepsin B activity. J. Biol. Chem. 1997, 272, 1197–1202. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; Wang, J.; Zhang, L.; Tang, P.; Zhai, S.; Guo, W.; Yu, N.; Zhao, L.; Liu, M. Expression and clinical significance of cathepsin B and stefin A in laryngeal cancer. Oncol. Rep. 2011, 26, 869–875. [Google Scholar] [CrossRef]
- Li, W.; Ding, F.; Zhang, L.; Liu, Z.; Wu, Y.; Luo, A.; Wu, M.; Wang, M.; Zhan, Q.; Liu, Z. Overexpression of stefin A in human esophageal squamous cell carcinoma cells inhibits tumor cell growth, angiogenesis, invasion, and metastasis. Clin. Cancer Res. 2005, 11, 8753–8762. [Google Scholar] [CrossRef]
- Syrocheva, A.O.; Ivanov, K.I.; Laktyushkin, V.S.; Gorokhovets, N.V.; Parodi, A.; Zamyatnin, A.A., Jr. Expression Interplay Between Cathepsin B and Its Natural Inhibitor Stefin A in Cancer and Embryonic Cell Lines. Cell Biol. Int. 2025. [Google Scholar] [CrossRef] [PubMed]
- Padamsey, Z.; McGuinness, L.; Bardo, S.J.; Reinhart, M.; Tong, R.; Hedegaard, A.; Hart, M.L.; Emptage, N.J. Activity-dependent exocytosis of lysosomes regulates the structural plasticity of dendritic spines. Neuron 2017, 93, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chen, X.; Liu, W.; Chen, S.; Yang, C.; Yang, J. CTSB is a negative prognostic biomarker and therapeutic target associated with immune cells infiltration and immunosuppression in gliomas. Sci. Rep. 2022, 12, 4295. [Google Scholar] [CrossRef] [PubMed]
- Reisenauer, A.; Eickelberg, O.; Wille, A.; Heimburg, A.; Reinhold, A.; Sloane, B.F.; Welte, T.; Bühling, F. Increased carcinogenic potential of myeloid tumor cells induced by aberrant TGF-β1-signaling and upregulation of cathepsin B. Biol. Chem. 2007, 388, 639–650. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Yu, H.; Xu, Y.; He, W.; Zhou, X.; Gou, X. Secretory autophagy-induced bladder tumour-derived extracellular vesicle secretion promotes angiogenesis by activating the TPX2-mediated phosphorylation of the AURKA-PI3K-AKT axis. Cancer Lett. 2021, 523, 10–28. [Google Scholar] [CrossRef]
- Vasiljeva, O.; Papazoglou, A.; KrügEr, A.; Brodoefel, H.; Korovin, M.; Deussing, J.; Augustin, N.; Nielsen, B.S.; Almholt, K.; Bogyo, M. Tumor cell–derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006, 66, 5242–5250. [Google Scholar] [CrossRef]
- Peng, S.; Yang, Q.; Li, H.; Pan, Y.; Wang, J.; Hu, P.; Zhang, N. CTSB knockdown inhibits proliferation and tumorigenesis in HL-60 cells. Int. J. Med. Sci. 2021, 18, 1484. [Google Scholar] [CrossRef]
- Takahashi, H.; Komatsu, N.; Ibe, M.; Ishida-Yamamoto, A.; Hashimoto, Y.; Iizuka, H. Cystatin A suppresses ultraviolet B-induced apoptosis of keratinocytes. J. Dermatol. Sci. 2007, 46, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Frolova, A.S.; Tikhomirova, N.K.; Kireev, I.I.; Zernii, E.Y.; Parodi, A.; Ivanov, K.I.; Zamyatnin, A.A., Jr. Expression, intracellular localization, and maturation of cysteine cathepsins in renal embryonic and cancer cell lines. Biochemistry 2023, 88, 1034–1044. [Google Scholar] [PubMed]
- Strojnik, T.; Zajc, I.; Bervar, A.; Židanik, B.; Golouh, R.; Kos, J.; Dolenc, V.; Lah, T. Cathepsin B and its inhibitor stefin A in brain tumors. Pflüg. Arch.-Eur. J. Physiol. 2000, 439, R122–R123. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhao, S.; Li, X.; Miao, Z.; Cao, J.; Chen, Y.; Shi, Z.; Zhang, J.; Wang, D.; Chen, S. Cathepsin B S-nitrosylation promotes ADAR1-mediated editing of its own mRNA transcript via an ADD1/MATR3 regulatory axis. Cell Res. 2023, 33, 546–561. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef]
- Yan, W.; Feng, Y.; Lei, Z.; Kuang, W.; Long, C. MicroRNA-214-3p Ameliorates LPS-Induced Cardiomyocyte Injury by Inhibiting Cathepsin B. Folia Biol. 2022, 68, 78–85. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, J.; Shi, J.; Liu, S.; Zou, H. MicroRNA-140-5p represses chondrocyte pyroptosis and relieves cartilage injury in osteoarthritis by inhibiting cathepsin B/Nod-like receptor protein 3. Bioengineered 2021, 12, 9933–9948. [Google Scholar] [CrossRef]
- An, Y.; Duan, H. The role of m6A RNA methylation in cancer metabolism. Mol. Cancer 2022, 21, 14. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, D.; Li, M.; Yang, M. MicroRNA-99 family in cancer: Molecular mechanisms for clinical applications. PeerJ 2025, 13, e19188. [Google Scholar] [CrossRef]
- Gole, B.; Huszthy, P.C.; Popović, M.; Jeruc, J.; Ardebili, Y.S.; Bjerkvig, R.; Lah, T.T. The regulation of cysteine cathepsins and cystatins in human gliomas. Int. J. Cancer 2012, 131, 1779–1789. [Google Scholar] [CrossRef]
- Nycander, M.; Estrada, S.; Mort, J.S.; Abrahamson, M.; Björk, I. Two-step mechanism of inhibition of cathepsin B by cystatin C due to displacement of the proteinase occluding loop. FEBS Lett. 1998, 422, 61–64. [Google Scholar] [CrossRef]
- Chwieralski, C.; Welte, T.; Bühling, F. Cathepsin-regulated apoptosis. Apoptosis 2006, 11, 143–149. [Google Scholar] [CrossRef]
- Hämälistö, S.; Stahl, J.L.; Favaro, E.; Yang, Q.; Liu, B.; Christoffersen, L.; Loos, B.; Guasch Boldú, C.; Joyce, J.A.; Reinheckel, T. Spatially and temporally defined lysosomal leakage facilitates mitotic chromosome segregation. Nat. Commun. 2020, 11, 229. [Google Scholar] [CrossRef]
- Li, C.; Sun, S.; Zhuang, Y.; Luo, Z.; Ji, G.; Liu, Z. CTSB nuclear translocation facilitates DNA damage and lysosomal stress to promote retinoblastoma cell death. Mol. Biotechnol. 2024, 66, 2583–2594. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syrocheva, A.O.; Kolesova, E.P.; Parodi, A.; Zamyatnin, A.A., Jr. Stefin A Regulation of Cathepsin B Expression and Localization in Cancerous and Non-Cancerous Cells. Int. J. Mol. Sci. 2025, 26, 9321. https://doi.org/10.3390/ijms26199321
Syrocheva AO, Kolesova EP, Parodi A, Zamyatnin AA Jr. Stefin A Regulation of Cathepsin B Expression and Localization in Cancerous and Non-Cancerous Cells. International Journal of Molecular Sciences. 2025; 26(19):9321. https://doi.org/10.3390/ijms26199321
Chicago/Turabian StyleSyrocheva, Anastasiia O., Ekaterina P. Kolesova, Alessandro Parodi, and Andrey A. Zamyatnin, Jr. 2025. "Stefin A Regulation of Cathepsin B Expression and Localization in Cancerous and Non-Cancerous Cells" International Journal of Molecular Sciences 26, no. 19: 9321. https://doi.org/10.3390/ijms26199321
APA StyleSyrocheva, A. O., Kolesova, E. P., Parodi, A., & Zamyatnin, A. A., Jr. (2025). Stefin A Regulation of Cathepsin B Expression and Localization in Cancerous and Non-Cancerous Cells. International Journal of Molecular Sciences, 26(19), 9321. https://doi.org/10.3390/ijms26199321









