Fe2+-Sensing α-Synuclein Iron-Responsive Messenger RNA/eIF4F Complex Binding and Regulating mRNA Translation Activation and Repression
Abstract
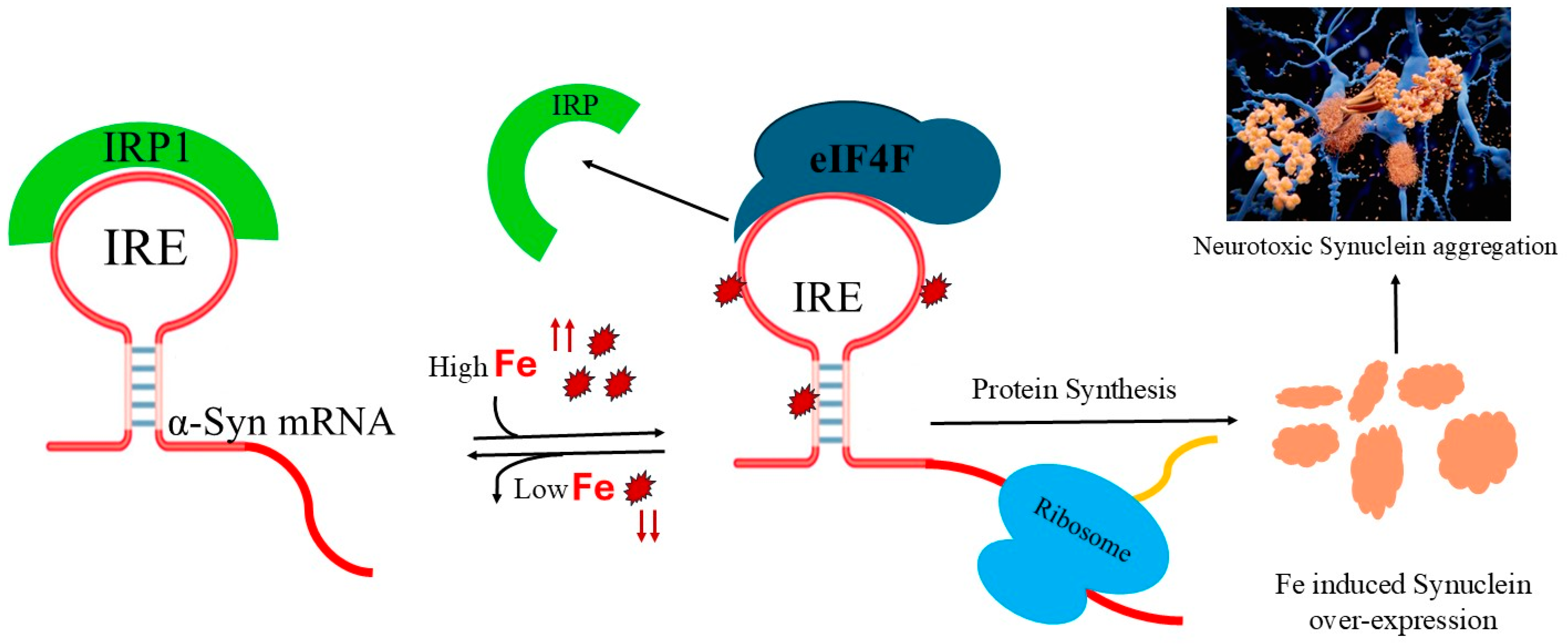
1. Introduction
2. Results
2.1. α-Syn IRE Binds Strongly to eIF4F
2.2. Fe2+ Affect Interaction of α-Syn IRE with eIF4F
2.3. Temperature Effects on α-Syn IRE/eIF4F Binding
2.4. Thermodynamic Analyses of eIF4F/α-Syn IRE Association
2.5. Competition of eIF4F and IRP1 for α-Syn IRE Binding
2.6. Effects of Fe2+ on α-Syn mRNA Translation
3. Discussion
4. Materials and Methods
4.1. Preparation of eIF4F, IRP1, and RNA
4.2. Fluorescence Spectroscopy Measurements
4.3. RNA Gel Shift Assay
4.4. Fe2+ Effect on α-Syn IRE/eIF4F Interaction
4.5. Temperature-Dependent α-Syn IRE/eIF4F Binding
4.6. Thermodynamic Measurements
4.7. Competitive Binding of IRP and eIF4F for α-Syn IRE
4.8. Protein Synthesis Assays
4.9. Statistical Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balestrino, R.; Schapira, A.H. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kågedal, K.; Halliday, G.M. Alpha-synuclein biology in Lewy body diseases. Alzheimer’s Res. Ther. 2014, 6, 73. [Google Scholar]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.M.-Y.; Trojanowski, J.Q. Mechanisms of Parkinson’s disease linked to pathological α-synuclein: New targets for drug discovery. Neuron 2006, 52, 33–38. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M.-Y. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar]
- Bok, J.; Ha, J.; Ahn, B.J.; Jang, Y. Disease-Modifying Effects of Non-Invasive Electroceuticals on beta-Amyloid Plaques and Tau Tangles for Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 679. [Google Scholar] [CrossRef]
- Singleton, A.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R. α-Synuclein locus triplication causes Parkinson’s disease. Science 2003, 302, 841. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Participation of Amyloid and Tau Protein in Neuronal Death and Neurodegeneration after Brain Ischemia. Int. J. Mol. Sci. 2020, 21, 4599. [Google Scholar] [CrossRef]
- Cao, D.-F.; Zhou, X.-Y.; Guo, Q.; Xiang, M.-Y.; Bao, M.-H.; He, B.-S.; Mao, X.-Y. Unveiling the role of histone deacetylases in neurological diseases: Focus on epilepsy. Biomark. Res. 2024, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Ostrerova-Golts, N.; Petrucelli, L.; Hardy, J.; Lee, J.M.; Farer, M.; Wolozin, B. The A53T α-synuclein mutation increases iron-dependent aggregation and toxicity. J. Neurosci. 2000, 20, 6048–6054. [Google Scholar] [CrossRef]
- Acosta-Cabronero, J.; Betts, M.J.; Cardenas-Blanco, A.; Yang, S.; Nestor, P.J. In Vivo MRI Mapping of Brain Iron Deposition across the Adult Lifespan. J. Neurosci. 2016, 36, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Everett, J.; Céspedes, E.; Shelford, L.R.; Exley, C.; Collingwood, J.F.; Dobson, J.; van der Laan, G.; Jenkins, C.A.; Arenholz, E.; Telling, N.D. Ferrous iron formation following the co-aggregation of ferric iron and the Alzheimer’s disease peptide β-amyloid (1-42). J. R. Soc. Interface 2014, 11, 20140165. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Z.; Zhang, L.; Meng, R.N.; Gao, J.; Jin, M.; Li, M.; Wang, X.P. Effect of metal ions on Alzheimer’s disease. Brain Behav. 2022, 12, e2527. [Google Scholar] [CrossRef]
- Xia, Y.; Saitoh, T.; Uéda, K.; Tanaka, S.; Chen, X.; Hashimoto, M.; Hsu, L.; Conrad, C.; Sundsmo, M.; Yoshimoto, M. Characterization of the human α-synuclein gene: Genomic structure, transcription start site, promoter region and polymorphisms. J. Alzheimer’s Dis. 2001, 3, 485–494. [Google Scholar] [CrossRef]
- Preiss, T.; Muckenthaler, M.; Hentze, M.W. Poly(A)-tail-promoted translation in yeast: Implications for translational control. RNA 1998, 4, 1321–1331. [Google Scholar] [CrossRef]
- Bordeleau, M.-E.; Matthews, J.; Wojnar, J.M.; Lindqvist, L.; Novac, O.; Jankowsky, E.; Sonenberg, N.; Northcote, P.; Teesdale-Spittle, P.; Pelletier, J. Stimulation of mammalian translation initiation factor eIF4A activity by a small molecule inhibitor of eukaryotic translation. Proc. Natl. Acad. Sci. USA 2005, 102, 10460–10465. [Google Scholar] [CrossRef]
- Hundsdoerfer, P.; Thoma, C.; Hentze, M.W. Eukaryotic translation initiation factor 4GI and p97 promote cellular internal ribosome entry sequence-driven translation. Proc. Natl. Acad. Sci. USA 2005, 102, 13421–13426. [Google Scholar] [CrossRef]
- Nie, M.; Htun, H. Different modes and potencies of translational repression by sequence-specific RNA–protein interaction at the 5′-UTR. Nucleic Acids Res. 2006, 34, 5528–5540. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Randall, J.D.; Cahill, C.M.; Eder, P.S.; Huang, X.; Gunshin, H.; Leiter, L.; McPhee, J.; Sarang, S.S.; Utsuki, T.; et al. An iron-responsive element type II in the 5’-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J. Biol. Chem. 2002, 277, 45518–45528. [Google Scholar] [CrossRef]
- Khan, M.A.; Mohammad, T.; Alalami, Z.S.; Hassan, M.I. Iron activates Alzheimer’s amyloid precursor protein synthesis through an iron responsive element (IRE) mRNA binding to translation initiation factor. Int. J. Biol. Macromol. 2025, 321, 146231. [Google Scholar] [CrossRef]
- Khan, M.A. Iron-Mediated Overexpression of Amyloid Precursor Protein via Iron Responsive mRNA in Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 5283. [Google Scholar] [CrossRef]
- McDowall, J.; Brown, D. Alpha-synuclein: Relating metals to structure, function and inhibition. Met. Integr. Biometal Sci. 2016, 8, 385–397. [Google Scholar]
- Zhou, Z.D.; Tan, E.-K. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 75. [Google Scholar] [CrossRef]
- Olivares, D.; Huang, X.; Branden, L.; Greig, N.H.; Rogers, J.T. Physiological and pathological role of alpha-synuclein in Parkinson’s disease through iron mediated oxidative stress; the role of a putative iron-responsive element. Int. J. Mol. Sci. 2009, 10, 1226–1260. [Google Scholar] [CrossRef]
- Castellani, R.J.; Siedlak, S.L.; Perry, G.; Smith, M.A. Sequestration of iron by Lewy bodies in Parkinson’s disease. Acta Neuropathol. 2000, 100, 111–114. [Google Scholar]
- Muckenthaler, M.; Gray, N.K.; Hentze, M.W. IRP-1 binding to ferritin mRNA prevents the recruitment of the small ribosomal subunit by the cap-binding complex eIF4F. Mol. Cell 1998, 2, 383–388. [Google Scholar]
- Ma, J.; Haldar, S.; Khan, M.A.; Sharma, S.D.; Merrick, W.C.; Theil, E.C.; Goss, D.J. Fe2+ binds iron responsive element-RNA, selectively changing protein-binding affinities and regulating mRNA repression and activation. Proc. Natl. Acad. Sci. USA 2012, 109, 8417–8422. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Mohammad, T.; Malik, A.; Hassan, M.I.; Domashevskiy, A.V. Iron response elements (IREs)-mRNA of Alzheimer’s amyloid precursor protein binding to iron regulatory protein (IRP1): A combined molecular docking and spectroscopic approach. Sci. Rep. 2023, 13, 5073. [Google Scholar] [CrossRef]
- Breuer, W.; Epsztejn, S.; Cabantchik, Z.I. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J. Biol. Chem. 1995, 270, 24209–24215. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A. α-Synuclein Iron-Responsive-Element RNA and Iron Regulatory Protein Affinity Is Specifically Reduced by Iron in Parkinson’s Disease. Biomolecules 2025, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Oerlemans, R.; Groves, M.R. Theory and applications of differential scanning fluorimetry in early-stage drug discovery. Biophys. Rev. 2020, 12, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Malik, A.; Domashevskiy, A.V.; San, A.; Khan, J.A. Interaction of ferritin iron responsive element (IRE) mRNA with translation initiation factor eIF4F. Spectrochim. Acta Part A Mol. Spectrosc. 2020, 243, 118776. [Google Scholar]
- Dix, D.J.; Lin, P.N.; Kimata, Y.; Theil, E.C. The iron regulatory region of ferritin mRNA is also a positive control element for iron-independent translation. Biochemistry 1992, 31, 2818–2822. [Google Scholar]
- Khan, M.A.; Walden, W.E.; Goss, D.J.; Theil, E.C. Direct Fe2+ sensing by iron-responsive messenger RNA: Repressor complexes weakens binding. J. Biol. Chem. 2009, 284, 30122–30128. [Google Scholar] [CrossRef]
- Ke, Y.; Wu, J.; Leibold, E.A.; Walden, W.E.; Theil, E.C. Loops and bulge/loops in iron-responsive element isoforms influence iron regulatory protein binding. Fine-tuning of mRNA regulation? J. Biol. Chem. 1998, 273, 23637–23640. [Google Scholar]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Shamsi, A.; Anwar, S.; Shahbaaz, M.; Mohammad, T.; Alajmi, M.F.; Hussain, A.; Hassan, I.; Ahmad, F.; Islam, A. Evaluation of Binding of Rosmarinic Acid with Human Transferrin and Its Impact on the Protein Structure: Targeting Polyphenolic Acid-Induced Protection of Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2020, 2020, 1245875. [Google Scholar] [CrossRef]
- Khan, M.A.; Miyoshi, H.; Gallie, D.R.; Goss, D.J. Potyvirus genome-linked protein, VPg, directly affects wheat germ in vitro translation: Interactions with translation initiation factors eIF4F and eIFiso4F. J. Biol. Chem. 2008, 283, 1340–1349. [Google Scholar] [CrossRef]
- Gallie, D. Cap-Independent translation conferred by the 5’ leader of tobacco etch virus is eukaryotic initiation factor 4G dependent. J. Virol. 2001, 75, 12141–12152. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hellen, C.U.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Cahill, C.M.; Lahiri, D.K.; Huang, X.; Rogers, J.T. Amyloid precursor protein and alpha synuclein translation, implications for iron and inflammation in neurodegenerative diseases. Biochim. Biophys. Acta 2009, 1790, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Febbraro, F.; Giorgi, M.; Caldarola, S.; Loreni, F.; Romero-Ramos, M. α-Synuclein expression is modulated at the translational level by iron. Neuroreport 2012, 23, 576–580. [Google Scholar] [CrossRef]
- Hintze, K.J.; Theil, E.C. DNA and mRNA elements with complementary responses to hemin, antioxidant inducers, and iron control ferritin-L expression. Proc. Natl. Acad. Sci. USA 2005, 102, 15048–15052. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Walden, W.E.; Theil, E.C.; Goss, D.J. Thermodynamic and kinetic analyses of iron response element (IRE)-mRNA binding to iron regulatory protein, IRP1. Sci. Rep. 2017, 7, 8532. [Google Scholar] [CrossRef]
- Khan, M.A. Ferritin iron responsive elements (IREs) mRNA interacts with eIF4G and activates in vitro translation. Front. Biosci. 2022, 14, 17. [Google Scholar] [CrossRef]
- Tayyab, S.; Sam, S.E.; Kabir, M.Z.; Ridzwan, N.F.W.; Mohamad, S.B. Molecular interaction study of an anticancer drug, ponatinib with human serum albumin using spectroscopic and molecular docking methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 199–206. [Google Scholar] [CrossRef]
- Kuntz, I.D.; Chen, K.; Sharp, K.A.; Kollman, P.A. The maximal affinity of ligands. Proc. Natl. Acad. Sci. USA 1999, 96, 9997–10002. [Google Scholar] [CrossRef]
- Wallander, M.L.; Leibold, E.A.; Eisenstein, R.S. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim. Biophys. Acta 2006, 1763, 668–689. [Google Scholar] [CrossRef]
- Khan, M.A.; Domashevskiy, A.V. Iron enhances the binding rates and translational efficiency of iron responsive elements (IREs) mRNA with initiation factor eIF4F. PLoS ONE 2021, 16, e0250374. [Google Scholar] [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.O.; Dore, L.C.; Valentine, E.; Shelat, S.G.; Hardison, R.C.; Ghosh, M.; Wang, W.; Eisenstein, R.S.; Costa, F.F.; Weiss, M.J. An iron responsive element-like stem-loop regulates alpha-hemoglobin-stabilizing protein mRNA. J. Biol. Chem. 2008, 283, 26956–26964. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Galy, B.; Muckenthaler, M.U.; Hentze, M.W. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat. Struct. Mol. Biol. 2007, 14, 420–426. [Google Scholar] [CrossRef]
- Casanova, F.; Tian, Q.; Atkins, J.L.; Wood, A.R.; Williamson, D.; Qian, Y.; Zweibaum, D.; Ding, J.; Melzer, D.; Ferrucci, L.; et al. Iron and risk of dementia: Mendelian randomisation analysis in UK Biobank. J. Med. Genet. 2024, 61, 435–442. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]

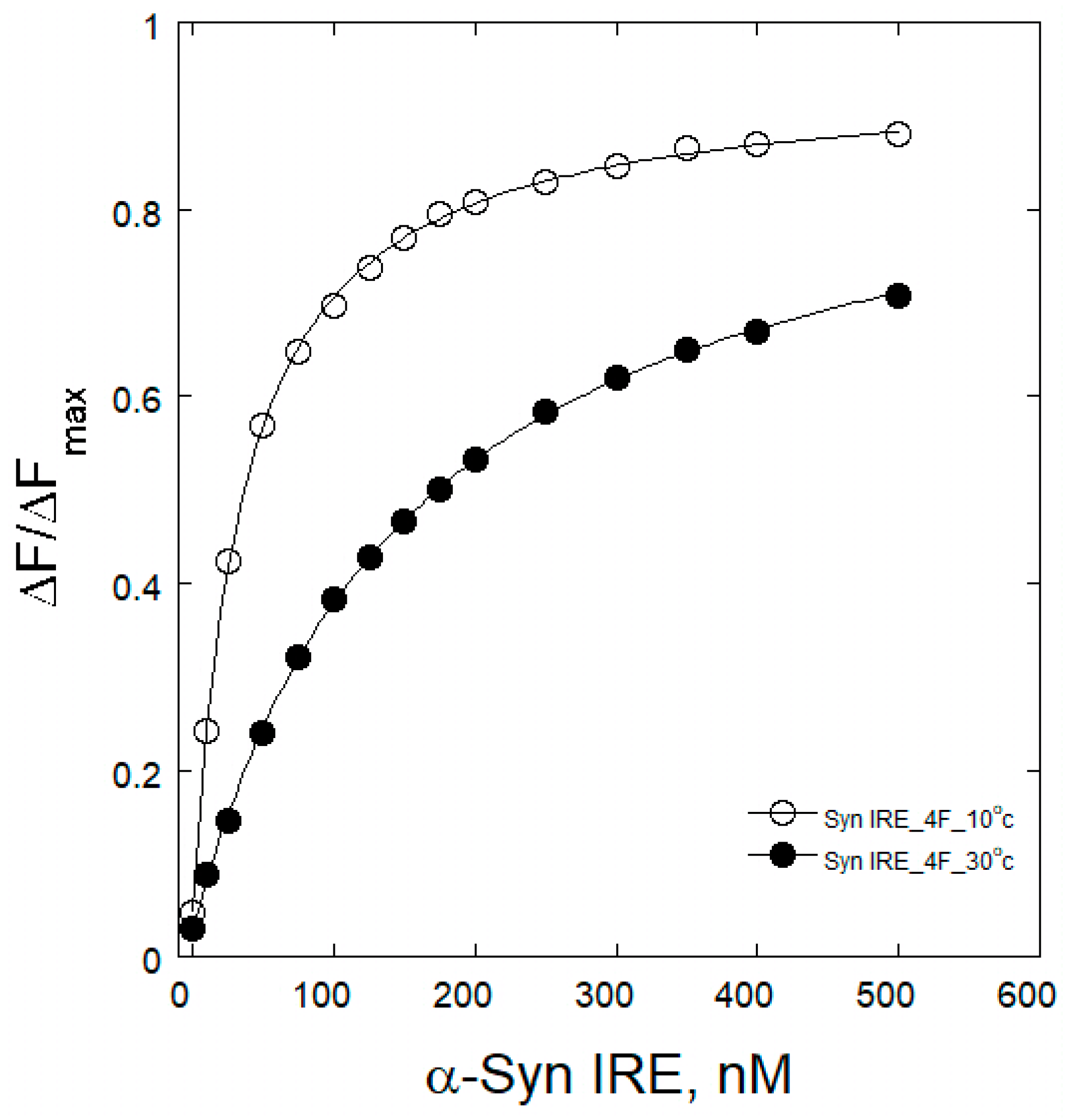
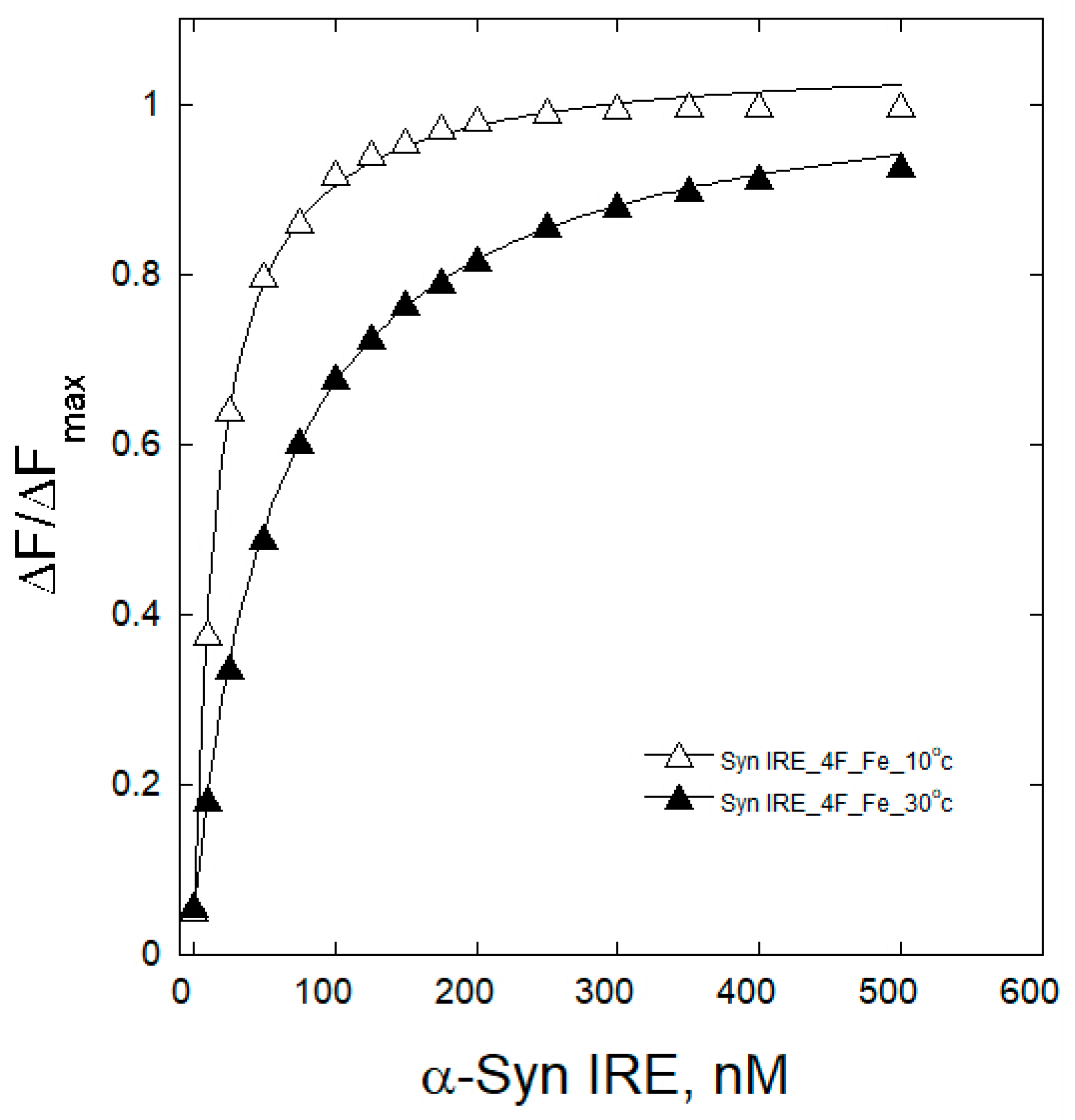

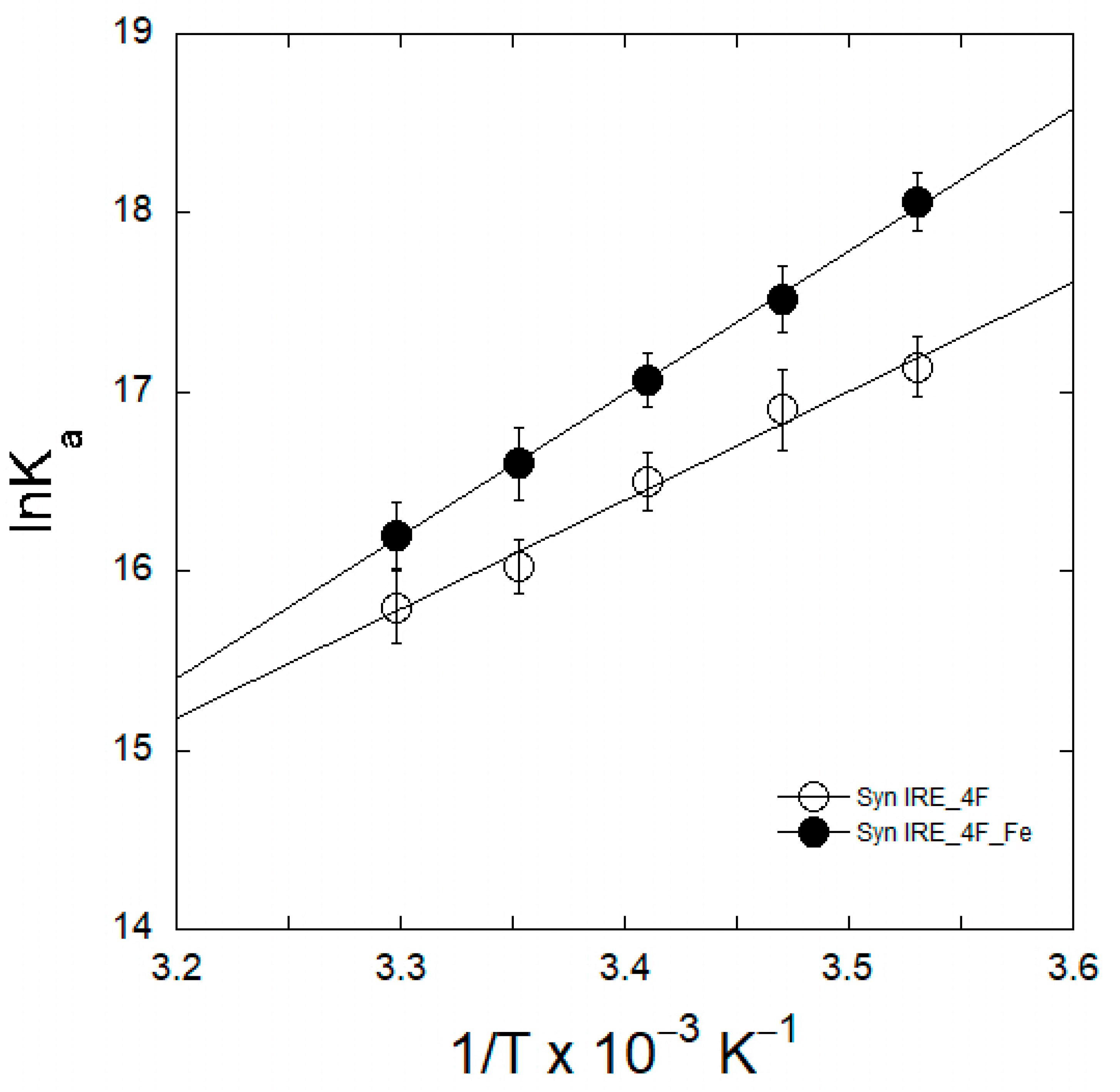

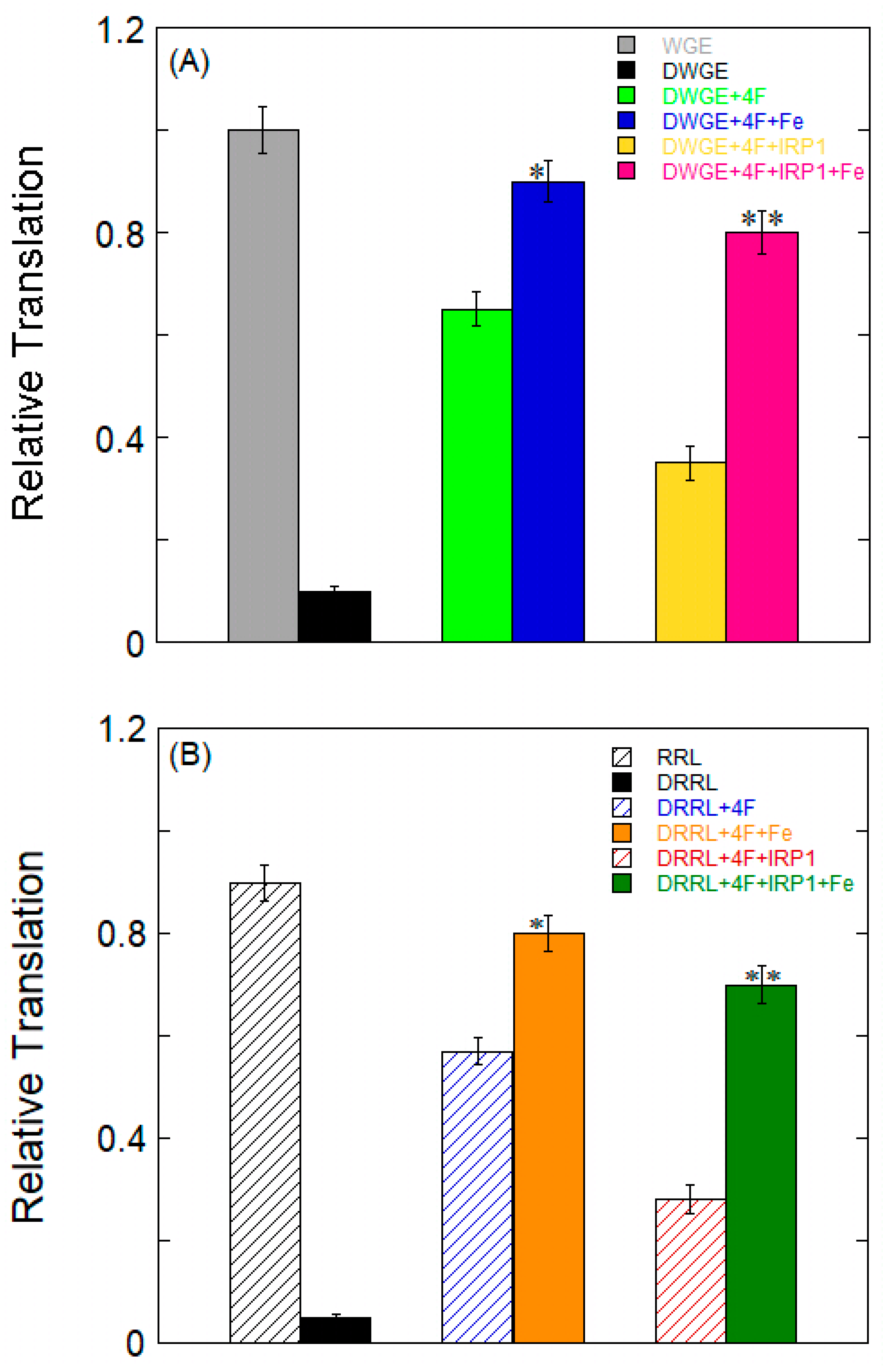
| Complex | KD (nM) | ||||
|---|---|---|---|---|---|
| 10 °C | 15 °C | 20 °C | 25 °C | 30 °C | |
| α-Syn IRE∙eIF4F | 35.8 ± 1.6 | 45.6 ± 2.1 | 69.8 ± 2.8 | 119.2 ± 6.4 | 158 ± 8.7 |
| α-Syn IRE∙eIF4F∙Fe2+ | 14.4 ± 0.7 | 24.6 ± 1.4 | 38.5 ± 1.9 | 43.7 ± 2.7 | 88.2 ± 2.8 |
| Complex | ΔH | ΔS | ΔG |
|---|---|---|---|
| kJ mol−1 | J mol−1 K−1 | kJ mol−1 | |
| α-Syn IRE∙4F | −45.6 ± 2.9 | −35.7 ± 3.4 | −33.2 ± 2.7 |
| α-Syn IRE∙4F-Fe2+ | −69.2 ± 3.5 | −83.9 ± 4.7 | −51.9 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A. Fe2+-Sensing α-Synuclein Iron-Responsive Messenger RNA/eIF4F Complex Binding and Regulating mRNA Translation Activation and Repression. Int. J. Mol. Sci. 2025, 26, 9320. https://doi.org/10.3390/ijms26199320
Khan MA. Fe2+-Sensing α-Synuclein Iron-Responsive Messenger RNA/eIF4F Complex Binding and Regulating mRNA Translation Activation and Repression. International Journal of Molecular Sciences. 2025; 26(19):9320. https://doi.org/10.3390/ijms26199320
Chicago/Turabian StyleKhan, Mateen A. 2025. "Fe2+-Sensing α-Synuclein Iron-Responsive Messenger RNA/eIF4F Complex Binding and Regulating mRNA Translation Activation and Repression" International Journal of Molecular Sciences 26, no. 19: 9320. https://doi.org/10.3390/ijms26199320
APA StyleKhan, M. A. (2025). Fe2+-Sensing α-Synuclein Iron-Responsive Messenger RNA/eIF4F Complex Binding and Regulating mRNA Translation Activation and Repression. International Journal of Molecular Sciences, 26(19), 9320. https://doi.org/10.3390/ijms26199320






