Organophosphate Chemical Nerve Agents, Oxidative Stress, and NADPH Oxidase Inhibitors: An Overview
Abstract
1. Introduction
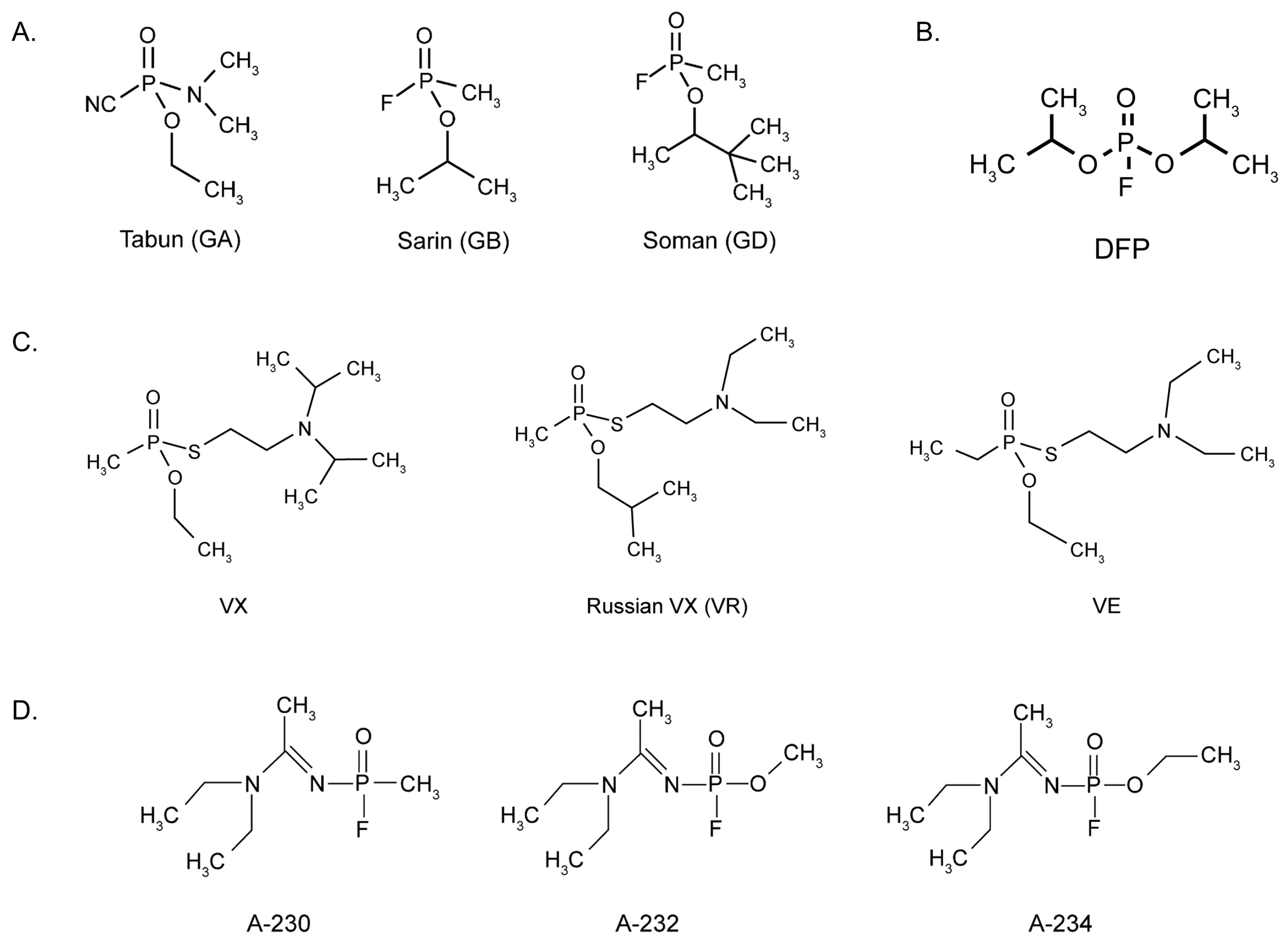
2. Historical Perspectives of Organophosphate Nerve Agents
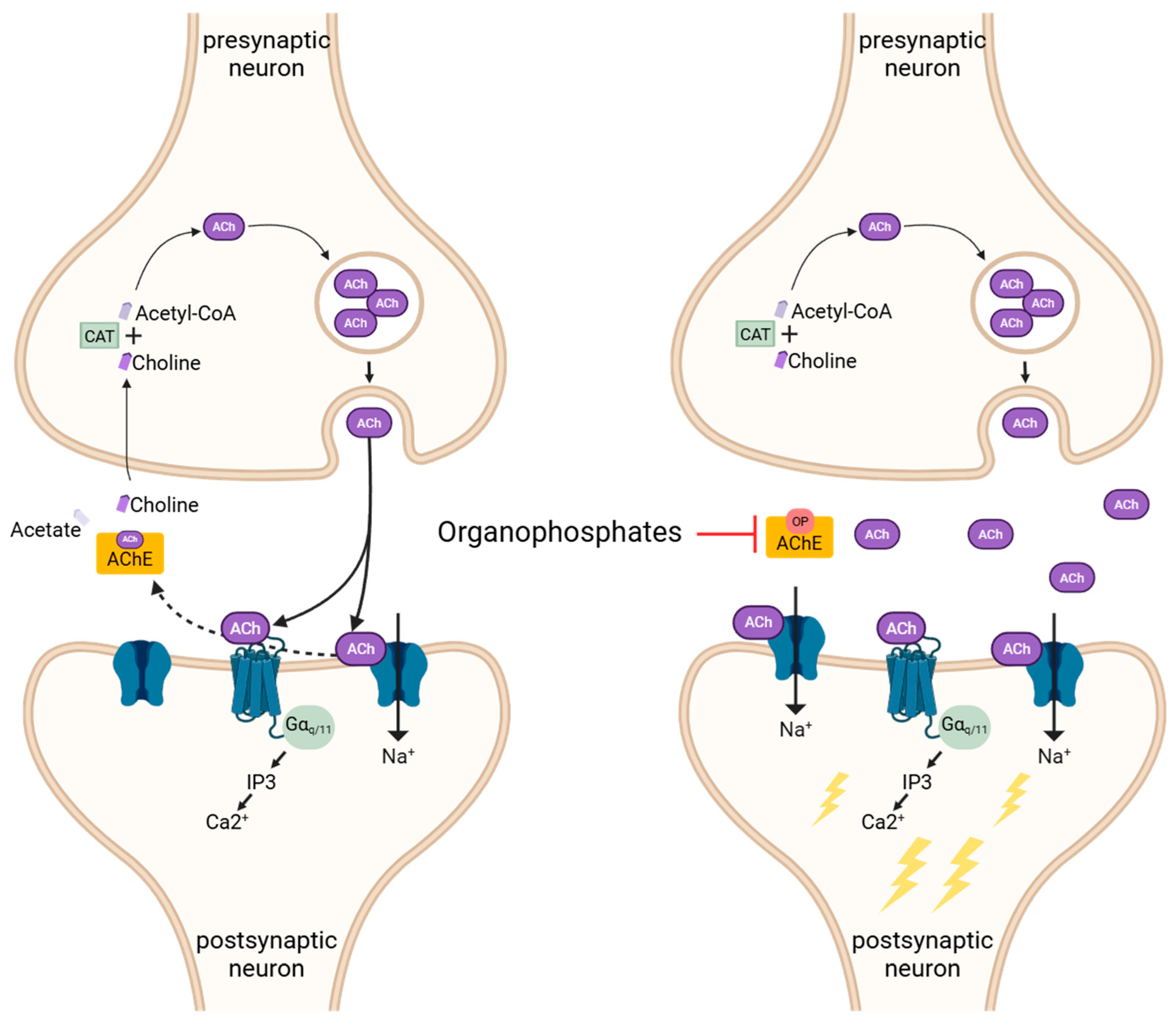
3. Mechanisms of Organophosphate Poisoning
3.1. Acute Cholinergic Crisis and Medical Countermeasures
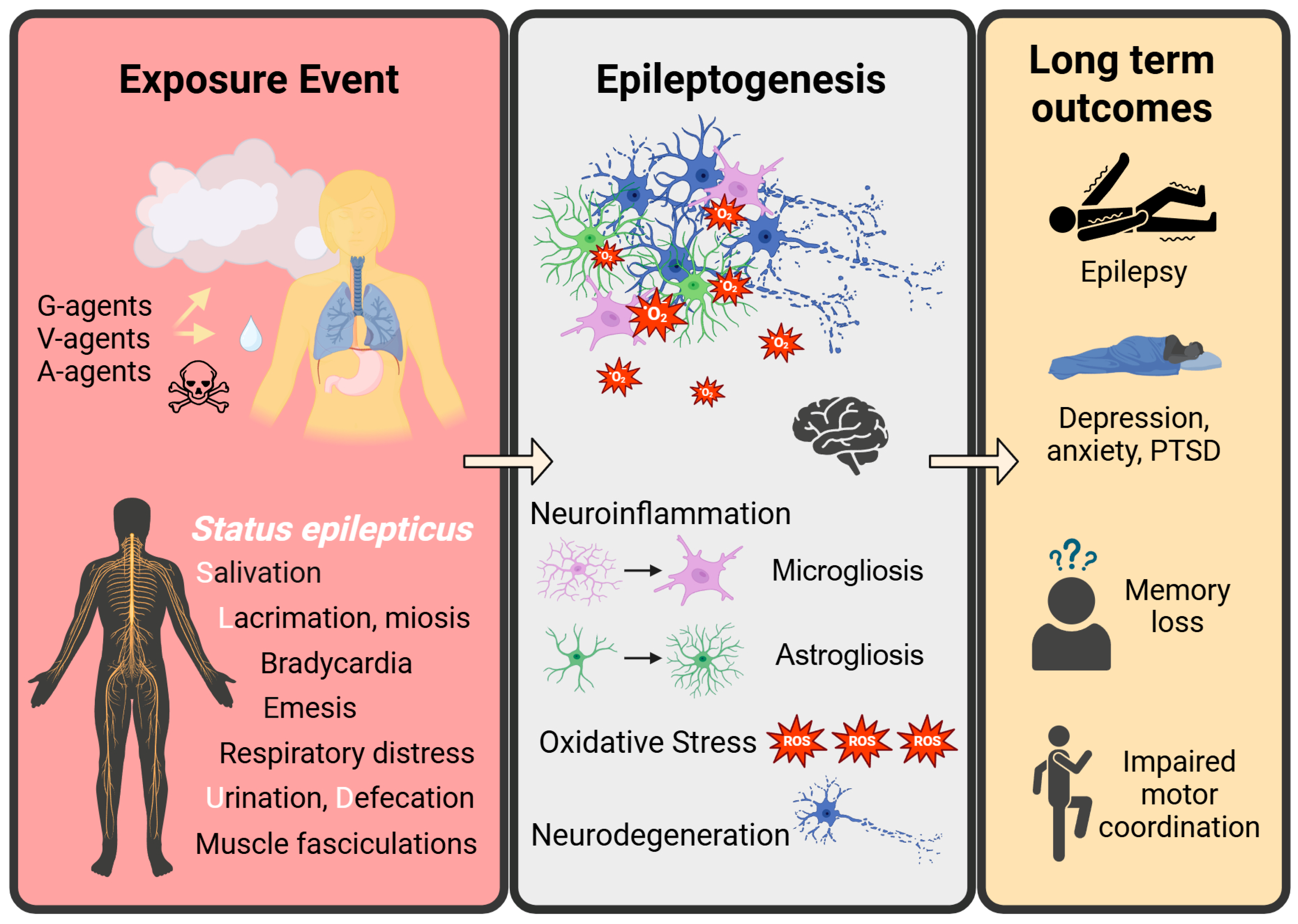
3.2. Status Epilepticus-Induced Epileptogenesis
3.3. Neuronal Hyperexcitation and Neurodegeneration
3.4. Gliosis
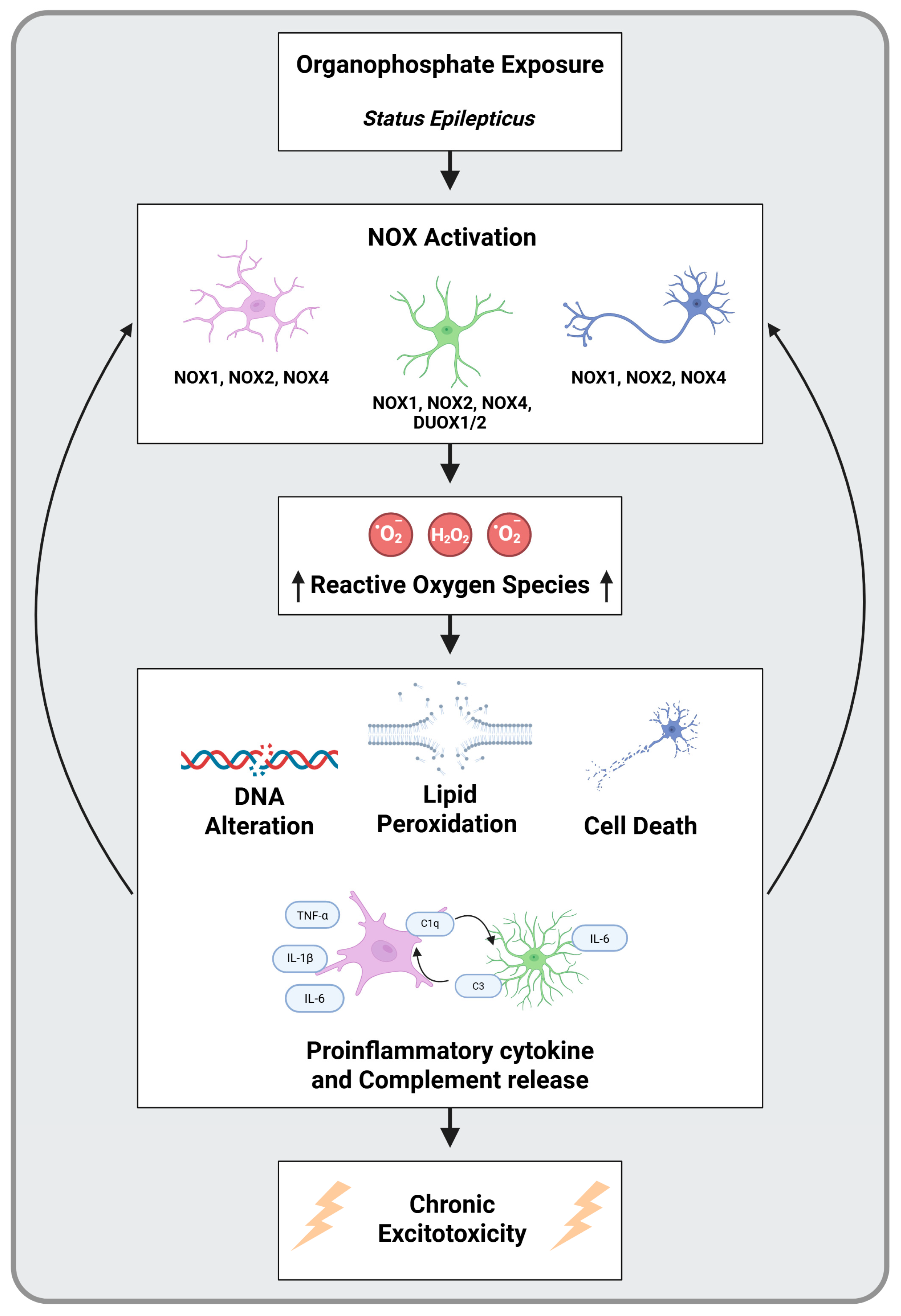
4. Oxidative Stress and NADPH Oxidase as a Key Pathological Mechanism
5. NOX Inhibitors as Therapeutic Agents in Epilepsy and Organophosphate Poisoning
5.1. Apocynin, Diapocynin, and Mitoapocynin
5.2. APO, DPO, and MPO as a Therapy for Excitotoxicity
5.3. Additional NOX Inhibitors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| AMPAR | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| APO | Apocynin |
| ATS | Atropine sulfate |
| BBB | Blood–brain barrier |
| C3 | Complement 3 |
| CAT | Choline acetyltransferase |
| CNS | Central nervous system |
| CWC | Chemical Weapons Convention |
| DAMPS | Damage-associated molecular patterns |
| DFP | Diisopropylfluorophosphate |
| DPO | Diapocynin |
| FAD | Flavin adenine dinucleotide |
| GABA | gamma-aminobutyric acid |
| GB | Sarin |
| GD | Soman |
| GF | Cyclosarin |
| hCMEC | Human brain microvascular endothelial cells |
| H2O2 | Hydrogen peroxide |
| KA | Kainic acid |
| Kir 4.1 | Inward rectifying potassium channel 4.1 |
| mAChR | Muscarinic acetylcholine receptors |
| MCM | Medical countermeasure |
| MDZ | Midazolam |
| MPO | Mitoapocynin |
| MRI | Magnetic resonance imaging |
| nAChR | Nicotinic acetylcholine receptor |
| NADPH | Nicotinamide adenine dinucleotide phosphate hydrogen |
| NMDAR | N-methyl-D-aspartate receptor |
| NOX | NADPH oxidase |
| •NO | Nitric oxide |
| O2•− | Superoxide anion |
| OP | Organophosphate |
| OPNA | Organophosphate nerve agent |
| PAMPS | Pathogen-associated molecular patterns |
| PET | Positron-emission tomography |
| PTZ | Pentylenetetrazol |
| RNS | Reactive nitrogen sepcies |
| ROS | Reactive oxygen species |
| SE | Status epilepticus |
| SLUD | Salivation, lacrimation, urination, defecation |
References
- Organophosphorous Compounds. In Experimental and Clinical Neurotoxicology; Spencer, P.S., Schaumburg, H.H., Ludolph, A.C., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 527–544. ISBN 978-0-19-508477-1. [Google Scholar]
- Adeyinka, A.; Muco, E.; Regina, A.C.; Pierre, L. Organophosphates. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mew, E.J.; Padmanathan, P.; Konradsen, F.; Eddleston, M.; Chang, S.-S.; Phillips, M.R.; Gunnell, D. The Global Burden of Fatal Self-Poisoning with Pesticides 2006-15: Systematic Review. J. Affect. Disord. 2017, 219, 93–104. [Google Scholar] [CrossRef]
- Tadesse, B.; Kibret, H.; Heluf, H.; Mesfin, S.; Alemu, Y. Pattern and Outcome of Acute Organophosphate Poisoning at Health Facilities of Harari Region, Eastern Ethiopia. SAGE Open Med. 2023, 11, 20503121231216603. [Google Scholar] [CrossRef] [PubMed]
- Munro, N. Toxicity of the Organophosphate Chemical Warfare Agents GA, GB, and VX: Implications for Public Protection. Environ. Health Perspect. 1994, 102, 18–37. [Google Scholar] [CrossRef]
- Diauudin, F.N.; Rashid, J.I.A.; Knight, V.F.; Yunus, W.M.Z.W.; Ong, K.K.; Kasim, N.A.M.; Halim, N.A.; Noor, S.A.M. A Review of Current Advances in the Detection of Organophosphorus Chemical Warfare Agents Based Biosensor Approaches. Sens. Bio-Sens. Res. 2019, 26, 100305. [Google Scholar] [CrossRef]
- Thiermann, H.; Worek, F.; Kehe, K. Limitations and Challenges in Treatment of Acute Chemical Warfare Agent Poisoning. Chem. -Biol. Interact. 2013, 206, 435–443. [Google Scholar] [CrossRef]
- Patel, H. Neurognostics Answer. J. Hist. Neurosci. 2010, 19, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Schmaltz, F. Neurosciences and Research on Chemical Weapons of Mass Destruction in Nazi Germany. J. Hist. Neurosci. 2006, 15, 186–209. [Google Scholar] [CrossRef]
- Petroianu, G.A. Pharmacists Adolf Schall and Ernst Ratzlaff and the Synthesis of Tabun-like Compounds: A Brief History. Pharmazie 2014, 69, 780–784. [Google Scholar] [CrossRef]
- Tucker, J. War of Nerves: Chemical Warfare from World War I to Al-Qaeda; Anchor Books: New York, NY, USA; Knopf Doubleday Publishing Group: New York, NY, USA, 2007. [Google Scholar]
- Schrader, G. Nr. 8—Arbeiten aus der Tabun-, Sarin- und Somanreihe (Dustbin) 1945. The National Archives, Kew, London, FO 1031/239. 13 October 1945. Available online: https://www.academia.edu/34433304/Ein_gescheitertes_Geheimprojekt_Die_Bunkeranlage_Falkenhagen_1938_45. (accessed on 26 February 2025).
- Johnson, N.H.; Larsen, J.C.; Meek, E.C. Historical Perspective of Chemical Warfare Agents. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–26. ISBN 978-0-12-819090-6. [Google Scholar]
- Soltaninejad, K.; Shadnia, S. History of the Use and Epidemiology of Organophosphorus Poisoning. In Basic and Clinical Toxicology of Organophosphorus Compounds; Balali-Mood, M., Abdollahi, M., Eds.; Springer: London, UK, 2014; pp. 25–43. ISBN 978-1-4471-5624-6. [Google Scholar]
- Saunders, B.C.; Stacey, G.J. 138. Esters Containing Phosphorus. Part IV. Diisopropyl Fluorophosphonate. J. Chem. Soc. 1948, 95, 69. [Google Scholar] [CrossRef] [PubMed]
- Sogorb, M.; Estevez, J.; Vilanova, E. Toxicokinetics and Toxicodynamics of DFP. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 921–944. ISBN 978-0-12-819090-6. [Google Scholar]
- Newmark, J. Nerve Agents. Neurol. 2007, 13, 20–32. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Saber, H. Recent Advances in the Treatment of Organophosphorous Poisonings. Iran. J. Med. Sci. 2012, 37, 74–91. [Google Scholar]
- Gunderson, C.H.; Lehmann, C.R.; Sidell, F.R.; Jabbari, B. Nerve Agents: A Review. Neurology 1992, 42, 946. [Google Scholar] [CrossRef] [PubMed]
- McCauley, L.A.; Rischitelli, G.; Lambert, W.E.; Lasarev, M.; Sticker, D.L.; Spencer, P.S. Symptoms of Gulf War Veterans Possibly Exposed to Organophosphate Chemical Warfare Agents at Khamisiyah, Iraq. Int. J. Occup. Environ. Health 2001, 7, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Proctor, S. Health Status of Persian Gulf War Veterans: Self-Reported Symptoms, Environmental Exposures and the Effect of Stress. Int. J. Epidemiol. 1998, 27, 1000–1010. [Google Scholar] [CrossRef]
- Steele, L.; Sastre, A.; Gerkovich, M.M.; Cook, M.R. Complex Factors in the Etiology of Gulf War Illness: Wartime Exposures and Risk Factors in Veteran Subgroups. Environ. Health Perspect. 2012, 120, 112–118. [Google Scholar] [CrossRef]
- Abu-Qare, A.W.; Abou-Donia, M.B. Combined Exposure to Sarin and Pyridostigmine Bromide Increased Levels of Rat Urinary 3-Nitrotyrosine and 8-Hydroxy-2′-Deoxyguanosine, Biomarkers of Oxidative Stress. Toxicol. Lett. 2001, 123, 51–58. [Google Scholar] [CrossRef]
- Abou-Donia, M.B. Neurotoxicity Resulting From Coexposure to Pyridostigmine Bromide, DEET, and Permethrin: Implications of Gulf War Chemical Exposures. J. Toxicol. Environ. Health 1996, 48, 35–56. [Google Scholar] [CrossRef]
- Okumura, T.; Takasu, N.; Ishimatsu, S.; Miyanoki, S.; Mitsuhashi, A.; Kumada, K.; Tanaka, K.; Hinohara, S. Report on 640 Victims of the Tokyo Subway Sarin Attack. Ann. Emerg. Med. 1996, 28, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Sekijima, Y.; Morita, H.; Yanagisawa, N. Follow-up of Sarin Poisoning in Matsumoto. Ann. Intern. Med. 1997, 127, 1042. [Google Scholar] [CrossRef]
- Morita, H.; Yanagisawa, N.; Nakajima, T.; Shimizu, M.; Hirabayashi, H.; Okudera, H.; Nohara, M.; Midorikawa, Y.; Mimura, S. Sarin Poisoning in Matsumoto, Japan. Lancet 1995, 346, 290–293. [Google Scholar] [CrossRef]
- Noga, M.; Michalska, A.; Jurowski, K. Review of Possible Therapies in Treatment of Novichoks Poisoning and HAZMAT/CBRNE Approaches: State of the Art. J. Clin. Med. 2023, 12, 2221. [Google Scholar] [CrossRef]
- Chai, P.R.; Boyer, E.W.; Al-Nahhas, H.; Erickson, T.B. Toxic Chemical Weapons of Assassination and Warfare: Nerve Agents VX and Sarin. Toxicol. Commun. 2017, 1, 21–23. [Google Scholar] [CrossRef]
- Haslam, J.D.; Russell, P.; Hill, S.; Emmett, S.R.; Blain, P.G. Chemical, Biological, Radiological, and Nuclear Mass Casualty Medicine: A Review of Lessons from the Salisbury and Amesbury Novichok Nerve Agent Incidents. Br. J. Anaesth. 2022, 128, e200–e205. [Google Scholar] [CrossRef]
- Kloske, M.; Witkiewicz, Z. Novichoks—The A Group of Organophosphorus Chemical Warfare Agents. Chemosphere 2019, 221, 672–682. [Google Scholar] [CrossRef]
- Steindl, D.; Boehmerle, W.; Körner, R.; Praeger, D.; Haug, M.; Nee, J.; Schreiber, A.; Scheibe, F.; Demin, K.; Jacoby, P.; et al. Novichok Nerve Agent Poisoning. Lancet 2021, 397, 249–252. [Google Scholar] [CrossRef] [PubMed]
- McElroy, R.J. The Geneva Protocol of 1925. In The Politics of Arms Control Treaty Ratification; Krepon, M., Caldwell, D., Eds.; Palgrave Macmillan US: New York, NY, USA, 1991; pp. 125–166. ISBN 978-1-349-60585-9. [Google Scholar]
- Voros, C.; Dias, J.; Timperley, C.M.; Nachon, F.; Brown, R.C.D.; Baati, R. The Risk Associated with Organophosphorus Nerve Agents: From Their Discovery to Their Unavoidable Threat, Current Medical Countermeasures and Perspectives. Chem.-Biol. Interact. 2024, 395, 110973. [Google Scholar] [CrossRef] [PubMed]
- Kelle, A. Adding Novichok Nerve Agents to the CWC Annex on Chemicals: A Technical Fix and Its Implications for the Chemical Weapons Prohibition Regime; WMD Compliance Enforcement Series; UNIDIR: Geneva, Switzerland, 2022. [Google Scholar]
- Bertrand, D.; Wallace, T.L. A Review of the Cholinergic System and Therapeutic Approaches to Treat Brain Disorders. In Behavioral Pharmacology of the Cholinergic System; Shoaib, M., Wallace, T.L., Eds.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2020; Volume 45, pp. 1–28. ISBN 978-3-030-56012-6. [Google Scholar]
- Berizzi, A.E.; Gentry, P.R.; Rueda, P.; Den Hoedt, S.; Sexton, P.M.; Langmead, C.J.; Christopoulos, A. Molecular Mechanisms of Action of M5 Muscarinic Acetylcholine Receptor Allosteric Modulators. Mol. Pharmacol. 2016, 90, 427–436. [Google Scholar] [CrossRef]
- Patocka, J.; Kuca, K.; Jun, D. Acetylcholinesterase and Butyrylcholinesterase—Important Enzymes of Human Body. Acta Medica 2004, 47, 215–228. [Google Scholar] [CrossRef]
- Sam, C.; Bordoni, B. Physiology, Acetylcholine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Boczkowski, M.; Popiel, S.; Nawała, J.; Suska, H. History of Organophosphorus Compounds in the Context of Their Use as Chemical Warfare Agents. Molecules 2025, 30, 1615. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Thiermann, H.; Szinicz, L.; Eyer, P. Kinetic Analysis of Interactions between Human Acetylcholinesterase, Structurally Different Organophosphorus Compounds and Oximes. Biochem. Pharmacol. 2004, 68, 2237–2248. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Serine Hydrolase Targets of Organophosphorus Toxicants. Chem.-Biol. Interact. 2005, 157–158, 277–283. [Google Scholar] [CrossRef]
- Marrs, T.C.; Maynard, R.L.; Sidell, F.R. Chemical Warfare Agents: Toxicology and Treatment, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-470-01359-5. [Google Scholar]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; De Araujo Furtado, M.; Pidoplichko, V.I.; Braga, M.F.M. Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition. Toxics 2023, 11, 866. [Google Scholar] [CrossRef]
- De Candole, C.A.; Douglas, W.W.; Evans, C.L.; Holmes, R.; Spencer, K.E.V.; Torrance, R.W.; Wilson, K.M. The Failure of Respiration in Death by Anticholinesterase Poisoning. Br. J. Pharmacol. Chemother. 1953, 8, 466–475. [Google Scholar] [CrossRef]
- Sinha, S.N.; Kumpati, R.K.; Ramavath, P.N.; Sangaraju, R.; Gouda, B.; Chougule, P. Investigation of Acute Organophosphate Poisoning in Humans Based on Sociodemographic and Role of Neurotransmitters with Survival Study in South India. Sci. Rep. 2022, 12, 16513. [Google Scholar] [CrossRef] [PubMed]
- Grob, D.; Harvey, J.C. Effects in Man of the Anticholinesterase Compound Sarin (Isopropyl Methyl Phosphonofluoridate). J. Clin. Investig. 1958, 37, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Edery, H.; Geyer, M.A.; Taylor, P.; Berman, H.A. Target sites for anticholinesterases on the ventral surface of the medulla oblongata: Hypotension elicited by organophosphorus agents. J. Auton. Pharmacol. 1986, 6, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Robineau, P.; Guittin, P. Effects of an Organophosphorous Compound on Cardiac Rhythm and Haemodynamics in Anaesthetized and Conscious Beagle Dogs. Toxicol. Lett. 1987, 37, 95–102. [Google Scholar] [CrossRef]
- Pouliot, W.; Bealer, S.L.; Roach, B.; Dudek, F.E. A Rodent Model of Human Organophosphate Exposure Producing Status Epilepticus and Neuropathology. NeuroToxicology 2016, 56, 196–203. [Google Scholar] [CrossRef]
- Gage, M.; Putra, M.; Gomez-Estrada, C.; Golden, M.; Wachter, L.; Gard, M.; Thippeswamy, T. Differential Impact of Severity and Duration of Status Epilepticus, Medical Countermeasures, and a Disease-Modifier, Saracatinib, on Brain Regions in the Rat Diisopropylfluorophosphate Model. Front. Cell. Neurosci. 2021, 15, 772868. [Google Scholar] [CrossRef]
- Rao, N.S.; Meyer, C.; Vasanthi, S.S.; Massey, N.; Samidurai, M.; Gage, M.; Putra, M.; Almanza, A.N.; Wachter, L.; Thippeswamy, T. DFP-Induced Status Epilepticus Severity in Mixed-Sex Cohorts of Adult Rats Housed in the Same Room: Behavioral and EEG Comparisons. Front. Cell Dev. Biol. 2022, 10, 895092. [Google Scholar] [CrossRef]
- French, R.N.E.; Walter, F.G. Atropine. In Critical Care Toxicology; Brent, J., Burkhart, K., Dargan, P., Hatten, B., Megarbane, B., Palmer, R., White, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 2725–2731. ISBN 978-3-319-17899-8. [Google Scholar]
- Buzyurova, D.N.; Pashirova, T.N.; Zueva, I.V.; Burilova, E.A.; Shaihutdinova, Z.M.; Rizvanov, I.K.; Babaev, V.M.; Petrov, K.A.; Souto, E.B. Surface Modification of Pralidoxime Chloride-Loaded Solid Lipid Nanoparticles for Enhanced Brain Reactivation of Organophosphorus-Inhibited AChE: Pharmacokinetics in Rat. Toxicology 2020, 444, 152578. [Google Scholar] [CrossRef] [PubMed]
- Lépinard, L.; Leterrier, S.; Jourdain, L.; Turri, L.; Belkebir, A.; Knoertzer, J.; Champault, A.; Bel, R.; Selingue, E.; Mériaux, S.; et al. Enhancing Oxime Efficacy into Brain Using Ultrasound to Counteract Nerve Agent Exposure. Biomed. Pharmacother. 2025, 187, 118120, Corrigendum in Biomed. Pharmacother. 2025, 188, 118233. https://doi.org/10.1016/j.biopha.2025.118233. [Google Scholar] [CrossRef] [PubMed]
- Sidell, F.R.; Groff, W.A. The Reactivatibility of Cholinesterase Inhibited by VX and Sarin in Man. Toxicol. Appl. Pharmacol. 1974, 27, 241–252. [Google Scholar] [CrossRef]
- Wu, X.; Kuruba, R.; Reddy, D.S. Midazolam-Resistant Seizures and Brain Injury after Acute Intoxication of Diisopropylfluorophosphate, an Organophosphate Pesticide and Surrogate for Nerve Agents. J. Pharmacol. Exp. Ther. 2018, 367, 302–321. [Google Scholar] [CrossRef]
- Supasai, S.; González, E.A.; Rowland, D.J.; Hobson, B.; Bruun, D.A.; Guignet, M.A.; Soares, S.; Singh, V.; Wulff, H.; Saito, N. Acute Administration of Diazepam or Midazolam Minimally Alters Long-Term Neuropathological Effects in the Rat Brain Following Acute Intoxication with Diisopropylfluorophosphate. Eur. J. Pharmacol. 2020, 886, 173538. [Google Scholar] [CrossRef] [PubMed]
- Putra, M.; Vasanthi, S.S.; Rao, N.S.; Meyer, C.; Van Otterloo, M.; Thangi, L.; Thedens, D.R.; Kannurpatti, S.S.; Thippeswamy, T. Inhibiting Inducible Nitric Oxide Synthase with 1400W Reduces Soman (GD)-Induced Ferroptosis in Long-Term Epilepsy-Associated Neuropathology: Structural and Functional Magnetic Resonance Imaging Correlations with Neurobehavior and Brain Pathology. J. Pharmacol. Exp. Ther. 2024, 388, 724–738. [Google Scholar] [CrossRef]
- Massey, N.; Vasanthi, S.S.; Gimenez-Lirola, L.G.; Tyler, H.; Thippeswamy, T. Proinflammatory Cytokines, Oxidative Stress, and Organ Function as Biomarkers of Soman (GD) Chronic Neurotoxicity. Sci. Rep. 2025, 15, 9021. [Google Scholar] [CrossRef]
- Pestana, R.R.; Kinjo, E.R.; Hernandes, M.S.; Britto, L.R. Reactive Oxygen Species Generated by NADPH Oxidase Are Involved in Neurodegeneration in the Pilocarpine Model of Temporal Lobe Epilepsy. Neurosci. Lett. 2010, 484, 187–191. [Google Scholar] [CrossRef]
- Chen, H.; Albertson, T.E.; Olson, K.R. Treatment of Drug-induced Seizures. Br. J. Clin. Pharmacol. 2016, 81, 412–419. [Google Scholar] [CrossRef]
- Williamson, J.; Singh, T.; Kapur, J. Neurobiology of Organophosphate-Induced Seizures. Epilepsy Behav. 2019, 101, 106426. [Google Scholar] [CrossRef]
- Fordington, S.; Manford, M. A Review of Seizures and Epilepsy Following Traumatic Brain Injury. J. Neurol. 2020, 267, 3105–3111. [Google Scholar] [CrossRef]
- Rao, N.S.; Putra, M.; Meyer, C.; Almanza, A.N.; Thippeswamy, T. The Effects of Src Tyrosine Kinase Inhibitor, Saracatinib, on the Markers of Epileptogenesis in a Mixed-Sex Cohort of Adult Rats in the Kainic Acid Model of Epilepsy. Front. Mol. Neurosci. 2023, 16, 1294514. [Google Scholar] [CrossRef]
- Blair, R.E.; Hawkins, E.; Lauren, R.P.; DeLorenzo, R.J.; Deshpande, L.S. Chronic Epilepsy and Mossy Fiber Sprouting Following Organophosphate-induced Status Epilepticus in Rats. J. Pharmacol. Exp. Ther. 2024, 388, 325–332. [Google Scholar] [CrossRef]
- Yanagisawa, N.; Morita, H.; Nakajima, T. Sarin Experiences in Japan: Acute Toxicity and Long-Term Effects. J. Neurol. Sci. 2006, 249, 76–85. [Google Scholar] [CrossRef]
- International League Against Epilepsy ILAE Epilepsy Classification 2024. Available online: https://www.epilepsydiagnosis.org/epilepsy/epilepsy-classification-groupoverview.html (accessed on 15 August 2025).
- Chuang, C.-S.; Yang, K.-W.; Yen, C.-M.; Lin, C.-L.; Kao, C.-H. Risk of Seizures in Patients with Organophosphate Poisoning: A Nationwide Population-Based Study. Int. J. Environ. Res. Public Health 2019, 16, 3147. [Google Scholar] [CrossRef] [PubMed]
- Enderlin, J.; Igert, A.; Auvin, S.; Nachon, F.; Dal Bo, G.; Dupuis, N. Characterization of Organophosphate-induced Brain Injuries in a Convulsive Mouse Model of Diisopropylfluorophosphate Exposure. Epilepsia 2020, 61, e54–e59. [Google Scholar] [CrossRef]
- Puttachary, S.; Sharma, S.; Tse, K.; Beamer, E.; Sexton, A.; Crutison, J.; Thippeswamy, T. Immediate Epileptogenesis after Kainate-Induced Status Epilepticus in C57BL/6J Mice: Evidence from Long Term Continuous Video-EEG Telemetry. PLoS ONE 2015, 10, e0131705. [Google Scholar] [CrossRef] [PubMed]
- Borowicz-Reutt, K.K.; Czuczwar, S.J. Role of Oxidative Stress in Epileptogenesis and Potential Implications for Therapy. Pharmacol. Rep. 2020, 72, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Gage, M.; Putra, M.; Wachter, L.; Dishman, K.; Gard, M.; Gomez-Estrada, C.; Thippeswamy, T. Saracatinib, a Src Tyrosine Kinase Inhibitor, as a Disease Modifier in the Rat DFP Model: Sex Differences, Neurobehavior, Gliosis, Neurodegeneration, and Nitro-Oxidative Stress. Antioxidants 2022, 11, 61. [Google Scholar] [CrossRef]
- Wolinski, P.; Ksiazek-Winiarek, D.; Glabinski, A. Cytokines and Neurodegeneration in Epileptogenesis. Brain Sci. 2022, 12, 380. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Zeng, Y.; Zheng, W. Neuroinflammation in Epileptogenesis: From Pathophysiology to Therapeutic Strategies. Front. Immunol. 2023, 14, 1269241. [Google Scholar] [CrossRef]
- Sidell, F.R. Soman and Sarin: Clinical Manifestations and Treatment of Accident of Accidental Poisoning by Organophosphates. Clin. Toxicol. 1974, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Proctor, S.P.; Heaton, K.J.; Heeren, T.; White, R.F. Effects of Sarin and Cyclosarin Exposure during the 1991 Gulf War on Neurobehavioral Functioning in US Army Veterans. Neurotoxicology 2006, 27, 931–939. [Google Scholar] [CrossRef]
- Toomey, R.; Alpern, R.; Vasterling, J.J.; Baker, D.G.; Reda, D.J.; Lyons, M.J.; Henderson, W.G.; Kang, H.K.; Eisen, S.A.; Murphy, F.M. Neuropsychological Functioning of U.S. Gulf War Veterans 10 Years after the War. J. Int. Neuropsychol. Soc. 2009, 15, 717–729. [Google Scholar] [CrossRef]
- Löscher, W.; Brandt, C. Prevention or Modification of Epileptogenesis after Brain Insults: Experimental Approaches and Translational Research. Pharmacol. Rev. 2010, 62, 668–700. [Google Scholar] [CrossRef]
- Vezzani, A.; Friedman, A.; Dingledine, R.J. The Role of Inflammation in Epileptogenesis. Neuropharmacology 2013, 69, 16–24. [Google Scholar] [CrossRef]
- Engel, J.; Pitkänen, A. Biomarkers for Epileptogenesis and Its Treatment. Neuropharmacology 2020, 167, 107735. [Google Scholar] [CrossRef]
- Vasanthi, S.S.; Rao, N.S.; Samidurai, M.; Massey, N.; Meyer, C.; Gage, M.; Kharate, M.; Almanza, A.; Wachter, L.; Mafuta, C.; et al. Disease-Modifying Effects of a Glial-Targeted Inducible Nitric Oxide Synthase Inhibitor (1400W) in Mixed-Sex Cohorts of a Rat Soman (GD) Model of Epilepsy. J. Neuroinflammation 2023, 20, 163. [Google Scholar] [CrossRef]
- Massey, N.; Vasanthi, S.S.; Holtkamp, C.; Meyer, C.; Rao, N.S.; Gimenez-Lirola, L.G.; Wang, C.; Im, H.; Bevoor, A.S.; Kannurpatti, S.; et al. Mitigating Organophosphate Nerve Agent, Soman (GD), Induced Long-Term Neurotoxicity: Saracatinib, a Src Tyrosine Kinase Inhibitor, as a Potential Countermeasure. J. Neuroinflammation 2025, 22, 199. [Google Scholar] [CrossRef] [PubMed]
- Alolayan, Y.S.; McKinley, K.; Bhatia, R.; Alkhachroum, A. Review and Updates on the Treatment of Refractory and Super Refractory Status Epilepticus. J. Clin. Med. 2021, 10, 3028. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, L.S.; Lou, J.K.; Mian, A.; Blair, R.E.; Sombati, S.; Attkisson, E.; DeLorenzo, R.J. Time Course and Mechanism of Hippocampal Neuronal Death in an in Vitro Model of Status Epilepticus: Role of NMDA Receptor Activation and NMDA Dependent Calcium Entry. Eur. J. Pharmacol. 2008, 583, 73–83. [Google Scholar] [CrossRef]
- Marx, M.; Haas, C.A.; Häussler, U. Differential Vulnerability of Interneurons in the Epileptic Hippocampus. Front. Cell. Neurosci. 2013, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Fahnestock, M.; Racine, R.J. Kindling and Status Epilepticus Models of Epilepsy: Rewiring the Brain. Prog. Neurobiol. 2004, 73, 1–60. [Google Scholar] [CrossRef]
- Gage, M.; Vasanthi, S.S.; Meyer, C.M.; Rao, N.S.; Thedens, D.R.; Kannurpatti, S.S.; Thippeswamy, T. Sex-based Structural and Functional MRI Outcomes in the Rat Brain after Soman (GD) Exposure-induced Status Epilepticus. Epilepsia Open 2023, 8, 399–410. [Google Scholar] [CrossRef]
- Hobson, B.A.; Rowland, D.J.; Dou, Y.; Saito, N.; Harmany, Z.T.; Bruun, D.A.; Harvey, D.J.; Chaudhari, A.J.; Garbow, J.R.; Lein, P.J. A Longitudinal MRI and TSPO PET-Based Investigation of Brain Region-Specific Neuroprotection by Diazepam versus Midazolam Following Organophosphate-Induced Seizures. Neuropharmacology 2024, 251, 109918. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Bohnert, S.; Bouchard, M.; Vair, C.; Farrell, J.S.; Teskey, G.C.; Mikler, J.; Dunn, J.F. Quantitative T2 MRI Is Predictive of Neurodegeneration Following Organophosphate Exposure in a Rat Model. Sci. Rep. 2020, 10, 13007. [Google Scholar] [CrossRef]
- Reddy, S.D.; Wu, X.; Kuruba, R.; Sridhar, V.; Reddy, D.S. Magnetic Resonance Imaging Analysis of Long-term Neuropathology after Exposure to the Nerve Agent Soman: Correlation with Histopathology and Neurological Dysfunction. Ann. N. Y. Acad. Sci. 2020, 1480, 116–135. [Google Scholar] [CrossRef]
- Nass, R.D.; Wagner, M.; Surges, R.; Holdenrieder, S. Time Courses of HMGB1 and Other Inflammatory Markers after Generalized Convulsive Seizures. Epilepsy Res. 2020, 162, 106301. [Google Scholar] [CrossRef]
- Shih, T.-M.; McDonough, J.H., Jr.; Koplovitz, I. Anticonvulsants for Soman-Induced Seizure Activity1. J. Biomed. Sci. 1999, 6, 86–96. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Brown, I.R. Differential Induction of Heat Shock mRNA in Oligodendrocytes, Microglia, and Astrocytes Following Hyperthermia. Mol. Brain Res. 1997, 45, 207–218. [Google Scholar] [CrossRef]
- Matsuda, T.; Murao, N.; Katano, Y.; Juliandi, B.; Kohyama, J.; Akira, S.; Kawai, T.; Nakashima, K. TLR9 Signalling in Microglia Attenuates Seizure-Induced Aberrant Neurogenesis in the Adult Hippocampus. Nat. Commun. 2015, 6, 6514. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Hei, Y.; Yi, X.; Baskys, A.; Liu, W.; Long, Q. High Mobility Group Box-1 (HMGB1) Antagonist BoxA Suppresses Status Epilepticus-Induced Neuroinflammatory Responses Associated with Toll-like Receptor 2/4 down-Regulation in Rats. Brain Res. 2019, 1717, 44–51. [Google Scholar] [CrossRef]
- Hu, Y.; Yao, Y.; Qi, H.; Yang, J.; Zhang, C.; Zhang, A.; Liu, X.; Zhang, C.; Gan, G.; Zhu, X. Microglia Sense and Suppress Epileptic Neuronal Hyperexcitability. Pharmacol. Res. 2023, 195, 106881. [Google Scholar] [CrossRef]
- Eyo, U.B.; Peng, J.; Swiatkowski, P.; Mukherjee, A.; Bispo, A.; Wu, L.-J. Neuronal Hyperactivity Recruits Microglial Processes via Neuronal NMDA Receptors and Microglial P2Y12 Receptors after Status Epilepticus. J. Neurosci. 2014, 34, 10528–10540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, C.; Huang, D. Microglial Activation: An Important Process in the Onset of Epilepsy. Am. J. Transl. Res. 2018, 10, 2877–2889. [Google Scholar]
- Putra, M.; Sharma, S.; Gage, M.; Gasser, G.; Hinojo-Perez, A.; Olson, A.; Gregory-Flores, A.; Puttachary, S.; Wang, C.; Anantharam, V. Inducible Nitric Oxide Synthase Inhibitor, 1400W, Mitigates DFP-Induced Long-Term Neurotoxicity in the Rat Model. Neurobiol. Dis. 2020, 133, 104443. [Google Scholar] [CrossRef] [PubMed]
- Barath, A.S.; Wu, L. Microglial Phagocytosis in Epilepsy: Mechanisms and Impact. J. Physiol. 2025, JP288573. [Google Scholar] [CrossRef]
- Rojas, A.; McCarren, H.S.; Wang, J.; Wang, W.; Abreu-Melon, J.; Wang, S.; McDonough, J.H.; Dingledine, R. Comparison of Neuropathology in Rats Following Status Epilepticus Induced by Diisopropylfluorophosphate and Soman. NeuroToxicology 2021, 83, 14–27. [Google Scholar] [CrossRef]
- Somkhit, J.; Yanicostas, C.; Soussi-Yanicostas, N. Microglia Remodelling and Neuroinflammation Parallel Neuronal Hyperactivation Following Acute Organophosphate Poisoning. Int. J. Mol. Sci. 2022, 23, 8240. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.C.; Morrison, E.H. Histology, Astrocytes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Schartz, N.D.; Wyatt-Johnson, S.K.; Price, L.R.; Colin, S.A.; Brewster, A.L. Status Epilepticus Triggers Long-Lasting Activation of Complement C1q-C3 Signaling in the Hippocampus That Correlates with Seizure Frequency in Experimental Epilepsy. Neurobiol. Dis. 2018, 109, 163–173. [Google Scholar] [CrossRef]
- Putra, M.; Gage, M.; Sharma, S.; Gardner, C.; Gasser, G.; Anantharam, V.; Thippeswamy, T. Diapocynin, an NADPH Oxidase Inhibitor, Counteracts Diisopropylfluorophosphate-induced Long-term Neurotoxicity in the Rat Model. Ann. N. Y. Acad. Sci. 2020, 1479, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Gage, M.; Gard, M.; Thippeswamy, T. Characterization of Cortical Glial Scars in the Diisopropylfluorophosphate (DFP) Rat Model of Epilepsy. Neuroinflammation Acquir. Epilepsy 2022, 10, 867949. [Google Scholar] [CrossRef]
- Gruber, V.; Luinenburg, M.J.; Colleselli, K.; Endmayr, V.; Anink, J.J.; Zimmer, T.S.; Jansen, F.; Gosselaar, P.; Coras, R.; Scholl, T.; et al. Increased Expression of Complement Components in Tuberous Sclerosis Complex and Focal Cortical Dysplasia Type 2B Brain Lesions. Epilepsia 2022, 63, 364–374. [Google Scholar] [CrossRef]
- Meyer, C.; Rao, N.S.; Vasanthi, S.S.; Pereira, B.; Gage, M.; Putra, M.; Holtkamp, C.; Huss, J.; Thippeswamy, T. Peripheral and Central Effects of NADPH Oxidase Inhibitor, Mitoapocynin, in a Rat Model of Diisopropylfluorophosphate (DFP) Toxicity. Front. Cell. Neurosci. 2023, 17, 1195843. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, T.; Bosco, D.B.; Xie, M.; Zheng, J.; Dheer, A.; Ying, Y.; Wu, Q.; Lennon, V.A.; Wu, L. The Complement C3-C3aR Pathway Mediates Microglia–Astrocyte Interaction Following Status Epilepticus. Glia 2021, 69, 1155–1169. [Google Scholar] [CrossRef]
- Schartz, N.D.; Aroor, A.; Li, Y.; Pinzón-Hoyos, N.; Brewster, A.L. Mice Deficient in Complement C3 Are Protected against Recognition Memory Deficits and Astrogliosis Induced by Status Epilepticus. Front. Mol. Neurosci. 2023, 16, 1265944. [Google Scholar] [CrossRef] [PubMed]
- Sarac, S.; Afzal, S.; Broholm, H.; Madsen, F.F.; Ploug, T.; Laursen, H. Excitatory Amino Acid Transporters EAAT-1 and EAAT-2 in Temporal Lobe and Hippocampus in Intractable Temporal Lobe Epilepsy. APMIS 2009, 117, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Heuser, K.; Eid, T.; Lauritzen, F.; Thoren, A.E.; Vindedal, G.F.; Taubøll, E.; Gjerstad, L.; Spencer, D.D.; Ottersen, O.P.; Nagelhus, E.A.; et al. Loss of Perivascular Kir4.1 Potassium Channels in the Sclerotic Hippocampus of Patients With Mesial Temporal Lobe Epilepsy. J. Neuropathol. Exp. Neurol. 2012, 71, 814–825. [Google Scholar] [CrossRef]
- Verhoog, Q.P.; Holtman, L.; Aronica, E.; Van Vliet, E.A. Astrocytes as Guardians of Neuronal Excitability: Mechanisms Underlying Epileptogenesis. Front. Neurol. 2020, 11, 591690. [Google Scholar] [CrossRef]
- Meyer, C.; Grego, E.; Vasanthi, S.S.; Rao, N.S.; Massey, N.; Holtkamp, C.; Huss, J.; Showman, L.; Narasimhan, B.; Thippeswamy, T. The NADPH Oxidase Inhibitor, Mitoapocynin, Mitigates DFP-Induced Reactive Astrogliosis in a Rat Model of Organophosphate Neurotoxicity. Antioxidants 2023, 12, 2061. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of Oxidative Stress in Neurodegenerative Disorders: A Review of Reactive Oxygen Species and Prevention by Antioxidants. Brain Commun. 2023, 6, fcad356. [Google Scholar] [CrossRef]
- Martínez, M.C.; Andriantsitohaina, R. Reactive Nitrogen Species: Molecular Mechanisms and Potential Significance in Health and Disease. Antioxid. Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible to Oxidative Stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Łukawski, K.; Czuczwar, S.J. Oxidative Stress and Neurodegeneration in Animal Models of Seizures and Epilepsy. Antioxidants 2023, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- MacMullin, P.; Hodgson, N.; Damar, U.; Lee, H.H.C.; Hameed, M.Q.; Dhamne, S.C.; Hyde, D.; Conley, G.M.; Morriss, N.; Qiu, J.; et al. Increase in Seizure Susceptibility After Repetitive Concussion Results from Oxidative Stress, Parvalbumin-Positive Interneuron Dysfunction and Biphasic Increases in Glutamate/GABA Ratio. Cereb. Cortex 2020, 30, 6108–6120. [Google Scholar] [CrossRef]
- Roy, A.; Jana, A.; Yatish, K.; Freidt, M.B.; Fung, Y.K.; Martinson, J.A.; Pahan, K. Reactive Oxygen Species Up-Regulate CD11b in Microglia via Nitric Oxide: Implications for Neurodegenerative Diseases. Free Radic. Biol. Med. 2008, 45, 686–699. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, X.; Liu, K.; Zhang, X.; Yang, F.; Zhang, H.; He, Y.; Zhu, T.; Li, F.; Shi, W.; et al. NOX2 Deficiency Ameliorates Cerebral Injury through Reduction of Complexin II-Mediated Glutamate Excitotoxicity in Experimental Stroke. Free Radic. Biol. Med. 2013, 65, 942–951. [Google Scholar] [CrossRef]
- Lambeth, J.D. NOX Enzymes and the Biology of Reactive Oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Bonekamp, N.A.; Völkl, A.; Fahimi, H.D.; Schrader, M. Reactive Oxygen Species and Peroxisomes: Struggling for Balance. BioFactors 2009, 35, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Pecchillo Cimmino, T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef]
- Magnani, F.; Mattevi, A. Structure and Mechanisms of ROS Generation by NADPH Oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef]
- Ogboo, B.C.; Grabovyy, U.V.; Maini, A.; Scouten, S.; Van Der Vliet, A.; Mattevi, A.; Heppner, D.E. Architecture of the NADPH Oxidase Family of Enzymes. Redox Biol. 2022, 52, 102298. [Google Scholar] [CrossRef]
- Guo, S.; Chen, X. The Human Nox4: Gene, Structure, Physiological Function and Pathological Significance. J. Drug Target. 2015, 23, 888–896. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.M.-C.; El-Benna, J. P47phox and NOXO1, the Organizer Subunits of the NADPH Oxidase 2 (Nox2) and NADPH Oxidase 1 (Nox1). In NADPH Oxidases Revisited: From Function to Structure; Pick, E., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 249–261. ISBN 978-3-031-23751-5. [Google Scholar]
- Eid, S.A.; Savelieff, M.G.; Eid, A.A.; Feldman, E.L. Nox, Nox, Are You There? The Role of NADPH Oxidases in the Peripheral Nervous System. Antioxid. Redox Signal. 2022, 37, 613–630. [Google Scholar] [CrossRef]
- Ibi, M.; Katsuyama, M.; Fan, C.; Iwata, K.; Nishinaka, T.; Yokoyama, T.; Yabe-Nishimura, C. NOX1/NADPH Oxidase Negatively Regulates Nerve Growth Factor-Induced Neurite Outgrowth. Free Radic. Biol. Med. 2006, 40, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Chéret, C.; Gervais, A.; Lelli, A.; Colin, C.; Amar, L.; Ravassard, P.; Mallet, J.; Cumano, A.; Krause, K.-H.; Mallat, M. Neurotoxic Activation of Microglia Is Promoted by a Nox1-Dependent NADPH Oxidase. J. Neurosci. 2008, 28, 12039–12051. [Google Scholar] [CrossRef] [PubMed]
- Olguín-Albuerne, M.; Domínguez, G.; Morán, J. Effect of Staurosporine in the Morphology and Viability of Cerebellar Astrocytes: Role of Reactive Oxygen Species and NADPH Oxidase. Oxidative Med. Cell. Longev. 2014, 2014, 678371. [Google Scholar] [CrossRef]
- Kuriakose, M.; Younger, D.; Ravula, A.R.; Alay, E.; Rama Rao, K.V.; Chandra, N. Synergistic Role of Oxidative Stress and Blood-Brain Barrier Permeability as Injury Mechanisms in the Acute Pathophysiology of Blast-Induced Neurotrauma. Sci. Rep. 2019, 9, 7717. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Hirko, A.C.; King, M.A.; Liu, B. Role of NADPH Oxidase in Cooperative Reactive Oxygen Species Generation in Dopaminergic Neurons Induced by Combined Treatment with Dieldrin and Lindane. Toxicol. Lett. 2018, 299, 47–55. [Google Scholar] [CrossRef]
- Choi, D.-H.; Cristóvão, A.C.; Guhathakurta, S.; Lee, J.; Joh, T.H.; Beal, M.F.; Kim, Y.-S. NADPH Oxidase 1-Mediated Oxidative Stress Leads to Dopamine Neuron Death in Parkinson’s Disease. Antioxid. Redox Signal. 2012, 16, 1033–1045. [Google Scholar] [CrossRef]
- Schiavone, S.; Jaquet, V.; Sorce, S.; Dubois-Dauphin, M.; Hultqvist, M.; Bäckdahl, L.; Holmdahl, R.; Colaianna, M.; Cuomo, V.; Trabace, L.; et al. NADPH Oxidase Elevations in Pyramidal Neurons Drive Psychosocial Stress-Induced Neuropathology. Transl. Psychiatry 2012, 2, e111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.-H.; Kim, Y.-D.; Seo, J.H. Ultrafine Diesel Exhaust Particles Induce Apoptosis of Oligodendrocytes by Increasing Intracellular Reactive Oxygen Species through NADPH Oxidase Activation. Antioxidants 2022, 11, 1031. [Google Scholar] [CrossRef]
- Ejlerskov, P.; Christensen, D.P.; Beyaie, D.; Burritt, J.B.; Paclet, M.-H.; Gorlach, A.; Van Deurs, B.; Vilhardt, F. NADPH Oxidase Is Internalized by Clathrin-Coated Pits and Localizes to a Rab27A/B GTPase-Regulated Secretory Compartment in Activated Macrophages. J. Biol. Chem. 2012, 287, 4835–4852. [Google Scholar] [CrossRef]
- Li, S.; Guo, Z.; Liu, J.; Ma, Y.; Zhang, X.; Hou, L.; Wang, Q.; Jiang, W.; Wang, Q. CD11b-NOX2 Mutual Regulation-Mediated Microglial Exosome Release Contributes to Rotenone-Induced Inflammation and Neurotoxicity in BV2 Microglia and Primary Cultures. Free Radic. Biol. Med. 2024, 224, 436–446. [Google Scholar] [CrossRef]
- Loth, M.K.; Guariglia, S.R.; Re, D.B.; Perez, J.; De Paiva, V.N.; Dziedzic, J.L.; Chambers, J.W.; Azzam, D.J.; Guilarte, T.R. A Novel Interaction of Translocator Protein 18 kDa (TSPO) with NADPH Oxidase in Microglia. Mol. Neurobiol. 2020, 57, 4467–4487. [Google Scholar] [CrossRef]
- Accetta, R.; Damiano, S.; Morano, A.; Mondola, P.; Paternò, R.; Avvedimento, E.V.; Santillo, M. Reactive Oxygen Species Derived from NOX3 and NOX5 Drive Differentiation of Human Oligodendrocytes. Front. Cell. Neurosci. 2016, 10, 146. [Google Scholar] [CrossRef]
- Saadi, A.; Sandouka, S.; Grad, E.; Singh, P.K.; Shekh-Ahmad, T. Spatial, Temporal, and Cell-Type-Specific Expression of NADPH Oxidase Isoforms Following Seizure Models in Rats. Free Radic. Biol. Med. 2022, 190, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.A.; Lively, S.; Schlichter, L.C. Complex Molecular and Functional Outcomes of Single versus Sequential Cytokine Stimulation of Rat Microglia. J. Neuroinflammation 2016, 13, 66. [Google Scholar] [CrossRef]
- Boonpraman, N.; Yoon, S.; Kim, C.Y.; Moon, J.-S.; Yi, S.S. NOX4 as a Critical Effector Mediating Neuroinflammatory Cytokines, Myeloperoxidase and Osteopontin, Specifically in Astrocytes in the Hippocampus in Parkinson’s Disease. Redox Biol. 2023, 62, 102698. [Google Scholar] [CrossRef]
- Shen, J.; Li, G.; Zhu, Y.; Xu, Q.; Zhou, H.; Xu, K.; Huang, K.; Zhan, R.; Pan, J. Foxo1-induced miR-92b Down-regulation Promotes Blood-brain Barrier Damage after Ischaemic Stroke by Targeting NOX4. J. Cell. Mol. Med. 2021, 25, 5269–5282. [Google Scholar] [CrossRef]
- Kuroda, J.; Ago, T.; Nishimura, A.; Nakamura, K.; Matsuo, R.; Wakisaka, Y.; Kamouchi, M.; Kitazono, T. Nox4 Is a Major Source of Superoxide Production in Human Brain Pericytes. J. Vasc. Res. 2014, 51, 429–438. [Google Scholar] [CrossRef]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.-S. NOX4 Promotes Ferroptosis of Astrocytes by Oxidative Stress-Induced Lipid Peroxidation via the Impairment of Mitochondrial Metabolism in Alzheimer’s Diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef] [PubMed]
- Gola, L.; Bierhansl, L.; Csatári, J.; Schroeter, C.B.; Korn, L.; Narayanan, V.; Cerina, M.; Abdolahi, S.; Speicher, A.; Hermann, A.M.; et al. NOX4-Derived ROS Are Neuroprotective by Balancing Intracellular Calcium Stores. Cell. Mol. Life Sci. 2023, 80, 127. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yang, Y.; Yang, Q.; Pang, B.; Sun, S.; Wang, Y.; Qiao, Q.; Guo, C.; Liu, H.; Pang, Q. NOX4-Derived ROS-Induced Overexpression of FOXM1 Regulates Aerobic Glycolysis in Glioblastoma. BMC Cancer 2021, 21, 1181. [Google Scholar] [CrossRef] [PubMed]
- Marqués, J.; Fernández-Irigoyen, J.; Ainzúa, E.; Martínez-Azcona, M.; Cortés, A.; Roncal, C.; Orbe, J.; Santamaría, E.; Zalba, G. NADPH Oxidase 5 (NOX5) Overexpression Promotes Endothelial Dysfunction via Cell Apoptosis, Migration, and Metabolic Alterations in Human Brain Microvascular Endothelial Cells (hCMEC/D3). Antioxidants 2022, 11, 2147. [Google Scholar] [CrossRef]
- Reinehr, R.; Görg, B.; Becker, S.; Qvartskhava, N.; Bidmon, H.J.; Selbach, O.; Haas, H.L.; Schliess, F.; Häussinger, D. Hypoosmotic Swelling and Ammonia Increase Oxidative Stress by NADPH Oxidase in Cultured Astrocytes and Vital Brain Slices. Glia 2007, 55, 758–771. [Google Scholar] [CrossRef]
- Kim, J.; Moon, J.-S. Molecular Roles of NADPH Oxidase-Mediated Oxidative Stress in Alzheimer’s Disease: Isoform-Specific Contributions. Int. J. Mol. Sci. 2024, 25, 12299. [Google Scholar] [CrossRef]
- Damiano, S.; Fusco, R.; Morano, A.; De Mizio, M.; Paternò, R.; De Rosa, A.; Spinelli, R.; Amente, S.; Frunzio, R.; Mondola, P.; et al. Reactive Oxygen Species Regulate the Levels of Dual Oxidase (Duox1-2) in Human Neuroblastoma Cells. PLoS ONE 2012, 7, e34405. [Google Scholar] [CrossRef] [PubMed]
- Herb, M. NADPH Oxidase 3: Beyond the Inner Ear. Antioxidants 2024, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.; Solas, M.; Pejenaute, Á.; Abellanas, M.A.; Garcia-Lacarte, M.; Aymerich, M.S.; Marqués, J.; Ramírez, M.J.; Zalba, G. Expression of Endothelial NOX5 Alters the Integrity of the Blood-Brain Barrier and Causes Loss of Memory in Aging Mice. Antioxidants 2021, 10, 1311. [Google Scholar] [CrossRef]
- Barati, A.; Masoudi, R.; Yousefi, R.; Monsefi, M.; Mirshafiey, A. Tau and Amyloid Beta Differentially Affect the Innate Immune Genes Expression in Drosophila Models of Alzheimer’s Disease and β- D Mannuronic Acid (M2000) Modulates the Dysregulation. Gene 2022, 808, 145972. [Google Scholar] [CrossRef]
- Choi, D.-H.; Kim, J.-H.; Lee, K.-H.; Kim, H.-Y.; Kim, Y.-S.; Choi, W.S.; Lee, J. Role of Neuronal NADPH Oxidase 1 in the Peri-Infarct Regions after Stroke. PLoS ONE 2015, 10, e0116814. [Google Scholar] [CrossRef]
- Deng, M.; Sun, J.; Peng, L.; Huang, Y.; Jiang, W.; Wu, S.; Zhou, L.; Chung, S.K.; Cheng, X. Scutellarin Acts on the AR-NOX Axis to Remediate Oxidative Stress Injury in a Mouse Model of Cerebral Ischemia/Reperfusion Injury. Phytomedicine 2022, 103, 154214. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, W.; Zhang, Y.; Feng, W.; Bai, B.; Ji, B.; Chen, J.; Cheng, B.; Yan, F. NOX1 Triggers Ferroptosis and Ferritinophagy, Contributes to Parkinson’s Disease. Free Radic. Biol. Med. 2024, 222, 331–343. [Google Scholar] [CrossRef]
- Boonpraman, N.; Yi, S.S. NADPH Oxidase 4 (NOX4) as a Biomarker and Therapeutic Target in Neurodegenerative Diseases. Neural Regen. Res. 2024, 19, 1961–1966. [Google Scholar] [CrossRef]
- Bano, N.; Khan, S.; Ahamad, S.; Dar, N.J.; Alanazi, H.H.; Nazir, A.; Bhat, S.A. Microglial NOX2 as a Therapeutic Target in Traumatic Brain Injury: Mechanisms, Consequences, and Potential for Neuroprotection. Ageing Res. Rev. 2025, 108, 102735. [Google Scholar] [CrossRef]
- Kumar, A.; Barrett, J.P.; Alvarez-Croda, D.-M.; Stoica, B.A.; Faden, A.I.; Loane, D.J. NOX2 Drives M1-like Microglial/Macrophage Activation and Neurodegeneration Following Experimental Traumatic Brain Injury. Brain Behav. Immun. 2016, 58, 291–309. [Google Scholar] [CrossRef]
- Tu, D.; Velagapudi, R.; Gao, Y.; Hong, J.-S.; Zhou, H.; Gao, H.-M. Activation of Neuronal NADPH Oxidase NOX2 Promotes Inflammatory Neurodegeneration. Free Radic. Biol. Med. 2023, 200, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, H.; Liu, S. Insight into the Role of Ferroptosis in Epilepsy. J. Integr. Neurosci. 2024, 23, 113. [Google Scholar] [CrossRef]
- Walker, M.C. Reactive Oxygen Species in Status Epilepticus. Epilepsia Open 2023, 8, S66–S72. [Google Scholar] [CrossRef]
- Pecorelli, A.; Natrella, F.; Belmonte, G.; Miracco, C.; Cervellati, F.; Ciccoli, L.; Mariottini, A.; Rocchi, R.; Vatti, G.; Bua, A.; et al. NADPH Oxidase Activation and 4-Hydroxy-2-Nonenal/Aquaporin-4 Adducts as Possible New Players in Oxidative Neuronal Damage Presents in Drug-Resistant Epilepsy. Biochim. Et. Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 507–519. [Google Scholar] [CrossRef]
- Wood, H.C. A study of Apocynum Cannabinum. JAMA 1904, XLIII, 1953. [Google Scholar] [CrossRef]
- Britto, G.F.; Subash, K.R.; Nagamani, N. Natural Treasures from Picrorhiza Kurrooa: A Computational Exploration of Drug-like Properties and Bioactivity of Kutkin, Cucurbitacin, Apocynin and Lupanine. Int. J. Basic. Clin. Pharmacol. 2024, 13, 223–227. [Google Scholar] [CrossRef]
- Savla, S.R.; Laddha, A.P.; Kulkarni, Y.A. Pharmacology of Apocynin: A Natural Acetophenone. Drug Metab. Rev. 2021, 53, 542–562. [Google Scholar] [CrossRef]
- Nascimento, A.L.F.; Medeiros, P.O.S.; Pedrão, L.F.A.T.; Queiroz, V.C.; Oliveira, L.M.; Novaes, L.S.; Caetano, A.L.; Munhoz, C.D.; Takakura, A.C.; Falquetto, B. Oxidative Stress Inhibition Via Apocynin Prevents Medullary Respiratory Neurodegeneration and Respiratory Pattern Dysfunction in a 6-Hydroxydopamine Animal Model of Parkinson’s Disease. Neuroscience 2022, 502, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tompkins, K.D.; Simonyi, A.; Korthuis, R.J.; Sun, A.Y.; Sun, G.Y. Apocynin Protects against Global Cerebral Ischemia–Reperfusion-Induced Oxidative Stress and Injury in the Gerbil Hippocampus. Brain Res. 2006, 1090, 182–189. [Google Scholar] [CrossRef]
- De Diego-Otero, Y.; El Bekay, R.; García-Guirado, F.; Sánchez-Salido, L.; Giráldez-Pérez, R.M. Apocynin, a Selective NADPH Oxidase (Nox2) Inhibitor, Ameliorates Behavioural and Learning Deficits in the Fragile X Syndrome Mouse Model. Biomedicines 2024, 12, 2887. [Google Scholar] [CrossRef]
- Yang, T.; Zang, D.-W.; Shan, W.; Guo, A.-C.; Wu, J.-P.; Wang, Y.-J.; Wang, Q. Synthesis and Evaluations of Novel Apocynin Derivatives as Anti-Glioma Agents. Front. Pharmacol. 2019, 10, 951. [Google Scholar] [CrossRef]
- Okamura, T.; Okada, M.; Kikuchi, T.; Wakizaka, H.; Zhang, M.-R. Kinetics and Metabolism of Apocynin in the Mouse Brain Assessed with Positron-Emission Tomography. Phytomedicine 2018, 38, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Ferriz, J.M.; Vinsova, J. Prodrug Design of Phenolic Drugs. CPD 2010, 16, 2033–2052. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, L.; Song, Y.; Ye, X.; Fu, S.; Jiang, J.; Li, S. Improvement of Pharmacokinetics Behavior of Apocynin by Nitrone Derivatization: Comparative Pharmacokinetics of Nitrone-Apocynin and Its Parent Apocynin in Rats. PLoS ONE 2013, 8, e70189. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Wan, S.; Wang, Y.; Yu, P. Protective Effects of Apocynin Nitrone on Acute Lung Injury Induced by Lipopolysaccharide in Rats. Int. Immunopharmacol. 2014, 20, 377–382. [Google Scholar] [CrossRef]
- Wang, Q.; Smith, R.E.; Luchtefeld, R.; Sun, A.Y.; Simonyi, A.; Luo, R.; Sun, G.Y. Bioavailability of Apocynin through Its Conversion to Glycoconjugate but Not to Diapocynin. Phytomedicine 2008, 15, 496–503. [Google Scholar] [CrossRef]
- Ismail, H.M.; Scapozza, L.; Ruegg, U.T.; Dorchies, O.M. Diapocynin, a Dimer of the NADPH Oxidase Inhibitor Apocynin, Reduces ROS Production and Prevents Force Loss in Eccentrically Contracting Dystrophic Muscle. PLoS ONE 2014, 9, e110708. [Google Scholar] [CrossRef]
- Luchtefeld, R.; Luo, R.; Stine, K.; Alt, M.L.; Chernovitz, P.A.; Smith, R.E. Dose Formulation and Analysis of Diapocynin. J. Agric. Food Chem. 2008, 56, 301–306. [Google Scholar] [CrossRef]
- Luchtefeld, R.; Dasari, M.S.; Richards, K.M.; Alt, M.L.; Crawford, C.F.P.; Schleiden, A.; Ingram, J.; Hamidou, A.A.A.; Williams, A.; Chernovitz, P.A.; et al. Synthesis of Diapocynin. J. Chem. Educ. 2008, 85, 411. [Google Scholar] [CrossRef]
- Chandasana, H.; Chhonker, Y.S.; Bala, V.; Prasad, Y.D.; Chaitanya, T.K.; Sharma, V.L.; Bhatta, R.S. Pharmacokinetic, Bioavailability, Metabolism and Plasma Protein Binding Evaluation of NADPH-Oxidase Inhibitor Apocynin Using LC–MS/MS. J. Chromatogr. B 2015, 985, 180–188. [Google Scholar] [CrossRef]
- Kanegae, M.P.P.; Condino-Neto, A.; Pedroza, L.A.; De Almeida, A.C.; Rehder, J.; Da Fonseca, L.M.; Ximenes, V.F. Diapocynin versus Apocynin as Pretranscriptional Inhibitors of NADPH Oxidase and Cytokine Production by Peripheral Blood Mononuclear Cells. Biochem. Biophys. Res. Commun. 2010, 393, 551–554. [Google Scholar] [CrossRef]
- Madretsma, G.; Vondijk, A.; Tak, C.; Donze, G.; Wilson, J.; Ziljstra, J. Nicotine Inhibits the Production of IL-2 and TNF-? By Peripheral Blood and Intestinal Mono-Nuclear Cells. Neth. J. Med. 1995, 47, A41. [Google Scholar] [CrossRef]
- Ghosh, A.; Kanthasamy, A.; Joseph, J.; Anantharam, V.; Srivastava, P.; Dranka, B.P.; Kalyanaraman, B.; Kanthasamy, A.G. Anti-Inflammatory and Neuroprotective Effects of an Orally Active Apocynin Derivative in Pre-Clinical Models of Parkinson’s Disease. J. Neuroinflammation 2012, 9, 241. [Google Scholar] [CrossRef]
- Ibrahim, W.W.; Abdel Rasheed, N.O. Diapocynin Neuroprotective Effects in 3-Nitropropionic Acid Huntington’s Disease Model in Rats: Emphasis on Sirt1/Nrf2 Signaling Pathway. Inflammopharmacology 2022, 30, 1745–1758. [Google Scholar] [CrossRef]
- Ghosh, A.; Langley, M.R.; Harischandra, D.S.; Neal, M.L.; Jin, H.; Anantharam, V.; Joseph, J.; Brenza, T.; Narasimhan, B.; Kanthasamy, A. Mitoapocynin Treatment Protects against Neuroinflammation and Dopaminergic Neurodegeneration in a Preclinical Animal Model of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2016, 11, 259–278. [Google Scholar] [CrossRef]
- Dranka, B.P.; Gifford, A.; McAllister, D.; Zielonka, J.; Joseph, J.; O’Hara, C.L.; Stucky, C.L.; Kanthasamy, A.G.; Kalyanaraman, B. A Novel Mitochondrially-Targeted Apocynin Derivative Prevents Hyposmia and Loss of Motor Function in the Leucine-Rich Repeat Kinase 2 (LRRK2R1441G) Transgenic Mouse Model of Parkinson’s Disease. Neurosci. Lett. 2014, 583, 159–164. [Google Scholar] [CrossRef]
- Langley, M.; Ghosh, A.; Charli, A.; Sarkar, S.; Ay, M.; Luo, J.; Zielonka, J.; Brenza, T.; Bennett, B.; Jin, H.; et al. Mito-Apocynin Prevents Mitochondrial Dysfunction, Microglial Activation, Oxidative Damage, and Progressive Neurodegeneration in MitoPark Transgenic Mice. Antioxid. Redox Signal. 2017, 27, 1048–1066. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, B.G.; Choi, B.Y.; Kim, H.S.; Sohn, M.; Chung, T.N.; Choi, H.C.; Song, H.K.; Suh, S.W. Post-Treatment of an NADPH Oxidase Inhibitor Prevents Seizure-Induced Neuronal Death. Brain Res. 2013, 1499, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, B.Y.; Kho, A.R.; Jeong, J.H.; Hong, D.K.; Kang, D.H.; Kang, B.S.; Song, H.K.; Choi, H.C.; Suh, S.W. Inhibition of NADPH Oxidase Activation by Apocynin Rescues Seizure-Induced Reduction of Adult Hippocampal Neurogenesis. Int. J. Mol. Sci. 2018, 19, 3087. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, K.; Bai, Y.; Zhang, A.; Xia, Z.; Chao, J.; Yao, H. NADPH Oxidase Activation Is Required for Pentylenetetrazole Kindling-Induced Hippocampal Autophagy. Free Radic. Biol. Med. 2016, 94, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, G.; Kumar, P. Neuroprotective Role of Apocynin against Pentylenetetrazole Kindling Epilepsy and Associated Comorbidities in Mice by Suppression of ROS/RNS. Behav. Brain Res. 2022, 419, 113699. [Google Scholar] [CrossRef]
- Liu, N.; Lin, M.-M.; Huang, S.-S.; Liu, Z.-Q.; Wu, J.-C.; Liang, Z.-Q.; Qin, Z.-H.; Wang, Y. NADPH and Mito-Apocynin Treatment Protects against Ka-Induced Excitotoxic Injury through Autophagy Pathway. Front. Cell Dev. Biol. 2021, 9, 612554. [Google Scholar] [CrossRef]
- Singh, P.K.; Saadi, A.; Sheeni, Y.; Shekh-Ahmad, T. Specific Inhibition of NADPH Oxidase 2 Modifies Chronic Epilepsy. Redox Biol. 2022, 58, 102549. [Google Scholar] [CrossRef]
- Yang, J.-J.; Liu, Y.-X.; Wang, Y.-F.; Ge, B.-Y.; Wang, Y.; Wang, Q.-S.; Li, S.; Zhang, J.-J.; Jin, L.-L.; Hong, J.-S.; et al. Anti-Epileptic and Neuroprotective Effects of Ultra-Low Dose NADPH Oxidase Inhibitor Dextromethorphan on Kainic Acid-Induced Chronic Temporal Lobe Epilepsy in Rats. Neurosci. Bull. 2024, 40, 577–593. [Google Scholar] [CrossRef]
- Malkov, A.; Ivanov, A.I.; Latyshkova, A.; Bregestovski, P.; Zilberter, M.; Zilberter, Y. Activation of Nicotinamide Adenine Dinucleotide Phosphate Oxidase Is the Primary Trigger of Epileptic Seizures in Rodent Models. Ann. Neurol. 2019, 85, 907–920. [Google Scholar] [CrossRef]
- Singh, P.K.; Maurya, S.; Saadi, A.; Shekh-Ahmad, T. Targeting NOX2 Mitigates Seizure Susceptibility, Oxidative Stress, and Neuroinflammation in the Pentylenetetrazol Seizure Model. Free Radic. Biol. Med. 2025, 235, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Parfenova, H. NOX1/NOX4 Inhibitor Setanaxib Reduces Brain Oxidative Stress and Prevents Cerebrovascular Disease Caused by Epileptic Seizures. Physiology 2025, 40, 0983. [Google Scholar] [CrossRef]
- Rojas, A.; Abreu-Melon, J.; Wang, S.; Dingledine, R. Time-Dependent Neuropathology in Rats Following Organophosphate-Induced Status Epilepticus. Neurotoxicology 2022, 91, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, P.N.; Hobson, B.A.; Huddleston, S.L.; Andrew, P.M.; MacMahon, J.A.; Saito, N.H.; Porter, V.A.; Bruun, D.A.; Harvey, D.J.; Garbow, J.R. Time-and Region-Dependent Blood-Brain Barrier Impairment in a Rat Model of Organophosphate-Induced Status Epilepticus. Neurobiol. Dis. 2023, 187, 106316. [Google Scholar] [CrossRef] [PubMed]
| Isoform | Cell Type | Subcellular Localization | Product |
|---|---|---|---|
| NOX1 | Neurons [134], microglia [135], astrocytes [136], neurovascular endothelial [137] | Phagosomal membrane [135], mitochondria [138], nucleus [139] | O2•− |
| NOX2 | Neurons [140], microglia [110], astrocytes [136], oligodendrocytes [141] | Plasma membrane [142,143], mitochondria-associated endoplasmic reticulum membrane [144] | O2•− |
| NOX3 | Oligodendrocyte precursor cells [145] | Unknown | O2•− |
| NOX4 | Neurons [146], microglia [147], astrocytes [148], microvascular endothelial [149], pericytes [150] | Mitochondria [151], mitochondria-endoplasmic reticulum contact sites [152], nucleus [152], cytoplasm [153] | H2O2 |
| NOX5 | Microvascular endothelial [154], oligodendrocyte precursor cells [145] | Unknown | O2•− |
| DUOX1 | Astrocytes [155], oligodendrocytes [156], neuroblastoma [157] | Unknown | H2O2 |
| DUOX2 | Astrocytes [155], oligodendrocytes [156], neuroblastoma [157] | Unknown | H2O2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, C.; Thippeswamy, T. Organophosphate Chemical Nerve Agents, Oxidative Stress, and NADPH Oxidase Inhibitors: An Overview. Int. J. Mol. Sci. 2025, 26, 9313. https://doi.org/10.3390/ijms26199313
Meyer C, Thippeswamy T. Organophosphate Chemical Nerve Agents, Oxidative Stress, and NADPH Oxidase Inhibitors: An Overview. International Journal of Molecular Sciences. 2025; 26(19):9313. https://doi.org/10.3390/ijms26199313
Chicago/Turabian StyleMeyer, Christina, and Thimmasettappa Thippeswamy. 2025. "Organophosphate Chemical Nerve Agents, Oxidative Stress, and NADPH Oxidase Inhibitors: An Overview" International Journal of Molecular Sciences 26, no. 19: 9313. https://doi.org/10.3390/ijms26199313
APA StyleMeyer, C., & Thippeswamy, T. (2025). Organophosphate Chemical Nerve Agents, Oxidative Stress, and NADPH Oxidase Inhibitors: An Overview. International Journal of Molecular Sciences, 26(19), 9313. https://doi.org/10.3390/ijms26199313






