Elevated Matrix Metalloproteinase Type 9 (MMP-9) Transcripts After Thymoglobulin Induction in Incident Kidney Transplant Recipients
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
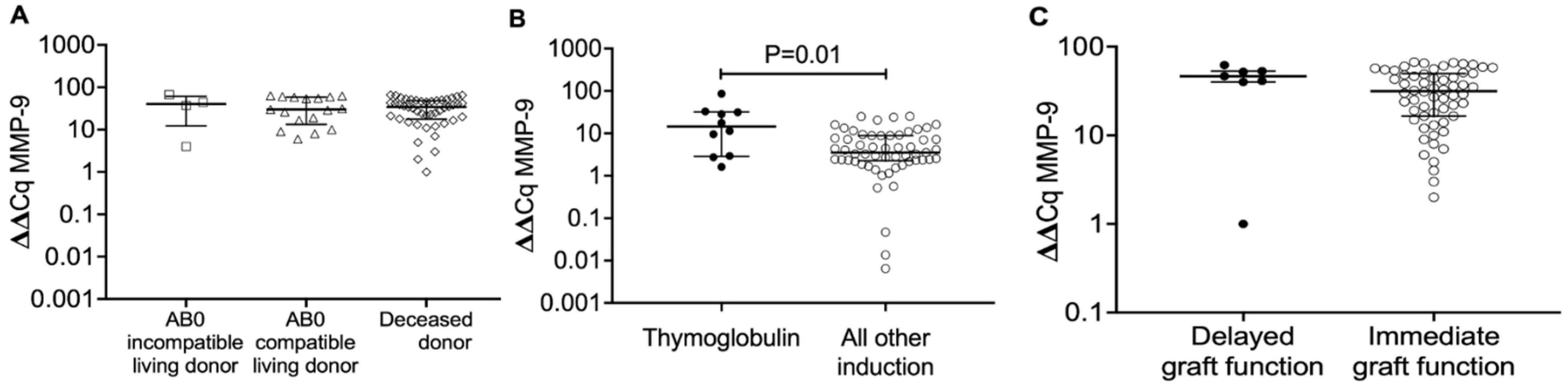
2.2. MMP-9 Transcripts in Blood Mononuclear Cells from Recipients, Who Obtained a Transplant from AB0-Incompatible Living Donors, AB0-Compatible Living Donors and Deceased Donors, 8 Days After Transplantation
2.3. MMP-9 Transcripts in Blood Mononuclear Cells from Kidney Transplant Recipients According to Type of Induction Therapy (Thymoglobulin vs. All Other Induction Therapies) 8 Days After Transplantation
2.4. MMP-9 Transcript Levels in Blood Mononuclear Cells from Kidney Transplant Recipients 8 Days After Kidney Transplantation in Delayed Versus Immediate Graft Function
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Study Cohort
4.3. Outcome Variables
4.4. Isolation of Peripheral Blood Mononuclear Cells
4.5. Purification of Total RNA and Synthesis of Complementary DNA
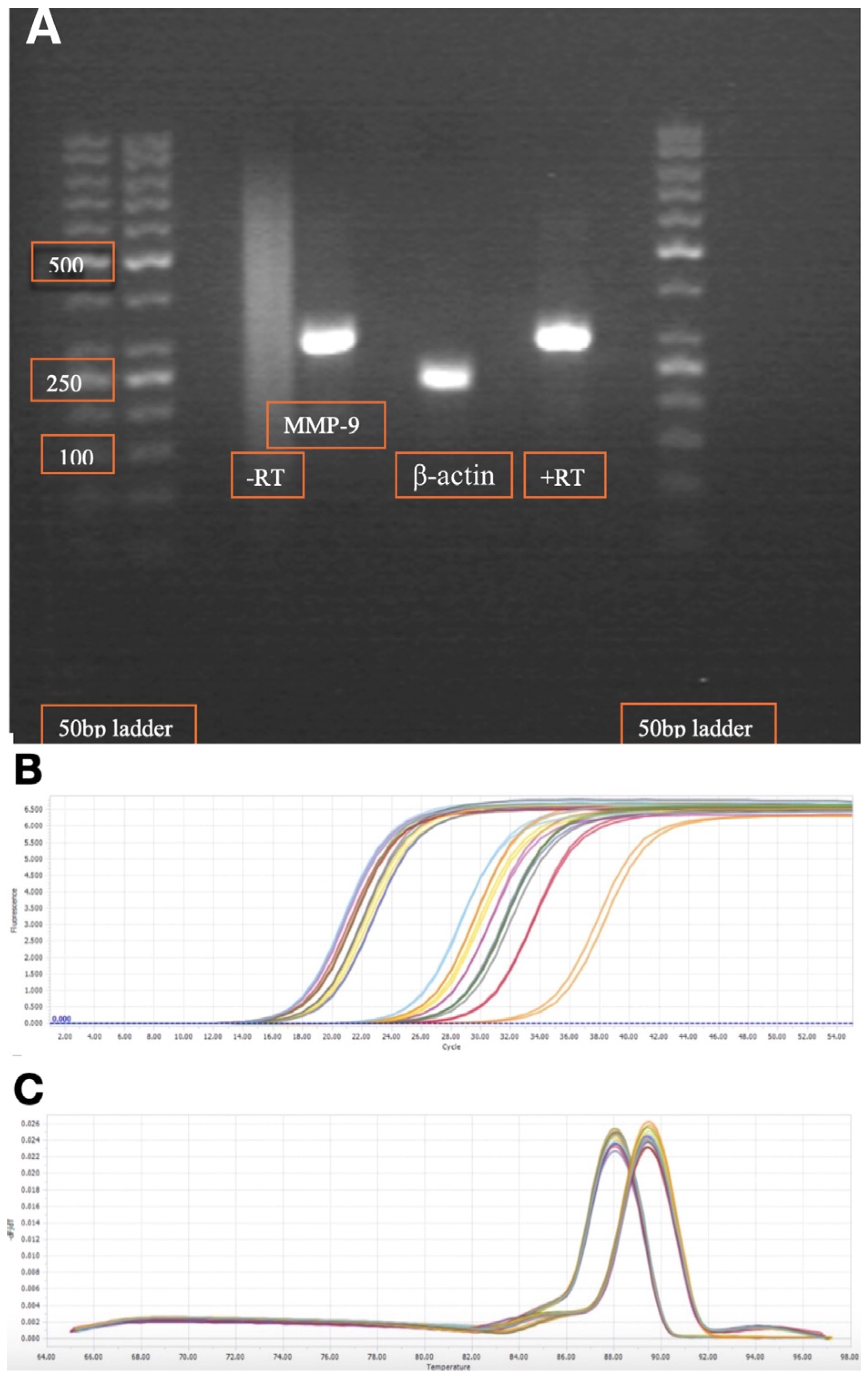
4.6. Primers and Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
- MMP-9 primer sequence
- β-actin primer sequence:
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DGF | delayed graft function |
| IQR | interquartile range |
| KTR | kidney transplant recipient |
| MMP-9 | matrix metalloproteinase type 9 |
| PCR | polymerase chain reaction |
References
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.-h.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Chaudhry, D.; Chaudhry, A.; Peracha, J.; Sharif, A. Survival for waitlisted kidney failure patients receiving transplantation versus remaining on waiting list: Systematic review and meta-analysis. BMJ 2022, 376, e068769. [Google Scholar] [CrossRef]
- Chen, H.-F.; Ali, H.; Marrero, W.J.; Parikh, N.D.; Lavieri, M.S.; Hutton, D.W. The Magnitude of the Health and Economic Impact of Increased Organ Donation on Patients with End-Stage Renal Disease. MDM Policy Pract. 2021, 6, 238146832110634. [Google Scholar] [CrossRef]
- Jensen, C.E.; Sørensen, P.; Petersen, K.D. In Denmark kidney transplantation is more cost-effective than dialysis. Dan. Med. J. 2014, 61, A4796. [Google Scholar]
- Lewis, A.; Koukoura, A.; Tsianos, G.-I.; Gargavanis, A.A.; Nielsen, A.A.; Vassiliadis, E. Organ donation in the US and Europe: The supply vs demand imbalance. Transplant. Rev. 2021, 35, 100585. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.C. Improving Long-Term Graft Survival as a Means to Reduce Retransplantation; Springer: Dordrecht, The Netherlands, 1997; pp. 277–287. [Google Scholar]
- Hariharan, S.; Israni, A.K.; Danovitch, G. Long-Term Survival after Kidney Transplantation. N. Engl. J. Med. 2021, 385, 729–743. [Google Scholar] [CrossRef]
- Sparding, N.; Genovese, F.; Rasmussen, D.G.K.; Karsdal, M.A.; Krogstrup, N.V.; Nielsen, M.B.; Hornum, M.; Nagarajah, S.; Birn, H.; The CONTEXT Study Group; et al. Endotrophin Levels Are Associated with Allograft Outcomes in Kidney Transplant Recipients. Biomolecules 2023, 13, 792. [Google Scholar] [CrossRef] [PubMed]
- La Russa, A.; Serra, R.; Faga, T.; Crugliano, G.; Bonelli, A.; Coppolino, G.; Bolignano, D.; Battaglia, Y.; Ielapi, N.; Costa, D.; et al. Kidney Fibrosis and Matrix Metalloproteinases (MMPs). Front. Biosci.-Landmark 2024, 29, 192. [Google Scholar] [CrossRef]
- Ahmed, A.K.; El Nahas, A.M.; Johnson, T.S. Changes in Matrix Metalloproteinases and Their Inhibitors in Kidney Transplant Recipients. Exp. Clin. Transplant. 2012, 10, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.J.; Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol.-Ren. Physiol. 2012, 302, F1351–F1361. [Google Scholar] [CrossRef]
- Zakiyanov, O.; Kalousová, M.; Zima, T.; Tesař, V. Matrix Metalloproteinases and Tissue Inhibitors of Matrix Metalloproteinases in Kidney Disease; Elsevier: Amsterdam, The Netherlands, 2021; pp. 141–212. [Google Scholar]
- Lobmann, R.; Ambrosch, A.; Schultz, G.; Waldmann, K.; Schiweck, S.; Lehnert, H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002, 45, 1011–1016. [Google Scholar] [CrossRef]
- Zakiyanov, O.; Kalousová, M.; Zima, T.; Tesař, V. Matrix Metalloproteinases in Renal Diseases: A Critical Appraisal. Kidney Blood Press. Res. 2019, 44, 298–330. [Google Scholar] [CrossRef] [PubMed]
- Akbari, T.; Fard, T.K.; Fadaei, R.; Rostami, R.; Moradi, N.; Movahedi, M.; Fallah, S. Evaluation of MMP-9, IL-6, TNF-α levels and peripheral blood mononuclear cells genes expression of MMP-9 and TIMP-1 in Iranian patients with coronary artery disease. J. Cardiovasc. Thorac. Res. 2023, 15, 223–230. [Google Scholar] [CrossRef]
- Thiyagarajan, U.M.; Ponnuswamy, A.; Bagul, A. Thymoglobulin and Its Use in Renal Transplantation: A Review. Am. J. Nephrol. 2013, 37, 586–601. [Google Scholar] [CrossRef]
- Turunen, A.J.; Lindgren, L.; Salmela, K.; Kyllönen, L.; Andersson, S.; Pesonen, E. Matrix Metalloproteinase-9 and Graft Preservation Injury in Clinical Renal Transplantation. Transplant. Proc. 2015, 47, 2831–2835. [Google Scholar] [CrossRef] [PubMed]
- Weerd, A.E.; van den Brand, J.A.; Bouwsma, H.; de Vries, A.P.; Dooper, I.P.; Sanders, J.S.; Christiaans, M.H.; van Reekum, F.E.; van Zuilen, A.D.; Bemelman, F.J.; et al. ABO-incompatible kidney transplantation in perspective of deceased donor transplantation and induction strategies: A propensity-matched analysis. Transplant. Int. 2021, 34, 2706–2719. [Google Scholar] [CrossRef]
- Eckardt, K.-U.; Kasiske, B.L.; Zeier, M.G. Special Issue: KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transplant. 2009, 9, S1–S155. [Google Scholar] [CrossRef]
- Tepel, M.; Alkaff, F.F.; Kremer, D.; Bakker, S.J.L.; Thaunat, O.; Nagarajah, S.; Saleh, Q.; Berger, S.P.; Born, J.v.D.; Krogstrup, N.V.; et al. Pretransplant endotrophin predicts delayed graft function after kidney transplantation. Sci. Sci. Reports 2022, 12, 4079. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Shi, B.-Y.; Qian, Y.-Y.; Bai, H.-W.; Xiao, L.; He, X.-Y. Clinical Significance of Monitoring Serum Level of Matrix Metalloproteinase 9 in Patients with Acute Kidney Allograft Rejection. Transplant. Proc. 2015, 47, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, D.; Kościelska-Kasprzak, K.; Mazanowska, O.; Żabińska, M.; Bartoszek, D.; Banasik, M.; Chudoba, P.; Lepiesza, A.; Gomułkiewicz, A.; Dzięgiel, P.; et al. Pretransplant Immune Interplay Between Donor and Recipient Influences Posttransplant Kidney Allograft Function. Transplant. Proc. 2018, 50, 1658–1661. [Google Scholar] [CrossRef]
- Wu, W.K.; Famure, O.; Li, Y.; Kim, S.J. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int. 2015, 88, 851–858. [Google Scholar] [CrossRef]
- Mogulla, M.R.; Bhattacharjya, S.; Clayton, P.A. Risk factors for and outcomes of delayed graft function in live donor kidney transplantation—A retrospective study. Transplant. Int. 2019, 32, 1151–1160. [Google Scholar] [CrossRef]
- Yarlagadda, S.G.; Coca, S.G.; Formica, R.N.; Poggio, E.D.; Parikh, C.R. Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2008, 24, 1039–1047. [Google Scholar] [CrossRef]
- Pipic, D.; Rasmussen, M.; Saleh, Q.W.; Tepel, M. Induction Therapies Determine the Distribution of Perforin and Granzyme B Transcripts in Kidney Transplant Recipients. Biomedicines 2024, 12, 1258. [Google Scholar] [CrossRef] [PubMed]
- Nagarajah, S.; Rasmussen, M.; Hoegh, S.V.; Tepel, M. Prospective Study of Long Noncoding RNA, MGAT3-AS1, and Viremia of BK Polyomavirus and Cytomegalovirus in Living Donor Renal Transplant Recipients. Kidney Int. Rep. 2020, 5, 2218–2227. [Google Scholar] [CrossRef]
- Nagarajah, S.; Xia, S.; Rasmussen, M.; Tepel, M. Endogenous intronic antisense long non-coding RNA, MGAT3-AS1, and kidney transplantation. Sci. Rep. 2019, 9, 14743. [Google Scholar] [CrossRef]
- Kondraganti, S.; Mohanam, S.; Chintala, S.K.; Kin, Y.; Jasti, S.L.; Nirmala, C.; Lakka, S.S.; Adachi, Y.; Kyritsis, A.P.; Ali-Osman, F.; et al. Selective suppression of matrix metalloproteinase-9 in human glioblastoma cells by antisense gene transfer impairs glioblastoma cell invasion. Cancer Res. 2000, 60, 6851–6855. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All Patients (N = 67) | AB0- Incompatible Living Donor (N = 4) | AB0- Compatible Living Donor (N = 17) | Deceased Donor (N = 46) |
|---|---|---|---|---|
| Age, years | 55 (45.5–62.5) | 54.5 (47.5–59.8) | 40 (31–56) | 56.5 (50.3–63.8) |
| Gender male, N (%) | 46 (69%) | 3 (75%) | 9 (53%) | 34 (74%) |
| Gender female, N (%) | 21 (31%) | 1 (25%) | 8 (47%) | 12 (26%) |
| Weight (kg) | 80.9 (65–92.4) | 78 (61.5–97.5) | 74 (61.7–88) | 84.2 (66.5–92.8) |
| Height (cm) | 176 (169.5–185) a | 185 (172.5–191) c | 174 (171.8–182.3) d | 177 (168.9–184.3) e |
| Body mass index (kg/m2) | 25.4 (22.6–28.4) a | 26.6 (23.3–28.3) c | 24.8 (22.9–25.7) d | 25.8 (22.6–28.8) e |
| Systolic blood pressure (mmHg) | 156 (137–164) | 163 (156–176) | 146 (136–169) | 154 (137–164) |
| Diastolic blood pressure (mmHg) | 87 (78–95) | 88 (79–103) | 88 (83–103) | 85 (78–94) |
| Delayed graft function, N (%) | 7 (10%) b | 0 (0%) | 1 (6%) | 6 (13%) i |
| Plasma creatinine preoperative (μmol/L) | 651 (571–895) b | 891 (634.3–1147.5) | 795 (623–919) | 640.5 (561.8–863) i |
| Plasma creatinine first postoperative day (μmol/L) | 180 (67–441) b | 393.5(326–440) | 387 (306–453) | 517 (392.5–712.5) i |
| Cause of kidney disease (%) | ||||
| Diabetic nephropathy | 9 (13.4%) | 0 (0%) | 0 (0%) | 9 (19.5%) |
| Hypertensive nephropathy | 4 (6%) | 0 (0%) | 1 (5.8%) | 3 (6.5%) |
| Glomerulonephritis | 27 (40%) | 2 (50%) | 9 (53%) | 16 (34.7%) |
| Polycystic kidney disease | 18 (26.8%) | 1 (25%) | 4 (23.5%) | 13 (28.3%) |
| Other/unknown | 9 (13.4%) | 1 (25%) | 3 (17.6%) | 5 (11%) |
| Induction therapy, N (%) | ||||
| Basiliximab | 56 (83.6%) | 3 (75%) | 14 (82%) | 39 (85%) |
| Rituximab | 4 (6%) | 3 (75%) | 0 (0%) | 1 (2.1%) |
| Prednisolone | 22 (32.8%) | 4 (100%) | 10 (59%) | 8 (17.4%) |
| Thymoglobulin | 10 (15%) | 1 (25%) | 3 (17.6%) | 6 (13%) |
| HLA total mismatch, range (0–6) | 3 (3–4) f | 3 (2–4) c | 3 (2–3) g | 3 (3–4) h |
| Recipient Characteristics | Thymoglobulin Induction Therapy (N = 10) | All Other Induction Therapies (N = 57) | p Value |
|---|---|---|---|
| Age, years | 56 (35.5–59) | 55 (46–63) | 0.4417 |
| Gender male, N (%) | 8 (80%) | 38 (66.6%) | 0.4869 |
| Gender female, N (%) | 2 (20%) | 19 (33.3%) | 0.4869 |
| Weight (kg) | 80.8 (71.7–95) | 80.9 (64.8–92.2) | 0.6808 |
| Height (cm) | 181 (172–185.8) | 176 (169–184) b | 0.4451 |
| Body mass index (kg/m2) | 24.8 (23.2–28.2) | 25.5 (22.1–28.5) b | 0.9287 |
| Systolic blood pressure (mmHg) | 143 (127–161) | 157 (138–165) | 0.2370 |
| Diastolic blood pressure (mmHg) | 84 (72–87) | 87 (79–96) | 0.2405 |
| Delayed graft function, N (%) | 1 (10%) | 6 (10.5%) c | >0.999 |
| Plasma creatinine preoperative (μmol/L) | 687 (496.8–843.8) | 651 (587–906) c | 0.6324 |
| Plasma creatinine first postoperative day (μmol/L) | 433 (346–594) a | 469.5 (352.8–639.3) d | 0.5783 |
| Cause of chronic kidney disease, N (%) | |||
| Diabetic nephropathy | 0 (0%) | 9 (15.8%) | |
| Hypertensive nephropathy | 0 (0%) | 4 (7%) | |
| Glomerulonephritis | 10 (100%) | 17 (30%) | <0.01 |
| Polycystic kidney disease | 0 (0%) | 18 (31.6%) | |
| Other/unknown | 0 (0%) | 9 (15.8%) | |
| Induction therapy, N (%) | |||
| Basiliximab | 0 (0%) | 56 (98.2%) | |
| Rituximab | 1 (10%) | 3 (5.3%) | |
| Prednisolone | 10 (100%) | 12 (21%) | |
| Thymoglobulin | 10 (100%) | 0 (0%) | |
| HLA total mismatch, range (0–6) | 3 (2–4) a | 3 (3–4) e | 0.9465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, T.B.B.; Nagarajah, S.; Tepel, M. Elevated Matrix Metalloproteinase Type 9 (MMP-9) Transcripts After Thymoglobulin Induction in Incident Kidney Transplant Recipients. Int. J. Mol. Sci. 2025, 26, 9310. https://doi.org/10.3390/ijms26199310
Petersen TBB, Nagarajah S, Tepel M. Elevated Matrix Metalloproteinase Type 9 (MMP-9) Transcripts After Thymoglobulin Induction in Incident Kidney Transplant Recipients. International Journal of Molecular Sciences. 2025; 26(19):9310. https://doi.org/10.3390/ijms26199310
Chicago/Turabian StylePetersen, Tor B. B., Subagini Nagarajah, and Martin Tepel. 2025. "Elevated Matrix Metalloproteinase Type 9 (MMP-9) Transcripts After Thymoglobulin Induction in Incident Kidney Transplant Recipients" International Journal of Molecular Sciences 26, no. 19: 9310. https://doi.org/10.3390/ijms26199310
APA StylePetersen, T. B. B., Nagarajah, S., & Tepel, M. (2025). Elevated Matrix Metalloproteinase Type 9 (MMP-9) Transcripts After Thymoglobulin Induction in Incident Kidney Transplant Recipients. International Journal of Molecular Sciences, 26(19), 9310. https://doi.org/10.3390/ijms26199310





