Study on the Molecular Mechanism of Arbuscular Mycorrhizal Symbiosis Regulating Polysaccharide Synthesis in Dendrobium officinale
Abstract
1. Introduction
2. Results
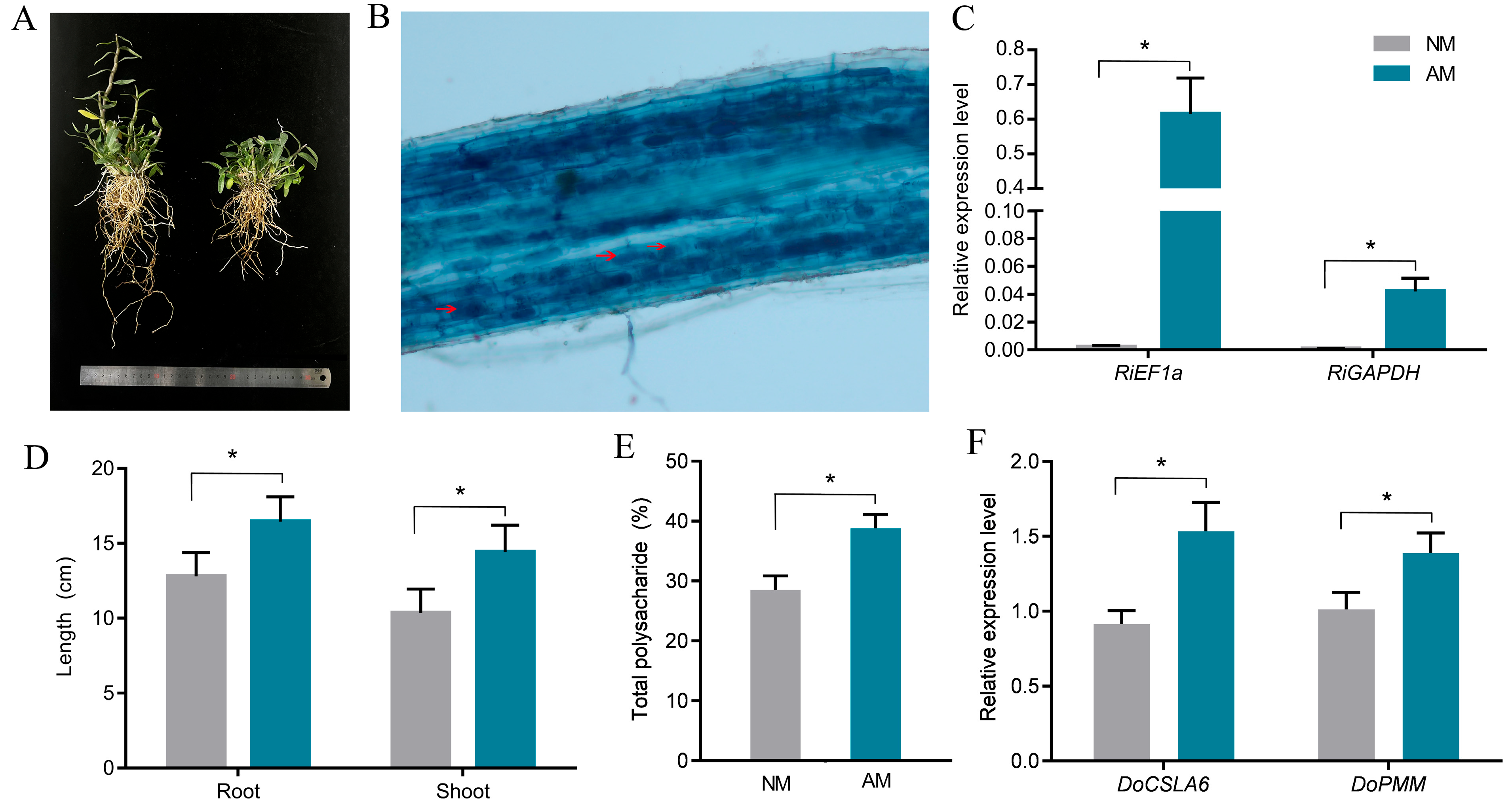
2.1. Influence of Mycorrhizal Fungi on Phenotypic Characteristics of D. officinale
2.2. RNA Sequencing and Assembly of the D. officinale Transcriptome
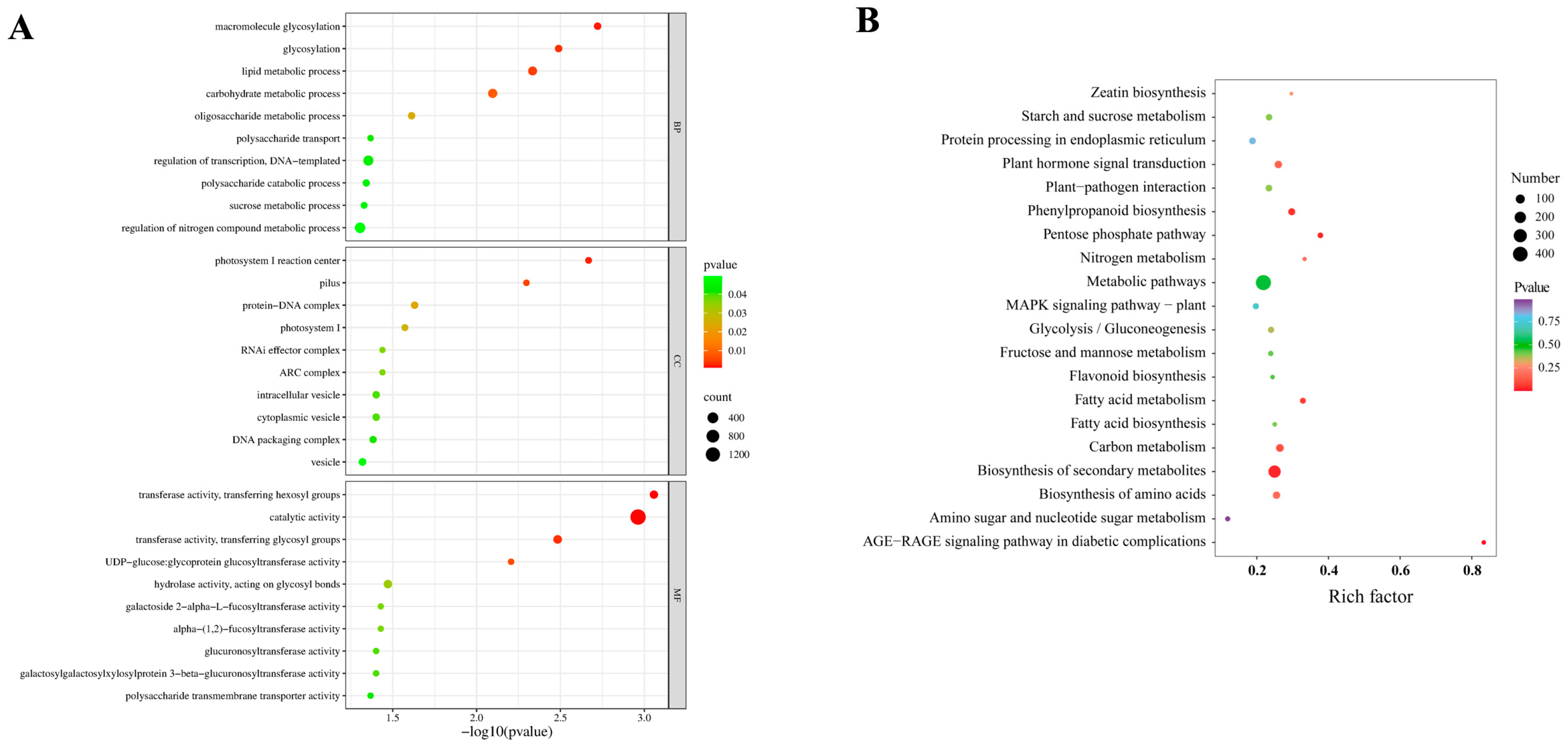
2.3. Key Genes and Metabolic Pathways Involved in Polysaccharide Biosynthesis in D. officinale
2.4. Identification of Transcription Factors Responsive to D. officinale Mycorrhizal Symbiosis
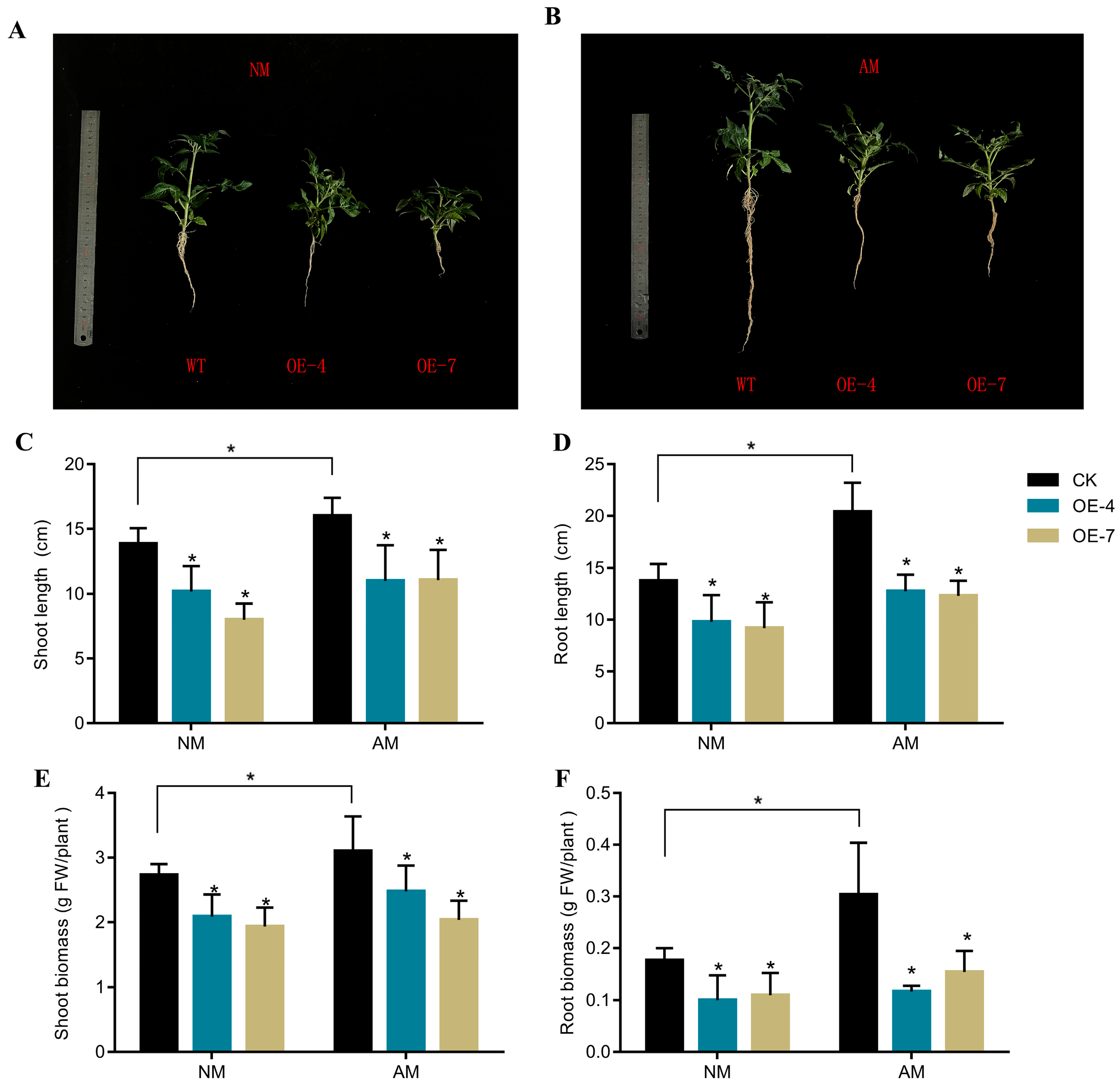
2.5. Overexpression of DoUGT83A1 Inhibits Tomato Growth and Reduces Polysaccharide Synthesis in Mycorrhizal Symbiosis
3. Discussion
3.1. Establishment of Mycorrhizal Symbiosis System in D. officinale Reveals Molecular Insights into Plant-Fungal Interactions
3.2. Transcriptomic Analysis Reveals AMF-Induced Modulation of Sugar Metabolism Pathways in D. officinale
3.3. Transcriptional Regulation of Symbiotic Responses and Stress Adaptation
3.4. DoUGT83A1 Functions as a Negative Regulator in Modulating AM Symbiosis
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design
4.3. Extraction of Total RNA and cDNA Synthesis from D. officinale
4.4. Transcriptome Sequencing
4.5. Screening and Functional Annotation of Differentially Expressed Genes (DEGs)
4.6. Construction of Binary Vectors and Generation of Transgenic Plants
4.7. RNA Extraction and Real-Time RT-PCR Analysis of Transgenic Tomato Lines
4.8. Mycorrhizal Quantification and Analysis of Arbuscule Populations
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Wing Sze, S.C.; Tong, Y.; Zhang, K.Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2019, 93, 1795–1803. [Google Scholar]
- Ding, G.; Li, X.; Ding, X.; Qian, L. Genetic diversity across natural populations of Dendrobium officinale, the endangered medicinal herb endemic to China, revealed by ISSR and RAPD markers. Russ. J. Genet. 2009, 45, 327–334. [Google Scholar] [CrossRef]
- Ye, Z.; Dai, J.R.; Zhang, C.G.; Lu, Y.; Wu, L.L.; Gong, A.G.; Wang, Z.T. Chemical differentiation of Dendrobium officinale and Dendrobium devonianum by using HPLC fingerprints, HPLC-ESI-MS, and HPTLC analyses. Evid.-Based Complement. Altern. Med. 2017, 2017, 8647212. [Google Scholar] [CrossRef]
- Wang, Z.D.; Dai, Y.F.; Luo, S.J.; Li, Y.W. Research Progress on Chemical Constituents and Pharmacological Effects of Dendrobium officinale. West China J. Pharm. Sci. 2022, 37, 472–476. [Google Scholar]
- Wu, J.; Wang, J.F.; Fang, L.; Wu, H.L.; Zhu, M.W.; Wang, R.W. Overview of Domestic Research on Dendrobium officinale. Chin. Pharm. J. 2013, 48, 1610–1613. [Google Scholar]
- Tian, L.X.; Chen, X.M.; Guo, S.X.; Shan, T.T. Study on Two Phenotypes of Dendrobium officinale. Chin. Pharm. J. 2018, 53, 98–103. [Google Scholar]
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; National Pharmacopoeia Commission: Ghaziabad, India, 2025; pp. 303–304. [Google Scholar]
- Nie, S.P.; Cai, H.L. Research Progress on Active Components and Functions of Dendrobium officinale. Food Sci. 2012, 33, 356–361. [Google Scholar]
- Liu, R.M.; Shi, S.S.; Xiong, S.; Su, J.; Gan, X.N.; Wu, J.J.; Wang, H.J.; Wang, S.C. Quality Markers of Dendrobium officinale by “Oligosaccharide-Spectrum-Effect” Relationships. Front. Nutr. 2022, 9, 914380. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhang, C.; Wang, N.; Xu, Y.; Tang, G.Y.; Xu, L.; Feng, Y.B. Bioactivities and Mechanism of Actions of Dendrobium officinale: A Comprehensive Review. Oxid Med. Cell. Longev. 2022, 2022, 6293355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, H.W.; Fan, J.H.; Jia, S.S.; Yi, X.; Han, Z.W.; Wang, R.L.; Qiu, H.W.; Lv, G.P. Study on Differences in Structure and Anti-Inflammatory Activity of Polysaccharides in Five Species of Dendrobium. Polymers 2025, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shao, G.G.; Song, M.Q.; Ye, Y.T.; Zhu, J.J.; Yang, X.N.; Song, X.S. Dynamic Changes in Functional Components of Dendrobium officinale and Their Applications in Food Science: A Review. Plant Foods Hum. Nutr. 2025, 80, 59. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, L.W.; Wang, D.J.; Wang, D.; Wen, C.W.; Han, B.X.; Ouyang, Z. Characterization and anti-tumor activity of a polysaccharide isolated from Dendrobium officinale grown in the Huoshan County. Chin Med. 2018, 13, 47. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Shi, J.C.; Wang, X.L.; Wang, E.T. Mycorrhizal symbiosis in plant growth and stress adaptation: From genes to ecosystems. Annu. Rev. Plant Biol. 2023, 74, 569–607. [Google Scholar] [CrossRef] [PubMed]
- Breuillin-Sessoms, F.; Floss, D.S.; Gomez, S.K.; Pumplin, N.; Ding, Y.; Levesque-Tremblay, V.; Noar, R.D.; Daniels, D.A.; Bravo, A.; Eaglesham, J.B. Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the ammonium transporter 2 family protein AMT2; 3. Plant Cell 2025, 27, 1352–1366. [Google Scholar] [CrossRef]
- Jin, H.R.; Liu, J.; Liu, J.; Huang, X.W. Forms of nitrogen uptake, translocation, and transfer via arbuscular mycorrhizal fungi: A review. Sci. China Life Sci. 2012, 55, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.; Doidy, J.; Zimmermann, S.D.; Wipf, D.; Courty, P.E. Take a trip through the plant and fungal transportome of mycorrhiza. Trends Plant Sci. 2016, 21, 937–950. [Google Scholar] [CrossRef]
- Liang, J.; Wu, Y.; Yuan, H.; Yang, Y.; Xiong, Q.; Liang, C.; Li, Z.; Li, C.; Zhang, G.; Li, X.; et al. Dendrobium officinale polysaccharides attenuate learning and memory disabilities via anti-oxidant and anti-inflammatory actions. Int. J. Biol. Macromol. 2019, 126, 414–426. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, Q.N.; Wang, B.J.; Zhang, N.; Qiu, R.; Yuan, Y.Y.; Yang, M.; Wang, F.D.; Mei, L.L.; Cui, G.W. Arbuscular mycorrhizal fungi communities and promoting the growth of alfalfa in saline ecosystems of northern China. Front. Plant Sci. 2024, 15, 1438771. [Google Scholar] [CrossRef]
- Duc, N.H.; Vo, A.T.; Haddidi, I.; Daood, H.; Posta, K. Arbuscular mycorrhizal fungi improve tolerance of the medicinal plant Eclipta prostrata (L.) L. and induce major changes in polyphenol profiles under salt stresses. Front. Plant Sci. 2021, 11, 612299. [Google Scholar] [CrossRef]
- Wu, Y.H.; Qin, Y.; Cai, Q.Q.; Liu, M.; He, D.M.; Chen, X.; Wang, Y.H.; Yan, Z.Y. Effect the accumulation of bioactive constituents of a medicinal plant (Salvia Miltiorrhiza Bge.) by arbuscular mycorrhizal fungi community. BMC Plant Biol. 2023, 23, 597. [Google Scholar] [CrossRef]
- Casieri, L.; Ait Lahmidi, N.A.; Doidy, J.; Veneault-Fourrey, C.; Migeon, A.; Bonneau, L.; Emmanuel Courty, P.; Garcia, K.; Charbonnier, M.; Delteil, A.; et al. Biotrophic transportome in mutualistic plant–fungal interactions. Mycorrhiza 2013, 23, 597–625. [Google Scholar] [CrossRef]
- Jiang, Y.N.; Wang, W.X.; Xie, Q.J.; Liu, N.; Liu, L.X.; Wang, D.P.; Zhang, X.W.; Yang, C.; Chen, X.Y.; Tang, D.Z.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; Van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef]
- Kretzschmar, T.; Kohlen, W.; Sasse, J.; Borghi, L.; Schlegel, M.; Bachelier, J.B.; Reinhardt, D.; Bours, R.; Bouwmeester, H.J.; Martinoia, E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012, 483, 341–344. [Google Scholar] [CrossRef]
- Floss, D.S.; Levy, J.G.; Lévesque-Tremblay, V.; Pumplin, N.; Harrison, M.J. DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2013, 110, E5025–E5034. [Google Scholar] [CrossRef]
- Martín-Rodríguez, J.Á.; Ocampo, J.A.; Molinero-Rosales, N.; Tarkowská, D.; Ruíz-Rivero, O.; García-Garrido, J.M. Role of gibberellins during arbuscular mycorrhizal formation in tomato: New insights revealed by endogenous quantification and genetic analysis of their metabolism in mycorrhizal roots. Physiol. Plant. 2015, 154, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Zhao, Q.C.; Xie, K.; Wang, M.N.; Li, L.C.; Zeng, D.C.; Wang, Q.; Wang, S.; Chen, A.; Xu, G.H. A Mycorrhiza-Induced UDP-Glucosyl Transferase Negatively Regulates the Arbuscular Mycorrhizal Symbiosis. Plant Cell Environ. 2025, 48, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Wu, Y.; Liu, Y.; Li, Y.; Tang, Y.E.; Chen, Y.S.; Yu, B.Y.; Liu, Y.J. Identification and Expression Analysis of Sucrose Synthase Family in Dendrobium catenatum Lindl. Mol. Plant Breed. 2022, 1–14. [Google Scholar]
- Feng, Z.Y.; Li, Y.W.; Zhang, S.X.; Song, J.J.; Xiang, H.X.; Huang, J.R.; Fan, H.H.; Liu, L. DoSPX1 and DoMYB37 regulate the expression of DoCSLA6 in Dendrobium officinale during phosphorus starvation. BMC Plant Biol. 2024, 24, 803. [Google Scholar] [CrossRef]
- He, C.M.; Zeng, S.J.; Teixeira da Silva, J.A.; Yu, Z.M.; Tan, J.W.; Duan, J. Molecular cloning and functional analysis of the phosphomannomutase (PMM) gene from Dendrobium officinale and evidence for the involvement of an abiotic stress response during germination. Protoplasma 2017, 254, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; An, H.Q.; Pei, W.; Wang, W.J. Cloning and Analysis of the UDP-Glucose Pyrophosphorylase Gene in Dendrobium officinale. Adv. Mod. Biomed. 2017, 17, 1215–1219+1232. [Google Scholar]
- He, C.M.; Wu, K.L.; Zhang, J.X.; Liu, X.C.; Zeng, S.J.; Yu, Z.M.; Zhang, X.H.; Teixeira da Silva, J.A.; Deng, R.F.; Tan, J.W.; et al. Cytochemical Localization of Polysaccharides in Dendrobium officinale and the Involvement of DoCSLA6 in the Synthesis of Mannan Polysaccharides. Front. Plant Sci. 2017, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.M.; Bai, T.J.; Liu, Y.L.; Guo, L.; Liu, X.G.; Zheng, S.Z.; Sun, D.Y. Research Progress on High Temperature Signal Transduction Pathways in Plants. J. Hebei Norm. Univ. 2024, 48, 433–440. [Google Scholar]
- Li, D.B.; Gao, Y.h.; Si, J.P.; Zhu, Y.Q. Cloning and cold stress expression analysis of the HSP70 gene in Dendrobium officinale. China J. Chin. Mater. Medica 2013, 38, 3446–3452. [Google Scholar]
- Zhang, K.H.; Gao, S.P.; Liu, S.L.; Li, Q.Z.; Zhang, K.Y. Effects of low temperature and exogenous NO on polysaccharide synthesis in protocorms of Dendrobium officinale. Acta Bot. Boreali-Occident. Sin. 2015, 35, 1626–1633. [Google Scholar]
- Zhang, J.X.; He, C.M.; Wu, K.L.; Teixeira da Silva, J.A.; Zeng, S.J.; Zhang, X.H.; Yu, Z.M.; Xia, H.Q.; Duan, J. Transcriptome analysis of Dendrobium officinale and its application to the identification of genes associated with polysaccharide synthesis. Front. Plant Sci. 2016, 7, 5. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Xu, Q.; Bian, C.; Tsai, W.C.; Yeh, C.M.; Liu, K.W.; Yoshida, K.; Zhang, L.-S.; Chang, S.-B.; Chen, F.; et al. The Dendrobium catenatum Lindl. genome sequence provides insights into polysaccharide synthase, floral development and adaptive evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef]
- Müller, L.M.; Flokova, K.; Schnabel, E.; Sun, X.; Fei, Z.; Frugoli, J.; Bouwmeester, H.J.; Harrison, M.J. A CLE–SUNN module regulates strigolactone content and fungal colonization in arbuscular mycorrhiza. Nat. Plants 2019, 5, 933–939. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.S.; Xie, Q.J.; Xia, Y.J.; Lu, L.; Wang, M.X.; Wang, G.; Long, S.Y.; Cai, Y.; Xu, L. Control of arbuscule development by a transcriptional negative feedback loop in Medicago. Nat. Commun. 2023, 14, 5743. [Google Scholar] [CrossRef]
- Liu, J.L.; Chen, J.D.; Xie, K.; Tian, Y.; Yan, N.; Liu, J.J.; Huang, Y.J.; Wang, S.S.; Zhu, Y.Y.; Chen, A.Q.; et al. A mycorrhiza-specific H+-ATPase is essential for arbuscule development and symbiotic phosphate and nitrogen uptake. Plant Cell Environ. 2020, 43, 1069–1083. [Google Scholar] [CrossRef]
- Yan, H.X.; Fu, D.Q.L.; Zhu, B.Z.; Liu, H.P.; Shen, X.Y.; Luo, Y.B. Sprout vacuum-infiltration: A simple and efficient agroinoculation method for virus-induced gene silencing in diverse solanaceous species. Plant Cell Rep. 2012, 31, 1713–1722. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.D.; Liao, D.H.; Ye, H.H.; Li, C.; Luo, Z.Z.; Yan, A.N.; Zhao, Q.; Xie, K.; Li, Y.; et al. Auxin-mediated regulation of arbuscular mycorrhizal symbiosis: A role of SlGH3.4 in tomato. Plant Cell Environ. 2022, 45, 955–968. [Google Scholar] [CrossRef]
- Liu, J.J.; Liu, J.L.; Liu, J.H.; Cui, M.M.; Huang, Y.J.; Tian, Y.; Chen, A.; Xu, G.H. The potassium transporter SlHAK10 is involved in mycorrhizal potassium uptake. Plant Physiol. 2019, 180, 465–479. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]




| Serial Number | Family Name | Number of Items | Serial Number | Family Name | Number of Items |
|---|---|---|---|---|---|
| 1 | bHLH | 218 | 29 | GATA | 21 |
| 2 | MYB_related | 121 | 30 | GeBP | 20 |
| 3 | NAC | 118 | 31 | Dof | 15 |
| 4 | WRKY | 98 | 32 | NF-YC | 15 |
| 5 | B3 | 94 | 33 | SBP | 13 |
| 6 | ERF | 94 | 34 | AP2 | 12 |
| 7 | bZIP | 88 | 35 | NF-YA | 12 |
| 8 | C2H2 | 86 | 36 | NF-X1 | 11 |
| 9 | MYB | 85 | 37 | BES1 | 10 |
| 10 | FAR1 | 84 | 38 | CO-like | 10 |
| 11 | G2-like | 68 | 39 | CPP | 9 |
| 12 | C3H | 60 | 40 | ARR-B | 8 |
| 13 | HD-ZIP | 54 | 41 | BBR-BPC | 8 |
| 14 | ARF | 52 | 42 | SRS | 8 |
| 15 | M-type | 49 | 43 | EIL | 7 |
| 16 | HSF | 48 | 44 | ZF-HD | 7 |
| 17 | GRAS | 39 | 45 | GRF | 6 |
| 18 | TCP | 38 | 46 | STAT | 6 |
| 19 | Nin-like | 36 | 47 | WOX | 6 |
| 20 | MIKC | 35 | 48 | DBB | 5 |
| 21 | S1Fa-like | 35 | 49 | HB-PHD | 4 |
| 22 | TALE | 33 | 50 | CAMTA | 3 |
| 23 | E2F/DP | 28 | 51 | LSD | 3 |
| 24 | Trihelix | 28 | 52 | HRT-like | 2 |
| 25 | HB-other | 25 | 53 | RAV | 2 |
| 26 | NF-YB | 24 | 54 | LFY | 1 |
| 27 | LBD | 23 | 55 | VOZ | 1 |
| 28 | YABBY | 23 | 56 | Whirly | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, Y.; Zhang, M.; Zhang, Z.; Liu, Y.; Duan, X.; Tao, Z.; Jiang, W. Study on the Molecular Mechanism of Arbuscular Mycorrhizal Symbiosis Regulating Polysaccharide Synthesis in Dendrobium officinale. Int. J. Mol. Sci. 2025, 26, 9298. https://doi.org/10.3390/ijms26199298
Chen J, Zhang Y, Zhang M, Zhang Z, Liu Y, Duan X, Tao Z, Jiang W. Study on the Molecular Mechanism of Arbuscular Mycorrhizal Symbiosis Regulating Polysaccharide Synthesis in Dendrobium officinale. International Journal of Molecular Sciences. 2025; 26(19):9298. https://doi.org/10.3390/ijms26199298
Chicago/Turabian StyleChen, Jiadong, Yiqun Zhang, Man Zhang, Ziyi Zhang, Yingying Liu, Xiaojing Duan, Zhengming Tao, and Wu Jiang. 2025. "Study on the Molecular Mechanism of Arbuscular Mycorrhizal Symbiosis Regulating Polysaccharide Synthesis in Dendrobium officinale" International Journal of Molecular Sciences 26, no. 19: 9298. https://doi.org/10.3390/ijms26199298
APA StyleChen, J., Zhang, Y., Zhang, M., Zhang, Z., Liu, Y., Duan, X., Tao, Z., & Jiang, W. (2025). Study on the Molecular Mechanism of Arbuscular Mycorrhizal Symbiosis Regulating Polysaccharide Synthesis in Dendrobium officinale. International Journal of Molecular Sciences, 26(19), 9298. https://doi.org/10.3390/ijms26199298






