Progressive Retinal Vascular and Neuronal Degeneration in BXD32 Mice: A Model for Age-Dependent Neurovascular Pathology
Abstract
1. Introduction
2. Results
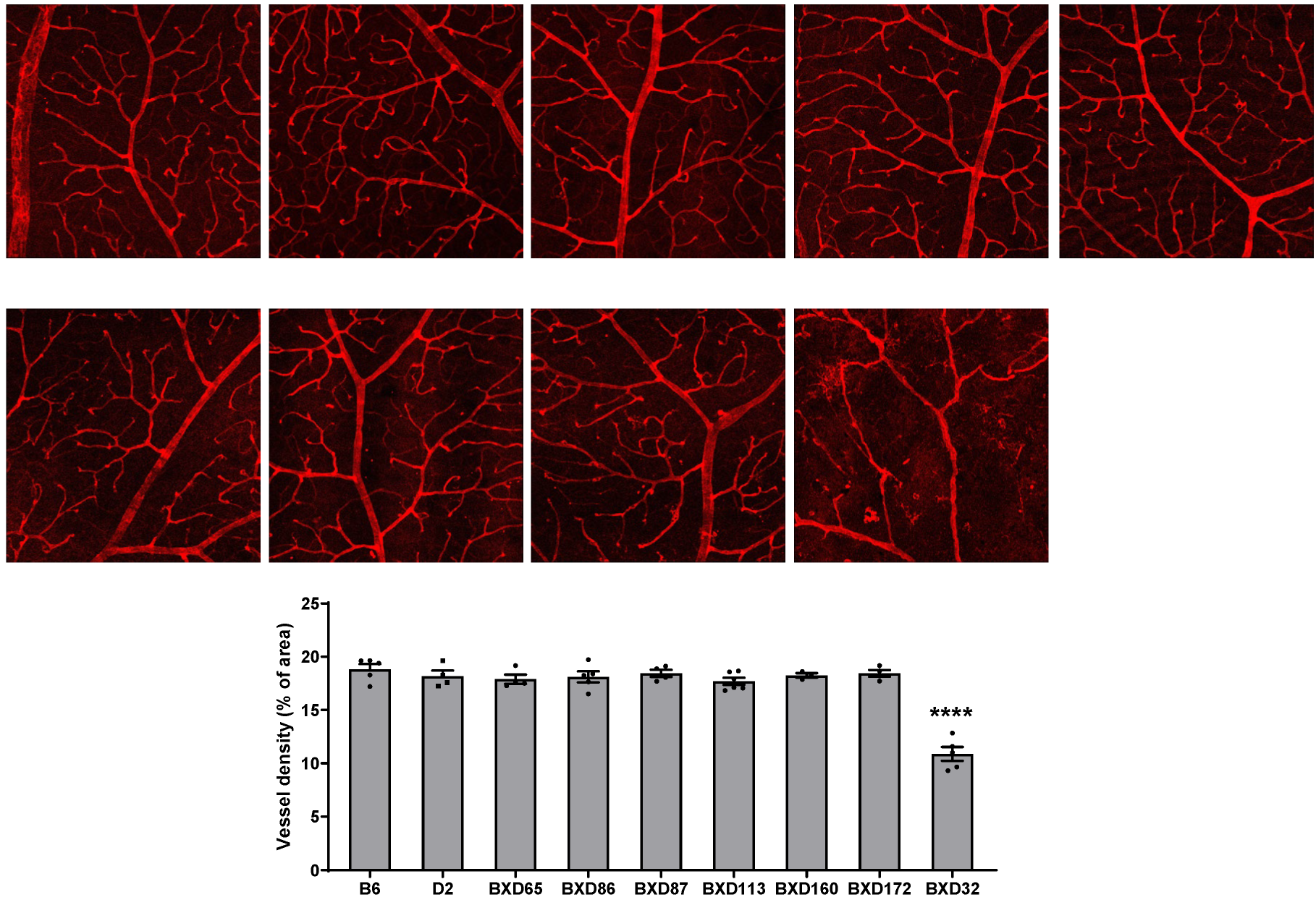
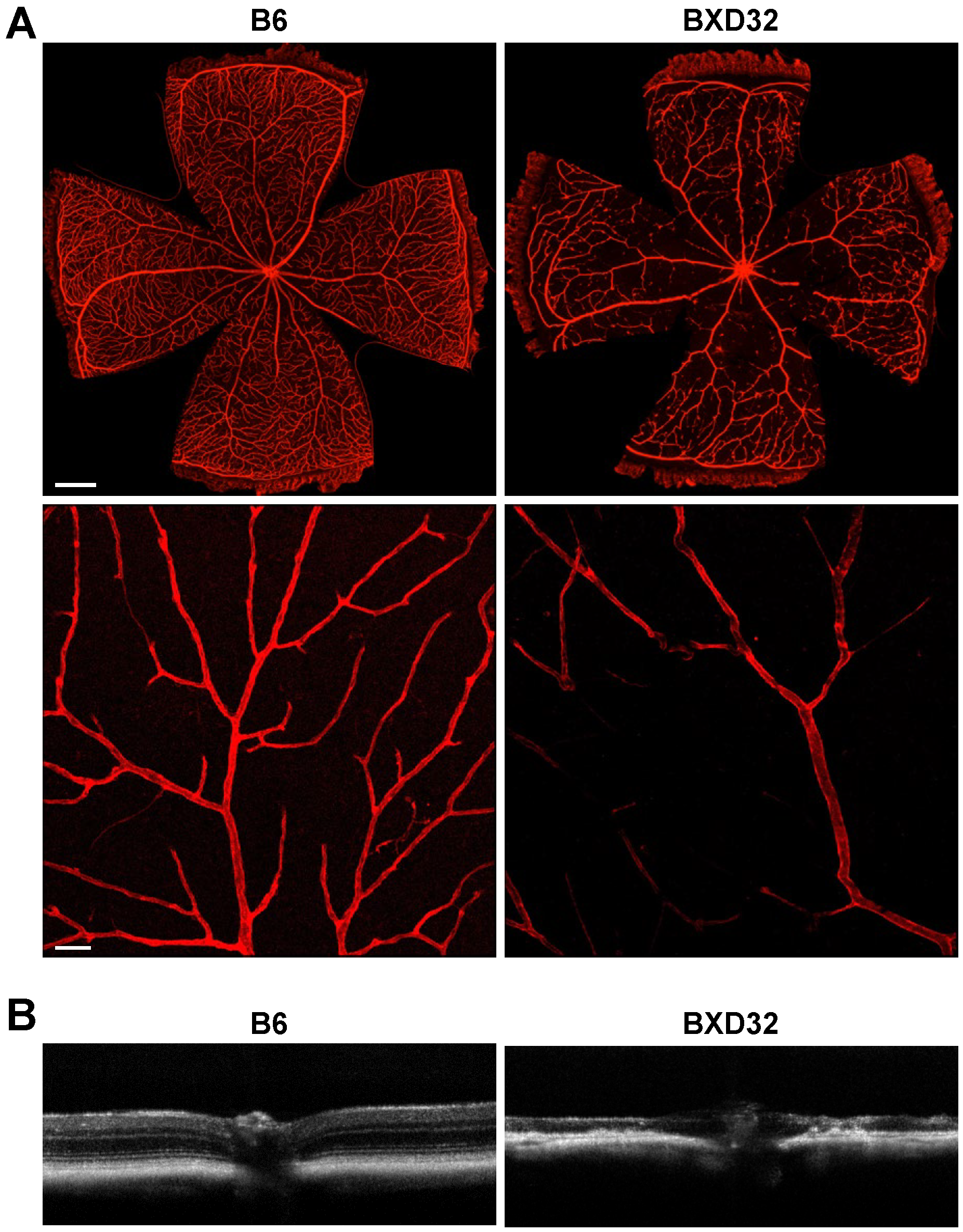
2.1. Identification of BXD32 as an Outlier with Markedly Reduced Retinal Vessel Density
2.2. Early Retinal Degeneration in Young BXD32 Mice
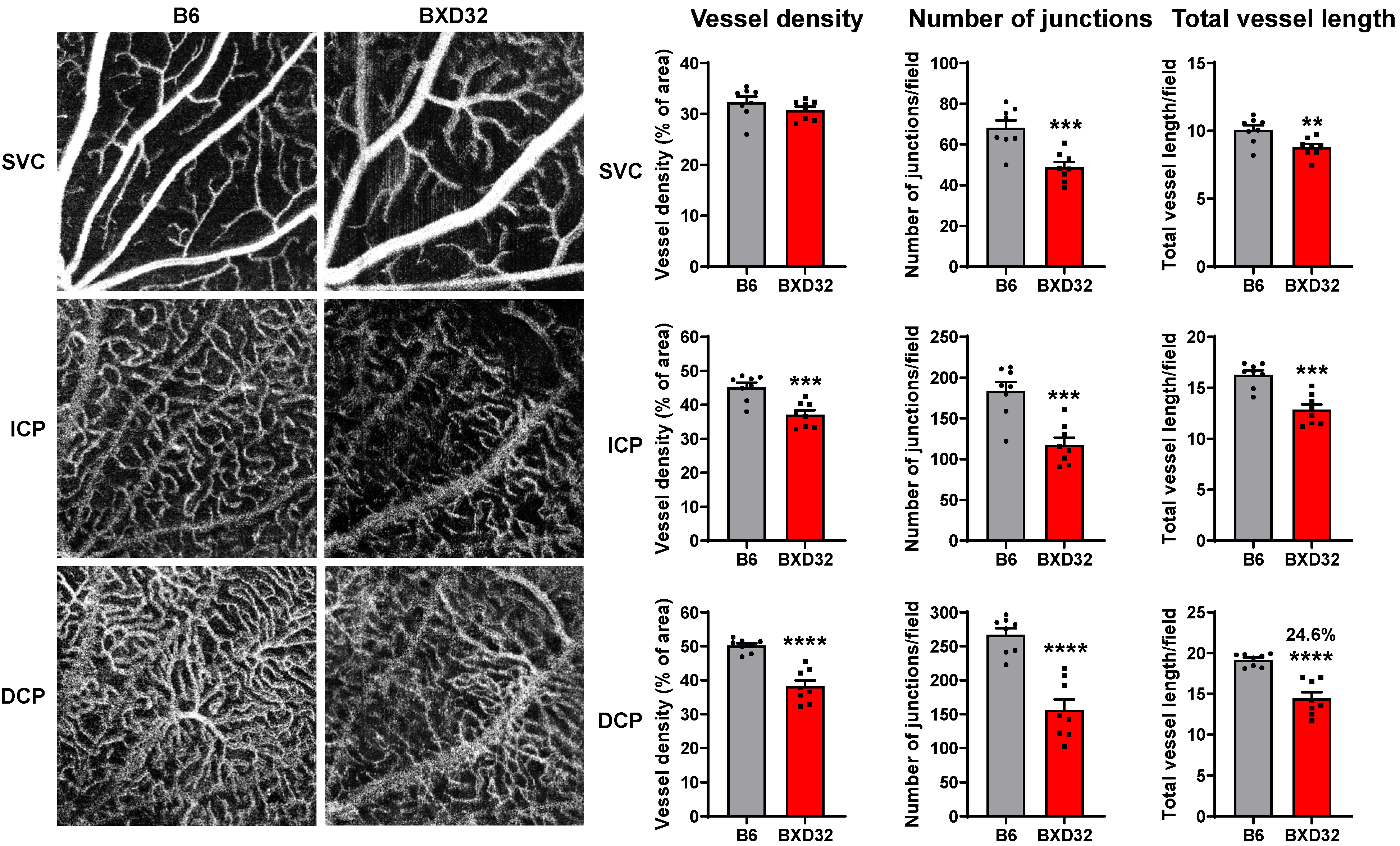
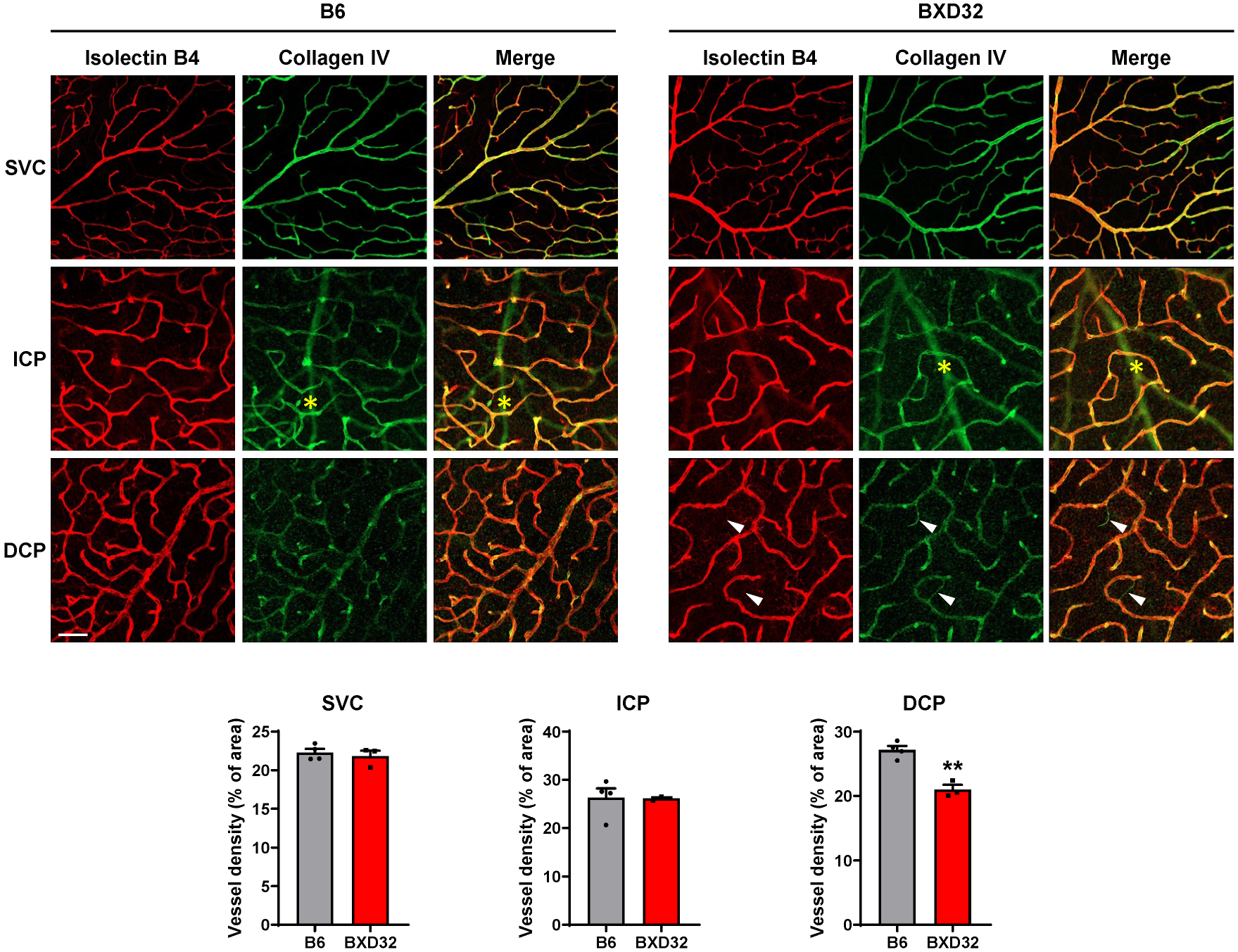
2.3. Progressive Retinal Degeneration in BXD32 Mice
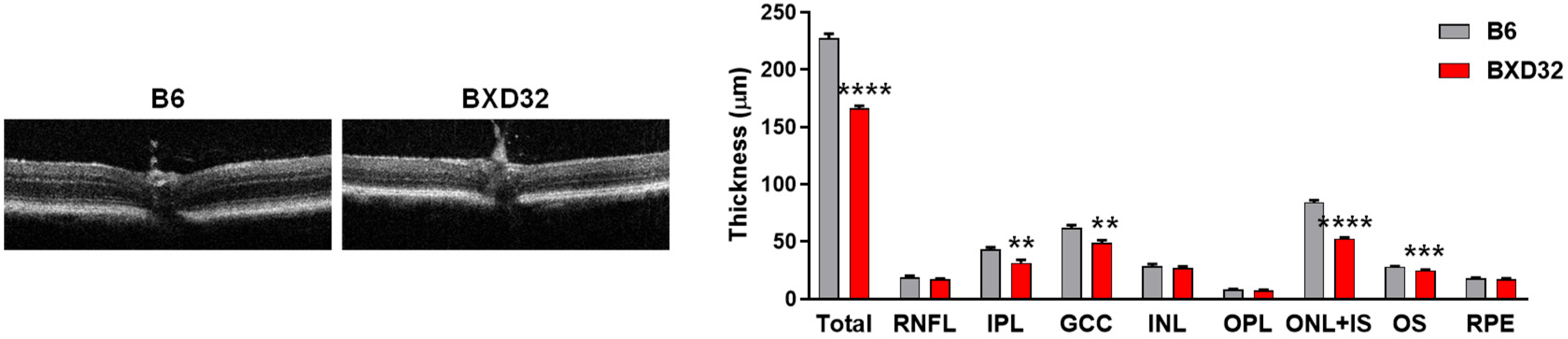
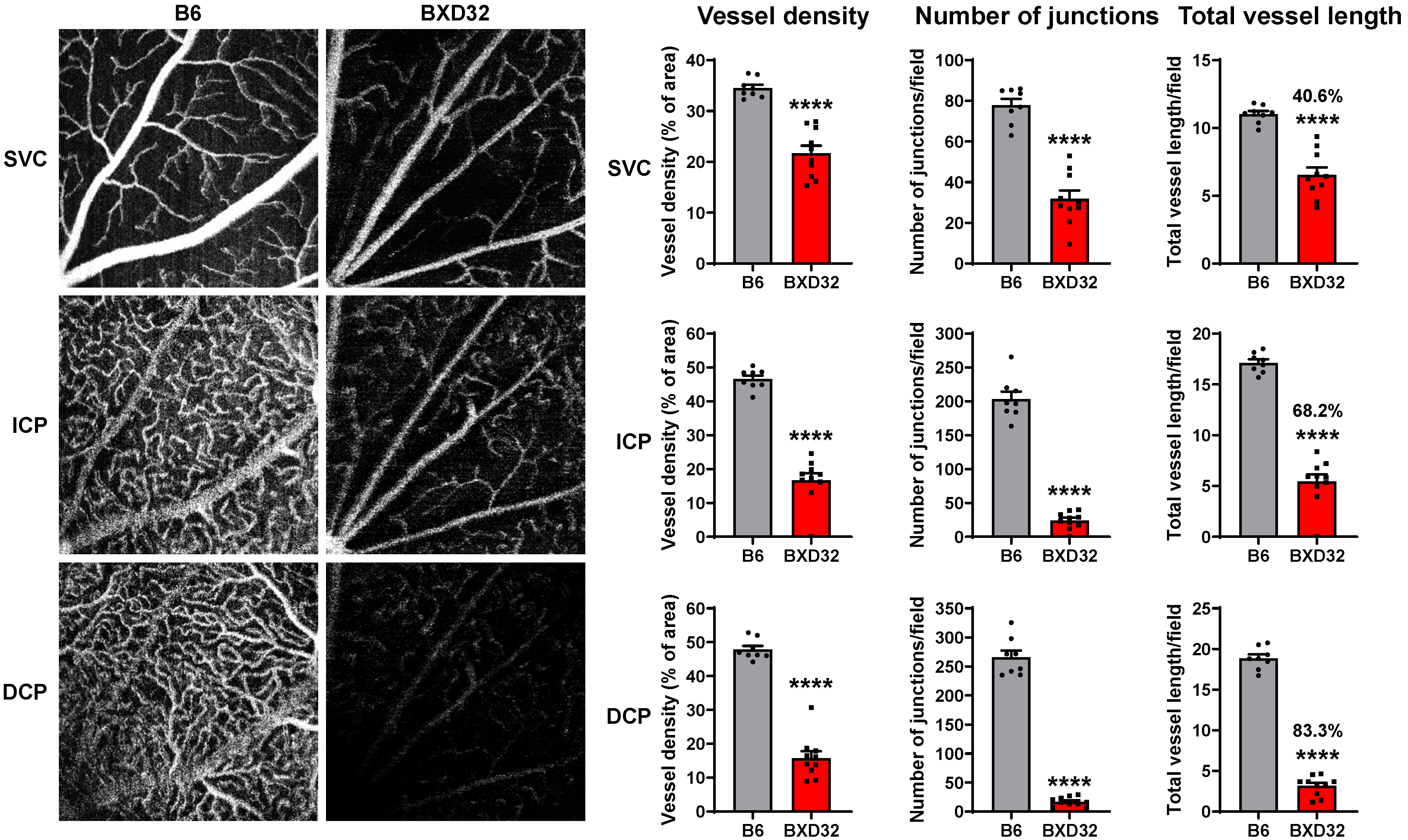
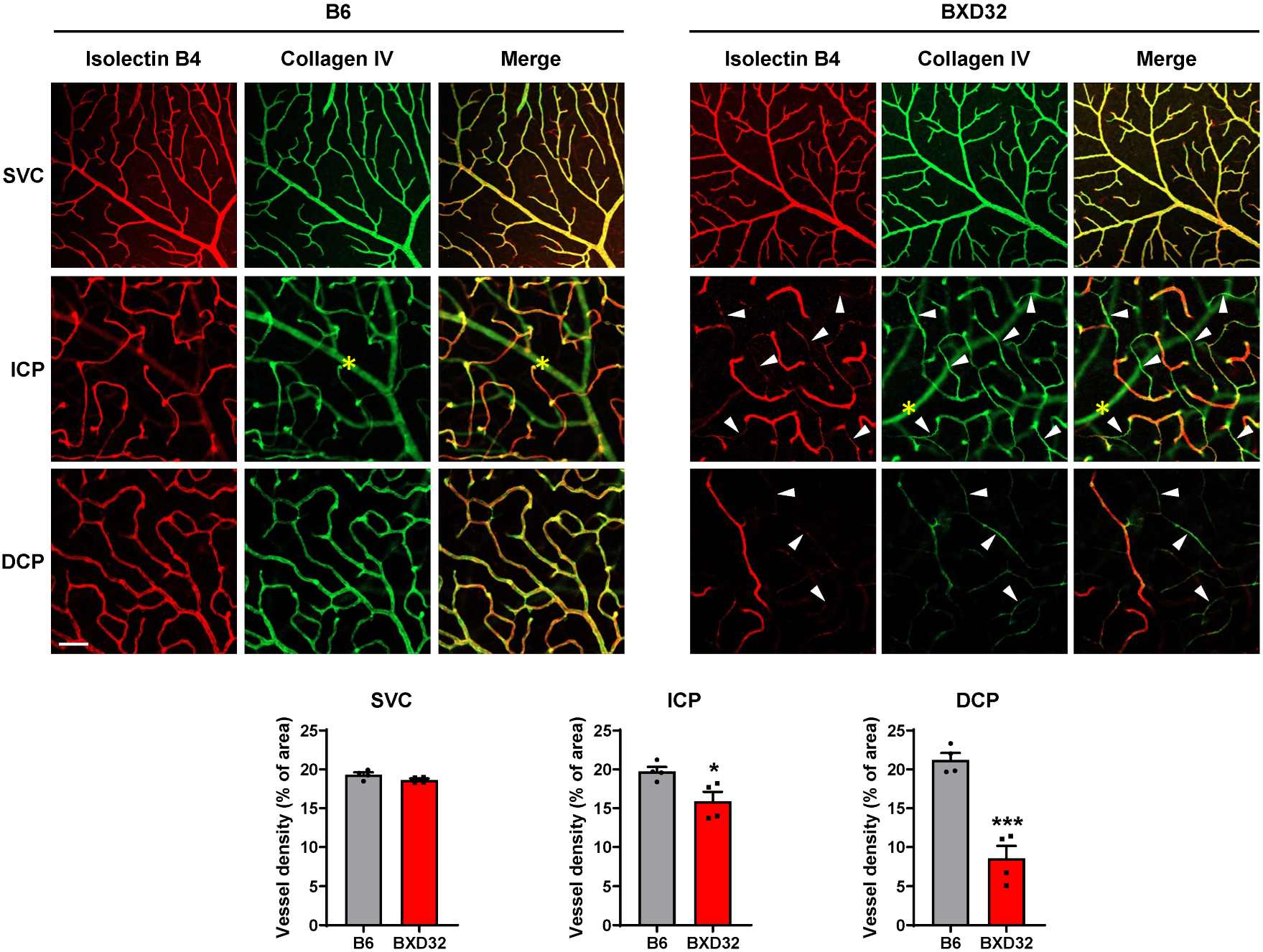
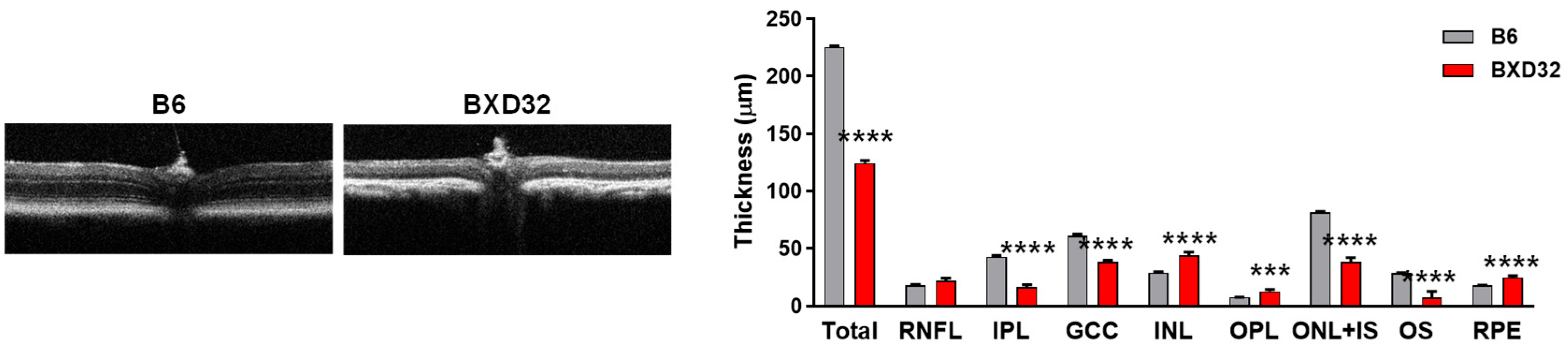
2.4. Severe Retinal and Vascular Degeneration in Aged BXD32 Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Optical Coherence Tomography (OCT) Imaging
4.3. Optical Coherence Tomography Angiography (OCTA) Imaging
4.4. Retinal Dissection and Flatmounts
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruan, Y.; Jiang, S.; Musayeva, A.; Gericke, A. Oxidative Stress and Vascular Dysfunction in the Retina: Therapeutic Strategies. Antioxidants 2020, 9, 761. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Rojas, M.; Caldwell, R.W.; Caldwell, R.B. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy 2011, 3, 609–628. [Google Scholar] [CrossRef]
- Tan, C.S.; Li, K.Z.; Sadda, S.R. Wide-field angiography in retinal vein occlusions. Int. J. Retina Vitreous 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jiang, H.; Liu, Y.; Rosa Gameiro, G.; Gregori, G.; Dong, C.; Rundek, T.; Wang, J. Age-Related Alterations in Retinal Tissue Perfusion and Volumetric Vessel Density. Investig. Ophthalmol. Vis. Sci. 2019, 60, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jiang, H.; Shi, Y.; Qu, D.; Gregori, G.; Zheng, F.; Rundek, T.; Wang, J. Age-Related Alterations in the Retinal Microvasculature, Microcirculation, and Microstructure. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3804–3817. [Google Scholar] [CrossRef]
- Civelek, M.; Lusis, A.J. Systems genetics approaches to understand complex traits. Nat. Rev. Genet. 2014, 15, 34–48. [Google Scholar] [CrossRef]
- Wainberg, M.; Sinnott-Armstrong, N.; Mancuso, N.; Barbeira, A.N.; Knowles, D.A.; Golan, D.; Ermel, R.; Ruusalepp, A.; Quertermous, T.; Hao, K.; et al. Opportunities and challenges for transcriptome-wide association studies. Nat. Genet. 2019, 51, 592–599. [Google Scholar] [CrossRef]
- Morse, H.C., 3rd; Chused, T.M.; Hartley, J.W.; Mathieson, B.J.; Sharrow, S.O.; Taylor, B.A. Expression of xenotropic murine leukemia viruses as cell-surface gp70 in genetic crosses between strains DBA/2 and C57BL/6. J. Exp. Med. 1979, 149, 1183–1196. [Google Scholar] [CrossRef]
- Geisert, E.E.; Williams, R.W. Using BXD mouse strains in vision research: A systems genetics approach. Mol. Vis. 2020, 26, 173–187. [Google Scholar]
- Li, X.; Morel, J.D.; Sulc, J.; De Masi, A.; Lalou, A.; Benegiamo, G.; Poisson, J.; Liu, Y.; Von Alvensleben, G.V.G.; Gao, A.W.; et al. Systems genetics of metabolic health in the BXD mouse genetic reference population. Cell Syst. 2024, 15, 497–509.e493. [Google Scholar] [CrossRef]
- Gurdon, B.; Yates, S.C.; Csucs, G.; Groeneboom, N.E.; Hadad, N.; Telpoukhovskaia, M.; Ouellette, A.; Ouellette, T.; O’Connell, K.M.S.; Singh, S.; et al. Detecting the effect of genetic diversity on brain composition in an Alzheimer’s disease mouse model. Commun. Biol. 2024, 7, 605. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Lopez-Granero, C.; Ferrer, B.; Tinkov, A.A.; Skalny, A.V.; Paoliello, M.M.B.; Aschner, M. BXD Recombinant Inbred Mice as a Model to Study Neurotoxicity. Biomolecules 2021, 11, 1762. [Google Scholar] [CrossRef]
- Li, Z.A.; Bajpai, A.K.; Wang, R.; Liu, Y.; Webby, R.J.; Wilk, E.; Gu, W.; Schughart, K.; Li, K.; Lu, L. Systems genetics of influenza A virus-infected mice identifies TRIM21 as a critical regulator of pulmonary innate immune response. Virus Res. 2024, 342, 199335. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Morel, J.D.; Benegiamo, G.; Poisson, J.; Bachmann, A.; Rapin, A.; Sulc, J.; Williams, E.; Perino, A.; Schoonjans, K.; et al. Genetic and dietary modulators of the inflammatory response in the gastrointestinal tract of the BXD mouse genetic reference population. eLife 2023, 12, RP87569. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Cho, K.S.; Wei, X.; Xu, F.; Lennikov, A.; Hu, G.; Tang, J.; Guo, S.; Chen, J.; Kriukov, E.; et al. IGFBPL1 is a master driver of microglia homeostasis and resolution of neuroinflammation in glaucoma and brain tauopathy. Cell Rep. 2023, 42, 112889. [Google Scholar] [CrossRef]
- Rajan, J.R.S.; Gill, K.; Chow, E.; Ashbrook, D.G.; Williams, R.W.; Zwicker, J.G.; Goldowitz, D. Investigating Motor Coordination Using BXD Recombinant Inbred Mice to Model the Genetic Underpinnings of Developmental Coordination Disorder. Genes. Brain Behav. 2025, 24, e70014. [Google Scholar] [CrossRef]
- Orgil, B.O.; Xu, F.; Li, N.; Bajpai, A.K.; Alberson, N.R.; Johnson, J.N.; Gu, Q.; Wetzel, G.T.; Towbin, J.A.; Lu, L.; et al. Genetic mapping of electrocardiographic parameters in BXD strains reveals Chromosome 3 loci to be associated with cardiac repolarization abnormalities. Physiol. Genomics 2025, 57, 456–469. [Google Scholar] [CrossRef]
- Braga, R.R.; Rocha, M.B.; Morelli, A.P.; da Costa, R.G.; Mori, M.A.S.; da Silva, A.S.R.; Pauli, J.R.; Cintra, D.E.; Ropelle, E.R. NAD replenishment restores mitochondrial function and thermogenesis in the brown adipose tissue of mice with obesity. J. Physiol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Xia, J.; Bajpai, A.K.; Liu, Y.; Yu, L.; Dong, Y.; Li, F.; Chen, F.; Lu, L.; Feng, S. Systems Genetics Reveals the Gene Regulatory Mechanisms of Arrb2 in the Development of Autism Spectrum Disorders. Genes. 2025, 16, 605. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, J.; Yang, X.; Yi, P.; Ruan, J.; Wu, Y.; Li, Y.; Tian, G.; Xu, F.; Mi, J.; et al. Morc4 is a novel functional gene associated with lipid metabolism in BXD recombinant inbred population. Front. Cardiovasc. Med. 2025, 12, 1570729. [Google Scholar] [CrossRef]
- Peirce, J.L.; Lu, L.; Gu, J.; Silver, L.M.; Williams, R.W. A new set of BXD recombinant inbred lines from advanced intercross populations in mice. BMC Genet. 2004, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.G.; Auwerx, J. The Convergence of Systems and Reductionist Approaches in Complex Trait Analysis. Cell 2015, 162, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Buscho, S.; Palacios, E.; Xia, F.; Shi, S.; Li, S.; Luisi, J.; Kayed, R.; Motamedi, M.; Zhang, W.; Liu, H. Longitudinal characterization of retinal vasculature alterations with optical coherence tomography angiography in a mouse model of tauopathy. Exp. Eye Res. 2022, 224, 109240. [Google Scholar] [CrossRef]
- McVicar, C.M.; Hamilton, R.; Colhoun, L.M.; Gardiner, T.A.; Brines, M.; Cerami, A.; Stitt, A.W. Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes 2011, 60, 2995–3005. [Google Scholar] [CrossRef]
- Chintalapudi, S.R.; Maria, D.; Di Wang, X.; Bailey, J.N.C.; Hysi, P.G.; Wiggs, J.L.; Williams, R.W.; Jablonski, M.M.; International Glaucoma Genetics consortium; NEIGHBORHOOD Consortium. Systems genetics identifies a role for Cacna2d1 regulation in elevated intraocular pressure and glaucoma susceptibility. Nat. Commun. 2017, 8, 1755. [Google Scholar] [CrossRef]
- Hollingsworth, T.J.; Wang, X.; White, W.A.; Simpson, R.N.; Jablonski, M.M. Chronic Proinflammatory Signaling Accelerates the Rate of Degeneration in a Spontaneous Polygenic Model of Inherited Retinal Dystrophy. Front. Pharmacol. 2022, 13, 839424. [Google Scholar] [CrossRef]
- Williams, R.W.; Strom, R.C.; Goldowitz, D. Natural variation in neuron number in mice is linked to a major quantitative trait locus on Chr 11. J. Neurosci. 1998, 18, 138–146. [Google Scholar] [CrossRef]
- Strom, R.C.; Williams, R.W. Cell production and cell death in the generation of variation in neuron number. J. Neurosci. 1998, 18, 9948–9953. [Google Scholar] [CrossRef]
- Cepko, C.L.; Austin, C.P.; Yang, X.; Alexiades, M.; Ezzeddine, D. Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA 1996, 93, 589–595. [Google Scholar] [CrossRef]
- Srejovic, J.V.; Muric, M.D.; Jakovljevic, V.L.; Srejovic, I.M.; Sreckovic, S.B.; Petrovic, N.T.; Todorovic, D.Z.; Bolevich, S.B.; Sarenac Vulovic, T.S. Molecular and Cellular Mechanisms Involved in the Pathophysiology of Retinal Vascular Disease-Interplay Between Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 11850. [Google Scholar] [CrossRef]
- Yue, T.; Shi, Y.; Luo, S.; Weng, J.; Wu, Y.; Zheng, X. The role of inflammation in immune system of diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Front. Immunol. 2022, 13, 1055087. [Google Scholar] [CrossRef]
- Gilead, N.; Chong, Y.J.; Yamaguchi, T.C.N.; Chan, H.H.; Fenner, B.J.; Ibrahim, F.I.; Sun, C.; Sivaprasad, S.; Gemmy Cheung, C.M. Incidence of Visual Loss in Patients with Diabetic Macular Ischemia (DMI) A 1- year Prospective Study. Retina 2025, 45, 1460–1468. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M. Impact of Glaucomatous Ganglion Cell Damage on Central Visual Function. Annu. Rev. Vis. Sci. 2024, 10, 425–453. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Phu, J.; Wang, H.; Sowmya, A.; Meijering, E.; Kalloniatis, M. Predicting visual field global and local parameters from OCT measurements using explainable machine learning. Sci. Rep. 2025, 15, 5685. [Google Scholar] [CrossRef] [PubMed]
- Garzone, D.; Finger, R.P.; Mauschitz, M.M.; Koch, A.; Reuter, M.; Breteler, M.M.B.; Aziz, N.A. Visual impairment and retinal and brain neurodegeneration: A population-based study. Hum. Brain Mapp. 2023, 44, 2701–2711. [Google Scholar] [CrossRef]
- Suleman, N. Current understanding on Retinitis Pigmentosa: A literature review. Front. Ophthalmol. 2025, 5, 1600283. [Google Scholar] [CrossRef]
- Ameri, H.; Hong, A.T.; Chwa, J. Loss of Peripheral Retinal Vessels in Retinitis Pigmentosa. Ophthalmol. Sci. 2025, 5, 100767. [Google Scholar] [CrossRef]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Liu, W.; Ha, Y.; Xia, F.; Zhu, S.; Li, Y.; Shi, S.; Mei, F.C.; Merkley, K.; Vizzeri, G.; Motamedi, M.; et al. Neuronal Epac1 mediates retinal neurodegeneration in mouse models of ocular hypertension. J. Exp. Med. 2020, 217, e20190930. [Google Scholar] [CrossRef]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A computational tool for quantitative analysis of vascular networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, F.; Shi, S.; Buscho, S.E.; Palacios, E.; McCarty, M.; Nazemi, M.; Lu, L.; Zhang, W.; Liu, H. Progressive Retinal Vascular and Neuronal Degeneration in BXD32 Mice: A Model for Age-Dependent Neurovascular Pathology. Int. J. Mol. Sci. 2025, 26, 9289. https://doi.org/10.3390/ijms26199289
Xia F, Shi S, Buscho SE, Palacios E, McCarty M, Nazemi M, Lu L, Zhang W, Liu H. Progressive Retinal Vascular and Neuronal Degeneration in BXD32 Mice: A Model for Age-Dependent Neurovascular Pathology. International Journal of Molecular Sciences. 2025; 26(19):9289. https://doi.org/10.3390/ijms26199289
Chicago/Turabian StyleXia, Fan, Shuizhen Shi, Seth E. Buscho, Erick Palacios, Melinda McCarty, Monia Nazemi, Lu Lu, Wenbo Zhang, and Hua Liu. 2025. "Progressive Retinal Vascular and Neuronal Degeneration in BXD32 Mice: A Model for Age-Dependent Neurovascular Pathology" International Journal of Molecular Sciences 26, no. 19: 9289. https://doi.org/10.3390/ijms26199289
APA StyleXia, F., Shi, S., Buscho, S. E., Palacios, E., McCarty, M., Nazemi, M., Lu, L., Zhang, W., & Liu, H. (2025). Progressive Retinal Vascular and Neuronal Degeneration in BXD32 Mice: A Model for Age-Dependent Neurovascular Pathology. International Journal of Molecular Sciences, 26(19), 9289. https://doi.org/10.3390/ijms26199289







