Curcumin Attenuates Liver Steatosis via Antioxidant and Anti-Inflammatory Pathways in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial
Abstract
1. Introduction
2. Results
2.1. Intervention Outcomes
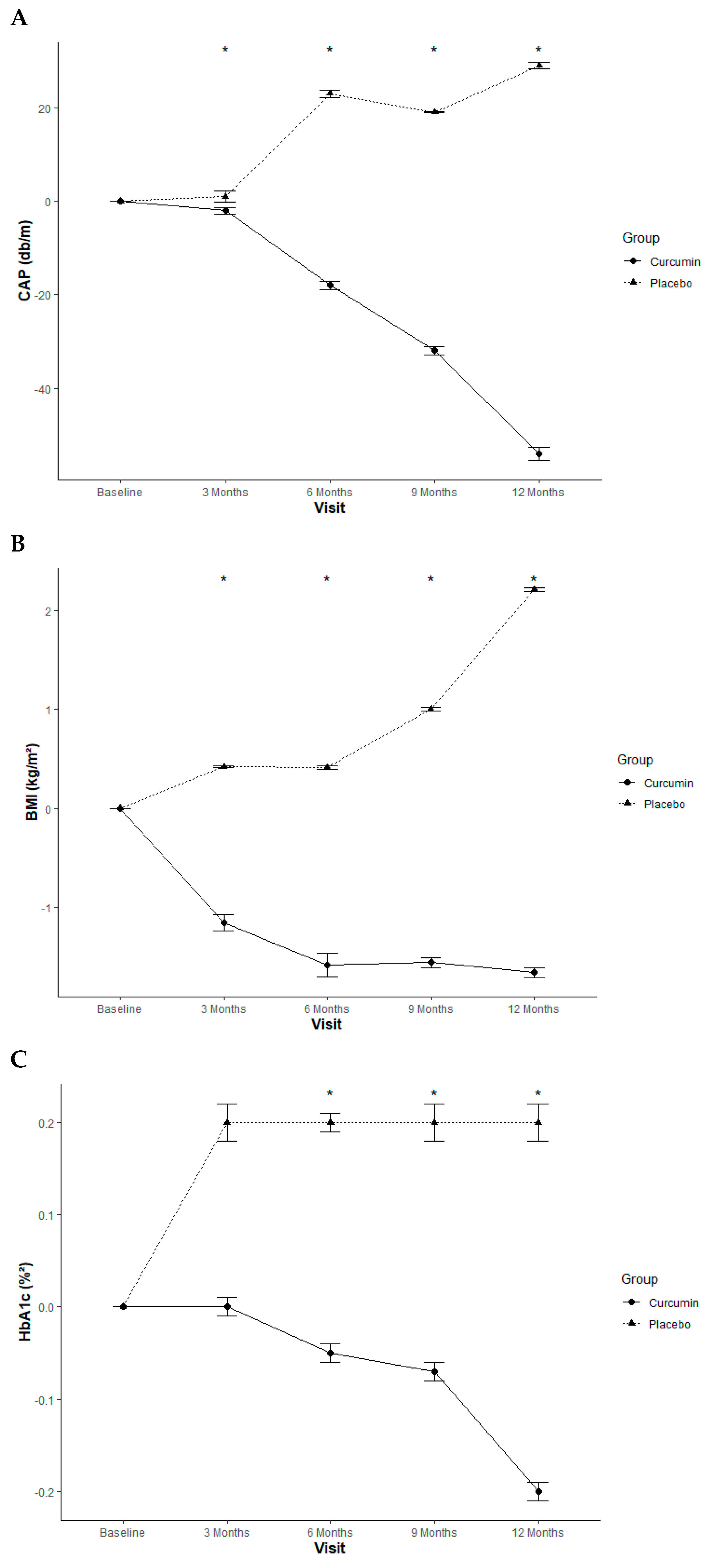
2.1.1. Curcumin Treatment Improved Liver Steatosis and Liver Stiffness
2.1.2. Antioxidant Effects
2.1.3. Anti-Inflammatory Effects
2.1.4. Anthropometric Measurement Effect
2.1.5. Glycemic Control Effect
2.1.6. Insulin Sensitivity Index
2.1.7. Insulin Resistance Index
2.1.8. Lipid Profiles
2.1.9. Non-Esterified Fatty Acid Levels
2.1.10. Adverse Effects
3. Discussion
4. Materials and Methods
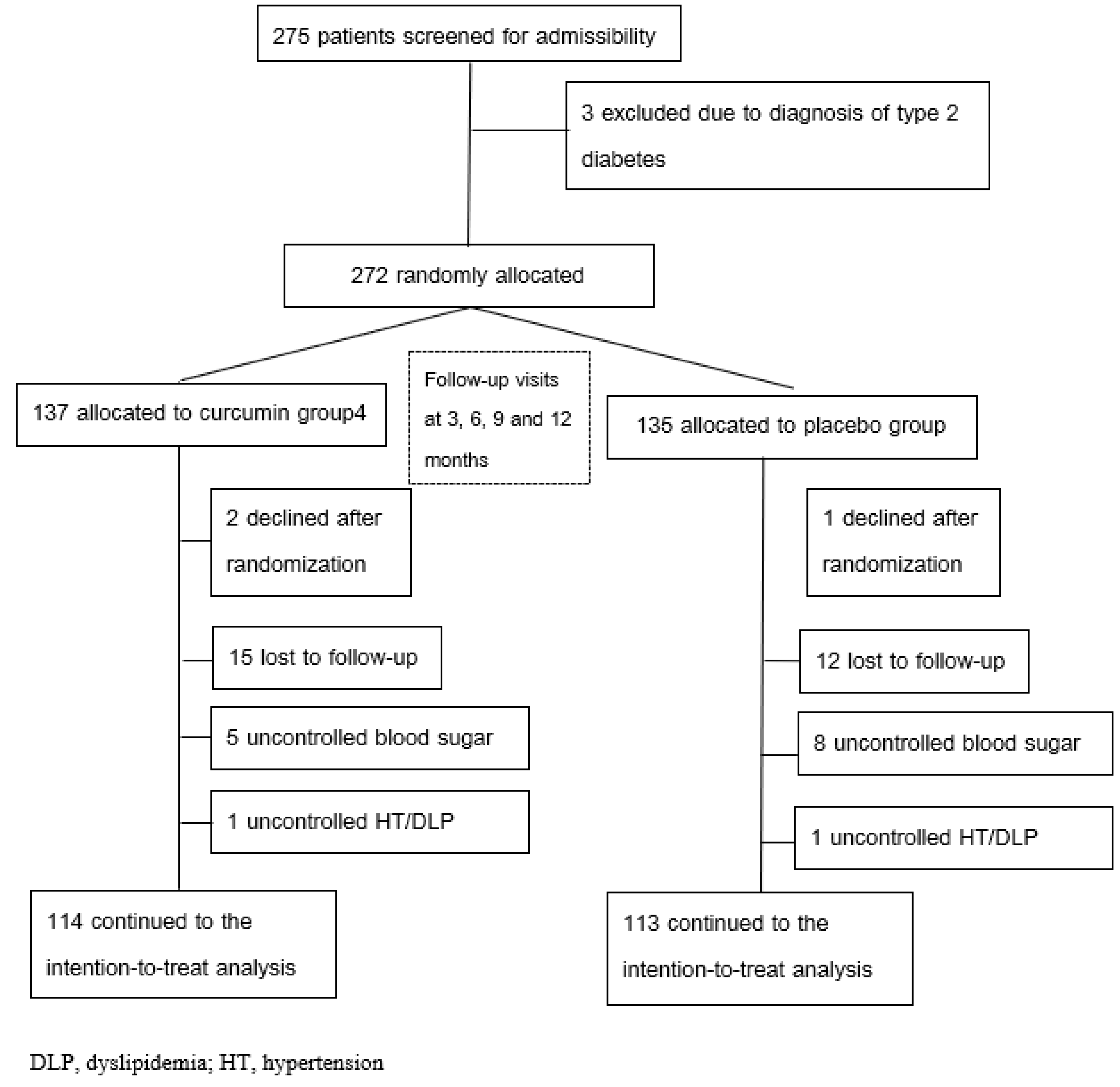
4.1. Study Design and Participants
4.2. Randomization Procedures
4.3. Blinding Procedures
4.4. Preparation of Curcuminoid Capsules
4.5. Intervention
4.6. Study Outcomes
4.7. Data Collection and Measurement Methods
4.8. Sample Size
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| BFP | body fat percentage |
| BMI | body mass index |
| CAP | controlled attenuation parameter |
| GPx | glutathione peroxidase |
| HbA1c | glycated hemoglobin |
| IL-1β | interleukin-1 beta |
| IQR | interquartile Range |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MDA | malondialdehyde |
| QUICKI | quantitative insulin sensitivity check index |
| SOD | superoxide dismutase |
| T2DM | type 2 diabetes mellitus |
| TBF | total body fat |
| TG | triglycerides |
| TNF-α | tumor necrosis factor-alpha |
| TyG-WC index | triglyceride glucose-waist circumference index |
References
- International Diabetes Federation. IDF Diabetes Atlas 2021; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar]
- Ntikoudi, A.; Papachristou, A.; Tsalkitzi, A.; Margari, N.; Evangelou, E.; Vlachou, E. Metabolic-Associated Steatotic Liver Disease (MASLD) and Type 2 Diabetes: Mechanisms, Diagnostic Approaches, and Therapeutic Interventions. Diabetology 2025, 6, 23. [Google Scholar] [CrossRef]
- Cusi, K.; Abdelmalek, M.F.; Apovian, C.M.; Balapattabi, K.; Bannuru, R.R.; Barb, D.; Bardsley, J.K.; Beverly, E.A.; Corbin, K.D.; ElSayed, N.A.; et al. Metabolic Dysfunction–Associated Steatotic Liver Disease (MASLD) in People with Diabetes: The Need for Screening and Early Intervention. A Consensus Report of the American Diabetes Association. Diabetes Care 2025, 48, 1057–1082. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.P.; Cusi, K. Role of Insulin Resistance in the Development of Nonalcoholic Fatty Liver Disease in People with Type 2 Diabetes: From Bench to Patient Care. Diabetes Spectr. 2024, 37, 20–28. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar]
- Svobodová, G.; Horní, M.; Velecká, E.; Boušová, I. Metabolic dysfunction-associated steatotic liver disease-induced changes in the antioxidant system: A review. Arch. Toxicol. 2025, 99, 1–22. [Google Scholar]
- Mohammadian, K.; Fakhar, F.; Keramat, S.; Stanek, A. The Role of Antioxidants in the Treatment of Metabolic Dysfunction-Associated Fatty Liver Disease: A Systematic Review. Antioxidants 2024, 13, 797. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.F.; She, Z.G.; Cai, J.J.; Li, H.L. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free. Radic. Biol. Med. 2020, 152, 116–141, Erratum in Free Radic Biol. Med. 2021, 162, 174. [Google Scholar] [CrossRef] [PubMed]
- Dattani, J.J.; Rajput, D.K.; Moid, N.; Highland, H.N.; George, L.B.; Desai, K.R. Ameliorative effect of curcumin on hepatotoxicity induced by chloroquine phosphate. Environ. Toxicol. Pharmacol. 2010, 30, 103–109. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, S.; Chen, A. Curcumin eliminates leptin’s effects on hepatic stellate cell activation via interrupting leptin signaling. Endocrinology 2009, 150, 3011–3020. [Google Scholar] [CrossRef]
- Jang, E.-M.; Choi, M.-S.; Jung, U.J.; Kim, M.-J.; Kim, H.-J.; Jeon, S.-M.; Shin, S.-K.; Seong, C.-N.; Lee, M.-K. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metabolism 2008, 57, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y. Curcumin targets multiple pathways to halt hepatic stellate cell activation: Updated mechanisms in vitro and in vivo. Dig. Dis. Sci. 2015, 60, 1554–1564. [Google Scholar] [CrossRef]
- Vizzutti, F.; Provenzano, A.; Galastri, S.; Milani, S.; Delogu, W.; Novo, E.; Caligiuri, A.; Zamara, E.; Arena, U.; Laffi, G.; et al. Curcumin limits the fibrogenic evolution of experimental steatohepatitis. Lab. Investig. 2010, 90, 104–115. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, M.C.; Ramirez-Tortosa, C.L.; Mesa, M.D.; Granados, S.; Gil, A.; Quiles, J.L. Curcumin ameliorates rabbits’s steatohepatitis via respiratory chain, oxidative stress, and TNF-alpha. Free Radic. Biol. Med. 2009, 47, 924–931. [Google Scholar] [CrossRef]
- Musso, G.; Pinach, S.; Mariano, F.; Saba, F.; De Michieli, F.; Framarin, L.; Berrutti, M.; Paschetta, E.; Parente, R.; Lizet Castillo, Y.; et al. Effect of phospholipid curcumin Meriva on liver histology and kidney disease in nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled trial. Hepatology 2025, 81, 560–575. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Li, Y.; Liang, Y.; Hong, T.; Yang, J.; Cao, Z.; Mai, H.; Yao, J.; Zhang, T.; et al. Curcumin supplementation alleviates hepatic fat content associated with modulation of gut microbiota-dependent bile acid metabolism in patients with nonalcoholic simple fatty liver disease: A randomized controlled trial. Am. J. Clin. Nutr. 2024, 120, 66–79. [Google Scholar] [CrossRef]
- Safari, Z.; Bagherniya, M.; Khoram, Z.; Ebrahimi Varzaneh, A.; Heidari, Z.; Sahebkar, A.; Askari, G. The effect of curcumin on anthropometric indices, blood pressure, lipid profiles, fasting blood glucose, liver enzymes, fibrosis, and steatosis in non-alcoholic fatty livers. Front. Nutr. 2023, 10, 1163950. [Google Scholar] [CrossRef]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem.—Asian J. 2024, 19, e202400380. [Google Scholar] [CrossRef] [PubMed]
- Leith, D.; Lin, Y.Y.; Brennan, P. Metabolic dysfunction-associated steatotic liver disease and type 2 diabetes: A deadly synergy. touchREVIEWS Endocrinol. 2024, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Garibay, V.M.; Chavez-Tapia, N.C. The rationale for the aggressive progression of MASLD in patients with type 2 diabetes. Ann. Hepatol. 2025, 30, 101778. [Google Scholar] [CrossRef]
- Ferdous, S.-E.; Ferrell, J.M. Pathophysiological relationship between type 2 diabetes mellitus and metabolic dysfunction-associated steatotic liver disease: Novel therapeutic approaches. Int. J. Mol. Sci. 2024, 25, 8731. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, T.; Wang, Z.; Shen, W.; Yuan, X.; Xie, J.; Zhang, Y. Role of curcumin in chronic liver diseases: A comprehensive review. Drug Des. Dev. Ther. 2025, 19, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- Sabini, J.H.; Timotius, K.H. Hepatoprotective and Fat-Accumulation-Reductive Effects of Curcumin on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Curr. Issues Mol. Biol. 2025, 47, 159. [Google Scholar]
- Tran, B.V.; Ujita, K.; Taketomi-Takahashi, A.; Hirasawa, H.; Suto, T.; Tsushima, Y. Reliability of ultrasound hepatorenal index and magnetic resonance imaging proton density fat fraction techniques in the diagnosis of hepatic steatosis, with magnetic resonance spectroscopy as the reference standard. PLoS ONE 2021, 16, e0255768. [Google Scholar]
- Hudson, D.; Afzaal, T.; Bualbanat, H.; AlRamdan, R.; Howarth, N.; Parthasarathy, P.; AlDarwish, A.; Stephenson, E.; Almahanna, Y.; Hussain, M.; et al. Modernizing metabolic dysfunction-associated steatotic liver disease diagnostics: The progressive shift from liver biopsy to noninvasive techniques. Ther. Adv. Gastroenterol. 2024, 17, 17562848241276334. [Google Scholar] [CrossRef]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.-G.; Mi, Y.-Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.-H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar]
- Rodrigues, H.C.N.; Martins, T.F.P.; Santana, N.C.F.e.S.; Braga, C.C.; Silva, M.A.C.; Cunha, L.C.d.; Sugizaki, C.S.d.A.; Freitas, A.T.V.d.S.; Costa, N.A.; Peixoto, M.d.R.G. Antioxidant and anti-inflammatory response to curcumin supplementation in hemodialysis patients: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN 2021, 44, 136–142. [Google Scholar]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin for Inflammation Control in Individuals with Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Randomized Controlled Trial. Nutrients 2025, 17, 1972. [Google Scholar] [CrossRef]
- Jin, W.; Botchway, B.O.A.; Liu, X. Curcumin Can Activate the Nrf2/HO-1 Signaling Pathway and Scavenge Free Radicals in Spinal Cord Injury Treatment. Neurorehabil. Neural Repair 2021, 35, 576–584. [Google Scholar] [PubMed]
- Lee, D.E.; Lee, S.J.; Kim, S.J.; Lee, H.S.; Kwon, O.S. Curcumin Ameliorates Nonalcoholic Fatty Liver Disease through Inhibition of O-GlcNAcylation. Nutrients 2019, 11, 2702. [Google Scholar] [CrossRef]
- Shapiro, H.; Bruck, R. Therapeutic potential of curcumin in non-alcoholic steatohepatitis. Nutr. Res. Rev. 2005, 18, 212–221. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Muriel, P. Pharmacological actions of curcumin in liver diseases or damage. Liver Int. 2009, 29, 1457–1466. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE 2012, 7, e28784. [Google Scholar] [PubMed]
- Mendoza, Y.P.; Rodrigues, S.G.; Delgado, M.G.; Murgia, G.; Lange, N.F.; Schropp, J.; Montani, M.; Dufour, J.F.; Berzigotti, A. Inflammatory activity affects the accuracy of liver stiffness measurement by transient elastography but not by two-dimensional shear wave elastography in non-alcoholic fatty liver disease. Liver Int. 2022, 42, 102–111. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Appiah-Opong, R.; Commandeur, J.N.; van Vugt-Lussenburg, B.; Vermeulen, N.P. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology 2007, 235, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Alquiraish, M.H.; Nguyen, P.; Hernandez, C.; Cepin, S.; Fortney, L.E.; Ajmera, V.; Bettencourt, R.; Collier, S.; Hooker, J. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018, 67, 1348–1359. [Google Scholar]
- Kuo, C.C.; Chuang, M.H.; Li, C.H.; Tsai, Y.W.; Huang, P.Y.; Kuo, H.T.; Lai, C.C. Glucagon-Like Peptide-1 Receptor Agonists and Liver Outcomes in Patients with MASLD and Type 2 Diabetes. Aliment. Pharmacol. Ther. 2025, 61, 1163–1174. [Google Scholar] [CrossRef]
- Suki, M.; Imam, A.; Amer, J.; Milgrom, Y.; Massarwa, M.; Hazou, W.; Tiram, Y.; Perzon, O.; Sharif, Y.; Sackran, J.; et al. SGLT2 Inhibitors in MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease) Associated with Sustained Hepatic Benefits, Besides the Cardiometabolic. Pharmaceuticals 2025, 18, 1118. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef]
- Association, A.D. Standards of medical care in diabetes—2017 abridged for primary care providers. Clin. Diabetes Publ. Am. Diabetes Assoc. 2017, 35, 5. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Bera, T.K. Bioelectrical impedance methods for noninvasive health monitoring: A review. J. Med. Eng. 2014, 2014, 381251. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Son, D.-H.; Baik, S.-J.; Cho, W.-J.; Lee, Y.-J. Triglyceride glucose-waist circumference (TyG-WC) is a reliable marker to predict non-alcoholic fatty liver disease. Biomedicines 2022, 10, 2251. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Pellegrini, N.; Colombi, B.; Bianchi, M.; Serafini, M.; Torta, F.; Tegoni, M.; Musci, M.; Brighenti, F. Rapid fluorimetric method to detect total plasma malondialdehyde with mild derivatization conditions. Clin. Chem. 2003, 49, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of non-alcoholic fatty liver disease with curcumin: A randomized placebo-controlled trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Placebo Median (IQR) n = 114 | Curcumin Median (IQR) n = 113 | p-Values * |
|---|---|---|---|
| Sex (M/F ratio) | 42/67 (0.63) | 50/65 (0.77) | 0.54 † |
| Age (years) | 63 (13) | 61 (12) | 0.07 |
| BMI (kg/m2) | 26.48 (4.90) | 26.56 (5.58) | 0.54 |
| Waist circumference (cm) | 93 (13) | 92 (12) | 0.29 |
| TAC (mmol/L) | 1.60 (0.17) | 1.59 (0.185) | 0.23 |
| Glutathione peroxidase (U/L) | 6534 (3152) | 6447 (3454) | 0.97 |
| Superoxide dismutase (U/mL) | 231 (62) | 231 (68) | 0.70 |
| Malonaldehyde (μmol/L) | 2.03 (0.63) | 1.98 (0.64) | 0.16 |
| IL1-beta (pg/mL) | 0.41 (0.35) | 0.40 (0.445) | 0.60 |
| TNF-α (pg/mL) | 5.28 (2.64) | 4.40 (2.64) | 0.17 |
| Glucose (mg/dL) | 120 (23) | 121 (22) | 0.67 |
| HbA1c (%) | 6.2 (0.7) | 6.2 (0.75) | 0.77 |
| QUICKI | 0.302 (0.02) | 0.302 (0.03) | 0.63 |
| TC (mg/dL) | 164 (40) | 165 (38.5) | 0.75 |
| TG (mg/dL) | 131 (88) | 116 (73.5) | 0.06 |
| LDL (mg/dL) | 99 (32) | 97 (31.5) | 0.88 |
| NEFA (μmol/L) | 0.80 (0.62) | 0.90 (0.615) | 0.21 |
| TyG-WC | 833.19 (153.43) | 811.10 (138.31) | 0.11 |
| Liver stiffness (kPa) | 5.4 (3.2) | 5.4 (2.6) | 0.65 |
| CAP (dB/m) | 230 (84) | 222 (58.5) | 0.70 |
| History of cerebrovascular disease | 7 (5.2%) | 5 (3.7%) | 0.30 † |
| History of coronary artery disease | 9 (6.7%) | 8 (5.9%) | 0.80 † |
| History of hypertension | 82 (61.2%) | 76 (51.2%) | 0.68 † |
| History of dyslipidemia | 104 (77.6%) | 101 (74.8%) | 0.84 † |
| Outcomes | Follow Up Period (mo) | Placebo | Curcumin | p-Values * |
|---|---|---|---|---|
| CAP (dB/m) | 0 | 230 (84) | 227 (58.5) | NS |
| 3 | 231 (68) | 225 (68.5) | 0.01 | |
| 6 | 253 (72) | 209 (71.5) | <0.001 | |
| 9 | 249 (85) | 195 (70) | <0.001 | |
| 12 | 259 (74) | 173 (78.5) | <0.001 | |
| Liver stiffness (kPa) | 0 | 5.4 (3.2) | 5.4 (2.6) | NS |
| 3 | 5.8 (2.2) | 5.6 (2.3) | NS | |
| 6 | 6.7 (2.2) | 5.3 (2.3) | <0.001 | |
| 9 | 6.9 (3.6) | 4.4 (1.4) | <0.001 | |
| 12 | 6.9 (2.8) | 3.9 (1.5) | <0.001 |
| Outcomes | Follow Up Period (mo) | Placebo | Curcumin | p-Values * |
|---|---|---|---|---|
| TAC (μmol trolox eq/l) | 0 | 1.60 (0.17) | 1.59 (0.19) | NS |
| 3 | 1.72 (0.26) | 1.75 (0.22) | 0.040 | |
| 6 | 1.66 (0.19) | 1.71 (0.22) | 0.02 | |
| 9 | 1.70 (0.32) | 1.80 (0.20) | 0.006 | |
| 12 | 1.63 (0.23) | 1.85 (0.16) | <0.001 | |
| GPx (U/mL) | 0 | 6534 (3152) | 6447 (3454) | NS |
| 3 | 6274 (2585) | 7143 (3005) | 0.01 | |
| 6 | 6748 (2472) | 8000 (3016.5) | <0.001 | |
| 9 | 5467 (1639) | 9912 (2261.5) | <0.001 | |
| 12 | 4837 (1159) | 12,587 (3586) | <0.001 | |
| SOD (U/mL) | 0 | 231 (62) | 231 (68) | NS |
| 3 | 232 (67) | 251 (45) | 0.035 | |
| 6 | 210 (39) | 268 (44.5) | <0.001 | |
| 9 | 206 (23) | 278 (37.5) | <0.001 | |
| 12 | 178 (30) | 315 (69.5) | <0.001 | |
| MDA (μmol/L) | 0 | 2.03 (0.63) | 1.98 (0.64) | NS |
| 3 | 1.90 (0.77) | 2.09 (0.70) | 0.03 | |
| 6 | 2.34 (0.59) | 1.95 (0.81) | <0.001 | |
| 9 | 2.32 (0.74) | 1.67 (0.68) | <0.001 | |
| 12 | 2.40 (0.80) | 1.32 (0.56) | <0.001 | |
| IL1-beta (pg/mL) | 0 | 0.41 (0.35) | 0.40 (0.45) | NS |
| 3 | 0.40 (0.42) | 0.49 (0.46) | NS | |
| 6 | 0.89 (0.20) | 0.52 (0.27) | <0.001 | |
| 9 | 0.92 (0.10) | 0.42 (0.24) | <0.001 | |
| 12 | 0.93 (0.07) | 0.31 (0.18) | <0.001 | |
| TNF-α (pg/mL) | 0 | 5.28 (2.64) | 4.40 (2.64) | NS |
| 3 | 5.28 (1.76) | 5.28 (2.64) | NS | |
| 6 | 5.65 (2.27) | 4.01 (1.76) | <0.001 | |
| 9 | 6.69 (2.53) | 3.98 (1.37) | <0.001 | |
| 12 | 6.99 (2.92) | 3.29 (1.17) | <0.001 |
| Outcomes | Follow Up Period (mo) | Placebo | Curcumin | p-Values * |
|---|---|---|---|---|
| BMI (kg/m2) | 0 | 26.48 (4.90) | 26.56 (5.58) | NS |
| 3 | 26.90 (4.82) | 25.40 (4.44) | 0.036 | |
| 6 | 26.89 (4.71) | 24.98 (3.96) | 0.008 | |
| 9 | 27.48 (5.24) | 25.00 (4.98) | <0.001 | |
| 12 | 28.69 (5.26) | 24.90 (4.93) | <0.001 | |
| WC (cm) | 0 | 93 (13) | 92 (12) | NS |
| 3 | 93 (13) | 90 (12.5) | 0.04 | |
| 6 | 94 (11) | 92 (11) | <0.001 | |
| 9 | 95 (14) | 90 (11) | <0.001 | |
| 12 | 95 (14) | 88 (11) | <0.001 | |
| TyG-WC | 0 | 833.192 (153.428) | 811.101 (138.313) | NS |
| 3 | 840.894 (161.246) | 801.300 (140.731) | 0.002 | |
| 6 | 891.349 (126.558) | 818.757 (101.063) | <0.001 | |
| 9 | 853.660 (150.686) | 783.073 (104.690) | <0.001 | |
| 12 | 865.939 (163.763) | 777.063 (105.613) | <0.001 | |
| Glucose (mg/dl) | 0 | 120 (23) | 121 (22) | NS |
| 3 | 126 (26) | 124 (22) | NS | |
| 6 | 125 (27) | 122 (27) | 0.02 | |
| 9 | 129 (21) | 117 (22.5) | <0.001 | |
| 12 | 133 (22) | 114 (24) | <0.001 | |
| HbA1c (%) | 0 | 6.2 (0.7) | 6.2 (0.8) | NS |
| 3 | 6.4 (0.7) | 6.2 (0.7) | NS | |
| 6 | 6.4 (0.7) | 6.1 (0.9) | 0.01 | |
| 9 | 6.4 (0.7) | 6.1 (0.9) | <0.001 | |
| 12 | 6.4 (0.9) | 6.0 (0.8) | <0.001 | |
| QUICKI | 0 | 0.302 (0.02) | 0.302 (0.03) | NS |
| 3 | 0.299 (0.02) | 0.305 (0.02) | 0.04 | |
| 6 | 0.300 (0.02) | 0.308 (0.02) | 0.009 | |
| 9 | 0.296 (0.02) | 0.304 (0.02) | <0.001 | |
| 12 | 0.294 (0.02) | 0.306 (0.03) | <0.001 | |
| HOMA-IR | 0 | 5.0 (2.5) | 5.10 (3.35) | NS |
| 3 | 5.5 (2.8) | 4.5 (2.85) | 0.02 | |
| 6 | 5.5 (2.8) | 4.4 (2.85) | 0.003 | |
| 9 | 5.9 (2.6) | 4.6 (2.90) | <0.001 | |
| 12 | 6.3 (2.6) | 4.4 (2.60) | <0.001 |
| Outcomes | Follow Up Period (mo) | Placebo | Curcumin | p-Values * |
|---|---|---|---|---|
| TC (mg/dl) | 0 | 164 (40) | 165 (38.5) | NS |
| 3 | 169 (40) | 163 (34) | NS | |
| 6 | 179 (45) | 161 (32.5) | <0.001 | |
| 9 | 181 (40) | 160 (35.5) | <0.001 | |
| 12 | 184 (43) | 159 (36) | <0.001 | |
| TG (mg/dl) | 0 | 131 (88) | 116 (73.5) | NS |
| 3 | 132 (94) | 111 (69.5) | 0.003 | |
| 6 | 148 (97) | 110 (66) | <0.001 | |
| 9 | 139 (96) | 107 (72.5) | <0.001 | |
| 12 | 132 (99) | 107 (66.5) | <0.001 | |
| LDL (mg/dl) | 0 | 99 (32) | 97 (31.5) | NS |
| 3 | 93 (31) | 91 (27) | NS | |
| 6 | 110 (40) | 98 (35) | 0.03 | |
| 9 | 99 (36) | 85 (28) | <0.001 | |
| 12 | 101 (37) | 87 (27.5) | <0.001 | |
| NEFA (μmol/L) | 0 | 0.80 (0.62) | 0.90 (0.62) | NS |
| 3 | 1.04 (0.45) | 1.15 (0.54) | NS | |
| 6 | 0.82 (0.48) | 1.16 (0.80) | <0.001 | |
| 9 | 1.10 (0.57) | 0.86 (0.51) | <0.001 | |
| 12 | 1.20 (0.70) | 0.76 (0.51) | <0.001 |
| Adverse Effects | Placebo (n = 114) * | Curcumin (n = 113) * |
|---|---|---|
| Abdominal pain | - | 13 (11.5) |
| Diarrhea | - | 8 (7.1) |
| Headache | 2 (1.7) | 5 (4.4) |
| Variables | Visit | Placebo | Curcumin | p Value | ||
|---|---|---|---|---|---|---|
| Mean (SEM) | Min-Max | Mean (SEM) | Min-Max | |||
| Creatinine (mg/dL) | Baseline | 0.87 (0.02) | 0.40–1.69 | 0.86 (0.02) | 0.45–1.6 | 0.77 |
| 3 mo | 0.88(0.02) | 0.45–1.81 | 0.91 (0.05) | 0.46–7.26 | 0.64 | |
| 6 mo | 0.94(0.02) | 0.47–2.04 | 0.92 (0.02) | 0.52–1.70 | 0.40 | |
| 9 mo | 0.94 (0.02) | 0.44–1.83 | 0.93 (0.02) | 0.54–1.81 | 0.51 | |
| 12 mo | 0.87(0.02) | 0.40–1.69 | 0.85(0.02) | 0.45–1.60 | 0.77 | |
| Aspartate aminotransferase (U/L) | Baseline | 25.01 (0.87) | 11–89 | 25.34 (0.80) | 13–67 | 0.58 |
| 3 mo | 22.45 (0.79) | 9–78 | 23.85(0.90) | 11–111 | 0.076 | |
| 6 mo | 23.53 (1.43) | 8–214 | 24.12 (0.98) | 10–89 | 0.47 | |
| 9 mo | 21.78 (0.63) | 11–76 | 24.29 (1.23) | 12–114 | 0.88 | |
| 12 mo | 25.01(0.87) | 11–89 | 25.41(0.81) | 13–67 | 0.54 | |
| Alanine aminotransferase (U/L) | Baseline | 27.58 (1.56) | 5–145 | 30.09 (1.50) | 5–118 | 0.08 |
| 3 mo | 24.08 (1.1) | 6–101 | 27.49 (1.7) | 6–214 | 0.08 | |
| 6 mo | 24.74 (1.15) | 7–98 | 28.16 (1.64) | 7–186 | 0.18 | |
| 9 mo | 23.01 (1.14) | 6–117 | 27.50 (1.80) | 6–129 | 0.36 | |
| 12 mo | 27.58(1.56) | 5–145 | 30.27(1.51) | 8–118 | 0.21 | |
| Medications | Placebo (n = 114) | Curcumin (n = 113) | p-Value † |
|---|---|---|---|
| Antihypertensive Medications * | |||
| Angiotensin receptor blockers | 80 (70.2) | 86 (76.1) | 0.39 |
| Calcium channel blockers | 26 (22.8) | 18 (15.9) | 0.25 |
| Beta blockers | 21 (18.4) | 17 (15.0) | 0.61 |
| Statins | 59 (51.8) | 55 (48.7) | 0.74 |
| Daily Intake of Nutrients | Placebo (n = 114) | Curcumin (n = 113) | p Value 2 | ||
|---|---|---|---|---|---|
| Baseline 1 | 12 mo | Baseline 1 | 12 mo | ||
| Energy (kcal/d) | 1857.60 ± 110.97 | 1893.74 ± 74.30 | 1864.21 ± 87.98 | 1881.16 ± 67.23 | 0.100 |
| Carbohydrate (% of energy) | 57.50 ± 2.57 | 58.07 ± 2.56 | 57.04 ± 1.52 | 58.08 ± 1.62 | 0.148 |
| Protein (% of energy) | 12.98 ± 2.13 | 13.21 ± 1.35 | 13.33 ± 1.28 | 13.44 ± 1.20 | 0.418 |
| FAT (% of energy) | 28.27 ± 2.12 | 28.91 ± 1.91 | 28.47 ± 2.42 | 28.44 ± 2.23 | 0.056 |
| Fiber (g/d) | 8.54 ± 1.16 | 8.46 ± 0.88 | 8.49 ± 0.82 | 8.39 ± 0.64 | 0.464 |
| Visit | Placebo | Curcumin | p Value | |||
|---|---|---|---|---|---|---|
| Number of Subjects Assessed | Number of Capsules Taken * | Number of Subjects Assessed | Number of Capsules Taken * | |||
| Consumption per 3 Months | 3 mo | 114 | 522.90 (54.59) | 113 | 514.80 (50.11) | 0.48 |
| 6 mo | 114 | 524.7 (21.61) | 113 | 512.10 (25.51) | 0.23 | |
| 9 mo | 114 | 517.16 (20.32) | 113 | 514.96 (21.33) | 0.43 | |
| 12 mo | 114 | 515.06 (20.82) | 113 | 514.11 (21.78) | 0.33 | |
| Consumption per Day | 3 mo | 114 | 5.81 (0.60) | 113 | 5.72 (0.58) | 0.57 |
| 6 mo | 114 | 5.83 (0.23) | 113 | 5.69 (0.28) | 0.13 | |
| 9 mo | 114 | 5.75 (0.22) | 113 | 5.72 (0.24) | 0.43 | |
| 12 mo | 114 | 5.72 (0.21) | 113 | 5.71 (0.25) | 0.33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaikwawong, M.; Kamdee, K.; Chuengsamarn, S. Curcumin Attenuates Liver Steatosis via Antioxidant and Anti-Inflammatory Pathways in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Int. J. Mol. Sci. 2025, 26, 9286. https://doi.org/10.3390/ijms26199286
Yaikwawong M, Kamdee K, Chuengsamarn S. Curcumin Attenuates Liver Steatosis via Antioxidant and Anti-Inflammatory Pathways in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. International Journal of Molecular Sciences. 2025; 26(19):9286. https://doi.org/10.3390/ijms26199286
Chicago/Turabian StyleYaikwawong, Metha, Khanittha Kamdee, and Somlak Chuengsamarn. 2025. "Curcumin Attenuates Liver Steatosis via Antioxidant and Anti-Inflammatory Pathways in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial" International Journal of Molecular Sciences 26, no. 19: 9286. https://doi.org/10.3390/ijms26199286
APA StyleYaikwawong, M., Kamdee, K., & Chuengsamarn, S. (2025). Curcumin Attenuates Liver Steatosis via Antioxidant and Anti-Inflammatory Pathways in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. International Journal of Molecular Sciences, 26(19), 9286. https://doi.org/10.3390/ijms26199286







