Coevolutionary Patterns in SOS1 and NHX1: Insights into Plant Ion Homeostasis Proteins
Abstract
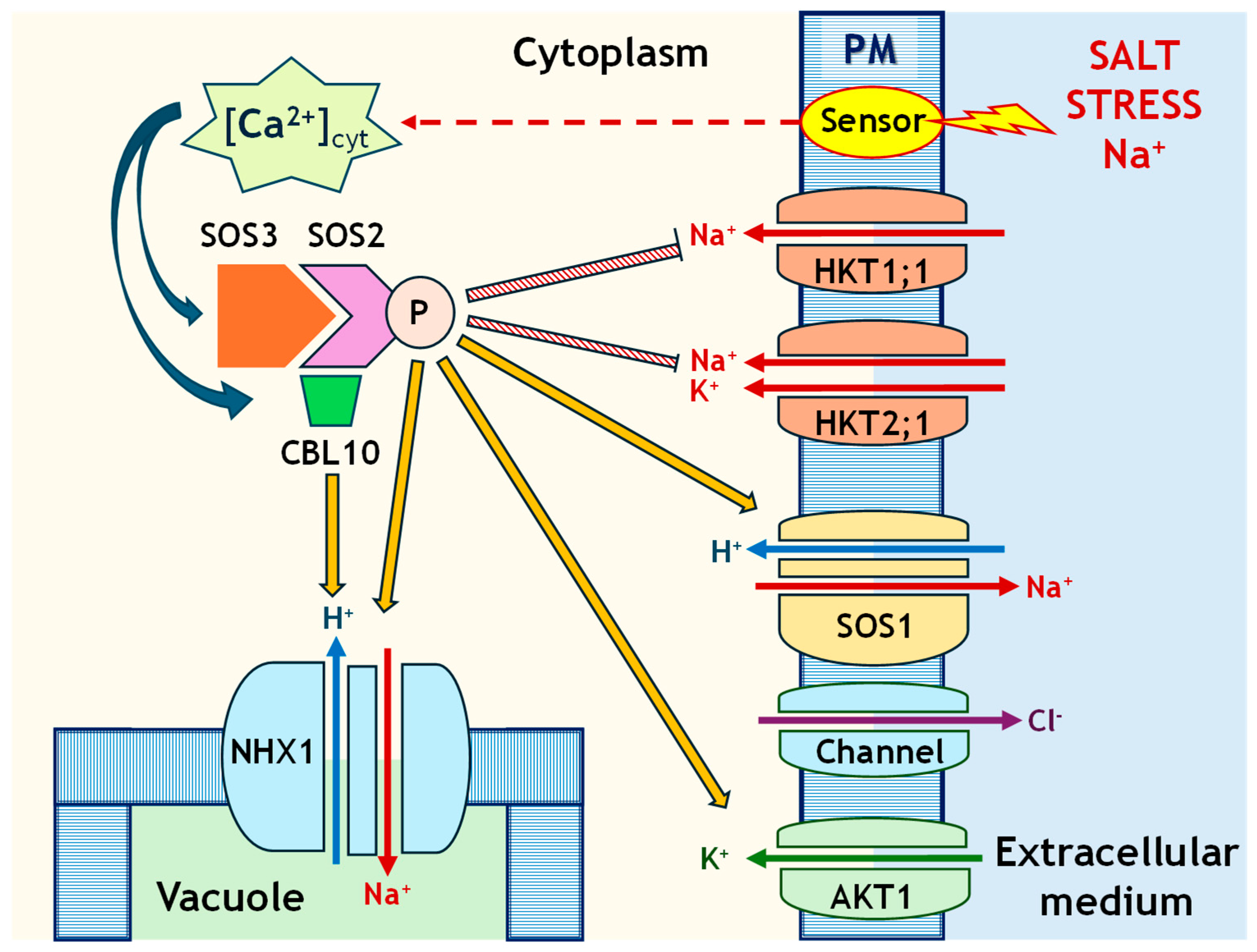
1. Introduction
2. Results
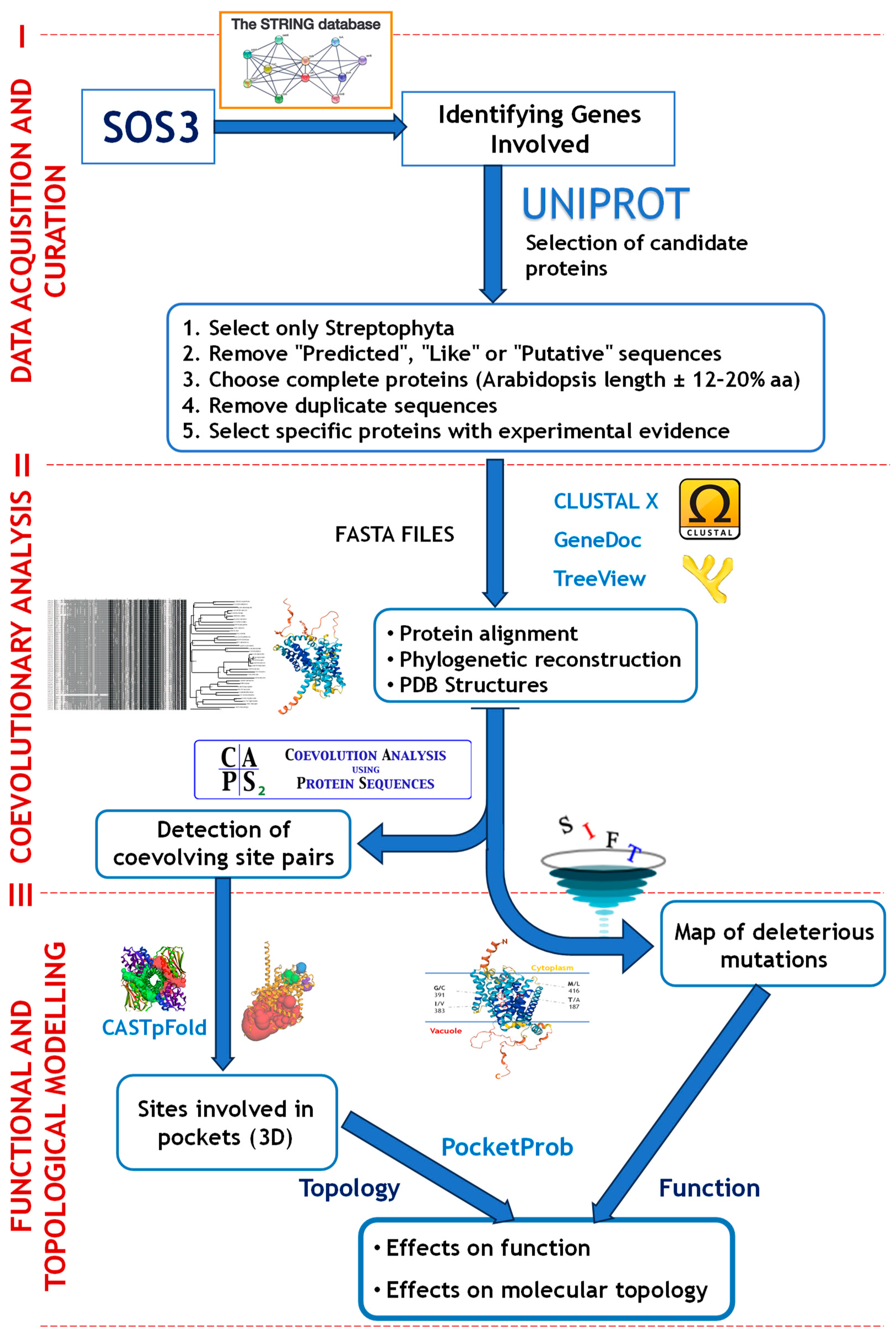
2.1. Data Acquisition and Sequence Curation
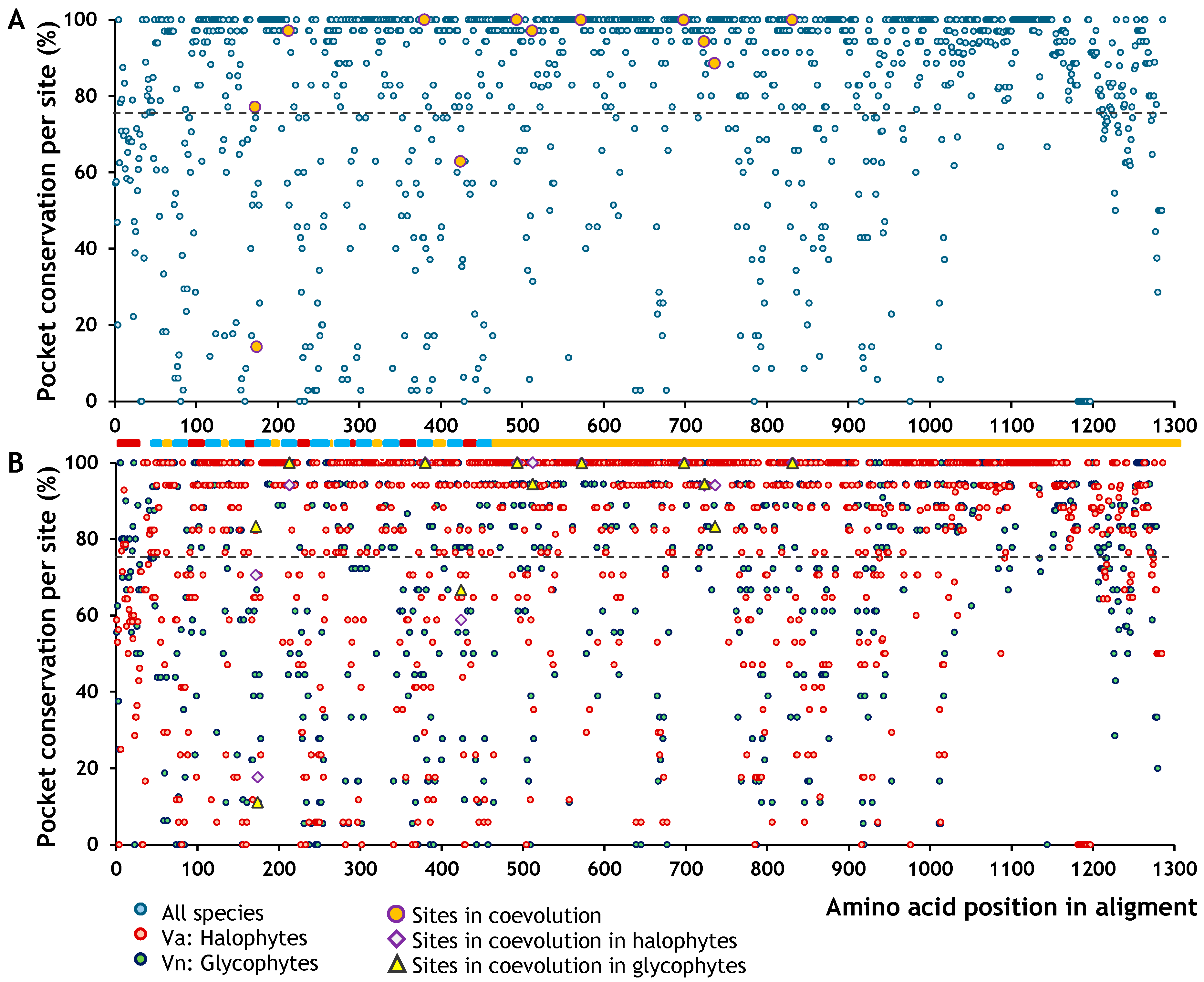
2.2. Intra- and Intermolecular Coevolutionary Analysis
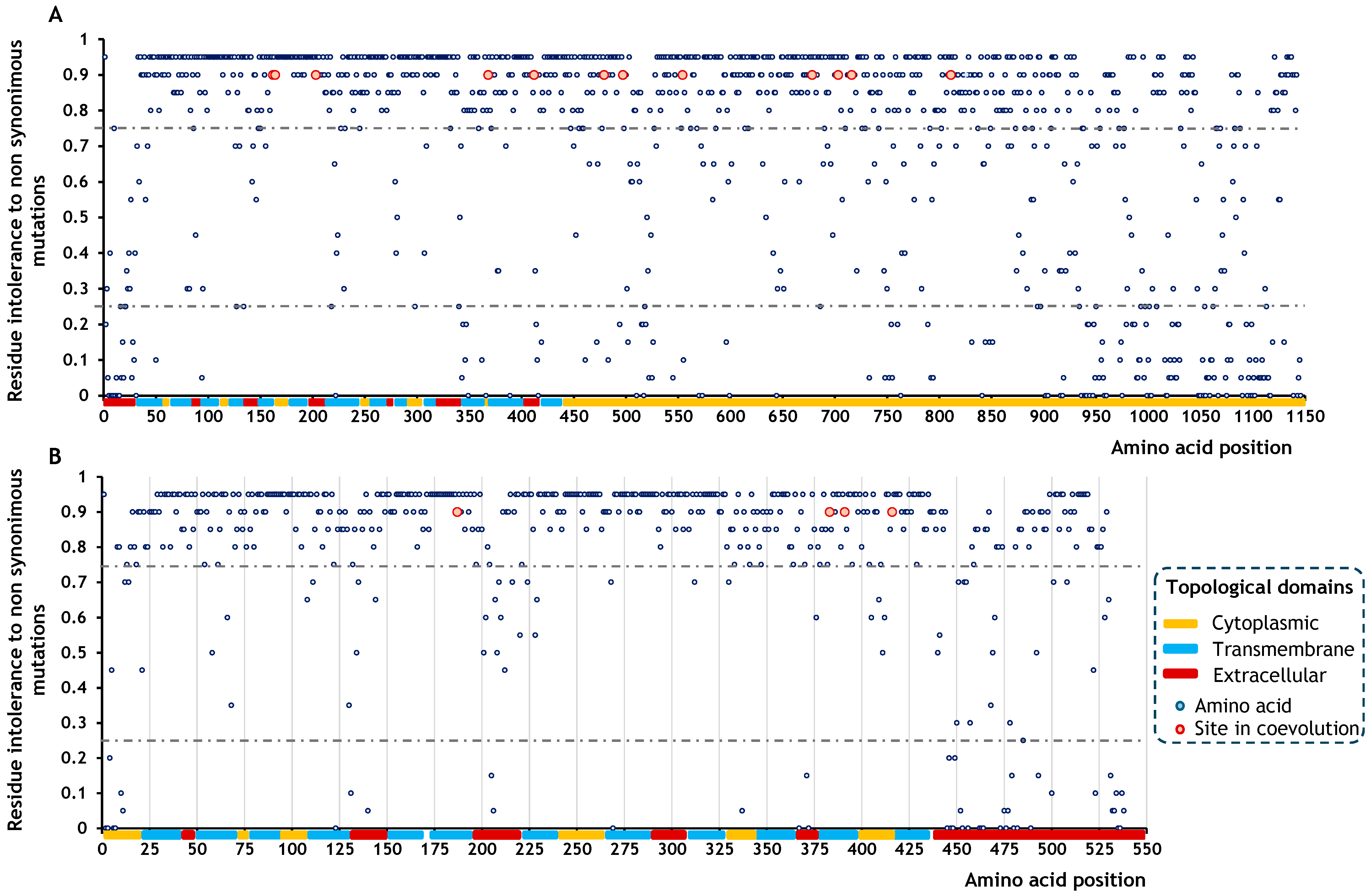
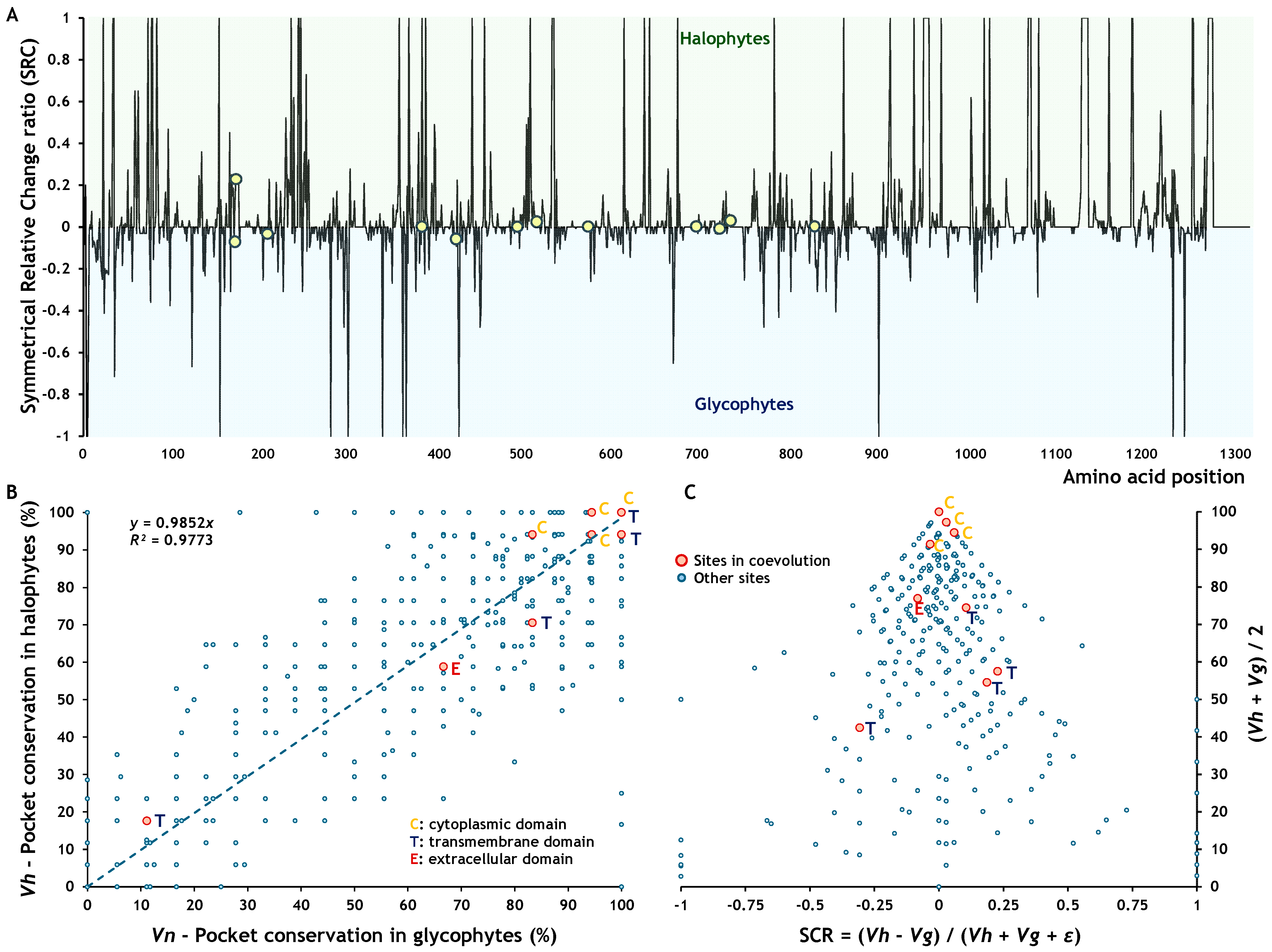
2.3. Effects of Amino Acid Residues on SOS1 and NHX1 Function
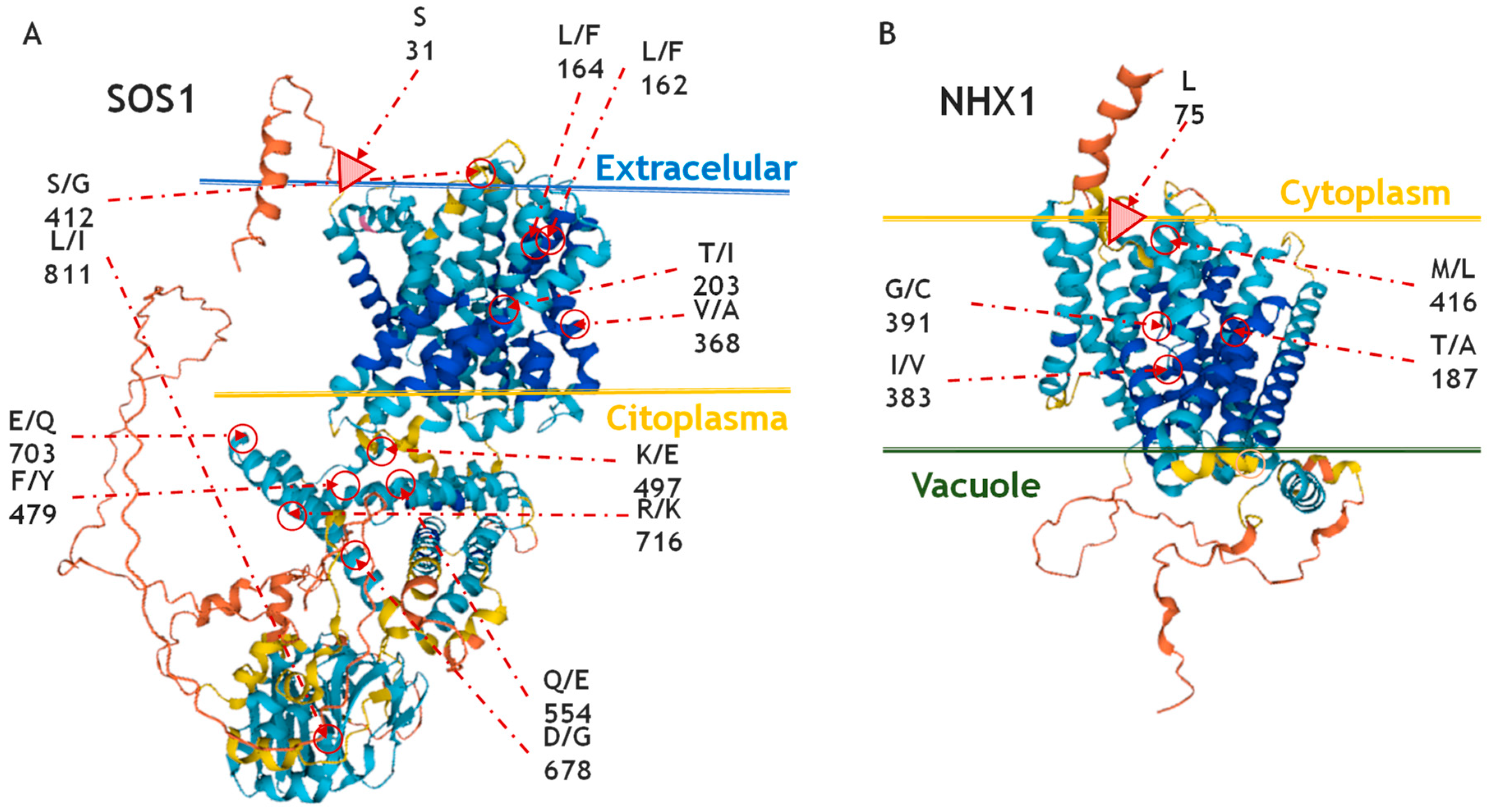
2.4. Effects of Amino Acid Residues on SOS1 and NHX1 Topology
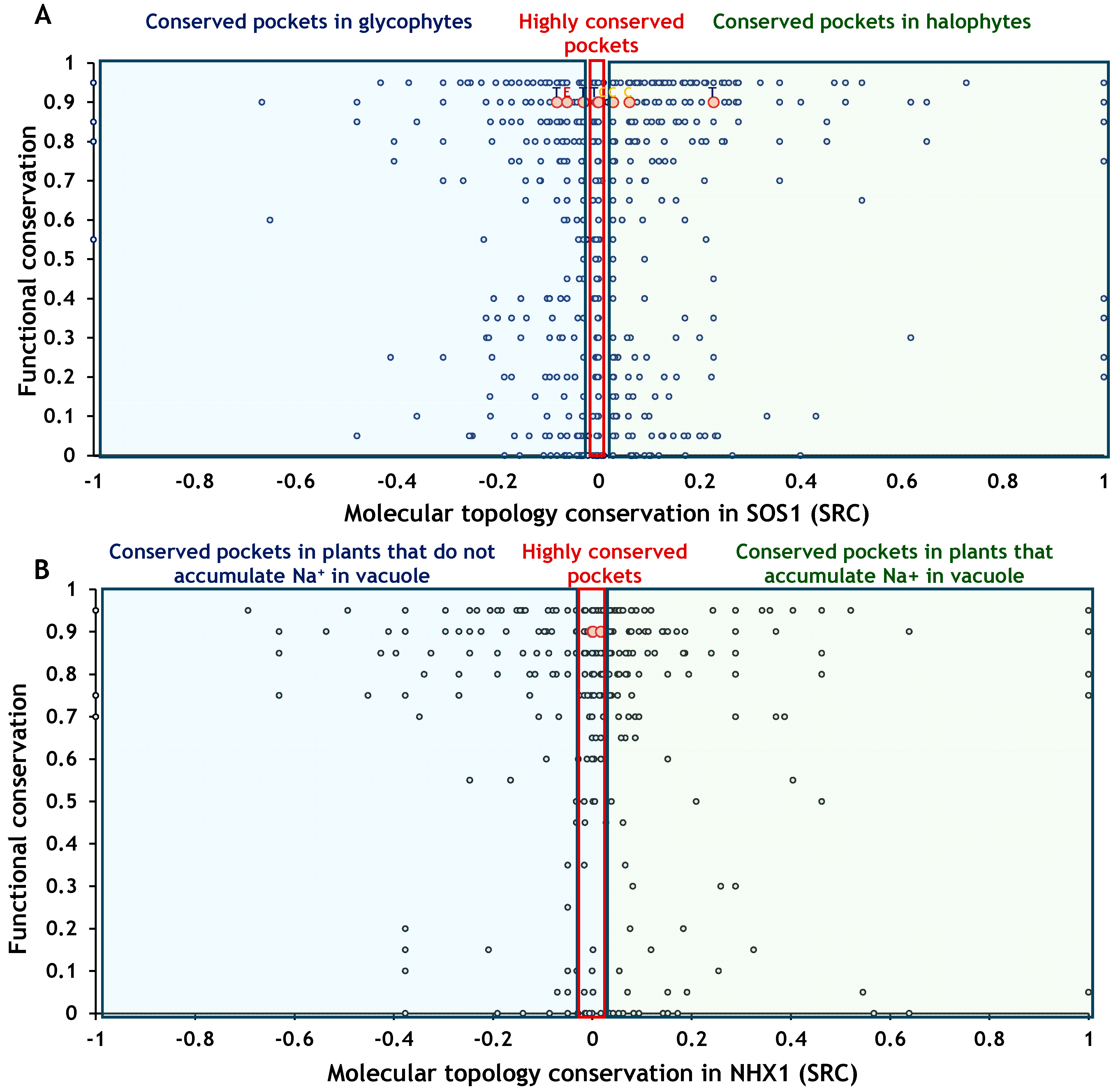
2.4.1. Effects of Amino Acid Residues on SOS1 Topology
2.4.2. Effects of Amino Acid Residues on NHX1 Topology
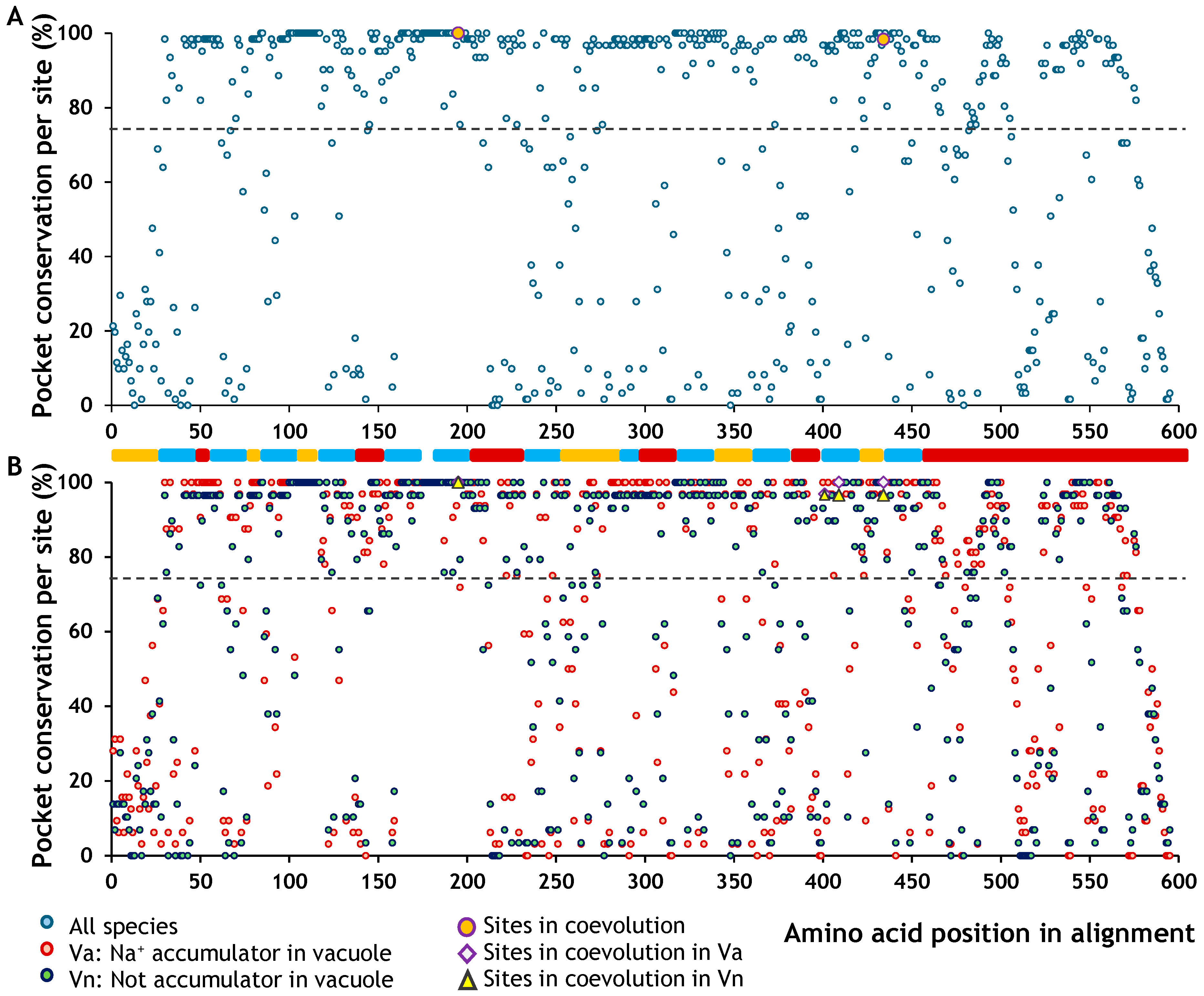

2.5. Overall Effects on Function and Topology
3. Discussion
4. Materials and Methods
4.1. Data Acquisition and Sequence Curation
4.2. Intra- and Intermolecular Coevolutionary Analysis
4.3. Effects of Amino Acid Residues on SOS1 and NHX1 Function
4.4. Effects of Amino Acid Residues on SOS1 and NHX1 Topology
4.5. Overall Effects on Function and Topology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC—Intergovernmental Panel on Climate Change. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022; 3068p. [Google Scholar]
- Rising, J.; Tedesco, M.; Piontek, F.; Stainforth, D.A. The missing risks of climate change. Nature 2022, 610, 643–651. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Res. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Grigore, M.-N.; Toma, C. Morphological and anatomical adaptations of halophytes: A review. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 1079–1221. [Google Scholar]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Ruiz-González, M.X.; Vicente, O. The Microbially Extended Phenotype of Plants, a Keystone against Abiotic Stress. EuroBiotech J. 2022, 6, 167–182. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Kumar, V.; Wani, S.H.; Suprasanna, P.; Tran, L.S.P. Salinity Responses and Tolerance in Plants, Volume 1: Targeting Sensory, Transport and Signaling Mechanisms; Springer: Cham, Switzerland, 2018; 408p. [Google Scholar]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Gámez-Arjona, F.; Park, H.J.; García, E.; Aman, R.; Villalta, I.; Raddatz, N.; Carranco, R.; Ali, A.; Ali, Z.; Zareen, S.; et al. Inverse regulation of SOS1 and HKT1 protein localization and stability by SOS3/CBL4 in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2024, 121, e2320657121. [Google Scholar] [CrossRef]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Dabravolski, S.A.; Pan, T.; Shabala, S. Phylogenetic Diversity and Physiological Roles of Plant Monovalent Cation/H+ Antiporters. Front. Plant Sci. 2020, 11, 573564. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. Preponderance of synonymous changes as evidence for the neutral theory of molecular evolution. Nature 1977, 267, 275–276. [Google Scholar] [CrossRef]
- Jordan, I.K.; Rogozin, I.B.; Wolf, Y.I.; Koonin, E.V. Essential genes are more evolutionarily conserved than are nonessential genes in bacteria. Genome Res. 2002, 12, 962–968. [Google Scholar] [CrossRef]
- Mintseris, J.; Weng, Z. Structure, function, and evolution of transient and obligate protein–protein interactions. Proc. Natl. Acad. Sci. USA 2005, 102, 10930–10935. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Van Valen, L. A New Evolutionary Law. Evol. Theory 1973, 1, 1–30. [Google Scholar]
- Thompson, J.N. The Geographic Mosaic of Coevolution; University of Chicago Press: Chicago, IL, USA, 2005; 443p. [Google Scholar]
- Fares, M.A.; Ruiz-González, M.X.; Labrador, J.P. Protein coadaptation and the design of novel approaches to identify protein-protein interactions. IUBMB Life 2011, 63, 264–271. [Google Scholar] [CrossRef]
- Atchley, W.R.; Wollenberg, K.R.; Fitch, W.M.; Terhalle, W.; Dress, A.W. Correlations among amino acid sites in bHLH protein domains: An information theoretic analysis. Mol. Biol. Evol. 2000, 17, 164–178. [Google Scholar] [CrossRef]
- Carmona, D.; Fitzpatrick, C.R.; Johnson, M.T. Fifty years of co-evolution and beyond: Integrating co-evolution from molecules to species. Mol. Ecol. 2015, 24, 5315–5329. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.L.; Alani, E.; Aquadro, C.F. Evolutionary rate covariation reveals shared functionality and coexpression of genes. Genome Res. 2012, 22, 714–720. [Google Scholar] [CrossRef]
- Ruiz-González, M.X.; Fares, M.A. Coevolution analyses illuminate the dependencies between amino acid sites in the chaperonin system GroES-L. BMC Evol. Biol. 2013, 13, 156. [Google Scholar]
- Noda-Garcia, L.; Liebermeister, W.; Tawfik, D.S. Metabolite-Enzyme Coevolution: From Single Enzymes to Metabolic Pathways and Networks. Annu. Rev. Biochem. 2018, 87, 187–216. [Google Scholar] [CrossRef]
- Lovell, S.C.; Robertson, D.L. An integrated view of molecular coevolution in protein-protein interactions. Mol. Biol. Evol. 2010, 27, 2567–2575. [Google Scholar] [CrossRef] [PubMed]
- De Juan, D.; Pazos, F.; Valencia, A. Emerging methods in protein co-evolution. Nat. Rev. Genet. 2013, 14, 249–261. [Google Scholar] [CrossRef]
- Pollock, D.D.; Thiltgen, G.; Goldstein, R.A. Amino acid coevolution induces an evolutionary Stokes shift. Proc. Natl. Acad. Sci. USA 2012, 109, E1352–E1359. [Google Scholar] [CrossRef]
- Backwell, L.; Marsh, J.A. Diverse Molecular Mechanisms Underlying Pathogenic Protein Mutations: Beyond the Loss-of-Function Paradigm. Annu. Rev. Genom. Hum. Genet. 2022, 23, 475–498. [Google Scholar] [CrossRef]
- Jacquier, H.; Birgy, A.; Le Nagard, H.; Mechulam, Y.; Schmitt, E.; Glodt, J.; Bercot, B.; Petit, E.; Poulain, J.; Barnaud, G.; et al. Capturing the mutational landscape of the β-lactamase TEM-1. Proc. Natl. Acad. Sci. USA 2013, 110, 13067–13072. [Google Scholar] [CrossRef]
- Poon, A.; Chao, L. The rate of compensatory mutation in the DNA bacteriophage phiX174. Genetics 2005, 170, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Salinas, V.H.; Ranganathan, R. Coevolution-based inference of amino acid interactions underlying protein function. eLife 2018, 7, e34300. [Google Scholar] [CrossRef]
- Wittmund, M.; Cadet, F.; Davari, M.D. Learning epistasis and residue coevolution patterns: Current trends and future perspectives for advancing enzyme engineering. ACS Catal. 2022, 12, 14243–14263. [Google Scholar] [CrossRef]
- Rombolá-Caldentey, B.; Mendoza, I.; Quintero, F.J.; Pardo, J.M. Structure-Guided Identification of Critical Residues in the Vacuolar Cation/Proton Antiporter NHX1 from Arabidopsis thaliana. Plants 2023, 12, 2778. [Google Scholar] [CrossRef]
- Zhang, W.D.; Wang, P.; Bao, Z.; Ma, Q.; Duan, L.J.; Bao, A.K.; Zhang, J.L.; Wang, S.M. SOS1, HKT1;5, and NHX1 Synergistically Modulate Na+ Homeostasis in the Halophytic Grass Puccinellia tenuiflora. Front. Plant Sci. 2017, 8, 576. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, A.; Sidhu, S.; Upadhyay, S.K. Na+/H+ antiporter (NHX) and salt stress tolerance. In Cation Transporters in Plants; Academic Press: London, UK, 2022; pp. 99–113. [Google Scholar]
- Zhang, Y.; Zhou, J.; Ni, X.; Wang, Q.; Jia, Y.; Xu, X.; Wu, H.; Fu, P.; Wen, H.; Guo, Y.; et al. Structural basis for the activity regulation of Salt Overly Sensitive 1 in Arabidopsis salt tolerance. Nat. Plants 2023, 9, 1915–1923. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Chen, Q.; Xie, Q.; Gao, Y.; He, L.; Li, Y.; Dong, Y.; Jiang, X.; Zhao, Y. Architecture and autoinhibitory mechanism of the plasma membrane Na+/H+ antiporter SOS1 in Arabidopsis. Nat. Commun. 2023, 14, 4487. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Panchenko, A.R. Structural and functional roles of coevolved sites in proteins. PLoS ONE 2010, 5, e8591. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Dutta, D.; Fliegel, L. Expression and characterization of the SOS1 Arabidopsis salt tolerance protein. Mol. Cell. Biochem. 2016, 415, 133–143. [Google Scholar] [CrossRef]
- Le Guilloux, V.; Schmidtke, P.; Tuffery, P. Fpocket: An open source platform for ligand pocket detection. BMC Bioinform. 2009, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Stank, A.; Kokh, D.B.; Fuller, J.C.; Wade, R.C. Protein Binding Pocket Dynamics. Acc. Chem. Res. 2016, 49, 809–815. [Google Scholar] [CrossRef]
- Calzone, A.; Cotrozzi, L.; Pellegrini, E.; Lorenzini, G.; Nali, C.; Maathuis, F. Can the transcriptional regulation of NHX1, SOS1 and HKT1 genes handle the response of two pomegranate cultivars to moderate salt stress?: Salt-tolerance of two pomegranate cultivars. Sci. Hortic. 2021, 288, 110309. [Google Scholar] [CrossRef]
- Peng, J.; Svetec, N.; Zhao, L. Intermolecular Interactions Drive Protein Adaptive and Coadaptive Evolution at Both Species and Population Levels. Mol. Biol. Evol. 2022, 39, msab350. [Google Scholar] [CrossRef]
- Maisnier-Patin, S.; Paulander, W.; Pennhag, A.; Andersson, D.I. Compensatory evolution reveals functional interactions between ribosomal proteins S12, L14 and L19. J. Mol. Biol. 2007, 366, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2018, 46, 2699. [Google Scholar] [CrossRef]
- Buslje, C.M.; Santos, J.; Delfino, J.M.; Nielsen, M. Correction for phylogeny, small number of observations and data redundancy improves the identification of coevolving amino acid pairs using mutual information. Bioinformatics 2009, 25, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Avila-Herrera, A.; Pollard, K.S. Coevolutionary analyses require phylogenetically deep alignments and better null models to accurately detect inter-protein contacts within and between species. BMC Bioinform. 2015, 16, 268. [Google Scholar] [CrossRef]
- Teppa, E.; Zea, D.J.; Oteri, F.; Carbone, A. COVTree: Coevolution in OVerlapped sequences by Tree analysis server. Nucleic Acids Res. 2020, 48, W558–W565. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Nicholas, K.B.; Nicholas, H.B., Jr.; Deerfield, D.W., II. GeneDoc: Analysis and Visualization of Genetic Variation. EMBNEW News 1997, 4, 14. [Google Scholar]
- Page, R.D. TreeView: An application to display phylogenetic trees on personal computers. CABIOS 1996, 12, 357–358. [Google Scholar] [PubMed]
- Fares, M.A.; McNally, D. CAPS: Coevolution analysis using protein sequences. Bioinformatics 2006, 22, 2821–2822. [Google Scholar] [CrossRef] [PubMed]
- Sim, N.L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef]
- Ye, B.; Tian, W.; Wang, B.; Liang, J. CASTpFold: Computed Atlas of Surface Topography of the universe of protein Folds. Nucleic Acids Res. 2024, 52, W194–W199. [Google Scholar] [CrossRef]
| Protein | Synonyms | Number of Sequences | Valid Sequences | Function |
|---|---|---|---|---|
| Direct interactions with SOS3 (CBL4) | ||||
| SOS3 | CBL4 | 37 | 15 | Calcineurin B-like 4 protein |
| AKT1 | At2g26650, F18A8.2 | 4776 | 10 | Potassium channel AKT1 |
| CBL10 | At4g33000, F26P21_120, SCABP8 | 40 | 10 | Calcineurin B-like 10 protein |
| CIPK1 | PKS13, SnRK3.16, At3g17510, MKP6.6 | 39 | 6 | Protein 1 CBL serine/threonine kinase |
| CIPK11 | PKS5, SIP4, SnRK3.22, At2g30360, T9D9.17 | 46 | 12 | Protein 11 CBL serine/threonine kinase |
| CIPK14 | PKS24, SnRK3.15, SR1, At5g01820, T20L15.90 | 55 | 14 | Protein 14 CBL serine/threonine kinase |
| SOS2 | CIPK24, SnRK3.11, At5g35410, F6I13.1, K21B8.3 | 47 | 16 | Protein 24 CBL Serine/Threonine-Kinase Interaction |
| CIPK6 | PKS4, SIP3, SnRK3.14, At4g30960, F6I18.130 | 43 | 8 | Protein 6 CBL serine/threonine kinase |
| NHX1 | At5g27150, T21B4.60 | 176 | 62 | Na+/H+ Antiporter 1 |
| SOS1 | NHX7, At2g01980, F14H20.5 | 356 | 36 | Na+/H+ Antiporter 7 |
| Second layer of interactions with SOS3 (CBL4) | ||||
| AKT2 | AKT3, At4g22200, T10I14.30 | 620 | 2 | AKT2/3 K+ Channel |
| CBL1 | SCABP5, At4g17615, dl4845w, FCAALL.122 | 65 | 14 | Calcineurin B-like protein 1 |
| CBL2 | SCABP1, At5g55990, MDA7.3 | 42 | 14 | Calcineurin B-like 2 protein |
| CBL3 | SCABP6, At4g26570, T15N24.20 | 57 | 13 | Calcineurin B-like 3 protein |
| CBL9 | At5g47100, K14A3.5 | 53 | 8 | Calcineurin B-like 9 protein |
| Position AA1 | Position AA2 | Corr. | Boots | AA Pairs | % | Taxonomic Groups |
|---|---|---|---|---|---|---|
| SOS1 | ||||||
| 162 (172) | 164 (174) | 0.95 | 1.00 | L-L F-F | 86.5 13.5 | All taxa except: Aegilops Triticum Group |
| 203 (213) | 368 (380) | 1.00 | 1.00 | T-V I-A | 94.6 5.4 | All taxa except: Caryophyllales: Bassia, Salicornia |
| 412 (424) | 811 (831) | 1.00 | 1.00 | S-L G-I | 94.6 5.4 | All taxa except: Malpighiales: Ricinus, Fabales: Glycine |
| 479 (493) | 703 (723) | 1.00 | 1.00 | F-E Y-Q | 94.6 5.4 | All taxa except: Caryophyllales: Fagopyrum |
| 497 (512) | 716 (736) | 1.00 | 1.00 | K-R E-K | 94.6 5.4 | All taxa except: Brassicales: Arabidopsis, Eutrema |
| 554 (572) | 678 (698) | 1.00 | 1.00 | Q-D E-G | 94.6 5.4 | All taxa except: Brassicales: Arabidopsis, Eutrema |
| NHX1 | ||||||
| 187 (195) | 416 (434) | 1.00 | 0.96 | T-M A-L | 96.77 3.23 | All taxa except: Saxifragales: Paeonia sp. Gentianales: Gentiana sp. |
| 383 (401) | 391 (409) | 1.00 | 0.87 | I-G V-C | 96.77 3.23 | All taxa except: Saxifragales: Paeonia sp. Fabales: Robinia sp. |
| Position SOS1 | Position NHX1 | Correlation | Bootstrap |
|---|---|---|---|
| 1043 (1087) | 69 (81) | 0.526 | 0.880 |
| 31 (38) | 75 (87) | 0.606 | 0.954 |
| 217 (224) | 456 (472) | 0.0378 | 0.826 |
| 257 (264) | 456 (472) | 0.023 | 0.807 |
| 384 (393) | 456 (472) | 0.507 | 0.795 |
| 911 (929) | 456 (472) | 0.0575 | 0.851 |
| 991 (1028) | 456 (472) | 0.0417 | 0.875 |
| SOS1 | NHX1 | |||
|---|---|---|---|---|
| Inactivation Ranks | # Residues | % Sites | # Residues | % Sites |
| 0.00 | 58 | 5.1 | 23 | 4.3 |
| 0.00 to 0.25 | 130 | 11.3 | 25 | 4.6 |
| 0.25 to 0.50 | 64 | 5.6 | 18 | 3.3 |
| 0.50 to 0.75 | 125 | 10.9 | 53 | 9.9 |
| 0.75 to 0.99 | 769 | 67.1 | 419 | 77.9 |
| 1.00 | 0 | 0.0 | 0 | 0.0 |
| Binding Pocket Conservation Range | Total | G | H | Total (%) | G (%) | H (%) |
|---|---|---|---|---|---|---|
| 0 | 24 | 29 | 32 | 1.87 | 2.37 | 2.50 |
| 0 to 25% | 80 | 68 | 79 | 6.22 | 5.55 | 6.17 |
| 25 to 50% | 87 | 87 | 72 | 6.77 | 7.10 | 5.63 |
| 50 to 75% | 156 | 136 | 154 | 12.13 | 11.09 | 12.03 |
| 75 to 99% | 497 | 324 | 415 | 38.65 | 26.43 | 32.42 |
| 100% | 442 | 582 | 528 | 34.37 | 47.47 | 41.25 |
| Binding Pocket Conservation Range | Total | PAV | PnAV | Total (%) | PAV (%) | PnAV (%) |
|---|---|---|---|---|---|---|
| 0 | 8 | 29 | 27 | 1.34 | 4.87 | 4.54 |
| 0 to 25% | 132 | 114 | 119 | 22.18 | 19.16 | 20.00 |
| 25 to 50% | 47 | 51 | 39 | 7.90 | 8.57 | 6.55 |
| 50 to 75% | 56 | 48 | 60 | 9.41 | 8.07 | 10.08 |
| 75 to 99% | 267 | 167 | 243 | 44.87 | 28.07 | 40.84 |
| 100% | 85 | 186 | 107 | 14.29 | 31.26 | 17.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-González, M.X.; Vélez-Mejía, A.; Vicente, O. Coevolutionary Patterns in SOS1 and NHX1: Insights into Plant Ion Homeostasis Proteins. Int. J. Mol. Sci. 2025, 26, 9276. https://doi.org/10.3390/ijms26199276
Ruiz-González MX, Vélez-Mejía A, Vicente O. Coevolutionary Patterns in SOS1 and NHX1: Insights into Plant Ion Homeostasis Proteins. International Journal of Molecular Sciences. 2025; 26(19):9276. https://doi.org/10.3390/ijms26199276
Chicago/Turabian StyleRuiz-González, Mario X., Antonio Vélez-Mejía, and Oscar Vicente. 2025. "Coevolutionary Patterns in SOS1 and NHX1: Insights into Plant Ion Homeostasis Proteins" International Journal of Molecular Sciences 26, no. 19: 9276. https://doi.org/10.3390/ijms26199276
APA StyleRuiz-González, M. X., Vélez-Mejía, A., & Vicente, O. (2025). Coevolutionary Patterns in SOS1 and NHX1: Insights into Plant Ion Homeostasis Proteins. International Journal of Molecular Sciences, 26(19), 9276. https://doi.org/10.3390/ijms26199276







