Hypoxia and Tissue Regeneration: Adaptive Mechanisms and Therapeutic Opportunities
Abstract
1. Introduction
2. Hypoxia Types and Systemic Adaptation
| Species or Group | Genes or Identified Variants | Type of Variant | Biological Pathway or Function | Phenotype or Adaptive Trait | Adaptive Physiological Effect | Ref. |
|---|---|---|---|---|---|---|
| Teleost fish | ||||||
| Wild-type Zebrafish (Danio rerio) | hif-1aa, hif-1ab, hif-2aa, hif-2ab, hif-3aa, hif-3ab | Coding; retained paralogs from teleost genome duplication | HIF signaling; oxygen sensing and erythropoiesis | Differential responses to hypoxia across tissues; Hif-3 paralogs are required for erythropoiesis | Improved hypoxia tolerance; sub-functionalized oxygen response | [26] |
| Wild-type Zebrafish (Danio rerio) | phd1, phd2, phd3 | Coding; genome duplication | Prolyl hydroxylation; regulation of HIF stability | Retention of multiple isoforms allows fine regulation | Enhanced capacity to modulate HIF degradation under variable oxygen levels | [26] |
| Birds | ||||||
| Andean house wren (Troglodytes aedon) | β-globin (avian HBB ortholog) | Missense mutation in oxygen-binding site | Hemoglobin oxygen-binding affinity | Increased hemoglobin O2 affinity in high-altitude populations | Increased oxygen uptake and delivery to tissues in hypoxic environments | [22] |
| Andean ducks (A. flavirostris, Spatula cyanoptera, A. georgica) | ND2, COI, ATP6 (mitochondrial genes) | Purifying selection on mitochondrial coding sequences | Oxidative phosphorylation efficiency | Enhanced ATP production under low oxygen availability | Efficient aerobic energy production in hypoxic high-altitude habitats | [28] |
| Bar-headed goose (Anser indicus) | α-globin, β-globin (HBA, HBB orthologs) | Amino acid substitutions with increased O2 affinity | Hemoglobin structure and gas transport | Efficient O2 transport in hypobaric conditions | Sustained aerobic flight at high altitude with low ambient oxygen pressure | [21] |
| Non-human mammals | ||||||
| Deer mice (Peromyscus maniculatu) | HIF-2 α (EPAS1), metabolic regulators | Functional SNP in knock-in models | Ventilatory drive and oxygen sensing | Reduced carotid body sensitivity; energy conservation | Energy conservation by reducing ventilation response to hypoxia | [29] |
| Yak (Bos grunniens) | EPAS1, EGLN1, ADAM17 | Regulatory variants; differential gene expression | Oxygen sensing, angiogenesis, erythropoiesis | Low hematocrit, reduced pulmonary vascular resistance, blunted sympathetic response | Protection against hypoxia-induced pulmonary hypertension and efficient oxygen delivery | [30] |
| Tibetan sheep (Ovis ammon hodgsoni) | EPAS1, EGLN1 | Functional polymorphisms; signals of positive selection | HIF pathway, ventilatory regulation | Enhanced ventilatory response, regulated hemoglobin concentration | Enhanced ventilation and stable blood oxygenation | [30] |
| American pika (Ochotona princeps) | Not specified (physiological traits documented) | Physiological adaptation; genetic basis not fully characterized | Pulmonary circulation and sympathetic regulation | Protection against pulmonary hypertension and cardiopulmonary stress | Resistance to pulmonary hypertension through reduced sympathetic tone | [24] |
| Humans | ||||||
| Tibetans (Homo sapiens) | EPAS1 | Introgressed non-coding SNPs | HIF regulation, erythropoiesis | Low hemoglobin concentration without hypoxia symptoms | Avoidance of chronic mountain sickness and erythrocytosis | [32] |
| Tibetans (Homo sapiens) | EGLN1 (C127S) | Coding SNP (missense mutation) | HIF hydroxylation, O2 homeostasis | Balanced erythropoiesis with reduced HIF activity | Fine-tuned erythropoiesis without excessive red blood cell production | [32] |
| Andeans (Homo sapiens) | PRKAA1, EDNRA | Regulatory polymorphisms | Cellular energy sensing and vascular regulation | Improved fetal outcomes and energy metabolism | Better fetal oxygen supply and vascular adaptation during pregnancy | [36] |
| Andeans (Homo sapiens) | NOS2A, EGLN1 | Regulatory variants and non-synonymous SNPs | Nitric oxide synthesis, vascular function | Enhanced vasodilation and oxygen delivery | Increased NO-mediated vasodilation and oxygen delivery | [35] |
| Ethiopians (Homo sapiens) | VAV3, BHLHE41 | Regulatory SNPs; signals of selection | Hematopoiesis, circadian regulation | Stable oxygen saturation at low Hb | Maintained oxygen saturation with lower hemoglobin levels | [37] |
| Ethiopians (Homo sapiens) | CBARA1 (MICU1) | Regulatory SNPs | Mitochondrial calcium uptake | Optimized mitochondrial metabolism in hypoxia | Improved mitochondrial calcium handling for energy efficiency | [38] |
3. Cellular Mechanisms Under Hypoxia
3.1. Cellular Mechanisms of Adaptation to Hypoxia Mediated by HIF
3.2. Non-HIF-Dependent Mechanisms of Adaptation to Hypoxia
4. Hypoxia in Regenerative Processes
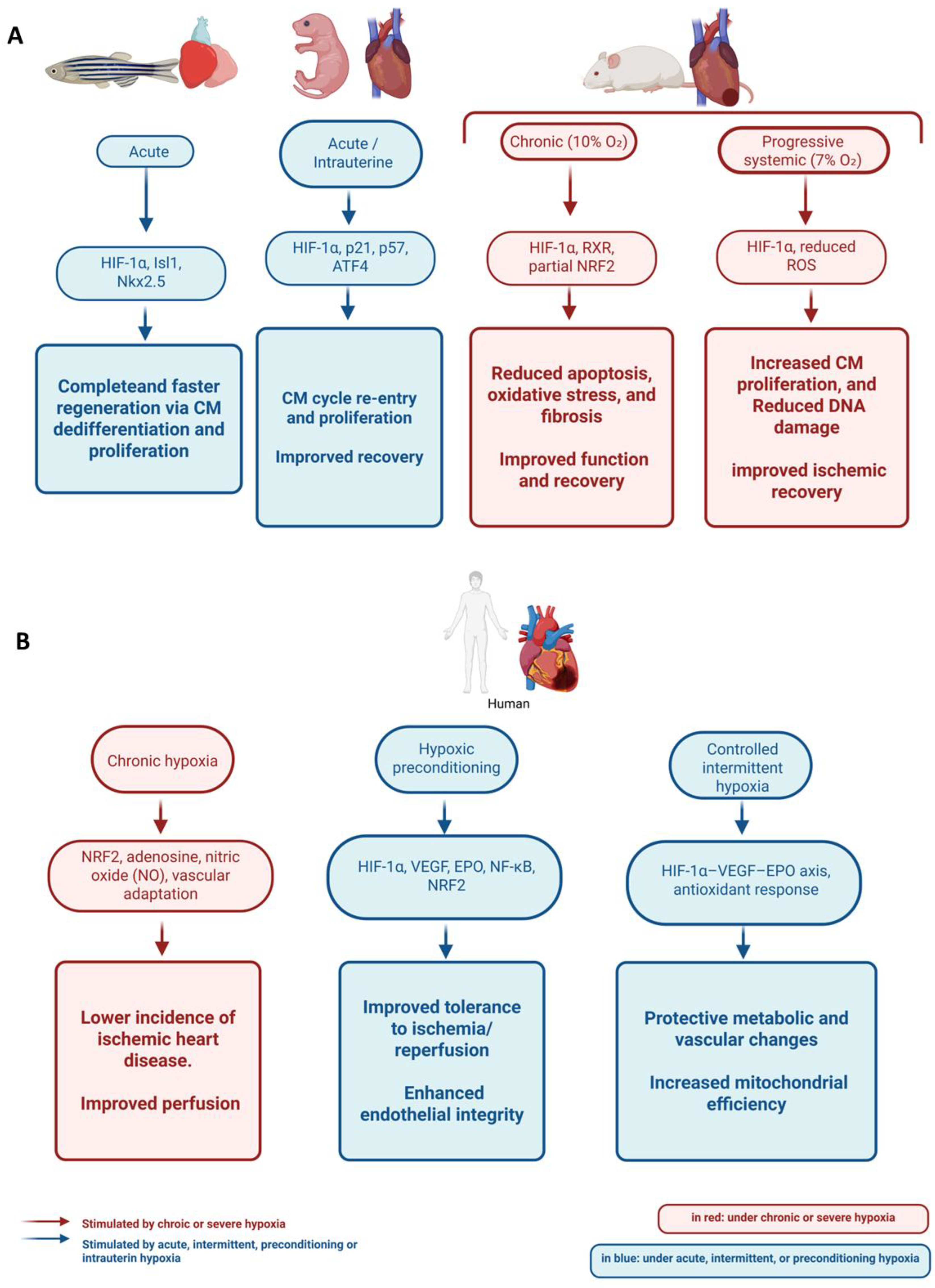
4.1. Cardiac Regeneration
4.2. Muscle Regeneration
4.3. Bone Regeneration
4.4. Hypoxia and Vascular Responses During Regeneration
4.5. Hypoxia in Appendage Regeneration
5. Hypoxia in Regenerative Medicine
5.1. Stem Cells and Bioengineering Applications
5.1.1. Heart
5.1.2. Muscle
5.1.3. Bone
5.1.4. Nervous System
5.2. Clinical Applications
6. Conclusions, Challenges, and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIH | Acute Intermittent Hypoxia |
| AKT | Protein Kinase B |
| ALP | Alkaline Phosphatase |
| AMPK | AMP-Activated Protein Kinase |
| Ang-1 | Angiopoietin-1 |
| AREs | Antioxidant Response Elements |
| ARNT | Aryl Hydrocarbon Receptor Nuclear Translocator |
| ATF-1 | Activating Transcription Factor 1 |
| ATF4 | Activating Transcription Factor 4 |
| ATP | ATP Synthase Subunit 6 |
| BCL2 | B-cell Lymphoma 2 |
| BMSCs | Bone Marrow-Derived Mesenchymal Stem Cells |
| Bmi1 | B-cell-specific Moloney murine leukemia virus integration site 1 |
| CASP-3 | Caspase-3 |
| CASP-9 | Caspase-9 |
| CAT | Catalase |
| CCN2 (CTGF) | Connective Tissue Growth Factor |
| CIH | Chronic Intermittent Hypoxia |
| circWhsc1 | Circular RNA derived from Whsc1 |
| COI | Cytochrome c Oxidase Subunit I |
| CpG | Cytosine-phosphate-Guanine dinucleotide |
| CREB-1 | cAMP Response Element-Binding Protein 1 |
| CPT2 | Carnitine Palmitoyltransferase 2 |
| C-TAD | C-Terminal Transactivation Domain |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 |
| CXCR4 | C-X-C Chemokine Receptor Type 4 |
| DAMPs | Damage-Associated Molecular Patterns |
| DMOG | Dimethyloxalylglycine |
| EGLN1 | EGL Nine Homolog 1 (Prolyl Hydroxylase Domain Protein 2) |
| EPAS1 (HIF-2α) | Endothelial PAS Domain-Containing Protein 1 |
| EPO | Erythropoietin |
| ERK1/2 | Extracellular Signal-Regulated Kinases 1 and 2 |
| FGF-1/FGF10/FGF20 | Fibroblast Growth Factors 1, 10, 20 |
| FiO2 | Fraction of Inspired Oxygen |
| FIH | Factor Inhibiting HIF |
| GAP-43 | Growth-Associated Protein 43 |
| GSTP1 | Glutathione S-Transferase Pi 1 |
| HBA | Hemoglobin Subunit Alpha |
| HBB | Hemoglobin Subunit Beta |
| HIF/HIF-1α/HIF-2α/HIF-3α | Hypoxia-Inducible Factor (alpha isoforms) |
| HMOX1 | Heme Oxygenase 1 |
| HREs | Hypoxia Response Elements |
| hiPSCs | Human Induced Pluripotent Stem Cells |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| IL-22 | Interleukin-22 |
| IPAS | Inhibitory PAS Domain Protein |
| iNOS | Inducible Nitric Oxide Synthase |
| Isl1 | ISL LIM Homeobox 1 |
| ISR | Integrated Stress Response |
| JAK2 | Janus Kinase 2 |
| KEAP1 | Kelch-like ECH-associated Protein 1 |
| kPa | Kilopascal |
| LDHA | Lactate Dehydrogenase A |
| MAPK | Mitogen-Activated Protein Kinase |
| MCL-1 | Myeloid Cell Leukemia-1 |
| MCU | Mitochondrial Calcium Uniporter |
| MICU1 | Mitochondrial Calcium Uptake 1 |
| miRNA/miRNAs | microRNA(s) |
| mmHg | Millimeters of Mercury |
| MPTP | Mitochondrial Permeability Transition Pore |
| m.a.s.l. | Metres Above Sea Level |
| mRNA | messenger RNA |
| mTOR | Mechanistic Target of Rapamycin |
| Ngb | Neuroglobin |
| NF-κB | Nuclear Factor κB |
| Nkx2.5 | NK2 Homeobox 5 |
| NO | Nitric Oxide |
| NRF2 | Nuclear factor erythroid 2–related factor 2 |
| NRVMs | Neonatal Rat Ventricular Myocytes |
| O2 | Molecular Oxygen |
| OCN | Osteocalcin |
| OSA | Obstructive Sleep Apnea |
| OXPHOS | Oxidative Phosphorylation |
| ND2 | NADH Dehydrogenase Subunit 2 |
| Pax7 | Paired Box 7 |
| pASCs | Adipose-Derived Stem Cells |
| PDHA1 | Pyruvate Dehydrogenase E1 Alpha 1 |
| PDGFRA | Platelet-Derived Growth Factor Receptor Alpha |
| PHDs | Prolyl Hydroxylase Domain-Containing Proteins |
| pfkfb3 | 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase 3 |
| PI3K | Phosphoinositide 3-Kinase |
| pO2 | Partial Pressure of Oxygen |
| PPARα | Peroxisome Proliferator-Activated Receptor Alpha |
| PRDX1/PRDX6 | Peroxiredoxin-1, -6 |
| RAAS | Renin–Angiotensin–Aldosterone System |
| RANKL | Receptor Activator of Nuclear Factor κB Ligand |
| RIPC | Remote Ischemic Preconditioning |
| ROS | Reactive Oxygen Species |
| RUNX2 | Runt-Related Transcription Factor 2 |
| RXR | Retinoid X Receptor |
| SAL | Salidroside |
| SaO2 | Arterial Oxygen Saturation |
| SDF-1 | Stromal Cell-Derived Factor 1 |
| SERCA | Sarco/Endoplasmic Reticulum Ca2+-ATPase |
| SMAD2 | SMAD Family Member 2 |
| SP7 (Osterix) | Sp7 Transcription Factor |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TAD | Transactivation Domain |
| TGF-β/TGF-β1 | Transforming Growth Factor Beta (1) |
| TGFBR2 | Transforming Growth Factor Beta Receptor 2 |
| TRD | Transrepression Domain |
| TRIM59 | Tripartite Motif-Containing Protein 59 |
| UCP2 | Uncoupling Protein 2 |
| USP20 | Ubiquitin-Specific Peptidase 20 |
| VEGF/VEGF-A | Vascular Endothelial Growth Factor (A) |
| VHL | Von Hippel–Lindau Protein |
| Wnt | Wingless/Integrated Signaling Pathway |
| Wnt5A | Wnt Family Member 5A |
| YAP1 | Yes-Associated Protein 1 |
| c-Fos | Cellular Oncogene Fos |
References
- Carreau, A.; Hafny-Rahbi, B.E.; Matejuk, A.; Grillon, C.; Kieda, C. Why Is the Partial Oxygen Pressure of Human Tissues a Crucial Parameter? Small Molecules and Hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef]
- Scott, G.R.; Milsom, W.K. Control of Breathing and Adaptation to High Altitude in the Bar-Headed Goose. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 293, R379–R391. [Google Scholar] [CrossRef]
- Zoccal, D.B.; Vieira, B.N.; Mendes, L.R.; Evangelista, A.B.; Leirão, I.P. Hypoxia Sensing in the Body: An Update on the Peripheral and Central Mechanisms. Exp. Physiol. 2024, 109, 461–469. [Google Scholar] [CrossRef]
- Tremblay, J.C.; Ainslie, P.N. Global and Country-Level Estimates of Human Population at High Altitude. Proc. Natl. Acad. Sci. USA 2021, 118, e2102463118. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, M.; Yang, G.; Tan, Q.; Chen, Y.; Li, G.; Zhang, Q.; Li, Y.; Wan, P.; Wu, J. Hypoxia Signaling in Human Health and Diseases: Implications and Prospects for Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 218. [Google Scholar] [CrossRef]
- Lucero García Rojas, E.Y.; Villanueva, C.; Bond, R.A. Hypoxia Inducible Factors as Central Players in the Pathogenesis and Pathophysiology of Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 709509. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and Maladaptive Cardiorespiratory Responses to Continuous and Intermittent Hypoxia Mediated by Hypoxia-Inducible Factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef]
- Lévy, P.; Pépin, J.-L.; Arnaud, C.; Tamisier, R.; Borel, J.-C.; Dematteis, M.; Godin-Ribuot, D.; Ribuot, C. Intermittent Hypoxia and Sleep-Disordered Breathing: Current Concepts and Perspectives. Eur. Respir. J. 2008, 32, 1082–1095. [Google Scholar] [CrossRef]
- Tan, J.; Virtue, S.; Norris, D.M.; Conway, O.J.; Yang, M.; Bidault, G.; Gribben, C.; Lugtu, F.; Kamzolas, I.; Krycer, J.R.; et al. Limited Oxygen in Standard Cell Culture Alters Metabolism and Function of Differentiated Cells. EMBO J. 2024, 43, 2127–2165. [Google Scholar] [CrossRef]
- Bakleh, M.Z.; Al Haj Zen, A. The Distinct Role of HIF-1α and HIF-2α in Hypoxia and Angiogenesis. Cells 2025, 14, 673. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Zubieta-Calleja, G. Redefining Chronic Mountain Sickness: Insights from High-Altitude Research and Clinical Experience. Med. Rev. 2025, 5, 44–65. [Google Scholar] [CrossRef]
- Wicks, E.E.; Semenza, G.L. Hypoxia-Inducible Factors: Cancer Progression and Clinical Translation. J. Clin. Investig. 2022, 132, e159839. [Google Scholar] [CrossRef]
- Yu, J.J.; Non, A.L.; Heinrich, E.C.; Gu, W.; Alcock, J.; Moya, E.A.; Lawrence, E.S.; Tift, M.S.; O’Brien, K.A.; Storz, J.F.; et al. Time Domains of Hypoxia Responses and -Omics Insights. Front. Physiol. 2022, 13, 885295. [Google Scholar] [CrossRef]
- Mallet, R.T.; Burtscher, J.; Pialoux, V.; Pasha, Q.; Ahmad, Y.; Millet, G.P.; Burtscher, M. Molecular Mechanisms of High-Altitude Acclimatization. Int. J. Mol. Sci. 2023, 24, 1698. [Google Scholar] [CrossRef]
- Taylor, C.T.; Scholz, C.C. The Effect of HIF on Metabolism and Immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef]
- Hochachka, P.W. Mechanism and Evolution of Hypoxia-Tolerance in Humans. J. Exp. Biol. 1998, 201, 1243–1254. [Google Scholar] [CrossRef]
- Sydykov, A.; Mamazhakypov, A.; Maripov, A.; Kosanovic, D.; Weissmann, N.; Ghofrani, H.A.; Sarybaev, A.S.; Schermuly, R.T. Pulmonary Hypertension in Acute and Chronic High Altitude Maladaptation Disorders. Int. J. Environ. Res. Public Health 2021, 18, 1692. [Google Scholar] [CrossRef]
- Pham, K.; Frost, S.; Parikh, K.; Puvvula, N.; Oeung, B.; Heinrich, E.C. Inflammatory Gene Expression during Acute High-altitude Exposure. J. Physiol. 2022, 600, 4169–4186. [Google Scholar] [CrossRef]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef]
- Ivy, C.M.; Scott, G.R. Control of Breathing and the Circulation in High-Altitude Mammals and Birds. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 186, 66–74. [Google Scholar] [CrossRef]
- Galen, S.C.; Natarajan, C.; Moriyama, H.; Weber, R.E.; Fago, A.; Benham, P.M.; Chavez, A.N.; Cheviron, Z.A.; Storz, J.F.; Witt, C.C. Contribution of a Mutational Hot Spot to Hemoglobin Adaptation in High-Altitude Andean House Wrens. Proc. Natl. Acad. Sci. USA 2015, 112, 13958–13963. [Google Scholar] [CrossRef]
- Storz, J.F.; Scott, G.R. Phenotypic Plasticity, Genetic Assimilation, and Genetic Compensation in Hypoxia Adaptation of High-Altitude Vertebrates. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 253, 110865. [Google Scholar] [CrossRef]
- Storz, J.F.; Cheviron, Z.A. Physiological Genomics of Adaptation to High-Altitude Hypoxia. Annu. Rev. Anim. Biosci. 2021, 9, 149–171. [Google Scholar] [CrossRef]
- Sharma, V.; Varshney, R.; Sethy, N.K. Human Adaptation to High Altitude: A Review of Convergence between Genomic and Proteomic Signatures. Hum. Genom. 2022, 16, 21. [Google Scholar] [CrossRef]
- Mandic, M.; Joyce, W.; Perry, S.F. The Evolutionary and Physiological Significance of the Hif Pathway in Teleost Fishes. J. Exp. Biol. 2021, 224, jeb231936. [Google Scholar] [CrossRef]
- Maina, J.N.; McCracken, K.G.; Chua, B.; York, J.M.; Milsom, W.K. Morphological and Morphometric Specializations of the Lung of the Andean Goose, Chloephaga Melanoptera: A Lifelong High-Altitude Resident. PLoS ONE 2017, 12, e0174395. [Google Scholar] [CrossRef]
- Graham, A.M.; Lavretsky, P.; Wilson, R.E.; McCracken, K.G. High-Altitude Adaptation Is Accompanied by Strong Signatures of Purifying Selection in the Mitochondrial Genomes of Three Andean Waterfowl. PLoS ONE 2024, 19, e0294842. [Google Scholar] [CrossRef]
- Ivy, C.M.; Velotta, J.P.; Cheviron, Z.A.; Scott, G.R. Genetic Variation in HIF-2α Attenuates Ventilatory Sensitivity and Carotid Body Growth in Chronic Hypoxia in High-altitude Deer Mice. J. Physiol. 2022, 600, 4207–4225. [Google Scholar] [CrossRef]
- Zhao, P.; Li, S.; He, Z.; Ma, X. Physiological and Genetic Basis of High-Altitude Indigenous Animals’ Adaptation to Hypoxic Environments. Animals 2024, 14, 3031. [Google Scholar] [CrossRef]
- Beall, C.M. Two Routes to Functional Adaptation: Tibetan and Andean High-Altitude Natives. Proc. Natl. Acad. Sci. USA 2007, 104, 8655–8660. [Google Scholar] [CrossRef]
- Simonson, T.S.; Yang, Y.; Huff, C.D.; Yun, H.; Qin, G.; Witherspoon, D.J.; Bai, Z.; Lorenzo, F.R.; Xing, J.; Jorde, L.B.; et al. Genetic Evidence for High-Altitude Adaptation in Tibet. Science 2010, 329, 72–75. [Google Scholar] [CrossRef]
- Yi, X.; Liang, Y.; Huerta-Sanchez, E.; Jin, X.; Cuo, Z.X.P.; Pool, J.E.; Xu, X.; Jiang, H.; Vinckenbosch, N.; Korneliussen, T.S.; et al. Sequencing of 50 Human Exomes Reveals Adaptation to High Altitude. Science 2010, 329, 75–78. [Google Scholar] [CrossRef]
- Villafuerte, F.C.; Simonson, T.S.; Bermudez, D.; León-Velarde, F. High-Altitude Erythrocytosis: Mechanisms of Adaptive and Maladaptive Responses. Physiology 2022, 37, 175–186. [Google Scholar] [CrossRef]
- Bigham, A.W.; Mao, X.; Mei, R.; Brutsaert, T.; Wilson, M.J.; Julian, C.G.; Parra, E.J.; Akey, J.M.; Moore, L.G.; Shriver, M.D. Identifying Positive Selection Candidate Loci for High-Altitude Adaptation in Andean Populations. Hum. Genom. 2009, 4, 79. [Google Scholar] [CrossRef]
- Crawford, J.E.; Amaru, R.; Song, J.; Julian, C.G.; Racimo, F.; Cheng, J.Y.; Guo, X.; Yao, J.; Ambale-Venkatesh, B.; Lima, J.A.; et al. Natural Selection on Genes Related to Cardiovascular Health in High-Altitude Adapted Andeans. Am. J. Hum. Genet. 2017, 101, 752–767. [Google Scholar] [CrossRef]
- Scheinfeldt, L.B.; Soi, S.; Thompson, S.; Ranciaro, A.; Woldemeskel, D.; Beggs, W.; Lambert, C.; Jarvis, J.P.; Abate, D.; Belay, G.; et al. Genetic Adaptation to High Altitude in the Ethiopian Highlands. Genome Biol. 2012, 13, R1. [Google Scholar] [CrossRef]
- Alkorta-Aranburu, G.; Beall, C.M.; Witonsky, D.B.; Gebremedhin, A.; Pritchard, J.K.; Di Rienzo, A. The Genetic Architecture of Adaptations to High Altitude in Ethiopia. PLoS Genet. 2012, 8, e1003110. [Google Scholar] [CrossRef]
- Bao, W.; Qin, P.; Needle, S.; Erickson-Miller, C.L.; Duffy, K.J.; Ariazi, J.L.; Zhao, S.; Olzinski, A.R.; Behm, D.J.; Teg Pipes, G.C.; et al. Chronic Inhibition of Hypoxia-Inducible Factor Prolyl 4-Hydroxylase Improves Ventricular Performance, Remodeling, and Vascularity After Myocardial Infarction in the Rat. J. Cardiovasc. Pharmacol. 2010, 56, 147–155. [Google Scholar] [CrossRef]
- Handzlik, M.K.; Constantin-Teodosiu, D.; Greenhaff, P.L.; Cole, M.A. Increasing Cardiac Pyruvate Dehydrogenase Flux during Chronic Hypoxia Improves Acute Hypoxic Tolerance. J. Physiol. 2018, 596, 3357–3369. [Google Scholar] [CrossRef]
- Richalet, J.-P.; Hermand, E.; Lhuissier, F.J. Cardiovascular Physiology and Pathophysiology at High Altitude. Nat. Rev. Cardiol. 2024, 21, 75–88. [Google Scholar] [CrossRef]
- Ostadal, B.; Kolar, F. Cardiac Adaptation to Chronic High-Altitude Hypoxia: Beneficial and Adverse Effects. Respir. Physiol. Neurobiol. 2007, 158, 224–236. [Google Scholar] [CrossRef]
- Faeh, D.; Gutzwiller, F.; Bopp, M. Lower Mortality From Coronary Heart Disease and Stroke at Higher Altitudes in Switzerland. Circulation 2009, 120, 495–501. [Google Scholar] [CrossRef]
- Ezzati, M.; Horwitz, M.E.M.; Thomas, D.S.K.; Friedman, A.B.; Roach, R.; Clark, T.; Murray, C.J.L.; Honigman, B. Altitude, Life Expectancy and Mortality from Ischaemic Heart Disease, Stroke, COPD and Cancers: National Population-Based Analysis of US Counties. J. Epidemiol. Community Health 2012, 66, e17. [Google Scholar] [CrossRef]
- Walshe, T.E.; D’Amore, P.A. The Role of Hypoxia in Vascular Injury and Repair. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 615–643. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; Robach, P.; Lundby, C. The Exercising Heart at Altitude. Cell. Mol. Life Sci. 2009, 66, 3601–3613. [Google Scholar] [CrossRef]
- West, J.B. Physiological Effects of Chronic Hypoxia. N. Engl. J. Med. 2017, 376, 1965–1971. [Google Scholar] [CrossRef]
- Doutreleau, S.; Ulliel-Roche, M.; Hancco, I.; Bailly, S.; Oberholzer, L.; Robach, P.; Brugniaux, J.V.; Pichon, A.; Stauffer, E.; Perger, E.; et al. Cardiac Remodelling in the Highest City in the World: Effects of Altitude and Chronic Mountain Sickness. Eur. J. Prev. Cardiol. 2022, 29, 2154–2162. [Google Scholar] [CrossRef]
- Pham, K.; Parikh, K.; Heinrich, E.C. Hypoxia and Inflammation: Insights From High-Altitude Physiology. Front. Physiol. 2021, 12, 676782. [Google Scholar] [CrossRef]
- Suresh, M.V.; Balijepalli, S.; Solanki, S.; Aktay, S.; Choudhary, K.; Shah, Y.M.; Raghavendran, K. Hypoxia-Inducible Factor 1α and Its Role in Lung Injury: Adaptive or Maladaptive. Inflammation 2023, 46, 491–508. [Google Scholar] [CrossRef]
- Paul, S.; Gangwar, A.; Bhargava, K.; Ahmad, Y. STAT3-RXR-Nrf2 Activates Systemic Redox and Energy Homeostasis upon Steep Decline in pO2 Gradient. Redox Biol. 2018, 14, 423–438. [Google Scholar] [CrossRef]
- Burtscher, J.; Citherlet, T.; Camacho-Cardenosa, A.; Camacho-Cardenosa, M.; Raberin, A.; Krumm, B.; Hohenauer, E.; Egg, M.; Lichtblau, M.; Müller, J.; et al. Mechanisms Underlying the Health Benefits of Intermittent Hypoxia Conditioning. J. Physiol. 2024, 602, 5757–5783. [Google Scholar] [CrossRef]
- Jarrard, C.P.; Nagel, M.J.; Stray-Gundersen, S.; Tanaka, H.; Lalande, S. Hypoxic Preconditioning Attenuates Ischemia-Reperfusion Injury in Young Healthy Adults. J. Appl. Physiol. 2021, 130, 846–852. [Google Scholar] [CrossRef]
- Saxena, K.; Jolly, M.K. Acute vs. Chronic vs. Cyclic Hypoxia: Their Differential Dynamics, Molecular Mechanisms, and Effects on Tumor Progression. Biomolecules 2019, 9, 339. [Google Scholar] [CrossRef]
- Arnaud, C.; Billoir, E.; De Melo Junior, A.F.; Pereira, S.A.; O’Halloran, K.D.; Monteiro, E.C. Chronic Intermittent Hypoxia-induced Cardiovascular and Renal Dysfunction: From Adaptation to Maladaptation. J. Physiol. 2023, 601, 5553–5577. [Google Scholar] [CrossRef]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological Mechanisms and Therapeutic Approaches in Obstructive Sleep Apnea Syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-Inducible Nuclear Factors Bind to an Enhancer Element Located 3′ to the Human Erythropoietin Gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, Z.-F.; Wang, S.-P.; Vong, C.-T.; Yu, B.; Wang, Y.-T. HIF-1: Structure, Biology and Natural Modulators. Chin. J. Nat. Med. 2021, 19, 521–527. [Google Scholar] [CrossRef]
- Wu, D.; Rastinejad, F. Structural Characterization of Mammalian bHLH-PAS Transcription Factors. Curr. Opin. Struct. Biol. 2017, 43, 1–9. [Google Scholar] [CrossRef]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. miRNAs Regulate the HIF Switch during Hypoxia: A Novel Therapeutic Target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef]
- Ivan, M.; Kaelin, W.G. The EGLN-HIF O2-Sensing System: Multiple Inputs and Feedbacks. Mol. Cell 2017, 66, 772–779. [Google Scholar] [CrossRef]
- Wenger, R.H.; Stiehl, D.P.; Camenisch, G. Integration of Oxygen Signaling at the Consensus HRE. Sci. STKE 2005, 2005, re12. [Google Scholar] [CrossRef]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HIF-1 versus HIF-2—Is One More Important than the Other? Vascul. Pharmacol. 2012, 56, 245–251. [Google Scholar] [CrossRef]
- Jaśkiewicz, M.; Moszyńska, A.; Króliczewski, J.; Cabaj, A.; Bartoszewska, S.; Charzyńska, A.; Gebert, M.; Dąbrowski, M.; Collawn, J.F.; Bartoszewski, R. The Transition from HIF-1 to HIF-2 during Prolonged Hypoxia Results from Reactivation of PHDs and HIF1A mRNA Instability. Cell. Mol. Biol. Lett. 2022, 27, 109. [Google Scholar] [CrossRef]
- Yang, S.-L.; Wu, C.; Xiong, Z.-F.; Fang, X. Progress on Hypoxia-Inducible Factor-3: Its Structure, Gene Regulation and Biological Function (Review). Mol. Med. Rep. 2015, 12, 2411–2416. [Google Scholar] [CrossRef]
- Bae, T.; Hallis, S.P.; Kwak, M.-K. Hypoxia, Oxidative Stress, and the Interplay of HIFs and NRF2 Signaling in Cancer. Exp. Mol. Med. 2024, 56, 501–514. [Google Scholar] [CrossRef]
- Kip, A.M.; Soons, Z.; Mohren, R.; Duivenvoorden, A.A.M.; Röth, A.A.J.; Cillero-Pastor, B.; Neumann, U.P.; Dejong, C.H.C.; Heeren, R.M.A.; Olde Damink, S.W.M.; et al. Proteomics Analysis of Human Intestinal Organoids during Hypoxia and Reoxygenation as a Model to Study Ischemia-Reperfusion Injury. Cell Death Dis. 2021, 12, 95. [Google Scholar] [CrossRef]
- Bukowska, J.; Słowińska, M.; Cierniak, P.; Kopcewicz, M.; Walendzik, K.; Frazier, T.; Gawrońska-Kozak, B. The Effect of Hypoxia on the Proteomic Signature of Pig Adipose-Derived Stromal/Stem Cells (pASCs). Sci. Rep. 2020, 10, 20035. [Google Scholar] [CrossRef]
- Dumbali, S.P.; Horton, P.D.; Moore, T.I.; Wenzel, P.L. Mitochondrial Permeability Transition Dictates Mitochondrial Maturation upon Switch in Cellular Identity of Hematopoietic Precursors. Commun. Biol. 2024, 7, 967. [Google Scholar] [CrossRef]
- Belosludtsev, K.; Dubinin, M.; Talanov, E.; Starinets, V.; Tenkov, K.; Zakharova, N.; Belosludtseva, N. Transport of Ca2+ and Ca2+-Dependent Permeability Transition in the Liver and Heart Mitochondria of Rats with Different Tolerance to Acute Hypoxia. Biomolecules 2020, 10, 114. [Google Scholar] [CrossRef]
- Venediktova, N.; Shigaeva, M.; Belova, S.; Belosludtsev, K.; Belosludtseva, N.; Gorbacheva, O.; Lezhnev, E.; Lukyanova, L.; Mironova, G. Oxidative Phosphorylation and Ion Transport in the Mitochondria of Two Strains of Rats Varying in Their Resistance to Stress and Hypoxia. Mol. Cell. Biochem. 2013, 383, 261–269. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Chen, D.; Tian, Y.; Liu, C.; Li, F. MICU1 Alleviates Hypobaric Hypoxia-Induced Myocardial Injury through Regulating Ca2+ Uptake to Inhibit Mitochondria-Dependent Apoptosis. Cell. Signal. 2025, 125, 111524. [Google Scholar] [CrossRef]
- Sasaki, H.; Nakagawa, I.; Furuta, T.; Yokoyama, S.; Morisaki, Y.; Saito, Y.; Nakase, H. Mitochondrial Calcium Uniporter (MCU) Is Involved in an Ischemic Postconditioning Effect Against Ischemic Reperfusion Brain Injury in Mice. Cell. Mol. Neurobiol. 2024, 44, 32. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, J.; Zhou, Q.; He, X.; Zheng, Z.; Wei, Y.; Zhou, K.; Lin, Y.; Yu, H.; Zhang, H.; et al. Hypoxia Induces Mitochondrial Protein Lactylation to Limit Oxidative Phosphorylation. Cell Res. 2024, 34, 13–30. [Google Scholar] [CrossRef]
- Mucci, S.; Isaja, L.; Rodríguez-Varela, M.S.; Ferriol-Laffouillere, S.L.; Marazita, M.; Videla-Richardson, G.A.; Sevlever, G.E.; Scassa, M.E.; Romorini, L. Acute Severe Hypoxia Induces Apoptosis of Human Pluripotent Stem Cells by a HIF-1α and P53 Independent Mechanism. Sci. Rep. 2022, 12, 18803. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation 2021, 143. [Google Scholar] [CrossRef]
- Garry, D.J.; Zhang, J.; Larson, T.A.; Sadek, H.A.; Garry, M.G. Networks That Govern Cardiomyocyte Proliferation to Facilitate Repair of the Injured Mammalian Heart. Methodist DeBakey Cardiovasc. J. 2023, 19, 16–25. [Google Scholar] [CrossRef]
- Kadota, S.; Pabon, L.; Reinecke, H.; Murry, C.E. In Vivo Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Neonatal and Adult Rat Hearts. Stem Cell Rep. 2017, 8, 278–289. [Google Scholar] [CrossRef]
- Lemcke, H.; Voronina, N.; Steinhoff, G.; David, R. Recent Progress in Stem Cell Modification for Cardiac Regeneration. Stem Cells Int. 2018, 2018, 1909346. [Google Scholar] [CrossRef]
- Coulombe, K.L.K.; Bajpai, V.K.; Andreadis, S.T.; Murry, C.E. Heart Regeneration with Engineered Myocardial Tissue. Annu. Rev. Biomed. Eng. 2014, 16, 1–28. [Google Scholar] [CrossRef]
- Miyakawa, M.; Katada, T.; Numa, Y.; Kinoshita, T. Transcriptional Regulatory Elements of Hif1α in a Distal Locus of Islet1 in Xenopus Laevis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 255, 110598. [Google Scholar] [CrossRef]
- Jopling, C.; Sleep, E.; Raya, M.; Martí, M.; Raya, A.; Belmonte, J.C.I. Zebrafish Heart Regeneration Occurs by Cardiomyocyte Dedifferentiation and Proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef]
- Jopling, C.; Suñé, G.; Faucherre, A.; Fabregat, C.; Izpisua Belmonte, J.C. Hypoxia Induces Myocardial Regeneration in Zebrafish. Circulation 2012, 126, 3017–3027. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient Regenerative Potential of the Neonatal Mouse Heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The Oxygen-Rich Postnatal Environment Induces Cardiomyocyte Cell-Cycle Arrest through DNA Damage Response. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef]
- Guimarães-Camboa, N.; Stowe, J.; Aneas, I.; Sakabe, N.; Cattaneo, P.; Henderson, L.; Kilberg, M.S.; Johnson, R.S.; Chen, J.; McCulloch, A.D.; et al. HIF1α Represses Cell Stress Pathways to Allow Proliferation of Hypoxic Fetal Cardiomyocytes. Dev. Cell 2015, 33, 507–521. [Google Scholar] [CrossRef]
- Kimura, W.; Xiao, F.; Canseco, D.C.; Muralidhar, S.; Thet, S.; Zhang, H.M.; Abderrahman, Y.; Chen, R.; Garcia, J.A.; Shelton, J.M.; et al. Hypoxia Fate Mapping Identifies Cycling Cardiomyocytes in the Adult Heart. Nature 2015, 523, 226–230. [Google Scholar] [CrossRef]
- Nakada, Y.; Canseco, D.C.; Thet, S.; Abdisalaam, S.; Asaithamby, A.; Santos, C.X.; Shah, A.M.; Zhang, H.; Faber, J.E.; Kinter, M.T.; et al. Hypoxia Induces Heart Regeneration in Adult Mice. Nature 2017, 541, 222–227. [Google Scholar] [CrossRef]
- Eckle, T.; Köhler, D.; Lehmann, R.; El Kasmi, K.C.; Eltzschig, H.K. Hypoxia-Inducible Factor-1 Is Central to Cardioprotection: A New Paradigm for Ischemic Preconditioning. Circulation 2008, 118, 166–175. [Google Scholar] [CrossRef]
- Hölscher, M.; Silter, M.; Krull, S.; Von Ahlen, M.; Hesse, A.; Schwartz, P.; Wielockx, B.; Breier, G.; Katschinski, D.M.; Zieseniss, A. Cardiomyocyte-Specific Prolyl-4-Hydroxylase Domain 2 Knock Out Protects from Acute Myocardial Ischemic Injury. J. Biol. Chem. 2011, 286, 11185–11194. [Google Scholar] [CrossRef]
- Lee, W.; Lin, S.-L.; Chiang, C.-S.; Chen, J.-Y.; Chieng, W.-W.; Huang, S.-R.; Chang, T.-Y.; Linju Yen, B.; Hung, M.-C.; Chang, K.-C.; et al. Role of HIF-1α-Activated IL-22/IL-22R1/Bmi1 Signaling Modulates the Self-Renewal of Cardiac Stem Cells in Acute Myocardial Ischemia. Stem Cell Rev. Rep. 2024, 20, 2194–2214. [Google Scholar] [CrossRef]
- Albendea-Gomez, T.; Mendoza-Tamajon, S.; Castro-Mecinas, R.; Escobar, B.; Ferreira Rocha, S.; Urra-Balduz, S.; Nicolas-Avila, J.A.; Oliver, E.; Villalba-Orero, M.; Martin-Puig, S. Vascular HIF2 Signaling Prevents Cardiomegaly, Alveolar Congestion, and Capillary Remodeling During Chronic Hypoxia. Arterioscler. Thromb. Vasc. Biol. 2025, 45, e78–e98. [Google Scholar] [CrossRef]
- Ali, S.R.; Nguyen, N.U.N.; Menendez-Montes, I.; Hsu, C.-C.; Elhelaly, W.; Lam, N.T.; Li, S.; Elnwasany, A.; Nakada, Y.; Thet, S.; et al. Hypoxia-Induced Stabilization of HIF2A Promotes Cardiomyocyte Proliferation by Attenuating DNA Damage. J. Cardiovasc. Aging 2024, 4, 11. [Google Scholar] [CrossRef]
- Bekeredjian, R.; Walton, C.B.; MacCannell, K.A.; Ecker, J.; Kruse, F.; Outten, J.T.; Sutcliffe, D.; Gerard, R.D.; Bruick, R.K.; Shohet, R.V. Conditional HIF-1α Expression Produces a Reversible Cardiomyopathy. PLoS ONE 2010, 5, e11693. [Google Scholar] [CrossRef]
- Kido, M.; Du, L.; Sullivan, C.C.; Li, X.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Hypoxia-Inducible Factor 1-Alpha Reduces Infarction and Attenuates Progression of Cardiac Dysfunction After Myocardial Infarction in the Mouse. J. Am. Coll. Cardiol. 2005, 46, 2116–2124. [Google Scholar] [CrossRef]
- Hölscher, M.; Schäfer, K.; Krull, S.; Farhat, K.; Hesse, A.; Silter, M.; Lin, Y.; Pichler, B.J.; Thistlethwaite, P.; El-Armouche, A.; et al. Unfavourable Consequences of Chronic Cardiac HIF-1α Stabilization. Cardiovasc. Res. 2012, 94, 77–86. [Google Scholar] [CrossRef]
- Johnson, J.; Yang, Y.; Bian, Z.; Schena, G.; Li, Y.; Zhang, X.; Eaton, D.M.; Gross, P.; Angheloiu, A.; Shaik, A.; et al. Systemic Hypoxemia Induces Cardiomyocyte Hypertrophy and Right Ventricular Specific Induction of Proliferation. Circ. Res. 2023, 132, 723–740. [Google Scholar] [CrossRef]
- Eschenhagen, T. Hypoxia to Stimulate Cardiomyocyte Proliferation After MI? A Word of Caution. Circ. Res. 2023, 132, 741–743. [Google Scholar] [CrossRef]
- Ye, L.; Qiu, L.; Feng, B.; Jiang, C.; Huang, Y.; Zhang, H.; Zhang, H.; Hong, H.; Liu, J. Role of Blood Oxygen Saturation During Post-Natal Human Cardiomyocyte Cell Cycle Activities. JACC Basic Transl. Sci. 2020, 5, 447–460. [Google Scholar] [CrossRef]
- Rigaud, V.O.C.; Zarka, C.; Kurian, J.; Harlamova, D.; Elia, A.; Kasatkin, N.; Johnson, J.; Behanan, M.; Kraus, L.; Pepper, H.; et al. UCP2 Modulates Cardiomyocyte Cell Cycle Activity, Acetyl-CoA, and Histone Acetylation in Response to Moderate Hypoxia. JCI Insight 2022, 7, e155475. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Fang, J.; Chen, X.; Xu, T.; Zhuang, T.; Peng, S.; Bao, W.; Wu, W.; Lu, Y.; et al. Cardiomyocyte Foxp1-Specific Deletion Promotes Post-injury Heart Regeneration via Targeting Usp20-HIF1α-Hand1 Signaling Pathway. Adv. Sci. 2025, 12, 2412124. [Google Scholar] [CrossRef]
- Elashry, M.I.; Kinde, M.; Klymiuk, M.C.; Eldaey, A.; Wenisch, S.; Arnhold, S. The Effect of Hypoxia on Myogenic Differentiation and Multipotency of the Skeletal Muscle-Derived Stem Cells in Mice. Stem Cell Res. Ther. 2022, 13, 56. [Google Scholar] [CrossRef]
- Archacka, K.; Grabowska, I.; Mierzejewski, B.; Graffstein, J.; Górzyńska, A.; Krawczyk, M.; Różycka, A.M.; Kalaszczyńska, I.; Muras, G.; Stremińska, W.; et al. Hypoxia Preconditioned Bone Marrow-Derived Mesenchymal Stromal/Stem Cells Enhance Myoblast Fusion and Skeletal Muscle Regeneration. Stem Cell Res. Ther. 2021, 12, 448. [Google Scholar] [CrossRef]
- Ono, Y.; Sensui, H.; Sakamoto, Y.; Nagatomi, R. Knockdown of Hypoxia-inducible Factor-1α by siRNA Inhibits C2C12 Myoblast Differentiation. J. Cell. Biochem. 2006, 98, 642–649. [Google Scholar] [CrossRef]
- Pircher, T.; Wackerhage, H.; Aszodi, A.; Kammerlander, C.; Böcker, W.; Saller, M.M. Hypoxic Signaling in Skeletal Muscle Maintenance and Regeneration: A Systematic Review. Front. Physiol. 2021, 12, 684899. [Google Scholar] [CrossRef]
- Xie, L.; Yin, A.; Nichenko, A.S.; Beedle, A.M.; Call, J.A.; Yin, H. Transient HIF2A Inhibition Promotes Satellite Cell Proliferation and Muscle Regeneration. J. Clin. Investig. 2018, 128, 2339–2355. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Skuli, N.; Mesquita, R.C.; Kim, M.N.; Yodh, A.G.; Nguyen-McCarty, M.; Simon, M.C. O2 Regulates Skeletal Muscle Progenitor Differentiation through Phosphatidylinositol 3-Kinase/AKT Signaling. Mol. Cell. Biol. 2012, 32, 36–49. [Google Scholar] [CrossRef]
- Santocildes, G.; Viscor, G.; Pagès, T.; Torrella, J.R. Simulated Altitude Is Medicine: Intermittent Exposure to Hypobaric Hypoxia and Cold Accelerates Injured Skeletal Muscle Recovery. J. Physiol. 2024, 602, 5855–5878. [Google Scholar] [CrossRef]
- Chaillou, T.; Koulmann, N.; Meunier, A.; Pugnière, P.; McCarthy, J.J.; Beaudry, M.; Bigard, X. Ambient Hypoxia Enhances the Loss of Muscle Mass after Extensive Injury. Pflüg. Arch.-Eur. J. Physiol. 2014, 466, 587–598. [Google Scholar] [CrossRef]
- Settelmeier, S.; Schreiber, T.; Mäki, J.; Byts, N.; Koivunen, P.; Myllyharju, J.; Fandrey, J.; Winning, S. Prolyl Hydroxylase Domain 2 Reduction Enhances Skeletal Muscle Tissue Regeneration after Soft Tissue Trauma in Mice. PLoS ONE 2020, 15, e0233261. [Google Scholar] [CrossRef]
- Billin, A.N.; Honeycutt, S.E.; McDougal, A.V.; Kerr, J.P.; Chen, Z.; Freudenberg, J.M.; Rajpal, D.K.; Luo, G.; Kramer, H.F.; Geske, R.S.; et al. HIF Prolyl Hydroxylase Inhibition Protects Skeletal Muscle from Eccentric Contraction-Induced Injury. Skelet. Muscle 2018, 8, 35. [Google Scholar] [CrossRef]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W.; Vandenburgh, H.H.; Mooney, D.J. Functional Muscle Regeneration with Combined Delivery of Angiogenesis and Myogenesis Factors. Proc. Natl. Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef]
- Messina, S.; Mazzeo, A.; Bitto, A.; Aguennouz, M.; Migliorato, A.; De Pasquale, M.G.; Minutoli, L.; Altavilla, D.; Zentilin, L.; Giacca, M.; et al. VEGF Overexpression via Adeno-associated Virus Gene Transfer Promotes Skeletal Muscle Regeneration and Enhances Muscle Function in Mdx Mice. FASEB J. 2007, 21, 3737–3746. [Google Scholar] [CrossRef]
- Valle-Tenney, R.; Rebolledo, D.; Acuña, M.J.; Brandan, E. HIF-Hypoxia Signaling in Skeletal Muscle Physiology and Fibrosis. J. Cell Commun. Signal. 2020, 14, 147–158. [Google Scholar] [CrossRef]
- Yin, A.; Fu, W.; Elengickal, A.; Kim, J.; Liu, Y.; Bigot, A.; Mamchaoui, K.; Call, J.A.; Yin, H. Chronic Hypoxia Impairs Skeletal Muscle Repair via HIF-2α Stabilization. J. Cachexia Sarcopenia Muscle 2024, 15, 631–645. [Google Scholar] [CrossRef]
- Dong, Q.; Fei, X.; Zhang, H.; Zhu, X.; Ruan, J. Effect of Dimethyloxalylglycine on Stem Cells Osteogenic Differentiation and Bone Tissue Regeneration—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3879. [Google Scholar] [CrossRef]
- Sathy, B.N.; Daly, A.; Gonzalez-Fernandez, T.; Olvera, D.; Cunniffe, G.; McCarthy, H.O.; Dunne, N.; Jeon, O.; Alsberg, E.; Donahue, T.L.H.; et al. Hypoxia Mimicking Hydrogels to Regulate the Fate of Transplanted Stem Cells. Acta Biomater. 2019, 88, 314–324. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Zhao, Y.; Chen, X.; Xiao, Y.; Bao, C. HIF Signaling: A New Propellant in Bone Regeneration. Biomater. Adv. 2022, 138, 212874. [Google Scholar] [CrossRef]
- Ding, H.; Gao, Y.-S.; Wang, Y.; Hu, C.; Sun, Y.; Zhang, C. Dimethyloxaloylglycine Increases the Bone Healing Capacity of Adipose-Derived Stem Cells by Promoting Osteogenic Differentiation and Angiogenic Potential. Stem Cells Dev. 2014, 23, 990–1000. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Z.; Wei, J.; Yu, Y.; Luo, J.; Zhou, J.; Li, Y.; Zheng, X.; Tang, W.; Liu, L.; et al. Repair of Critical-Sized Mandible Defects in Aged Rat Using Hypoxia Preconditioned BMSCs with Up-Regulation of Hif-1α. Int. J. Biol. Sci. 2018, 14, 449–460. [Google Scholar] [CrossRef]
- Shang, L.; Liu, Z.; Ma, B.; Shao, J.; Wang, B.; Ma, C.; Ge, S. Dimethyloxallyl Glycine/Nanosilicates-Loaded Osteogenic/Angiogenic Difunctional Fibrous Structure for Functional Periodontal Tissue Regeneration. Bioact. Mater. 2021, 6, 1175–1188. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, J.; Chen, Y.; Jin, X.; Li, Z.; Wen, X.; Xia, Q.; Wang, Y. Salidroside Improves Angiogenesis-Osteogenesis Coupling by Regulating the HIF-1α/VEGF Signalling Pathway in the Bone Environment. Eur. J. Pharmacol. 2020, 884, 173394. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Liu, W.; Wang, Y.; Liu, Z.; Rong, M. Impact of High-Altitude Hypoxia on Bone Defect Repair: A Review of Molecular Mechanisms and Therapeutic Implications. Front. Med. 2022, 9, 842800. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; Rigual, R.; Ruiz-Mambrilla, M.; Fernández-Gómez, J.-M.; Dueñas, A.; Pérez-Castrillón, J.L. Molecular Mechanisms Involved in Hypoxia-Induced Alterations in Bone Remodeling. Int. J. Mol. Sci. 2022, 23, 3233. [Google Scholar] [CrossRef]
- Utting, J.C.; Robins, S.P.; Brandao-Burch, A.; Orriss, I.R.; Behar, J.; Arnett, T.R. Hypoxia Inhibits the Growth, Differentiation and Bone-Forming Capacity of Rat Osteoblasts. Exp. Cell Res. 2006, 312, 1693–1702. [Google Scholar] [CrossRef]
- Zou, W.; Yang, S.; Zhang, T.; Sun, H.; Wang, Y.; Xue, H.; Zhou, D. Hypoxia Enhances Glucocorticoid-Induced Apoptosis and Cell Cycle Arrest via the PI3K/Akt Signaling Pathway in Osteoblastic Cells. J. Bone Miner. Metab. 2015, 33, 615–624. [Google Scholar] [CrossRef]
- Hulley, P.A.; Bishop, T.; Vernet, A.; Schneider, J.E.; Edwards, J.R.; Athanasou, N.A.; Knowles, H.J. Hypoxia-inducible Factor 1-alpha Does Not Regulate Osteoclastogenesis but Enhances Bone Resorption Activity via Prolyl-4-hydroxylase 2. J. Pathol. 2017, 242, 322–333. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, K.H.; Yu, H.-G.; Kook, E.; Song, W.-H.; Lee, G.; Koh, J.-T.; Shin, H.-I.; Choi, J.-Y.; Huh, Y.H.; et al. Controlling Hypoxia-Inducible Factor-2α Is Critical for Maintaining Bone Homeostasis in Mice. Bone Res. 2019, 7, 14. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, L.; Guo, J.; Bao, K.; Hu, J.; Zhang, Y.; Hou, Z.; Zhang, L. Chronic Intermittent Hypobaric Hypoxia Enhances Bone Fracture Healing. Front. Endocrinol. 2021, 11, 582670. [Google Scholar] [CrossRef]
- Oikonomidou, P.R.; Casu, C.; Yang, Z.; Crielaard, B.; Shim, J.H.; Rivella, S.; Vogiatzi, M.G. Polycythemia Is Associated with Bone Loss and Reduced Osteoblast Activity in Mice. Osteoporos. Int. 2016, 27, 1559–1568. [Google Scholar] [CrossRef]
- Hiram-Bab, S.; Liron, T.; Deshet-Unger, N.; Mittelman, M.; Gassmann, M.; Rauner, M.; Franke, K.; Wielockx, B.; Neumann, D.; Gabet, Y. Erythropoietin Directly Stimulates Osteoclast Precursors and Induces Bone Loss. FASEB J. 2015, 29, 1890–1900. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y.; Sun, H.; Joseph, J.; Mishra, A.; Shiozawa, Y.; Wang, J.; Krebsbach, P.H.; Taichman, R.S. Erythropoietin Mediated Bone Formation Is Regulated by mTOR Signaling. J. Cell. Biochem. 2012, 113, 220–228. [Google Scholar] [CrossRef]
- Garcia, P.; Speidel, V.; Scheuer, C.; Laschke, M.W.; Holstein, J.H.; Histing, T.; Pohlemann, T.; Menger, M.D. Low Dose Erythropoietin Stimulates Bone Healing in Mice. J. Orthop. Res. 2011, 29, 165–172. [Google Scholar] [CrossRef]
- Holstein, J.H.; Orth, M.; Scheuer, C.; Tami, A.; Becker, S.C.; Garcia, P.; Histing, T.; Mörsdorf, P.; Klein, M.; Pohlemann, T.; et al. Erythropoietin Stimulates Bone Formation, Cell Proliferation, and Angiogenesis in a Femoral Segmental Defect Model in Mice. Bone 2011, 49, 1037–1045. [Google Scholar] [CrossRef]
- Rafique, M.; Wei, T.; Sun, Q.; Midgley, A.C.; Huang, Z.; Wang, T.; Shafiq, M.; Zhi, D.; Si, J.; Yan, H.; et al. The Effect of Hypoxia-Mimicking Responses on Improving the Regeneration of Artificial Vascular Grafts. Biomaterials 2021, 271, 120746. [Google Scholar] [CrossRef]
- Dirscherl, K.; Schläpfer, M.; Roth Z’graggen, B.; Wenger, R.H.; Booy, C.; Flury-Frei, R.; Fatzer, R.; Aloman, C.; Bartosch, B.; Parent, R.; et al. Hypoxia Sensing by Hepatic Stellate Cells Leads to VEGF-Dependent Angiogenesis and May Contribute to Accelerated Liver Regeneration. Sci. Rep. 2020, 10, 4392. [Google Scholar] [CrossRef]
- Qing, Z.; Huang, H.; Yang, S.; Lin, J.; Zeng, Z.; Duan, J.; Yuan, B.; Ming, T. Hypoxia Maintains the Fenestration of Liver Sinusoidal Endothelial Cells and Promotes Their Proliferation through the SENP1/HIF-1α/VEGF Signaling Axis. Biochem. Biophys. Res. Commun. 2021, 540, 42–50. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Wang, R.; Li, W.; He, W.; Luo, X.; Ye, Y. Expression of Notch–Hif-1α Signaling Pathway in Liver Regeneration of Rats. J. Int. Med. Res. 2020, 48, 0300060520943790. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W.; Ai, X.; Kilic, E.; Hermann, D.M.; Venkataramani, V.; Bähr, M.; Doeppner, T.R. Extracellular Vesicles from Hypoxia-Preconditioned Microglia Promote Angiogenesis and Repress Apoptosis in Stroke Mice via the TGF-β/Smad2/3 Pathway. Cell Death Dis. 2021, 12, 1068. [Google Scholar] [CrossRef]
- Ge, L.; Xun, C.; Li, W.; Jin, S.; Liu, Z.; Zhuo, Y.; Duan, D.; Hu, Z.; Chen, P.; Lu, M. Extracellular Vesicles Derived from Hypoxia-Preconditioned Olfactory Mucosa Mesenchymal Stem Cells Enhance Angiogenesis via miR-612. J. Nanobiotechnol. 2021, 19, 380. [Google Scholar] [CrossRef]
- Ma, T.; Hao, Y.; Li, S.; Xia, B.; Gao, X.; Zheng, Y.; Mei, L.; Wei, Y.; Yang, C.; Lu, L.; et al. Sequential Oxygen Supply System Promotes Peripheral Nerve Regeneration by Enhancing Schwann Cells Survival and Angiogenesis. Biomaterials 2022, 289, 121755. [Google Scholar] [CrossRef]
- Patel, J.H.; Schattinger, P.A.; Takayoshi, E.E.; Wills, A.E. Hif1α and Wnt Are Required for Posterior Gene Expression during Xenopus Tropicalis Tail Regeneration. Dev. Biol. 2022, 483, 157–168. [Google Scholar] [CrossRef]
- Novianti, T.; Juniantito, V.; Jusuf, A.A.; Arida, E.A.; Jusman, S.W.A.; Sadikin, M. Expression and Role of HIF-1α and HIF-2α in Tissue Regeneration: A Study of Hypoxia in House Gecko Tail Regeneration. Organogenesis 2019, 15, 69–84. [Google Scholar] [CrossRef]
- Tsissios, G.; Leleu, M.; Hu, K.; Demirtas, A.E.; Hu, H.; Kawanishi, T.; Skoufa, E.; Valente, A.; Herrera, A.; Mery, A.; et al. Species-Specific Oxygen Sensing Governs the Initiation of Vertebrate Limb Regeneration. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ferreira, F.; Raghunathan, V.; Luxardi, G.; Zhu, K.; Zhao, M. Early Redox Activities Modulate Xenopus Tail Regeneration. Nat. Commun. 2018, 9, 4296. [Google Scholar] [CrossRef]
- Carbonell-M, B.; Zapata Cardona, J.; Delgado, J.P. Post-Amputation Reactive Oxygen Species Production Is Necessary for Axolotls Limb Regeneration. Front. Cell Dev. Biol. 2022, 10, 921520. [Google Scholar] [CrossRef]
- Bobis-Wozowicz, S.; Paw, M.; Sarna, M.; Kędracka-Krok, S.; Nit, K.; Błażowska, N.; Dobosz, A.; Hammad, R.; Cathomen, T.; Zuba-Surma, E.; et al. Hypoxic Extracellular Vesicles from hiPSCs Protect Cardiomyocytes from Oxidative Damage by Transferring Antioxidant Proteins and Enhancing Akt/Erk/NRF2 Signaling. Cell Commun. Signal. 2024, 22, 356. [Google Scholar] [CrossRef]
- Paw, M.; Kusiak, A.A.; Nit, K.; Litewka, J.J.; Piejko, M.; Wnuk, D.; Sarna, M.; Fic, K.; Stopa, K.B.; Hammad, R.; et al. Hypoxia Enhances Anti-Fibrotic Properties of Extracellular Vesicles Derived from hiPSCs via the miR302b-3p/TGFβ/SMAD2 Axis. BMC Med. 2023, 21, 412. [Google Scholar] [CrossRef]
- Peters, M.M.C.; Sampaio-Pinto, V.; Da Costa Martins, P.A. Non-Coding RNAs in Endothelial Cell Signalling and Hypoxia during Cardiac Regeneration. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2020, 1867, 118515. [Google Scholar] [CrossRef]
- Deng, K.; Hua, Y.; Gao, Y.; Zheng, H.; Jiang, Y.; Wang, Y.; Gao, C.; Ren, T.; Zhu, Y. Thermosensitive Hydrogel with Programmable, Self-Regulated HIF-1α Stabilizer Release for Myocardial Infarction Treatment. Adv. Sci. 2024, 11, 2408013. [Google Scholar] [CrossRef]
- Du, J.; Wang, T.; Xu, L. HIF-1α Overexpression Improves the Efficacy of Human Induced Pluripotent Stem Cell-derived Cardiomyocytes for Cardiac Repair Following Myocardial Infarction. Mol. Med. Rep. 2024, 31, 40. [Google Scholar] [CrossRef]
- Yang, X.; Yang, S.; Wang, C.; Kuang, S. The Hypoxia-Inducible Factors HIF1α and HIF2α Are Dispensable for Embryonic Muscle Development but Essential for Postnatal Muscle Regeneration. J. Biol. Chem. 2017, 292, 5981–5991. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Y.; Wu, S.; Chiang, E.; Hsu, S.; Tseng, T.; Hung, S. Mesenchymal Stem Cells from a Hypoxic Culture Improve Nerve Regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 1804–1814. [Google Scholar] [CrossRef]
- Yue, W.; Cunlin, G.; Lu, H.; Yuanqing, Z.; Yanjun, T.; Qiong, W. Neuroprotective Effect of Intermittent Hypobaric Hypoxia Preconditioning on Cerebral Ischemia/Reperfusion in Rats. Int. J. Clin. Exp. Pathol. 2020, 13, 2860–2869. [Google Scholar]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and Brain Aging: Neurodegeneration or Neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef]
- Zhang, Z.; Kalra, H.; Delzell, M.C.; Jedlicka, C.R.; Vasilyev, M.; Vasileva, A.; Tomasson, M.H.; Bates, M.L. CORP: Sources and Degrees of Variability in Whole Animal Intermittent Hypoxia Experiments. J. Appl. Physiol. 2023, 134, 1207–1215. [Google Scholar] [CrossRef]
- Christiansen, L.; Chen, B.; Lei, Y.; Urbin, M.A.; Richardson, M.S.A.; Oudega, M.; Sandhu, M.; Rymer, W.Z.; Trumbower, R.D.; Mitchell, G.S.; et al. Acute Intermittent Hypoxia Boosts Spinal Plasticity in Humans with Tetraplegia. Exp. Neurol. 2021, 335, 113483. [Google Scholar] [CrossRef]
- Hornby, T.G.; Plawecki, A.; Lotter, J.K.; Shoger, L.H.; Voigtmann, C.J.; Inks, E.; Henderson, C.E. Acute Intermittent Hypoxia With High-Intensity Gait Training in Chronic Stroke: A Phase II Randomized Crossover Trial. Stroke 2024, 55, 1748–1757. [Google Scholar] [CrossRef]
- Pearcey, G.E.P.; Barry, A.J.; Sandhu, M.S.; Carroll, T.J.; Roth, E.J.; Rymer, W.Z. Acute Intermittent Hypoxia in People Living With Chronic Stroke: A Case Series. Stroke 2025, 56, 1054–1057. [Google Scholar] [CrossRef]
- Sandhu, M.S.; Perez, M.A.; Oudega, M.; Mitchell, G.S.; Rymer, W.Z. Efficacy and Time Course of Acute Intermittent Hypoxia Effects in the Upper Extremities of People with Cervical Spinal Cord Injury. Exp. Neurol. 2021, 342, 113722. [Google Scholar] [CrossRef]
- Alekseeva, T.M.; Topuzova, M.P.; Kulikov, V.P.; Kovzelev, P.D.; Kosenko, M.G.; Tregub, P.P. Hypercapnic Hypoxia as a Rehabilitation Method for Patients after Ischemic Stroke. Neurol. Res. 2024, 46, 695–705. [Google Scholar] [CrossRef]
- Vose, A.K.; Welch, J.F.; Nair, J.; Dale, E.A.; Fox, E.J.; Muir, G.D.; Trumbower, R.D.; Mitchell, G.S. Therapeutic Acute Intermittent Hypoxia: A Translational Roadmap for Spinal Cord Injury and Neuromuscular Disease. Exp. Neurol. 2022, 347, 113891. [Google Scholar] [CrossRef]
- Mallet, R.T.; Manukhina, E.B.; Ruelas, S.S.; Caffrey, J.L.; Downey, H.F. Cardioprotection by Intermittent Hypoxia Conditioning: Evidence, Mechanisms, and Therapeutic Potential. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H216–H232. [Google Scholar] [CrossRef]
- Meybohm, P.; Bein, B.; Brosteanu, O.; Cremer, J.; Gruenewald, M.; Stoppe, C.; Coburn, M.; Schaelte, G.; Böning, A.; Niemann, B.; et al. A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N. Engl. J. Med. 2015, 373, 1397–1407. [Google Scholar] [CrossRef]
- Law, Y.M.; Hsu, C.; Hingorani, S.R.; Richards, M.; McMullan, D.M.; Jefferies, H.; Himmelfarb, J.; Katz, R. Randomized Controlled Trial of Remote Ischemic Preconditioning in Children Having Cardiac Surgery. J. Cardiothorac. Surg. 2024, 19, 5. [Google Scholar] [CrossRef]
- Bartolo, K.; Hill, E.A. The Association between Obstructive Sleep Apnoea and Wound Healing: A Systematic Review. Sleep Breath. 2023, 27, 775–787. [Google Scholar] [CrossRef]
- Panza, G.S.; Puri, S.; Lin, H.-S.; Badr, M.S.; Mateika, J.H. Daily Exposure to Mild Intermittent Hypoxia Reduces Blood Pressure in Male Patients with Obstructive Sleep Apnea and Hypertension. Am. J. Respir. Crit. Care Med. 2022, 205, 949–958. [Google Scholar] [CrossRef]
- Chen, P.-S.; Chiu, W.-T.; Hsu, P.-L.; Lin, S.-C.; Peng, I.-C.; Wang, C.-Y.; Tsai, S.-J. Pathophysiological Implications of Hypoxia in Human Diseases. J. Biomed. Sci. 2020, 27, 63. [Google Scholar] [CrossRef]
- Rees, B.B.; Matute, L.A. Repeatable Interindividual Variation in Hypoxia Tolerance in the Gulf Killifish, Fundulus Grandis. Physiol. Biochem. Zool. 2018, 91, 1046–1056. [Google Scholar] [CrossRef]
- Salyha, N.; Oliynyk, I. Hypoxia Modeling Techniques: A Review. Heliyon 2023, 9, e13238. [Google Scholar] [CrossRef]
- Al-Ani, A.; Toms, D.; Kondro, D.; Thundathil, J.; Yu, Y.; Ungrin, M. Oxygenation in Cell Culture: Critical Parameters for Reproducibility Are Routinely Not Reported. PLoS ONE 2018, 13, e0204269. [Google Scholar] [CrossRef]
- Pavlacky, J.; Polak, J. Technical Feasibility and Physiological Relevance of Hypoxic Cell Culture Models. Front. Endocrinol. 2020, 11, 57. [Google Scholar] [CrossRef]
- Semenov, D.G.; Belyakov, A.V.; Rybnikova, E.A. Experimental Modeling of Damaging and Protective Hypoxia of the Mammalian Brain. J. Evol. Biochem. Physiol. 2022, 58, 2021–2034. [Google Scholar] [CrossRef]
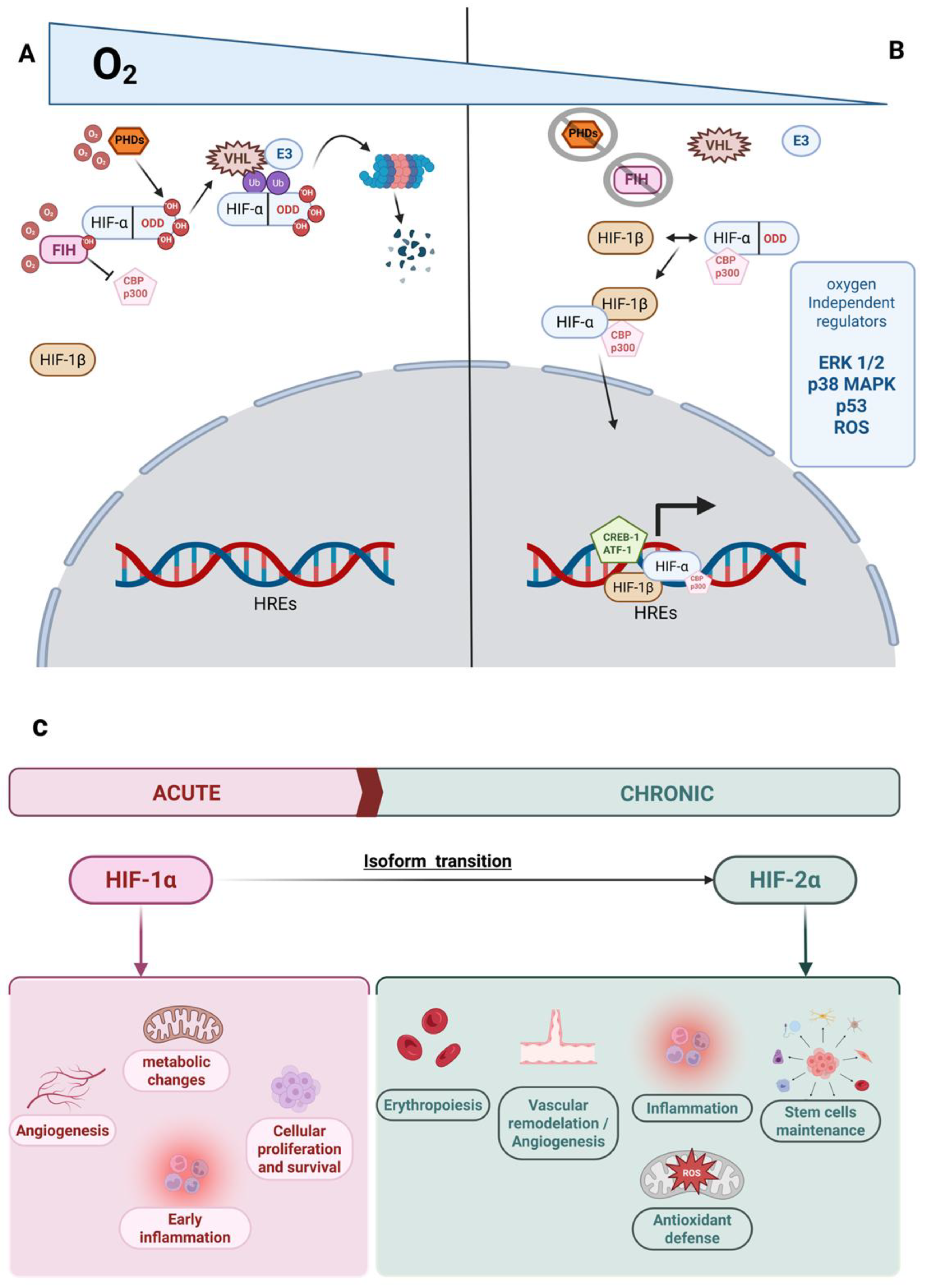


| Hypoxia Type | Regulator | Target Isoform | Effect | Ref. |
|---|---|---|---|---|
| Acute | Sirtuin 1 (SIRT1) | HIF-1α | Deacetylates HIF-1α, enhancing its transcriptional activity on target genes | [60] |
| Heat shock protein 90 (HSP90) | HIF-1α | Stabilizes and enhances HIF-1α function | [60] | |
| PHD3 stimulated by HIF-1α | HIF-2α | Preferentially increases HIF-2α degradation | [64] | |
| Chronic | NF-κB essential modulator (NEMO) | HIF-2α | Increases HIF-2α stability and availability (via STAT3 coactivation) | [63] |
| Ets-1 transcription factor (E-twenty-six oncogene) | HIF-2α | Acts as a coactivator | [63] | |
| HAF/SART1 (Hypoxia-associated factor) | HIF-1α | Promotes proteasomal degradation | [63] | |
| HIF-2α | Stimulates HIF-2α activity | [60,63,64] | ||
| aHIF (antisense transcript of HIF-1α) | HIF-1α | Binds HIF-1α mRNA and promotes its degradation | [63] | |
| FIH-1 (Factor Inhibiting HIF-1α) | HIF-1α | Hydroxylates HIF-1α and blocks cofactor binding | [60] | |
| Hsp70/CHIP (Heat shock protein 70/C-terminus of Hsc70-interacting protein) | HIF-1α | Ubiquitination and degradation | [60] | |
| RACK1 (Receptor for Activated C Kinase 1) | HIF-1α | Proteasomal degradation/inhibits HSP90 | [60] | |
| KLF2 (Krüppel-like factor 2) | HIF-1α | Inhibits HIF-1α interaction with HSP90 | [60] | |
| Reduced stability of HIF-1α mRNA | HIF-2α and HIF-3α | Predominance of HIF-2α and HIF-3α isoforms | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez Vélez, I.C.; Charris Domínguez, C.M.; Fernández Sánchez, M.J.; Garavito-Aguilar, Z.V. Hypoxia and Tissue Regeneration: Adaptive Mechanisms and Therapeutic Opportunities. Int. J. Mol. Sci. 2025, 26, 9272. https://doi.org/10.3390/ijms26199272
Vásquez Vélez IC, Charris Domínguez CM, Fernández Sánchez MJ, Garavito-Aguilar ZV. Hypoxia and Tissue Regeneration: Adaptive Mechanisms and Therapeutic Opportunities. International Journal of Molecular Sciences. 2025; 26(19):9272. https://doi.org/10.3390/ijms26199272
Chicago/Turabian StyleVásquez Vélez, Isabel Cristina, Carlos Mario Charris Domínguez, María José Fernández Sánchez, and Zayra Viviana Garavito-Aguilar. 2025. "Hypoxia and Tissue Regeneration: Adaptive Mechanisms and Therapeutic Opportunities" International Journal of Molecular Sciences 26, no. 19: 9272. https://doi.org/10.3390/ijms26199272
APA StyleVásquez Vélez, I. C., Charris Domínguez, C. M., Fernández Sánchez, M. J., & Garavito-Aguilar, Z. V. (2025). Hypoxia and Tissue Regeneration: Adaptive Mechanisms and Therapeutic Opportunities. International Journal of Molecular Sciences, 26(19), 9272. https://doi.org/10.3390/ijms26199272






