Imaging Flow Cytometry as a Molecular Biology Tool: From Cell Morphology to Molecular Mechanisms
Abstract
1. Introduction
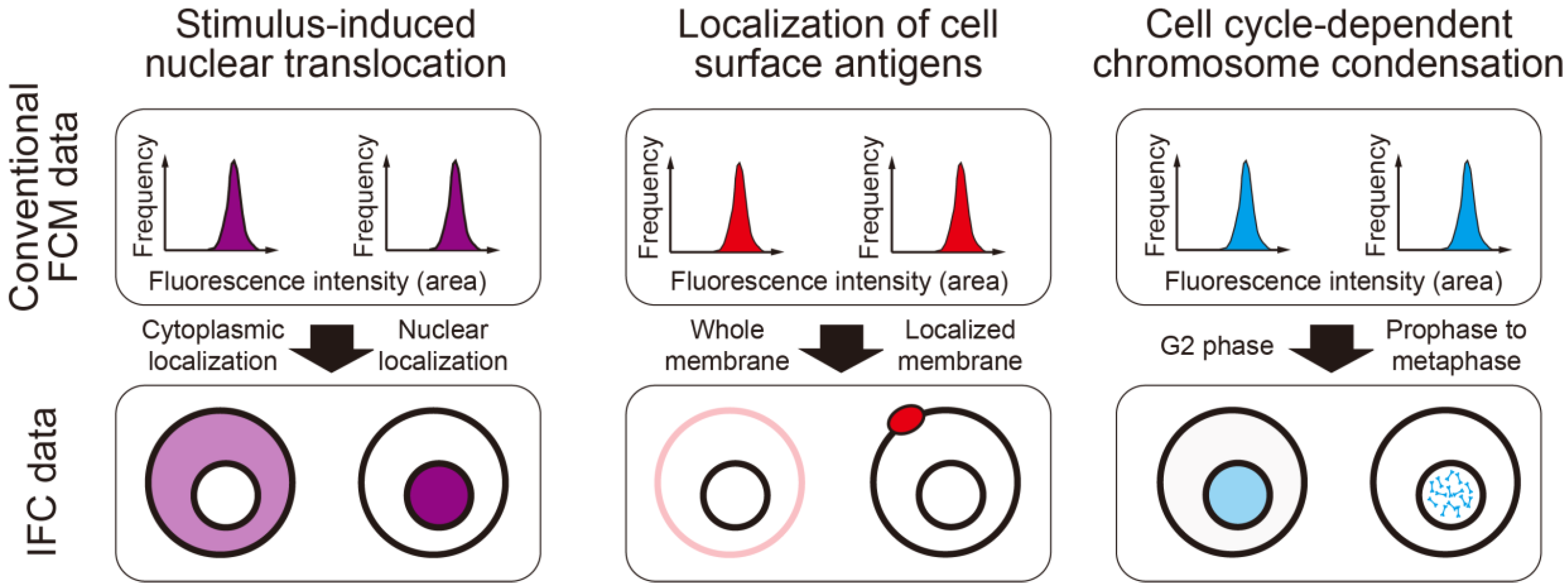
2. Advances and Current Applications of Imaging Flow Cytometry (IFC)
2.1. History of IFC
2.2. Current Applications of IFC
3. Practical Applications of IFC in Basic Medical and Life Science Research
3.1. Cell Cycle Analysis
3.2. Analysis of Protein Localization
3.3. Analysis of the Immunological Synapse
3.4. Detection of Leukemic Cells
4. Future Directions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CML | Chronic myeloid leukemia |
| cSMAC | Central supramolecular activation cluster |
| CTC | Circulating tumor cell |
| FISH | Fluorescence in situ hybridization |
| FCM | Flow cytometry |
| IFC | Imaging flow cytometry |
| IKK | IκB kinase |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| MPM-2 | Mitotic Protein Monoclonal-2 |
| MRD | Minimal residual disease |
| PMA/I | Phorbol 12-myristate 13-acetate/ionomycin |
| TCR | T cell receptor |
| Teff | Effector T cell |
| Treg | Regulatory T cell |
References
- Altschuler, S.J.; Wu, L.F. Cellular Heterogeneity: Do Differences Make a Difference? Cell 2010, 141, 559–563. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-Wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef]
- Buenrostro, J.D.; Wu, B.; Litzenburger, U.M.; Ruff, D.; Gonzales, M.L.; Snyder, M.P.; Chang, H.Y.; Greenleaf, W.J. Single-Cell Chromatin Accessibility Reveals Principles of Regulatory Variation. Nature 2015, 523, 486–490. [Google Scholar] [CrossRef]
- Stoeckius, M.; Hafemeister, C.; Stephenson, W.; Houck-Loomis, B.; Chattopadhyay, P.K.; Swerdlow, H.; Satija, R.; Smibert, P. Simultaneous Epitope and Transcriptome Measurement in Single Cells. Nat. Methods 2017, 14, 865–868. [Google Scholar] [CrossRef]
- Bendall, S.C.; Simonds, E.F.; Qiu, P.; Amir, E.D.; Krutzik, P.O.; Finck, R.; Bruggner, R.V.; Melamed, R.; Trejo, A.; Ornatsky, O.I.; et al. Single-Cell Mass Cytometry of Differential Immune and Drug Responses Across a Human Hematopoietic Continuum. Science 2011, 332, 687–696. [Google Scholar] [CrossRef]
- Thompson, D.W. On Growth and Form; Cambridge University Press: Cambridge, UK, 1917. [Google Scholar]
- Lepekhin, E.A.; Walmod, P.S.; Berezin, A.; Berezin, V.; Bock, E. Evaluation of Cell Morphology. In Cytoskeleton Methods and Protocols; Gavin, R.H., Ed.; Humana Press: New Jersey, NJ, USA, 2001; pp. 85–100. ISBN 978-1-59259-051-3. [Google Scholar] [CrossRef]
- Alizadeh, E.; Castle, J.; Quirk, A.; Taylor, C.D.L.; Xu, W.; Prasad, A. Cellular Morphological Features Are Predictive Markers of Cancer Cell State. Comput. Biol. Med. 2020, 126, 104044. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chen, X.; Gao, C.; Wang, J.; Huo, X.; Chen, J. Recent Developments (After 2020) in Flow Cytometry Worldwide and Within China. Biosensors 2025, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gu, Y.; Zhang, A.C.; Lo, Y.-H. Review: Imaging Technologies for Flow Cytometry. Lab Chip 2016, 16, 4639–4647. [Google Scholar] [CrossRef] [PubMed]
- Hybel, T.E.; Jensen, S.H.; Rodrigues, M.A.; Hybel, T.E.; Pedersen, M.N.; Qvick, S.H.; Enemark, M.H.; Bill, M.; Rosenberg, C.A.; Ludvigsen, M. Imaging Flow Cytometry and Convolutional Neural Network-Based Classification Enable Discrimination of Hematopoietic and Leukemic Stem Cells in Acute Myeloid Leukemia. IJMS 2024, 25, 6465. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Nakatsuka, R.; Fujioka, T. Automatic Discrimination of Human Hematopoietic Tumor Cell Lines Using a Combination of Imaging Flow Cytometry and Convolutional Neural Network. Hum. Cell 2021, 34, 1021–1024. [Google Scholar] [CrossRef]
- Filby, A.; Perucha, E.; Summers, H.; Rees, P.; Chana, P.; Heck, S.; Lord, G.M.; Davies, D. An Imaging Flow Cytometric Method for Measuring Cell Division History and Molecular Symmetry during Mitosis. Cytometry 2011, 79A, 496–506. [Google Scholar] [CrossRef]
- Blasi, T.; Hennig, H.; Summers, H.D.; Theis, F.J.; Cerveira, J.; Patterson, J.O.; Davies, D.; Filby, A.; Carpenter, A.E.; Rees, P. Label-Free Cell Cycle Analysis for High-Throughput Imaging Flow Cytometry. Nat. Commun. 2016, 7, 10256. [Google Scholar] [CrossRef]
- Eulenberg, P.; Köhler, N.; Blasi, T.; Filby, A.; Carpenter, A.E.; Rees, P.; Theis, F.J.; Wolf, F.A. Reconstructing Cell Cycle and Disease Progression Using Deep Learning. Nat. Commun. 2017, 8, 463. [Google Scholar] [CrossRef]
- Hui, H.; Fuller, K.A.; Chuah, H.; Liang, J.; Sidiqi, H.; Radeski, D.; Erber, W.N. Imaging Flow Cytometry to Assess Chromosomal Abnormalities in Chronic Lymphocytic Leukaemia. Methods 2018, 134–135, 32–40. [Google Scholar] [CrossRef]
- Hui, H.Y.L.; Clarke, K.M.; Fuller, K.A.; Stanley, J.; Chuah, H.H.; Ng, T.F.; Cheah, C.; McQuillan, A.; Erber, W.N. “Immuno-flowFISH” for the Assessment of Cytogenetic Abnormalities in Chronic Lymphocytic Leukemia. Cytometry 2019, 95, 521–533. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Kinoshita, M.; Yamada, K.; Ito, H.; Yamaguchi, T.; Chinen, Y.; Mizutani, S.; Fujino, T.; Kobayashi, T.; Shimura, Y.; et al. Imaging Flow Cytometry-Based Multiplex FISH for Three IGH Translocations in Multiple Myeloma. J. Hum. Genet. 2023, 68, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Hui, H.; Erber, W.; Clynick, B.; Fuller, K. Analysis of Human Chromosomes by Imaging Flow Cytometry. Cytom. Part B Clin. 2021, 100, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.A.; Bennett, S.; Hui, H.; Chakera, A.; Erber, W.N. Development of a Robust immuno-S-FISH Protocol Using Imaging Flow Cytometry. Cytometry 2016, 89, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Ranjbaran, R.; Behzad Behbahani, A.; Afshari, K.T.; Okhovat, M.A.; Tamadon, G.; Sharifzadeh, S. Development of Flow Cytometry-Fluorescent In Situ Hybridization (Flow-FISH) Method for Detection of PML/RARa Chromosomal Translocation in Acute Promyelocytic Leukemia Cell Line. Avicenna J. Med. Biotechnol. 2017, 9, 104–108. [Google Scholar]
- Grimwade, L.F.; Fuller, K.A.; Erber, W.N. Applications of Imaging Flow Cytometry in the Diagnostic Assessment of Acute Leukaemia. Methods 2017, 112, 39–45. [Google Scholar] [CrossRef]
- Simpson, A.P.A.; George, C.E.; Hui, H.Y.L.; Doddi, R.; Kotecha, R.S.; Fuller, K.A.; Erber, W.N. Imaging Flow Cytometric Identification of Chromosomal Defects in Paediatric Acute Lymphoblastic Leukaemia. Cells 2025, 14, 114. [Google Scholar] [CrossRef]
- Maguire, O.; Wallace, P.K.; Minderman, H. Fluorescent In Situ Hybridization in Suspension by Imaging Flow Cytometry. In Imaging Flow Cytometry; Barteneva, N., Vorobjev, I., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1389, pp. 111–126. [Google Scholar] [CrossRef]
- Durdik, M.; Kosik, P.; Jakl, L.; Kozackova, M.; Markova, E.; Vigasova, K.; Beresova, K.; Jakubikova, J.; Horvathova, E.; Zastko, L.; et al. Imaging Flow Cytometry and Fluorescence Microscopy in Assessing Radiation Response in Lymphocytes from Umbilical Cord Blood and Cancer Patients. Cytometry 2021, 99, 1198–1208. [Google Scholar] [CrossRef]
- Durdik, M.; Kosik, P.; Gursky, J.; Vokalova, L.; Markova, E.; Belyaev, I. Imaging Flow Cytometry as a Sensitive Tool to Detect Low-dose-induced DNA Damage by Analyzing 53BP1 and γH2AX Foci in Human Lymphocytes. Cytometry 2015, 87, 1070–1078. [Google Scholar] [CrossRef]
- Pärnamaa, T.; Parts, L. Accurate Classification of Protein Subcellular Localization from High-Throughput Microscopy Images Using Deep Learning. G3 Genes Genomes Genet. 2017, 7, 1385–1392. [Google Scholar] [CrossRef]
- Muffels, I.J.J.; Waterham, H.R.; D’Alessandro, G.; Zagnoli-Vieira, G.; Sacher, M.; Lefeber, D.J.; Van Der Vinne, C.; Roifman, C.M.; Gassen, K.L.I.; Rehmann, H.; et al. Imaging Flow Cytometry-Based Cellular Screening Elucidates Pathophysiology in Individuals with Variants of Uncertain Significance. Genome Med. 2025, 17, 12. [Google Scholar] [CrossRef]
- Schraivogel, D.; Kuhn, T.M.; Rauscher, B.; Rodríguez-Martínez, M.; Paulsen, M.; Owsley, K.; Middlebrook, A.; Tischer, C.; Ramasz, B.; Ordoñez-Rueda, D.; et al. High-Speed Fluorescence Image–Enabled Cell Sorting. Science 2022, 375, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Horisaki, R.; Kawamura, Y.; Ugawa, M.; Sato, I.; Hashimoto, K.; Kamesawa, R.; Setoyama, K.; Yamaguchi, S.; Fujiu, K.; et al. Ghost Cytometry. Science 2018, 360, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, S.; Dova, L.; Kotsianidis, I.; Hatzimichael, E.; Kapsali, E.; Markopoulos, G.S. Imaging Flow Cytometry: Development, Present Applications, and Future Challenges. Methods Protoc. 2024, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhou, Z.; Lv, Q.; Min, Q.; Jiang, L.; Chen, Q.; Peng, J.; Zhou, H.; Zhou, J.; Dai, Q.; et al. Imaging Flow Cytometry: From High-Resolution Morphological Imaging to Innovation in High-Throughput Multidimensional Biomedical Analysis. Front. Bioeng. Biotechnol. 2025, 13, 1580749. [Google Scholar] [CrossRef]
- Cambier, J.L.; Kay, D.B.; Wheeless, L.L. A Multidimensional Slit-Scan Flow System. J. Histochem. Cytochem. 1979, 27, 321–324. [Google Scholar] [CrossRef]
- Shapiro, H.M. Practical Flow Cytometry, 4th ed.; Wiley: Hoboken, NJ, USA, 2003; ISBN 978-0-471-41125-3. [Google Scholar]
- George, T.C.; Basiji, D.A.; Hall, B.E.; Lynch, D.H.; Ortyn, W.E.; Perry, D.J.; Seo, M.J.; Zimmerman, C.A.; Morrissey, P.J. Distinguishing Modes of Cell Death Using the ImageStream® Multispectral Imaging Flow Cytometer. Cytometry 2004, 59A, 237–245. [Google Scholar] [CrossRef]
- Ortyn, W.E.; Hall, B.E.; George, T.C.; Frost, K.; Basiji, D.A.; Perry, D.J.; Zimmerman, C.A.; Coder, D.; Morrissey, P.J. Sensitivity Measurement and Compensation in Spectral Imaging. Cytometry 2006, 69A, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Basiji, D.A.; Ortyn, W.E.; Liang, L.; Venkatachalam, V.; Morrissey, P. Cellular Image Analysis and Imaging by Flow Cytometry. Clin. Lab. Med. 2007, 27, 653–670. [Google Scholar] [CrossRef]
- Basiji, D.A. Principles of Amnis Imaging Flow Cytometry. In Imaging Flow Cytometry; Barteneva, N., Vorobjev, I., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1389, pp. 29–42. [Google Scholar] [CrossRef]
- Han, Y.; Tang, R.; Gu, Y.; Zhang, A.C.; Cai, W.; Castor, V.; Cho, S.H.; Alaynick, W.; Lo, Y.-H. Cameraless High-Throughput Three-Dimensional Imaging Flow Cytometry. Optica 2019, 6, 1297. [Google Scholar] [CrossRef]
- Holzner, G.; Mateescu, B.; Van Leeuwen, D.; Cereghetti, G.; Dechant, R.; Stavrakis, S.; deMello, A. High-Throughput Multiparametric Imaging Flow Cytometry: Toward Diffraction-Limited Sub-Cellular Detection and Monitoring of Sub-Cellular Processes. Cell Rep. 2021, 34, 108824. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lo, Y.-H. Imaging Cells in Flow Cytometer Using Spatial-Temporal Transformation. Sci. Rep. 2015, 5, 13267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mei, L.; Yu, M.; Ma, X.; Hou, D.; Yin, Z.; Liu, X.; Ding, Y.; Yang, K.; Xiao, R.; et al. Imaging Flow Cytometry with a Real-Time Throughput beyond 1,000,000 Events per Second. Light Sci. Appl. 2025, 14, 76. [Google Scholar] [CrossRef]
- Jagannadh, V.K.; Mackenzie, M.D.; Pal, P.; Kar, A.K.; Gorthi, S.S. Imaging Flow Cytometry With Femtosecond Laser-Micromachined Glass Microfluidic Channels. IEEE J. Sel. Top. Quantum Electron. 2015, 21, 370–375. [Google Scholar] [CrossRef]
- Nitta, N.; Sugimura, T.; Isozaki, A.; Mikami, H.; Hiraki, K.; Sakuma, S.; Iino, T.; Arai, F.; Endo, T.; Fujiwaki, Y.; et al. Intelligent Image-Activated Cell Sorting. Cell 2018, 175, 266–276.e13. [Google Scholar] [CrossRef]
- Lin, S.; Li, R.; Weng, Y.; Mei, L.; Wei, C.; Song, C.; Wei, S.; Yao, Y.; Ruan, X.; Zhou, F.; et al. Optical Time-stretch Imaging Flow Cytometry in the Compressed Domain. J. Biophotonics 2023, 16, e202300096. [Google Scholar] [CrossRef]
- Göröcs, Z.; Baum, D.; Song, F.; De Haan, K.; Ceylan Koydemir, H.; Qiu, Y.; Cai, Z.; Skandakumar, T.; Peterman, S.; Tamamitsu, M.; et al. Label-Free Detection of Giardia Lamblia Cysts Using a Deep Learning-Enabled Portable Imaging Flow Cytometer. Lab Chip 2020, 20, 4404–4412. [Google Scholar] [CrossRef] [PubMed]
- Ugawa, M.; Ota, S. High-Throughput Parallel Optofluidic 3D-Imaging Flow Cytometry. Small Sci. 2022, 2, 2100126. [Google Scholar] [CrossRef] [PubMed]
- Mikami, H.; Kawaguchi, M.; Huang, C.-J.; Matsumura, H.; Sugimura, T.; Huang, K.; Lei, C.; Ueno, S.; Miura, T.; Ito, T.; et al. Virtual-Freezing Fluorescence Imaging Flow Cytometry. Nat. Commun. 2020, 11, 1162. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image Analysis Software for Identifying and Quantifying Cell Phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef]
- Lamprecht, M.R.; Sabatini, D.M.; Carpenter, A.E. CellProfilerTM: Free, Versatile Software for Automated Biological Image Analysis. BioTechniques 2007, 42, 71–75. [Google Scholar] [CrossRef]
- Otto, O.; Rosendahl, P.; Mietke, A.; Golfier, S.; Herold, C.; Klaue, D.; Girardo, S.; Pagliara, S.; Ekpenyong, A.; Jacobi, A.; et al. Real-time deformability cytometry: On-the-fly cell mechanical phenotyping. Nat. Methods 2015, 12, 199–202. [Google Scholar] [CrossRef]
- Oliferenko, S.; Paiha, K.; Harder, T.; Gerke, V.; Schwärzler, C.; Schwarz, H.; Beug, H.; Günthert, U.; Huber, L.A. Analysis of Cd44-Containing Lipid Rafts. J. Cell Biol. 1999, 146, 843–854. [Google Scholar] [CrossRef]
- Maguire, O.; Collins, C.; O’Loughlin, K.; Miecznikowski, J.; Minderman, H. Quantifying Nuclear P65 as a Parameter for NF-κB Activation: Correlation between ImageStream Cytometry, Microscopy, and Western Blot. Cytom. Part A 2011, 79A, 461–469. [Google Scholar] [CrossRef]
- George, T.C.; Fanning, S.L.; Fitzgeral-Bocarsly, P.; Medeiros, R.B.; Highfill, S.; Shimizu, Y.; Hall, B.E.; Frost, K.; Basiji, D.; Ortyn, W.E.; et al. Quantitative Measurement of Nuclear Translocation Events Using Similarity Analysis of Multispectral Cellular Images Obtained in Flow. J. Immunol. Methods 2006, 311, 117–129. [Google Scholar] [CrossRef]
- Hritzo, M.K.; Courneya, J.-P.; Golding, A. Imaging Flow Cytometry: A Method for Examining Dynamic Native FOXO1 Localization in Human Lymphocytes. J. Immunol. Methods 2018, 454, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Monks, C.R.F.; Freiberg, B.A.; Kupfer, H.; Sciaky, N.; Kupfer, A. Three-Dimensional Segregation of Supramolecular Activation Clusters in T Cells. Nature 1998, 395, 82–86. [Google Scholar] [CrossRef]
- Grakoui, A.; Bromley, S.K.; Sumen, C.; Davis, M.M.; Shaw, A.S.; Allen, P.M.; Dustin, M.L. The Immunological Synapse: A Molecular Machine Controlling T Cell Activation. Science 1999, 285, 221–227. [Google Scholar] [CrossRef]
- Nicolaou, S.A.; Neumeier, L.; Steckly, A.; Kucher, V.; Takimoto, K.; Conforti, L. Localization of Kv1.3 Channels in the Immunological Synapse Modulates the Calcium Response to Antigen Stimulation in T Lymphocytes. J. Immunol. 2009, 183, 6296–6302. [Google Scholar] [CrossRef]
- Philipsen, L.; Engels, T.; Schilling, K.; Gurbiel, S.; Fischer, K.-D.; Tedford, K.; Schraven, B.; Gunzer, M.; Reichardt, P. Multimolecular Analysis of Stable Immunological Synapses Reveals Sustained Recruitment and Sequential Assembly of Signaling Clusters. Mol. Cell. Proteom. 2013, 12, 2551–2567. [Google Scholar] [CrossRef] [PubMed]
- Juvet, S.C.; Whatcott, C.J.; Bushell, A.; Wood, K.J. Measurement of T Cell Alloreactivity Using Imaging Flow Cytometry. J. Vis. Exp. 2017, 122, e55283. [Google Scholar] [CrossRef] [PubMed]
- Markey, K.A.; Gartlan, K.H.; Kuns, R.D.; MacDonald, K.P.A.; Hill, G.R. Imaging the Immunological Synapse between Dendritic Cells and T Cells. J. Immunol. Methods 2015, 423, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Shetab Boushehri, S.; Essig, K.; Chlis, N.-K.; Herter, S.; Bacac, M.; Theis, F.J.; Glasmacher, E.; Marr, C.; Schmich, F. Explainable Machine Learning for Profiling the Immunological Synapse and Functional Characterization of Therapeutic Antibodies. Nat. Commun. 2023, 14, 7888. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Tang, R.; Zhu, Y.; Guo, H.; Qu, Y.; Xie, P.; Lian, I.Y.; Wang, Y.; Lo, Y.-H. Interpretable Unsupervised Learning Enables Accurate Clustering with High-Throughput Imaging Flow Cytometry. Sci. Rep. 2023, 13, 20533. [Google Scholar] [CrossRef]
- Lannigan, J.; Erdbruegger, U. Imaging flow cytometry for the characterization of extracellular vesicles. Methods 2017, 112, 55–67. [Google Scholar] [CrossRef]
- Mizuta, R.; Sasaki, Y.; Katagiri, K.; Sawada, S.; Akiyoshi, K. Reversible conjugation of biomembrane vesicles with magnetic nanoparticles using a self-assembled nanogel interface: Single particle analysis using imaging flow cytometry. Nanoscale Adv. 2022, 4, 1999–2010. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuoka, Y. Imaging Flow Cytometry as a Molecular Biology Tool: From Cell Morphology to Molecular Mechanisms. Int. J. Mol. Sci. 2025, 26, 9261. https://doi.org/10.3390/ijms26199261
Matsuoka Y. Imaging Flow Cytometry as a Molecular Biology Tool: From Cell Morphology to Molecular Mechanisms. International Journal of Molecular Sciences. 2025; 26(19):9261. https://doi.org/10.3390/ijms26199261
Chicago/Turabian StyleMatsuoka, Yoshikazu. 2025. "Imaging Flow Cytometry as a Molecular Biology Tool: From Cell Morphology to Molecular Mechanisms" International Journal of Molecular Sciences 26, no. 19: 9261. https://doi.org/10.3390/ijms26199261
APA StyleMatsuoka, Y. (2025). Imaging Flow Cytometry as a Molecular Biology Tool: From Cell Morphology to Molecular Mechanisms. International Journal of Molecular Sciences, 26(19), 9261. https://doi.org/10.3390/ijms26199261





