The Role of Prenatal Microglial Activation and Its Sex Differences in the Development of Neuropsychiatric Disorders and Neurodegenerative Diseases
Abstract
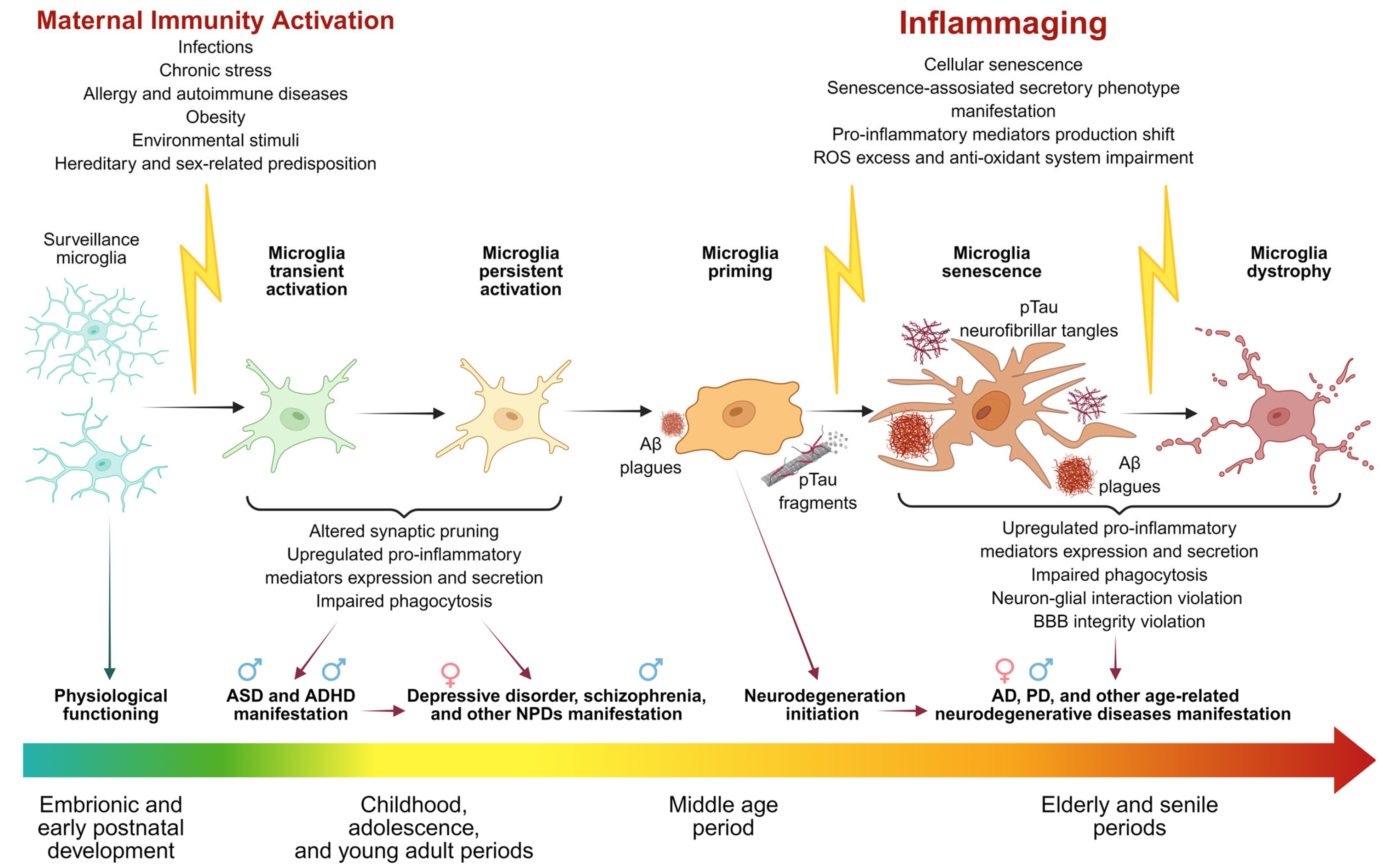
1. Introduction
2. Literature Search Strategy
3. Maternal Immune Activation (MIA)
3.1. Epidemiological Data Regarding the Role of MIA in the Development of Neuropsychiatric Disorders
3.2. Approaches to MIA Modeling
3.2.1. MIA and Poly I:C
3.2.2. MIA and LPS
4. Microglia
4.1. Microglia Features and Functions
4.2. Sex Differences Microglia Under Physiological and Pathological Conditions
5. The Role of Microglia in the Development of Neuropsychiatric and Neurodegenerative Diseases
5.1. Autism Spectrum Disorder (ASD)
5.2. Schizophrenia
5.3. Major Depressive Disorder (MDD)
5.4. Parkinson’s Disease (PD)
5.5. Alzheimer’s Disease (AD)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease. |
| ADHD | Attention deficit hyperactivity disorder. |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid. |
| APP | Amyloid precursor protein. |
| ARM | Age-Related Microglia. |
| ASD | Autism spectrum disorder. |
| ATF-4 | Activating transcription factor. |
| Aβ | Amyloid-beta. |
| BDNF | Brain-Derived Neurotrophic Factor. |
| Bmp2 | Bone morphogenetic protein. |
| CCL2 | C-C motif ligand 2. |
| Clec7a | C-type lectin domain family 7 member A. |
| CNS | Central nervous system. |
| CX3CR1 | C-X3-C Motif Chemokine Receptor 1. |
| CXCL8 | C-X-C Motif Chemokine Ligand 8. |
| DAM | Disease-Associated Microglia. |
| DAMP | Damage-Associated Molecular Patterns. |
| DM | Dark Microglia. |
| DOHaD | Developmental Origins of Health and Disease. |
| dsRNA | Double-stranded Ribonucleic acid. |
| ERK | Extracellular signal-regulated kinase. |
| ERRα | Estrogen receptor-related receptor. |
| FGF2 | Fibroblast Growth Factor. |
| GD | Gestational day. |
| GFAP | Glial Fibrillary Acidic Protein. |
| GM-CSF | Granulocyte–Macrophage Colony-Stimulating Factor. |
| Gpx3 | Glutathione peroxidase 3. |
| HIF-1 | Hypoxia-inducible factor 1. |
| HPAA | Hypothalamus–pituitary–adrenal axis. |
| IDE | Insulin-degrading enzyme. |
| IGF1 | Insulin-like grown factor. |
| IKK | Complex IκB kinase complex. |
| iNOS | Inducible NO synthase. |
| IRF3 | Interferon Regulatory Factor 3. |
| JNK | c-Jun N-terminal kinases. |
| LDAM | Late Disease-Associated Microglia. |
| LPS | Lipopolysaccharide. |
| LTP | Long-term potentiation. |
| LTD | Long-term depression. |
| MAPK | Mitogen-activated protein kinase. |
| MCP-1 | Monocyte Chemoattractant Protein 1. |
| MD-2 | Myeloid Differentiation Factor 2). |
| MeCP2 | Methyl-CpG-binding Protein 2. |
| MGnD | Microglial Neurodegenerative Phenotype. |
| MHC | Major histocompatibility complex. |
| MIA | Maternal immune activation. |
| MyD88 | Myeloid differentiation primary response gene 88. |
| NF-κB | Nuclear factor-kappa B. |
| NLRP3 inflammasome | NOD-like receptor family, pyrin domain-containing 3 inflammasome. |
| NMDA | N-methyl-D-aspartate. |
| NPCs | Neural progenitor cells. |
| NR3C1 | Nuclear receptor subfamily 3 group C member 1. |
| NSCs | Neural stem cells. |
| P2RY12 | Purinergic Receptor 12. |
| p75NTR | p75 Neurotrophin Receptor. |
| PAMP | Pathogen-Associated Molecular Patterns. |
| PD | Parkinson’s disease. |
| PDGF | Platelet-derived growth factor. |
| Poly I:C | Polyinosinic–polycytidylic acid. |
| PPARγ | Peroxisome proliferator-activated receptor. |
| PTEN | Phosphatase and Tensin Homolog. |
| RAGE | Receptor for Advanced Glycation End Products. |
| ROS | Reactive oxygen species. |
| Sall1 | Spalt-Like transcription factor 1. |
| STAT3 | Signal Transducer and Activator of Transcription 3. |
| SUMO | Small Ubiquitin-like Modifier. |
| TAK1 | Transforming Growth Factor β-Activated Kinase 1. |
| TBK1 | TANK-binding kinase 1. |
| TGF-β1 | Transforming growth factor beta 1. |
| TLR | Toll-Like Receptor. |
| TMEM119 | Transmembrane Protein 119. |
| TNF-α | Tumor necrosis factor. |
| TORCH | Toxoplasma, Other, Rubella, Cytomegalovirus, Herpes. |
| TRAF | TNF receptor-associated factor. |
| TREM2 | Triggering Receptor Expressed On Myeloid Cells. |
| TRIF | TIR domain-containing adapter-inducing interferon-β. |
| UPR | Unfolded stress response. |
| VEGFα | Vascular Endothelial Growth Factor A. |
| WAM | White Matter-Associated Microglia. |
References
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Pronovost, G.N.; Hsiao, E.Y. Perinatal Interactions between the Microbiome, Immunity, and Neurodevelopment. Immunity 2019, 50, 18–36. [Google Scholar] [CrossRef] [PubMed]
- VanRyzin, J.W.; Pickett, L.A.; McCarthy, M.M. Microglia: Driving critical periods and sexual differentiation of the brain. Dev. Neurobiol. 2018, 78, 580–592. [Google Scholar] [CrossRef]
- Sentyabreva, A.V. Neuronal reprogramming: Approaches, challenges, and prospects. Morphology 2024, 162, 436–453. [Google Scholar] [CrossRef]
- Weber-Stadlbauer, U. Epigenetic and transgenerational mechanisms in infection-mediated neurodevelopmental disorders. Transl. Psychiatry 2017, 7, e1113. [Google Scholar] [CrossRef]
- Richetto, J.; Massart, R.; Weber-Stadlbauer, U.; Szyf, M.; Riva, M.A.; Meyer, U. Genome-wide DNA Methylation Changes in a Mouse Model of Infection-Mediated Neurodevelopmental Disorders. Biol. Psychiatry 2017, 81, 265–276. [Google Scholar] [CrossRef]
- Butovsky, O.; Ziv, Y.; Schwartz, A.; Landa, G.; Talpalar, A.E.; Pluchino, S.; Martino, G.; Schwartz, M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006, 31, 149–160. [Google Scholar] [CrossRef]
- Nakanishi, M.; Niidome, T.; Matsuda, S.; Akaike, A.; Kihara, T.; Sugimoto, H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur. J. Neurosci. 2007, 25, 649–658. [Google Scholar] [CrossRef]
- Mattei, D.; Ivanov, A.; Ferrai, C.; Jordan, P.; Guneykaya, D.; Buonfiglioli, A.; Schaafsma, W.; Przanowski, P.; Deuther-Conrad, W.; Brust, P.; et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl. Psychiatry 2017, 7, e1120. [Google Scholar] [CrossRef]
- Southey, B.R.; Zhang, P.; Keever, M.R.; Rymut, H.E.; Johnson, R.W.; Sweedler, J.V.; Rodriguez-Zas, S.L. Effects of maternal immune activation in porcine transcript isoforms of neuropeptide and receptor genes. J. Integr. Neurosci. 2021, 20, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Canetta, S.; Bolkan, S.; Padilla-Coreano, N.; Song, L.J.; Sahn, R.; Harrison, N.L.; Gordon, J.A.; Brown, A. Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol. Psychiatry 2016, 21, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Mednick, S.A.; Machon, R.A.; Huttunen, M.O.; Bonett, D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry 1988, 45, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Derkits, E.J. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef]
- Brown, A.S.; Begg, M.D.; Gravenstein, S.; Schaefer, C.A.; Wyatt, R.J.; Bresnahan, M.; Babulas, V.P.; Susser, E.S. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry 2004, 61, 774–780. [Google Scholar] [CrossRef]
- Brown, A.S.; Sourander, A.; Hinkka-Yli-Salomäki, S.; McKeague, I.W.; Sundvall, J.; Surcel, H.M. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol. Psychiatry 2014, 19, 259–264. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Zimbron, J.; Lewis, G.; Jones, P.B. Prenatal maternal infection, neurodevelopment and adult schizophrenia: A systematic review of population-based studies. Psychol. Med. 2013, 43, 239–257. [Google Scholar] [CrossRef]
- Cheslack-Postava, K.; Brown, A.S. Prenatal infection and schizophrenia: A decade of further progress. Schizophr. Res. 2022, 247, 7–15. [Google Scholar] [CrossRef]
- Kępińska, A.P.; Iyegbe, C.O.; Vernon, A.C.; Yolken, R.; Murray, R.M.; Pollak, T.A. Schizophrenia and Influenza at the Centenary of the 1918–1919 Spanish Influenza Pandemic: Mechanisms of Psychosis Risk. Front. Psychiatry 2020, 11, 72. [Google Scholar] [CrossRef]
- Patel, S.; Dale, R.C.; Rose, D.; Heath, B.; Nordahl, C.W.; Rogers, S.; Guastella, A.J.; Ashwood, P. Maternal immune conditions are increased in males with autism spectrum disorders and are associated with behavioural and emotional but not cognitive co-morbidity. Transl. Psychiatry 2020, 10, 286. [Google Scholar] [CrossRef]
- Patel, S.; Masi, A.; Dale, R.C.; O Whitehouse, A.J.; Pokorski, I.; A Alvares, G.; Hickie, I.B.; Breen, E.; Guastella, A.J. Social impairments in autism spectrum disorder are related to maternal immune history profile. Mol. Psychiatry 2018, 23, 1794–1797. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Xu, L.-L.; Shao, L.; Xia, R.-M.; Yu, Z.-H.; Ling, Z.-X.; Yang, F.; Deng, M.; Ruan, B. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav. Immun. 2016, 58, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.M.; Antonson, A.M. At the crux of maternal immune activation: Viruses, microglia, microbes, and IL-17A. Immunol. Rev. 2022, 311, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Atladóttir, H.Ó.; Thorsen, P.; Østergaard, L.; Schendel, D.E.; Lemcke, S.; Abdallah, M.; Parner, E.T. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2010, 40, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Bresnahan, M.A.; Che, X.; Schultz, A.F.; Ukaigwe, J.E.; Eddy, M.L.; Hirtz, D.; Gunnes, N.; Lie, K.K.; Magnus, P.; et al. Prenatal fever and autism risk. Mol. Psychiatry 2018, 23, 759–766. [Google Scholar] [CrossRef]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U.; Pearce, B.D.; Pletnikov, M.V.; Yolken, R.H. Maternal immune activation: Reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef]
- Meyer, U.; Feldon, J.; Fatemi, S.H. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci. Biobehav. Rev. 2009, 33, 1061–1079. [Google Scholar] [CrossRef]
- Malkova, N.V.; Yu, C.Z.; Hsiao, E.Y.; Moore, M.J.; Patterson, P.H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 2012, 26, 607–616. [Google Scholar] [CrossRef]
- Kumar, M.; Saadaoui, M.; Al Khodor, S. Infections and Pregnancy: Effects on Maternal and Child Health. Front. Cell. Infect. Microbiol. 2022, 12, 873253. [Google Scholar] [CrossRef]
- Megli, C.J.; Coyne, C.B. Infections at the maternal-fetal interface: An overview of pathogenesis and defence. Nat. Rev. Microbiol. 2022, 20, 67–82. [Google Scholar] [CrossRef]
- Müllegger, R.R.; Glatz, M. Skin infections in pregnancy. Hautarzt 2010, 61, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Pineda, D.A.; Palacio, L.G.; Puerta, I.C.; Merchán, V.; Arango, C.P.; Galvis, A.Y.; Gómez, M.; Aguirre, D.C.; Lopera, F.; Arcos-Burgos, M. Environmental influences that affect attention deficit/hyperactivity disorder: Study of a genetic isolate. Eur. Child. Adolesc. Psychiatry 2007, 16, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.R.; McDermott, S. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J. Atten. Disord. 2011, 15, 667–673. [Google Scholar] [CrossRef]
- Silva, D.; Colvin, L.; Hagemann, E.; Bower, C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 2014, 133, e14–e22. [Google Scholar] [CrossRef] [PubMed]
- Mataix-Cols, D.; Frans, E.; Pérez-Vigil, A.; Kuja-Halkola, R.; Gromark, C.; Isomura, K.; Fernández de la Cruz, L.; Serlachius, E.; Leckman, J.F.; Crowley, J.J.; et al. A total-population multigenerational family clustering study of autoimmune diseases in obsessive-compulsive disorder and Tourette’s/chronic tic disorders. Mol. Psychiatry 2018, 23, 1652–1658. [Google Scholar] [CrossRef]
- Dalsgaard, S.; Waltoft, B.L.; Leckman, J.F.; Mortensen, P.B. Maternal history of autoimmune disease and later development of Tourette syndrome in offspring. J. Am. Acad. Child. Adolesc. Psychiatry 2015, 54, 495–501.e1. [Google Scholar] [CrossRef]
- Chen, S.-W.; Zhong, X.-S.; Jiang, L.-N.; Zheng, X.-Y.; Xiong, Y.-Q.; Ma, S.-J.; Qiu, M.; Huo, S.-T.; Ge, J.; Chen, Q. Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. Behav. Brain Res. 2016, 296, 61–69. [Google Scholar] [CrossRef]
- Nielsen, T.C.; Nassar, N.; Shand, A.W.; Jones, H.; Guastella, A.J.; Dale, R.C.; Lain, S.J. Association of Maternal Autoimmune Disease with Attention-Deficit/Hyperactivity Disorder in Children. JAMA Pediatr. 2021, 175, e205487. [Google Scholar] [CrossRef]
- Instanes, J.T.; Halmøy, A.; Engeland, A.; Haavik, J.; Furu, K.; Klungsøyr, K. Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers with Inflammatory and Immune System Diseases. Biol. Psychiatry 2017, 81, 452–459. [Google Scholar] [CrossRef]
- Gong, T.; Lundholm, C.; Rejnö, G.; Bölte, S.; Larsson, H.; D’ONofrio, B.M.; Lichtenstein, P.; Almqvist, C. Parental asthma and risk of autism spectrum disorder in offspring: A population and family-based case-control study. Clin. Exp. Allergy J. 2019, 49, 883–891. [Google Scholar] [CrossRef]
- Hisle-Gorman, E.; Susi, A.; Stokes, T.; Gorman, G.; Erdie-Lalena, C.; Nylund, C.M. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr. Res. 2018, 84, 190–198. [Google Scholar] [CrossRef]
- Liu, X.; Dalsgaard, S.; Munk-Olsen, T.; Li, J.; Wright, R.J.; Momen, N.C. Parental asthma occurrence, exacerbations and risk of attention-deficit/hyperactivity disorder. Brain Behav. Immun. 2019, 82, 302–308. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Kim, J.Y.; Son, M.J.; Son, C.Y.; Radua, J.; Eisenhut, M.; Gressier, F.; Koyanagi, A.; Carvalho, A.F.; Stubbs, B.; Solmi, M.; et al. Environmental risk factors and biomarkers for autism spectrum disorder: An umbrella review of the evidence. Lancet Psychiatry 2019, 6, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lagerberg, T.; Chang, Z.; Cortese, S.; A Rosenqvist, M.; Almqvist, C.; D’oNofrio, B.M.; Hegvik, T.-A.; Hartman, C.; Chen, Q.; et al. Maternal pre-pregnancy overweight/obesity and the risk of attention-deficit/hyperactivity disorder in offspring: A systematic review, meta-analysis and quasi-experimental family-based study. Int. J. Epidemiol. 2020, 49, 857–875. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, S.; Xu, S.; Weng, S.; Liu, Z. Maternal Body Mass Index and Risk of Autism Spectrum Disorders in Offspring: A Meta-analysis. Sci. Rep. 2016, 6, 34248. [Google Scholar] [CrossRef]
- Miller, L.L.; Scharf, J.M.; Mathews, C.A.; Ben-Shlomo, Y. Tourette syndrome and chronic tic disorder are associated with lower socio-economic status: Findings from the Avon Longitudinal Study of Parents and Children cohort. Dev. Med. Child. Neurol. 2014, 56, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.E.; Ford, T.; Williams, R.; Russell, G. The Association Between Socioeconomic Disadvantage and Attention Deficit/Hyperactivity Disorder (ADHD): A Systematic Review. Child. Psychiatry Hum. Dev. 2016, 47, 440–458. [Google Scholar] [CrossRef]
- Manzari, N.; Matvienko-Sikar, K.; Baldoni, F.; O’Keeffe, G.W.; Khashan, A.S. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 1299–1309. [Google Scholar] [CrossRef]
- Scola, G.; Duong, A. Prenatal maternal immune activation and brain development with relevance to psychiatric disorders. Neuroscience 2017, 346, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.B.; Willis, D.E.; Rodriguez, E.L.; Schwarz, J.M. Maternal immune activation as an epidemiological risk factor for neurodevelopmental disorders: Considerations of timing, severity, individual differences, and sex in human and rodent studies. Front. Neurosci. 2023, 17, 1135559. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, L.; Samsom, J.N.; Ujic, D.; Liu, F. PolyI: C Maternal Immune Activation on E9.5 Causes the Deregulation of Microglia and the Complement System in Mice, Leading to Decreased Synaptic Spine Density. Int. J. Mol. Sci. 2024, 25, 5480. [Google Scholar] [CrossRef]
- Schafer, D.P.; Heller, C.T.; Gunner, G.; Heller, M.; Gordon, C.; Hammond, T.; Wolf, Y.; Jung, S.; Stevens, B. Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression. eLife 2016, 5, e15224. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- Reed, M.D.; Yim, Y.S.; Wimmer, R.D.; Kim, H.; Ryu, C.; Welch, G.M.; Andina, M.; King, H.O.; Waisman, A.; Halassa, M.M.; et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 2020, 577, 249–253. [Google Scholar] [CrossRef]
- Casado, M.E.; Collado-Pérez, R.; Frago, L.M.; Barrios, V. Recent Advances in the Knowledge of the Mechanisms of Leptin Physiology and Actions in Neurological and Metabolic Pathologies. Int. J. Mol. Sci. 2023, 24, 1422. [Google Scholar] [CrossRef]
- Sun, J.; Gao, Y.; Yao, T.; Huang, Y.; He, Z.; Kong, X.; Yu, K.-J.; Wang, R.-T.; Guo, H.; Yan, J.; et al. Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons. Mol. Metab. 2016, 5, 882–891. [Google Scholar] [CrossRef]
- Prince, C.S.; Maloyan, A.; Myatt, L. Maternal obesity alters brain derived neurotrophic factor (BDNF) signaling in the placenta in a sexually dimorphic manner. Placenta 2017, 49, 55–63. [Google Scholar] [CrossRef]
- Wang, S.-D.; Wang, X.; Zhao, Y.; Xue, B.-H.; Wang, X.-T.; Chen, Y.-X.; Zhang, Z.-Q.; Tian, Y.-R.; Xie, F.; Qian, L.-J. Homocysteine-Induced Disturbances in DNA Methylation Contribute to Development of Stress-Associated Cognitive Decline in Rats. Neurosci. Bull. 2022, 38, 887–900. [Google Scholar] [CrossRef]
- Louwies, T.; Greenwood-Van Meerveld, B. Chronic stress increases DNA methylation of the GR promoter in the central nucleus of the amygdala of female rats. Neurogastroenterol. Motil. 2022, 34, e14377. [Google Scholar] [CrossRef]
- Verma, R.; Balhara, Y.P.S.; Gupta, C.S. Gender differences in stress response: Role of developmental and biological determinants. Ind. Psychiatry J. 2011, 20, 4–10. [Google Scholar] [CrossRef]
- Torrisi, S.A.; Rizzo, S.; Laudani, S.; Ieraci, A.; Drago, F.; Leggio, G.M. Acute stress alters recognition memory and AMPA/NMDA receptor subunits in a sex-dependent manner. Neurobiol. Stress 2023, 25, 100545. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kikkawa, S.; Kohase, M.; Miyake, K.; Seya, T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem. Biophys. Res. Commun. 2002, 293, 1364–1369. [Google Scholar] [CrossRef]
- Matsumoto, M.; Oshiumi, H.; Seya, T. Antiviral responses induced by the TLR3 pathway. Rev. Med. Virol. 2011, 21, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Burfeind, K.G.; Zhu, X.; Levasseur, P.R.; Michaelis, K.A.; Norgard, M.A.; Marks, D.L. TRIF is a key inflammatory mediator of acute sickness behavior and cancer cachexia. Brain Behav. Immun. 2018, 73, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Abak, A.; Fattahi, F.; Hussen, B.M.; Bahroudi, Z.; Shoorei, H.; Taheri, M. The interaction between miRNAs/lncRNAs and nuclear factor-κB (NF-κB) in human disorders. Biomed. Pharmacother. 2021, 138, 111519. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Chen, T.-Y.; Tsai, K.-J.; Lin, M.-W.; Hsu, C.-Y.; Wu, D.-C.; Tsai, E.-M.; Hsieh, T.-H. NF-κB/miR-18a-3p and miR-4286/BZRAP1 axis may mediate carcinogenesis in Helicobacter pylori-Associated gastric cancer. Biomed. Pharmacother. 2020, 132, 110869. [Google Scholar] [CrossRef]
- Jin, S.; Li, X.; Dai, Y.; Li, C.; Wang, D. NF-κB-mediated miR-650 plays oncogenic roles and activates AKT/ERK/NF-κB pathways by targeting RERG in glioma cells. Cell. Oncol. 2020, 43, 1035–1048. [Google Scholar] [CrossRef]
- Liu, B.S.; Cao, Y.; Huizinga, T.W.; Hafler, D.A.; Toes, R.E.M. TLR-mediated STAT3 and ERK activation controls IL-10 secretion by human B cells. Eur. J. Immunol. 2014, 44, 2121–2129. [Google Scholar] [CrossRef]
- Schnöder, L.; Hao, W.; Qin, Y.; Liu, S.; Tomic, I.; Liu, X.; Fassbender, K.; Liu, Y. Deficiency of Neuronal p38α MAPK Attenuates Amyloid Pathology in Alzheimer Disease Mouse and Cell Models through Facilitating Lysosomal Degradation of BACE1. J. Biol. Chem. 2016, 291, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Colié, S.; Sarroca, S.; Palenzuela, R.; Garcia, I.; Matheu, A.; Corpas, R.; Dotti, C.G.; Esteban, J.A.; Sanfeliu, C.; Nebreda, A.R. Neuronal p38α mediates synaptic and cognitive dysfunction in an Alzheimer’s mouse model by controlling β-amyloid production. Sci. Rep. 2017, 7, 45306. [Google Scholar] [CrossRef]
- Longhi, M.P.; Trumpfheller, C.; Idoyaga, J.; Caskey, M.; Matos, I.; Kluger, C.; Salazar, A.M.; Colonna, M.; Steinman, R.M. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 2009, 206, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Möller, I.; Michel, K.; Frech, N.; Burger, M.; Pfeifer, D.; Frommolt, P.; Veelken, H.; Thomas-Kaskel, A.-K. Dendritic cell maturation with poly(I:C)-based versus PGE2-based cytokine combinations results in differential functional characteristics relevant to clinical application. J. Immunother. 2008, 31, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Hameete, B.C.; Fernández-Calleja, J.M.; de Groot, M.W.; Oppewal, T.R.; Tiemessen, M.M.; Hogenkamp, A.; de Vries, R.B.; Groenink, L. The poly(I:C)-induced maternal immune activation model; a systematic review and meta-analysis of cytokine levels in the offspring. Brain Behav.Immun.—Health 2021, 11, 100192. [Google Scholar] [CrossRef]
- Shimizu, Y.; Tsukada, T.; Sakata-Haga, H.; Sakai, D.; Shoji, H.; Saikawa, Y.; Hatta, T. Exposure to Maternal Immune Activation Causes Congenital Unfolded Protein Response Defects and Increases the Susceptibility to Postnatal Inflammatory Stimulation in Offspring. J. Inflamm. Res. 2021, 14, 355–365. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Hu, S.; Qu, S.-T.; Lv, M.-D.; Wang, J.-J.; Liu, X.-T.; Yao, J.-H.; Ding, Y.-Y.; Xu, G.-Y. Inhibition of NKCC1 Ameliorates Anxiety and Autistic Behaviors Induced by Maternal Immune Activation in Mice. Curr. Issues Mol. Biol. 2024, 46, 1851–1864. [Google Scholar] [CrossRef]
- Zeng, X.; Fan, L.; Li, M.; Qin, Q.; Pang, X.; Shi, S.; Zheng, D.; Jiang, Y.; Wang, H.; Wu, L.; et al. Resveratrol regulates Thoc5 to improve maternal immune activation-induced autism-like behaviors in adult mouse offspring. J. Nutr. Biochem. 2024, 129, 109638. [Google Scholar] [CrossRef]
- Ding, S.; Hu, Y.; Luo, B.; Cai, Y.; Hao, K.; Yang, Y.; Zhang, Y.; Wang, X.; Ding, M.; Zhang, H.; et al. Age-related changes in neuroinflammation and prepulse inhibition in offspring of rats treated with Poly I:C in early gestation. Behav. Brain Funct. 2019, 15, 3. [Google Scholar] [CrossRef]
- Sun, W.; Mei, Y.; Li, X.; Yang, Y.; An, L. Maternal immune activation-induced proBDNF-mediated neural information processing dysfunction at hippocampal CA3-CA1 synapses associated with memory deficits in offspring. Front. Cell. Dev. Biol. 2022, 10, 1018586. [Google Scholar] [CrossRef]
- Vasudevan, S.O.; Russo, A.J.; Kumari, P.; Vanaja, S.K.; Rathinam, V.A. A TLR4-independent critical role for CD14 in intracellular LPS sensing. Cell Rep. 2022, 39, 110755. [Google Scholar] [CrossRef]
- Gray, P.; Michelsen, K.S.; Sirois, C.M.; Lowe, E.; Shimada, K.; Crother, T.R.; Chen, S.; Brikos, C.; Bulut, Y.; Latz, E.; et al. Identification of a novel human MD-2 splice variant that negatively regulates Lipopolysaccharide-induced TLR4 signaling. J. Immunol. 2010, 184, 6359–6366. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, N.J.; Vladimer, G.I.; Stenvik, J.; Orning, M.P.A.; Zeid-Kilani, M.V.; Bugge, M.; Bergstroem, B.; Conlon, J.; Husebye, H.; Hise, A.G.; et al. A role for the adaptor proteins TRAM and TRIF in toll-like receptor 2 signaling. J. Biol. Chem. 2015, 290, 3209–3222. [Google Scholar] [CrossRef]
- O’Keefe, M.E.; Dubyak, G.R.; Abbott, D.W. Post-translational control of NLRP3 inflammasome signaling. J. Biol. Chem. 2024, 300, 107386. [Google Scholar] [CrossRef]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Li, H.; Wang, F.; Yao, S. Toll-like receptor 4, A potential therapeutic target for multiple human diseases. Biomed. Pharmacother. 2023, 166, 115338. [Google Scholar] [CrossRef]
- Obadia, N.; Andrade, G.; Leardini-Tristão, M.; Albuquerque, L.; Garcia, C.; Lima, F.; Daleprane, J.; Castro-Faria-Neto, H.C.; Tibiriçá, E.; Estato, V. TLR4 mutation protects neurovascular function and cognitive decline in high-fat diet-fed mice. J. Neuroinflamm. 2022, 19, 104. [Google Scholar] [CrossRef]
- McLarnon, J.G.; Ryu, J.K. Relevance of abeta1-42 intrahippocampal injection as an animal model of inflamed Alzheimer’s disease brain. Curr. Alzheimer Res. 2008, 5, 475–480. [Google Scholar] [CrossRef]
- Miron, J.; Picard, C.; Frappier, J.; Dea, D.; Théroux, L.; Poirier, J. TLR4 Gene Expression and Pro-Inflammatory Cytokines in Alzheimer’s Disease and in Response to Hippocampal Deafferentation in Rodents. J. Alzheimers Dis. 2018, 63, 1547–1556. [Google Scholar] [CrossRef]
- Arnaboldi, F.; Sommariva, M.; Opizzi, E.; Rasile, M.; Camelliti, S.; Busnelli, M.; Menegola, E.; Di Renzo, F.; Menon, A.; Barajon, I. Expression of Toll-like receptors 4 and 7 in murine peripheral nervous system development. Ann. Anat.—Anat. Anz. 2020, 231, 151526. [Google Scholar] [CrossRef]
- Grasselli, C.; Ferrari, D.; Zalfa, C.; Soncini, M.; Mazzoccoli, G.; Facchini, F.A.; Marongiu, L.; Granucci, F.; Copetti, M.; Vescovi, A.L.; et al. Toll-like receptor 4 modulation influences human neural stem cell proliferation and differentiation. Cell Death Dis. 2018, 9, 280. [Google Scholar] [CrossRef]
- Connolly, M.G.; Potter, O.V.; Sexton, A.R.; Kohman, R.A. Effects of Toll-like receptor 4 inhibition on spatial memory and cell proliferation in male and female adult and aged mice. Brain Behav. Immun. 2021, 97, 383–393. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, R.; Yan, M.; Zhou, M.; Liu, H.; Wang, X.; Lu, C.; Li, Q.; Mo, Y.; Zhang, P.; et al. Sex-Specific Behavioral and Molecular Responses to Maternal Lipopolysaccharide-Induced Immune Activation in a Murine Model: Implications for Neurodevelopmental Disorders. Int. J. Mol. Sci. 2024, 25, 9885. [Google Scholar] [CrossRef]
- Lyamtsev, A.S.; Sentyabreva, A.V.; Tsvetkov, I.S.; Miroshnichenko, E.A.; Kosyreva, A.M. Morphological and Molecular Biological Changes in the Hippocampus and Prefrontal Cortex of the Brain of Newborn Male and Female Wistar Rats after LPS-Induced Activation of the Maternal Immune Response. Bull. Exp. Biol. Med. 2025, 178, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Purves-Tyson, T.D.; Weber-Stadlbauer, U.; Richetto, J.; A Rothmond, D.; Labouesse, M.A.; Polesel, M.; Robinson, K.; Weickert, C.S.; Meyer, U. Increased levels of midbrain immune-related transcripts in schizophrenia and in murine offspring after maternal immune activation. Mol. Psychiatry 2021, 26, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Loayza, M.; Lin, S.; Carter, K.; Ojeda, N.; Fan, L.-W.; Ramarao, S.; Bhatt, A.; Pang, Y. Maternal immune activation alters fetal and neonatal microglia phenotype and disrupts neurogenesis in mice. Pediatr. Res. 2023, 93, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Z.Z.; Cao, L.; Yang, Q.G.; Lu, Q.F.; Chen, G.H. Lipopolysaccharide exposure during late embryogenesis triggers and drives Alzheimer-like behavioral and neuropathological changes in CD-1 mice. Brain Behav. 2020, 10, e01546. [Google Scholar] [CrossRef]
- Kar, F.; Hacioglu, C.; Kar, E.; Donmez, D.B.; Kanbak, G. Probiotics ameliorates LPS induced neuroinflammation injury on Aβ 1-42, APP, γ-β secretase and BDNF levels in maternal gut microbiota and fetal neurodevelopment processes. Metab. Brain Dis. 2022, 37, 1387–1399. [Google Scholar] [CrossRef]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161. [Google Scholar] [CrossRef]
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170. [Google Scholar] [CrossRef]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Navascués, J.; Calvente, R.; Marín-Teva, J.L.; Cuadros, M.A. Entry, dispersion and differentiation of microglia in the developing central nervous system. An. Acad. Bras. Cienc. 2000, 72, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Barger, N.; Keiter, J.; Kreutz, A.; Krishnamurthy, A.; Weidenthaler, C.; Martínez-Cerdeño, V.; Tarantal, A.F.; Noctor, S.C. Microglia: An Intrinsic Component of the Proliferative Zones in the Fetal Rhesus Monkey (Macaca mulatta) Cerebral Cortex. Cereb. Cortex 2019, 29, 2782–2796. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Swinnen, N.; Smolders, S.; Avila, A.; Notelaers, K.; Paesen, R.; Ameloot, M.; Brône, B.; Legendre, P.; Rigo, J. Complex invasion pattern of the cerebral cortex bymicroglial cells during development of the mouse embryo. Glia 2013, 61, 150–163. [Google Scholar] [CrossRef]
- Squarzoni, P.; Oller, G.; Hoeffel, G.; Pont-Lezica, L.; Rostaing, P.; Low, D.; Bessis, A.; Ginhoux, F.; Garel, S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014, 8, 1271–1279. [Google Scholar] [CrossRef]
- Yang, I.; Han, S.J.; Kaur, G.; Crane, C.; Parsa, A.T. The role of microglia in central nervous system immunity and glioma immunology. J. Clin. Neurosci. 2010, 17, 6–10. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Germelli, L.; Da Pozzo, E.; Giacomelli, C.; Tremolanti, C.; Marchetti, L.; Wetzel, C.H.; Barresi, E.; Taliani, S.; Da Settimo, F.; Martini, C.; et al. De novo Neurosteroidogenesis in Human Microglia: Involvement of the 18 kDa Translocator Protein. Int. J. Mol. Sci. 2021, 22, 3115. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Nishihara, T.; Yorozuya, T.; Tanaka, J. Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems. Cells 2020, 9, 2132. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K.; Ruthazer, E.S. Microglial trogocytosis and the complement system regulate axonal pruning in vivo. eLife 2021, 10, e62167. [Google Scholar] [CrossRef]
- Andoh, M.; Koyama, R. Comparative Review of Microglia and Monocytes in CNS Phagocytosis. Cells 2021, 10, 2555. [Google Scholar] [CrossRef]
- Mattei, D.; Ivanov, A.; Hammer, J.; Ugursu, B.; Schalbetter, S.; Richetto, J.; Weber-Stadlbauer, U.; Mueller, F.; Scarborough, J.; A Wolf, S.; et al. Microglia undergo molecular and functional adaptations to dark and light phases in male laboratory mice. Brain Behav. Immun. 2024, 120, 571–583. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Kiani, A.A.; Elyasi, H.; Ghoreyshi, S.; Nouri, N.; Safarzadeh, A.; Nafari, A. Study on hypoxia-inducible factor and its roles in immune system. Immunol. Med. 2021, 44, 223–236. [Google Scholar] [CrossRef]
- Barclay, K.M.; Abduljawad, N.; Cheng, Z.; Kim, M.W.; Zhou, L.; Yang, J.; Rustenhoven, J.; Mazzitelli, J.A.; Smyth, L.C.; Kapadia, D.; et al. An inducible genetic tool to track and manipulate specific microglial states reveals their plasticity and roles in remyelination. Immunity 2024, 57, 1394–1412.e8. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef]
- Srinivasan, K.; Friedman, B.A.; Etxeberria, A.; Huntley, M.A.; van der Brug, M.P.; Foreman, O.; Paw, J.S.; Modrusan, Z.; Beach, T.G.; Serrano, G.E.; et al. Alzheimer’s Patient Microglia Exhibit Enhanced Aging and Unique Transcriptional Activation. Cell Rep. 2020, 31, 107843. [Google Scholar] [CrossRef]
- Seshadri, S.; Wolf, P.A.; Beiser, A.; Au, R.; McNulty, K.; White, R.; D’AGostino, R.B. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology 1997, 49, 1498–1504. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Mielke, M.M. Sex Differences in Alzheimer’s Disease. Neurol. Clin. 2023, 41, 343–358. [Google Scholar] [CrossRef]
- Baldereschi, M.; Di Carlo, A.; Rocca, W.A.; Vanni, P.; Maggi, S.; Perissinotto, E.; Grigoletto, F.; Amaducci, L.; Inzitari, D. Parkinson’s disease and parkinsonism in a longitudinal study: Two-fold higher incidence in men. ILSA Working Group. Neurol. J. 2000, 55, 1358–1363. [Google Scholar] [CrossRef]
- Elbaz, A.; Bower, J.H.; Maraganore, D.M.; McDonnell, S.K.; Peterson, B.J.; Ahlskog, J.; Schaid, D.J.; A Rocca, W. Risk tables for parkinsonism and Parkinson’s disease. J. Clin. Epidemiol. 2002, 55, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Roy-O’Reilly, M.; McCullough, L.D. Age and Sex Are Critical Factors in Ischemic Stroke Pathology. Endocrinology 2018, 159, 3120–3131. [Google Scholar] [CrossRef] [PubMed]
- Altemus, M.; Sarvaiya, N.; Neill Epperson, C. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014, 35, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef]
- McGrath, J.; Saha, S.; Welham, J.; El Saadi, O.; MacCauley, C.; Chant, D. A systematic review of the incidence of schizophrenia: The distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004, 2, 13. [Google Scholar] [CrossRef]
- Arnold, A.P. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009, 55, 570–578. [Google Scholar] [CrossRef]
- Gorski, R.A.; Gordon, J.H.; Shryne, J.E.; Southam, A.M. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978, 148, 333–346. [Google Scholar] [CrossRef]
- Joel, D.; McCarthy, M.M. Incorporating Sex As a Biological Variable in Neuropsychiatric Research: Where Are We Now and Where Should We Be? Neuropsychopharmacology 2017, 42, 379–385. [Google Scholar] [CrossRef]
- Joel, D.; Berman, Z.; Tavor, I.; Wexler, N.; Gaber, O.; Stein, Y.; Shefi, N.; Pool, J.; Urchs, S.; Margulies, D.S.; et al. Sex beyond the genitalia: The human brain mosaic. Proc. Natl. Acad. Sci. USA 2015, 112, 15468–15473. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; McCarthy, M.M. Steroid-induced sexual differentiation of the developing brain: Multiple pathways, one goal. J. Neurochem. 2008, 105, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Fan, Y.; Zhou, K.; Blomgren, K.; Harris, R.A. Uncovering sex differences of rodent microglia. J. Neuroinflamm. 2021, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Bordt, E.A.; Ceasrine, A.M.; Bilbo, S.D. Microglia and sexual differentiation of the developing brain: A focus on ontogeny and intrinsic factors. Glia 2020, 68, 1085–1099. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, L.; Li, H.; Tang, Y.; Wu, Z.; Chen, Y.; Wang, Y.; Qian, Q. Interactions between MAOA and SYP polymorphisms were associated with symptoms of attention-deficit/hyperactivity disorder in Chinese Han subjects. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 45–53. [Google Scholar] [CrossRef]
- van Rijn, S.; Swaab, H. Executive dysfunction and the relation with behavioral problems in children with 47,XXY and 47,XXX. Genes Brain Behav. 2015, 14, 200–208. [Google Scholar] [CrossRef]
- Casali, B.T.; Lin, L.; Benedict, O.; Zuppe, H.; Marsico, E.; Reed, E.G. Sex chromosomes and gonads modify microglial-mediated pathology in a mouse model of Alzheimer’s disease. J. Neuroinflamm. 2025, 22, 81. [Google Scholar] [CrossRef]
- Pinheiro, I.; Dejager, L.; Libert, C. X-chromosome-located microRNAs in immunity: Might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays 2011, 33, 791–802. [Google Scholar] [CrossRef]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef]
- Sharon, A.; Erez, H.; Spira, M.E. Significant Sex Differences in the Efficacy of the CSF1R Inhibitor-PLX5622 on Rat Brain Microglia Elimination. Pharm. Basel Switz. 2022, 15, 569. [Google Scholar] [CrossRef]
- Prengel, T.M.; Brunne, B.; Habiballa, M.; Rune, G.M. Sexually differentiated microglia and CA1 hippocampal synaptic connectivity. J. Neuroendocrinol. 2023, 35, e13276. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Ince, L.M.; Darling, J.S.; Sanchez, K.; Bell, K.S.; Melbourne, J.K.; Davis, L.K.; Nixon, K.; Gaudet, A.D.; Fonken, L. Sex differences in microglia function in aged rats underlie vulnerability to cognitive decline. Brain Behav. Immun. 2023, 114, 438–452. [Google Scholar] [CrossRef]
- Guneykaya, D.; Ugursu, B.; Logiacco, F.; Popp, O.; Feiks, M.A.; Meyer, N.; Wendt, S.; Semtner, M.; Cherif, F.; Gauthier, C.; et al. Sex-specific microglia state in the Neuroligin-4 knock-out mouse model of autism spectrum disorder. Brain Behav. Immun. 2023, 111, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Delorme, T.C.; Arcego, D.M.; Penichet, D.; O’tOole, N.; Huebener, N.; Silveira, P.P.; Srivastava, L.K.; Cermakian, N. Large-scale effects of prenatal inflammation and early life circadian disruption in mice: Implications for neurodevelopmental disorders. Brain Behav. Immun. 2025, 127, 409–422. [Google Scholar] [CrossRef]
- Liu, L.L.; Li, J.M.; Su, W.J.; Wang, B.; Jiang, C.L. Sex differences in depressive-like behaviour may relate to imbalance of microglia activation in the hippocampus. Brain Behav. Immun. 2019, 81, 188–197. [Google Scholar] [CrossRef]
- Minakova, E.; Warner, B.B. Maternal immune activation, central nervous system development and behavioral phenotypes. Birth Defects Res. 2018, 110, 1539–1550. [Google Scholar] [CrossRef]
- de Cossío, L.F.; Guzmán, A.; van der Veldt, S.; Luheshi, G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017, 63, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Ruiz, R.; Trujillo-Villarreal, L.A.; Montalvo-Martínez, L.; Mercado-Gómez, O.F.; Arriaga-Ávila, V.; Garza-Ocañas, L.; Ortiz-López, R.; Garza-Villarreal, E.A.; Guevara-Guzmán, R.; Camacho-Morales, A. MCP-1 Signaling Disrupts Social Behavior by Modulating Brain Volumetric Changes and Microglia Morphology. Mol. Neurobiol. 2022, 59, 932–949. [Google Scholar] [CrossRef] [PubMed]
- Dutra, M.L.; Dias, P.; Freiberger, V.; Ventura, L.; Comim, C.M.; Martins, D.F.; Bobinski, F. Maternal immune activation induces autism-like behavior and reduces brain-derived neurotrophic factor levels in the hippocampus and offspring cortex of C57BL/6 mice. Neurosci. Lett. 2023, 793, 136974. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sugihara, G.; Ouchi, Y.; Nakamura, K.; Futatsubashi, M.; Takebayashi, K.; Yoshihara, Y.; Omata, K.; Matsumoto, K.; Tsuchiya, K.J.; et al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry 2013, 70, 49–58. [Google Scholar] [CrossRef]
- Morgan, J.T.; Chana, G.; Pardo, C.A.; Achim, C.; Semendeferi, K.; Buckwalter, J.; Courchesne, E.; Everall, I.P. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry 2010, 68, 368–376. [Google Scholar] [CrossRef]
- Tetreault, N.A.; Hakeem, A.Y.; Jiang, S.; Williams, B.A.; Allman, E.; Wold, B.J.; Allman, J.M. Microglia in the cerebral cortex in autism. J. Autism Dev. Disord. 2012, 42, 2569–2584. [Google Scholar] [CrossRef]
- Zantomio, D.; Chana, G.; Laskaris, L.; Testa, R.; Everall, I.; Pantelis, C.; Skafidas, E. Convergent evidence for mGluR5 in synaptic and neuroinflammatory pathways implicated in ASD. Neurosci. Biobehav. Rev. 2015, 52, 172–177. [Google Scholar] [CrossRef]
- Sciara, A.N.; Beasley, B.; Crawford, J.D.; Anderson, E.P.; Carrasco, T.; Zheng, S.; Ordway, G.A.; Chandley, M.J. Neuroinflammatory Gene Expression Alterations in Anterior Cingulate Cortical White and Gray Matter of Males with Autism Spectrum Disorder. Autism Res. 2020, 13, 870–884. [Google Scholar] [CrossRef]
- Tsilioni, I.; Patel, A.B.; Pantazopoulos, H.; Berretta, S.; Conti, P.; Leeman, S.E.; Theoharides, T.C. IL-37 is increased in brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin. Proc. Natl. Acad. Sci. USA 2019, 116, 21659–21665. [Google Scholar] [CrossRef]
- Sarn, N.; Thacker, S.; Lee, H.; Eng, C. Germline nuclear-predominant Pten murine model exhibits impaired social and perseverative behavior, microglial activation, and increased oxytocinergic activity. Mol. Autism 2021, 12, 41. [Google Scholar] [CrossRef]
- Meng, J.; Han, L.; Zheng, N.; Wang, T.; Xu, H.; Jiang, Y.; Wang, Z.; Liu, Z.; Zheng, Q.; Zhang, X.; et al. Microglial Tmem59 Deficiency Impairs Phagocytosis of Synapse and Leads to Autism-Like Behaviors in Mice. J. Neurosci. 2022, 42, 4958–4979. [Google Scholar] [CrossRef]
- Lin, P.; Nicholls, L.; Assareh, H.; Fang, Z.; Amos, T.G.; Edwards, R.J.; Assareh, A.A.; Voineagu, I. Transcriptome analysis of human brain tissue identifies reduced expression of complement complex C1Q Genes in Rett syndrome. BMC Genom. 2016, 17, 427. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Chen, Y.-W.; Tsai, S.-F.; Wu, S.-N.; Shih, Y.-H.; Jiang-Shieh, Y.-F.; Yang, T.-T.; Kuo, Y.-M. Estrogen ameliorates microglial activation by inhibiting the Kir2.1 inward-rectifier K(+) channel. Sci. Rep. 2016, 6, 22864. [Google Scholar] [CrossRef] [PubMed]
- Loiola, R.A.; Wickstead, E.S.; Solito, E.; McArthur, S. Estrogen Promotes Pro-resolving Microglial Behavior and Phagocytic Cell Clearance Through the Actions of Annexin A1. Front. Endocrinol. 2019, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Kuşman, A. Aetiology and Risk Factors of Schizophrenia. In New Approaches to the Management and Diagnosis of Schizophrenia; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef]
- Birnbaum, R.; Weinberger, D.R. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 2017, 18, 727–740. [Google Scholar] [CrossRef]
- Jahangir, M.; Zhou, J.S.; Lang, B.; Wang, X.P. GABAergic System Dysfunction and Challenges in Schizophrenia Research. Front. Cell Dev. Biol. 2021, 9, 663854. [Google Scholar] [CrossRef]
- Sonnenschein, S.F.; Gomes, F.V.; Grace, A.A. Dysregulation of Midbrain Dopamine System and the Pathophysiology of Schizophrenia. Front. Psychiatry 2020, 11, 613. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhang, W.W.; Chen, F.; Hu, S.S.; Jiang, H.Y. Maternal infection exposure and the risk of psychosis in the offspring: A systematic review and meta-analysis. J. Psychiatr. Res. 2021, 135, 28–36. [Google Scholar] [CrossRef]
- Wolff, A.R.; Bilkey, D.K. Prenatal immune activation alters hippocampal place cell firing characteristics in adult animals. Brain Behav. Immun. 2015, 48, 232–243. [Google Scholar] [CrossRef]
- Crum, W.R.; Sawiak, S.J.; Chege, W.; Cooper, J.D.; Williams, S.C.R.; Vernon, A.C. Evolution of structural abnormalities in the rat brain following in utero exposure to maternal immune activation: A longitudinal in vivo MRI study. Brain Behav. Immun. 2017, 63, 50–59. [Google Scholar] [CrossRef]
- Vasistha, N.A.; Pardo-Navarro, M.; Gasthaus, J.; Weijers, D.; Müller, M.K.; García-González, D.; Malwade, S.; Korshunova, I.; Pfisterer, U.; von Engelhardt, J.; et al. Maternal inflammation has a profound effect on cortical interneuron development in a stage and subtype-specific manner. Mol. Psychiatry 2020, 25, 2313–2329. [Google Scholar] [CrossRef]
- Dickerson, D.D.; Overeem, K.A.; Wolff, A.R.; Williams, J.M.; Abraham, W.C.; Bilkey, D.K. Association of aberrant neural synchrony and altered GAD67 expression following exposure to maternal immune activation, a risk factor for schizophrenia. Transl. Psychiatry 2014, 4, e418. [Google Scholar] [CrossRef]
- Hanson, K.L.; Grant, S.E.; Funk, L.H.; Schumann, C.M.; Bauman, M.D. Impact of Maternal Immune Activation on Nonhuman Primate Prefrontal Cortex Development: Insights for Schizophrenia. Biol. Psychiatry 2022, 92, 460–469. [Google Scholar] [CrossRef]
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M. Major depressive disorder. Nat. Rev. Dis. Primers 2023, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019, A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Jama Patient Page Screening for Depression. JAMA 2016, 315, 428. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Cruceanu, C.; Chen, G.G.; Turecki, G.; Mechawar, N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014, 42, 50–59. [Google Scholar] [CrossRef]
- Steiner, J.; Walter, M.; Gos, T.; Guillemin, G.J.; Bernstein, H.-G.; Sarnyai, Z.; Mawrin, C.; Brisch, R.; Bielau, H.; zu Schwabedissen, L.M.; et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: Evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflamm. 2011, 8, 94. [Google Scholar] [CrossRef]
- Holmes, S.E.; Hinz, R.; Conen, S.; Gregory, C.J.; Matthews, J.C.; Anton-Rodriguez, J.M.; Gerhard, A.; Talbot, P.S. Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biol. Psychiatry 2018, 83, 61–69. [Google Scholar] [CrossRef]
- Li, H.; Sagar, A.P.; Kéri, S. Translocator protein (18kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Wei, J.; Dilley, G.; Pittman, S.D.; Meltzer, H.Y.; Overholser, J.C.; Roth, B.L.; A Stockmeier, C. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 1999, 45, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Bowley, M.P.; Drevets, W.C.; Ongür, D.; Price, J.L. Low glial numbers in the amygdala in major depressive disorder. Biol. Psychiatry 2002, 52, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Cotter, D.; Mackay, D.; Landau, S.; Kerwin, R.; Everall, I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry 2001, 58, 545–553. [Google Scholar] [CrossRef]
- Schnieder, T.P.; Trencevska, I.; Rosoklija, G.; Stankov, A.; Mann, J.J.; Smiley, J.; Dwork, A.J. Microglia of prefrontal white matter in suicide. J. Neuropathol. Exp. Neurol. 2014, 73, 880–890. [Google Scholar] [CrossRef]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015, 72, 268–275. [Google Scholar] [CrossRef]
- Quagliato, L.A.; de Matos, U.; Nardi, A.E. Maternal immune activation generates anxiety in offspring: A translational meta-analysis. Transl. Psychiatry 2021, 11, 245. [Google Scholar] [CrossRef]
- Sheng, J.A.; Tobet, S.A. Maternal immune activation with toll-like receptor 7 agonist during mid-gestation alters juvenile and adult developmental milestones and behavior. J. Neuroendocrinol. 2024, 36, e13417. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Zaltieri, M.; Grigoletto, J.; Longhena, F.; Navarria, L.; Favero, G.; Castrezzati, S.; Colivicchi, M.A.; Della Corte, L.; Rezzani, R.; Pizzi, M.; et al. α-synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J. Cell Sci. 2015, 128, 2231–2243. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.; Kim, S.; Jung, U.J.; Kim, S.R. Alpha-Synuclein and Microglia in Parkinson’s Disease: From Pathogenesis to Therapeutic Prospects. J. Clin. Med. 2024, 13, 7243. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Condello, C.; Yuan, P.; Schain, A.; Grutzendler, J. Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat. Commun. 2015, 6, 6176. [Google Scholar] [CrossRef]
- Shimizu, Y.; Sakata-Haga, H.; Saikawa, Y.; Hatta, T. Influence of Immune System Abnormalities Caused by Maternal Immune Activation in the Postnatal Period. Cells 2023, 12, 741. [Google Scholar] [CrossRef]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef]
- Schaafsma, W.; Basterra, L.B.; Jacobs, S.; Brouwer, N.; Meerlo, P.; Schaafsma, A.; Boddeke, E.W.; Eggen, B.J. Maternal inflammation induces immune activation of fetal microglia and leads to disrupted microglia immune responses, behavior, and learning performance in adulthood. Neurobiol. Dis. 2017, 106, 291–300. [Google Scholar] [CrossRef]
- Zhang, X.; Kracht, L.; Lerario, A.M.; Dubbelaar, M.L.; Brouwer, N.; Wesseling, E.M.; Boddeke, E.W.G.M.; Eggen, B.J.L.; Kooistra, S.M. Epigenetic regulation of innate immune memory in microglia. J. Neuroinflamm. 2022, 19, 111. [Google Scholar] [CrossRef]
- Soteros, B.M.; Sia, G.M. Complement and microglia dependent synapse elimination in brain development. WIREs Mech. Dis. 2022, 14, e1545. [Google Scholar] [CrossRef]
- Sunwoo, J.; Jeon, D.; Lee, S.; Moon, J.; Yu, J.; Park, D.; Bae, J.; Lee, D.Y.; Kim, S.; Jung, K.; et al. Maternal immune activation alters brain microRNA expression in mouse offspring. Ann. Clin. Transl. Neurol. 2018, 5, 1264–1276. [Google Scholar] [CrossRef]


| Impact | Type of Study | Effect Size | Population | Reference |
|---|---|---|---|---|
| 1957 influenza A2 epidemic. | Retrospective cohort study | The risk of schizophrenia in offspring was doubled in those who were in their second trimester of pregnancy during the influenza A2 epidemic in Finland in 1957 | Children who were in utero during the 1957 influenza A2 epidemic in Finland | [15] |
| Signs of threatened miscarriage, premature birth, respiratory viral infections, moderate and severe illnesses, prenatal exposure to alcohol and nicotine, neonatal seizures, asphyxia, severe illnesses during pregnancy | Epidemiological, case–control | ADHD with a variety of factors (odds ratio, OR ≈ 2) | 486 children (200 with ADHD and 286 healthy), aged 6–11 years | [34] |
| Genitourinary infections and preeclampsia in the mother | Retrospective cohort study | Genitourinary infections in the mother were significantly associated with an increased risk of ADHD in the child (odds ratio, OR ≈ 1.3). Preeclampsia was also an independent risk factor for ADHD (OR ≈ 1.4) | More than 84,000 children born between 1996 and 2002; children diagnosed with ADHD between the ages of 5 and 11. Medicaid billing data for pregnant women and their children in South Carolina | [35] |
| Family socioeconomic status (SES) and risk of developing Tourette’s syndrome (TS) and chronic tic disorder (CTD) | Prospective cohort study | Children from families with low SES had a 2–3 times higher risk of developing TS or CTD | More than 6800 children observed from birth to adolescence. UK, from the Avon Longitudinal Study of Parents and Children | [49] |
| Autoimmune diseases in mothers | Population-based, nested case–control | Risk of ADHD Maternal disease Multiple sclerosis (OR)1.8 95% CI 1.2–2.5 Rheumatoid arthritis(OR)1.7 95% CI 1.5–1.9 Type 1 diabetes mellitus (OR)1.6 95% CI 1.3–2.0 Bronchial asthma (OR)1.5 95% CI 1.4–1.6 Hypothyroidism (OR)1.2 95% CI 1–1.4 | Children treated for ADHD in 2004–2012 (n = 47,944); control group—other children (n = 2,274,713), 1967–2008, Norwegian national registries | [41] |
| Autoimmune diseases in mothers | Population cohort study | Children whose mothers had any autoimmune disease had a 30% higher risk of developing CD (hazard ratio HR ≈ 1.3). rheumatoid arthritis (HR ≈ 1.6), systemic lupus erythematosus (HR ≈ 2.0) | More than 2.1 million children born in Denmark between 1978 and 2007. | [38] |
| Viral, bacterial, TORCH infections | Meta-analysis | Overall relative risk of developing ASD (RR): 1.13 (95% CI: 1.03–1.24), Bacterial infections were associated with a higher risk of developing ASD (RR = 1.19) than viral infections (RR = 1.09). | 15 studies with a total of more than 40,000 cases of ASD (PubMed, Embase, Web of Science (before January 2016)) | [23] |
| The mother’s body mass index (BMI) before pregnancy | Meta-analysis | Maternal overweight was associated with a moderately increased risk of ASD in the child: Relative risk (RR) = 1.28 (95% CI: 1.16–1.41), Maternal obesity increased the risk: RR = 1.36 (95% CI: 1.08–1.71) | 6 cohort studies (5 prospective and 1 retrospective) and 1 case–control study. There were 8403 cases and 509,167 participants. While 5 studies were conducted in the US, 2 studies were conducted in Europe (Norway and Sweden) | [48] |
| Socioeconomic disadvantage (SED) and ADHD | Systematic review | 35 out of 42 studies—children from families with low SES were, on average, 1.85–2.21 times more likely to have ADHD than children from families with high SES | 42 studies; studies with valid ADHD diagnosis and SES measurement (income, education, occupation, marital status) | [50] |
| Autoimmune diseases in the mother | A population-based, multigenerational, family cohort study | Patients with OCD and SAD/HTR had high comorbidity with AID: 43% and 36%, respectively. The risk of AID was increased in first-degree relatives (parents, siblings) of patients with OCD and SAD/HTR | 7,465,455 individuals born between 1940 and 2007; Swedish National Patient Register; 30,082 cases of obsessive–compulsive disorder (OCD) and 7292 cases of TD/CTD | [37] |
| Prenatal: Maternal depression, Gestational diabetes, Hypertension Perinatal: Cesarean section, Premature birth, Low birth weight Neonatal: Neonatal infection, Seizures in the neonatal period; hypoxia | Retrospective cohort study | Factors reliably associated with an increased risk of ASD: Prenatal: Maternal depression (OR ≈ 1.4) Gestational diabetes (OR ≈ 1.2) Hypertension (OR ≈ 1.3) Perinatal: Cesarean section (OR ≈ 1.2) Premature birth (OR ≈ 1.5) Low birth weight (OR ≈ 1.3) Neonatal: Neonatal infection (OR ≈ 1.6) Seizures in the neonatal period (OR ≈ 1.8) Hypoxia (OR ≈ 1.4) | 8760 children with ASD and more than 26,280 children without ASD (control group), medical records from the US Department of Defense health care system | [43] |
| Validity of proposed environmental risk factors and biomarkers for autism spectrum disorder (ASD). | Umbrella review of 46 meta-analyses | Maternal overweight RR = 1.28 95% CI 1.19–1.36. Use of selective serotonin reuptake inhibitors (SSRIs) during pregnancy OR = 1.84 95% CI 1.60–2.11 | 67 environmental factors (544,212 cases of ASD; 81,708,787 participants). 52 biomarkers (15,614 cases; 15,417 controls). Sources: PubMed, Embase, and Cochrane until October 17, 2018 | [46] |
| Asthma risk ADHD | Population cohort study | Asthma in mother, HR 1.41 95% CI 36–1.46 Asthma in father, HR 1.13 95% CI 1.08–1.18 | Danish national registries, 961,202 live-born singleton children (1997–2012). Follow-up period: until 2016 or until ADHD diagnosis | [44] |
| Asthma | Population-based and family nested case–control study | Maternal asthma was associated with an increased risk of ADHD in children: OR = 1.43 (95% CI: 1.38–1.49). Asthma in the father was also associated with ASD, but to a lesser extent: OR = 1.17 (95% CI: 1.11–1.23) | 1,579,263 children born in Sweden between 1992 and 2007, 22,894 cases of ASD and 228,940 controls, as well as relatives with varying degrees of kinship (full and half siblings, cousins) | [42] |
| Association between maternal prenatal stress and risk of (ASD)and (ADHD) in offspring | Systematic review and meta-analysis | ADHD: combined odds ratio (OR) = 1.64 (95% CI: 1.15–2.34), heterogeneity I2 = 90%. ADHD: OR = 1.72 (95% CI: 1.27–2.34), I2 = 85% | 15 studies on ASD, 12 studies on ADHD. PubMed, PsycINFO, Web of Science, EMBASE, SCOPUS | [51] |
| Overweight/obesity before pregnancy—risk of developing ADHD | Systematic review, meta-analysis | ADHD Meta-analysis: Overweight: RR = 1.31 (95% CI: 1.25–1.38) Obesity: RR = 1.92 (95% CI: 1.84–2.00), Cohort study: Unadjusted models: HR (overweight) = 1.30 HR (obesity) = 1.92 Adjusted models: HR (overweight) = 1.21 HR (obese) = 1.60 | 8 cohort studies covering 784,804 mother–child pairs; 971,501 children born in Sweden between 1992 and 2004 | [47] |
| Autoimmune diseases in the mother—risk of developing ADHD | Cohort | Cohort analysis: Any autoimmune disease: HR = 1.30 (95% CI: 1.15–1.46) Type 1 diabetes mellitus: HR = 2.23 (95% CI: 1.66–3.00) Psoriasis: HR = 1.66 (95% CI: 1.02–2.70) Rheumatic fever/carditis: HR = 1.75 (95% CI: 1.06–2.89) Meta-analysis: Any autoimmune disease: HR = 1.20 (95% CI: 1.03–1.38) Type 1 diabetes mellitus: HR = 1.53 (95% CI: 1.27–1.85) Hyperthyroidism: HR = 1.15 (95% CI: 1.06–1.26) Psoriasis: HR = 1.31 (95% CI: 1.10–1.56) | Cohort study: 63,050 children born in New South Wales (Australia) between 2000 and 2010. Meta-analysis: 5 studies included, including the current one | [40] |
| Species | Sex and Age | MIA Inducer | Dose and Gestational Period of the Inducer | The Primal Target Receptor of the Inducer | Outcomes in MIA Offspring Relative to Control | Reference |
|---|---|---|---|---|---|---|
| CD-1 mice | Male offspring, 2, 3, 6, 8, and 12 weeks old | PolyI:C | 20 mg/kg IP, 9.5 GD | TLR3 |
| [54] |
| C57BL/6J mice | Offspring sex not specified; 4 weeks old | PolyI:C | 20 mg/kg IP, 12.5 GD | TLR3 |
| [78] |
| C57BL6/N mice | Male and female offspring, PND120 | PolyI:C | 5 mg/kg IV, 17 GD | TLR3 |
| [97] |
| Sprague–Dawley rats | Male offspring, 6 and 8 weeks old | PolyI:C | 10 mg/kg IV, 9 GD | TLR3 |
| [80] |
| Sprague-Dawley rats | Offspring sex not specified; 8 weeks old | PolyI:C | 4 mg/kg IV, 15 GD | TLR3 |
| [81] |
| C57BL/6J mice | Male and female offspring; PND0 and 8 weeks old | LPS | 60 μg/kg IP, 12.5 GD | TLR4 |
| [95] |
| C57BL/6J mice | Male and female offspring, E15.5, PND1, PND4, and PND21 | LPS | 50 μg/kg IP, 12.5 GD | TLR4 |
| [98] |
| CD-1 mice | Male and female offspring; 1, 6, 12, 18, and 22 months old | LPS | 25 or 50 μg/kg IP, 15-17 GD | TLR4 |
| [99] |
| Wistar rats | Offspring sex not specified; PND0 | LPS | 100 μg/kg IP, 17 GD | TLR4 |
| [100] |
| Wistar rats | Male and female offspring; PND0 | LPS | 100 μg/kg IP, 17 GD | TLR4 |
| [96] |
| Species | Age | Brain Region | Impact | Sex Differences in Microglia | Reference |
| C57BL/6J mice | 20 weeks | Hippocampus (CA3) | Neuroligin-4 gene knockout (Nlgn4−/−) | Males—increased expression of genes and proteins associated with antigen-presenting function MHC-II, CD74, purinergic receptors P2ry12 and P2rx4. Higher microglia density Females—higher expression of genes associated with interferon signaling Ifit1, Ifit3, immune regulation Cxcl10 | [148] |
| C57BL/6J mice | 7 weeks | Hippocampus (CA1 and dentate gyrus), prefrontal cortex | MIA (poly I:C + 24 h constant lighting) | Males—1055 differentially expressed (DE) transcripts vs. physiological control (590 down, 465 up). Male hippocampal transcriptome responds more strongly to MIA than female Females—very few DE; noticeable changes only with poly I:C + constant lightning combination: 83 DE (70 down, 13 up) | [149] |
| Sprague-Dawley rats | 8 weeks | Hippocampus (CA1, CA3, and dentate gyrus) | Chronic unpredictable mild stress (CUMS) | Females—higher microglial activation: increase in the number of Iba1+ cells with amoeboid morphology. Il1β and Tnfα mRNA expression was significantly higher in females after stress than in males Males—microglial activation was less pronounced | [150] |
| Sprague-Dawley rats | 12 weeks | Hippocampus, cerebral cortex, amygdala, striatum, cerebellum, olfactory bulb | Colony-stimulating factor 1 receptor (CSF1R) inhibitor PLX5622 | Males—the initial density of microglia is higher compared to females After PLX5622 administration microglial density decreased by 40–60% Females—after PLX5622 reduction in microglial density was significantly higher than in males | [144] |
| Sprague-Dawley rats | 24 months | Hippocampus (CA1 and dentate gyrus), prefrontal cortex | MIA (LPS) | Males—more pronounced M1-like activation phenotype; increased expression of pro-inflammatory genes in microglia Il1b, Tnf, Ccl2, increased markers of microglia activation Iba1, CD68 Females—increased expression of genes associated with inflammation, regulation, and repair Il10, Arg1 | [146] |
| Wistar rats | PND1 | Hippocampus (CA1, CA3, and dentate gyrus); prefrontal cortex | MIA (LPS) | Males—increase in the total number of microglial cells; increased expression of Hif1α (neuroprotective effect) Females—decrease in the number of ramified microglial cells Both sexes—increase in Nfκb in the prefrontal cortex. | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyamtsev, A.S.; Sentyabreva, A.V.; Kosyreva, A.M. The Role of Prenatal Microglial Activation and Its Sex Differences in the Development of Neuropsychiatric Disorders and Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 9250. https://doi.org/10.3390/ijms26189250
Lyamtsev AS, Sentyabreva AV, Kosyreva AM. The Role of Prenatal Microglial Activation and Its Sex Differences in the Development of Neuropsychiatric Disorders and Neurodegenerative Diseases. International Journal of Molecular Sciences. 2025; 26(18):9250. https://doi.org/10.3390/ijms26189250
Chicago/Turabian StyleLyamtsev, Alexander Sergeevich, Alexandra Vladislavovna Sentyabreva, and Anna Mikhailovna Kosyreva. 2025. "The Role of Prenatal Microglial Activation and Its Sex Differences in the Development of Neuropsychiatric Disorders and Neurodegenerative Diseases" International Journal of Molecular Sciences 26, no. 18: 9250. https://doi.org/10.3390/ijms26189250
APA StyleLyamtsev, A. S., Sentyabreva, A. V., & Kosyreva, A. M. (2025). The Role of Prenatal Microglial Activation and Its Sex Differences in the Development of Neuropsychiatric Disorders and Neurodegenerative Diseases. International Journal of Molecular Sciences, 26(18), 9250. https://doi.org/10.3390/ijms26189250






