High-Fat Diet Alters Behavior and Hippocampal Gene Expression
Abstract
1. Introduction
2. Results
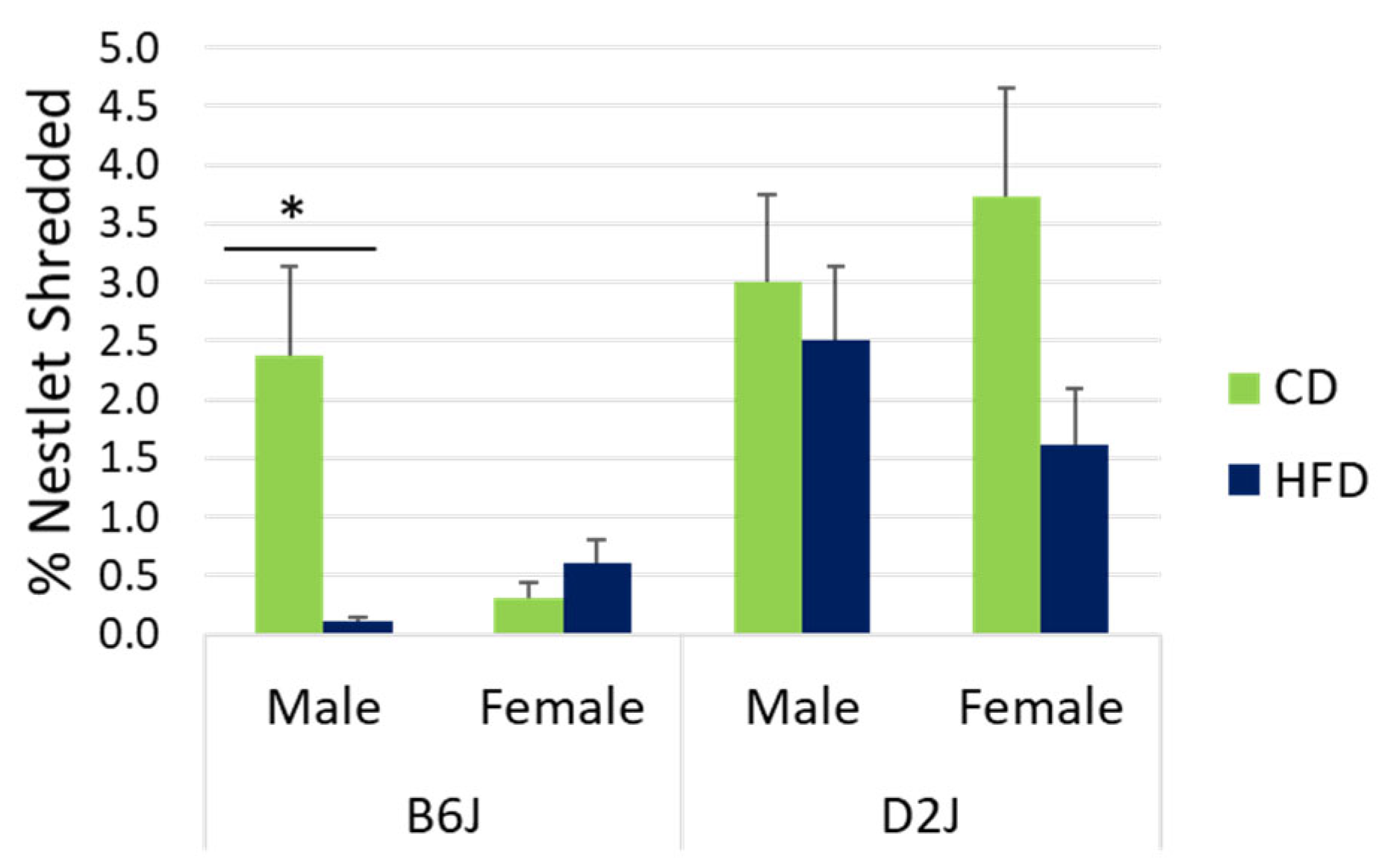
2.1. Male B6J Mice Fed a HFD Had Significantly Lower Levels of Nestlet Shredding
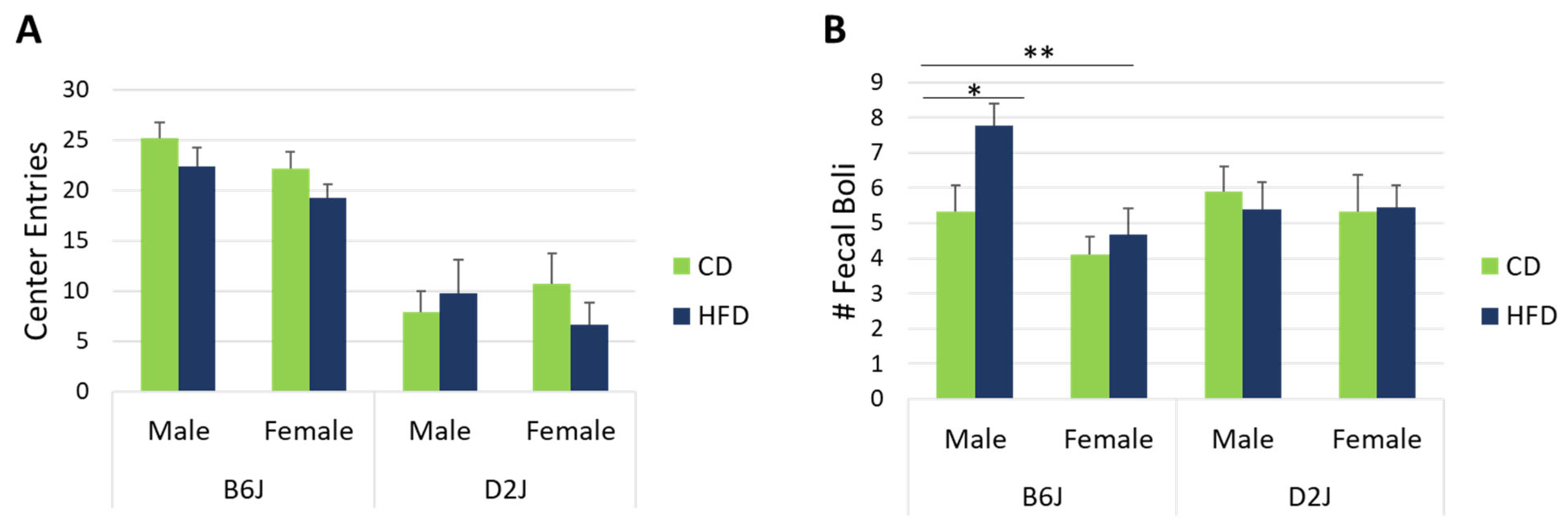
2.2. HFD Did Not Impact Center Entries in the Open Field but Did Impact Fecal Boli in Male B6J Mice
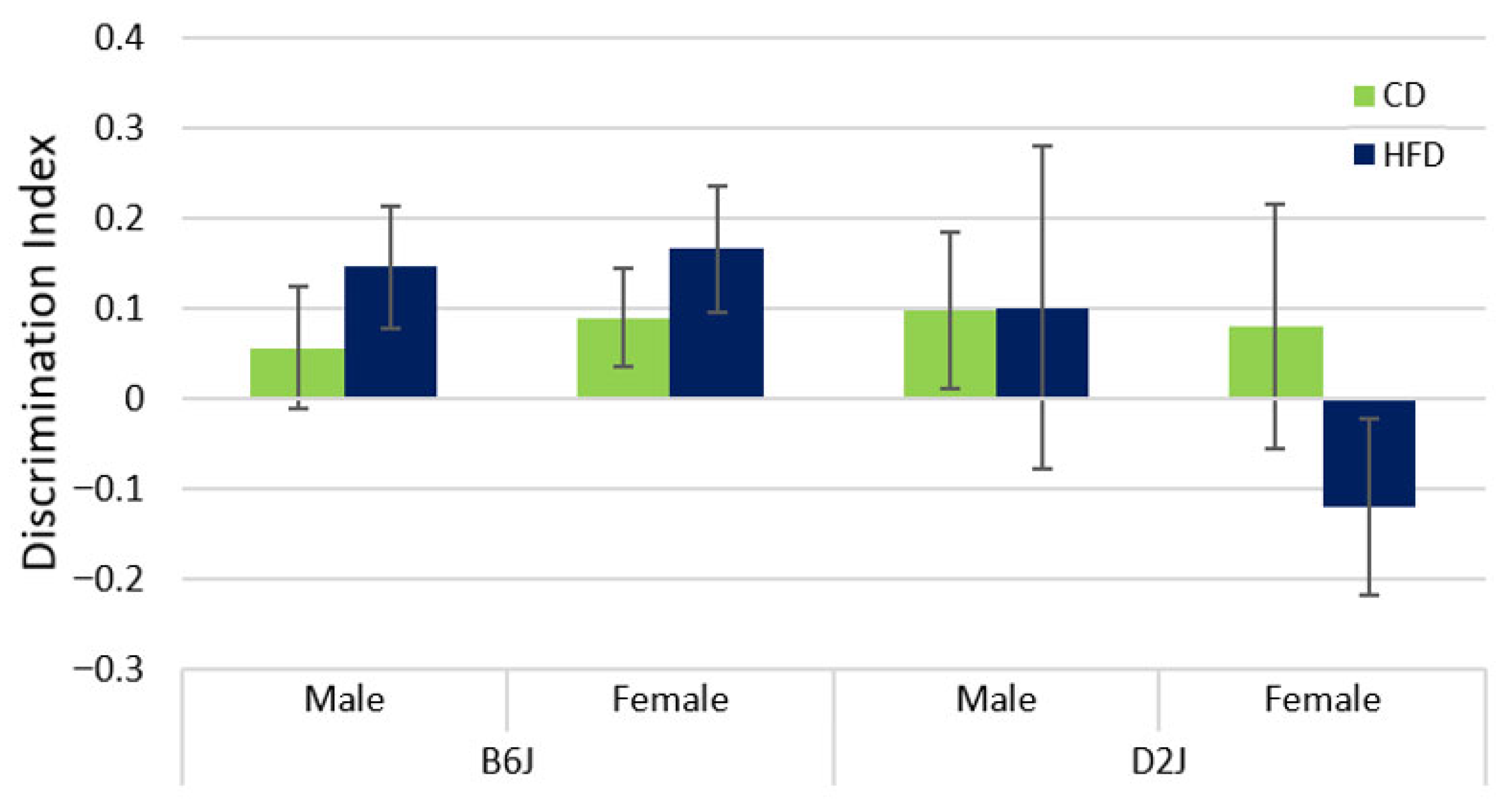
2.3. HFD Did Not Impact Memory as Assessed by Novel Object Recognition
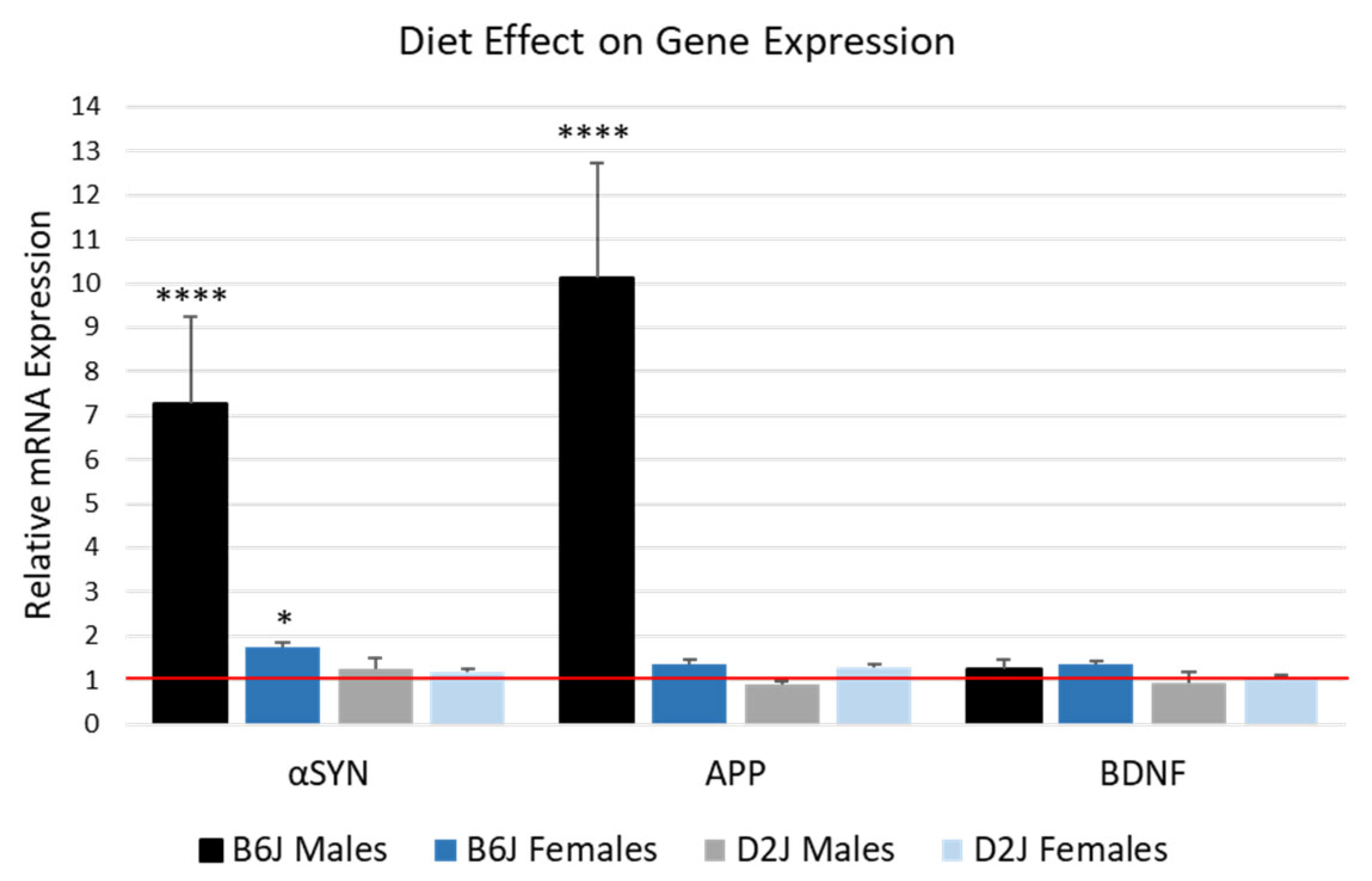
2.4. HFD Significantly Upregulated αSYN and APP mRNA Expression in the Hippocampus of B6J Male Mice
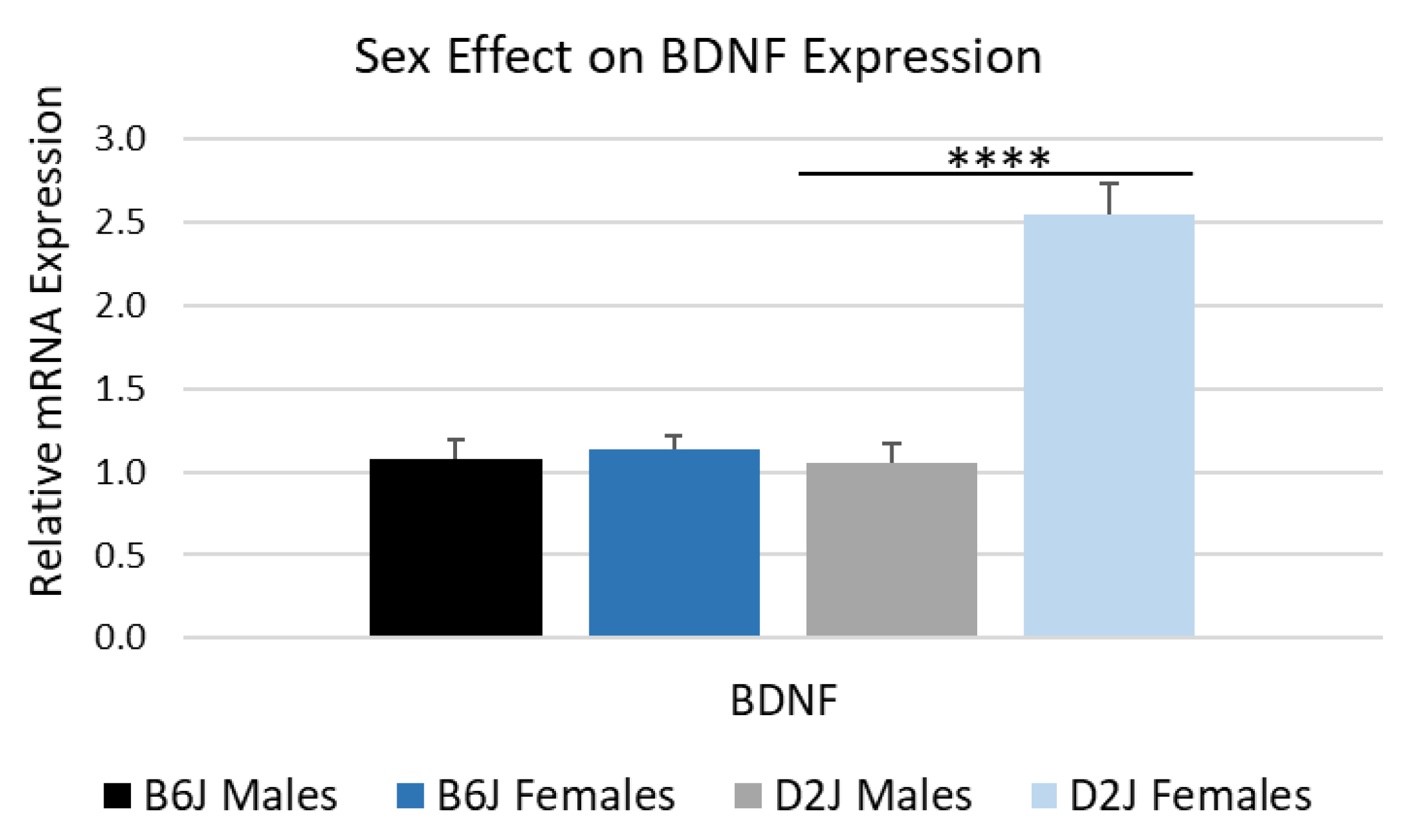
2.5. Female D2J Mice Express Significantly More BDNF in the Hippocampus Compared to D2J Males
3. Discussion
4. Materials and Methods
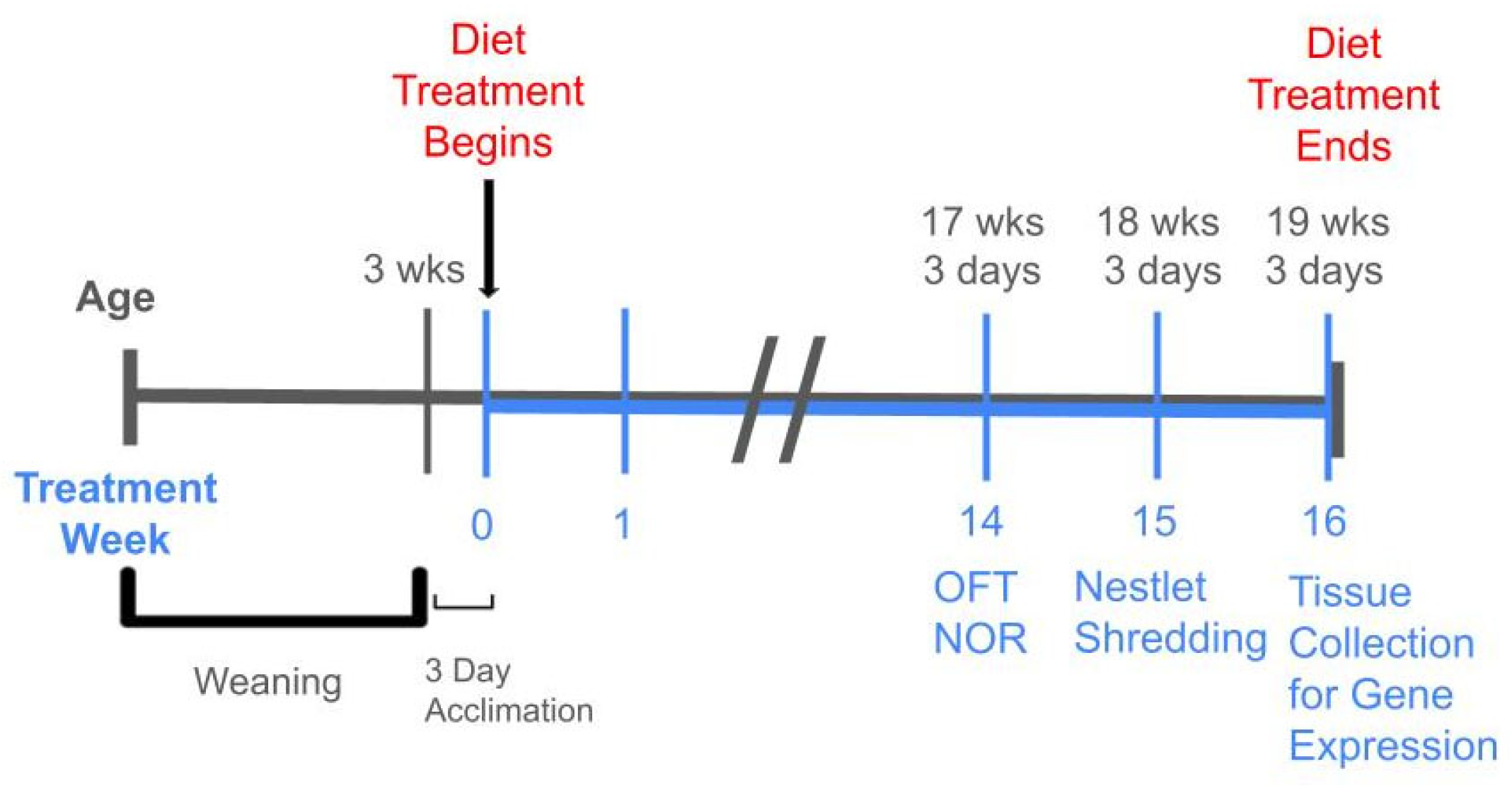
4.1. Animals and Diet
4.2. Behavior Testing
4.2.1. Nestlet Shredding
4.2.2. Open Field Test
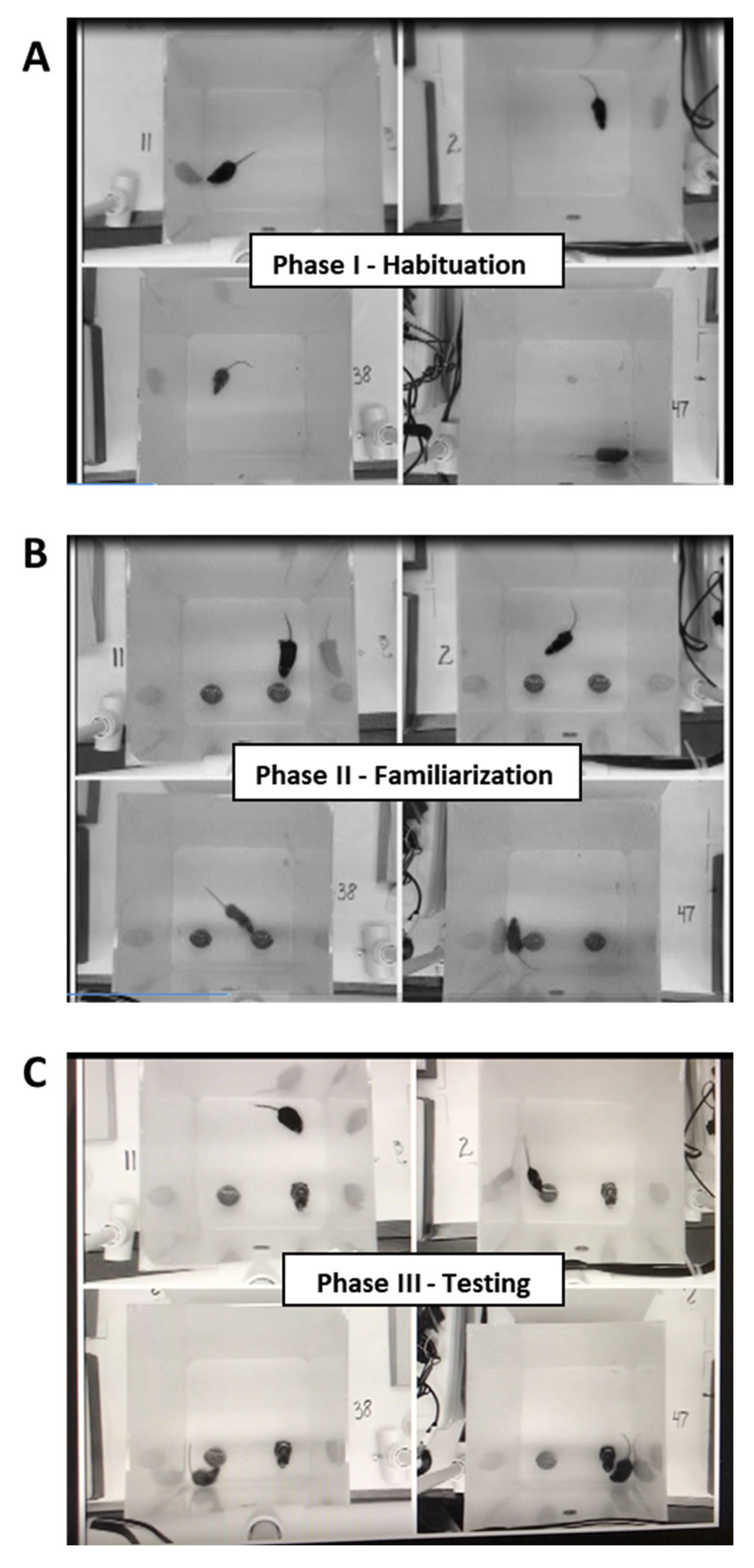
4.2.3. Novel Object Recognition
4.3. Tissue Collection
4.4. RNA Isolation and cDNA Synthesis
4.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APP | amyloid precursor protein |
| αSYN | alpha synuclein |
| BDNF | brain-derived neurotrophic factor |
| B6J | C57BL/6J |
| CD | control diet |
| D2J | DBA/2J |
| HFD | high-fat diet |
References
- Atak, S.; Boye, A.; Peciña, S.; Liu, Z.-X. High-Fat-Sugar Diet Is Associated with Impaired Hippocampus-Dependent Memory in Humans. Physiol. Behav. 2023, 268, 114225. [Google Scholar] [CrossRef]
- Fadó, R.; Molins, A.; Rojas, R.; Casals, N. Feeding the Brain: Effect of Nutrients on Cognition, Synaptic Function, and AMPA Receptors. Nutrients 2022, 14, 4137. [Google Scholar] [CrossRef]
- Zhuang, H.; Yao, X.; Li, H.; Li, Q.; Yang, C.; Wang, C.; Xu, D.; Xiao, Y.; Gao, Y.; Gao, J.; et al. Long-Term High-Fat Diet Consumption by Mice throughout Adulthood Induces Neurobehavioral Alterations and Hippocampal Neuronal Remodeling Accompanied by Augmented Microglial Lipid Accumulation. Brain Behav. Immun. 2022, 100, 155–171. [Google Scholar] [CrossRef]
- Davidson, T.L.; Tracy, A.L.; Schier, L.A.; Swithers, S.E. A View of Obesity as a Learning and Memory Disorder. J. Exp. Psychol. Anim. Learn. Cogn. 2014, 40, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.K.; Saw, N.L.; Woods, C.E.; Vidano, L.M.; Blumenfeld, S.E.; Lam, R.K.; Chu, E.K.; Reading, C.; Shamloo, M. Impact of High-Fat Diet on Cognitive Behavior and Central and Systemic Inflammation with Aging and Sex Differences in Mice. Brain Behav. Immun. 2024, 118, 334–354. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.D.; Loughman, A.; Spencer, S.J.; Reichelt, A.C. The Impact of Obesity and Hypercaloric Diet Consumption on Anxiety and Emotional Behavior across the Lifespan. Neurosci. Biobehav. Rev. 2017, 83, 173–182. [Google Scholar] [CrossRef]
- Nichols, J.N.; Deshane, A.S.; Niedzielko, T.L.; Smith, C.D.; Floyd, C.L. Greater Neurobehavioral Deficits Occur in Adult Mice after Repeated, as Compared to Single, Mild Traumatic Brain Injury (mTBI). Behav. Brain Res. 2016, 298, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Mazon, J.N.; De Mello, A.H.; Ferreira, G.K.; Rezin, G.T. The Impact of Obesity on Neurodegenerative Diseases. Life Sci. 2017, 182, 22–28. [Google Scholar] [CrossRef]
- Lin, L.; Basu, R.; Chatterjee, D.; Templin, A.T.; Flak, J.N.; Johnson, T.S. Disease-Associated Astrocytes and Microglia Markers Are Upregulated in Mice Fed High Fat Diet. Sci. Rep. 2023, 13, 12919. [Google Scholar] [CrossRef]
- Han, J.; Plummer, J.; Liu, L.; Byrd, A.; Aschner, M.; Erikson, K.M. The Impact of Obesity on Brain Iron Levels and α-Synuclein Expression Is Regionally Dependent. Nutr. Neurosci. 2017, 22, 335–343. [Google Scholar] [CrossRef]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The Role of Adult Hippocampal Neurogenesis in Brain Health and Disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Adamowicz, D.H.; Roy, S.; Salmon, D.P.; Galasko, D.R.; Hansen, L.A.; Masliah, E.; Gage, F.H. Hippocampal α-Synuclein in Dementia with Lewy Bodies Contributes to Memory Impairment and Is Consistent with Spread of Pathology. J. Neurosci. 2017, 37, 1675–1684. [Google Scholar] [CrossRef]
- Roher, A.E.; Kokjohn, T.A.; Clarke, S.G.; Sierks, M.R.; Maarouf, C.L.; Serrano, G.E.; Sabbagh, M.S.; Beach, T.G. APP/Aβ Structural Diversity and Alzheimer’s Disease Pathogenesis. Neurochem. Int. 2017, 110, 1–13. [Google Scholar] [CrossRef]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-Specific Neurotrophin Imbalances in Alzheimer Disease: Decreased Levels of Brain-Derived Neurotrophic Factor and Increased Levels of Nerve Growth Factor in Hippocampus and Cortical Areas. Arch. Neurol. 2000, 57, 846. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Bridi, J.C.; Hirth, F. Mechanisms of α-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef]
- Carrillo-Mora, P.; Luna, R.; Colín-Barenque, L. Amyloid Beta: Multiple Mechanisms of Toxicity and Only Some Protective Effects? Oxidative Med. Cell. Longev. 2014, 2014, 795375. [Google Scholar] [CrossRef]
- Zhang, Y.; Thompson, R.; Zhang, H.; Xu, H. APP Processing in Alzheimer’s Disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-Derived Neurotrophic Factor and Its Clinical Implications. Arch. Med. Sci. 2015, 6, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Dakanalis, A.; Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Vasios, G.K.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. The Association of Emotional Eating with Overweight/Obesity, Depression, Anxiety/Stress, and Dietary Patterns: A Review of the Current Clinical Evidence. Nutrients 2023, 15, 1173. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, G.; Nitka, D.; Schmitz, N. The Association between Obesity and Anxiety Disorders in the Population: A Systematic Review and Meta-Analysis. Int. J. Obes. 2010, 34, 407–419. [Google Scholar] [CrossRef]
- Strine, T.W.; Mokdad, A.H.; Dube, S.R.; Balluz, L.S.; Gonzalez, O.; Berry, J.T.; Manderscheid, R.; Kroenke, K. The Association of Depression and Anxiety with Obesity and Unhealthy Behaviors among Community-Dwelling US Adults. Gen. Hosp. Psychiatry 2008, 30, 127–137. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Zhou, Y.; Du, H.; Zhang, C.; Zhao, Z.; Chen, Y.; Zhou, Z.; Mei, J.; Wu, W.; et al. High Fat Diet-Induced Obesity Leads to Depressive and Anxiety-like Behaviors in Mice via AMPK/mTOR-Mediated Autophagy. Exp. Neurol. 2022, 348, 113949. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Suhett, C.P.; Graham, A.; Chen, Y.; Deuster, P. Behavioral Changes in Male Mice Fed a High-Fat Diet Are Associated with IL-1β Expression in Specific Brain Regions. Physiol. Behav. 2017, 169, 130–140. [Google Scholar] [CrossRef]
- Krishna, S.; Lin, Z.; De La Serre, C.B.; Wagner, J.J.; Harn, D.H.; Pepples, L.M.; Djani, D.M.; Weber, M.T.; Srivastava, L.; Filipov, N.M. Time-Dependent Behavioral, Neurochemical, and Metabolic Dysregulation in Female C57BL/6 Mice Caused by Chronic High-Fat Diet Intake. Physiol. Behav. 2016, 157, 196–208. [Google Scholar] [CrossRef]
- Moradi, M.; Mozaffari, H.; Askari, M.; Azadbakht, L. Association between Overweight/Obesity with Depression, Anxiety, Low Self-Esteem, and Body Dissatisfaction in Children and Adolescents: A Systematic Review and Meta-Analysis of Observational Studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 555–570. [Google Scholar] [CrossRef]
- Tsai, S.-F.; Wu, H.-T.; Chen, P.-C.; Chen, Y.-W.; Yu, M.; Wang, T.-F.; Wu, S.-Y.; Tzeng, S.-F.; Kuo, Y.-M. High-Fat Diet Suppresses the Astrocytic Process Arborization and Downregulates the Glial Glutamate Transporters in the Hippocampus of Mice. Brain Res. 2018, 1700, 66–77. [Google Scholar] [CrossRef]
- Araujo, D.; Marquezin, M.; Barbosa, T.; Fonseca, F.; Fegadolli, C.; Castelo, P. Assessment of Quality of Life, Anxiety, Socio-economic Factors and Caries Experience in Brazilian Children with Overweight and Obesity. Int. J. Dent. Hyg. 2017, 15, e156–e162. [Google Scholar] [CrossRef] [PubMed]
- Gelineau, R.R.; Arruda, N.L.; Hicks, J.A.; Monteiro De Pina, I.; Hatzidis, A.; Seggio, J.A. The Behavioral and Physiological Effects of High-fat Diet and Alcohol Consumption: Sex Differences in C57 BL 6/J Mice. Brain Behav. 2017, 7, e00708. [Google Scholar] [CrossRef]
- Tee, J.Y.H.; Gan, W.Y.; Tan, K.-A.; Chin, Y.S. Obesity and Unhealthy Lifestyle Associated with Poor Executive Function among Malaysian Adolescents. PLoS ONE 2018, 13, e0195934. [Google Scholar] [CrossRef]
- Coppin, G.; Nolan-Poupart, S.; Jones-Gotman, M.; Small, D.M. Working Memory and Reward Association Learning Impairments in Obesity. Neuropsychologia 2014, 65, 146–155. [Google Scholar] [CrossRef]
- Clark, D.O.; Xu, H.; Callahan, C.M.; Unverzagt, F.W. Does Body Mass Index Modify Memory, Reasoning, and Speed of Processing Training Effects in Older Adults. Obesity 2016, 24, 2319–2326. [Google Scholar] [CrossRef]
- Cordner, Z.A.; Tamashiro, K.L.K. Effects of High-Fat Diet Exposure on Learning & Memory. Physiol. Behav. 2015, 152, 363–371. [Google Scholar] [CrossRef]
- Totten, M.S.; Pierce, D.M.; Erikson, K.M. The Influence of Sex and Strain on Trace Element Dysregulation in the Brain Due to Diet-Induced Obesity. J. Trace Elem. Med. Biol. 2021, 63, 126661. [Google Scholar] [CrossRef]
- Totten, M.S.; Wallace, C.W.; Pierce, D.M.; Fordahl, S.C.; Erikson, K.M. The Impact of a High-Fat Diet on Physical Activity and Dopamine Neurochemistry in the Striatum Is Sex and Strain Dependent in C57BL/6J and DBA/2J Mice. Nutr. Neurosci. 2022, 25, 2601–2615. [Google Scholar] [CrossRef] [PubMed]
- Totten, M.S.; Howell, J.M.; Tomberlin, J.A.; Erikson, K.M. Relationship Between a High-Fat Diet, Reduced Mobility, and Trace Element Overload in the Olfactory Bulbs of C57BL/6J and DBA/2J Mice. Biol. Trace Elem. Res. 2024, 202, 3215–3224. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse Strain-Dependent Variation in Obesity and Glucose Homeostasis in Response to High-Fat Feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Mozhui, K.; Karlsson, R.-M.; Kash, T.L.; Ihne, J.; Norcross, M.; Patel, S.; Farrell, M.R.; Hill, E.E.; Graybeal, C.; Martin, K.P.; et al. Strain Differences in Stress Responsivity Are Associated with Divergent Amygdala Gene Expression and Glutamate-Mediated Neuronal Excitability. J. Neurosci. 2010, 30, 5357–5367. [Google Scholar] [CrossRef]
- Philip, V.M.; Duvvuru, S.; Gomero, B.; Ansah, T.A.; Blaha, C.D.; Cook, M.N.; Hamre, K.M.; Lariviere, W.R.; Matthews, D.B.; Mittleman, G.; et al. High-throughput Behavioral Phenotyping in the Expanded Panel of BXD Recombinant Inbred Strains. Genes Brain Behav. 2010, 9, 129–159. [Google Scholar] [CrossRef]
- Alexander, J.; Chang, G.Q.; Dourmashkin, J.T.; Leibowitz, S.F. Distinct Phenotypes of Obesity-Prone AKR/J, DBA2J and C57BL/6J Mice Compared to Control Strains. Int. J. Obes. 2006, 30, 50–59. [Google Scholar] [CrossRef] [PubMed]
- West, D.B.; Boozer, C.N.; Moody, D.L.; Atkinson, R.L. Dietary Obesity in Nine Inbred Mouse Strains. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1992, 262, R1025–R1032. [Google Scholar] [CrossRef]
- Totten, M.S.; Pierce, D.M.; Erikson, K.M. Diet-Induced Obesity Disrupts Trace Element Homeostasis and Gene Expression in the Olfactory Bulb. Nutrients 2020, 12, 3909. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble Burying and Nestlet Shredding as Tests of Repetitive, Compulsive-like Behaviors in Mice. J. Vis. Exp. 2013, 82, 50978. [Google Scholar] [CrossRef]
- Gaskill, B.N.; Karas, A.Z.; Garner, J.P.; Pritchett-Corning, K.R. Nest Building as an Indicator of Health and Welfare in Laboratory Mice. J. Vis. Exp. 2013, 82, 51012. [Google Scholar] [CrossRef]
- Li, X.; Morrow, D.; Witkin, J.M. Decreases in Nestlet Shredding of Mice by Serotonin Uptake Inhibitors: Comparison with Marble Burying. Life Sci. 2006, 78, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Jirkof, P.; Fleischmann, T.; Cesarovic, N.; Rettich, A.; Vogel, J.; Arras, M. Assessment of Postsurgical Distress and Pain in Laboratory Mice by Nest Complexity Scoring. Lab Anim. 2013, 47, 153–161. [Google Scholar] [CrossRef]
- Deacon, R.M.J.; Rawlins, J.N.P. Hippocampal Lesions, Species-Typical Behaviours and Anxiety in Mice. Behav. Brain Res. 2005, 156, 241–249. [Google Scholar] [CrossRef]
- Zou, L.; Tian, Y.; Wang, Y.; Chen, D.; Lu, X.; Zeng, Z.; Chen, Z.; Lin, C.; Liang, Y. High-Cholesterol Diet Promotes Depression- and Anxiety-like Behaviors in Mice by Impact Gut Microbe and Neuroinflammation. J. Affect. Disord. 2023, 327, 425–438. [Google Scholar] [CrossRef]
- Shi, H.-J.; Wang, S.; Wang, X.-P.; Zhang, R.-X.; Zhu, L.-J. Hippocampus: Molecular, Cellular, and Circuit Features in Anxiety. Neurosci. Bull. 2023, 39, 1009–1026. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Vis. Exp. 2015, 96, 52434. [Google Scholar] [CrossRef]
- Keleher, M.R.; Zaidi, R.; Patel, K.; Ahmed, A.; Bettler, C.; Pavlatos, C.; Shah, S.; Cheverud, J.M. The Effect of Dietary Fat on Behavior in Mice. J. Diabetes Metab. Disord. 2018, 17, 297–307. [Google Scholar] [CrossRef]
- Grover, L.; Sklioutovskaya-Lopez, K.; Parkman, J.K.; Wang, K.; Hendricks, E.; Adams-Duffield, J.; Kim, J.H. Diet, sex, and genetic predisposition to obesity and type 2 diabetes modulate motor and anxiety-related behaviors in mice, and alter cerebellar gene expression. Behav. Brain Res. 2023, 445, 114376. [Google Scholar] [CrossRef]
- Zilkha, N.; Kuperman, Y.; Kimchi, T. High-Fat Diet Exacerbates Cognitive Rigidity and Social Deficiency in the BTBR Mouse Model of Autism. Neuroscience 2017, 345, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Keralapurath, M.M.; Lin, Z.; Wagner, J.J.; De La Serre, C.B.; Harn, D.A.; Filipov, N.M. Neurochemical and Electrophysiological Deficits in the Ventral Hippocampus and Selective Behavioral Alterations Caused by High-Fat Diet in Female C57BL/6 Mice. Neuroscience 2015, 297, 170–181. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Neuromethods; Humana Press: Totowa, NJ, USA, 2009; Volume 42, pp. 1–20. [Google Scholar] [CrossRef]
- Agrimi, J.; Spalletti, C.; Baroni, C.; Keceli, G.; Zhu, G.; Caragnano, A.; Matteucci, M.; Chelko, S.; Ramirez-Correa, G.A.; Bedja, D.; et al. Obese Mice Exposed to Psychosocial Stress Display Cardiac and Hippocampal Dysfunction Associated with Local Brain-Derived Neurotrophic Factor Depletion. EBioMedicine 2019, 47, 384–401. [Google Scholar] [CrossRef]
- Bax, E.N.; Cochran, K.E.; Mao, J.; Wiedmeyer, C.E.; Rosenfeld, C.S. Opposing Effects of S-Equol Supplementation on Metabolic and Behavioral Parameters in Mice Fed a High-Fat Diet. Nutr. Res. 2019, 64, 39–48. [Google Scholar] [CrossRef]
- Eskelinen, M.H.; Ngandu, T.; Helkala, E.; Tuomilehto, J.; Nissinen, A.; Soininen, H.; Kivipelto, M. Fat Intake at Midlife and Cognitive Impairment Later in Life: A Population-based CAIDE Study. Int. J. Geriat. Psychiatry 2008, 23, 741–747. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Eberstein, J.A. Metabolic Syndrome and the Role of Dietary Lifestyles in Alzheimer’s Disease. J. Neurochem. 2008, 106, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, M.M.; Machaj, A.S.; Chiu, G.S.; Lawson, M.A.; Gainey, S.J.; York, J.M.; Meling, D.D.; Martin, S.A.; Kwakwa, K.A.; Newman, A.F.; et al. Methylphenidate Prevents High-Fat Diet (HFD)-Induced Learning/Memory Impairment in Juvenile Mice. Psychoneuroendocrinology 2013, 38, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.N.; Gomes, S.M.; Shukitt-Hale, B. Blueberry Supplementation Improves Memory in Middle-Aged Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2014, 62, 3972–3978. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.R.; Godbey, S.J.; Thiebaud, N.; Fadool, D.A. Olfactory Ability and Object Memory in Three Mouse Models of Varying Body Weight, Metabolic Hormones, and Adiposity. Physiol. Behav. 2012, 107, 424–432. [Google Scholar] [CrossRef]
- Lenselink, A.M.; Rotaru, D.C.; Li, K.W.; Van Nierop, P.; Rao-Ruiz, P.; Loos, M.; Van Der Schors, R.; Gouwenberg, Y.; Wortel, J.; Mansvelder, H.D.; et al. Strain Differences in Presynaptic Function. J. Biol. Chem. 2015, 290, 15635–15645. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, A.; Brum, P.O.; Ribeiro, C.T.; Gasparotto, J.; Bortolin, R.C.; De Vargas, A.R.; Heimfarth, L.; De Almeida, R.F.; Moreira, J.C.F.; De Oliveira, J.; et al. High Fat Diet-Induced Obesity Causes a Reduction in Brain Tyrosine Hydroxylase Levels and Non-Motor Features in Rats through Metabolic Dysfunction, Neuroinflammation and Oxidative Stress. Nutr. Neurosci. 2022, 25, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Muscat, S.M.; Butler, M.J.; Mackey-Alfonso, S.E.; Barrientos, R.M. Young Adult and Aged Female Rats Are Vulnerable to Amygdala-Dependent, but Not Hippocampus-Dependent, Memory Impairment Following Short-Term High-Fat Diet. Brain Res. Bull. 2023, 195, 145–156. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Asai, M.; Hara, T. High-Fat Diet Enhances Working Memory in the Y-Maze Test in Male C57BL/6J Mice with Less Anxiety in the Elevated Plus Maze Test. Nutrients 2020, 12, 2036. [Google Scholar] [CrossRef]
- Chen, K.; Yuan, R.; Geng, S.; Zhang, Y.; Ran, T.; Kowalski, E.; Liu, J.; Li, L. Toll-Interacting Protein Deficiency Promotes Neurodegeneration via Impeding Autophagy Completion in High-Fat Diet-Fed ApoE−/− Mouse Model. Brain Behav. Immun. 2017, 59, 200–210. [Google Scholar] [CrossRef]
- Rotermund, C.; Truckenmüller, F.M.; Schell, H.; Kahle, P.J. Diet-induced Obesity Accelerates the Onset of Terminal Phenotypes in A-synuclein Transgenic Mice. J. Neurochem. 2014, 131, 848–858. [Google Scholar] [CrossRef]
- Schommer, J.; Marwarha, G.; Nagamoto-Combs, K.; Ghribi, O. Palmitic Acid-Enriched Diet Increases α-Synuclein and Tyrosine Hydroxylase Expression Levels in the Mouse Brain. Front. Neurosci. 2018, 12, 552. [Google Scholar] [CrossRef]
- Bienkowski, M.S.; Bowman, I.; Song, M.Y.; Gou, L.; Ard, T.; Cotter, K.; Zhu, M.; Benavidez, N.L.; Yamashita, S.; Abu-Jaber, J.; et al. Integration of Gene Expression and Brain-Wide Connectivity Reveals the Multiscale Organization of Mouse Hippocampal Networks. Nat. Neurosci. 2018, 21, 1628–1643. [Google Scholar] [CrossRef]
- Wang, S.; Bolós, M.; Clark, R.; Cullen, C.L.; Southam, K.A.; Foa, L.; Dickson, T.C.; Young, K.M. Amyloid β Precursor Protein Regulates Neuron Survival and Maturation in the Adult Mouse Brain. Mol. Cell. Neurosci. 2016, 77, 21–33. [Google Scholar] [CrossRef]
- Busquets, O.; Ettcheto, M.; Pallàs, M.; Beas-Zarate, C.; Verdaguer, E.; Auladell, C.; Folch, J.; Camins, A. Long-Term Exposition to a High Fat Diet Favors the Appearance of β-Amyloid Depositions in the Brain of C57BL/6J Mice. A Potential Model of Sporadic Alzheimer’s Disease. Mech. Ageing Dev. 2017, 162, 38–45, Erratum in Mech. Ageing Dev. 2022, 201, 111615. [Google Scholar] [CrossRef]
- Puig, K.L.; Floden, A.M.; Adhikari, R.; Golovko, M.Y.; Combs, C.K. Amyloid Precursor Protein and Proinflammatory Changes Are Regulated in Brain and Adipose Tissue in a Murine Model of High Fat Diet-Induced Obesity. PLoS ONE 2012, 7, e30378. [Google Scholar] [CrossRef]
- Bao, J.; Cao, C.; Zhang, X.; Jiang, F.; Nicosia, S.V.; Bai, W. Suppression of β-Amyloid Precursor Protein Signaling into the Nucleus by Estrogens Mediated through Complex Formation between the Estrogen Receptor and Fe65. Mol. Cell. Biol. 2007, 27, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.T.; Thakur, M.K. In the cerebral cortex of female and male mice, amyloid precursor protein (APP) promoter methylation is higher in females and differentially regulated by sex steroids. Brain Res. 2006, 1067, 43–47. [Google Scholar] [CrossRef]
- Seemiller, L.R.; Goldberg, L.R.; Sebastian, A.; Siegel, S.R.; Praul, C.; Zeid, D.; Albert, I.; Beierle, J.; Bryant, C.D.; Gould, T.J. Alcohol and Fear Conditioning Produce Strain-specific Changes in the Dorsal Hippocampal Transcriptome of Adolescent C57BL/6J and DBA/2J Mice. Alcohol Clin. Exp. Res. 2024, 48, 2022–2034. [Google Scholar] [CrossRef]
- Chan, C.B.; Ye, K. Sex Differences in Brain-derived Neurotrophic Factor Signaling and Functions. J. Neurosci. Res. 2017, 95, 328–335, Erratum in J. Neurosci. Res. 2020, 98, 404.. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Nakasone, H.; Kasahara, R.; Fukuchi, M. Expression Profiles of Brain-Derived Neurotrophic Factor Splice Variants in the Hippocampus of Alzheimer’s Disease Model Mouse. Biol. Pharm. Bull. 2024, 47, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.B.; Bihelek, N.; Mychasiuk, R.; Dyck, R.H. Brain-derived Neurotrophic Factor and TrkB Levels in Mice that Lack Vesicular Zinc: Effects of Age and Sex. Neuroscience 2020, 425, 90–100. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Yin, H.; Zhang, G.; Yu, L.; Kong, X.; Yuan, H.; Fang, X.; Liu, Q.; Liu, C.; et al. Reduced Neurotrophic Factor Level Is the Early Event before the Functional Neuronal Deficiency in High-Fat Diet Induced Obese Mice. Metab. Brain Dis. 2017, 32, 247–257. [Google Scholar] [CrossRef]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive Impairment Following High Fat Diet Consumption Is Associated with Brain Inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A High-Fat, Refined Sugar Diet Reduces Hippocampal Brain-Derived Neurotrophic Factor, Neuronal Plasticity, and Learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef]
- Dias, C.T.; Curi, H.T.; Payolla, T.B.; Lemes, S.F.; Betim Pavan, I.C.; Torsoni, M.A.; Simabuco, F.M.; Lambertucci, R.H.; Mendes Da Silva, C. Maternal High-Fat Diet Stimulates Proinflammatory Pathway and Increases the Expression of Tryptophan Hydroxylase 2 (TPH2) and Brain-Derived Neurotrophic Factor (BDNF) in Adolescent Mice Hippocampus. Neurochem. Int. 2020, 139, 104781. [Google Scholar] [CrossRef] [PubMed]
- Genzer, Y.; Dadon, M.; Burg, C.; Chapnik, N.; Froy, O. Effect of Dietary Fat and the Circadian Clock on the Expression of Brain-Derived Neurotrophic Factor (BDNF). Mol. Cell. Endocrinol. 2016, 430, 49–55. [Google Scholar] [CrossRef]
- Gan, L.; England, E.; Yang, J.-Y.; Toulme, N.; Ambati, S.; Hartzell, D.L.; Meagher, R.B.; Baile, C.A. A 72-Hour High Fat Diet Increases Transcript Levels of the Neuropeptide Galanin in the Dorsal Hippocampus of the Rat. BMC Neurosci. 2015, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.B.; Dias, C.T.; Chaaban, A.F.A.; Beserra-Filho, J.I.A.; Ribeiro, A.M.; Lambertucci, R.H.; Mendes-da-Silva, C. Improving Dietary Patterns in Obese Mice: Effects on Body Weight, Adiposity, Anhedonia-like Behavior, pro-BDNF Expression and 5-HT System. Brain Res. 2024, 1838, 148996. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.T.D.S.; Andrade, A.V.D.D.; Moura Melo, P.K.; Júnior, R.R.D.S.; Souza, D.L.S.D.; Tavares, É.A.F.; Sena, I.G.D.; Fernandes, T.A.A.D.M.; Morais, P.L.A.D.G.; Fonseca, I.A.T.; et al. The Effects of the Association Between a High-Fat Diet and Physical Exercise on BDNF Expression in the Hippocampus: A Comprehensive Review. Life 2025, 15, 945. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, J.L.; Wu, Q.Y.; Li, J.X.; Liu, Y.; Wu, L.W.; Huang, J.L.; Wu, X.W.; Wang, M.H.; Chen, N. Swimming Suppresses Cognitive Decline of HFD-Induced Obese Mice through Reversing Hippocampal Inflammation, Insulin Resistance, and BDNF Level. Nutrients 2022, 164, 2432. [Google Scholar] [CrossRef]
- Wankhade, T.; Thakre, N.; Tadas, M.; Katariya, R.; Umekar, M.; Kotagale, N.; Taksande, B. Sex-specific neuroprotection: Does BDNF shield girls from autism? Mol. Cell. Neurosci. 2025, 134, 104028. [Google Scholar] [CrossRef]
- Gellért, L.; Varga, D. Locomotion Activity Measurement in an Open Field for Mice. Bio-Protocol 2016, 6, e1857. [Google Scholar] [CrossRef]
- Bridgewater, L.C.; Zhang, C.; Wu, Y.; Hu, W.; Zhang, Q.; Wang, J.; Li, S.; Zhao, L. Gender-Based Differences in Host Behavior and Gut Microbiota Composition in Response to High Fat Diet and Stress in a Mouse Model. Sci. Rep. 2017, 7, 10776. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The Novel Object Recognition Memory: Neurobiology, Test Procedure, and Its Modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Paola, V. The Object Recognition Task: A New Proposal for the Memory Performance Study; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Berlyne, D.E. Novelty and curiosity as determinants of exploratory behavior. Br. J. Psych. 1950, 41, 68–80. [Google Scholar] [CrossRef]
| Macronutrients | CD (10% kcal from Fat) D12450J | HFD (60% kcal from Fat) D12492 |
|---|---|---|
| Fat | 10% kcal | 60% kcal |
| Carbohydrate | 70% kcal | 20% kcal |
| Protein | 20% kcal | 20% kcal |
| Energy Density | 3.82 kcal/g | 5.21 kcal/g |
| Strain | C57BL/6J | DBA/2J | ||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | ||||
| Diet | CD | HFD | CD | HFD | CD | HFD | CD | HFD |
| αSYN | 4 | 3 | 4 | 5 | 5 | 5 | 5 | 5 |
| APP | 5 | 3 | 5 | 5 | 5 | 5 | 5 | 5 |
| BDNF | 5 | 5 | 5 | 5 | 5 | 3 | 5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totten, M.S.; Peterson, A.L.; Pierce, D.M.; Erikson, K.M. High-Fat Diet Alters Behavior and Hippocampal Gene Expression. Int. J. Mol. Sci. 2025, 26, 9241. https://doi.org/10.3390/ijms26189241
Totten MS, Peterson AL, Pierce DM, Erikson KM. High-Fat Diet Alters Behavior and Hippocampal Gene Expression. International Journal of Molecular Sciences. 2025; 26(18):9241. https://doi.org/10.3390/ijms26189241
Chicago/Turabian StyleTotten, Melissa S., Ava L. Peterson, Derek M. Pierce, and Keith M. Erikson. 2025. "High-Fat Diet Alters Behavior and Hippocampal Gene Expression" International Journal of Molecular Sciences 26, no. 18: 9241. https://doi.org/10.3390/ijms26189241
APA StyleTotten, M. S., Peterson, A. L., Pierce, D. M., & Erikson, K. M. (2025). High-Fat Diet Alters Behavior and Hippocampal Gene Expression. International Journal of Molecular Sciences, 26(18), 9241. https://doi.org/10.3390/ijms26189241









