Calprotectin Expression in Adventitial Layer of Cattle and Sheep Echinococcus granulosus sensu stricto Cysts
Abstract
1. Introduction
2. Results
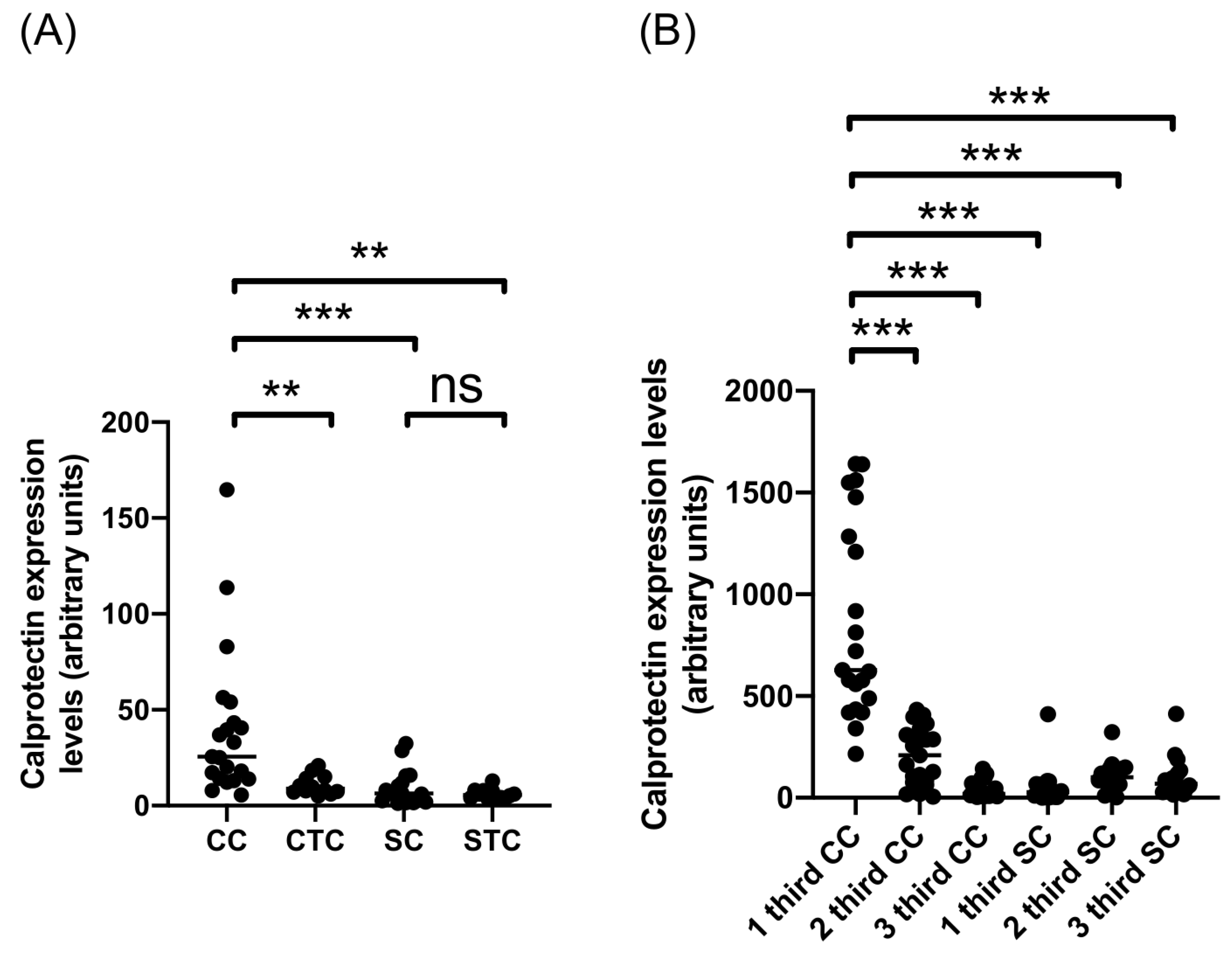
2.1. Calprotectin Expression in CE Cysts from Cattle and Sheep
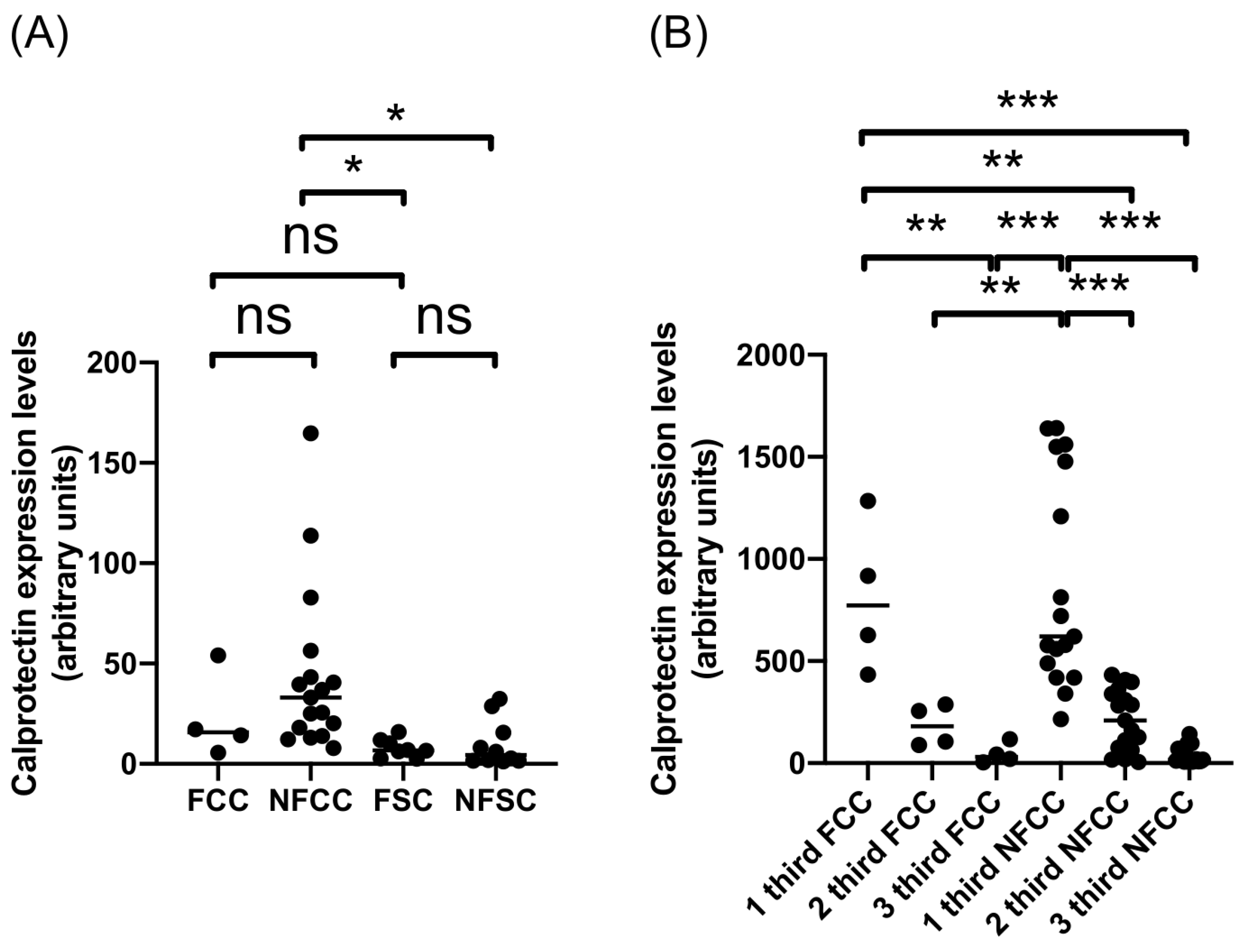
2.2. Calprotectin Expression in Fertile and Non-Fertile CE Cysts from Cattle and Sheep
2.3. Calprotectin Expression in CE Cysts from Liver and Lung of Cattle and Sheep
3. Discussion
4. Materials and Methods
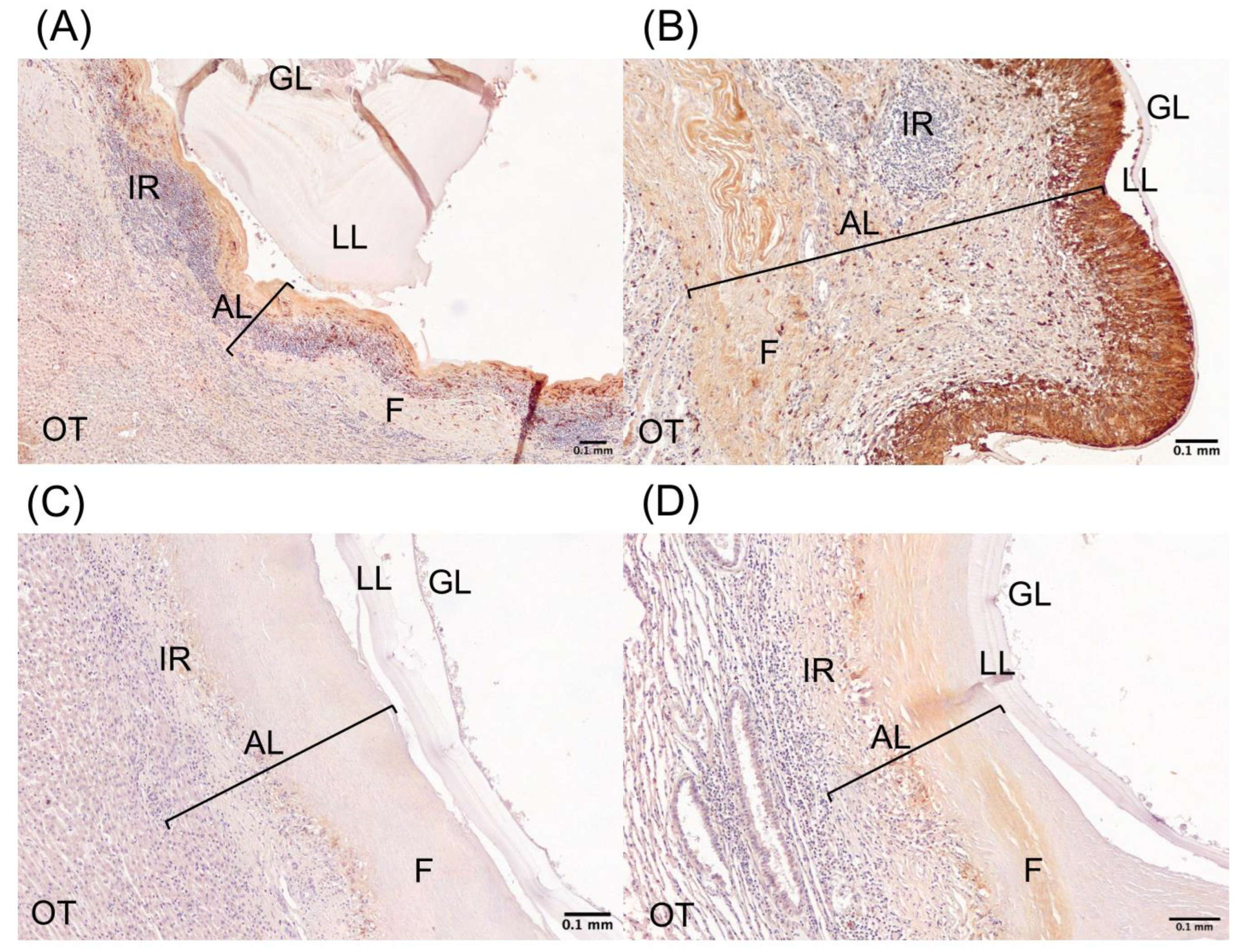
4.1. Samples and Processing
4.2. Immunohistochemical Procedure
4.3. Imaging and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CE | cystic echinococcosis |
| s.s. | sensu stricto |
| s.l. | sensu lato |
| PSCs | protoscoleces |
| WB | Western blot |
| IHC | immunohistochemistry |
| MS | mass spectrometry |
| AL | adventitial layer |
| LL | laminated layer |
| GL | germinal layer |
| IR | immune reaction |
| F | fibrosis |
| OT | organ tissue |
| CC | cattle cyst |
| CTC | cattle tissue control |
| SC | sheep cyst |
| STC | sheep tissue control |
| ns | non-significant |
| FCC | fertile cattle cyst |
| NFCC | non-fertile cattle cyst |
| FSC | fertile sheep cyst |
| NFSC | non-fertile sheep cyst |
Appendix A
Appendix A.1
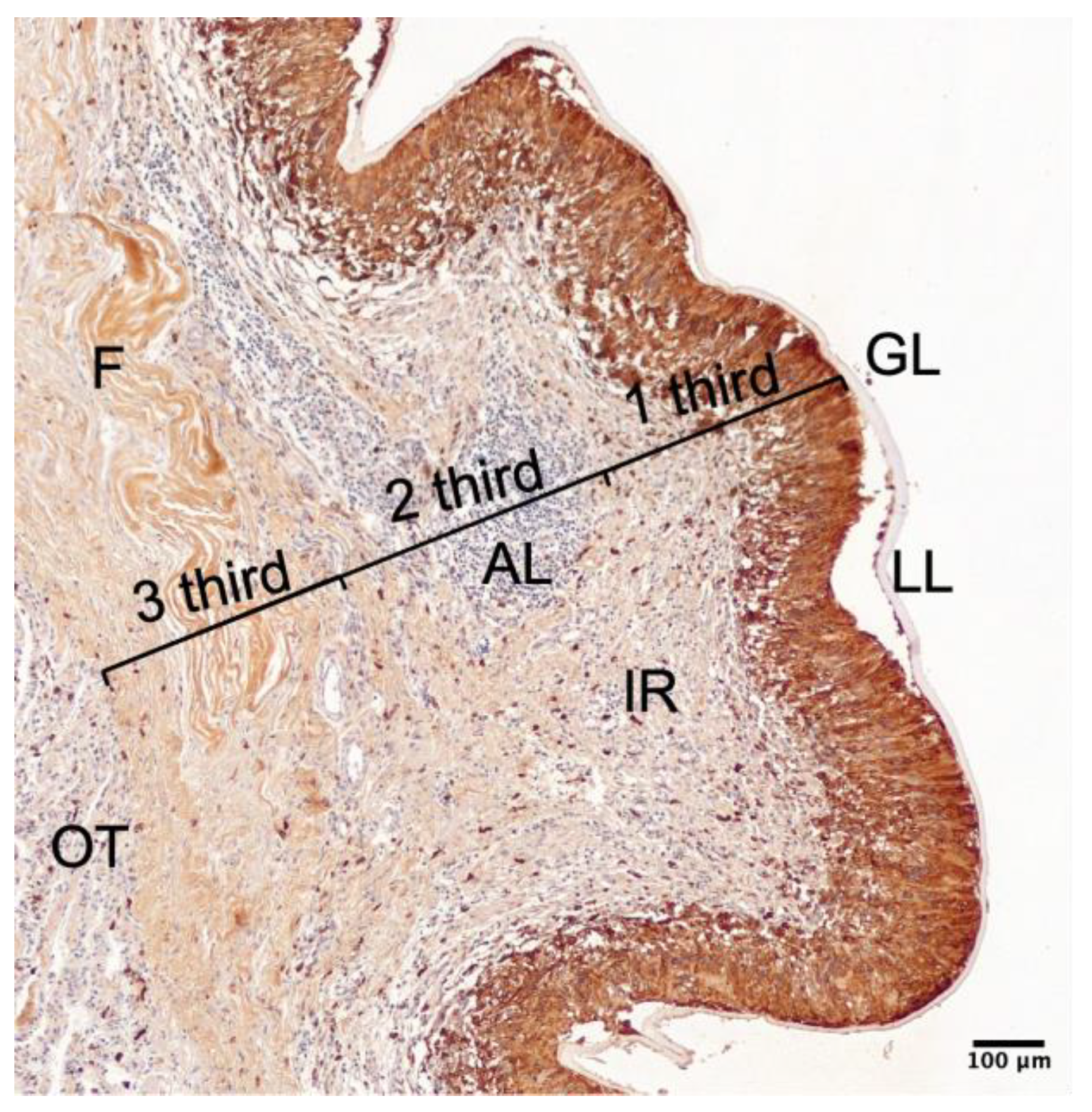
Appendix A.2

References
- Grosso, G.; Gruttadauria, S.; Blondi, A.; Marventano, S.; Mistretta, A. Worldwide epodemiology of liver hydatidosis including the mediterranean area. World J. Gastroenterol. 2012, 18, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Vuitton, D.A.; McManus, D.P.; Rogan, M.T.; Romig, T.; Gottstein, B.; Naidich, A.; Tuxun, T.; Wen, H.; Menezes da Silva, A. International consensus on terminology to be used in the field of echinococcoses. Parasite 2020, 27, 41. [Google Scholar] [CrossRef] [PubMed]
- Romig, T.; Ebi, D.; Wassermann, M. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet. Parasitol. 2015, 213, 76–84. [Google Scholar] [CrossRef]
- Hidalgo, C.; Stoore, C.; Pereira, I.; Paredes, R.; Alvarez Rojas, C.A. Multiple haplotypes of Echinococcus granulosus sensu stricto in single naturally infected intermediate hosts. Parasitol. Res. 2020, 119, 763–770. [Google Scholar] [CrossRef]
- Alvarez Rojas, C.A.; Ebi, D.; Paredes, R.; Acosta-Jamett, G.; Urriola, N.; Roa, J.C.; Manterola, C.; Cortes, S.; Romig, T.; Scheerlinck, J.P.; et al. High intraspecific variability of Echinococcus granulosus sensu stricto in Chile. Parasitol. Int. 2017, 66, 112–115. [Google Scholar] [CrossRef]
- Correa, F.; Stoore, C.; Horlacher, P.; Jimenez, M.; Hidalgo, C.; Alvarez Rojas, C.A.; Figueiredo Barros, G.; Bunselmeyer Ferreira, H.; Hernandez, M.; Cabrera, G.; et al. First description of Echinococcus ortleppi and cystic echinococcosis infection status in Chile. PLoS ONE 2018, 13, e0197620. [Google Scholar] [CrossRef]
- Manterola, C.; Benavente, F.; Melo, A.; Vial, M.; Roa, J.C. Description of Echinococcus granulosus genotypes in human hydatidosis in a region of southern Chile. Parasitol. Int. 2008, 57, 342–346. [Google Scholar] [CrossRef]
- Alvarez Rojas, C.A.; Romig, T.; Lightowlers, M.W. Echinococcus granulosus sensu lato genotypes infecting humans--review of current knowledge. Int. J. Parasitol. 2014, 44, 9–18. [Google Scholar] [CrossRef]
- Thompson, R.C. Biology and Systematics of Echinococcus. Adv. Parasitol. 2017, 95, 65–109. [Google Scholar] [CrossRef]
- Hidalgo, C.; Stoore, C.; Strull, K.; Franco, C.; Correa, F.; Jimenez, M.; Hernandez, M.; Lorenzatto, K.; Ferreira, H.B.; Galanti, N.; et al. New insights of the local immune response against both fertile and infertile hydatid cysts. PLoS ONE 2019, 14, e0211542. [Google Scholar] [CrossRef] [PubMed]
- Negash, K.; Beyene, D.; Kumsa, B. Cystic echinococcosis in cattle slaughtered at Shashemanne Municipal Abattoir, south central Oromia, Ethiopia: Prevalence, cyst distribution and fertility. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Pednekar, R.P.; Gatne, M.L.; Thompson, R.C.; Traub, R.J. Molecular and morphological characterisation of Echinococcus from food producing animals in India. Vet. Parasitol. 2009, 165, 58–65. [Google Scholar] [CrossRef]
- Balbinotti, H.; Santos, G.B.; Badaraco, J.; Arend, A.C.; Graichen, D.A.; Haag, K.L.; Zaha, A. Echinococcus ortleppi (G5) and Echinococcus granulosus sensu stricto (G1) loads in cattle from Southern Brazil. Vet. Parasitol. 2012, 188, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Omer, R.A.; Dinkel, A.; Romig, T.; Mackenstedt, U.; Elnahas, A.A.; Aradaib, I.E.; Ahmed, M.E.; Elmalik, K.H.; Adam, A. A molecular survey of cystic echinococcosis in Sudan. Vet. Parasitol. 2010, 169, 340–346. [Google Scholar] [CrossRef]
- González, H.; Plaza, J.; Ábalos, P. Fertilidad del quiste hidatídico entres especies animales en Chile y estudio de la vitalidad de sus escólices. Bol. Chile Parasitol. 1981, 36, 14–19. [Google Scholar]
- Moudgil, A.D.; Moudgil, P.; Asrani, R.K.; Agnihotri, R.K. Hydatidosis in slaughtered sheep and goats in India: Prevalence, genotypic characterization and pathological studies. J. Helminthol. 2020, 94, e27. [Google Scholar] [CrossRef] [PubMed]
- Brik, K.; Hassouni, T.; Youssir, S.; Baroud, S.; Elkharrim, K.; Belghyti, D. Epidemiological study of Echinococcus granulosus in sheep in the Gharb plain (North-West of Morocco). J. Parasit. Dis. 2018, 42, 505–510. [Google Scholar] [CrossRef]
- Abdel-Baki, A.S.; Almalki, E.; Al-Quarishy, S. Prevalence and characterization of hydatidosis in Najdi sheep slaughtered in Riyadh city, Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 1375–1379. [Google Scholar] [CrossRef]
- Odongo, D.O.; Tiampati, C.M.; Mulinge, E.; Mbae, C.K.; Bishop, R.P.; Zeyhle, E.; Magambo, J.; Wasserman, M.; Kern, P.; Romig, T. Prevalence and genotyping of Echinococcus granulosus in sheep in Narok County, Kenya. Parasitol. Res. 2018, 117, 2065–2073. [Google Scholar] [CrossRef]
- Pereira, I.; Paludo, G.P.; Hidalgo, C.; Stoore, C.; Baquedano, M.S.; Cabezas, C.; Cancela, M.; Ferreira, H.B.; Bastias, M.; Riveros, A.; et al. Weighted gene co-expression network analysis reveals immune evasion related genes in Echinococcus granulosus sensu stricto. Exp. Biol. Med. 2024, 249, 10126. [Google Scholar] [CrossRef]
- Zeghir-Bouteldja, R.; Amri, M.; Bouaziz, S.; Mezioug, D.; Touil-Boukoffa, C. Comparative study of nitric oxide (NO) production during human hydatidosis: Relationship with cystic fluid fertility. Parasitol. Res. 2013, 112, 649–654. [Google Scholar] [CrossRef]
- Paredes, R.; Jimenez, V.; Cabrera, G.; Iraguen, D.; Galanti, N. Apoptosis as a possible mechanism of infertility in Echinococcus granulosus hydatid cysts. J. Cell Biochem. 2007, 100, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.; Stoore, C.; Hidalgo, C.; Correa, F.; Hernandez, M.; Benavides, J.; Ferreras, M.C.; Saenz, L.; Paredes, R. Lymphocyte Populations in the Adventitial Layer of Hydatid Cysts in Cattle: Relationship With Cyst Fertility Status and Fasciola Hepatica Co-Infection. Vet. Pathol. 2020, 57, 108–114. [Google Scholar] [CrossRef]
- Hidalgo, C.; Stoore, C.; Baquedano, M.S.; Pereira, I.; Franco, C.; Hernandez, M.; Paredes, R. Response patterns in adventitial layer of Echinococcus granulosus sensu stricto cysts from naturally infected cattle and sheep. Vet. Res. 2021, 52, 66. [Google Scholar] [CrossRef]
- Barnes, T.S.; Hinds, L.A.; Jenkins, D.J.; Bielefeldt-Ohmann, H.; Lightowlers, M.W.; Coleman, G.T. Comparative pathology of pulmonary hydatid cysts in macropods and sheep. J. Comp. Pathol. 2011, 144, 113–122. [Google Scholar] [CrossRef]
- Diaz, A.; Sagasti, C.; Casaravilla, C. Granulomatous responses in larval taeniid infections. Parasite Immunol. 2018, 40, e12523. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef]
- Basika, T.; Munoz, N.; Casaravilla, C.; Irigoin, F.; Batthyany, C.; Bonilla, M.; Salinas, G.; Pacheco, J.P.; Roth, J.; Duran, R.; et al. Phagocyte-specific S100 proteins in the local response to the Echinococcus granulosus larva. Parasitology 2012, 139, 271–283. [Google Scholar] [CrossRef]
- Fernandez, M.; Benavides, J.; Castano, P.; Elguezabal, N.; Fuertes, M.; Munoz, M.; Royo, M.; Ferreras, M.C.; Perez, V. Macrophage Subsets Within Granulomatous Intestinal Lesions in Bovine Paratuberculosis. Vet. Pathol. 2017, 54, 82–93. [Google Scholar] [CrossRef]
- Albermani, Z.M.; Al-Dabhawi, A.H. Gross and histopathological changes in liver and lung of cattle and sheep infected with hydatid cyst. Kufa J. Vet. Med. Sci. 2022, 13, 24–33. [Google Scholar] [CrossRef]
- Pereira, I.; Hidalgo, C.; Stoore, C.; Baquedano, M.S.; Cabezas, C.; Bastias, M.; Riveros, A.; Meneses, C.; Cancela, M.; Ferreira, H.B.; et al. Transcriptome analysis of Echinococcus granulosus sensu stricto protoscoleces reveals differences in immune modulation gene expression between cysts found in cattle and sheep. Vet. Res. 2022, 53, 8. [Google Scholar] [CrossRef] [PubMed]
- Siles-Lucas, M.; Sanchez-Ovejero, C.; Gonzalez-Sanchez, M.; Gonzalez, E.; Falcon-Perez, J.M.; Boufana, B.; Fratini, F.; Casulli, A.; Manzano-Roman, R. Isolation and characterization of exosomes derived from fertile sheep hydatid cysts. Vet. Parasitol. 2017, 236, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, W.; Cao, S.; Yin, J.; Zhang, J.; Cao, J.; Shen, Y. Comprehensive Analysis of Non-coding RNA Profiles of Exosome-Like Vesicles From the Protoscoleces and Hydatid Cyst Fluid of Echinococcus granulosus. Front. Cell Infect. Microbiol. 2020, 10, 316. [Google Scholar] [CrossRef]
- Sejersen, K.; Eriksson, M.B.; Larsson, A.O. Calprotectin as a Biomarker for Infectious Diseases: A Comparative Review with Conventional Inflammatory Markers. Int. J. Mol. Sci. 2025, 26, 6476. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Moreau, E.; Chauvin, A. Immunity against helminths: Interactions with the host and the intercurrent infections. J. Biomed. Biotechnol. 2010, 2010, 428593. [Google Scholar] [CrossRef]
- Amri, M.; Touil-Boukoffa, C. A protective effect of the laminated layer on Echinococcus granulosus survival dependent on upregulation of host arginase. Acta Trop. 2015, 149, 186–194. [Google Scholar] [CrossRef]
- Atmaca, H.T. Determination of macrophage types by immunohistochemical methods in the local immune response to liver hydatid cysts in sheep. Acta Trop. 2022, 229, 106364. [Google Scholar] [CrossRef]
- Seoane, P.I.; Ruckerl, D.; Casaravilla, C.; Barrios, A.A.; Pittini, A.; MacDonald, A.S.; Allen, J.E.; Diaz, A. Particles from the Echinococcus granulosus laminated layer inhibit IL-4 and growth factor-driven Akt phosphorylation and proliferative responses in macrophages. Sci. Rep. 2016, 6, 39204. [Google Scholar] [CrossRef]
- Silva-Alvarez, V.; Folle, A.M.; Ramos, A.L.; Zamarreno, F.; Costabel, M.D.; Garcia-Zepeda, E.; Salinas, G.; Corsico, B.; Ferreira, A.M. Echinococcus granulosus antigen B: A Hydrophobic Ligand Binding Protein at the host-parasite interface. Prostaglandins Leukot. Essent. Fat. Acids 2015, 93, 17–23. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.S.; Fang, B.B.; Li, Z.D.; Li, L.; Bi, X.J.; Li, W.D.; Zhang, N.; Lin, R.Y.; Wen, H. Thioredoxin peroxidase secreted by Echinococcus granulosus (sensu stricto) promotes the alternative activation of macrophages via PI3K/AKT/mTOR pathway. Parasit. Vectors 2019, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.R.; Yue, W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio-Protocol 2019, 9, e3465. [Google Scholar] [CrossRef] [PubMed]

| Study Group | Calprotectin Expression Levels 1 | Number of Samples |
|---|---|---|
| Cattle CE cysts | 39.92 | 21 |
| 1 third CC | 861.7 | 21 |
| 2 third CC | 207.2 | 21 |
| 3 third CC | 39.61 | 21 |
| Cattle tissue control | 10.86 | 12 |
| Sheep CE cysts | 6.378 | 16 |
| 1 third SC | 56.57 | 16 |
| 2 third SC | 110.3 | 16 |
| 3 third SC | 96.98 | 16 |
| Sheep tissue control | 6.140 | 12 |
| Study Group | Calprotectin Expression Levels 1 | Number of Samples |
|---|---|---|
| Fertile cattle CE cysts | 22.78 | 4 |
| 1 third FCC | 815.9 | 4 |
| 2 third FCC | 184.1 | 4 |
| 3 third FCC | 46.10 | 4 |
| Non-fertile cattle CE cysts | 43.96 | 17 |
| 1 third NFCC | 872.5 | 17 |
| 2 third NFCC | 212.6 | 17 |
| 3 third NFCC | 38.08 | 17 |
| Fertile sheep CE cysts | 7.890 | 8 |
| 1 third FSC | 33.05 | 8 |
| 2 third FSC | 102.5 | 8 |
| 3 third FSC | 120.0 | 8 |
| Fertile sheep CE cysts | 4.865 | 8 |
| 1 third NFSC | 80.08 | 8 |
| 2 third NFSC | 118.1 | 8 |
| 3 third NFSC | 73.92 | 8 |
| Study Group | Calprotectin Expression Levels 1 | Number of Samples |
|---|---|---|
| Lung cattle CE cysts | 55.36 | 12 |
| 1 third lung CC | 921.8 | 12 |
| 2 third lung CC | 246.6 | 12 |
| 3 third lung CC | 44.85 | 12 |
| Liver cattle CE cysts | 12.21 | 9 |
| 1 third liver CC | 727.0 | 9 |
| 2 third liver CC | 154.6 | 9 |
| 3 third liver CC | 32.63 | 9 |
| Lung sheep CE cysts | 1.589 | 9 |
| 1 third lung SC | 43.45 | 9 |
| 2 third lung SC | 117.7 | 9 |
| 3 third lung SC | 63.89 | 9 |
| Liver sheep CE cysts | 1.226 | 7 |
| 1 third liver SC | 67.08 | 7 |
| 2 third liver SC | 100.7 | 7 |
| 3 third liver SC | 139.5 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baquedano, M.S.; Stoore, C.; Hidalgo, C.; Pereira, I.; Paredes, R. Calprotectin Expression in Adventitial Layer of Cattle and Sheep Echinococcus granulosus sensu stricto Cysts. Int. J. Mol. Sci. 2025, 26, 9236. https://doi.org/10.3390/ijms26189236
Baquedano MS, Stoore C, Hidalgo C, Pereira I, Paredes R. Calprotectin Expression in Adventitial Layer of Cattle and Sheep Echinococcus granulosus sensu stricto Cysts. International Journal of Molecular Sciences. 2025; 26(18):9236. https://doi.org/10.3390/ijms26189236
Chicago/Turabian StyleBaquedano, María Soledad, Caroll Stoore, Christian Hidalgo, Ismael Pereira, and Rodolfo Paredes. 2025. "Calprotectin Expression in Adventitial Layer of Cattle and Sheep Echinococcus granulosus sensu stricto Cysts" International Journal of Molecular Sciences 26, no. 18: 9236. https://doi.org/10.3390/ijms26189236
APA StyleBaquedano, M. S., Stoore, C., Hidalgo, C., Pereira, I., & Paredes, R. (2025). Calprotectin Expression in Adventitial Layer of Cattle and Sheep Echinococcus granulosus sensu stricto Cysts. International Journal of Molecular Sciences, 26(18), 9236. https://doi.org/10.3390/ijms26189236






