Inhibitory Effect of Fucoidan Analogs on Highly Metastatic Gastric Cancer Cells via Galectin-4 Inhibition
Abstract
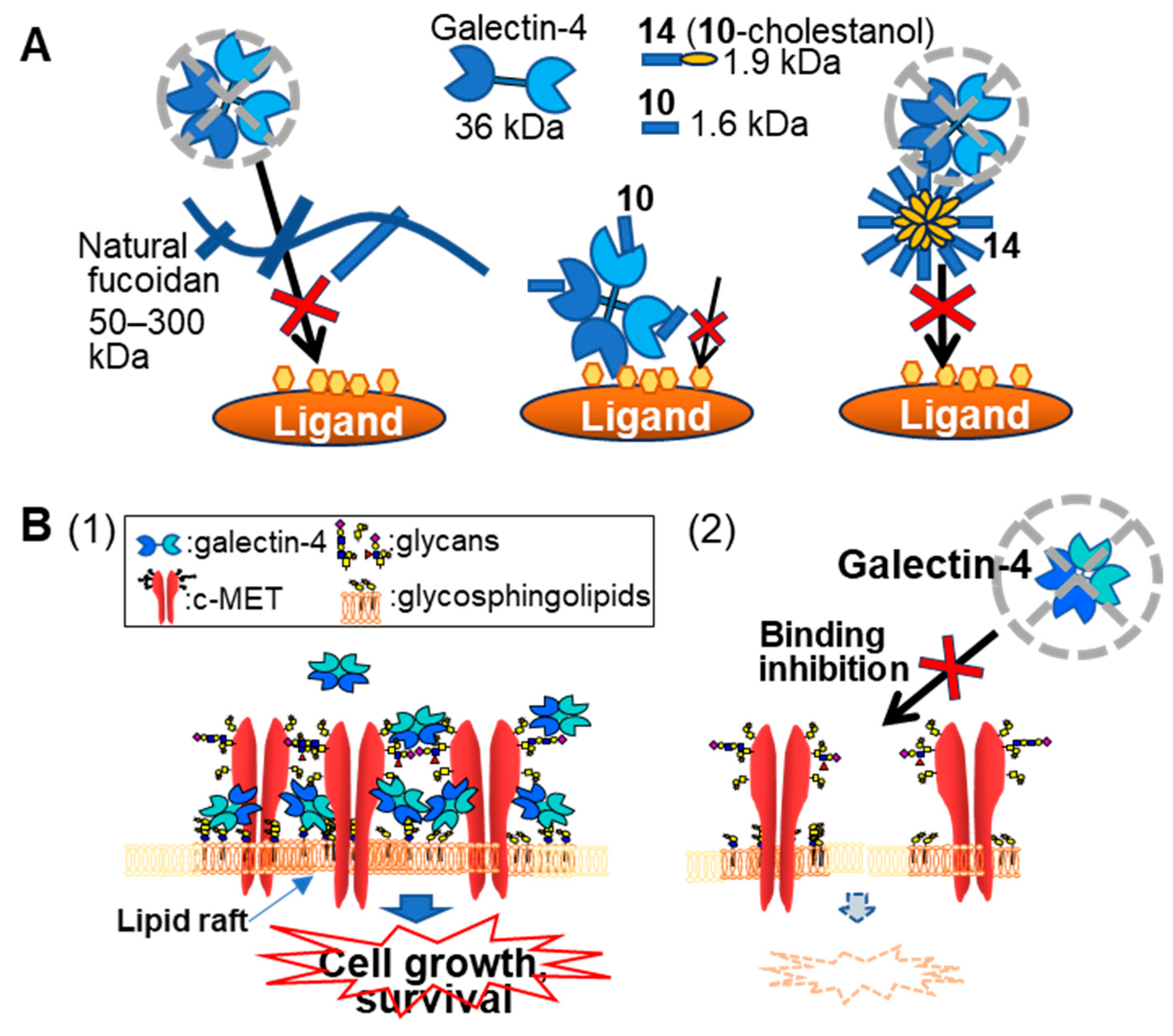
1. Introduction
2. Results
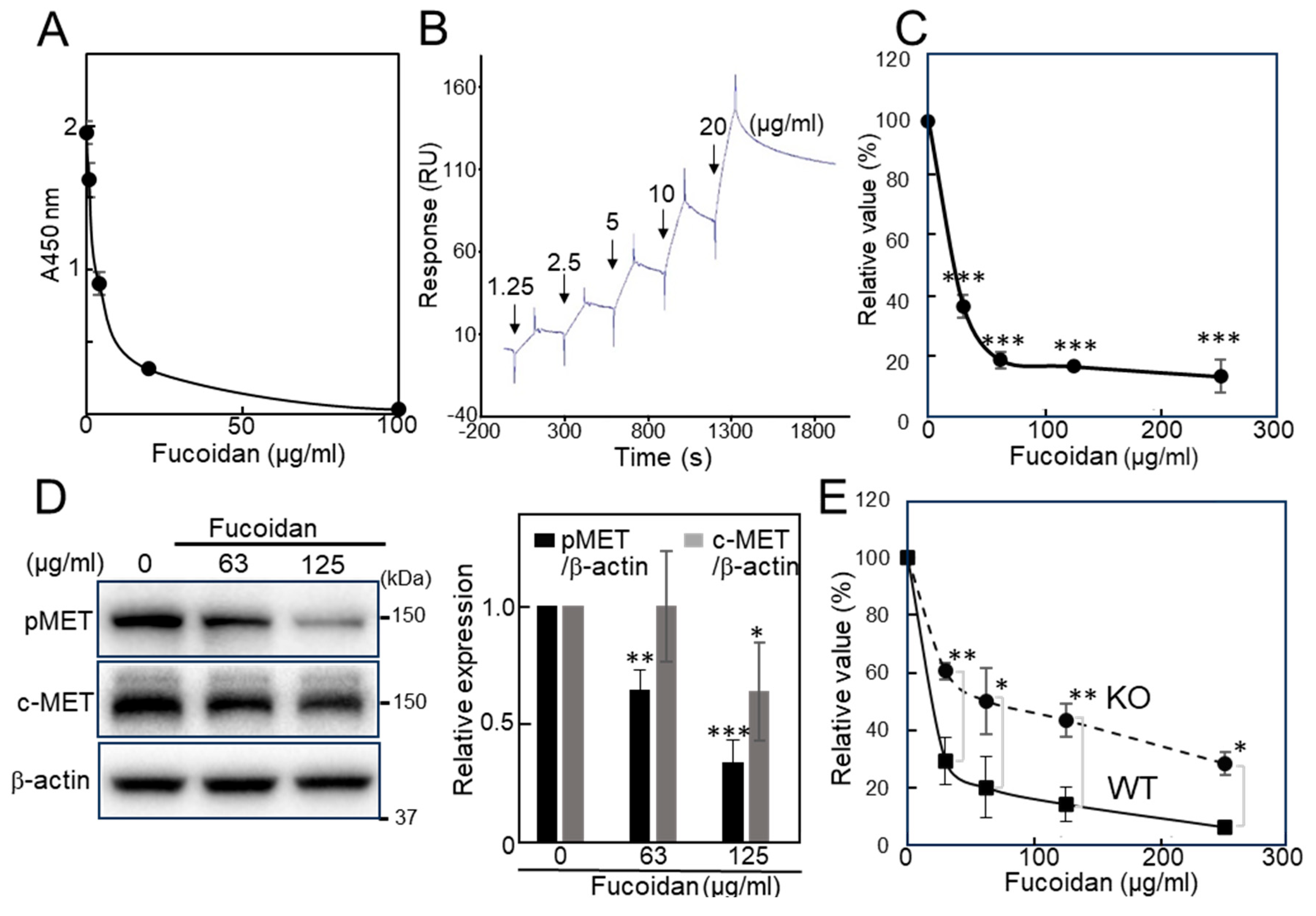
2.1. Evaluation of the Inhibitory Activity of Natural Fucoidan Toward Galectin-4 and Cell Proliferation
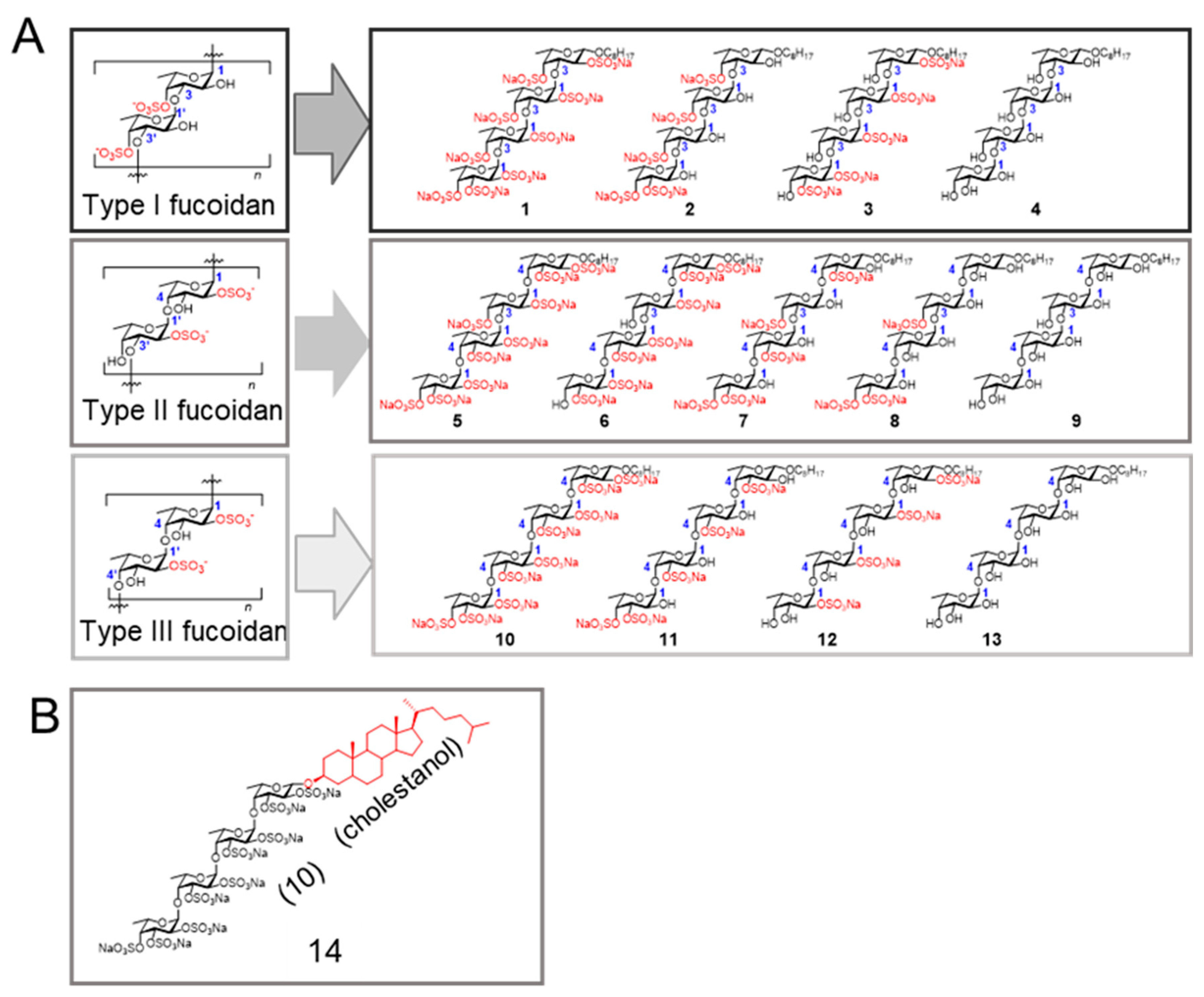
2.2. Chemical Structures of the Fucoidan Analogs and Their Inhibitory Activity
2.2.1. Chemical Structures of the Fucoidan Analogs
2.2.2. Inhibitory Activity of the Fucoidan Analogs
2.3. Properties of Fucoidan Analogs
2.3.1. Fucoidan Analogs Inhibit the Galectin-4 Binding
2.3.2. Downregulation of pMET by Fucoidan Analogs
2.3.3. Caspase-3 Activation Was Not Observed in Cells Treated with Fucoidan Analogs
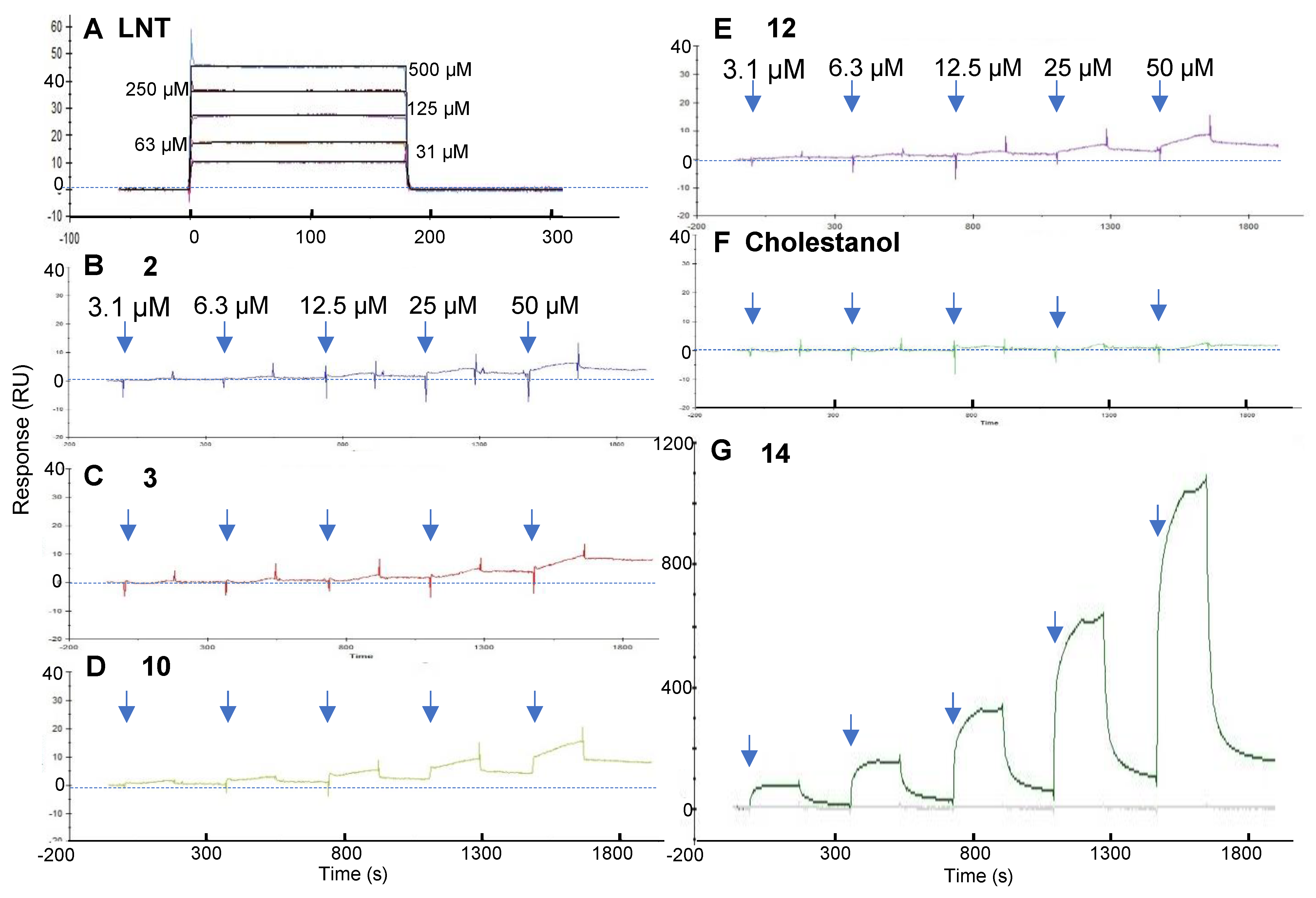
2.4. SPR Reveals the Binding Behaviors of Fucoidan Analogs Toward Galectin-4
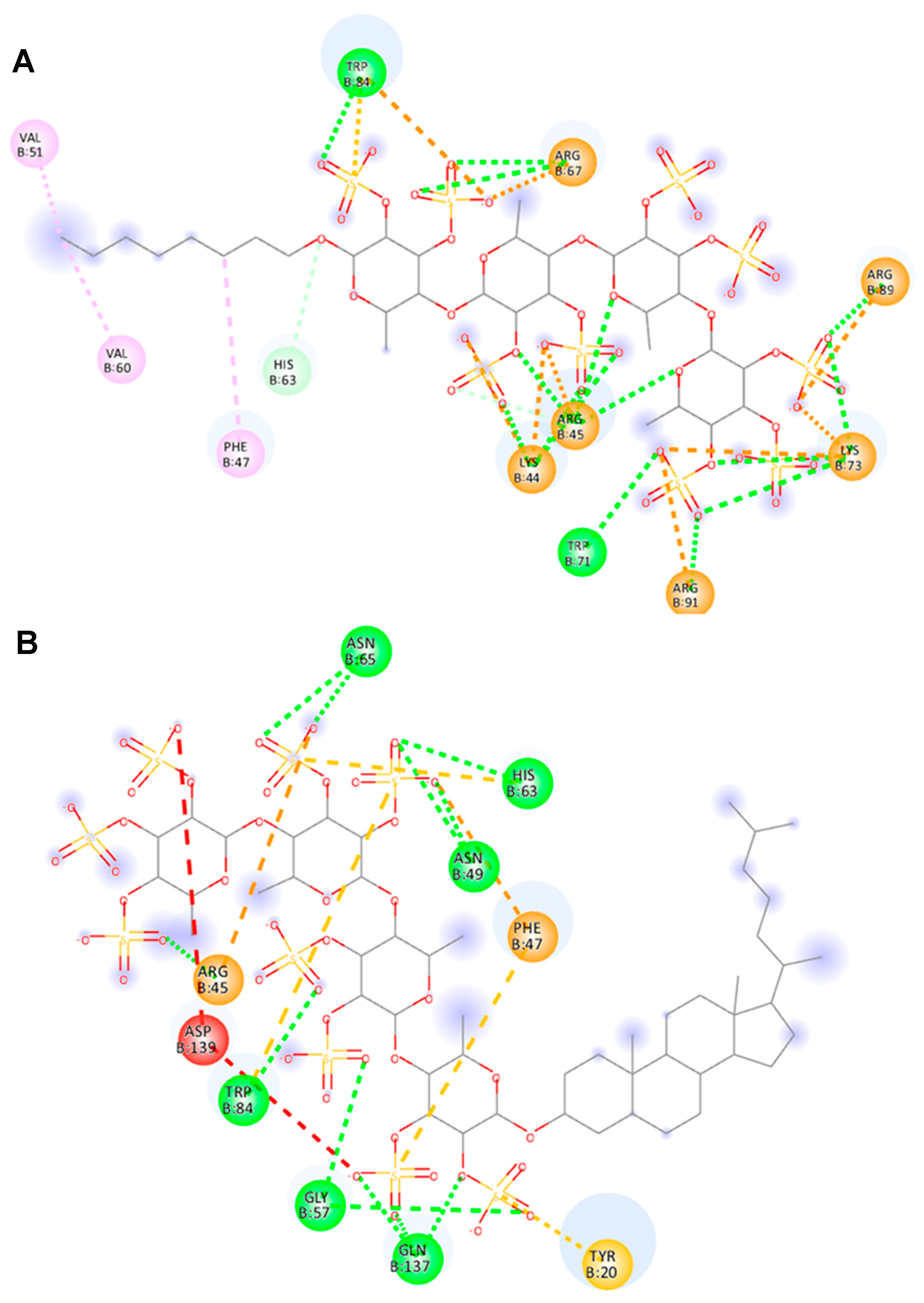
2.5. Molecular Docking of 10 and 14 with Galectin-4N Domain
2.6. Arg45 of Galectin-4 Is Important for Binding to Fucoidan Analogs
3. Discussion
4. Materials and Methods
4.1. Materials and Antibodies
4.2. Galectin-4 Preparation
4.3. Cell Lines and Cell Culture
4.4. Cell Proliferation Assay for Growth Inhibition Experiments
4.5. ELISA for Binding of Galectin-4
4.6. ELISA-Based Inhibition Assay
4.7. SPR Measurements
4.8. Western Blot Analysis
4.9. Synthesis of Fucoidan Analogs
4.10. Molecular Docking
4.11. CMC Determination
4.12. Immunofluorescence Studies
4.13. Co-Sedimentation Assays
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRD | Carbohydrate recognition domain |
| ELISA | Enzyme-linked immunosorbent assay |
| SPR | Surface plasmon resonance |
| KD | Dissociation constant |
| ATP | Adenosine triphosphate |
| pMET | Phospho-c-MET |
| KO | Knockout |
| WT | Wild-type |
| FCS | Fetal calf serum |
| RU | Response unit |
| Cho | Cholestanol |
| CC-3 | Cleaved-caspase-3 |
| LNT | Lacto-N-tetraose |
| CMC | Critical micelle concentration |
| NPN | N-phenyl-1-napthylamine |
| PDB | Protein data bank |
| RMSD | Root-mean-square deviation |
| ADME | Absorption, distribution, metabolism, and excretion |
| BSA | Bovine serum albumin |
| PVDF | Polyvinylidene difluoride |
| PFA | Paraformaldehyde |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| HRP | Horseradish peroxidase |
| IPTG | Isopropyl-β-D-thiogalactopyranoside |
| GST | Glutathione S-transferases |
| PBS | Phosphate-buffered saline |
| EDTA | Ethylenediaminetetraacetic acid |
| TBS | Tris-buffered saline |
| FITC | Fluorescein isothiocyanate |
References
- Pihlak, R.; Fong, C.; Starling, N. Targeted Therapies and Developing Precision Medicine in Gastric Cancer. Cancers 2023, 15, 3248. [Google Scholar] [CrossRef]
- Ideo, H.; Tsuchida, A.; Takada, Y.; Kinoshita, J.; Inaki, N.; Minamoto, T. Suppression of galectin-4 attenuates peritoneal metastasis of poorly differentiated gastric cancer cells. Gastric Cancer 2023, 26, 352–363. [Google Scholar] [CrossRef]
- Cao, Z.-Q.; Guo, X.-L. The role of galectin-4 in physiology and diseases. Protein Cell 2016, 7, 314–324. [Google Scholar] [CrossRef]
- Huflejt, M.E.; Leffler, H. Galectin-4 in normal tissues and cancer. Glycoconj. J. 2003, 20, 247–255. [Google Scholar] [CrossRef]
- Hayashi, T.; Saito, T.; Fujimura, T.; Hara, K.; Takamochi, K.; Mitani, K.; Mineki, R.; Kazuno, S.; Oh, S.; Ueno, T.; et al. Galectin-4, a Novel Predictor for Lymph Node Metastasis in Lung Adenocarcinoma. PLoS ONE 2013, 8, e81883. [Google Scholar] [CrossRef]
- Lidström, T.; Cumming, J.; Gaur, R.; Frängsmyr, L.; Pateras, I.S.; Mickert, M.J.; Franklin, O.; Forsell, M.N.; Arnberg, N.; Dongre, M.; et al. Extracellular Galectin 4 Drives Immune Evasion and Promotes T-cell Apoptosis in Pancreatic Cancer. Cancer Immunol. Res. 2022, 11, 72–92. [Google Scholar] [CrossRef]
- Ideo, H.; Seko, A.; Ohkura, T.; Matta, K.L.; Yamashita, K. High-affinity binding of recombinant human galectin-4 to SO3-->3Gal 1->3GalNAc pyranoside. Glycobiology 2002, 12, 199–208. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Kwak, J.-Y. Fucoidan as a Marine Anticancer Agent in Preclinical Development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and Cancer: A Multifunctional Molecule with Anti-Tumor Potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef]
- Ideo, H.; Seko, A.; Yamashita, K. Recognition Mechanism of Galectin-4 for Cholesterol 3-Sulfate. J. Biol. Chem. 2007, 282, 21081–21089. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.-R.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Yokozaki, H. Molecular characteristics of eight gastric cancer cell lines established in Japan. Pathol. Int. 2000, 50, 767–777. [Google Scholar] [CrossRef]
- Koike, T.; Sugimoto, A.; Kosono, S.; Komaba, S.; Kanno, Y.; Kitamura, T.; Anzai, I.; Watanabe, T.; Takahashi, D.; Toshima, K. Synthesis of low-molecular weight fucoidan derivatives and their binding abilities to SARS-CoV-2 spike proteins. RSC Med. Chem. 2021, 12, 2016–2021. [Google Scholar] [CrossRef]
- Sugimoto, A.; Koike, T.; Kuboki, Y.; Komaba, S.; Kosono, S.; Aswathy, M.; Anzai, I.; Watanabe, T.; Toshima, K.; Takahashi, D. Synthesis of Low-Molecular-Weight Fucoidan Analogue and Its Inhibitory Activities against Heparanase and SARS-CoV-2 Infection. Angew. Chem. Int. Ed. Engl. 2024, 64, e202411760. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Tsuchida, A.; Hachisu, K.; Mizuno, M.; Takada, Y.; Ideo, H. High expression of B3GALT5 suppresses the galectin-4-mediated peritoneal dissemination of poorly differentiated gastric cancer cells. Glycobiology 2024, 34, cwae064. [Google Scholar] [CrossRef]
- Cagnoni, A.J.; Massaro, M.; Cutine, A.M.; Gimeno, A.; Pérez-Sáez, J.M.; Cocco, M.N.M.; Maller, S.M.; Di Lella, S.; Jiménez-Barbero, J.; Ardá, A.; et al. Exploring galectin interactions with human milk oligosaccharides and blood group antigens identifies BGA6 as a functional galectin-4 ligand. J. Biol. Chem. 2024, 300, 107573. [Google Scholar] [CrossRef]
- Ferous, S.; Siafakas, N.; Boufidou, F.; Patrinos, G.P.; Tsakris, A.; Anastassopoulou, C. Investigating ABO Blood Groups and Secretor Status in Relation to SARS-CoV-2 Infection and COVID-19 Severity. J. Pers. Med. 2024, 14, 346. [Google Scholar] [CrossRef]
- Arafuka, S.; Koshiba, N.; Takahashi, D.; Toshima, K. Systematic synthesis of sulfated oligofucosides and their effect on breast cancer MCF-7 cells. Chem. Commun. 2014, 50, 9831–9834. [Google Scholar] [CrossRef]
- Yang, Y.; Wislez, M.; Fujimoto, N.; Prudkin, L.; Izzo, J.G.; Uno, F.; Ji, L.; Hanna, A.E.; Langley, R.R.; Liu, D.; et al. A selective small molecule inhibitor of c-Met, PHA-665752, reverses lung premalignancy induced by mutant K-ras. Mol. Cancer Ther. 2008, 7, 952–960. [Google Scholar] [CrossRef]
- Christensen, J.G.; Schreck, R.; Burrows, J.; Kuruganti, P.; Chan, E.; Le, P.; Chen, J.; Wang, X.; Ruslim, L.; Blake, R.; et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003, 63, 7345–7355. [Google Scholar]
- Brito, R.M.; Vaz, W.L. Determination of the critical micelle concentration of surfactants using the fluorescent probe N-phenyl-1-naphthylamine. Anal. Biochem. 1986, 152, 250–255. [Google Scholar] [CrossRef]
- Bum-Erdene, K.; Leffler, H.; Nilsson, U.J.; Blanchard, H. Structural characterisation of human galectin-4 N-terminal carbohydrate recognition domain in complex with glycerol, lactose, 3′-sulfo-lactose and 2′-fucosyllactose. Sci. Rep. 2016, 6, 20289. [Google Scholar] [CrossRef]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef]
- Turrini, E.; Maffei, F.; Fimognari, C. Ten Years of Research on Fucoidan and Cancer: Focus on Its Antiangiogenic and Antimetastatic Effects. Mar. Drugs 2023, 21, 307. [Google Scholar] [CrossRef]
- Xu, L.; Liu, F.; Li, C.; Li, S.; Wu, H.; Guo, B.; Gu, J.; Wang, L. Fucoidan suppresses the gastric cancer cell malignant phenotype and production of TGF-β1 via CLEC-2. Glycobiology 2019, 30, 301–311. [Google Scholar] [CrossRef]
- Mak, W.; Wang, S.K.; Liu, T.; Hamid, N.; Li, Y.; Lu, J.; White, W.L. Anti-Proliferation Potential and Content of Fucoidan Extracted from Sporophyll of New Zealand Undaria pinnatifida. Front. Nutr. 2014, 1, 9. [Google Scholar] [CrossRef]
- Wu, S.-C.; Arthur, C.M.; Wang, J.; Verkerke, H.; Josephson, C.D.; Kalman, D.; Roback, J.D.; Cummings, R.D.; Stowell, S.R. The SARS-CoV-2 receptor-binding domain preferentially recognizes blood group A. Blood Adv. 2021, 5, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Arthur, C.M.; Jan, H.-M.; Garcia-Beltran, W.F.; Patel, K.R.; Rathgeber, M.F.; Verkerke, H.P.; Cheedarla, N.; Jajosky, R.P.; Paul, A.; et al. Blood group A enhances SARS-CoV-2 infection. Blood 2023, 142, 742–747. [Google Scholar] [CrossRef]
- Hahismoto, S.; Yazawa, S.; Asao, T.; Faried, A.; Nishimura, T.; Tsuboi, K.; Nakagawa, T.; Yamauchi, T.; Koyama, N.; Umehara, K.; et al. Novel sugar-cholestanols as anticancer agents against peritoneal dissemination of tumor cells. Glycoconj. J. 2008, 25, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Mariño, K.V.; Cagnoni, A.J.; Croci, D.O.; Rabinovich, G.A. Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat. Rev. Drug Discov. 2023, 22, 295–316. [Google Scholar] [CrossRef]
- Martínez-Bailén, M.; Rojo, J.; Ramos-Soriano, J. Multivalent glycosystems for human lectins. Chem. Soc. Rev. 2022, 52, 536–572. [Google Scholar] [CrossRef]
- Slámová, K.; Červený, J.; Mészáros, Z.; Friede, T.; Vrbata, D.; Křen, V.; Bojarová, P. Oligosaccharide Ligands of Galectin-4 and Its Subunits: Multivalency Scores Highly. Molecules 2023, 28, 4039. [Google Scholar] [CrossRef]
- Tibbitts, J.; Canter, D.; Graff, R.; Smith, A.; Khawli, L.A. Key factors influencing ADME properties of therapeutic proteins: A need for ADME characterization in drug discovery and development. mAbs 2015, 8, 229–245. [Google Scholar] [CrossRef]
- Rustiguel, J.K.; Soares, R.O.S.; Meisburger, S.P.; Davis, K.M.; Malzbender, K.L.; Ando, N.; Dias-Baruffi, M.; Nonato, M.C. Full-length model of the human galectin-4 and insights into dynamics of inter-domain communication. Sci. Rep. 2016, 6, 33633. [Google Scholar] [CrossRef] [PubMed]
- Garner, O.B.; Baum, L.G. Galectin–glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem. Soc. Trans. 2008, 36, 1472–1477. [Google Scholar] [CrossRef]
- Tanikawa, R.; Tanikawa, T.; Okada, Y.; Nakano, K.; Hirashima, M.; Yamauchi, A.; Hosokawa, R.; Tanaka, Y. Interaction of Galectin-9 With Lipid Rafts Induces Osteoblast Proliferation Through the c-Src/ERK Signaling Pathway. J. Bone Miner. Res. 2008, 23, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Partridge, E.A.; Le Roy, C.; Di Guglielmo, G.M.; Pawling, J.; Cheung, P.; Granovsky, M.; Nabi, I.R.; Wrana, J.L.; Dennis, J.W. Regulation of Cytokine Receptors by Golgi N-Glycan Processing and Endocytosis. Science 2004, 306, 120–124. [Google Scholar] [CrossRef]
- Brewer, C. Clusters, bundles, arrays and lattices: Novel mechanisms for lectin–saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 2002, 12, 616–623. [Google Scholar] [CrossRef]
- Ideo, H.; Seko, A.; Yamashita, K. Galectin-4 Binds to Sulfated Glycosphingolipids and Carcinoembryonic Antigen in Patches on the Cell Surface of Human Colon Adenocarcinoma Cells. J. Biol. Chem. 2005, 280, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chan, H.S.; Hu, Z. Using PyMOL as a platform for computational drug design. WIREs Comput. Mol. Sci. 2017, 7, e1298. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, S.; Aswathy, M.; Kuboki, Y.; Takada, Y.; Toshima, K.; Takahashi, D.; Ideo, H. Inhibitory Effect of Fucoidan Analogs on Highly Metastatic Gastric Cancer Cells via Galectin-4 Inhibition. Int. J. Mol. Sci. 2025, 26, 9228. https://doi.org/10.3390/ijms26189228
Ji S, Aswathy M, Kuboki Y, Takada Y, Toshima K, Takahashi D, Ideo H. Inhibitory Effect of Fucoidan Analogs on Highly Metastatic Gastric Cancer Cells via Galectin-4 Inhibition. International Journal of Molecular Sciences. 2025; 26(18):9228. https://doi.org/10.3390/ijms26189228
Chicago/Turabian StyleJi, Shuting, Maniyamma Aswathy, Yuya Kuboki, Yoshio Takada, Kazunobu Toshima, Daisuke Takahashi, and Hiroko Ideo. 2025. "Inhibitory Effect of Fucoidan Analogs on Highly Metastatic Gastric Cancer Cells via Galectin-4 Inhibition" International Journal of Molecular Sciences 26, no. 18: 9228. https://doi.org/10.3390/ijms26189228
APA StyleJi, S., Aswathy, M., Kuboki, Y., Takada, Y., Toshima, K., Takahashi, D., & Ideo, H. (2025). Inhibitory Effect of Fucoidan Analogs on Highly Metastatic Gastric Cancer Cells via Galectin-4 Inhibition. International Journal of Molecular Sciences, 26(18), 9228. https://doi.org/10.3390/ijms26189228





