Transcriptomic Analysis of Osmotic Stress-Tolerant Somatic Embryos of Coffea arabica L. Mediated by the Coffee Antisense Trehalase Gene: A Marker-Free Approach
Abstract
1. Introduction
2. Results
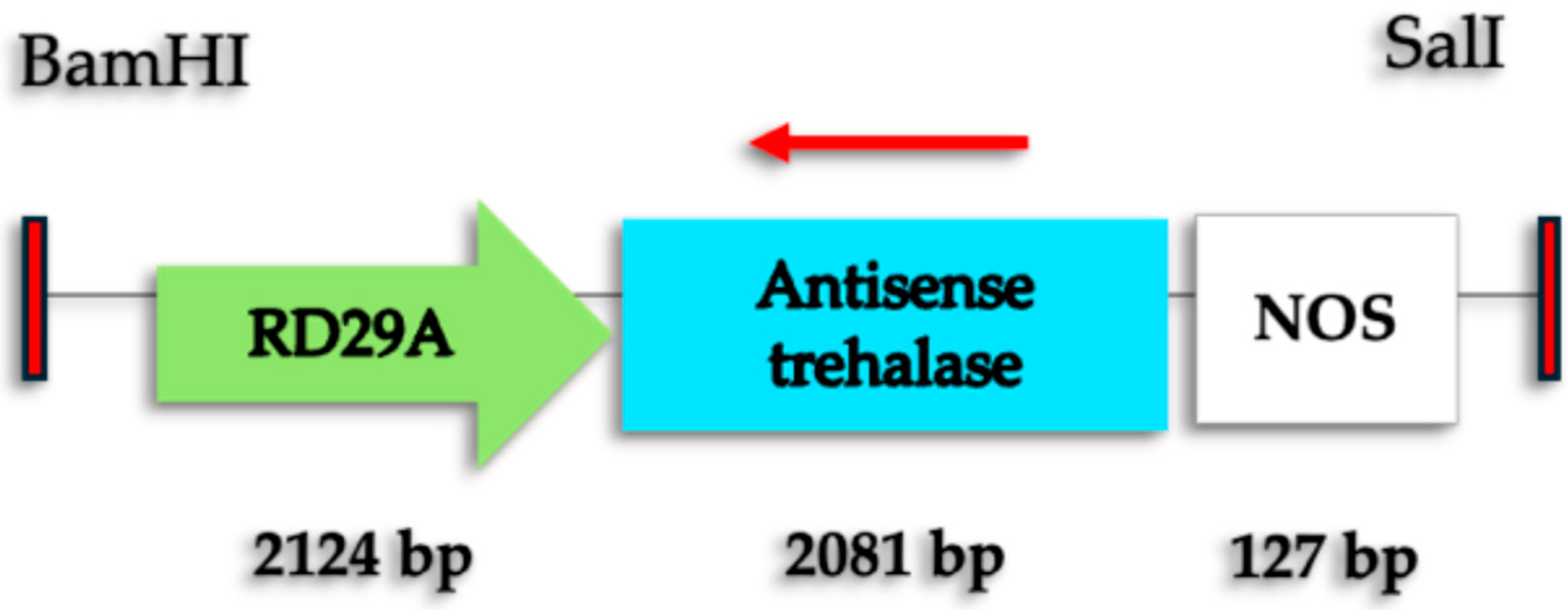
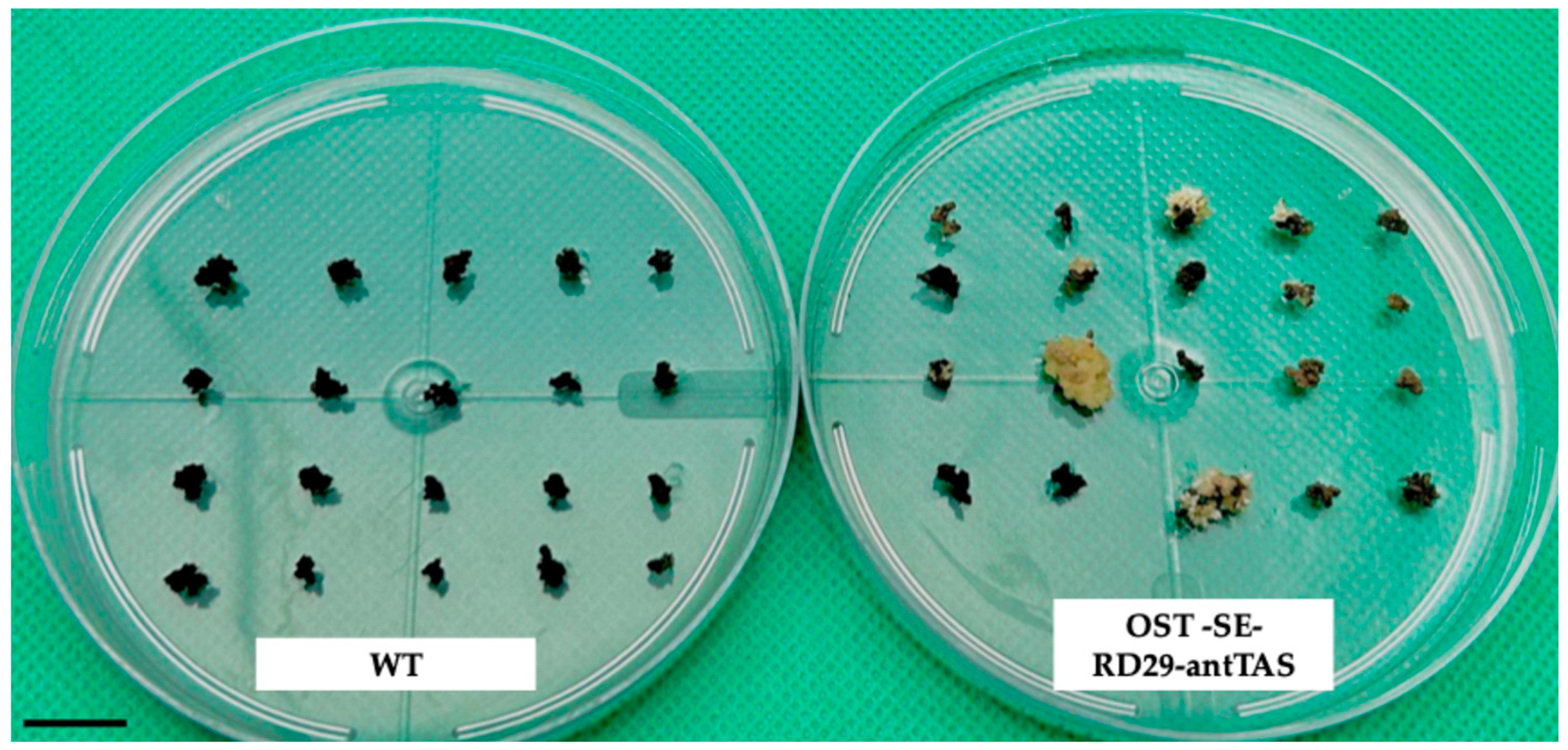
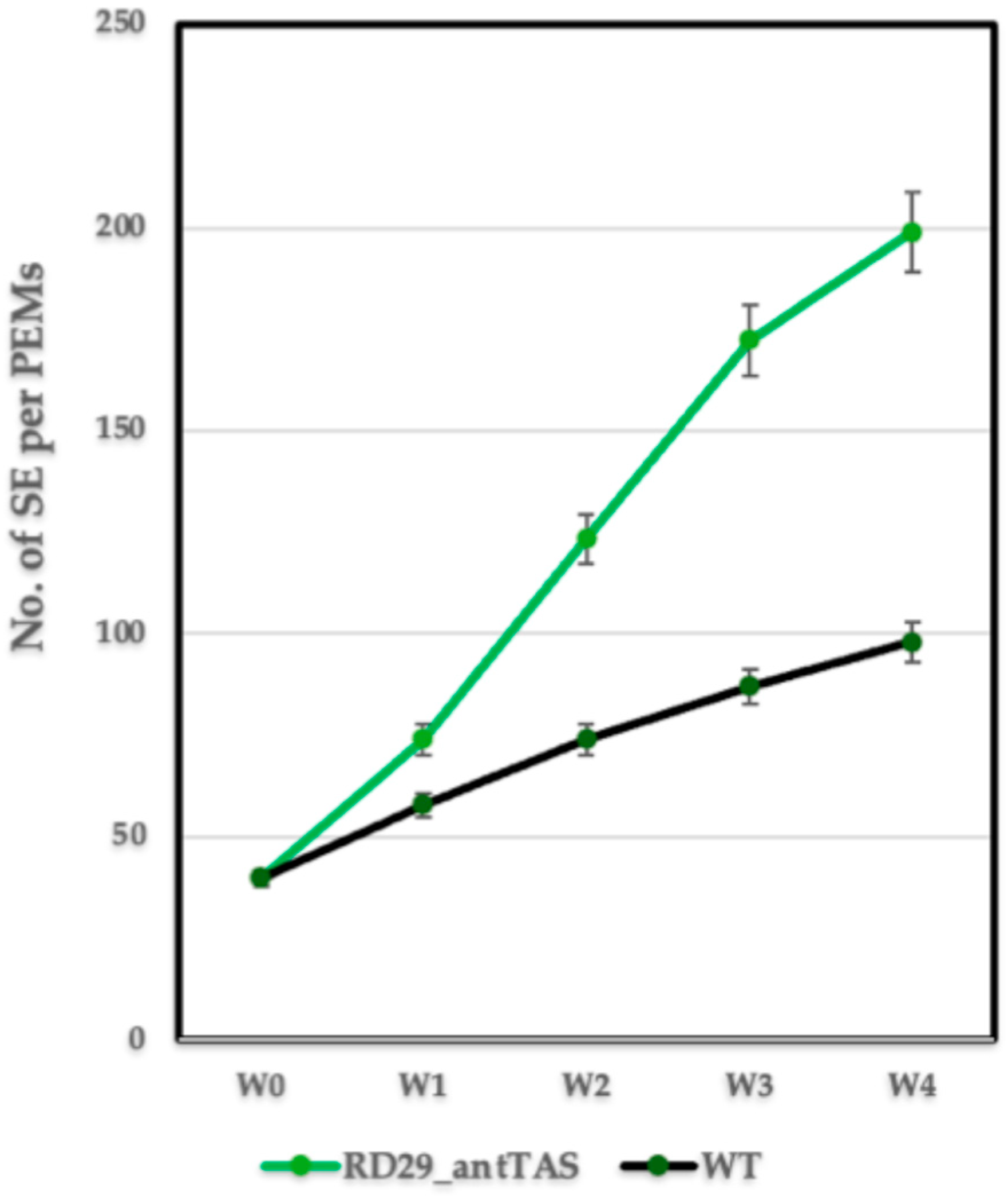
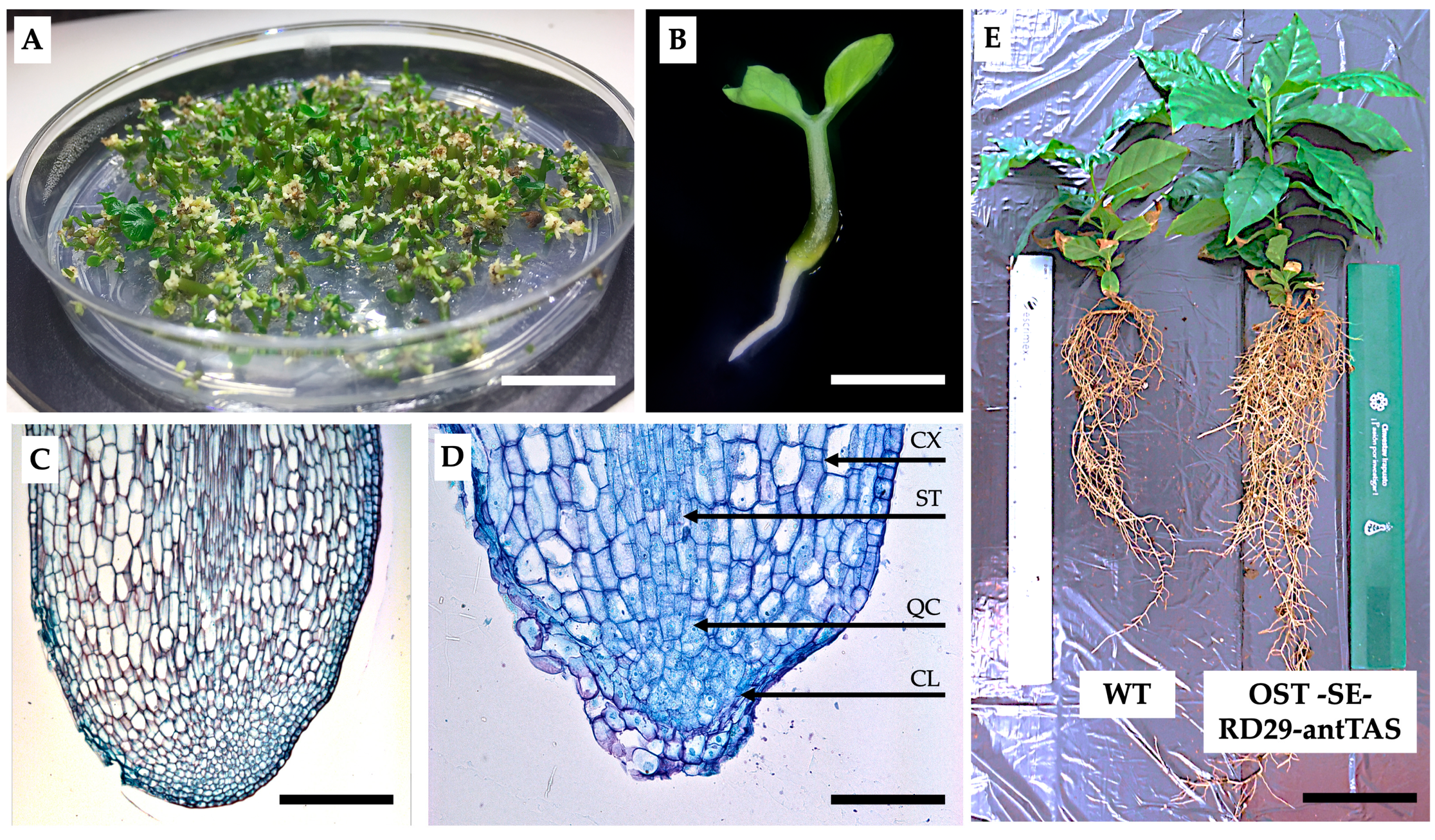
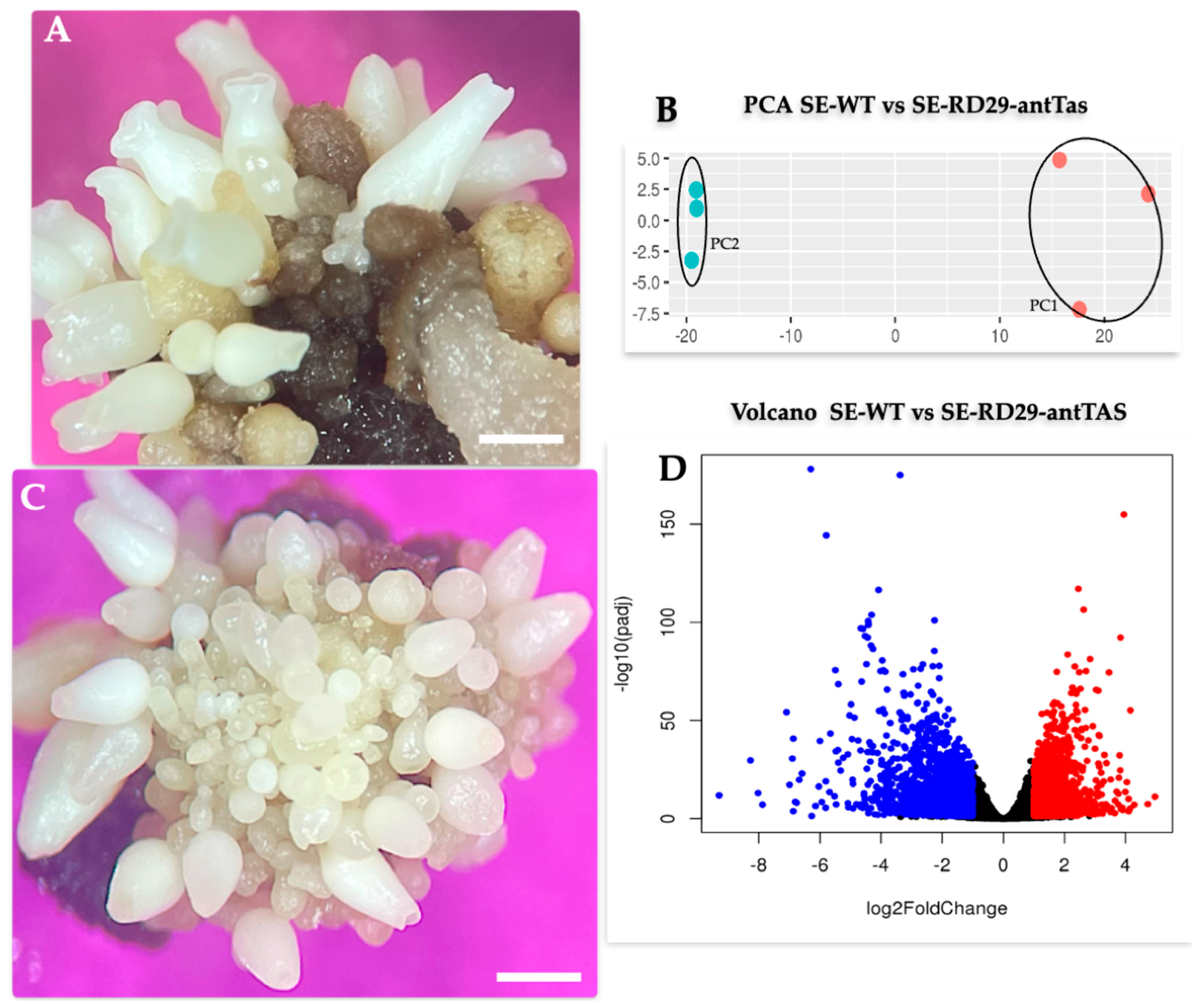
2.1. Genetic Modification of Coffee C. arabica L.
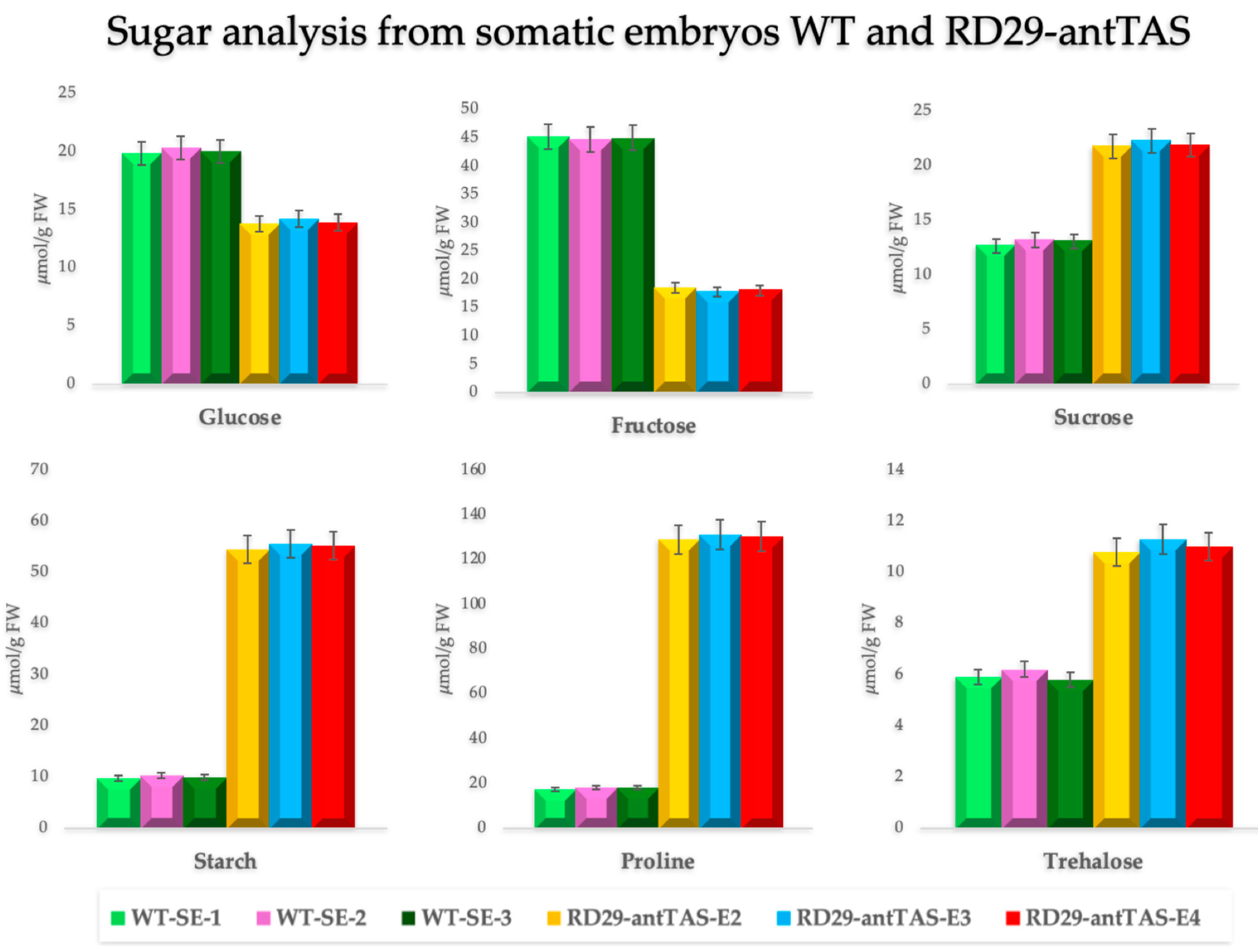
2.2. Quantification of Sugars and Proline in RD29-antTAS and WT-SE Lines
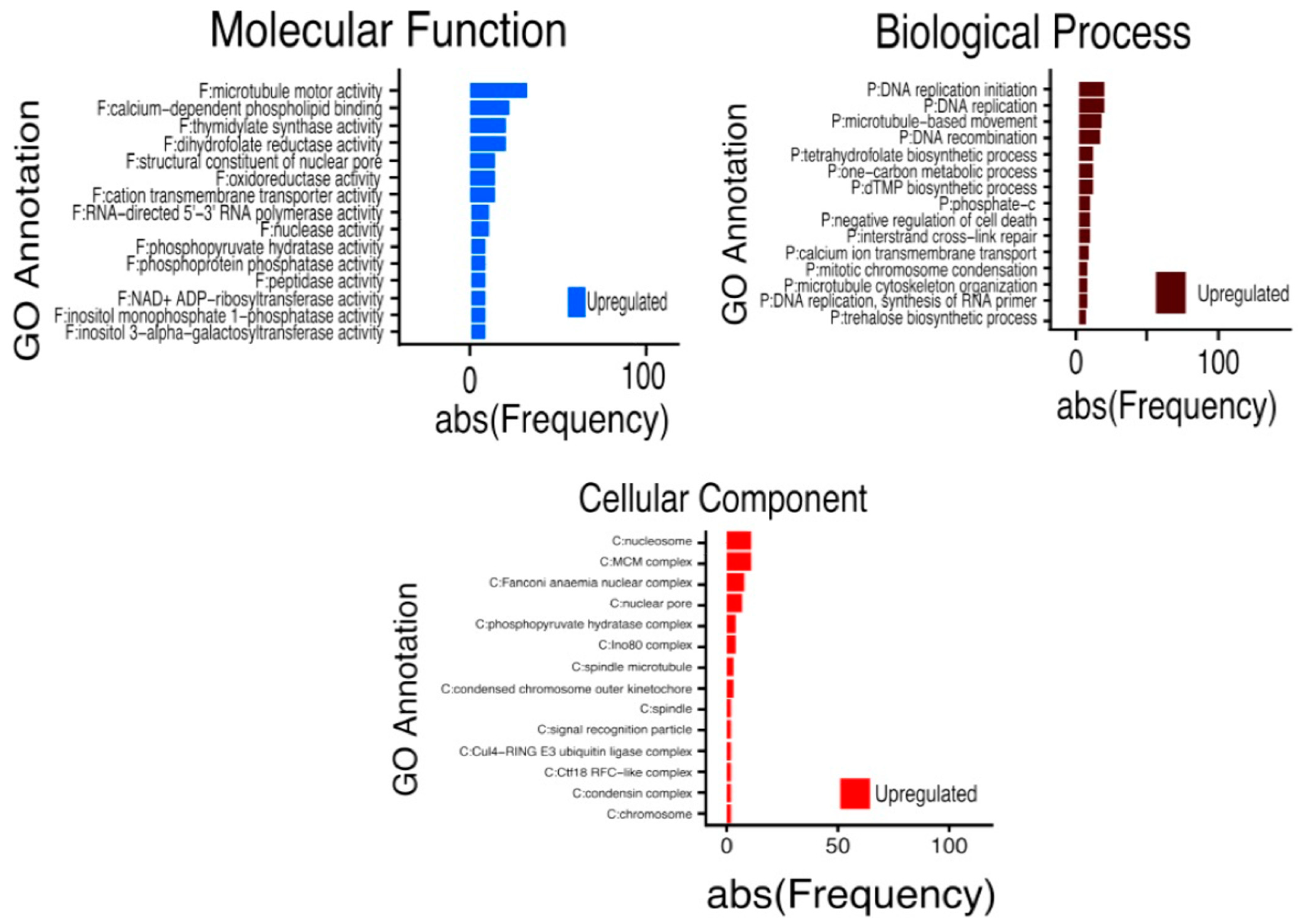
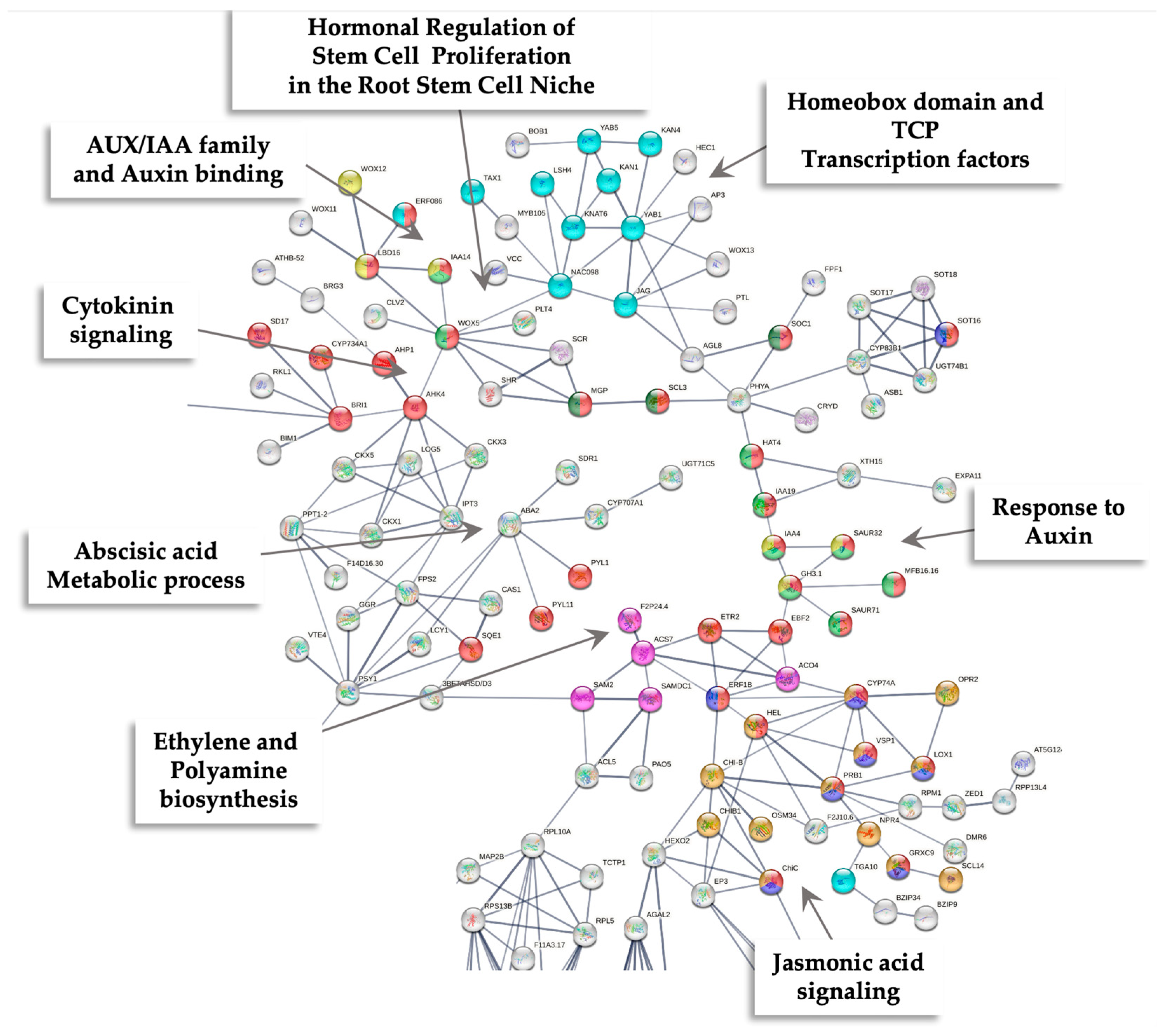
2.3. Transcriptomic-Wide Analysis of RD29-antTAS Line
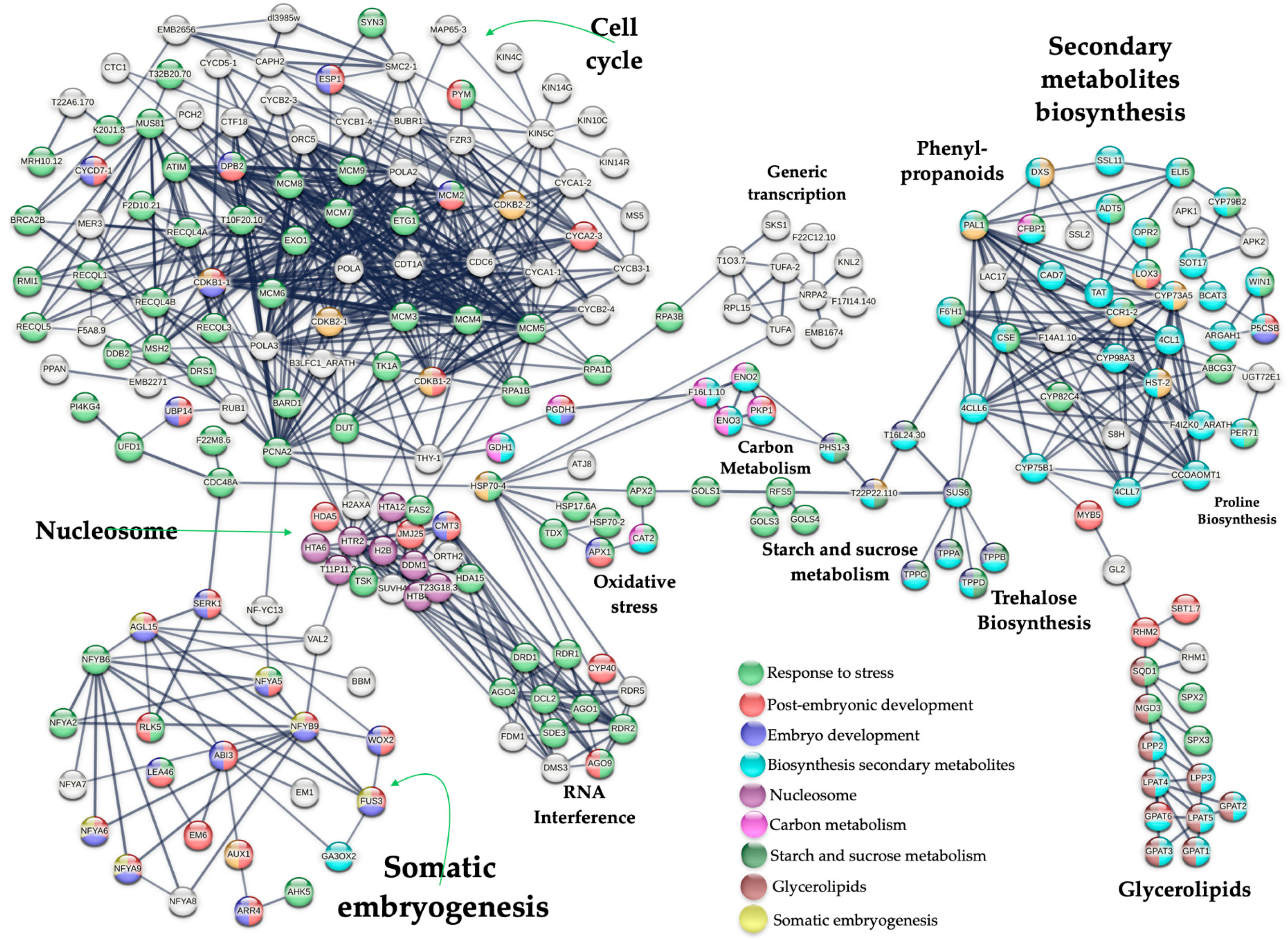
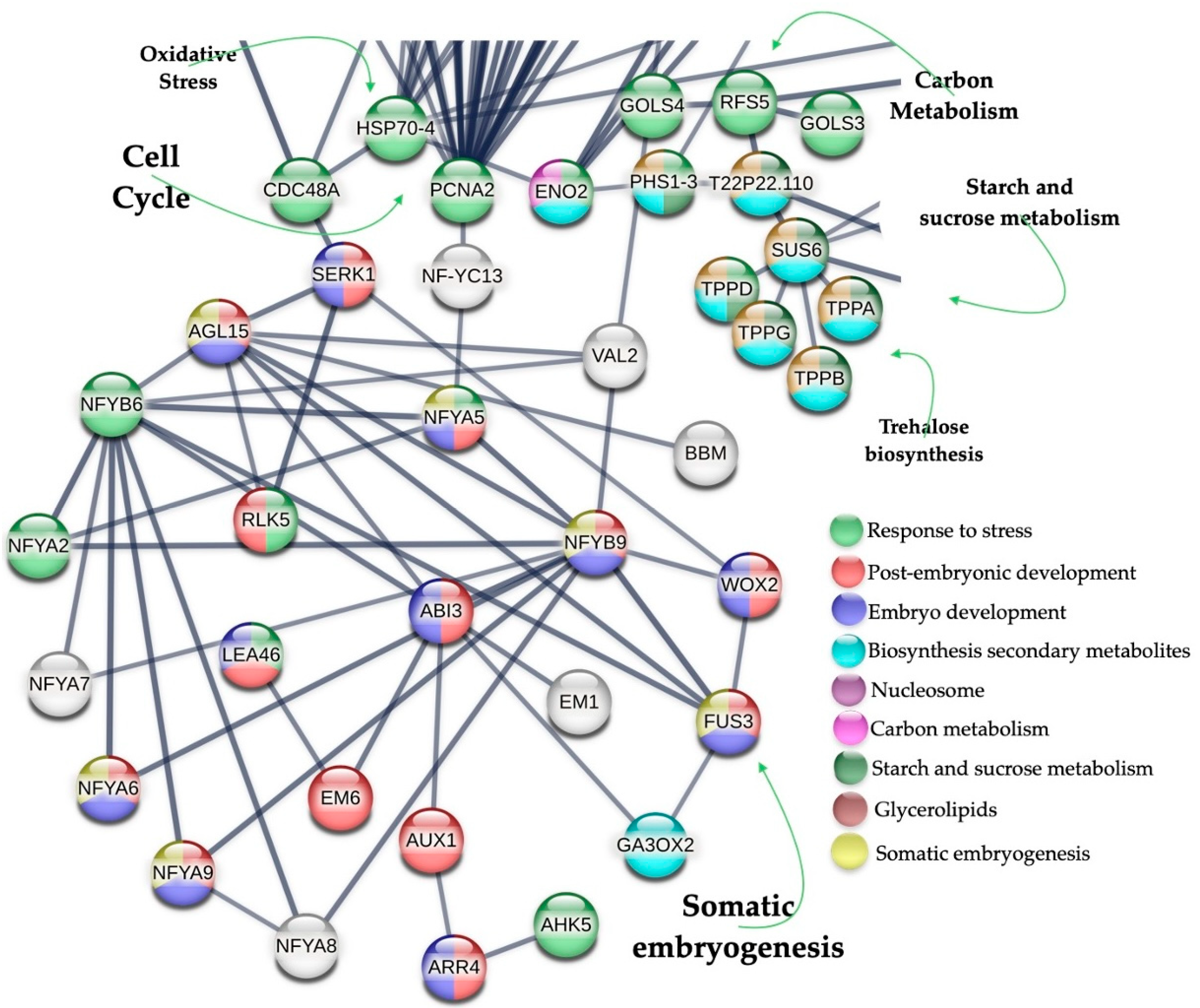
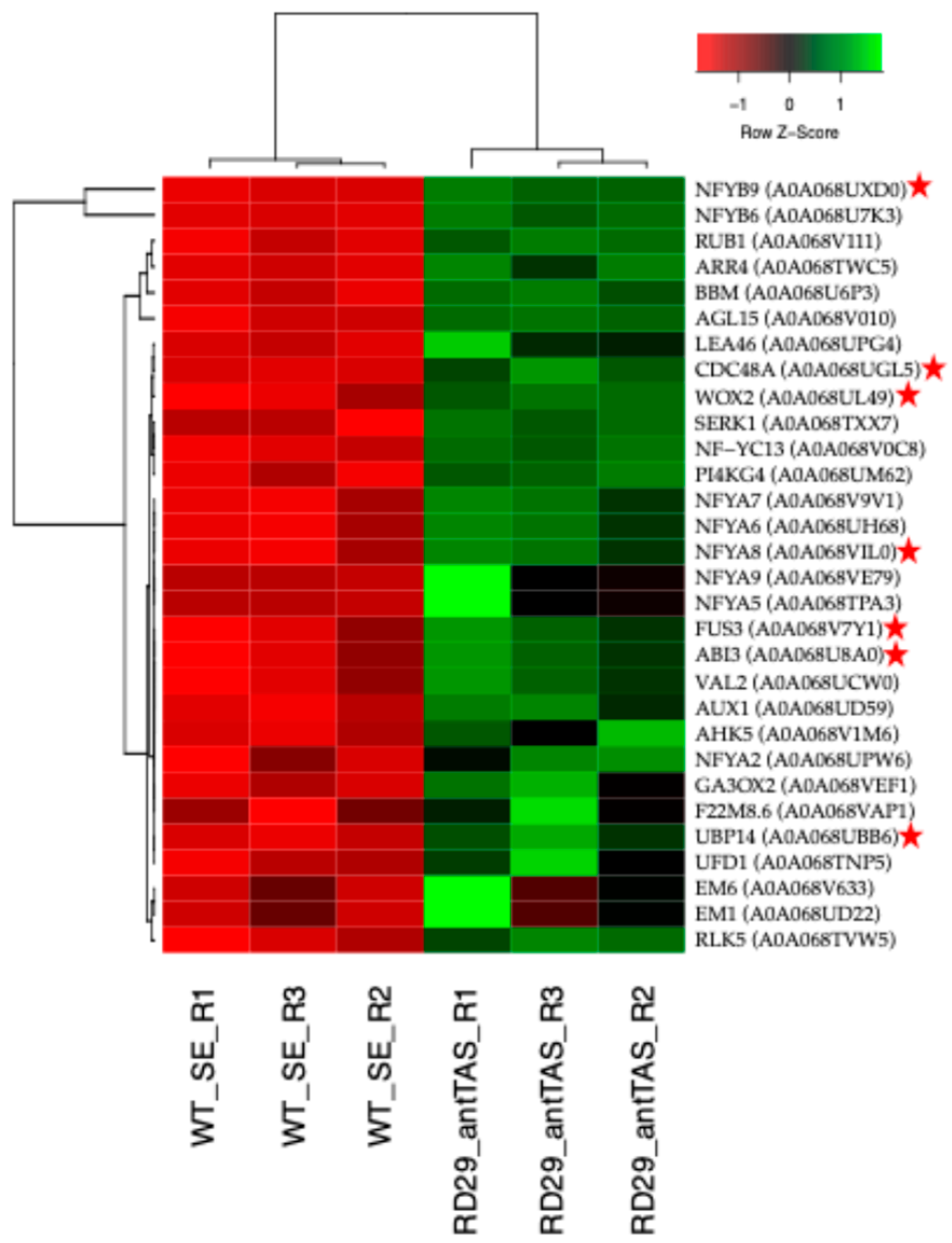
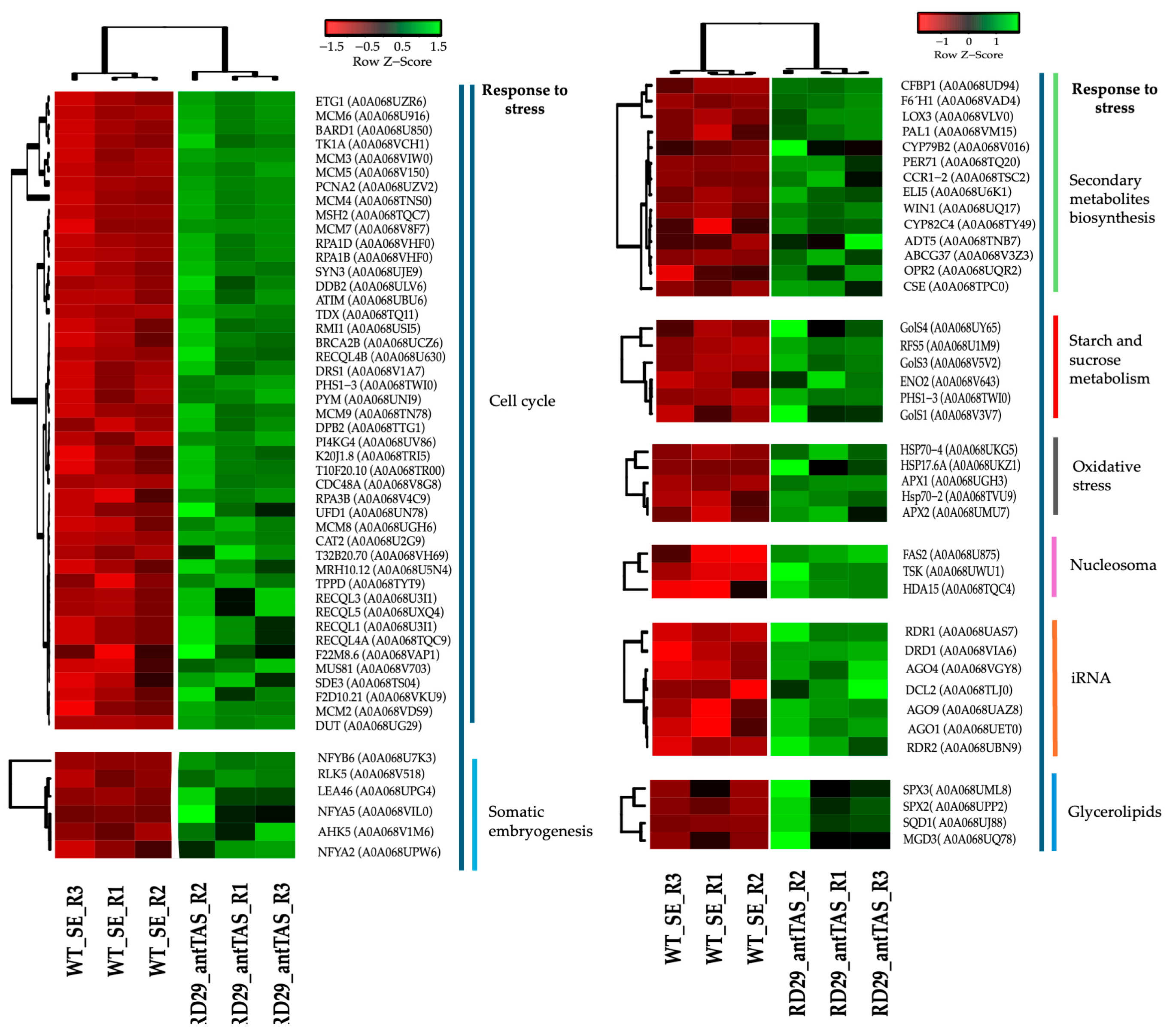
2.4. Up-Regulated Genes from a SE-RD29-antTAS Line
2.5. Up-Regulated Genes Related to SE in SE-RD29-antTAS
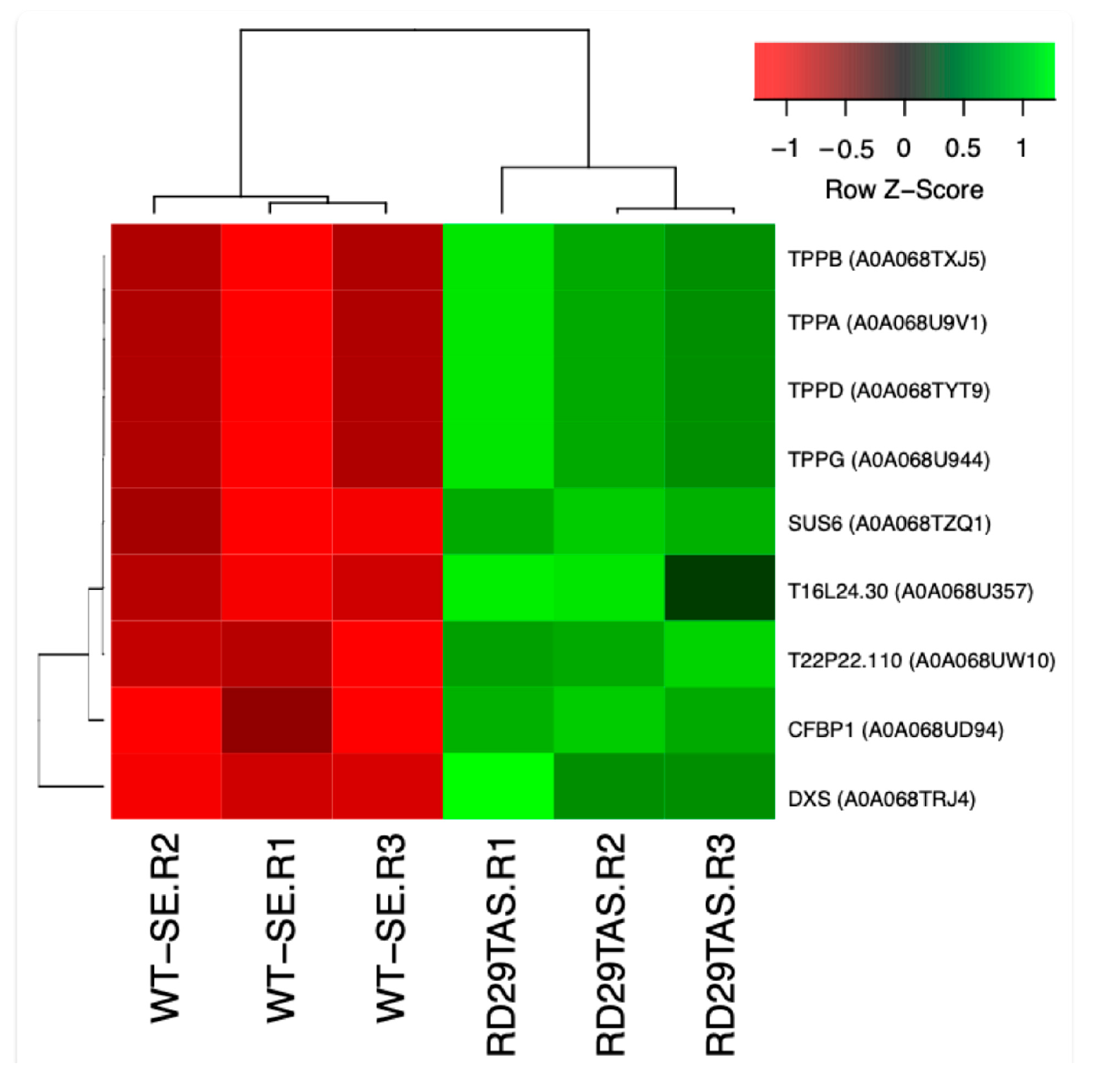
2.6. Up-Regulated Genes Related to Trehalose Biosynthesis in SE-RD29-antTAS
2.7. Up-Regulated Genes Related to Response to Stress in SE-RD29-antTAS
2.8. Down-Regulated Genes from a SE-RD29-antTAS Line
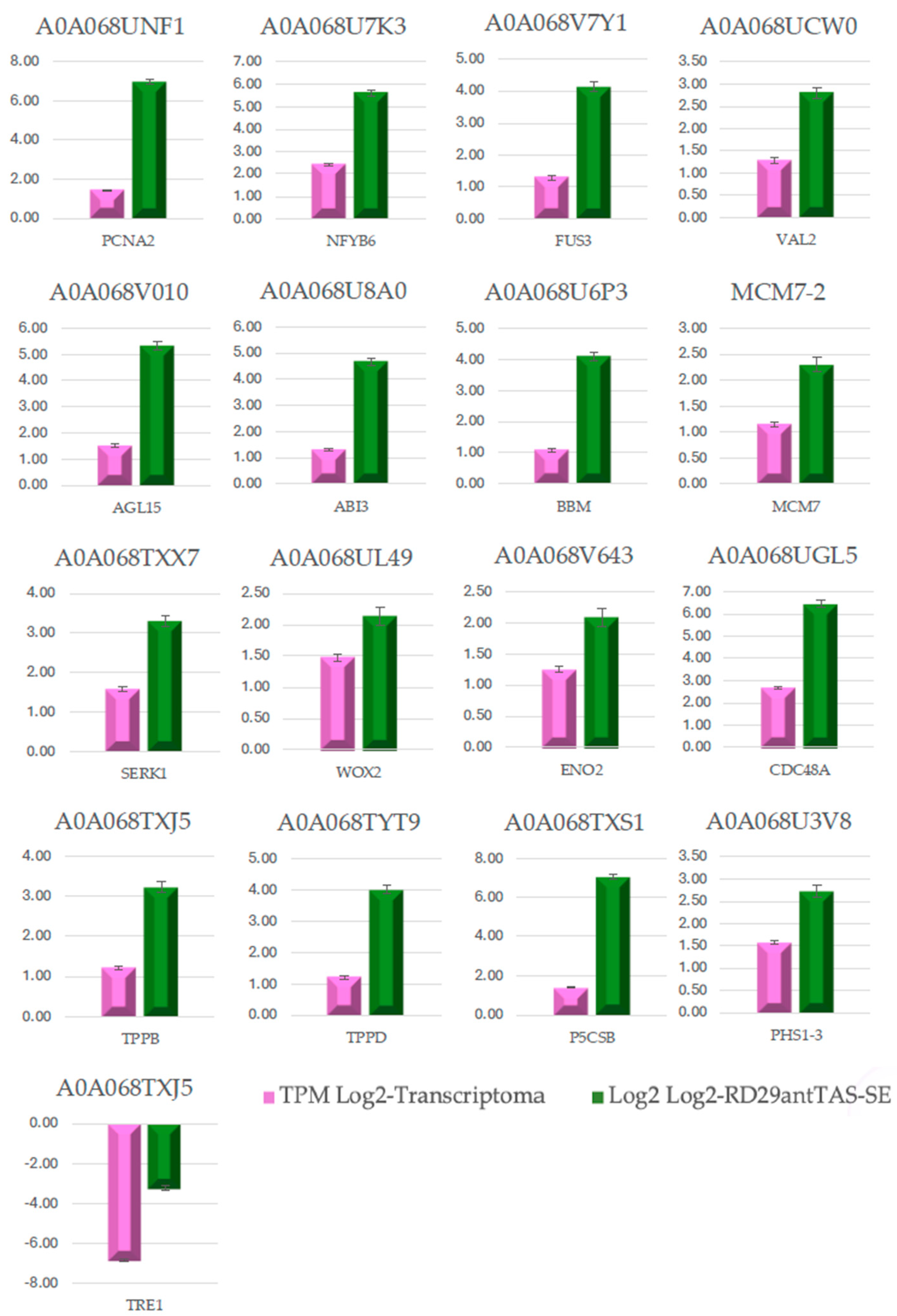
2.9. Validation of the Transcriptome-Wide Analysis
3. Discussion
3.1. Up-Regulated Genes in SE-RD29-antTAS
3.2. Interaction with Cell Cycle
3.3. PCNA2 Interacts with the Somatic Embryogenesis Gene Module
3.4. Proline Biosynthesis Module
3.5. Down-Regulated Genes in SE-RD29-antTAS
4. Materials and Methods
4.1. Generation of Marker-Free SE-RD29-antTAS of Coffee C. arabica L. var Typica
4.2. Histological Analysis of SEs
4.3. RNA Isolation and qPCR Analysis
4.4. Aligned and Analysis of DEG and GO in the SE-RD29-antTAS Line of Coffee C. arabica L.
4.5. PPI Analysis of Up-Regulated Genes Found in SE-RD29-antTAS Line
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TRE | Trehalose |
| RD29-antTAS | RD29 antisense TAS |
| TAS | Trehalase |
| RD29-antTAS | Osmotic-stress somatic embryos |
| WT-SE | Wild-type somatic embryogenesis |
References
- Pinheiro, H.A.; Da Matta, F.M.; Chaves, A.R.; Loureiro, M.E.; Ducatti, C. Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora. Ann. Bot. 2005, 96, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bunn, C.; Läderach, P.; Pérez Jimenez, J.G.; Montagnon, C.; Schilling, T. Multiclass classification of agroecological zones for Arabica coffee: An improved understanding of the impacts of climate change. PLoS ONE 2015, 10, e0140490. [Google Scholar] [CrossRef]
- ICO—International Coffee Organization. Coffee Price Continues in November Reaching a 10-Year High. Coffee Market Report. 2021. Available online: https://www.ico.org/documents/cy2021-22/cmr-1121-e.pdf (accessed on 1 June 2023).
- Assad, E.D.; Pinto, H.; Junior, J.Z.; Ávila, A.M.H. Impacto das mudanças climáticas no zoneamento agroclimático do café no Brasil. Pesqui Agropecu. Bras. 2004, 39, 1057–1064. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ramalho, J.D.C. Impacts of drought and temperature stress on coffee physiology and production: A review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Bertrand, B.; Marraccini, P.; Villain, L.; Breitler, J.C.; Etienne, H. Healthy tropical plants to mitigate the impact of climate change—As exemplified in coffee. Clim. Change Agric. Worldw. 2016, 1, 83–95. [Google Scholar] [CrossRef]
- Mofatto, L.S.; Carneiro, F.D.A.; Vieira, N.G.; Duarte, K.E.; Vidal, R.O.; Alekcevetch, J.C.; Cotta, M.G.; Verdeil, J.-L.; Lapeyre-Montes, F.; Lartaud, M.; et al. Identification of candidate genes for drought tolerance in coffee by high-throughput sequencing in the shoot apex of different Coffea arabica cultivars. BMC Plant Biol. 2016, 16, 94. [Google Scholar] [CrossRef]
- Menezes-Silva, P.E.; Sanglard, L.M.; Ávila, R.T.; Morais, L.E.; Martins, S.C.; Nobres, P.; Patreze, C.M.; Ferreira, M.A.; Araújo, W.L.; Fernie, A.R.; et al. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017, 68, 4309–4322. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Avila, R.T.; Cardoso, A.A.; Martins, S.C.; Ramalho, J.C. Physiological and agronomic performance of the coffee crop in the context of climate change and global warming: A review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef] [PubMed]
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The impact of climate change and variability on coffee production: A systematic review. Clim. Change 2019, 156, 609–630. [Google Scholar] [CrossRef]
- Ahmed, S.; Brinkley, S.; Smith, E.; Sela, A.; Theisen, M.; Thibodeau, C.; Warne, T.; Anderson, E.; Van Dusen, N.; Giuliano, P.; et al. Climate change and coffee quality: Systematic review on the effects of environmental and management variation on secondary metabolites and sensory attributes of Coffea arabica and Coffea canephora. Front. Plant Sci. 2021, 12, 708013. [Google Scholar] [CrossRef]
- Marques, I.; Gouveia, D.; Gaillard, J.C.; Martins, S.; Semedo, M.C.; Lidon, F.C.; DaMatta, F.M.; Ribeiro-Barros, A.I.; Armengaud, J.; Ramalho, J.C. Next-generation proteomics reveals a greater antioxidative response to drought in Coffea arabica than in Coffea canephora. Agronomy 2022, 12, 148. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, X.; Cheng, L.; Hu, L.; Zhu, L.; Cao, J.; Guo, X. A draft gene regulatory network for cellular totipotency reprogramming during plant somatic embryogenesis. Genomics 2007, 90, 620–628. [Google Scholar] [CrossRef]
- Karami, O.; Saidi, A. The molecular basis for stress-induced acquisition of somatic embryogenesis. Mol. Biol. Rep. 2010, 37, 2493–2507. [Google Scholar] [CrossRef]
- Elhiti, M.; Stasolla, C.; Wang, A. Molecular regulation of plant somatic embryogenesis. Vitr. Cell. Dev. Biol. Plant. 2013, 49, 631–642. [Google Scholar] [CrossRef]
- Horstman, A.; Bemer, M.; Boutilier, K. A transcriptional view on somatic embryogenesis. Regeneration 2017, 4, 201–216. [Google Scholar] [CrossRef]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-La-Peña, C.; Loyola-Vargas, V.M. Signaling overview of plant somatic embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef]
- Gulzar, B.; Mujib, A.; Malik, M.Q.; Sayeed, R.; Mamgain, J.; Ejaz, B. Genes, proteins and other networks regulating somatic embryogenesis in plants. J. Genet. Eng. Biotechnol. 2020, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Salaün, C.; Lepiniec, L.; Dubreucq, B. Genetic and molecular control of somatic embryogenesis. Plants 2021, 10, 1467. [Google Scholar] [CrossRef] [PubMed]
- de Silva, K.K.; Dunwell, J.M.; Wickramasuriya, A.M. Weighted Gene Correlation Network Analysis (WGCNA) of Arabidopsis Somatic Embryogenesis (SE) and Identification of Key Gene Modules to Uncover SE-Associated Hub Genes. Int. J. Genom. 2022, 4, 7471063. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Kagale, S.; Ferrie, A.M.R. Multifaceted roles of transcription factors during plant embryogenesis. Front. Plant Sci. 2024, 14, 1322728. [Google Scholar] [CrossRef]
- Tonietto, Â.; Sato, J.H.; Teixeira, J.B.; de Souza, E.M.; Pedrosa, F.O.; Franco, O.L.; Mehta, A. Proteomic analysis of developing somatic embryos of Coffea arabica. Plant Mol. Biol. Rep. 2012, 30, 1393–1399. [Google Scholar] [CrossRef]
- Pinto, R.T.; Freitas, N.C.; Máximo, W.P.F.; Cardoso, T.B.; Prudente, D.D.O.; Paiva, L.V. Genome-wide analysis, transcription factor network approach and gene expression profile of GH3 genes over early somatic embryogenesis in Coffea spp. BMC Genom. 2019, 20, 812. [Google Scholar] [CrossRef]
- Quintana-Escobar, A.O.; Nic-Can, G.I.; Avalos, R.M.G.; Loyola-Vargas, V.M.; Gongora-Castillo, E. Transcriptome analysis of the induction of somatic embryogenesis in Coffea canephora and the participation of ARF and Aux/IAA genes. PeerJ 2019, 7, e7752. [Google Scholar] [CrossRef]
- Quintana-Escobar, A.O.; Bojórquez-Velázquez, E.; Ruiz-May, E.; Loyola-Vargas, V.M. Proteomic Approach during the Induction of Somatic Embryogenesis in Coffea canephora. Plants 2023, 12, 4095. [Google Scholar] [CrossRef]
- Méndez-Hernández, H.A.; Quintana-Escobar, A.O.; Uc-Chuc, M.A.; Loyola-Vargas, V.M. Genome-wide analysis, modeling, and identification of amino acid binding motifs suggest the involvement of GH3 genes during somatic embryogenesis of Coffea canephora. Plants 2021, 10, 2034. [Google Scholar] [CrossRef] [PubMed]
- Awada, R.; Lepelley, M.; Breton, D.; Charpagne, A.; Campa, C.; Berry, V.; Georget, F.; Breitler, J.-C.; Léran, S.; Djerrab, D.; et al. Global transcriptome profiling reveals differential regulatory, metabolic and hormonal networks during somatic embryogenesis in Coffea arabica. BMC Genom. 2023, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Birch, G.G. Trehaloses. Adv. Carbohydr. Chem. 1963, 18, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Avonce, N.; Mendoza-Vargas, A.; Morett, E.; Iturriaga, G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006, 6, 109. [Google Scholar] [CrossRef]
- Müller, J.; Aeschbacher, R.A.; Wingler, A.; Boller, T.; Wiemken, A. Trehalose and trehalase in Arabidopsis. Plant Physiol. 2001, 125, 1086–1093. [Google Scholar] [CrossRef]
- Gámez Escobedo, I.A.; Cabrera Ponce, J.L.; Herrera Estrella, L.R.; Hernández Luna, C.E.; Montes de Oca Luna, R. Mejora del crecimiento de plantas de tabaco mediante la inhibición del gen de la trehalasa. Cienc. UANL 2004, 7, 4. Available online: http://eprints.uanl.mx/1463/ (accessed on 10 June 2025).
- Kenny Alejandra Agreda Laguna, J.L.; Cabrera Ponce, A.; Gamez Escobedo, L.R.; Herrera Estrella, R.; Montes De Oca Luna, R.; Ruíz Medrano, B.; Xoconostle Cázares, L.A. Methods To Obtain Drought Resistant Plants (Fr) Procédés Pour Obtenir Des Plantes Résistantes À La Sécheresse [Methods To Obtain Drought Resistant Plants]. WO2012085806, January 2012. Available online: http://patentscope.wipo.int/search/en/WO2012085806 (accessed on 10 June 2025).
- Agreda-Laguna, K.A.; Cabrera-Ponce, J.L.; Medrano, R.R.; Garzon-Tiznado, J.A.; Xoconostle-Cazares, B. Trehalose accumulation provides drought tolerance to genetically modified maize in open field trials. Pak. J. Agric. Sci. 2018, 55, 1009–1020. [Google Scholar]
- Valencia-Lozano, E.; Ibarra, J.E.; Herrera-Ubaldo, H.; De Folter, S.; Cabrera-Ponce, J.L. Osmotic stress-induced somatic embryo maturation of coffee Coffea arabica L., shoot and root apical meristems development and robustness. Sci. Rep. 2021, 11, 9661. [Google Scholar] [CrossRef]
- Byrareddy, V.; Kouadio, L.; Mushtaq, S.; Kath, J.; Stone, R. Coping with drought: Lessons learned from robusta coffee growers in Vietnam. Clim. Serv. 2021, 22, 100229. [Google Scholar] [CrossRef]
- de Freitas Guedes, F.A.; Nobres, P.; Ferreira, D.C.R.; Menezes-Silva, P.E.; Ribeiro-Alves, M.; Correa, R.L.; DaMatta, F.M.; Alves-Ferreira, M. Transcriptional memory contributes to drought tolerance in coffee (Coffea canephora) plants. Environ. Exp. Bot. 2018, 147, 220–233. [Google Scholar] [CrossRef]
- Kriegshauser, L.; Knosp, S.; Grienenberger, E.; Tatsumi, K.; Gütle, D.D.; Sørensen, I.; Herrgott, L.; Zumsteg, J.; Rose, J.K.C.; Reski, R.; et al. Function of the Hydroxycinnamoyl-Coa: Shikimate Hydroxycinnamoyl Transferase is evolutionarily conserved in embryophytes. Plant Cell 2021, 33, 1472–1491. [Google Scholar] [CrossRef] [PubMed]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Gao, G.; Qin, K.; Chen, Y.; Wang, H.; Li, X.; Liang, G.; Wang, C.; Mu, C.; Su, Q. Multiple low salinity stress modes provided novel insight into the metabolic response of Scylla paramamosain adapting to inland saline-alkaline water. Front. Mar. Sci. 2022, 9, 977599. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboiñska, M.; Labudda, M.; Morkunas, I. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Streb, S.; Zeeman, S.C. Starch metabolism in Arabidopsis. Arab. Book 2012, 10, e0160. [Google Scholar] [CrossRef]
- Ashraf, M.J.B.A. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Zrenner, R.; Salanoubat, M.; Willmitzer, L.; Sonnewald, U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants Solanum tuberosum L. Plant J. 1995, 7, 97–107. [Google Scholar] [CrossRef]
- Craig, J.; Barratt, P.; Tatge, H.; Déjardin, A.; Handley, L.; Gardner, C.D.; Barber, L.; Wang, T.; Hedley, C.; Martin, C.; et al. Mutations at the rug4 locus alter the carbon and nitrogen metabolism of pea plants through an effect on sucrose synthase. Plant J. 1999, 17, 353–362. [Google Scholar] [CrossRef]
- Zeeman, S.C.; Thorneycroft, D.; Schupp, N.; Chapple, A.; Weck, M.; Dunstan, H.; Haldimann, P.; Bechtold, N.; Smith, A.M.; Smith, S.M. Plastidial α-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiol. 2004, 135, 849–858. [Google Scholar] [CrossRef]
- Geshi, N.; Johansen, J.N.; Dilokpimol, A.; Rolland, A.; Belcram, K.; Verger, S.; Kotake, T.; Tsumuraya, Y.; Kaneko, S.; Tryfona, T.; et al. A galactosyltransferase acting on arabinogalactan protein glycans is essential for embryo development in Arabidopsis. Plant J. 2013, 76, 128–137. [Google Scholar] [CrossRef]
- Eremina, M.; Rozhon, W.; Yang, S.; Poppenberger, B. ENO2 activity is required for the development and reproductive success of plants and is feedback-repressed by AtMBP-1. Plant J. 2015, 81, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, H.; Bing, J.; Zhang, G. Integrative transcriptomic and TMT-based proteomic analysis reveals the mechanism by which AtENO2 affects seed germination under salt stress. Front. Plant Sci. 2022, 13, 1035750. [Google Scholar] [CrossRef]
- Mu, J.; Tan, H.; Zheng, Q.; Fu, F.; Liang, Y.; Zhang, J.; Yang, X.; Wang, T.; Chong, K.; Wang, X.-J.; et al. Leafy Cotyledon1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol. 2008, 148, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.T.; Ge, L.F.; Liang, R.Q.; Li, W.; Ruan, K.C.; Lin, H.X.; Jin, Y.X. Members of miR-169 family are induced by high salinity and transiently inhibit the NF-YA transcription factor. BMC Mol. Biol. 2009, 10, 29–41. [Google Scholar] [CrossRef]
- Mu, J.; Tan, H.; Hong, S.; Liang, Y.; Zuo, J. Arabidopsis transcription factor genes NF-YA1, 5, 6, and 9 play redundant roles in male gametogenesis, embryogenesis, and seed development. Mol. Plant. 2013, 6, 188–201. [Google Scholar] [CrossRef] [PubMed]
- West, M.A.; Yee, K.M.; Danao, J.; Zimmerman, J.L.; Fischer, R.L.; Goldberg, R.B.; Harada, J.J. Leafy Cotyledon1 is an essential regulator of late embryogenesis and cotyledon identity in Arabidopsis. Plant Cell 1994, 6, 1731–1745. [Google Scholar] [CrossRef]
- Lotan, T.; Ohto, M.A.; Yee, K.M.; West, M.A.; Lo, R.; Kwong, R.W.; Yamagishi, K.; Fischer, R.L.; Goldberg, R.B.; Harada, J.J. Arabidopsis Leafy Cotyledon1 is sufficient to induce embryo development in vegetative cells. Cell 1998, 93, 1195–1205. [Google Scholar] [CrossRef]
- Gaj, M.D.; Zhang, S.; Harada, J.J.; Lemaux, P.G. Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 2005, 222, 977–988. [Google Scholar] [CrossRef]
- Fornari, M.; Calvenzani, V.; Masiero, S.; Tonelli, C.; Petroni, K. The Arabidopsis NF-YA3 and NF-YA8 genes are functionally redundant and are required in early embryogenesis. PLoS ONE 2013, 8, e82043. [Google Scholar] [CrossRef] [PubMed]
- Thakare, D.; Tang, W.; Hill, K.; Perry, S.E. The MADS-domain transcriptional regulator AGAMOUS-LIKE15 promotes somatic embryo development in Arabidopsis and soybean. Plant Physiol. 2008, 146, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Morończyk, J.; Wójcik, A.; Gaj, M.D. AGL15 controls the embryogenic reprogramming of somatic cells in Arabidopsis through the histone acetylation-mediated repression of the miRNA biogenesis genes. Int. J. Mol. Sci. 2020, 21, 6733. [Google Scholar] [CrossRef]
- Suzuki, M.; Wang, H.H.Y.; McCarty, D.R. Repression of the Leafy Cotyledon 1/B3 regulatory network in plant embryo development by VP1/Abscisic Acid Insensitive 3-Like B3 genes. Plant physiol. 2007, 143, 902–911. [Google Scholar] [CrossRef]
- Kagale, S.; Rozwadowski, K. EAR motif-mediated transcriptional repression in plants: An underlying mechanism for epigenetic regulation of gene expression. Epigenetics 2011, 6, 141–146. [Google Scholar] [CrossRef]
- Jia, H.; McCarty, D.R.; Suzuki, M. Distinct roles of LAFL network genes in promoting the embryonic seedling fate in the absence of VAL repression. Plant Physiol. 2013, 163, 1293–1305. [Google Scholar] [CrossRef]
- Freitas, N.C.; Barreto, H.G.; Torres, L.F.; Freire, L.L.; Rodrigues, L.A.Z.; Diniz, L.E.C.; Beijo, L.A.; Paiva, L.V. In silico and in vivo analysis of ABI3 and VAL2 genes during somatic embryogenesis of Coffea arabica: Competence acquisition and developmental marker genes. Plant Cell Tissue Organ Cult. PCTOC 2019, 137, 599–611. [Google Scholar] [CrossRef]
- Boutilier, K.; Offringa, R.; Sharma, V.K.; Kieft, H.; Ouellet, T.; Zhang, L.; Hattori, J.; Liu, C.-M.; van Lammeren, A.A.M.; Miki, B.L.A.; et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 2002, 14, 1737–1749. [Google Scholar] [CrossRef]
- Hecht, V.; Vielle-Calzada, J.P.; Hartog, M.V.; Schmidt, E.D.L.; Boutilier, K.; Grossniklaus, U.; De Vries, S.C. The Arabidopsis somatic embryogenesis receptor kinase 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001, 127, 803–816. [Google Scholar] [CrossRef][Green Version]
- Albrecht, C.; Russinova, E.; Hecht, V.; Baaijens, E.; de Vries, S. The Arabidopsis thaliana Somatic Embryogenesis Receptor-Like Kinases1 and 2 control male sporogenesis. Plant Cell 2005, 17, 3337–3349. [Google Scholar] [CrossRef]
- Mahdavi-Darvari, F.; Noor, N.M.; Ismanizan, I. Epigenetic regulation and gene markers as signals of early somatic embryogenesis. Plant Cell Tissue Organ Cult. PCTOC 2015, 120, 407–422. [Google Scholar] [CrossRef]
- Harada, J.J.; Belmonte, M.F.; Kwong, R.W. Plant Embryogenesis (Zygotic and Somatic); John Wiley & Sons Ltd.: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Stone, S.L.; Kwong, L.W.; Yee, K.M.; Pelletier, J.; Lepiniec, L.; Fischer, R.L.; Goldberg, R.B.; Harada, J.J. Leafy Cotyledon2 encodes a B3 domain transcription factor that induces embryo development. Proc. Natl. Acad. Sci. USA 2001, 98, 11806–11811. [Google Scholar] [CrossRef]
- Nambara, E.; Keith, K.; McCourt, P.; Naito, S. A regulatory role for the ABI3 gene in the establishment of embryo maturation in Arabidopsis thaliana. Development 1995, 121, 629–636. [Google Scholar] [CrossRef]
- Keith, K.; Kraml, M.; Dengler, N.G.; McCourt, P. Fusca3: A heterochronic mutation affecting late embryo development in Arabidopsis. Plant Cell 1994, 6, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.K.; DeSimone, N.A.; Lingle, W.L.; Dure, L., 3rd. Cellular concentrations and uniformity of cell-type accumulation of two lea proteins in cotton embryos. Plant Cell 1993, 5, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Stasolla, C.; Bozhkov, P.V.; Chu, T.M.; Van Zyl, L.; Egertsdotter, U.; Suarez, M.F.; Craig, D.; Wolfinger, R.D.; Von Arnold, S.; Sederoff, R.R. Variation in transcript abundance during somatic embryogenesis in gymnosperms. Tree Physiol. 2004, 24, 1073–1085. [Google Scholar] [CrossRef]
- Müller, B.; Sheen, J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 2008, 453, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, J.M.; Cortizo, M.; Ordás, R.J. Characterization of a type-A response regulator differentially expressed during adventitious caulogenesis in Pinus pinaster. J. Plant. Physiol. 2012, 169, 1807–1814. [Google Scholar] [CrossRef]
- Mitsuhashi, W.; Toyomasu, T.; Masui, H.; Katho, T.; Nakaminami, K.; Kashiwagi, Y.; Akutsu, M.; Kenmoku, H.; Sassa, T.; Yamaguchi, S.; et al. Gibberellin is essentially required for carrot Daucus carota L. somatic embryogenesis: Dynamic regulation of gibberellin 3-oxidase gene expressions. Biosci. Biotechnol. Biochem. 2003, 67, 2438–2447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Curaba, J.; Moritz, T.; Blervaque, R.; Parcy, F.; Raz, V.; Herzog, M.; Vachon, G. AtGA3ox2, a key gene responsible for bioactive gibberellin biosynthesis, is regulated during embryogenesis by Leafy Cotyledon2 and FUSCA3 in Arabidopsis. Plant Physiol. 2004, 136, 3660–3669. [Google Scholar] [CrossRef]
- Haecker, A.; Groß-Hardt, R.; Geiges, B.; Sarkar, A.; Breuninger, H.; Herrmann, M.; Laux, T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arab. thaliana. Development 2004, 131, 657–668. [Google Scholar] [CrossRef]
- Park, S.; Rancour, D.M.; Bednarek, S.Y. In planta analysis of the cell cycle-dependent localization of AtCDC48A and its critical roles in cell division, expansion, and differentiation. Plant Physiol. 2008, 148, 246–258. [Google Scholar] [CrossRef]
- Wehmeyer, N.; Hernandez, L.D.; Finkelstein, R.R.; Vierling, E. Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol. 1996, 112, 747–757. [Google Scholar] [CrossRef]
- Su, P.H.; Li, H.M. Arabidopsis stromal 70-kD heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol. 2008, 146, 1231–1241. [Google Scholar] [CrossRef]
- Leng, L.; Liang, Q.; Jiang, J.; Zhang, C.; Hao, Y.; Wang, X.; Su, W. A subclass of HSP70s regulate development and abiotic stress responses in Arab. thaliana. J. Plant Res. 2017, 130, 349–363. [Google Scholar] [CrossRef]
- Sun, W.; Bernard, C.; Van De Cotte, B.; Van Montagu, M.; Verbruggen, N. At-HSP17. 6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. Plant J. 2001, 27, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.Y.; Kaplan, F.; Guy, C.L. Plant Hsp70 molecular chaperones: Protein structure, gene family, expression and function. Physiol. Plant 2001, 113, 443–451. [Google Scholar] [CrossRef]
- Mattioli, R.; Marchese, D.; D’Angeli, S.; Altamura, M.M.; Costantino, P.; Trovato, M. Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol. Biol. 2008, 66, 277–288. [Google Scholar] [CrossRef]
- Funck, D.; Winter, G.; Baumgarten, L.; Forlani, G. Requirement of proline synthesis during Arabidopsis reproductive development. BMC Plant Biol. 2012, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Biancucci, M.; Mattioli, R.; Forlani, G.; Funck, D.; Costantino, P.; Trovato, M. Role of proline and GABA in sexual reproduction of angiosperms. Front. Plant Sci. 2015, 6, 680. [Google Scholar] [CrossRef]
- Sahai, M.A.; Kehoe, T.A.; Koo, J.C.; Setiadi, D.H.; Chass, G.A.; Viskolcz, B.; Penke, B.; Pai, E.F.; Csizmadia, I.G. First principle computational study on the full conformational space of l-proline diamides. J. Phys. Chem. 2005, 109, 2660–2679. [Google Scholar] [CrossRef]
- Valencia-Lozano, E.; Cabrera-Ponce, J.L.; Noa-Carrazana, J.C.; Ibarra, J.E. Coffea arabica L. resistant to coffee berry borer Hypothenemus hampei mediated by expression of the Bacillus thuringiensis Cry10Aa protein. Front. Plant Sci. 2021, 12, 765292. [Google Scholar] [CrossRef]
- Valencia-Lozano, E.; Cabrera-Ponce, J.L.; Gómez-Lim, M.A.; Ibarra, J.E. Development of an efficient protocol to obtain transgenic coffee, Coffea arabica L., expressing the Cry10Aa toxin of Bacillus thuringiensis. Int. J. Mol. Sci. 2019, 20, 5334. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Freitas, N.C.; Barreto, H.G.; Fernandes-Brum, C.N.; Moreira, R.O.; Chalfun-Junior, A.; Paiva, L.V. Validation of reference genes for qPCR analysis of Coffea arabica L. somatic embryogenesis-related tissues. Plant Cell Tissue Organ Cult. PCTOC 2017, 128, 663–678. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general-purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Annika, G.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.M.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Bombarded Plates | RD29-antTAS Lines | RD29-antTAS Lines/Plate | Plant Conversion (%) |
|---|---|---|---|---|
| Mannitol 0.15 M + Sorbitol 0.15 M | 30 | 33 | 1.1 | 25% |
| Mannitol 0.3 M + Sorbitol 0.3 M | 30 | 85 | 2.8 | 90% |
| Mannitol 0.45 M + Sorbitol 0.45 M | 30 | 6 | 0.4 | 15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Lozano, E.; Barraza, A.; Ibarra, J.; Délano-Frier, J.P.; Martínez-Gallardo, N.A.; Gámez-Escobedo, I.A.; Cabrera-Ponce, J.L. Transcriptomic Analysis of Osmotic Stress-Tolerant Somatic Embryos of Coffea arabica L. Mediated by the Coffee Antisense Trehalase Gene: A Marker-Free Approach. Int. J. Mol. Sci. 2025, 26, 9224. https://doi.org/10.3390/ijms26189224
Valencia-Lozano E, Barraza A, Ibarra J, Délano-Frier JP, Martínez-Gallardo NA, Gámez-Escobedo IA, Cabrera-Ponce JL. Transcriptomic Analysis of Osmotic Stress-Tolerant Somatic Embryos of Coffea arabica L. Mediated by the Coffee Antisense Trehalase Gene: A Marker-Free Approach. International Journal of Molecular Sciences. 2025; 26(18):9224. https://doi.org/10.3390/ijms26189224
Chicago/Turabian StyleValencia-Lozano, Eliana, Aarón Barraza, Jorge Ibarra, John P. Délano-Frier, Norma A. Martínez-Gallardo, Idalia Analí Gámez-Escobedo, and José Luis Cabrera-Ponce. 2025. "Transcriptomic Analysis of Osmotic Stress-Tolerant Somatic Embryos of Coffea arabica L. Mediated by the Coffee Antisense Trehalase Gene: A Marker-Free Approach" International Journal of Molecular Sciences 26, no. 18: 9224. https://doi.org/10.3390/ijms26189224
APA StyleValencia-Lozano, E., Barraza, A., Ibarra, J., Délano-Frier, J. P., Martínez-Gallardo, N. A., Gámez-Escobedo, I. A., & Cabrera-Ponce, J. L. (2025). Transcriptomic Analysis of Osmotic Stress-Tolerant Somatic Embryos of Coffea arabica L. Mediated by the Coffee Antisense Trehalase Gene: A Marker-Free Approach. International Journal of Molecular Sciences, 26(18), 9224. https://doi.org/10.3390/ijms26189224







