Are Algae a Good Source of Antioxidants? Mechanistic Insights into Antiradical Activity of Eckol
Abstract
1. Introduction
2. Results and Discussion
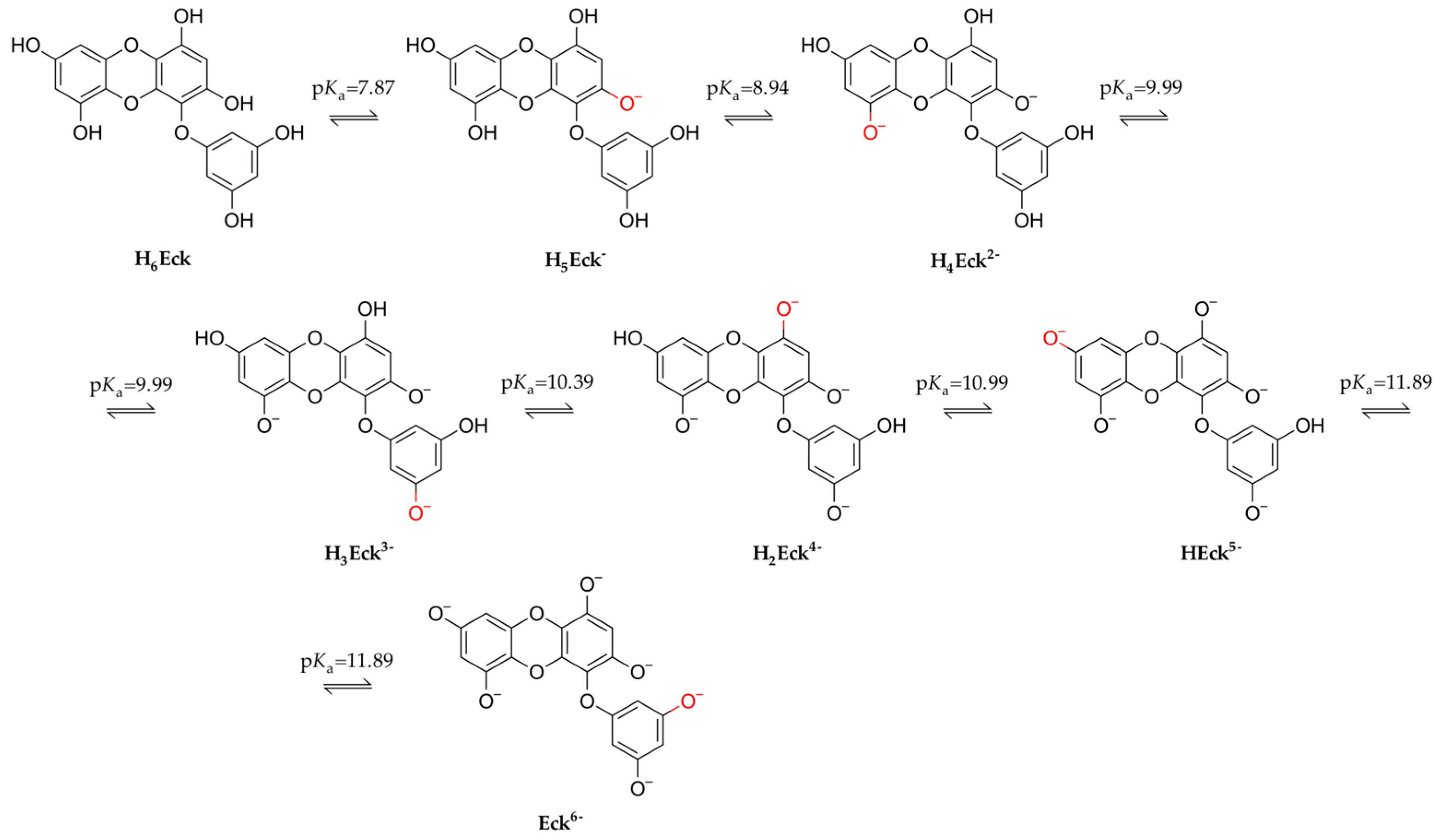
2.1. Eckol Speciation in Water
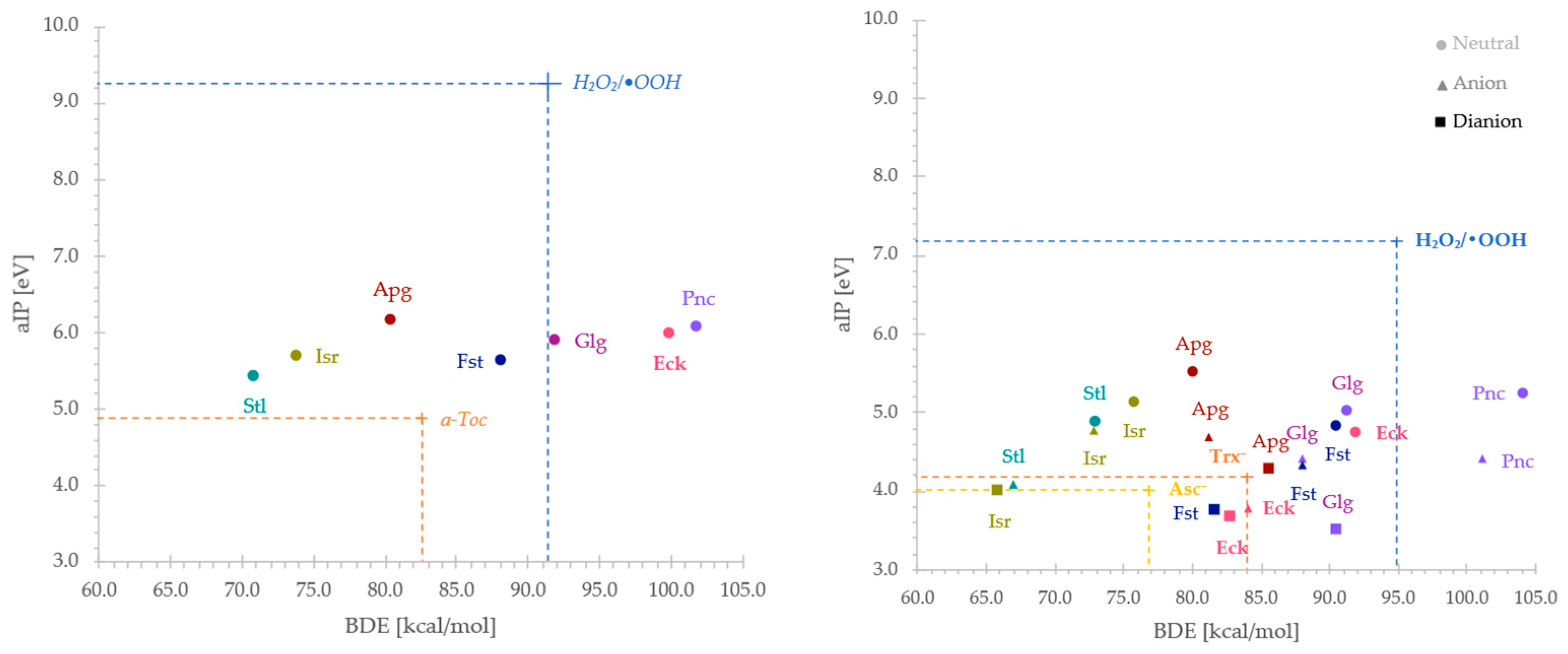
2.2. Reactivity Indices
2.3. Thermochemistry and Kinetics of Type I Antioxidative Reactivity
3. Materials and Methods
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halliwell, B. Reactive Oxygen Species (ROS), Oxygen Radicals and Antioxidants: Where Are We Now, Where Is the Field Going and Where Should We Go? Biochem. Biophys. Res. Commun. 2022, 633, 17–19. [Google Scholar] [CrossRef]
- Weng, M.; Xie, X.; Liu, C.; Lim, K.L.; Zhang, C.; Li, L. The Sources of Reactive Oxygen Species and Its Possible Role in the Pathogenesis of Parkinson’s Disease. Park. Dis. 2018, 2018, 9163040. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Puli, L.; Patil, C.R. Role of Reactive Oxygen Species in the Progression of Alzheimer’s Disease. Drug Discov. Today 2021, 26, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef]
- Taverne, Y.J.H.J.; Bogers, A.J.J.C.; Duncker, D.J.; Merkus, D. Reactive Oxygen Species and the Cardiovascular System. Oxidative Med. Cell. Longev. 2013, 2013, 862423. [Google Scholar] [CrossRef]
- Akhigbe, R.; Ajayi, A. The Impact of Reactive Oxygen Species in the Development of Cardiometabolic Disorders: A Review. Lipids Health Dis. 2021, 20, 23. [Google Scholar] [CrossRef]
- Paola Rosanna, D.; Salvatore, C. Reactive Oxygen Species, Inflammation, and Lung Diseases. CPD 2012, 18, 3889–3900. [Google Scholar] [CrossRef]
- Boukhenouna, S.; Wilson, M.A.; Bahmed, K.; Kosmider, B. Reactive Oxygen Species in Chronic Obstructive Pulmonary Disease. Oxidative Med. Cell. Longev. 2018, 2018, 5730395. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. A Computational Methodology for Accurate Predictions of Rate Constants in Solution: Application to the Assessment of Primary Antioxidant Activity. J. Comput. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kwon, O.I.; Hwang, H.J.; Shin, H.C.; Yang, S. Therapeutic Effects of Phlorotannins in the Treatment of Neurodegenerative Disorders. Front. Mol. Neurosci. 2023, 16, 1193590. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A Review on Biosynthesis, Chemistry and Bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Potential Pharmacological Applications of Polyphenolic Derivatives from Marine Brown Algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Shibata, T.; Nagayama, K.; Sugiura, S.; Makino, S.; Ueda, M.; Tamaru, Y. Analysis on Composition and Antioxidative Properties of Phlorotannins Isolated from Japanese Eisenia and Ecklonia Species. AJPS 2015, 06, 2510–2521. [Google Scholar] [CrossRef]
- Manandhar, B.; Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing Eckol as a Therapeutic Aid: A Systematic Review. Mar. Drugs 2019, 17, 361. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Kim, S.; Kim, J.; Hyun, J.W.; Lee, N.H.; Park, J.W.; Shin, T. Protective Effect of Phlorotannin Components Phloroglucinol and Eckol on Radiation-induced Intestinal Injury in Mice. Phytother. Res. 2008, 22, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Zhen, A.X.; Hyun, Y.J.; Piao, M.J.; Fernando, P.D.S.M.; Kang, K.A.; Ahn, M.J.; Yi, J.M.; Kang, H.K.; Koh, Y.S.; Lee, N.H.; et al. Eckol Inhibits Particulate Matter 2.5-Induced Skin Keratinocyte Damage via MAPK Signaling Pathway. Mar. Drugs 2019, 17, 444. [Google Scholar] [CrossRef]
- Galano, A.; Pérez-González, A.; Castañeda-Arriaga, R.; Muñoz-Rugeles, L.; Mendoza-Sarmiento, G.; Romero-Silva, A.; Ibarra-Escutia, A.; Rebollar-Zepeda, A.M.; León-Carmona, J.R.; Hernández-Olivares, M.A.; et al. Empirically Fitted Parameters for Calculating pKa Values with Small Deviations from Experiments Using a Simple Computational Strategy. J. Chem. Inf. Model. 2016, 56, 1714–1724. [Google Scholar] [CrossRef]
- Bell, R.P. The Theory of Reactions Involving Proton Transfers. Proc. R. Soc. Lond. A 1936, 154, 414–429. [Google Scholar] [CrossRef]
- Evans, M.G.; Polanyi, M. Further Considerations on the Thermodynamics of Chemical Equilibria and Reaction Rates. Trans. Faraday Soc. 1936, 32, 1333. [Google Scholar] [CrossRef]
- Walton-Raaby, M.; Floen, T.; Mora-Diez, N. Modelling the Repair of Carbon-Centered Protein Radicals by Phenolic Antioxidants. Antioxidants 2024, 13, 1368. [Google Scholar] [CrossRef] [PubMed]
- Morales-García, B.C.; Pérez-González, A.; Álvarez-Idaboy, J.R.; Galano, A. Computer-Aided Design of Caffeic Acid Derivatives: Free Radical Scavenging Activity and Reaction Force. J. Mol. Model. 2024, 31, 30. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Sroka, Z. Quantum-Mechanical Characteristics of Apigenin: Antiradical, Metal Chelation and Inhibitory Properties in Physiologically Relevant Media. Fitoterapia 2023, 164, 105352. [Google Scholar] [CrossRef]
- Spiegel, M.; Ciardullo, G.; Marino, T.; Russo, N. Computational Investigation on the Antioxidant Activities and on the Mpro SARS-CoV-2 Non-Covalent Inhibition of Isorhamnetin. Front. Chem. 2023, 11, 1122880. [Google Scholar] [CrossRef]
- Spiegel, M. Unveiling the Antioxidative Potential of Galangin: Complete and Detailed Mechanistic Insights through Density Functional Theory Studies. J. Org. Chem. 2024, 89, 8676–8690. [Google Scholar] [CrossRef]
- Spiegel, M. Theoretical Insights into the Oxidative Stress-Relieving Properties of Pinocembrin─An Isolated Flavonoid from Honey and Propolis. J. Phys. Chem. B 2023, 127, 8769–8779. [Google Scholar] [CrossRef]
- Spiegel, M. Fisetin as a Blueprint for Senotherapeutic Agents—Elucidating Geroprotective and Senolytic Properties with Molecular Modeling. Chem. A Eur. J. 2025, 31, e202403755. [Google Scholar] [CrossRef]
- Spiegel, M.; Marino, T.; Prejanò, M.; Russo, N. On the Scavenging Ability of Scutellarein against the OOH Radical in Water and Lipid-like Environments: A Theoretical Study. Antioxidants 2022, 11, 224. [Google Scholar] [CrossRef]
- Spiegel, M.; Prejanò, M.; Russo, N.; Marino, T. Primary Antioxidant Power and Mpro SARS-CoV-2 Non-Covalent Inhibition Capabilities of Miquelianin. Chem. Asian J. 2024, 19, e202400079. [Google Scholar] [CrossRef]
- Spiegel, M.; Kowalczyk, A. Aglycone, Glycoside, or Glucuronide? Experimental and Mechanistic Insights into the Antioxidative Potential of Gossypetin, Gossypin, and Hibifolin. J. Phys. Chem. B 2025, 129, 7593–7601. [Google Scholar] [CrossRef]
- Van, C.A.; Hai, T.Q.; Ha, N.X.; Hanh, N.T.; Linh, N.N.; Son, N.T. Antiradical Potency of Diphlorethol: DFT (Density Functional Theory), Molecular Docking, and ADMET Profile. J. Phys. Org. Chem. 2025, 38, e70029. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, version 16; Revision C.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Pracht, P.; Bohle, F.; Grimme, S. Automated Exploration of the Low-Energy Chemical Space with Fast Quantum Chemical Methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Pracht, P.; Grimme, S.; Bannwarth, C.; Bohle, F.; Ehlert, S.; Feldmann, G.; Gorges, J.; Müller, M.; Neudecker, T.; Plett, C.; et al. CREST—A Program for the Exploration of Low-Energy Molecular Chemical Space. J. Chem. Phys. 2024, 160, 114110. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient Diffuse Function-augmented Basis Sets for Anion Calculations. III. The 3-21+G Basis Set for First-row Elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Exploring the Limit of Accuracy of the Global Hybrid Meta Density Functional for Main-Group Thermochemistry, Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2008, 4, 1849–1868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. A New Local Density Functional for Main-Group Thermochemistry, Transition Metal Bonding, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor Chem Acc. 2007, 120, 215–241. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. How Well Can New-Generation Density Functionals Describe the Energetics of Bond-Dissociation Reactions Producing Radicals? J. Phys. Chem. A 2008, 112, 1095–1099. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Fukui, K. The Path of Chemical Reactions-the IRC Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Marcus, R.A. On the Theory of Oxidation-Reduction Reactions Involving Electron Transfer. II. Applications to Data on the Rates of Isotopic Exchange Reactions. J. Chem. Phys. 1957, 26, 867–871. [Google Scholar] [CrossRef]
- Marcus, R.A. Chemical and Electrochemical Electron-Transfer Theory. Annu. Rev. Phys. Chem. 1964, 15, 155–196. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron Transfer Reactions in Chemistry. Theory and Experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. Kinetics of Radical-molecule Reactions in Aqueous Solution: A Benchmark Study of the Performance of Density Functional Methods. J. Comput. Chem. 2014, 35, 2019–2026. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef]
- Guzman-Lopez, E.; Reina, M.; Perez-Gonzalez, A.; Francisco-Marquez, M.; Hernandez-Ayala, L.; Castañeda-Arriaga, R.; Galano, A. CADMA-Chem: A Computational Protocol Based on Chemical Properties Aimed to Design Multifunctional Antioxidants. Int. J. Mol. Sci. 2022, 23, 13246. [Google Scholar] [CrossRef]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational Strategies for Predicting Free Radical Scavengers’ Protection against Oxidative Stress: Where Are We and What Might Follow? Int J Quantum Chem. 2018, 119, e25665. [Google Scholar] [CrossRef]
- Isaza Martínez, J.H.; Torres Castañeda, H.G. Preparation and Chromatographic Analysis of Phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of Phlorotannins Isolated from Ecklonia Cava on Melanogenesis and Their Protective Effect against Photo-Oxidative Stress Induced by UV-B Radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Whitman, S.L.; Ehlig, J.M. Differences in Herbivore Preferences, Phlorotannin Production, and Nutritional Quality between Juvenile and Adult Tissues from Marine Brown Algae. Mar. Biol. 2001, 139, 201–210. [Google Scholar] [CrossRef]

| Mechanism | H6EckPET | |||
|---|---|---|---|---|
| ΔG | ΔG≠ | k | Γ | |
| f-HAT | ||||
| C1 | 9.2 | 25.2 | 2.25 × 10−4 | 100.0 |
| C3 | 10.6 | |||
| C3′ | 49.6 | |||
| C5′ | 16.2 | |||
| C6 | 12.9 | |||
| C8 | 11.0 | |||
| RAF | ||||
| C1 | 25.6 | |||
| C1′ | 27.9 | |||
| C2 | 27.8 | |||
| C2′ | 28.7 | |||
| C3 | 25.2 | |||
| C3′ | 31.5 | |||
| C4 | 26.5 | |||
| C4a | 24.2 | |||
| C4b | 22.5 | |||
| C4′ | 28.7 | |||
| C5 | 28.8 | |||
| C5′ | 31.5 | |||
| C6 | 28.8 | |||
| C6′ | 28.9 | |||
| C7 | 27.1 | |||
| C8 | 61.8 | |||
| C8a | 26.0 | |||
| C8b | 22.8 | |||
| \ | H6Eck | H5Eck− | H4Eck2− | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔG | ΔG≠ | k | Γ | ΔG | ΔG≠ | k | Γ | ΔG | ΔG≠ | k | Γ | |
| f-HAT | ||||||||||||
| C1 | −3.5 | 15.3 | 5.39 × 104 | 26.12 | −11.4 | 0.0 3 | 4.23×109 | 24.98 | −13.4 | 0.0 3 | 4.24×109 | 22.21 |
| C3 | −4.4 | 15.4 | 6.08 × 104 | 29.47 | ||||||||
| C3′ | 17.7 | −2.0 | 0.0 3 | 4.23 × 109 | 24.98 | −4.3 | 0.0 3 | 4.24 × 109 | 22.21 | |||
| C5′ | 17.7 | −2.2 | 0.0 3 | 4.23 × 109 | 24.98 | −4.7 | 0.0 3 | 4.24 × 109 | 22.21 | |||
| C6 | −2.4 | 16.4 | 5.68 × 104 | 27.52 | −2.4 | 0.0 3 | 4.23 × 109 | 24.98 | ||||
| C8 | −2.2 | 16.0 | 3.17 × 104 | 15.36 | −2.6 | 17.7 | 6.36 × 103 | 0.00 | −10.3 | 0.0 3 | 4.24 × 109 | 22.21 |
| RAF | ||||||||||||
| C1 | 11.9 | 12.4 | 10.7 | |||||||||
| C1′ | 12.3 | 15.2 | 15.1 | |||||||||
| C2 | 13.0 | 9.0 | 13.9 | 4.26 × 102 | 0.00 | 8.8 | 13.5 | 2.66 × 103 | 0.00 | |||
| C2′ | 12.9 | 13.5 | 13.3 | |||||||||
| C3 | 10.0 | 11.9 | 11.4 | |||||||||
| C3′ | 15.0 | 15.5 | 14.7 | |||||||||
| C4 | 10.4 | 1.9 | 11.1 | 5.01 × 104 | 0.00 | 1.5 | 10.2 | 1.63 × 106 | 0.01 | |||
| C4a | 8.9 | 19.0 | 1.03 × 10−1 | 0.00 | 7.1 | 15.7 | 1.91 × 101 | 0.00 | 6.4 | 12.2 | 6.56 × 104 | 0.00 |
| C4b | 7.0 | 12.8 | 3.12 × 103 | 1.51 | 7.2 | 13.3 | 1.26 × 103 | 0.00 | 1.9 | 6.2 | 8.88 × 108 | 4.65 |
| C4′ | 13.6 | 14.0 | 14.6 | |||||||||
| C5 | 12.5 | 12.1 | 12.6 | |||||||||
| C5′ | 15.6 | 15.2 | 15.7 | |||||||||
| C6 | 14.3 | 13.8 | 11.3 | |||||||||
| C6′ | 15.0 | 14.8 | 15.0 | |||||||||
| C7 | 12.0 | 11.8 | 9.8 | 16.9 | 4.10 × 101 | 0.00 | ||||||
| C8 | 44.0 | 40.6 | 33.9 | |||||||||
| C8a | 9.6 | 19.2 | 7.90 × 10−2 | 0.00 | 9.9 | 19.8 | 2.77 × 102 | 0.00 | 8.2 | 15.8 | 3.07 × 102 | 0.00 |
| C8b | 7.0 | 15.7 | 2.22 × 101 | 0.01 | −1.4 | 7.8 | 1.35 × 107 | 0.08 | −0.3 | 6.2 | 1.23 × 109 | 6.43 |
| SET | ||||||||||||
| 26.0 | 22.3 | 3.66 × 10−7 | 0.00 | 4.8 | 9.4 | 7.39 × 105 | 0.00 | 1.4 | 7.8 | 1.17 × 107 | 0.06 | |
| ki | 2.06 × 105 | 1.69 × 1010 | 1.91 × 1010 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiegel, M. Are Algae a Good Source of Antioxidants? Mechanistic Insights into Antiradical Activity of Eckol. Int. J. Mol. Sci. 2025, 26, 9223. https://doi.org/10.3390/ijms26189223
Spiegel M. Are Algae a Good Source of Antioxidants? Mechanistic Insights into Antiradical Activity of Eckol. International Journal of Molecular Sciences. 2025; 26(18):9223. https://doi.org/10.3390/ijms26189223
Chicago/Turabian StyleSpiegel, Maciej. 2025. "Are Algae a Good Source of Antioxidants? Mechanistic Insights into Antiradical Activity of Eckol" International Journal of Molecular Sciences 26, no. 18: 9223. https://doi.org/10.3390/ijms26189223
APA StyleSpiegel, M. (2025). Are Algae a Good Source of Antioxidants? Mechanistic Insights into Antiradical Activity of Eckol. International Journal of Molecular Sciences, 26(18), 9223. https://doi.org/10.3390/ijms26189223






