Bioactive Compounds and Pharmacological Properties of the Polypore Fomes fomentarius, a Medicinal Wild Mushroom Collected from Morocco
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield and Bioactive Compounds Contents in F. fomentarius
2.2. Biomolecules Profiles of F. fomentarius by GC-MS
2.3. Individual Polyphenols of F. fomentarius by LC–MS Analysis
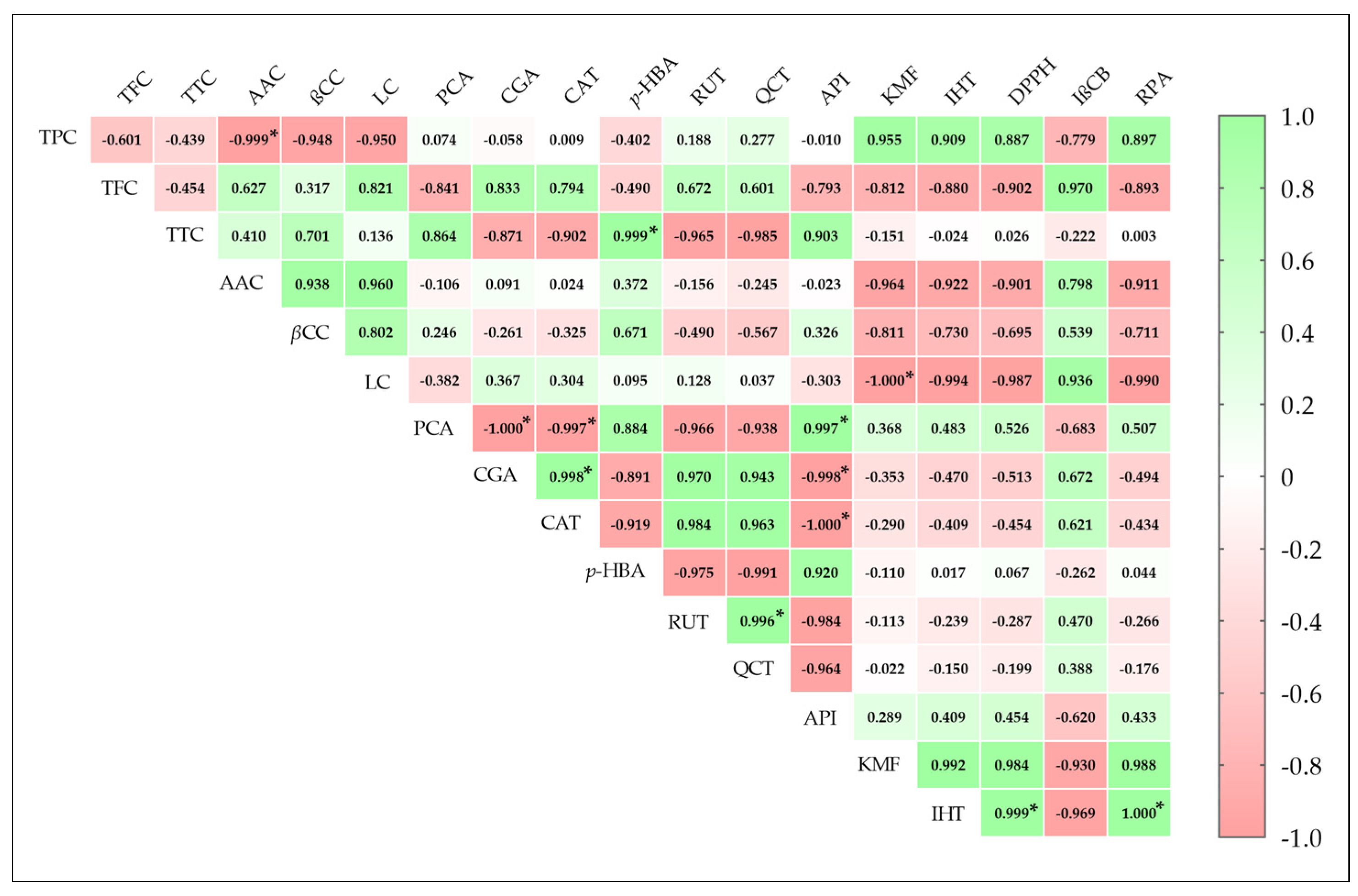
2.4. Antioxidant Properties of F. fomentarius
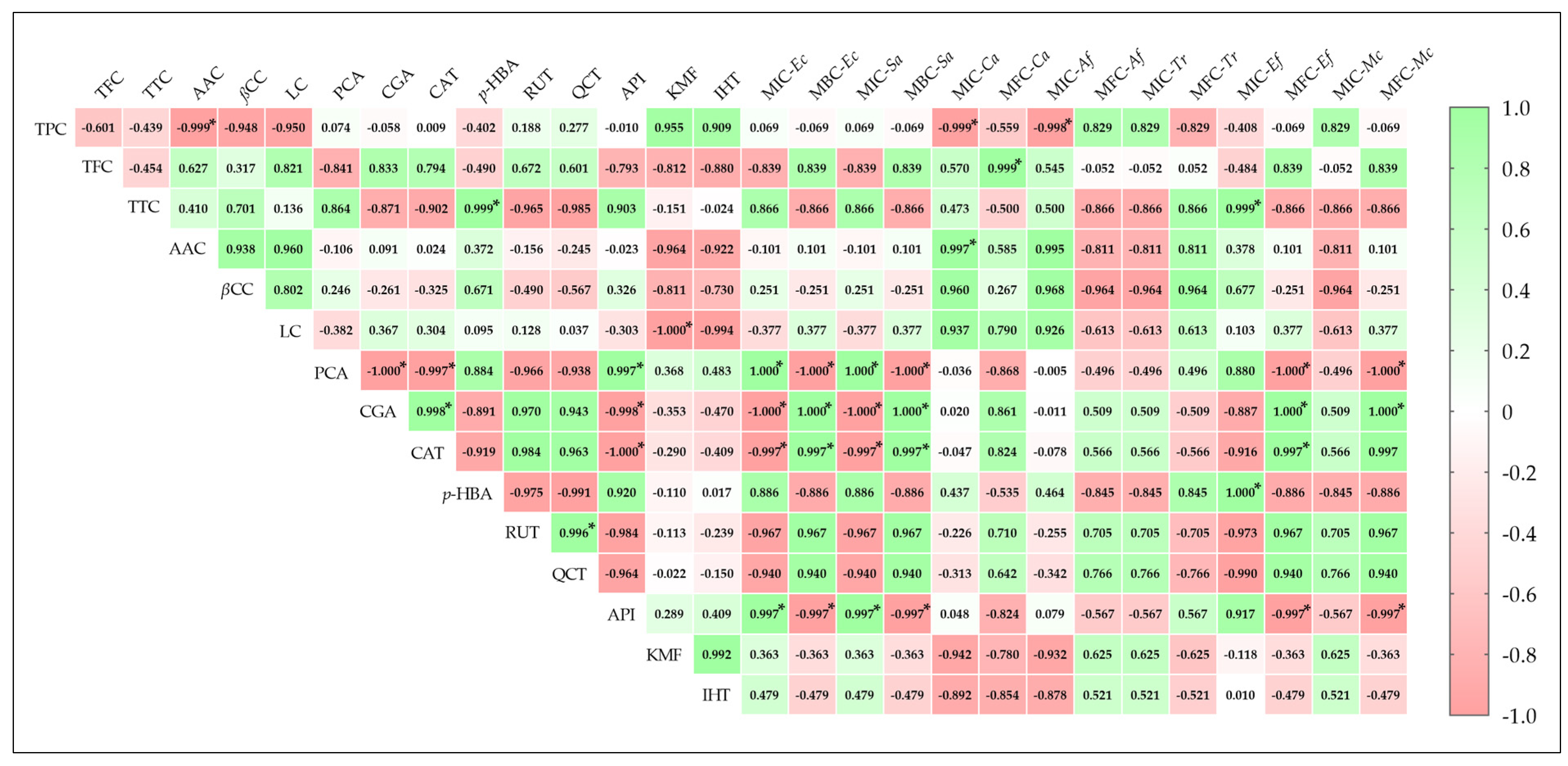
2.5. Antimicrobial Properties of F. fomentarius
3. Materials and Methods
3.1. Chemical Reagents and Standards
3.2. Mushroom Material
3.3. Determination of Bioactive Compound Contents
3.4. Biomolecules Analysis of F. fomentarius Methanolic Extract by GC-MS
3.5. Characterization of Individual Polyphenols by LC-MS Analysis
3.6. Determination of Antioxidant Properties
3.6.1. DPPH Radical-Scavenging Activity (RSA)
3.6.2. Inhibition of β-Carotene Bleaching (IβCB)
3.6.3. Reducing Power Assay (RPA)
3.7. Evaluation of Antimicrobial Properties
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, M. Chapter 13—Fungal Strains as Source of Bioactive Compounds and Their Potential Application. In Volatiles and Metabolites of Microbes; Kumar, A., Singh, J., Samuel, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 257–282. ISBN 978-0-12-824523-1. [Google Scholar]
- Doskocil, I.; Havlik, J.; Verlotta, R.; Tauchen, J.; Vesela, L.; Macakova, K.; Opletal, L.; Kokoska, L.; Rada, V. In Vitro Immunomodulatory Activity, Cytotoxicity and Chemistry of Some Central European Polypores. Pharm. Biol. 2016, 54, 2369–2376. [Google Scholar] [CrossRef]
- Grienke, U.; Zöll, M.; Peintner, U.; Rollinger, J.M. European Medicinal Polypores—A Modern View on Traditional Uses. J. Ethnopharmacol. 2014, 154, 564–583. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Amina, B.; Kaoutar, A.; Saidi, R.; Lamrani, Z.; Pinto, E.; Esteves da Silva, J.C.G.; Maouni, A.; Pinto da Silva, L. Chemical Characterization and Evaluation of Antimicrobial Properties of the Wild Medicinal Mushroom Ganoderma Lucidum Growing in Northern Moroccan Forests. Life 2023, 13, 1217. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Špirović-Trifunović, B.; Miletić, S.; Lazić, V.; Žižak, Ž.; Vunduk, J. Bioprospecting of Selected Species of Polypore Fungi from the Western Balkans. Molecules 2024, 29, 314. [Google Scholar] [CrossRef]
- Zjawiony, J.K. Biologically Active Compounds from Aphyllophorales (Polypore) Fungi. J. Nat. Prod. 2004, 67, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, N.; Tosti, T.; Srbljak, I.; Đurić, A.; Kosanic, M. Chemical Composition and Bioctivity of the Giant Polypore or Black-Staining Mushroom, Meripilus giganteus (Agaricomycetes), from Serbia. Int. J. Med. Mushrooms 2022, 24, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Maaloul, A.; Portillo-Lemus, L.; Vitou, M.; Rapior, S.; Morel, S.; Fons, F. Antioxidant Potential of Several Polypores Mushrooms from the South of France. Int. J. Med. Mushrooms 2023, 25, 1–10. [Google Scholar] [CrossRef]
- Pirronitto, S.; Teng, F.; Verheyen, C.; Gaucet, V.; Henin, J.-M.; Jourez, B.; Schmitz, S.; Chandelier, A. Characterization of Fomes fomentarius s.s. and F. inzengae in Belgian Beech Forests. Forests 2024, 15, 221. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Shi, C.; Sun, L.-T.; Yang, H.-X.; He, J.; Li, Z.-H.; Feng, T.; Liu, J.-K. Chemical Constituents and Their Biological Activities from the Mushroom Pyropolyporus fomentarius. Phytochemistry 2021, 183, 112625. [Google Scholar] [CrossRef]
- Morales, D. Fomes fomentarius: An Underexplored Mushroom as Source of Bioactive Compounds. Food Biosci. 2024, 61, 104781. [Google Scholar] [CrossRef]
- Park, C.-G.; Lim, H.-B. Evaluation of Antimutagenic and Antioxidant Properties in Fomes fomentarius L.: Potential Development as Functional Food. Appl. Sci. 2024, 14, 3927. [Google Scholar] [CrossRef]
- Kolundžić, M.; Grozdanić, N.Đ.; Dodevska, M.; Milenković, M.; Sisto, F.; Miani, A.; Farronato, G.; Kundaković, T. Antibacterial and Cytotoxic Activities of Wild Mushroom Fomes fomentarius (L.) Fr., Polyporaceae. Ind. Crops Prod. 2016, 79, 110–115. [Google Scholar] [CrossRef]
- Evidence Based Validation of Traditional Medicines: A Comprehensive Approach; Mandal, S.C., Chakraborty, R., Sen, S., Eds.; Springer: Singapore, 2021; ISBN 978-981-15-8126-7. [Google Scholar]
- Alvandi, H.; Hatamian-Zarmi, A.; Mokhtari-Hosseini, Z.B.; Webster, T.J.; Ebrahimi Hosseinzadeh, B. Selective Biological Effects of Natural Selenized Polysaccharides from Fomes fomentarius Mycelia Loaded Solid Lipid Nanoparticles on Bacteria and Gastric Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 77, 103900. [Google Scholar] [CrossRef]
- Karaman, M.; Stahl, M.; Vulic, J.; Vesic, M.; Canadanović-Brunet, J.C. Wild-Growing Lignicolous Mushroom Species as Sources of Novel Agents with Antioxidative and Antibacterial Potentials. Int. J. Food Sci. Nutr. 2013, 65, 1465–3478. [Google Scholar] [CrossRef] [PubMed]
- Mircea, C.; Cioanc, O.; Iancu, C. In Vitro Antioxidant Activity of Some Extracts Obtained from Agaricus Bisporus Brown, Pleurotus Ostreatus and Fomes fomentarius. FARMACIA 2015, 63, 927–933. [Google Scholar]
- Khadhri, A.; Aouadhi, C.; Aschi-Smiti, S. Screening of Bioactive Compounds of Medicinal Mushrooms Collected on Tunisian Territory. Int. J. Med. Mushrooms 2017, 19, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, Antiradical Potential and Phenolic Compounds of Thirty-One Polish Mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef] [PubMed]
- Kalitukha, L.; Sari, M. Chemical Composition and Ultraviolet Absorption Activity of an Aqueous Alkali Extract from the Fruiting Bodies of the Tinder Conk Mushroom, Fomes fomentarius (Agaricomycetes). Int. J. Med. Mushrooms 2021, 23, 23–37. [Google Scholar] [CrossRef]
- Abugri, D.A.; McElhenney, W.H. Extraction of Total Phenolic and Flavonoids from Edible Wild and Cultivated Medicinal Mushrooms as Affected by Different Solvents. J. Nat. Prod. Plant Resour. 2013, 3, 37–42. [Google Scholar]
- Ferreira, I.; Barros, L.; Abreu, R. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Nowacka-Jechalke, N.; Olech, M.; Nowak, R. Mushroom Polyphenols as Chemopreventive Agents. In Polyphenols: Prevention and Treatment of Human Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 137–150. ISBN 978-0-12-813008-7. [Google Scholar]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A Comprehensive Review on Phenolic Compounds from Edible Mushrooms: Occurrence, Biological Activity, Application and Future Prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef] [PubMed]
- Marella, A.; Tanwar, O.P.; Saha, R.; Ali, M.R.; Srivastava, S.; Akhter, M.; Shaquiquzzaman, M.; Alam, M.M. Quinoline: A Versatile Heterocyclic. Saudi Pharm. J. 2013, 21, 1–12. [Google Scholar] [CrossRef]
- Huang, T.-Z.; Du, D.-Y.; Chen, Y.-Q.; Yuan, B.; Ju, X.-Y.; Feng, Y.-J.; Wang, L.; Jiang, J.-H. Chemical Constituents and Antitumor Activity of Fruiting Body of Fomes fomentarius. Mycosystema 2012, 5, 775–783. [Google Scholar]
- Lee, S.-O.; Lee, M.-H.; Lee, K.-R.; Lee, E.-O.; Lee, H.-J. Fomes fomentarius Ethanol Extract Exerts Inhibition of Cell Growth and Motility Induction of Apoptosis via Targeting AKT in Human Breast Cancer MDA-MB-231 Cells. Int. J. Mol. Sci. 2019, 20, 1147. [Google Scholar] [CrossRef]
- Kurchenko, V.P.; Sushinskaya, N.V.; Kiseleva, I.S.; Ermoshin, A.A. Biologically Active Substances in Fruit Bodies of Wood Decomposing Fungi. AIP Conf. Proc. 2022, 2390, 030045. [Google Scholar] [CrossRef]
- Du, D.; Chen, C.-C.; Chen, X.; Wang, L.; FENG, Y.; Jiang, J. Analysis of Chemical Constituents of Petroleum Ether Fraction from the Fruiting Body of Fomes fomentarius and Its Antitumor Effect in Vitro. Chin. J. Pharm. Anal. 2011, 31, 261–265. [Google Scholar]
- Dutta, A.; Panchali, T.; Khatun, A.; Jarapala, S.R.; Das, K.; Ghosh, K.; Chakrabarti, S.; Pradhan, S. Anti-Cancer Potentiality of Linoelaidic Acid Isolated from Marine Tapra Fish Oil (Ophisthopterus tardoore) via ROS Generation and Caspase Activation on MCF-7 Cell Line. Sci. Rep. 2023, 13, 14125. [Google Scholar] [CrossRef]
- Prasad, L.; Kundu, A.; Bahukhandi, D. Comparative Analysis of Volatile Fractions of Fomes fomentarius and F. rhabarbarinus. Indian Phytopathol. 2018, 71, 25–31. [Google Scholar] [CrossRef]
- Devappa, R.K.; Rakshit, S.K.; Dekker, R.F.H. Forest Biorefinery: Potential of Poplar Phytochemicals as Value-Added Co-Products. Biotechnol. Adv. 2015, 33, 681–716. [Google Scholar] [CrossRef] [PubMed]
- Shahruzzaman, M.; Hossain, S.; Ahmed, T.; Kabir, S.F.; Minhajul Islam, M.; Rahman, A.; Sazedul Islam, M.; Sultana, S.; Rahman, M.M. Chapter 7—Biological Macromolecules as Antimicrobial Agents. In Biological Macromolecules; Nayak, A.K., Dhara, A.K., Pal, D., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 165–202. ISBN 978-0-323-85759-8. [Google Scholar]
- Aro, A. FATTY ACIDS|Trans-Fatty Acids: Health Effects. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 2324–2330. ISBN 978-0-12-227055-0. [Google Scholar]
- Shahvaroughi Farahani, A.; Kurepaz-mahmoodabadi, M.; Manayi, A. Evaluation of Cytotoxic Activity of Fomes fomentarius Extracts against Brine Shrimp Larvae and Colon Adenocarcinoma Cell Line (SW742). Adv. Pharmacol. Ther. J. 2022, 1, 77–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Xiao, Y.; Wang, X.; Yang, S.; Liu, Q. Chemical Compositions and Antiproliferation Activities of the Chloroform Fraction from Pyropolyporus Fomentarius in K562 Cells. Hum. Exp. Toxicol. 2015, 34, 732–743. [Google Scholar] [CrossRef]
- Doğan, H.H.; Kars, M.D.; Özdemir, Ö.; Gündüz, U. Fomes fomentarius and Tricholoma anatolicum (Agaricomycetes) Extracts Exhibit Significant Multiple Drug-Resistant Modulation Activity in Drug-Resistant Breast Cancer Cells. Int. J. Med. Mushrooms 2020, 22, 105–114. [Google Scholar] [CrossRef]
- Bal, C.; Akgul, H.; Sevindik, M.; Akata, I.; Yumrutas, O. Determination of the Anti-Oxidative Activities of Six Mushrooms. Fresenius Environ. Bull. 2017, 26, 6246–6252. [Google Scholar]
- Dundar, A.; Okumus, V.; Ozdemir, S.; Celik, K.S.; Boğa, M.; Ozcagli, E. Determination of Cytotoxic, Anticholinesterase, Antioxidant and Antimicrobial Activities of Some Wild Mushroom Species. Cogent Food Agric. 2016, 2, 1178060. [Google Scholar] [CrossRef]
- Rašeta, M.; Kebert, M.; Pintać Šarac, D.; Mišković, J.; Berežni, S.; Kulmány, Á.E.; Zupkó, I.; Karaman, M.; Jovanović-Šanta, S. Bioactive Potential of Balkan Fomes fomentarius Strains: Novel Insights into Comparative Mycochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Antiproliferative Activities. Microorganisms 2025, 13, 1210. [Google Scholar] [CrossRef] [PubMed]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The Importance of Antioxidants and Place in Today’s Scientific and Technological Studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef]
- İrez, E.İ.; Doğru, N.H.; Demir, N. Fomes fomentarius (L.) Fr. Extracts as Sources of an Antioxidant, Antimicrobial and Antibiofilm Agents. Biol. Nyssana 2021, 12, 55–62. [Google Scholar] [CrossRef]
- Darkal, A.K.; Zuraik, M.M.; Ney, Y.; Nasim, M.J.; Jacob, C. Unleashing the Biological Potential of Fomes fomentarius via Dry and Wet Milling. Antioxidants 2021, 10, 303. [Google Scholar] [CrossRef]
- Sveta, I. Biological Activity of Fomes fomentarius. J. Pharm. Pharmacol. 2022, 10, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Ferreira, I.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on Antimicrobial Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Slama, A.; Mezni, F.; Ayari, F.; Khaldi, A. Antifungal Activity of Basidiomycete Carpophores Fungi Against Mycotoxigenic Species. IJRASET 2022, 10, 1790–1793. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Bouchra, B.; da Silva, L.P.; Lamrani, Z.; Pinto, E.; da Silva, J.C.G.E.; Maouni, A. Chemical Composition and Antioxidant and Antimicrobial Activities of Lactarius sanguifluus, a Wild Edible Mushroom from Northern Morocco. Euro-Mediterr. J. Environ. Integr. 2021, 6, 43. [Google Scholar] [CrossRef]
- Ettakifi, H.; Abbassi, K.; Maouni, S.; Erbiai, E.H.; Rahmouni, A.; Legssyer, M.; Saidi, R.; Lamrani, Z.; Esteves da Silva, J.C.G.; Pinto, E.; et al. Chemical Characterization and Antifungal Activity of Blue Tansy (Tanacetum annuum) Essential Oil and Crude Extracts against Fusarium oxysporum f. sp. Albedinis, an Agent Causing Bayoud Disease of Date Palm. Antibiotics 2023, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Malençon, G.; Bertault, R. Flore des Champignons Superieurs du Maroc: Tome I; Travaux de l’Institut Scientifique Chérifien et de la Faculté des Sciences de Rabat. Série Botanique et Biologie Végétale; Institut Scientifique Chérifien: Rabat, Morocco, 1970; Volume 1. [Google Scholar]
- Régis, C. Les Champignons de France—Guide Encyclopédique; Eclectis: Paris, France, 1994; Volume 1, ISBN 978-2-908975-19-2. [Google Scholar]
- Erbiai, E.H.; da Silva, L.P.; Saidi, R.; Lamrani, Z.; Esteves da Silva, J.C.G.; Maouni, A. Chemical Composition, Bioactive Compounds, and Antioxidant Activity of Two Wild Edible Mushrooms Armillaria Mellea and Macrolepiota Procera from Two Countries (Morocco and Portugal). Biomolecules 2021, 11, 575. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Maouni, A.; Pinto da Silva, L.; Saidi, R.; Legssyer, M.; Lamrani, Z.; Esteves da Silva, J.C.G. Antioxidant Properties, Bioactive Compounds Contents, and Chemical Characterization of Two Wild Edible Mushroom Species from Morocco: Paralepista flaccida (Sowerby) Vizzini and Lepista nuda (Bull.) Cooke. Molecules 2023, 28, 1123. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols Composition of Portuguese Wild Mushrooms with Antioxidant Capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef]
- CLSI Document M27–S3; CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Third Informational Supplement. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28.
- CLSI Document M27–A3; CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard-Third Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28.
- CLSI Document M38–A2; CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Approved Standard-Second Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28.
- CLSI Document M07–A8; CLSI Method for Dilution Antimicrobial Susceptibility Tests of Bacteria That Grow Aerobically; Approved Standard-Eighth Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; Volume 29.


| Bioactive Compounds Content | F. fomentarius |
|---|---|
| Extraction yield (%) | 7.59 ± 0.15 1 |
| Total phenolic content (mg GAE/g dme) | 75.83 ± 0.33 |
| Total flavonoid content (mg CE/g dme) | 37.62 ± 1.09 |
| Total tannin content (mg CE/g dw) | 0.95 ± 0.07 |
| Ascorbic acid content (mg AAE/g dw) | 3.43 ± 0.07 |
| β-carotene content (mg/g dme) | 0.59 ± 0.00 |
| Lycopene content (mg/g dme) | 0.19 ± 0.00 |
| Groups of Biomolecules | Dilution in Chloroform | Derivatized with BSTFA | ||
|---|---|---|---|---|
| Area (%) | Number of Molecules | Area (%) | Number of Molecules | |
| Alcohols | 1.06 | 5 | 3.27 | 5 |
| Alkaloids | 11.55 | 2 | - | - |
| Alkanes | 1.66 | 7 | - | - |
| Amino acids | - | - | 1.08 | 6 |
| Fatty acids | 15.80 | 12 | 14.72 | 11 |
| Organic acids | 3.09 | 2 | 5.36 | 7 |
| Phenols | 40.30 | 3 | 0.14 | 1 |
| Steroids | 0.31 | 1 | 3.75 | 3 |
| Sugar compositions | - | - | 64.63 | 24 |
| Other groups | 32.99 | 7 | 7.03 | 13 |
| Total | 98.65 | 39 | 99.98 | 70 |
| Peak No. | Rt (min) | MW | Recorded m/z | Individual Polyphenols | Content (µg/g dw) |
|---|---|---|---|---|---|
| 1 | 5.05 | 170.02 | 169.67 | Gallic acid | 25.42 ± 0.06 |
| 2 | 8.60 | 154.12 | 153.10 | Protocatechuic acid | 98.61 ± 0.61 |
| 3 | 9.90 | 354.31 | 352.78 | Chlorogenic acid | 150.80 ± 0.86 |
| 4 | 12.31 | 290.08 | 289.51 | Catechin | 116.90 ± 2.43 |
| 5 | 13.42 | 138.03 | 137.45 | p-Hydroxybenzoic acid | 409.00 ± 2.46 |
| 6 | 14.35 | 180.04 | 179.01 | Caffeic acid | 4.96 ± 0.06 |
| 7 | 14.96 | 168.04 | 167.86 | Vanillic acid | 2.19 ± 0.06 |
| 8 | - | - | - | Syringic acid | ND |
| 9 | 15.78 | 610.15 | 608.78 | Rutin | 95.27 ± 0.25 |
| 10 | 16.84 | 302.20 | 303.01 | Ellagic acid | 5.56 ± 0.15 |
| 11 | 17.31 | 448.37 | 448.32 | Luteolin 7-glucoside | 21.85 ± 0.16 |
| 12 | 17.47 | 164.05 | 164.97 | p-Coumaric acid | 5.78 ± 0.05 |
| 13 | 18.17 | 152.05 | 151.07 | Vanillin | 15.81 ± 0.03 |
| 14 | 18.98 | 194.19 | 193.17 | Ferulic acid | 3.34 ± 0.02 |
| 15 | 19.30 | 580.18 | 578.18 | Naringin | 2.51 ± 0.03 |
| 16 | 20.14 | 432.11 | 432.61 | Apigenin 7-glucoside | 26.26 ± 0.14 |
| 17 | 21.92 | 360.08 | 361.03 | Rosmarinic acid | 17.15 ± 0.55 |
| 18 | 22.56 | 138.03 | 137.03 | Salicylic acid | 31.90 ± 0.13 |
| 19 | 23.13 | 152.05 | 151.94 | Methyl paraben | 21.46 ± 0.13 |
| 20 | 28.08 | 286.23 | 285.12 | Luteolin | 45.34 ± 0.34 |
| 21 | 28.96 | 302.04 | 301.35 | Quercetin | 99.47 ± 1.09 |
| 22 | 29.78 | 148.05 | 148.14 | Cinnamic acid | 11.02 ± 0.31 |
| 23 | 33.27 | 270.05 | 269.15 | Apigenin | 295.10 ± 0.24 |
| 24 | 33.71 | 286.05 | 285.25 | Kaempferol | 351.10 ± 0.56 |
| 25 | 34.19 | 316.27 | 315.26 | Isorhamnetin | 2734.00 ± 7.33 |
| Antioxidant Assay | F. fomentarius | Trolox |
|---|---|---|
| DPPH radical-scavenging activity | 114.40 ± 2.57 Ca | 19.17 ± 0.99 Bb |
| β-carotene bleaching inhibition | 174.50 ± 1.17 Ba | 3.84 ± 0.70 Cb |
| Ferric ion-reducing power | 250.70 ± 1.75 Aa | 80.11 ± 2.37 Ab |
| Bacteria Strains | F. fomentarius Extract (mg/mL) | Gentamicin (mg/mL) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Escherichia coli | 8.00 ± 0.00 Ab | 16.00 ± 0.00 Ab | 0.0020 ± 0.00 Ac | >0.032 |
| Staphylococcus aureus | 4.00 ± 0.00 Bb | 8.00 ± 0.00 Ba | 0.0004 ± 0.00 Bd | 0.016 ± 0.00 c |
| Fungal Pathogen Strains | F. fomentarius Extract (mg/mL) | Voriconazole (mg/mL) | Terbinafine (mg/mL) | |||
|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | |
| Candida albicans | 32.00 ± 0.00 Ab | 53.33 ± 18.48 Aa | 0.00038 ± 0.0 Ac | >0.004 | - | - |
| Aspergillus fumigatus | 26.67 ± 9.24 Ba | ≥64 | 0.00025 ± 0.0 Bc | 0.00075 ± 0.29 Bb | - | - |
| Trichophyton rubrum | 2.00 ± 0.00 Db | 7.00 ± 1.14 Ba | 0.00013 ± 0.0 Cd | 0.0015 ± 0.58 Ac | 0.00001 ± 0.0 Ce | 0.000129 ± 0.0 Ce |
| Epidermophyton floccosum | 2.00 ± 0.00 Db | 4.00 ± 0.00 Ca | 0.00003 ± 0.0 De | 0.00009 ± 0.04 Dd | 0.00002 ± 0.0 Bf | 0.000038 ± 0.0 Be |
| Microsporum canis | 4.00 ± 0.00 Cb | 8.00 ± 0.00 Ba | 0.00013 ± 0.0 Ce | 0.00038 ± 0.14 Cd | 0.00012 ± 0.0 Ae | 0.00075 ± 0.0 Ac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erbiai, E.H.; Maouni, S.; Pinto da Silva, L.; Saidi, R.; Lamrani, Z.; Esteves da Silva, J.C.G.; Maouni, A.; Pinto, E. Bioactive Compounds and Pharmacological Properties of the Polypore Fomes fomentarius, a Medicinal Wild Mushroom Collected from Morocco. Int. J. Mol. Sci. 2025, 26, 9215. https://doi.org/10.3390/ijms26189215
Erbiai EH, Maouni S, Pinto da Silva L, Saidi R, Lamrani Z, Esteves da Silva JCG, Maouni A, Pinto E. Bioactive Compounds and Pharmacological Properties of the Polypore Fomes fomentarius, a Medicinal Wild Mushroom Collected from Morocco. International Journal of Molecular Sciences. 2025; 26(18):9215. https://doi.org/10.3390/ijms26189215
Chicago/Turabian StyleErbiai, El Hadi, Safae Maouni, Luís Pinto da Silva, Rabah Saidi, Zouhaire Lamrani, Joaquim C. G. Esteves da Silva, Abdelfettah Maouni, and Eugénia Pinto. 2025. "Bioactive Compounds and Pharmacological Properties of the Polypore Fomes fomentarius, a Medicinal Wild Mushroom Collected from Morocco" International Journal of Molecular Sciences 26, no. 18: 9215. https://doi.org/10.3390/ijms26189215
APA StyleErbiai, E. H., Maouni, S., Pinto da Silva, L., Saidi, R., Lamrani, Z., Esteves da Silva, J. C. G., Maouni, A., & Pinto, E. (2025). Bioactive Compounds and Pharmacological Properties of the Polypore Fomes fomentarius, a Medicinal Wild Mushroom Collected from Morocco. International Journal of Molecular Sciences, 26(18), 9215. https://doi.org/10.3390/ijms26189215










