Evidence for Dietary Management of Histamine Intolerance
Abstract
1. Introduction
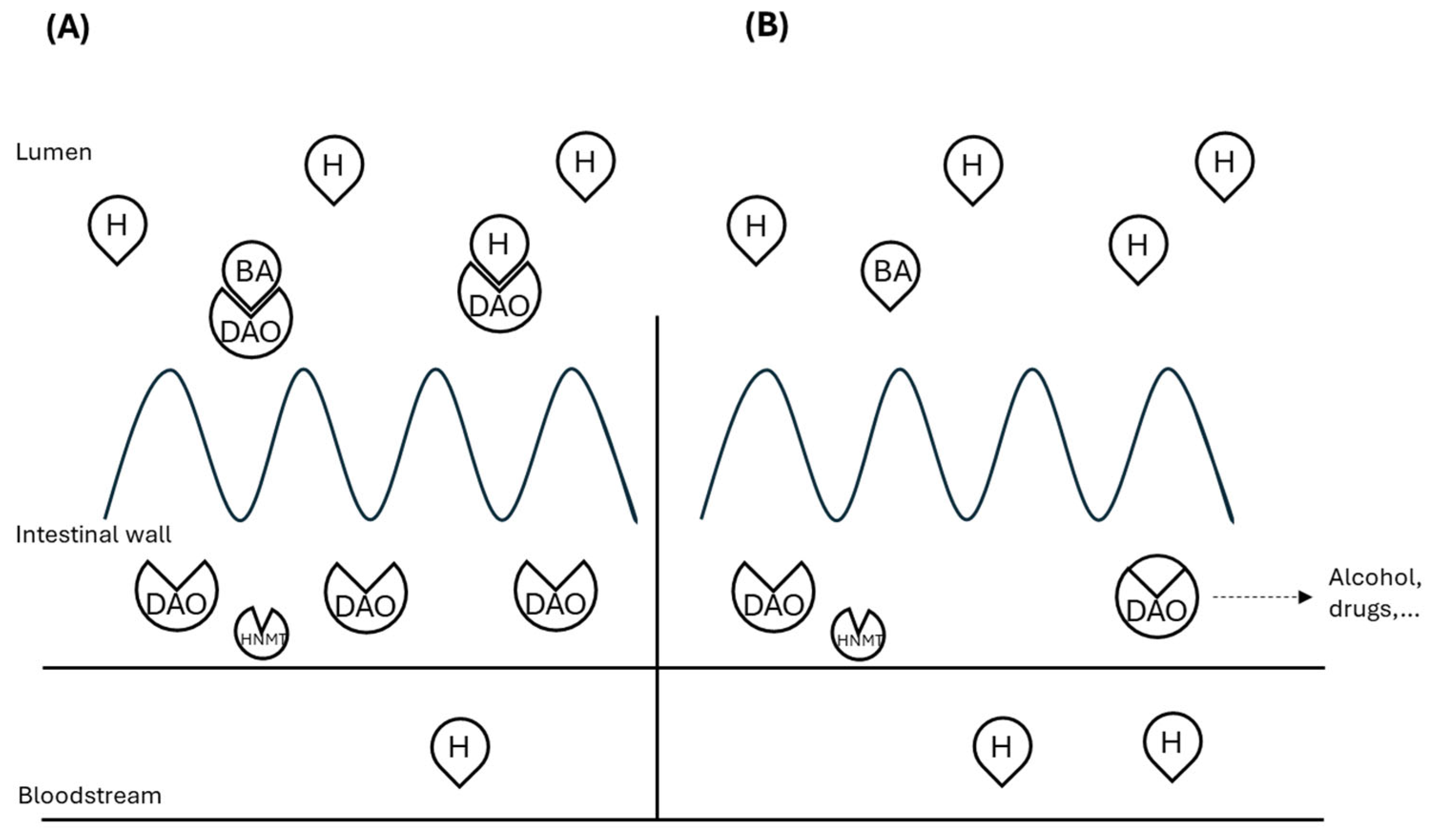
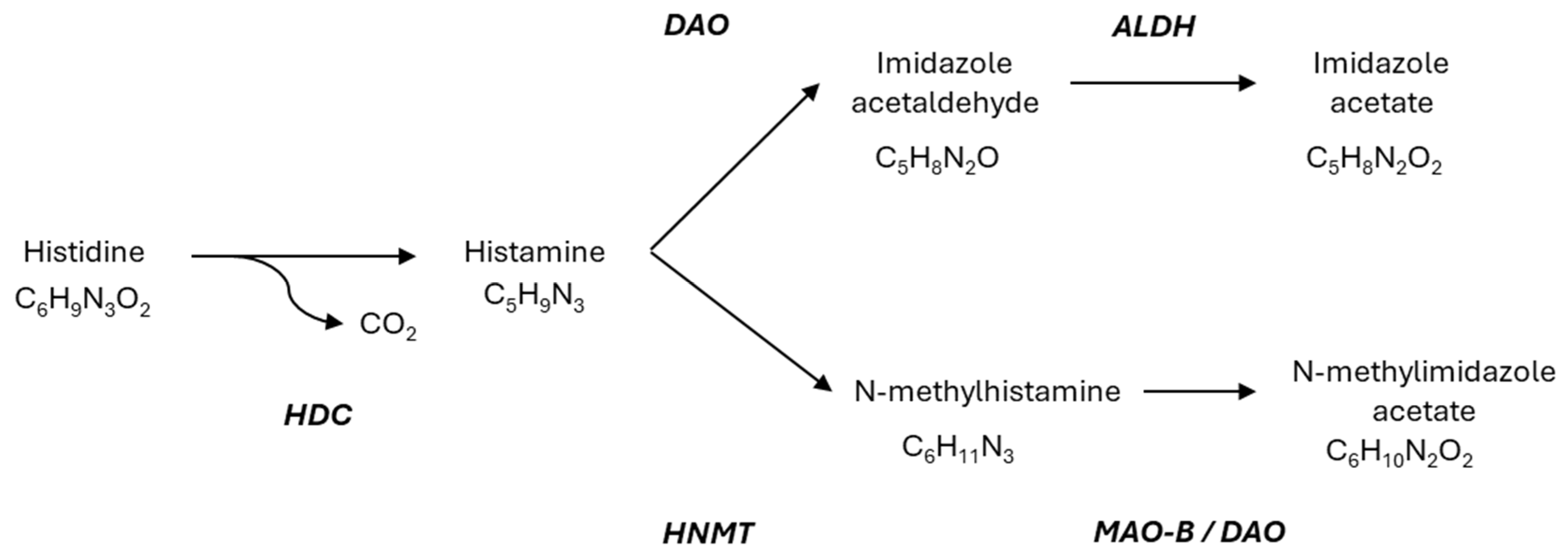
2. Molecular Aspect
3. Diagnostic Approach
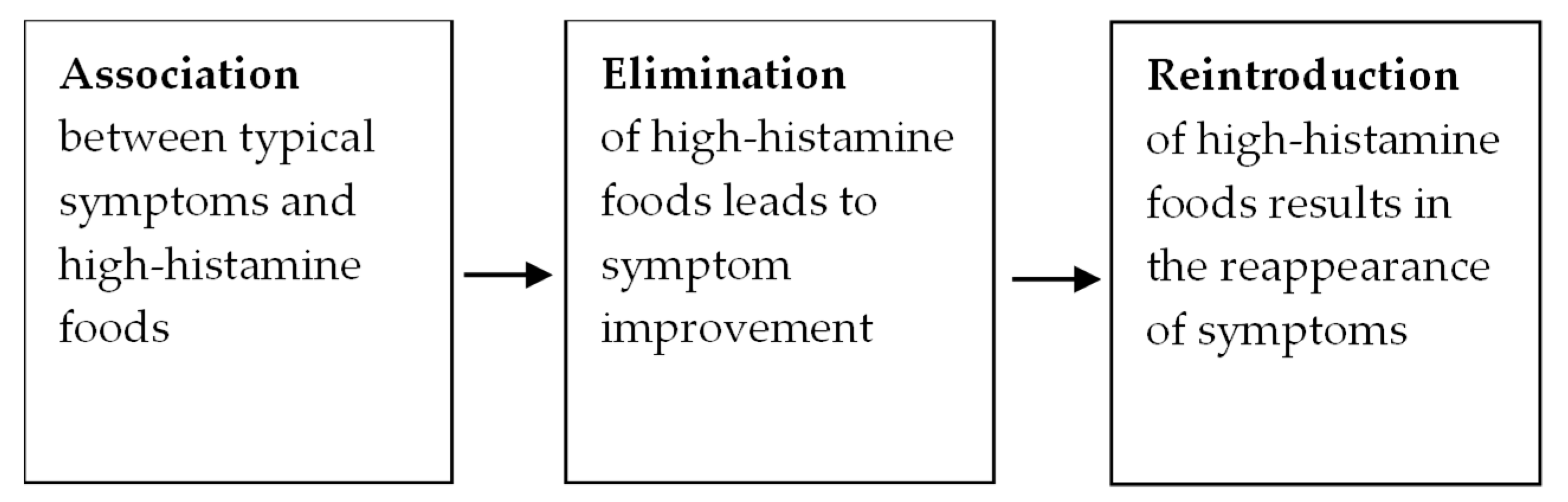
3.1. HIT Diagnosis
3.2. Disordered Eating
4. Therapeutic Approaches
4.1. Dietary Management
- PHASE 1: A 4-week dietary restriction of foods which may increase histamine levels in the body.
- PHASE 2: 1–2 week reintroduction of small portions of higher histamine-containing foods.
- PHASE 3: (if phase 2 is tolerated) 1–2 week trial of larger portions of histamine containing foods.
- PHASE 4: Continue with tolerated histamine intake and add histamine-releasing and -inhibiting foods.
4.2. DAO Supplementation
4.3. Pharmacological Interventions
4.4. Psychosocial Considerations
- Healthier diet: Patients with a poorly balanced diet (skipping meals, consuming highly processed foods, etc.) may pay more attention and adopt healthier patterns when they start an elimination diet.
- Placebo effect: Expecting benefit from a treatment is a powerful neurobiological influence.
- Treatment effect: Patients often feel better when they receive personalized support from a caring health provider.
- Natural fluctuations: HIT symptoms may wax and wane for unknown reasons. Patients usually make changes (such as an elimination diet) when their symptoms peak, and they may have felt better without intervention.
- Sense of control: Having a treatment plan (such as an elimination diet) can give patients a sense of control over difficult-to-manage symptoms, which can reduce anxiety and improve well-being.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lomer, M.C. Review article: The aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment. Pharmacol. Ther. 2015, 41, 262–275. [Google Scholar] [CrossRef]
- Turnbull, J.L.; Adams, H.N.; Gorard, D.A. Review article: The diagnosis and management of food allergy and food intolerances. Aliment. Pharmacol. Ther. 2015, 41, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.J.; Biesiekierski, J.R.; Schmid-Grendelmeier, P.; Pohl, D. Food Intolerances. Nutrients 2019, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Stoneham, M.D.; Petruckevitch, A.; Barton, J.; Rona, R. A population study of food intolerance. Lancet 1994, 343, 1127–1130. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Jin, H.; Chen, L.; Ji, J.; Zhang, Z. Histamine Intolerance-A Kind of Pseudoallergic Reaction. Biomolecules 2022, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Hrubisko, M.; Danis, R.; Huorka, M.; Wawruch, M. Histamine Intolerance—The More We Know the Less We Know. A Review. Nutrients 2021, 13, 2228. [Google Scholar] [CrossRef]
- Ji, Y.; Sakata, Y.; Li, X.; Zhang, C.; Yang, Q.; Xu, M.; Wollin, A.; Langhans, W.; Tso, P. Lymphatic diamine oxidase secretion stimulated by fat absorption is linked with histamine release. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G732–G740. [Google Scholar] [CrossRef]
- Bieganski, T.; Kusche, J.; Lorenz, W.; Hesterberg, R.; Stahlknecht, C.D.; Feussner, K.D. Distribution and properties of human intestinal diamine oxidase and its relevance for the histamine catabolism. Biochim. Biophys. Acta 1983, 756, 196–203. [Google Scholar] [CrossRef]
- Bieganski, T.; Kusche, J.; Feussner, K.D.; Hesterberg, R.; Richter, H.; Lorenz, W. Human intestinal diamine oxidase: Substrate specificity and comparative inhibitor study. Agents Actions 1980, 10, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Boehm, T.; Reiter, B.; Ristl, R.; Petroczi, K.; Sperr, W.; Stimpfl, T.; Valent, P.; Jilma, B. Massive release of the histamine-degrading enzyme diamine oxidase during severe anaphylaxis in mastocytosis patients. Allergy 2019, 74, 583–593. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Lackner, S.; Enko, D.; Schenk, M.; Holasek, S.J.; Mangge, H. Evaluation of symptoms and symptom combinations in histamine intolerance. Intest. Res. 2019, 17, 427–433. [Google Scholar] [CrossRef]
- Kucher, A.N. Association of Polymorphic Variants of Key Histamine Metabolism Genes and Histamine Receptor Genes with Multifactorial Diseases. Russ. J. Genet. 2019, 55, 794–814. [Google Scholar] [CrossRef]
- Garcia-Martin, E.; Garcia-Menaya, J.; Sanchez, B.; Martinez, C.; Rosendo, R.; Agundez, J.A. Polymorphisms of histamine-metabolizing enzymes and clinical manifestations of asthma and allergic rhinitis. Clin. Exp. Allergy 2007, 37, 1175–1182. [Google Scholar] [CrossRef]
- García-Martín, E.; Ayuso, P.; Martínez, C.; Agúndez, J.A. Improved analytical sensitivity reveals the occurrence of gender-related variability in diamine oxidase enzyme activity in healthy individuals. Clin. Biochem. 2007, 40, 1339–1341. [Google Scholar] [CrossRef]
- Ayuso, P.; García-Martín, E.; Martínez, C.; Agúndez, J.A. Genetic variability of human diamine oxidase: Occurrence of three nonsynonymous polymorphisms and study of their effect on serum enzyme activity. Pharmacogenet Genom. 2007, 17, 687–693. [Google Scholar] [CrossRef]
- Fukudome, I.; Kobayashi, M.; Dabanaka, K.; Maeda, H.; Okamoto, K.; Okabayashi, T.; Baba, R.; Kumagai, N.; Oba, K.; Fujita, M.; et al. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med. Mol. Morphol. 2014, 47, 100–107. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Duelo, A.; Veciana-Nogués, M.T.; Berlanga, M.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Intestinal Dysbiosis in Patients with Histamine Intolerance. Nutrients 2022, 14, 1774. [Google Scholar] [CrossRef] [PubMed]
- Kovacova-Hanuskova, E.; Buday, T.; Gavliakova, S.; Plevkova, J. Histamine, histamine intoxication and intolerance. Allergol. Immunopathol. 2015, 43, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- Enko, D.; Meinitzer, A.; Mangge, H.; Kriegshäuser, G.; Halwachs-Baumann, G.; Reininghaus, E.Z.; Bengesser, S.A.; Schnedl, W.J. Concomitant Prevalence of Low Serum Diamine Oxidase Activity and Carbohydrate Malabsorption. Can. J. Gastroenterol. Hepatol. 2016, 2016, 4893501. [Google Scholar] [CrossRef]
- Leitner, R.; Zoernpfenning, E.; Missbichler, A. Evaluation of the inhibitory effect of various drugs/active ingredients on the activity of human diamine oxidase in vitro. Clin. Transl. Allergy 2014, 4, P23. [Google Scholar] [CrossRef]
- Fortes Marin, E.; Carrera Marcolin, L.; Martí Melero, L.; Tintoré Gazulla, M.; Beltran Porres, M. The Prevalence of Single Nucleotide Polymorphisms of the AOC1 Gene Associated with Diamine Oxidase (DAO) Enzyme Deficiency in Healthy Newborns: A Prospective Population-Based Cohort Study. Genes 2025, 16, 141. [Google Scholar] [CrossRef] [PubMed]
- Klocker, J.; Perkmann, R.; Klein-Weigel, P.; Mörsdorf, G.; Drasche, A.; Klingler, A.; Fraedrich, G.; Schwelberger, H.G. Continuous administration of heparin in patients with deep vein thrombosis can increase plasma levels of diamine oxidase. Vasc. Pharmacol. 2004, 40, 293–300. [Google Scholar] [CrossRef]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Vidal-Carou, M.C.; Soler-Singla, L. Low serum diamine oxidase (DAO) activity levels in patients with migraine. J. Physiol. Biochem. 2018, 74, 93–99. [Google Scholar] [CrossRef]
- Hamada, Y.; Shinohara, Y.; Yano, M.; Yamamoto, M.; Yoshio, M.; Satake, K.; Toda, A.; Hirai, M.; Usami, M. Effect of the menstrual cycle on serum diamine oxidase levels in healthy women. Clin. Biochem. 2013, 46, 99–102. [Google Scholar] [CrossRef]
- Pavlov, V.; Dimitrov, O. Estrogenic and androgenic regulation of diamine oxidase in the mouse kidney. Biog. Amines 2000, 15, 601–612. [Google Scholar]
- Sessa, A.; Desiderio, M.A.; Perin, A. Estrogenic regulation of diamine oxidase activity in rat uterus. Agents Actions 1990, 29, 162–166. [Google Scholar] [CrossRef]
- Zaitsu, M.; Narita, S.; Lambert, K.C.; Grady, J.J.; Estes, D.M.; Curran, E.M.; Brooks, E.G.; Watson, C.S.; Goldblum, R.M.; Midoro-Horiuti, T. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol. Immunol. 2007, 44, 1977–1985. [Google Scholar] [CrossRef]
- Kalogeromitros, D.; Katsarou, A.; Armenaka, M.; Rigopoulos, D.; Zapanti, M.; Stratigos, I. Influence of the menstrual cycle on skin-prick test reactions to histamine, morphine and allergen. Clin. Exp. Allergy 1995, 25, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Camps, A.; Zibetti, S.; Pérez-Esteban, G.; Vila-Vidal, M.; Ferrés-Ramis, L.; García-Teresa-García, E. Histamine intolerance as a cause of chronic digestive complaints in pediatric patients. Rev. Esp. Enferm. Dig. 2013, 105, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Nazar, W.; Plata-Nazar, K.; Sznurkowska, K.; Szlagatys-Sidorkiewicz, A. Histamine Intolerance in Children: A Narrative Review. Nutrients 2021, 13, 1486. [Google Scholar] [CrossRef]
- Vlieg-Boerstra, B.J.; van der Heide, S.; Oude Elberink, J.N.; Kluin-Nelemans, J.C.; Dubois, A.E. Mastocytosis and adverse reactions to biogenic amines and histamine-releasing foods: What is the evidence? Neth. J. Med. 2005, 63, 244–249. [Google Scholar] [PubMed]
- Comas-Basté, O.; Latorre-Moratalla, M.L.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. In vitro determination of diamine oxidase activity in food matrices by an enzymatic assay coupled to UHPLC-FL. Anal. Bioanal. Chem. 2019, 411, 7595–7602. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Low-Histamine Diets: Is the Exclusion of Foods Justified by Their Histamine Content? Nutrients 2021, 13, 1395. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Schenk, M.; Lackner, S.; Enko, D.; Mangge, H.; Forster, F. Diamine oxidase supplementation improves symptoms in patients with histamine intolerance. Food Sci. Biotechnol. 2019, 28, 1779–1784. [Google Scholar] [CrossRef]
- Manzotti, G.; Breda, D.; Di Gioacchino, M.; Burastero, S.E. Serum diamine oxidase activity in patients with histamine intolerance. Int. J. Immunopathol. Pharmacol. 2016, 29, 105–111. [Google Scholar] [CrossRef]
- Komericki, P.; Klein, G.; Reider, N.; Hawranek, T.; Strimitzer, T.; Lang, R.; Kranzelbinder, B.; Aberer, W. Histamine intolerance: Lack of reproducibility of single symptoms by oral provocation with histamine: A randomised, double-blind, placebo-controlled cross-over study. Wien. Klin. Wochenschr. 2011, 123, 15–20. [Google Scholar] [CrossRef]
- Patel, R.H.; Mohiuddin, S.S. Biochemistry, Histamine. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.; Stark, H.; Thurmond, R.L.; Haas, H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar] [CrossRef]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Kitamura, Y.; Takeda, N.; Fukui, H. Molecular Signaling and Transcriptional Regulation of Histamine H(1) Receptor Gene. Curr. Top. Behav. Neurosci. 2022, 59, 91–110. [Google Scholar] [CrossRef]
- Miyoshi, K.; Das, A.K.; Fujimoto, K.; Horio, S.; Fukui, H. Recent advances in molecular pharmacology of the histamine systems: Regulation of histamine H1 receptor signaling by changing its expression level. J. Pharmacol. Sci. 2006, 101, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Ohta, K.; Kangawa, K.; Kikkawa, U.; Ogino, S.; Fukui, H. Identification of protein kinase C phosphorylation sites involved in phorbol ester-induced desensitization of the histamine H1 receptor. Mol. Pharmacol. 1999, 55, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.E.; Lorentz, A.; Sellge, G.; Coëffier, M.; Neipp, M.; Veres, T.; Frieling, T.; Meier, P.N.; Manns, M.P.; Bischoff, S.C. Selective expression of histamine receptors H1R, H2R, and H4R, but not H3R, in the human intestinal tract. Gut 2006, 55, 498–504. [Google Scholar] [CrossRef]
- Pillot, C.; Heron, A.; Cochois, V.; Tardivel-Lacombe, J.; Ligneau, X.; Schwartz, J.C.; Arrang, J.M. A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience 2002, 114, 173–193. [Google Scholar] [CrossRef]
- Liu, C.; Ma, X.; Jiang, X.; Wilson, S.J.; Hofstra, C.L.; Blevitt, J.; Pyati, J.; Li, X.; Chai, W.; Carruthers, N.; et al. Cloning and pharmacological characterization of a fourth histamine receptor (H(4)) expressed in bone marrow. Mol. Pharmacol. 2001, 59, 420–426. [Google Scholar] [CrossRef]
- Shulpekova, Y.O.; Nechaev, V.M.; Popova, I.R.; Deeva, T.A.; Kopylov, A.T.; Malsagova, K.A.; Kaysheva, A.L.; Ivashkin, V.T. Food Intolerance: The Role of Histamine. Nutrients 2021, 13, 3207. [Google Scholar] [CrossRef]
- Karer, M.; Rager-Resch, M.; Haider, T.; Petroczi, K.; Gludovacz, E.; Borth, N.; Jilma, B.; Boehm, T. Diamine oxidase knockout mice are not hypersensitive to orally or subcutaneously administered histamine. Inflamm. Res. 2022, 71, 497–511. [Google Scholar] [CrossRef]
- Rentzos, G.; Weisheit, A.; Ekerljung, L.; van Odijk, J. Measurement of diamine oxidase (DAO) during low-histamine or ordinary diet in patients with histamine intolerance. Eur. J. Clin. Nutr. 2024, 78, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Reese, I.; Ballmer-Weber, B.; Beyer, K.; Fuchs, T.; Kleine-Tebbe, J.; Klimek, L.; Lepp, U.; Niggemann, B.; Saloga, J.; Schäfer, C.; et al. German guideline for the management of adverse reactions to ingested histamine. Allergo J. Int. 2017, 26, 72–79. [Google Scholar] [CrossRef]
- Jochum, C. Histamine Intolerance: Symptoms, Diagnosis, and Beyond. Nutrients 2024, 16, 1219. [Google Scholar] [CrossRef]
- Lackner, S.; Malcher, V.; Enko, D.; Mangge, H.; Holasek, S.J.; Schnedl, W.J. Histamine-reduced diet and increase of serum diamine oxidase correlating to diet compliance in histamine intolerance. Eur. J. Clin. Nutr. 2019, 73, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, K.; Catsos, P.; Smith, J. From a Dietitian‘s Perspective, Diets for Irritable Bowel Syndrome Are Not One Size Fits All. Clin. Gastroenterol. Hepatol. 2020, 18, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Zickgraf, H.F.; Ellis, J.M. Initial validation of the Nine Item Avoidant/Restrictive Food Intake disorder screen (NIAS): A measure of three restrictive eating patterns. Appetite 2018, 123, 32–42. [Google Scholar] [CrossRef]
- Zickgraf, H.F.; Loftus, P.; Gibbons, B.; Cohen, L.C.; Hunt, M.G. “If I could survive without eating, it would be a huge relief”: Development and initial validation of the Fear of Food Questionnaire. Appetite 2022, 169, 105808. [Google Scholar] [CrossRef]
- Simons, M.; Taft, T.H.; Doerfler, B.; Ruddy, J.S.; Bollipo, S.; Nightingale, S.; Siau, K.; van Tilburg, M.A.L. Narrative review: Risk of eating disorders and nutritional deficiencies with dietary therapies for irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14188. [Google Scholar] [CrossRef]
- Bjeldanes, L.F.; Schutz, D.E.; Morris, M.M. On the aetiology of scombroid poisoning: Cadaverine potentiation of histamine toxicity in the guinea-pig. Food Cosmet. Toxicol. 1978, 16, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Lieber, E.R. In vitro inhibition of rat intestinal histamine-metabolizing enzymes. Food Cosmet. Toxicol. 1979, 17, 237–240. [Google Scholar] [CrossRef]
- Wagner, N.; Dirk, D.; Peveling-Oberhag, A.; Reese, I.; Rady-Pizarro, U.; Mitzel, H.; Staubach, P. A Popular myth—Low-histamine diet improves chronic spontaneous urticaria—Fact or fiction? J. Eur. Acad. Dermatol. Venereol. 2017, 31, 650–655. [Google Scholar] [CrossRef]
- Worm, M.; Fiedler, E.M.; Dölle, S.; Schink, T.; Hemmer, W.; Jarisch, R.; Zuberbier, T. Exogenous histamine aggravates eczema in a subgroup of patients with atopic dermatitis. Acta Derm. Venereol. 2009, 89, 52–56. [Google Scholar] [CrossRef]
- Steinbrecher, I.; Jarisch, R. Histamine and headache. Allergologie 2005, 28, 85–91. [Google Scholar] [CrossRef]
- Guida, B.; De Martino, C.D.; De Martino, S.D.; Tritto, G.; Patella, V.; Trio, R.; D’Agostino, C.; Pecoraro, P.; D’Agostino, L. Histamine plasma levels and elimination diet in chronic idiopathic urticaria. Eur. J. Clin. Nutr. 2000, 54, 155–158. [Google Scholar] [CrossRef]
- Son, J.H.; Chung, B.Y.; Kim, H.O.; Park, C.W. A Histamine-Free Diet Is Helpful for Treatment of Adult Patients with Chronic Spontaneous Urticaria. Ann. Dermatol. 2018, 30, 164–172. [Google Scholar] [CrossRef]
- Mušič, E.; Korošec, P.; Šilar, M.; Adamič, K.; Košnik, M.; Rijavec, M. Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wien. Klin. Wochenschr. 2013, 125, 239–243. [Google Scholar] [CrossRef]
- King, W.; McCargar, L.; Joneja, J.M.; Barr, S.I. Benefits of a histamine-reducing diet for some patients with chronic urticaria and angiodema. Can. J. Diet. Pract. Res. 2000, 61, 24–26. [Google Scholar]
- Taylor, S.L. Histamine food poisoning: Toxicology and clinical aspects. Crit. Rev. Toxicol. 1986, 17, 91–128. [Google Scholar] [CrossRef] [PubMed]
- Tsiasioti, A.; Tzanavaras, P.D. Simple and Reliable Determination of the Histamine Content of Selected Greek Vegetables and Related Products in the Frame of “Low Histamine Diet”. Foods 2022, 11, 3234. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Yoza, K.-I.; Nogata, Y.; Kusumoto, K.-I.; Voragen, A.G.J.; Ohta, H. Putrescine accumulation in banana fruit with ripening during storage. Phytochemistry 1997, 46, 57–60. [Google Scholar] [CrossRef]
- John, J.; Kodama, T.; Siegel, J.M. Caffeine promotes glutamate and histamine release in the posterior hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R704–R710. [Google Scholar] [CrossRef]
- Zimatkin, S.M.; Anichtchik, O.V. Alcohol-histamine interactions. Alcohol. Alcohol. 1999, 34, 141–147. [Google Scholar] [CrossRef]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food--existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef]
- San Mauro Martin, I.; Brachero, S.; Garicano Vilar, E. Histamine intolerance and dietary management: A complete review. Allergol. Immunopathol. 2016, 44, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Tobajas, Y.; Alemany-Fornés, M.; Samarra, I.; Romero-Giménez, J.; Tintoré, M.; Del Pino, A.; Canela, N.; Del Bas, J.M.; Ortega-Olivé, N.; de Lecea, C.; et al. Diamine Oxidase Interactions with Anti-Inflammatory and Anti-Migraine Medicines in the Treatment of Migraine. J. Clin. Med. 2023, 12, 7502. [Google Scholar] [CrossRef]
- Jonassen, F.; Granerus, G.; Wetterqvist, H. Histamine metabolism during the menstrual cycle. Acta Obs. Gynecol. Scand. 1976, 55, 297–304. [Google Scholar] [CrossRef]
- Schink, M.; Konturek, P.C.; Tietz, E.; Dieterich, W.; Pinzer, T.C.; Wirtz, S.; Neurath, M.F.; Zopf, Y. Microbial patterns in patients with histamine intolerance. J. Physiol. Pharmacol. 2018, 69, 579–593. [Google Scholar] [CrossRef]
- Kettner, L.; Seitl, I.; Fischer, L. Toward Oral Supplementation of Diamine Oxidase for the Treatment of Histamine Intolerance. Nutrients 2022, 14, 2621. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Martin, P.G.E.; Solano-Romaní, L.; Martínez-Puig, D.; Velasco, J. Evaluation of the activity of the DAO enzyme in marketed nutritional supplements. Nutr. Hosp. 2023, 40, 17–145. [Google Scholar]
- Jumarie, C.; Séïde, M.; Marcocci, L.; Pietrangeli, P.; Mateescu, M.A. Diamine Oxidase from White Pea (Lathyrus sativus) Combined with Catalase Protects the Human Intestinal Caco-2 Cell Line from Histamine Damage. Appl. Biochem. Biotechnol. 2017, 182, 1171–1181. [Google Scholar] [CrossRef]
- Kettner, L.; Braun, C.; Seitl, I.; Pross, E.; Fischer, L. Production and characterization of a new diamine oxidase from Yarrowia lipolytica. J. Biotechnol. 2021, 340, 39–46. [Google Scholar] [CrossRef]
- Gálvez-Martín, P.; Gallego, E.; Soriano-Romaní, L.; Martinez-Puig, D.; Velasco, J. In Vitro Evaluation of The Capacity to Degrade Histamine of DAO From Pig Kidney Combined with Vitamin C. J. Acad. Nutr. Diet. 2022, 122, A43. [Google Scholar] [CrossRef]
- Naila, A.; Flint, S.; Fletcher, G.C.; Bremer, P.J.; Meerdink, G.; Morton, R.H. Prediction of the amount and rate of histamine degradation by diamine oxidase (DAO). Food Chem. 2012, 135, 2650–2660. [Google Scholar] [CrossRef]
- Galvez-Martin, P.; Gallego, E.; Solano-Romaní, L.; Martínez-Puig, D.; Cabañas, J.; Castells, G.; Velasco, J. In vitro characterization, evaluation of enzymatic activity and capacity of intestinal absorption of daogest (Dao extract from pig kidney). Clin. Nutr. ESPEN 2023, 58, 512. [Google Scholar] [CrossRef]
- O’Connor, M.E.; Terradillos-Guillén, A.; Gálvez-Martín, P.; Martínez-Puig, D. Observational study to assess the efficacy and safety of diaminooxidase (DAO) supplementation in patients with histamine intolerance (HIT). Clin. Nutr. ESPEN 2023, 58, 741. [Google Scholar] [CrossRef]
- Yacoub, M.R.; Ramirez, G.A.; Berti, A.; Mercurio, G.; Breda, D.; Saporiti, N.; Burastero, S.; Dagna, L.; Colombo, G. Diamine Oxidase Supplementation in Chronic Spontaneous Urticaria: A Randomized, Double-Blind Placebo-Controlled Study. Int. Arch. Allergy Immunol. 2018, 176, 268–271. [Google Scholar] [CrossRef]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Soler-Singla, L.; Vidal-Carou, M.C. Diamine oxidase (DAO) supplement reduces headache in episodic migraine patients with DAO deficiency: A randomized double-blind trial. Clin. Nutr. 2019, 38, 152–158. [Google Scholar] [CrossRef]
- Okutan, G.; Sánchez Niño, G.M.; Terrén Lora, A.; López Oliva, S.; San Mauro Martín, I. Exogenous Supplementation with DAO Enzyme in Women with Fibromyalgia: A Double-Blind Placebo-Controlled Clinical Trial. J. Clin. Med. 2023, 12, 6449. [Google Scholar] [CrossRef] [PubMed]
- Töndury, B.; Wüthrich, B.; Schmid-Grendelmeier, P.; Seifert, B.; Ballmer-Weber, B.K. Histamine intolerance: Is the determination of diamine oxidase activity in the serum useful in routine clinical practice? Allergologie 2008, 31, 350–356. [Google Scholar] [CrossRef]
- Kofler, H.; Aberer, W.; Deibl, M.; Hawranek, T.; Klein, G.; Reider, N.; Fellner, N. Diamine oxidase (DAO) serum activity: Not a useful marker for diagnosis of histamine intolerance. Allergologie 2009, 32, 105–109. [Google Scholar] [CrossRef]
- Wantke, F.; Proud, D.; Siekierski, E.; Kagey-Sobotka, A. Daily variations of serum diamine oxidase and the influence of H1 and H2 blockers: A critical approach to routine diamine oxidase assessment. Inflamm. Res. 1998, 47, 396–400. [Google Scholar] [CrossRef]
- Vögele, A. Antihistaminika bei Histaminunverträglichkeit? Z. Allg. 2018, 94, 99–101. [Google Scholar] [CrossRef]
- Lorenz, W.; Ennis, M.; Doenicke, A.; Dick, W. Perioperative uses of histamine antagonists. J. Clin. Anesth. 1990, 2, 345–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
| High Histamine Foods | Histamine Liberator Foods | DAO Inhibitor Foods | |
|---|---|---|---|
| Fruits and vegetables | Tomato, spinach, fermented vegetables, eggplant, avocado | Lemons, limes, pineapples, kiwis, and papayas are often culprits | Bananas, citrus fruits, strawberries, pineapple |
| Proteins | Fermented, cured, smoked or dried meat. Blue fish, canned or preserved fish | Egg white, cow’s milk, seafood | - |
| Drinks | Alcohol-containing drinks | Coffee, tea, alcohol-containing drinks | Coffee, tea, alcohol-containing drinks, energy drinks |
| Other | Soy fermented products, yoghurt, vinegars, cheeses (cured, semi-cured or smoked) | Chocolate, cocoa, licorice, peanuts and walnuts | Chocolate |
| Symptom Profile | Design | Intervention Duration (Days) | Sample Size | Main Results Reported | Reference |
|---|---|---|---|---|---|
| General HIT symptoms | Randomized double-blind placebo-controlled cross-over study | - | 39 | Reduction in histamine-associated symptoms compared to placebo | [39] |
| General HIT symptoms | Retrospective observational study | 14 | 14 | 13 out of 14 patients reported improvement in at least one of the HIT symptoms | [38] |
| Chronic Spontaneous Urticaria (CSU) | Randomized double-blind placebo-controlled cross-over study | 30 | 20 | Reduction of 7-Day Urticaria Activity Score (UAS-7). Reduction in antihistamine dose | [86] |
| Episodic migraine | Randomized double-blind placebo-controlled study | 30 | 100 | Reduction in migraine episodes duration and triptan intake | [87] |
| General HIT symptoms | Open-label interventional pilot study | 28 | 28 | Reduction in all HIT-related symptoms | [37] |
| General HIT symptoms | Open-label interventional study | 28 | 82 | Reduction in all HIT-related symptoms | [85] |
| Fibromyalgia | Randomized double-blind placebo-controlled study | 56 | 100 | Reduction in fatigue, anxiety, depression, burning, rumination, magnification and helplessness compared to baseline | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, K.; Busse, W.; Gálvez-Martín, P.; Terradillos, A.; Martínez-Puig, D. Evidence for Dietary Management of Histamine Intolerance. Int. J. Mol. Sci. 2025, 26, 9198. https://doi.org/10.3390/ijms26189198
Jackson K, Busse W, Gálvez-Martín P, Terradillos A, Martínez-Puig D. Evidence for Dietary Management of Histamine Intolerance. International Journal of Molecular Sciences. 2025; 26(18):9198. https://doi.org/10.3390/ijms26189198
Chicago/Turabian StyleJackson, Kirsten, Wendy Busse, Patricia Gálvez-Martín, Andrea Terradillos, and Daniel Martínez-Puig. 2025. "Evidence for Dietary Management of Histamine Intolerance" International Journal of Molecular Sciences 26, no. 18: 9198. https://doi.org/10.3390/ijms26189198
APA StyleJackson, K., Busse, W., Gálvez-Martín, P., Terradillos, A., & Martínez-Puig, D. (2025). Evidence for Dietary Management of Histamine Intolerance. International Journal of Molecular Sciences, 26(18), 9198. https://doi.org/10.3390/ijms26189198






