A Bioactive Lipid Nanoparticle Integrating Arachidonic Acid Enables High-Efficiency mRNA Delivery and Potent CAR-Macrophage Engineering
Abstract
1. Introduction
2. Results
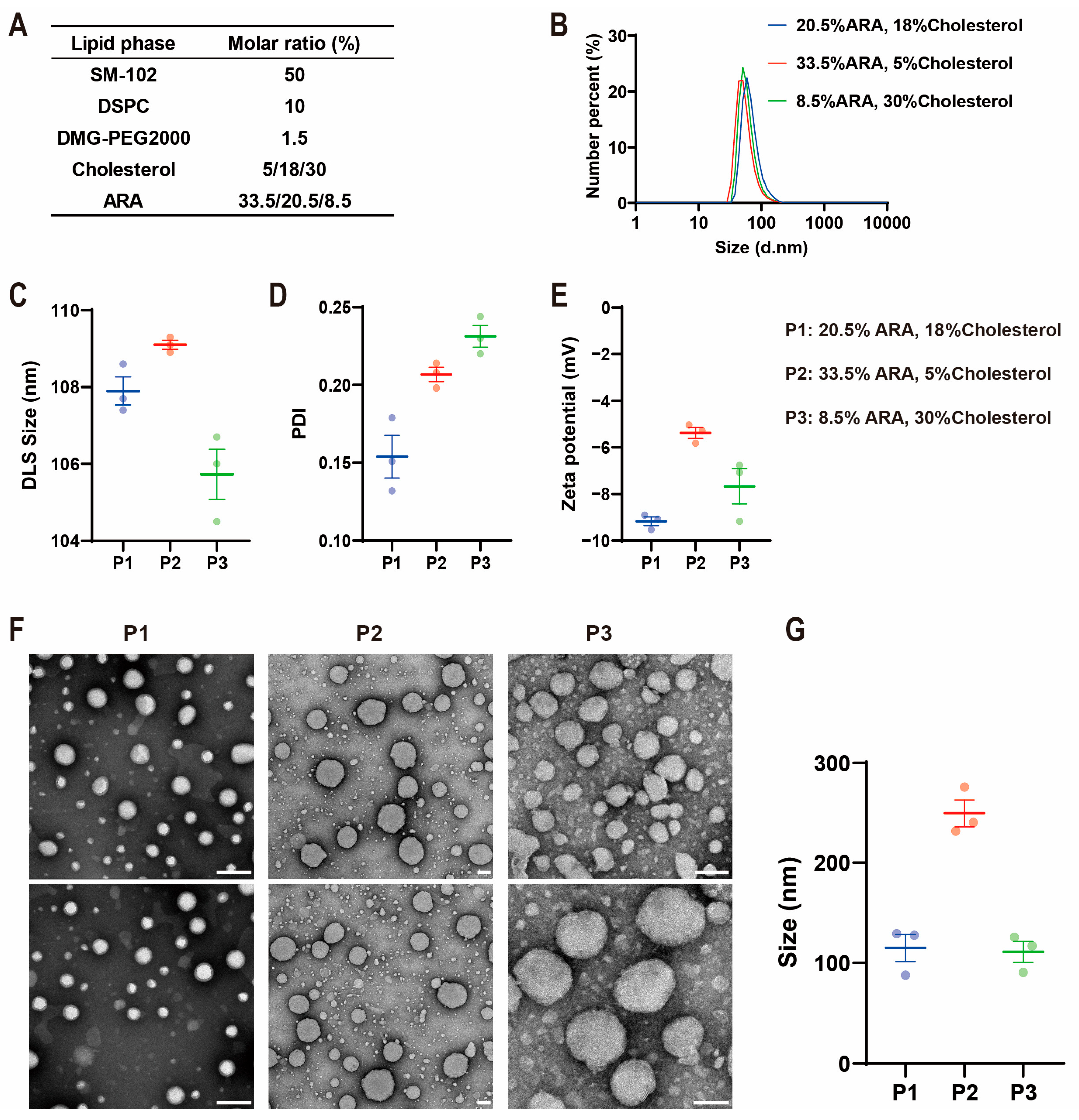
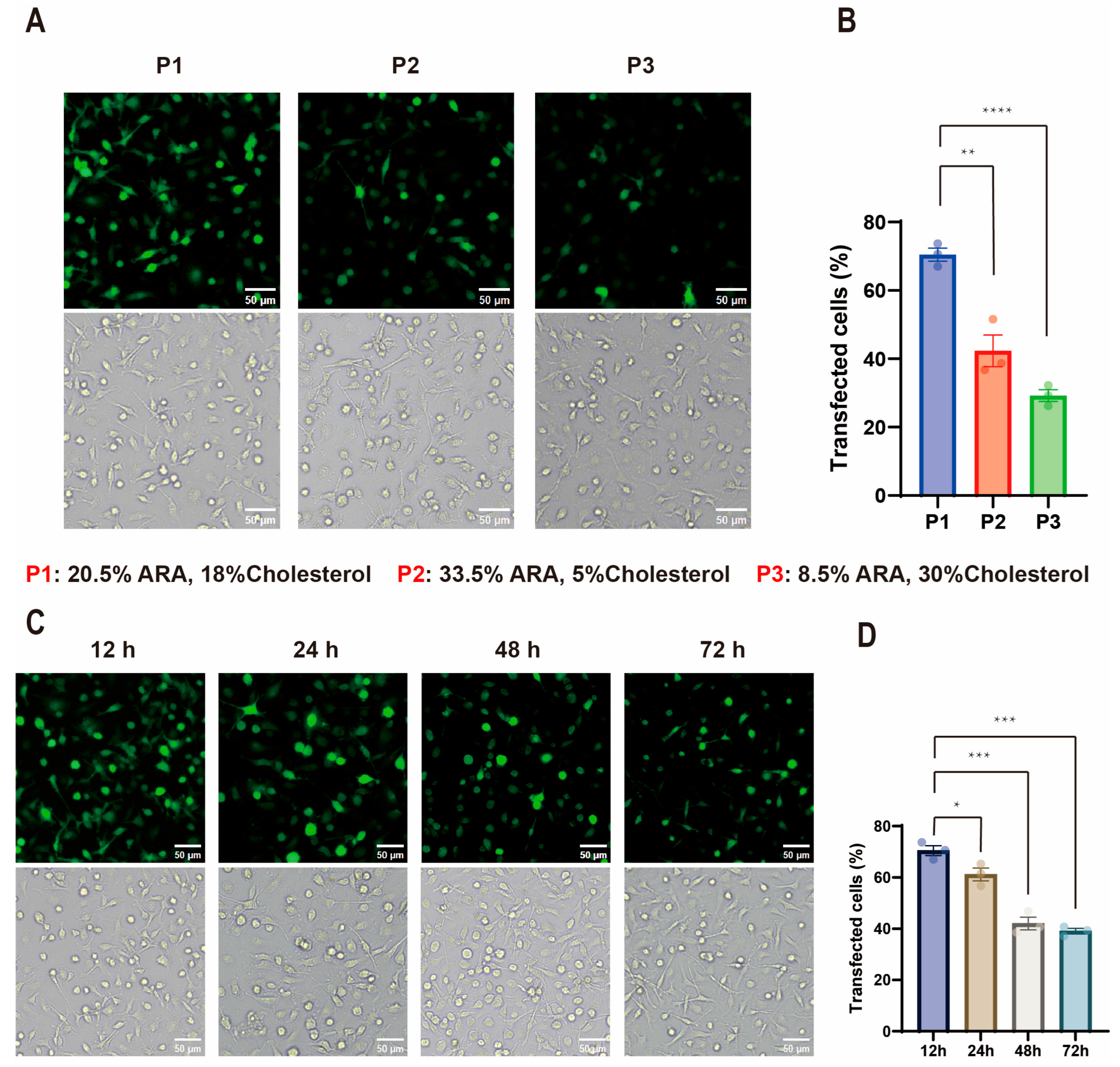
2.1. Influence of Lipid Composition on ARA-LNP Physicochemical Properties and Transfection Efficacy
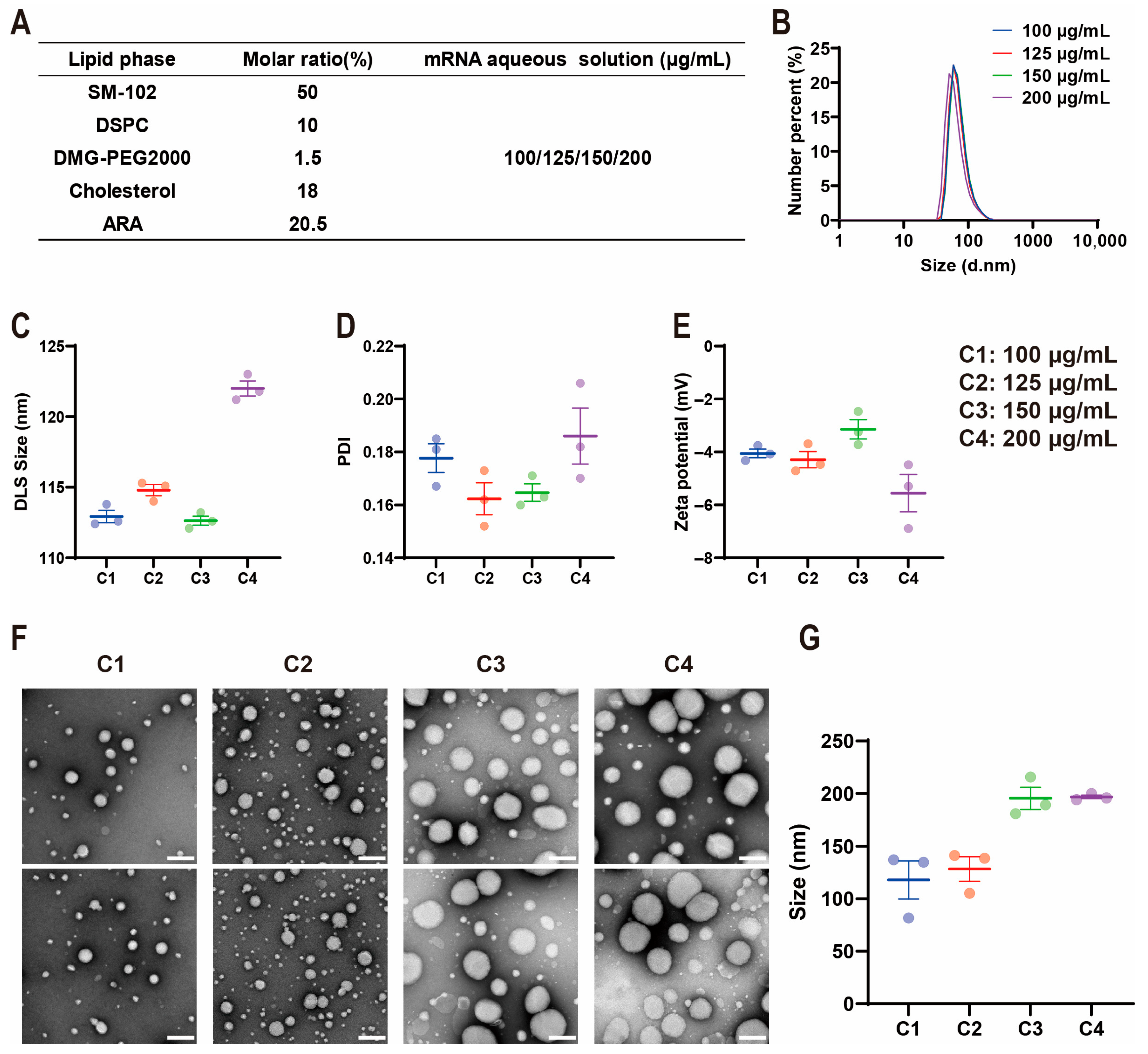
2.2. Influence of mRNA Concentration on ARA-LNP Properties and Transfection Efficacy
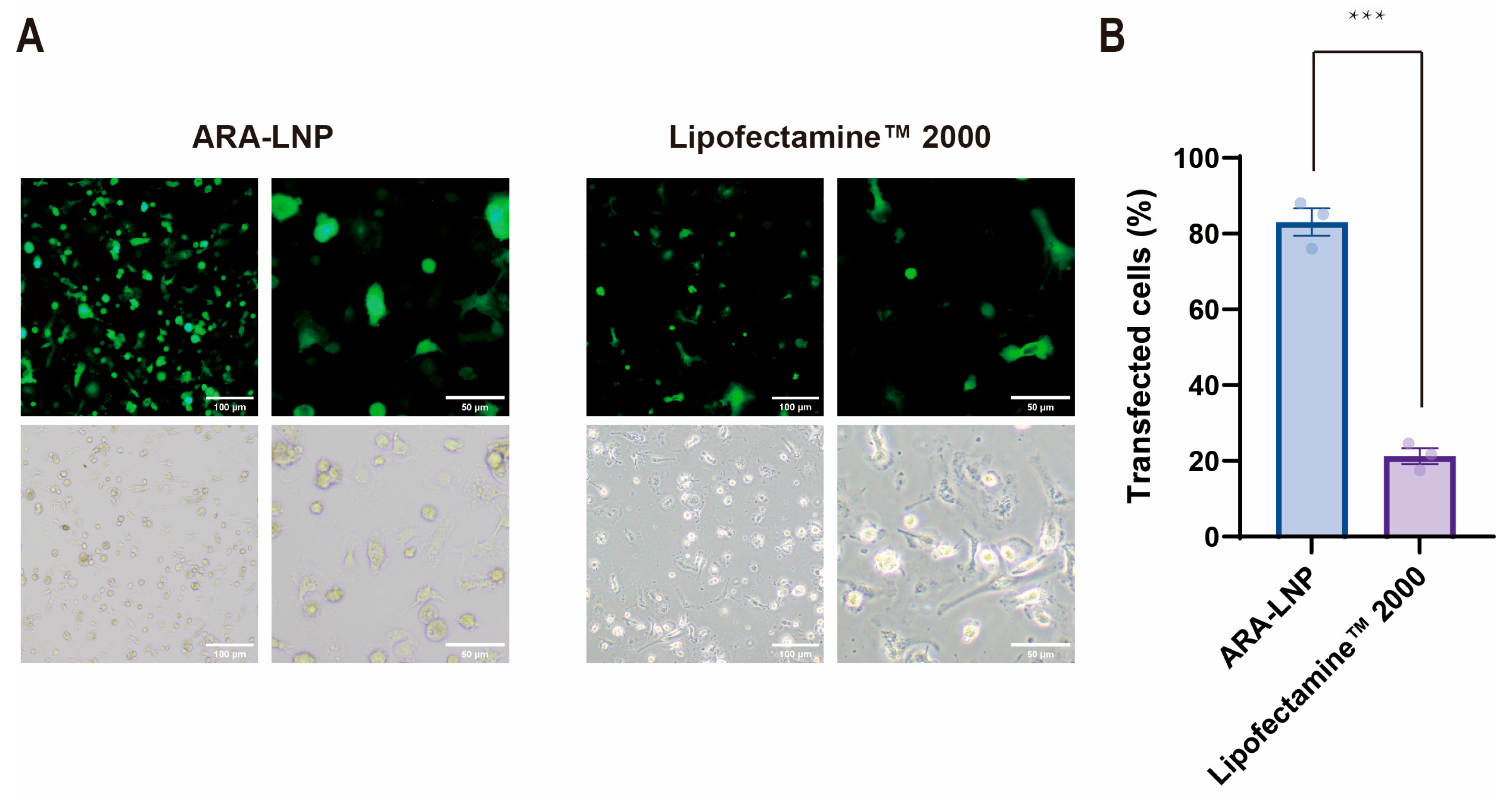
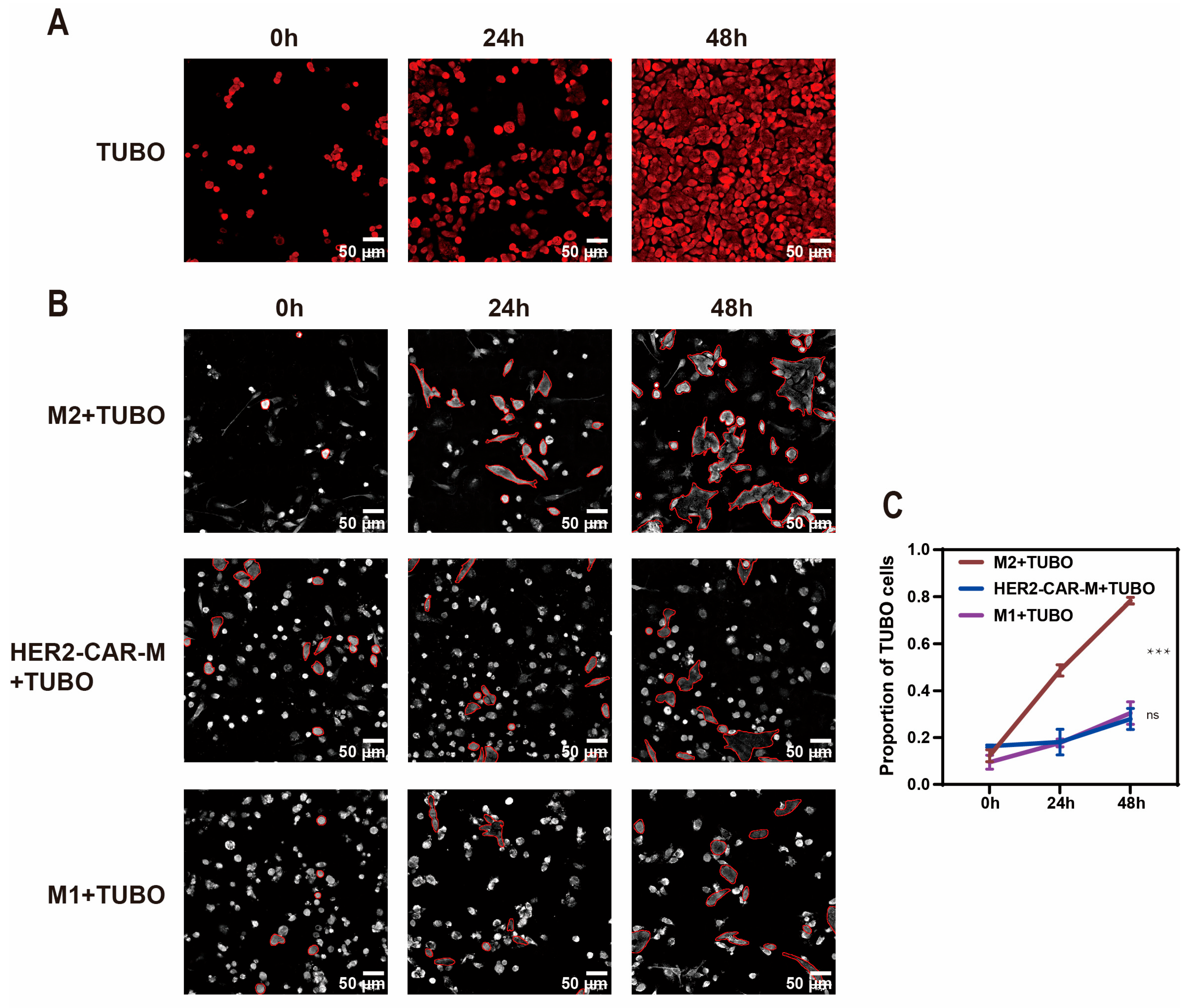
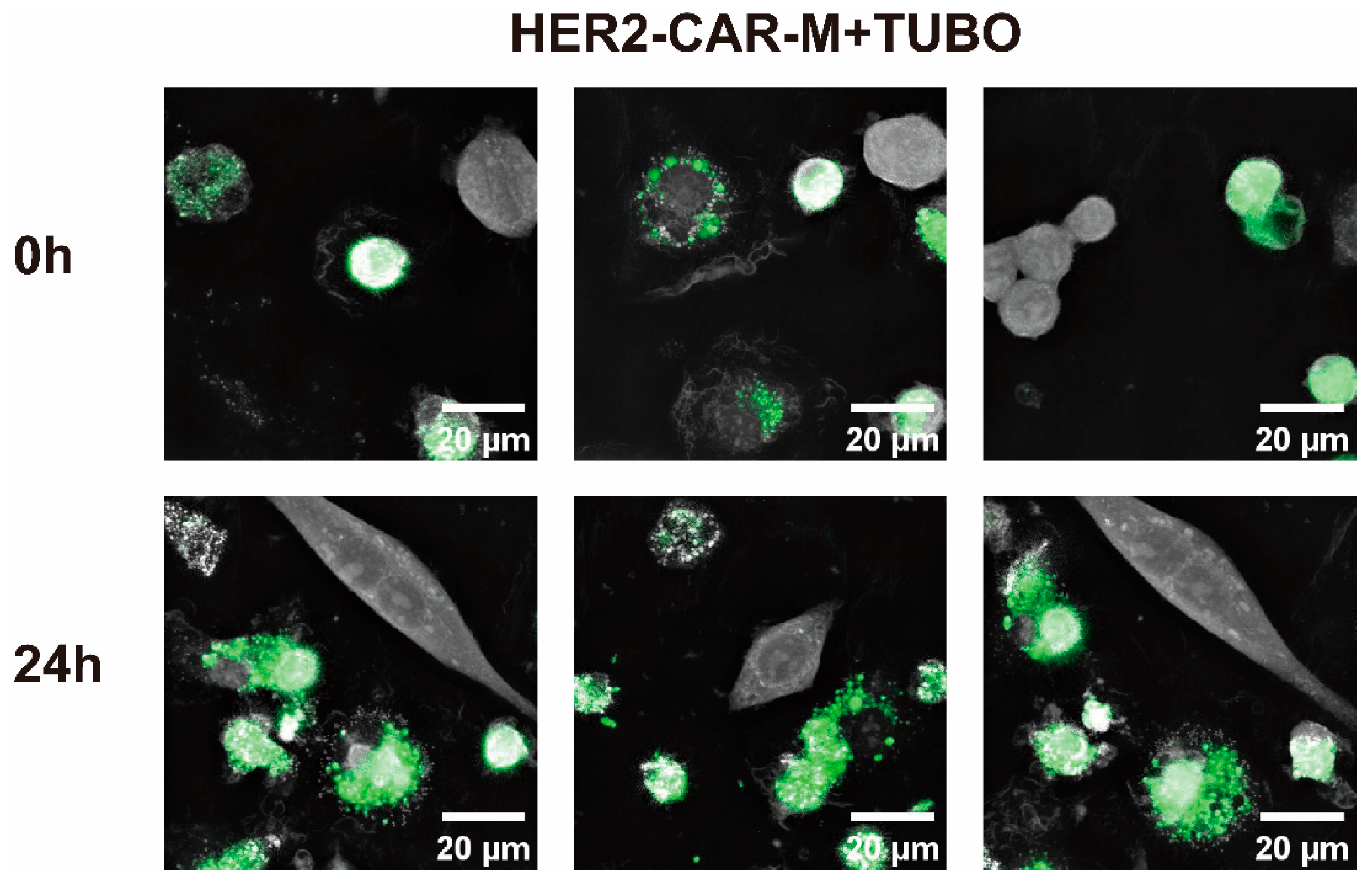
2.3. In Vitro Generation and Anti-Tumor Efficacy Validation of CAR-M Macrophages via ARA-LNP Delivery
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. The Preparation of LNP
4.3. The Characterization of LNP
4.4. Isolation, Polarization, and Expansion Culture of Primary Bone Marrow-Derived Monocytes
4.5. Validation of LNP Transfection Efficiency
4.6. Development of an ARA-LNP-Based In Vitro CAR-M Platform and Validation of Its Anti-Tumor Efficacy
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, Y.; Jiang, H.; Yin, Y.; Zhou, X.; Shao, Y.; Li, Y.; Rao, J. Macrophage Signaling Pathways in Health and Disease: From Bench to Bedside Applications. Med. Comm. 2025, 6, e70256. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- van Eijk, M.; Aerts, J. The Unique Phenotype of Lipid-Laden Macrophages. Int. J. Mol. Sci. 2021, 22, 4039. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int. J. Mol. Sci. 2021, 22, 6526. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, Y.Z.; Wang, L. Shaping Polarization of Tumor-Associated Macrophages in Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Shi, L.; Zhang, Y.; Guo, Y.; Gu, H. Mannose and Hyaluronic Acid Dual-Modified Iron Oxide Enhances Neoantigen-Based Peptide Vaccine Therapy by Polarizing Tumor-Associated Macrophages. Cancers 2022, 14, 5107. [Google Scholar] [CrossRef] [PubMed]
- Binnemars-Postma, K.; Storm, G.; Prakash, J. Nanomedicine Strategies to Target Tumor-Associated Macrophages. Int. J. Mol. Sci. 2017, 18, 979. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, X.; Liu, H.; Wang, C.; Chen, Y.; Wang, H.; Fang, Y.; Wu, X.; Xu, Y.; Li, C.; et al. The application of HER2 and CD47 CAR-macrophage in ovarian cancer. J. Transl. Med. 2023, 21, 654–666. [Google Scholar] [CrossRef]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Sureka, N.; Ahuja, S.; Aden, D.; Zaheer, S.; Zaheer, S. Tumor-associated macrophages: A sentinel of innate immune system in tumor microenvironment gone haywire. Int. J. Cell Biol. 2024, 48, 1406–1449. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Xu, L.; Song, Y. Chimeric antigen receptor-immune cells against solid tumors: Structures, mechanisms, recent advances, and future developments. Chin. Med. J. 2024, 137, 1285–1302. [Google Scholar] [CrossRef]
- Huang, T.; Bei, C.; Hu, Z.; Li, Y. CAR-macrophage: Breaking new ground in cellular immunotherapy. Front. Cell Dev. Biol. 2024, 12, 1464218. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Z.; Tan, X.; Jiang, H.; Xu, Z.; Fang, Y.; Han, D.; Hong, W.; Wei, W.; Tu, J. CAR-macrophage: A new immunotherapy candidate against solid tumors. Biomed. Pharmacother. 2021, 139, 111605. [Google Scholar] [CrossRef]
- Abdin, S.M.; Paasch, D.; Morgan, M.; Lachmann, N. CARs and beyond: Tailoring macrophage-based cell therapeutics to combat solid malignancies. J. Immunother. Cancer 2021, 9, e002741. [Google Scholar] [CrossRef]
- Ramishetti, S.; Landesman-Milo, D.; Peer, D. Advances in RNAi therapeutic delivery to leukocytes using lipid nanoparticles. J. Drug Target. 2016, 24, 780–786. [Google Scholar] [CrossRef]
- Moradian, H.; Roch, T.; Lendlein, A.; Gossen, M. mRNA Transfection-Induced Activation of Primary Human Monocytes and Macrophages: Dependence on Carrier System and Nucleotide Modification. Sci. Rep. 2020, 10, 4181. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Zhang, T.; Gu, H. Emerging advances in nanobiomaterials-assisted chimeric antigen receptor (CAR)-macrophages for tumor immunotherapy. Front. Bioeng. Biotechnol. 2023, 11, 1211687. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, X.Y.; Lu, A.; Wang, X.Y.; Jiang, L.X.; Wang, J.C. Non-viral vectors for RNA delivery. J. Control Release 2022, 342, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Chen, J.; Zhao, X.; Li, Y.; Harmon, J.; Huang, C.; Chen, J.; Xu, Q. In Vitro Engineering Chimeric Antigen Receptor Macrophages and T Cells by Lipid Nanoparticle-Mediated mRNA Delivery. ACS Biomater. Sci. Eng. 2022, 8, 722–733. [Google Scholar] [CrossRef]
- Tang, C.; Jing, W.; Han, K.; Yang, Z.; Zhang, S.; Liu, M.; Zhang, J.; Zhao, X.; Liu, Y.; Shi, C.; et al. mRNA-Laden Lipid-Nanoparticle-Enabled in Situ CAR-Macrophage Engineering for the Eradication of Multidrug-Resistant Bacteria in a Sepsis Mouse Model. ACS Nano 2024, 18, 2261–2278. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Horng, T. Lipid Metabolism in Regulation of Macrophage Functions. Trends Cell Biol. 2020, 30, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Seow, B.Y.L.; Bae, K.H.; Zhang, Y.; Liao, K.C.; Wan, Y.; Yang, Y.Y. Role of PEGylated lipid in lipid nanoparticle formulation for in vitro and in vivo delivery of mRNA vaccines. J. Control Release 2025, 380, 108–124. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Lingesh, S.; Krishnan, N.B.; Loong, B.S.Y.; Liu, M.; Chen, Q.; Yang, Y.Y. Cholesterol-Derived Mannosylated Polypeptide-Formed Lipid Nanoparticles for Efficient in Vivo mRNA Delivery. Small Methods 2025, 9, e2401712. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Raji, I.O.; Gordon, A.G.R.; Sun, L.; Raimondo, T.M.; Oladimeji, F.A.; Jiang, A.Y.; Varley, A.; Langer, R.S.; Anderson, D.G. Accelerating ionizable lipid discovery for mRNA delivery using machine learning and combinatorial chemistry. Nat. Mater. 2024, 23, 1002–1008. [Google Scholar] [CrossRef]
- Jung, O.; Jung, H.Y.; Thuy, L.T.; Choi, M.; Kim, S.; Jeon, H.G.; Yang, J.; Kim, S.M.; Kim, T.D.; Lee, E.; et al. Modulating Lipid Nanoparticles with Histidinamide-Conjugated Cholesterol for Improved Intracellular Delivery of mRNA. Adv. Healthc. Mater. 2024, 13, e2303857. [Google Scholar] [CrossRef]
- Guo, Z.; Zeng, C.; Shen, Y.; Hu, L.; Zhang, H.; Li, Z.; Dong, W.; Wang, Q.; Liu, Q.; Wang, Y.; et al. Helper Lipid-Enhanced mRNA Delivery for Treating Metabolic Dysfunction-Associated Fatty Liver Disease. Nano Lett. 2024, 24, 6743–6752. [Google Scholar] [CrossRef]
- Ma, H.; Gao, L.; Chang, R.; Zhai, L.; Zhao, Y. Crosstalk between macrophages and immunometabolism and their potential roles in tissue repair and regeneration. Heliyon 2024, 10, e38018. [Google Scholar] [CrossRef]
- Schoeniger, A.; Adolph, S.; Fuhrmann, H.; Schumann, J. The impact of membrane lipid composition on macrophage activation in the immune defense against Rhodococcus equi and Pseudomonas aeruginosa. Int. J. Mol. Sci. 2011, 12, 7510–7528. [Google Scholar] [CrossRef]
- Maurício, T.; Aveiro, S.; Guedes, S.; Lopes, D.; Melo, T.; Neves, B.M.; Domingues, R.; Domingues, P. Multi-Omic Profiling of Macrophages Treated with Phospholipids Containing Omega-3 and Omega-6 Fatty Acids Reveals Complex Immunomodulatory Adaptations at Protein, Lipid and Metabolic Levels. Int. J. Mol. Sci. 2022, 23, 2139. [Google Scholar] [CrossRef]
- Machado, R.M.; Nakandakare, E.R.; Quintao, E.C.; Cazita, P.M.; Koike, M.K.; Nunes, V.S.; Ferreira, F.D.; Afonso, M.S.; Bombo, R.P.; Machado-Lima, A.; et al. Omega-6 polyunsaturated fatty acids prevent atherosclerosis development in LDLr-KO mice, in spite of displaying a pro-inflammatory profile similar to trans fatty acids. Atherosclerosis 2012, 224, 66–74, Erratum to Atherosclerosis 2013, 226, 301–302. [Google Scholar] [CrossRef]
- Kain, V.; Halade, G.V. Immune responsive resolvin D1 programs peritoneal macrophages and cardiac fibroblast phenotypes in diversified metabolic microenvironment. J. Cell Physiol. 2019, 234, 3910–3920. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Li, S.; Hu, Y.; Li, A.; Lin, J.; Hsieh, K.; Schneiderman, Z.; Zhang, P.; Zhu, Y.; Qiu, C.; Kokkoli, E.; et al. Payload distribution and capacity of mRNA lipid nanoparticles. Nat. Commun. 2022, 13, 5561. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi Sözener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [PubMed]
- de Lima, T.M.; de Sa Lima, L.; Scavone, C.; Curi, R. Fatty acid control of nitric oxide production by macrophages. FEBS. Lett. 2006, 580, 3287–3295. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Zhang, Y.; Lv, Y.; Li, R.; Gu, H.; Yang, J. A Bioactive Lipid Nanoparticle Integrating Arachidonic Acid Enables High-Efficiency mRNA Delivery and Potent CAR-Macrophage Engineering. Int. J. Mol. Sci. 2025, 26, 9199. https://doi.org/10.3390/ijms26189199
Fu J, Zhang Y, Lv Y, Li R, Gu H, Yang J. A Bioactive Lipid Nanoparticle Integrating Arachidonic Acid Enables High-Efficiency mRNA Delivery and Potent CAR-Macrophage Engineering. International Journal of Molecular Sciences. 2025; 26(18):9199. https://doi.org/10.3390/ijms26189199
Chicago/Turabian StyleFu, Jia, Yanan Zhang, Yifan Lv, Ruilin Li, Hongchen Gu, and Jingxing Yang. 2025. "A Bioactive Lipid Nanoparticle Integrating Arachidonic Acid Enables High-Efficiency mRNA Delivery and Potent CAR-Macrophage Engineering" International Journal of Molecular Sciences 26, no. 18: 9199. https://doi.org/10.3390/ijms26189199
APA StyleFu, J., Zhang, Y., Lv, Y., Li, R., Gu, H., & Yang, J. (2025). A Bioactive Lipid Nanoparticle Integrating Arachidonic Acid Enables High-Efficiency mRNA Delivery and Potent CAR-Macrophage Engineering. International Journal of Molecular Sciences, 26(18), 9199. https://doi.org/10.3390/ijms26189199





