The Pivotal Role of the Western Diet, Hyperinsulinemia, Ectopic Fat, and Diacylglycerol-Mediated Insulin Resistance in Type 2 Diabetes
Abstract
1. Introduction
2. Fasting Hyperinsulinemia May Precede Insulin Resistance
3. Fasting Hyperinsulinemia Is a Premature Sign of Type 2 Diabetes
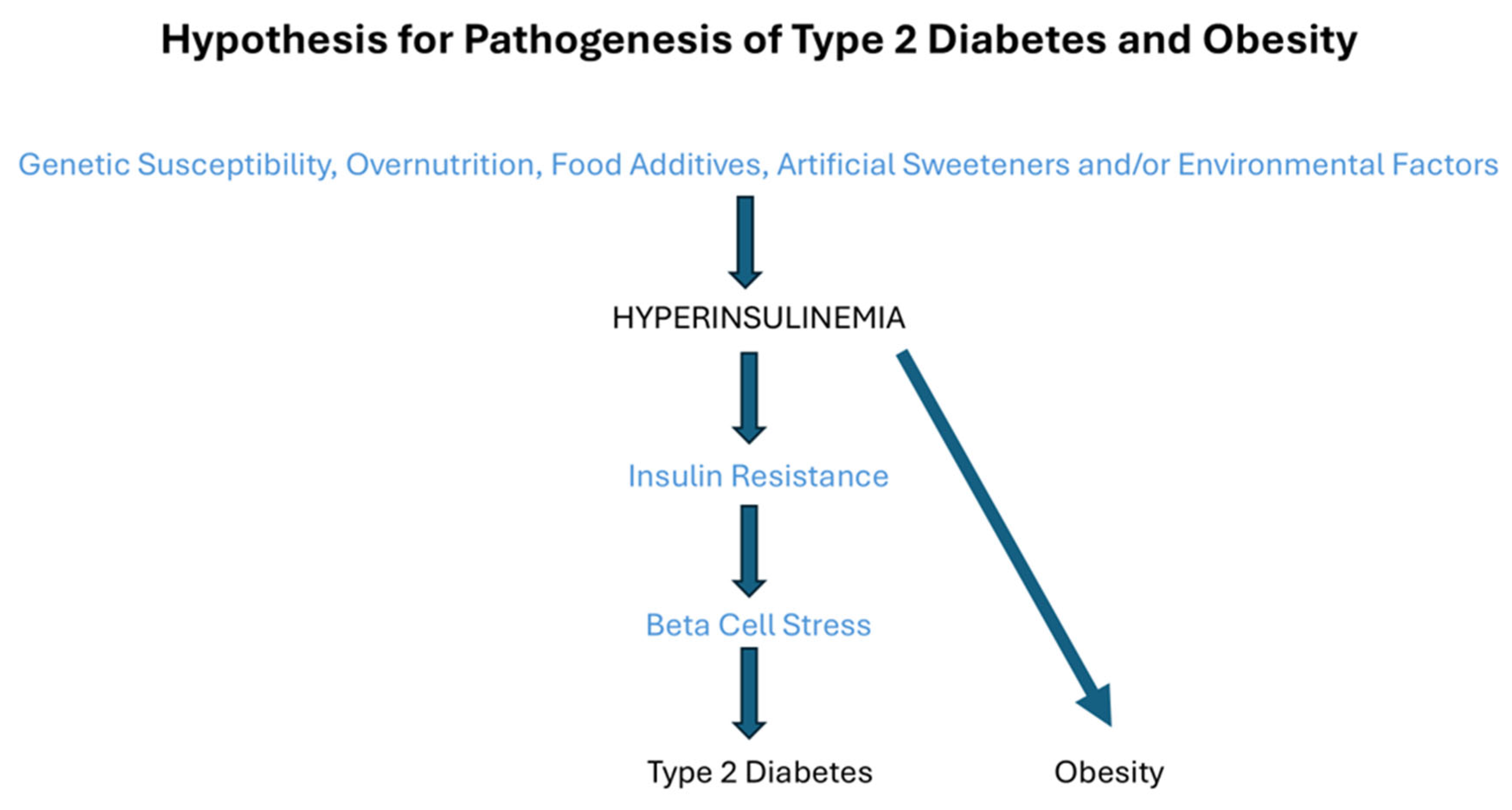
4. What Is the Cause of Hyperinsulinemia?
5. Hyperinsulinemia and Obesity
6. The Modulation of Food Intake by Central Insulin Action and Central Insulin Resistance
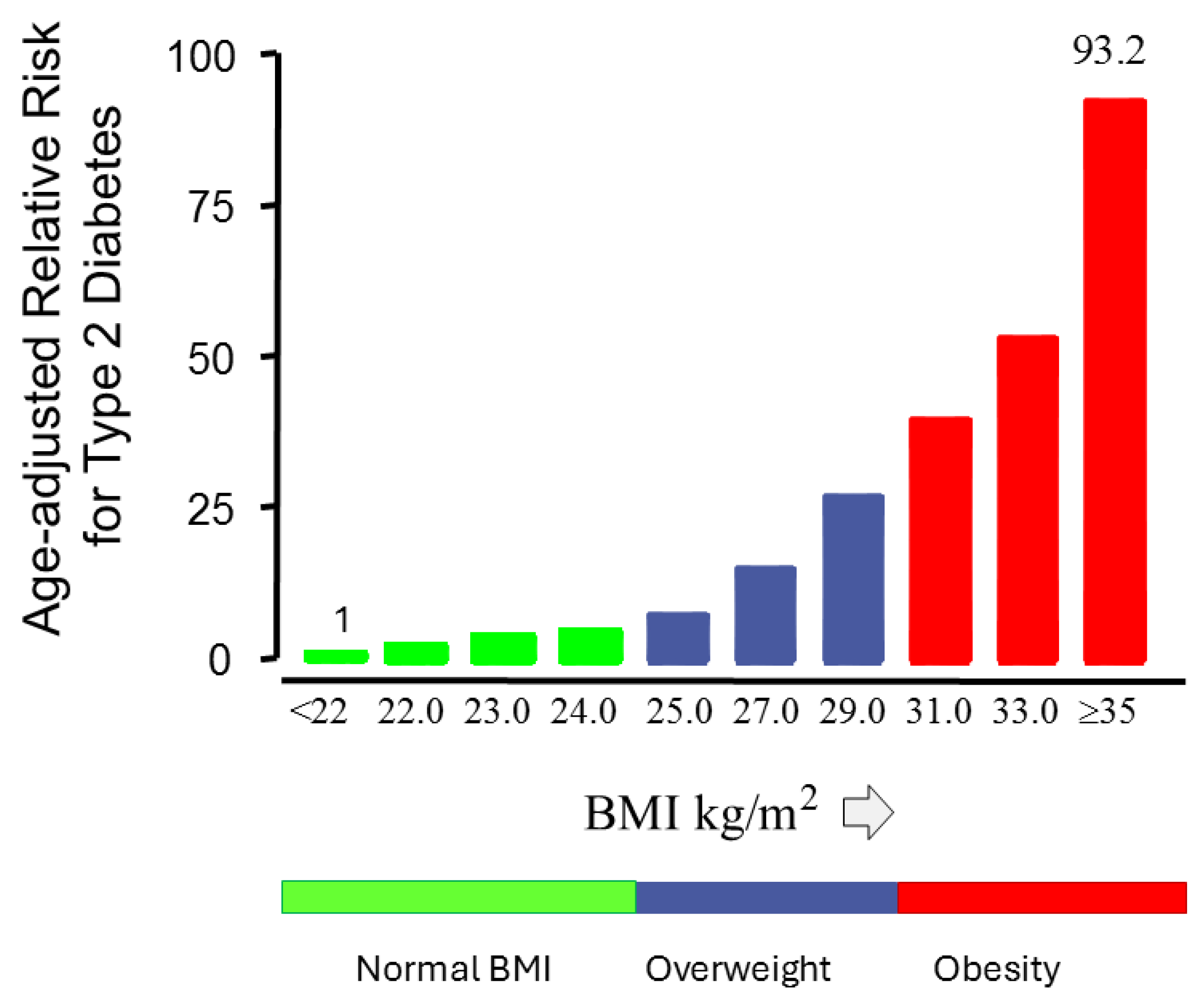
7. The Relationship Between Obesity and Type 2 Diabetes
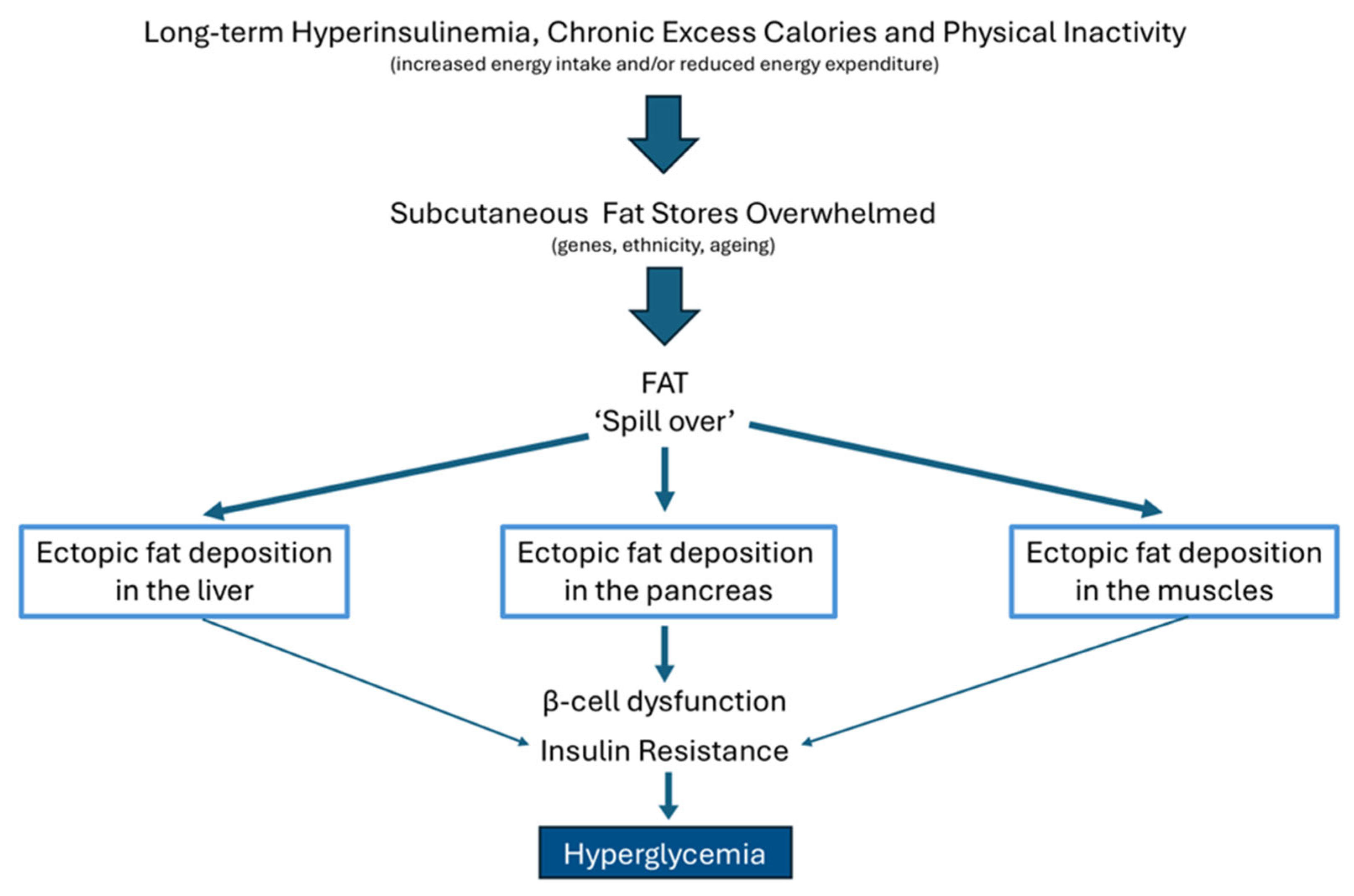
8. The Relationship Between Ectopic Fat Storage, Insulin Resistance, and Type 2 Diabetes Mellitus
9. Fat Cell Hypertrophy Correlates with Insulin Resistance
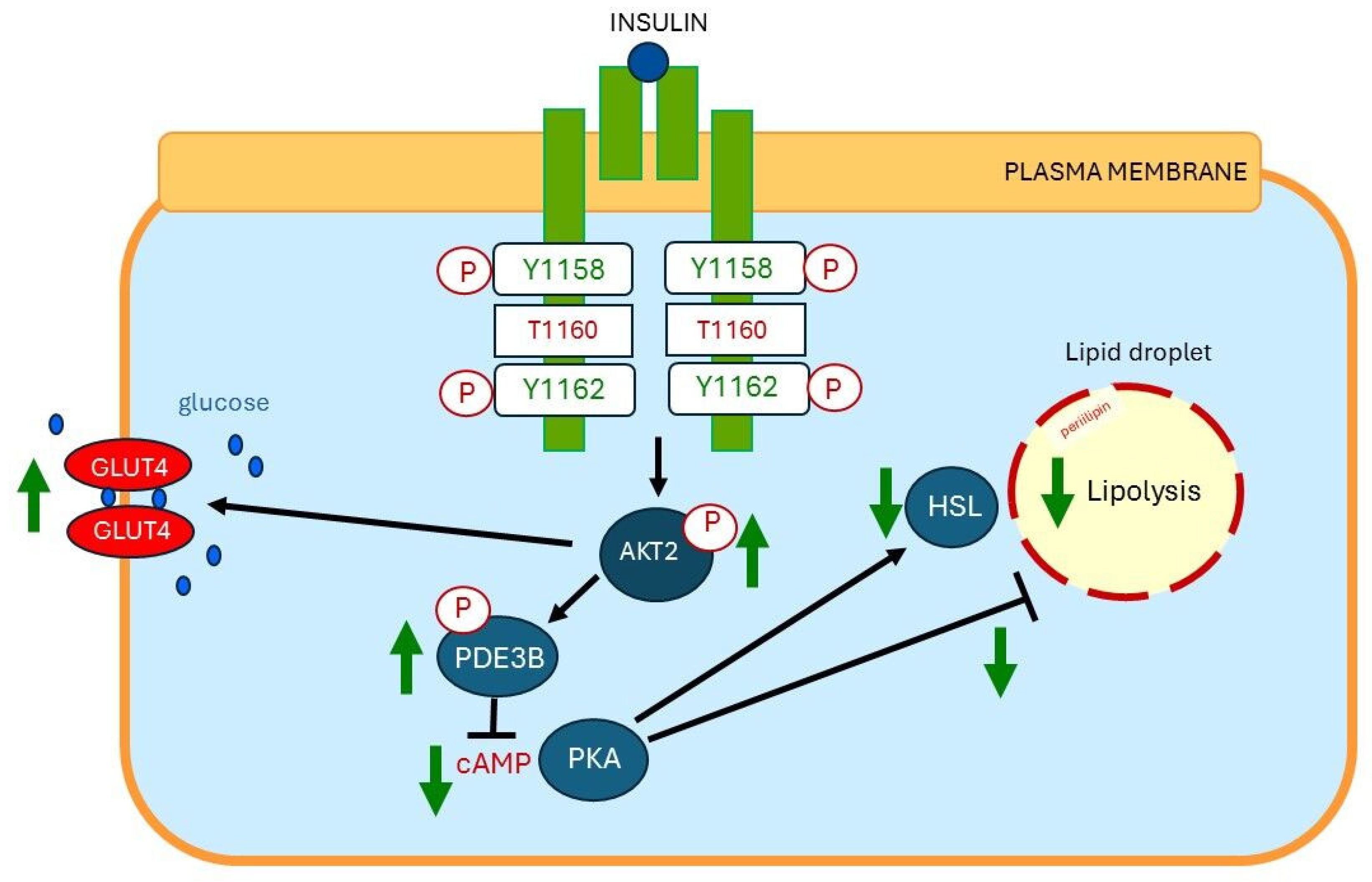
10. Insulin-Mediated Effects on Lipolysis, Glucose Metabolism, and Ectopic Fat Deposition
11. The Role of Hyperinsulinemia on Tissue-Level Inflammation
12. Lipotoxicity May Play a Key Role in the Development of Insulin Resistance in Type 2 Diabetes
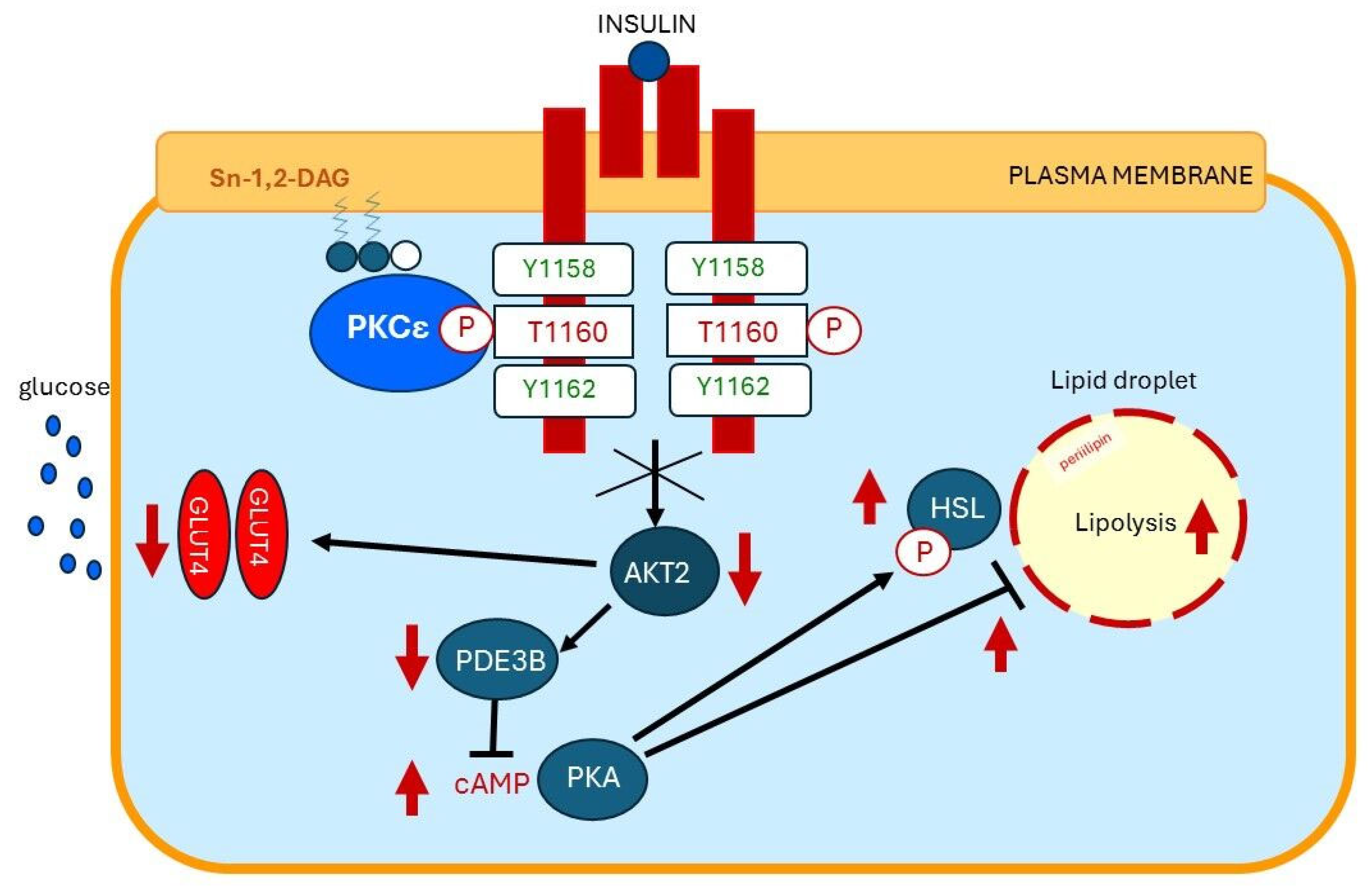
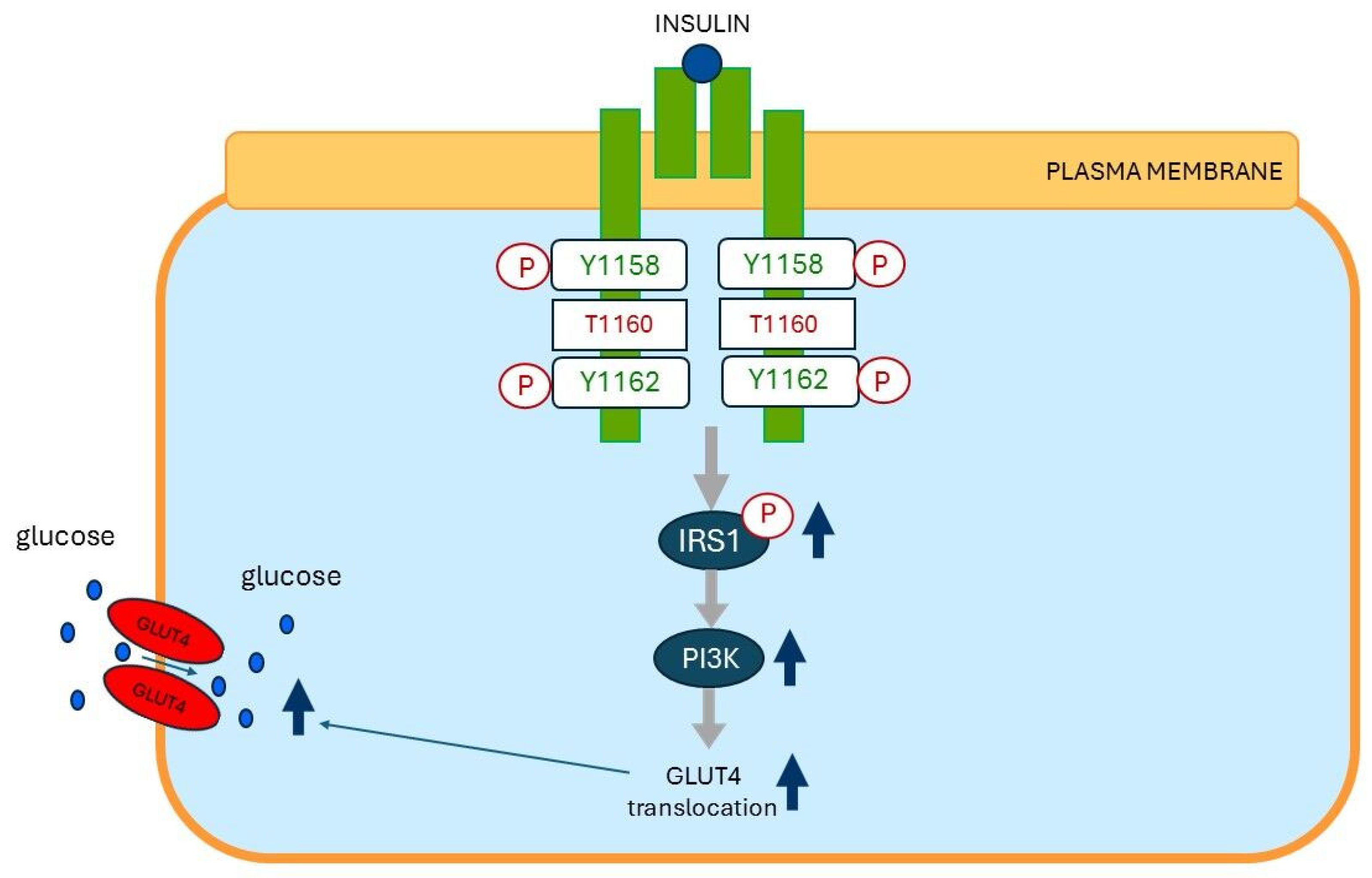
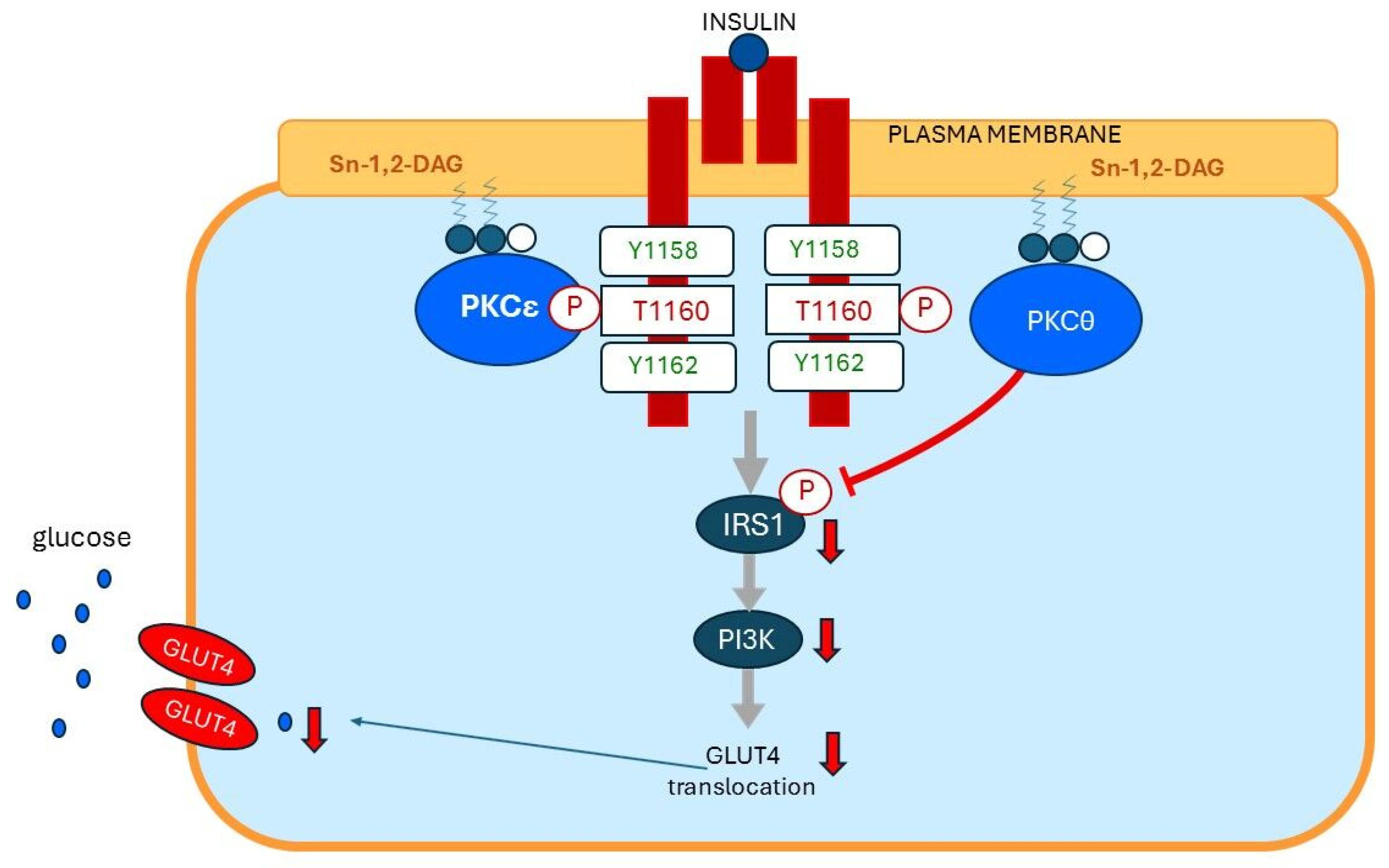
13. Plasma Membrane Sn-1,2-diacylglycerol Plays a Critical Role in Insulin Resistance of Fat Cells
14. Cause of Increased Diacylglycerol Content in Liver and Skeletal Muscles
15. Plasma Membrane Sn-1,2-diacylglycerols Play a Critical Role in Insulin Resistance of Skeletal Muscles and Liver
15.1. Insulin Resistance of Skeletal Muscles
15.2. Insulin Resistance of Hepatocytes
16. Ectopic Fat and the Pancreas
17. Correction of Hyperinsulinemia and Prompt Remission of Type 2 Diabetes After Bariatric Surgery
18. How Does Bariatric Surgery Result in Quick Remission of Type 2 Diabetes?
19. Reversal of Type 2 Diabetes After a Very-Low-Calorie-Diet (VLCD)
20. How Does a Very-Low-Calorie-Diet Result in Quick Remission of Type 2 Diabetes?
21. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pories, W.J.; Dohm, G.L. Diabetes: Have we got it all wrong? Hyperinsulinism as the culprit: Surgery provides the evidence. Diabetes Care 2012, 35, 2438–2442. [Google Scholar] [CrossRef]
- Stumvoll, M.; Haring, H.; Fritsche, A. For debate: Starling’s curve of the pancreas-overuse of a concept? Horm. Metab. Res. 2003, 35, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Sevilla-Gonzalez, M.; Smith, K.; Wang, N.; Jensen, A.E.; Litkowski, E.M.; Kim, H.; DiCorpo, D.A.; Hsu, S.; Cui, J.; Liu, C.T.; et al. Heterogeneous effects of genetic variants and traits associated with fasting insulin on cardiometabolic outcomes. Nat. Commun. 2025, 16, 2569. [Google Scholar] [CrossRef]
- Rizza, R.A.; Mandarino, L.J.; Genest, J.; Baker, B.A.; Gerich, J.E. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia 1985, 28, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, A.; DeFronzo, R.A. Insulin Resistance and Atherosclerosis: Implications for Insulin-Sensitizing Agents. Endocr. Rev. 2019, 40, 1447–1467. [Google Scholar] [CrossRef] [PubMed]
- Ramlo-Halsted, B.A.; Edelman, S.V. The natural history of type 2 diabetes. Implications for clinical practice. Prim. Care 1999, 26, 771–789. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef]
- Gerich, J.E. Is insulin resistance the principal cause of type 2 diabetes? Diabetes Obes. Metab. 1999, 1, 257–263. [Google Scholar] [CrossRef]
- Janssen, J. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef]
- Thomsen, S.K.; Gloyn, A.L. The pancreatic beta cell: Recent insights from human genetics. Trends Endocrinol. Metab. 2014, 25, 425–434. [Google Scholar] [CrossRef]
- Voight, B.F.; Scott, L.J.; Steinthorsdottir, V.; Morris, A.P.; Dina, C.; Welch, R.P.; Zeggini, E.; Huth, C.; Aulchenko, Y.S.; Thorleifsson, G.; et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010, 42, 579–589, Erratum in Nat. Genet. 2011, 43, 388. [Google Scholar] [CrossRef]
- Florez, J.C. Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: Where are the insulin resistance genes? Diabetologia 2008, 51, 1100–1110. [Google Scholar] [CrossRef]
- Ferrannini, E.; Gastaldelli, A.; Miyazaki, Y.; Matsuda, M.; Mari, A.; DeFronzo, R.A. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: A new analysis. J. Clin. Endocrinol. Metab. 2005, 90, 493–500. [Google Scholar] [CrossRef]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin resistance and hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31 (Suppl. 2), S262–S268. [Google Scholar] [CrossRef]
- Corkey, B.E. Banting lecture 2011: Hyperinsulinemia: Cause or consequence? Diabetes 2012, 61, 4–13. [Google Scholar] [CrossRef]
- Taegtmeyer, H.; Beauloye, C.; Harmancey, R.; Hue, L. Insulin resistance protects the heart from fuel overload in dysregulated metabolic states. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1693–H1697. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Ruderman, N.B.; Kahn, S.E.; Pedersen, O.; Prentki, M. Insulin resistance as a physiological defense against metabolic stress: Implications for the management of subsets of type 2 diabetes. Diabetes 2015, 64, 673–686. [Google Scholar] [CrossRef]
- Houston, E.J.; Templeman, N.M. Reappraising the relationship between hyperinsulinemia and insulin resistance in PCOS. J. Endocrinol. 2025, 265, e240269. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, Y.; Maddux, B.A.; McDonald, A.R.; Logsdon, C.D.; Williams, J.A.; Goldfine, I.D. Mechanisms of insulin-induced insulin-receptor downregulation. Decrease of receptor biosynthesis and mRNA levels. Diabetes 1989, 38, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Ronnett, G.V.; Knutson, V.P.; Lane, M.D. Insulin-induced down-regulation of insulin receptors in 3T3-L1 adipocytes. Altered rate of receptor inactivation. J. Biol. Chem. 1982, 257, 4285–4291. [Google Scholar] [CrossRef]
- Weyer, C.; Hanson, R.L.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: Evidence for a pathogenic role of relative hyperinsulinemia. Diabetes 2000, 49, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Trico, D.; Natali, A.; Arslanian, S.; Mari, A.; Ferrannini, E. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight 2018, 3, e124912. [Google Scholar] [CrossRef]
- Hamley, S.; Kloosterman, D.; Duthie, T.; Dalla Man, C.; Visentin, R.; Mason, S.A.; Ang, T.; Selathurai, A.; Kaur, G.; Morales-Scholz, M.G.; et al. Mechanisms of hyperinsulinaemia in apparently healthy non-obese young adults: Role of insulin secretion, clearance and action and associations with plasma amino acids. Diabetologia 2019, 62, 2310–2324. [Google Scholar] [CrossRef]
- Hansen, B.C.; Bodkin, N.L. Beta-cell hyperresponsiveness: Earliest event in development of diabetes in monkeys. Am. J. Physiol. 1990, 259, R612–R617. [Google Scholar] [CrossRef]
- Ferrannini, E.; Balkau, B. Insulin: In search of a syndrome. Diabet. Med. 2002, 19, 724–729. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; DeFronzo, R.A. Insulin Resistance and Hyperinsulinemia: The Egg and the Chicken. J. Clin. Endocrinol. Metab. 2021, 106, e1897–e1899. [Google Scholar] [CrossRef]
- Pories, W.J.; MacDonald, K.G., Jr.; Morgan, E.J.; Sinha, M.K.; Dohm, G.L.; Swanson, M.S.; Barakat, H.A.; Khazanie, P.G.; Leggett-Frazier, N.; Long, S.D. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am. J. Clin. Nutr. 1992, 55, 582S–585S. [Google Scholar] [CrossRef]
- Dankner, R.; Chetrit, A.; Shanik, M.H.; Raz, I.; Roth, J. Basal state hyperinsulinemia in healthy normoglycemic adults heralds dysglycemia after more than two decades of follow up. Diabetes Metab. Res. Rev. 2012, 28, 618–624. [Google Scholar] [CrossRef]
- Zimmet, P.Z.; Collins, V.R.; Dowse, G.K.; Knight, L.T. Hyperinsulinaemia in youth is a predictor of type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1992, 35, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Stern, M.P.; Mitchell, B.D.; Hazuda, H.P.; Patterson, J.K. Incidence of type II diabetes in Mexican Americans predicted by fasting insulin and glucose levels, obesity, and body-fat distribution. Diabetes 1990, 39, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Carnethon, M.R.; Palaniappan, L.P.; Burchfiel, C.M.; Brancati, F.L.; Fortmann, S.P. Serum insulin, obesity, and the incidence of type 2 diabetes in black and white adults: The atherosclerosis risk in communities study: 1987–1998. Diabetes Care 2002, 25, 1358–1364. [Google Scholar] [CrossRef]
- Ghasemi, A.; Tohidi, M.; Derakhshan, A.; Hasheminia, M.; Azizi, F.; Hadaegh, F. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta Diabetol. 2015, 52, 905–915. [Google Scholar] [CrossRef]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef] [PubMed]
- Odeleye, O.E.; de Courten, M.; Pettitt, D.J.; Ravussin, E. Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes 1997, 46, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Dowse, G.K.; Zimmet, P.Z.; Collins, V.R. Insulin levels and the natural history of glucose intolerance in Nauruans. Diabetes 1996, 45, 1367–1372. [Google Scholar] [CrossRef]
- Hannon, T.S.; Bacha, F.; Lin, Y.; Arslanian, S.A. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: A reflection of upregulated beta-cell function? Diabetes Care 2008, 31, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- Svec, F.; Nastasi, K.; Hilton, C.; Bao, W.; Srinivasan, S.R.; Berenson, G.S. Black-white contrasts in insulin levels during pubertal development. The Bogalusa Heart Study. Diabetes 1992, 41, 313–317. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-Insulin Model of Obesity: Beyond “Calories In, Calories Out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef]
- Kim, S.P.; Ellmerer, M.; Kirkman, E.L.; Bergman, R.N. Beta-cell “rest” accompanies reduced first-pass hepatic insulin extraction in the insulin-resistant, fat-fed canine model. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1581–E1589. [Google Scholar] [CrossRef]
- Schulz, L.O.; Bennett, P.H.; Ravussin, E.; Kidd, J.R.; Kidd, K.K.; Esparza, J.; Valencia, M.E. Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care 2006, 29, 1866–1871. [Google Scholar] [CrossRef]
- Schousboe, K.; Visscher, P.M.; Henriksen, J.E.; Hopper, J.L.; Sorensen, T.I.; Kyvik, K.O. Twin study of genetic and environmental influences on glucose tolerance and indices of insulin sensitivity and secretion. Diabetologia 2003, 46, 1276–1283. [Google Scholar] [CrossRef]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Dimas, A.S.; Lagou, V.; Barker, A.; Knowles, J.W.; Magi, R.; Hivert, M.F.; Benazzo, A.; Rybin, D.; Jackson, A.U.; Stringham, H.M.; et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes 2014, 63, 2158–2171. [Google Scholar] [CrossRef] [PubMed]
- DiCorpo, D.; Gaynor, S.M.; Russell, E.M.; Westerman, K.E.; Raffield, L.M.; Majarian, T.D.; Wu, P.; Sarnowski, C.; Highland, H.M.; Jackson, A.; et al. Whole genome sequence association analysis of fasting glucose and fasting insulin levels in diverse cohorts from the NHLBI TOPMed program. Commun. Biol. 2022, 5, 756. [Google Scholar] [CrossRef]
- Meier, J.J.; Veldhuis, J.D.; Butler, P.C. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 2005, 54, 1649–1656. [Google Scholar] [CrossRef]
- Bojsen-Moller, K.N.; Lundsgaard, A.M.; Madsbad, S.; Kiens, B.; Holst, J.J. Hepatic Insulin Clearance in Regulation of Systemic Insulin Concentrations-Role of Carbohydrate and Energy Availability. Diabetes 2018, 67, 2129–2136. [Google Scholar] [CrossRef]
- Bergman, R.N.; Piccinini, F.; Kabir, M.; Kolka, C.M.; Ader, M. Hypothesis: Role of Reduced Hepatic Insulin Clearance in the Pathogenesis of Type 2 Diabetes. Diabetes 2019, 68, 1709–1716, Erratum in Diabetes 2019, 68, 2350. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cui, J.; Jones, M.R.; Haritunians, T.; Xiang, A.H.; Chen, Y.D.; Taylor, K.D.; Buchanan, T.A.; Davis, R.C.; Hsueh, W.A.; et al. Insulin clearance: Confirmation as a highly heritable trait, and genome-wide linkage analysis. Diabetologia 2012, 55, 2183–2192. [Google Scholar] [CrossRef]
- Watada, H.; Tamura, Y. Impaired insulin clearance as a cause rather than a consequence of insulin resistance. J. Diabetes Investig. 2017, 8, 723–725. [Google Scholar] [CrossRef]
- Templeman, N.M.; Skovso, S.; Page, M.M.; Lim, G.E.; Johnson, J.D. A causal role for hyperinsulinemia in obesity. J. Endocrinol. 2017, 232, R173–R183. [Google Scholar] [CrossRef]
- Czech, M.P.; Tencerova, M.; Pedersen, D.J.; Aouadi, M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 2013, 56, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Y.; Rong, X.; Zheng, C.; Guo, J. Anti-Lipolysis Induced by Insulin in Diverse Pathophysiologic Conditions of Adipose Tissue. Diabetes Metab. Syndr. Obes. 2020, 13, 1575–1585. [Google Scholar] [CrossRef]
- Unger, R.H. Lipid overload and overflow: Metabolic trauma and the metabolic syndrome. Trends Endocrinol. Metab. 2003, 14, 398–403. [Google Scholar] [CrossRef]
- Tamura, Y. Ectopic fat, insulin resistance and metabolic disease in non-obese Asians: Investigating metabolic gradation. Endocr. J. 2019, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Trico, D.; Chiriaco, M.; Nouws, J.; Vash-Margita, A.; Kursawe, R.; Tarabra, E.; Galderisi, A.; Natali, A.; Giannini, C.; Hellerstein, M.; et al. Alterations in Adipose Tissue Distribution, Cell Morphology, and Function Mark Primary Insulin Hypersecretion in Youth with Obesity. Diabetes 2024, 73, 941–952. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Johnson, J.D.; Solinas, G.; Jansson, P.A. Insulin Hypersecretion as Promoter of Body Fat Gain and Hyperglycemia. Diabetes 2024, 73, 837–843. [Google Scholar] [CrossRef]
- Perng, W.; Kelsey, M.M.; Sauder, K.A.; Dabelea, D. How does exposure to overnutrition in utero lead to childhood adiposity? Testing the insulin hypersecretion hypothesis in the EPOCH cohort. Diabetologia 2021, 64, 2237–2246. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, J.P.; Jiang, Y.Y.; Li, H.; Hu, Y.H.; Lee, K.O.; Li, G.W. Fasting Plasma Insulin at 5 Years of Age Predicted Subsequent Weight Increase in Early Childhood over a 5-Year Period—The Da Qing Children Cohort Study. PLoS ONE 2015, 10, e0127389. [Google Scholar] [CrossRef]
- Astley, C.M.; Todd, J.N.; Salem, R.M.; Vedantam, S.; Ebbeling, C.B.; Huang, P.L.; Ludwig, D.S.; Hirschhorn, J.N.; Florez, J.C. Genetic Evidence That Carbohydrate-Stimulated Insulin Secretion Leads to Obesity. Clin. Chem. 2018, 64, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Le Stunff, C.; Fallin, D.; Schork, N.J.; Bougneres, P. The insulin gene VNTR is associated with fasting insulin levels and development of juvenile obesity. Nat. Genet. 2000, 26, 444–446, Erratum in Nat. Genet. 2001, 28, 97. [Google Scholar] [CrossRef] [PubMed]
- Page, M.M.; Johnson, J.D. Mild Suppression of Hyperinsulinemia to Treat Obesity and Insulin Resistance. Trends Endocrinol. Metab. 2018, 29, 389–399. [Google Scholar] [CrossRef]
- Woods, S.C.; Porte, D., Jr.; Bobbioni, E.; Ionescu, E.; Sauter, J.F.; Rohner-Jeanrenaud, F.; Jeanrenaud, B. Insulin: Its relationship to the central nervous system and to the control of food intake and body weight. Am. J. Clin. Nutr. 1985, 42, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Isganaitis, E.; Lustig, R.H. Fast food, central nervous system insulin resistance, and obesity. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2451–2462. [Google Scholar] [CrossRef]
- Chen, W.; Balland, E.; Cowley, M.A. Hypothalamic Insulin Resistance in Obesity: Effects on Glucose Homeostasis. Neuroendocrinology 2017, 104, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, U. Where does insulin resistance start? The brain. Diabetes Care 2009, 32 (Suppl. 2), S174–S177. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Jarvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Johnson, A.M.; Olefsky, J.M. The origins and drivers of insulin resistance. Cell 2013, 152, 673–684. [Google Scholar] [CrossRef]
- Taylor, R.; Holman, R.R. Normal weight individuals who develop type 2 diabetes: The personal fat threshold. Clin. Sci. 2015, 128, 405–410. [Google Scholar] [CrossRef]
- Hollenbeck, C.B.; Chen, Y.D.; Reaven, G.M. A comparison of the relative effects of obesity and non-insulin-dependent diabetes mellitus on in vivo insulin-stimulated glucose utilization. Diabetes 1984, 33, 622–626. [Google Scholar] [CrossRef]
- Lara-Castro, C.; Garvey, W.T. Diet, insulin resistance, and obesity: Zoning in on data for Atkins dieters living in South Beach. J. Clin. Endocrinol. Metab. 2004, 89, 4197–4205. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia 1991, 34, 877–890. [Google Scholar]
- Lin, B.; Coleman, R.L.; Bragg, F.; Maddaloni, E.; Holman, R.R.; Adler, A.I. Younger-onset compared with later-onset type 2 diabetes: An analysis of the UK Prospective Diabetes Study (UKPDS) with up to 30 years of follow-up (UKPDS 92). Lancet Diabetes Endocrinol. 2024, 12, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Willett, W.C.; Rotnitzky, A.; Manson, J.E. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med. 1995, 122, 481–486. [Google Scholar] [CrossRef]
- Thomas, E.L.; Frost, G.; Taylor-Robinson, S.D.; Bell, J.D. Excess body fat in obese and normal-weight subjects. Nutr. Res. Rev. 2012, 25, 150–161. [Google Scholar] [CrossRef]
- Yaghootkar, H.; Whitcher, B.; Bell, J.D.; Thomas, E.L. Ethnic differences in adiposity and diabetes risk-insights from genetic studies. J. Intern. Med. 2020, 288, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Smith, S.R. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2002, 967, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Smith, U. Abdominal obesity: A marker of ectopic fat accumulation. J. Clin. Investig. 2015, 125, 1790–1792. [Google Scholar] [CrossRef]
- Jensen, M.D.; Cardin, S.; Edgerton, D.; Cherrington, A. Splanchnic free fatty acid kinetics. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E1140–E1148. [Google Scholar] [CrossRef]
- Nielsen, S.; Guo, Z.; Johnson, C.M.; Hensrud, D.D.; Jensen, M.D. Splanchnic lipolysis in human obesity. J. Clin. Investig. 2004, 113, 1582–1588. [Google Scholar] [CrossRef]
- Miles, J.M.; Jensen, M.D. Counterpoint: Visceral adiposity is not causally related to insulin resistance. Diabetes Care 2005, 28, 2326–2328. [Google Scholar] [CrossRef]
- Abate, N.; Garg, A.; Peshock, R.M.; Stray-Gundersen, J.; Grundy, S.M. Relationships of generalized and regional adiposity to insulin sensitivity in men. J. Clin. Investig. 1995, 96, 88–98. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Thaete, F.L.; Simoneau, J.A.; Kelley, D.E. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997, 46, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; Zhang, D.; Song, J.; Li, X.; Perry, R.J.; Samuel, V.T.; Shulman, G.I. Short-term overnutrition induces white adipose tissue insulin resistance through sn-1,2-diacylglycerol/PKCepsilon/insulin receptor Thr1160 phosphorylation. JCI Insight 2021, 6, e139946. [Google Scholar] [CrossRef]
- Danforth, E., Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat. Genet. 2000, 26, 13. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Barnes, A.C.; Hollingsworth, K.G.; Irvine, K.M.; Solovyova, A.S.; Clark, L.; Kelly, T.; Martin-Ruiz, C.; Romeres, D.; Koulman, A.; et al. Aetiology of Type 2 diabetes in people with a ‘normal’ body mass index: Testing the personal fat threshold hypothesis. Clin. Sci. 2023, 137, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, N.; Reynolds, J.C.; Brychta, R.J.; Chen, K.Y.; Auh, S.; Gharib, A.M.; Startzell, M.; Cochran, E.K.; Brown, R.J. Visceral fat does not contribute to metabolic disease in lipodystrophy. Obes. Sci. Pract. 2019, 5, 75–82. [Google Scholar] [CrossRef]
- Agrawal, S.; Luan, J.; Cummings, B.B.; Weiss, E.J.; Wareham, N.J.; Khera, A.V. Relationship of Fat Mass Ratio, a Biomarker for Lipodystrophy, with Cardiometabolic Traits. Diabetes 2024, 73, 1099–1111. [Google Scholar] [CrossRef]
- Lotta, L.A.; Gulati, P.; Day, F.R.; Payne, F.; Ongen, H.; van de Bunt, M.; Gaulton, K.J.; Eicher, J.D.; Sharp, S.J.; Luan, J.; et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 2017, 49, 17–26, Erratum in Nat. Genet. 2017, 49, 317. [Google Scholar] [CrossRef]
- Lotta, L.A.; Wittemans, L.B.L.; Zuber, V.; Stewart, I.D.; Sharp, S.J.; Luan, J.; Day, F.R.; Li, C.; Bowker, N.; Cai, L.; et al. Association of Genetic Variants Related to Gluteofemoral vs Abdominal Fat Distribution with Type 2 Diabetes, Coronary Disease, and Cardiovascular Risk Factors. JAMA 2018, 320, 2553–2563. [Google Scholar] [CrossRef]
- Janssen, J. The Causal Role of Ectopic Fat Deposition in the Pathogenesis of Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 13238. [Google Scholar] [CrossRef]
- Yip, R.G.; Wolfe, M.M. GIP biology and fat metabolism. Life Sci. 2000, 66, 91–103. [Google Scholar] [CrossRef]
- Patriti, A.; Facchiano, E.; Sanna, A.; Gulla, N.; Donini, A. The enteroinsular axis and the recovery from type 2 diabetes after bariatric surgery. Obes. Surg. 2004, 14, 840–848. [Google Scholar] [CrossRef]
- Collier, G.R.; Greenberg, G.R.; Wolever, T.M.; Jenkins, D.J. The acute effect of fat on insulin secretion. J. Clin. Endocrinol. Metab. 1988, 66, 323–326. [Google Scholar] [CrossRef]
- Gasbjerg, L.S.; Bergmann, N.C.; Stensen, S.; Christensen, M.B.; Rosenkilde, M.M.; Holst, J.J.; Nauck, M.; Knop, F.K. Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides 2020, 125, 170183. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Raja, A. Physiology, Gastric Inhibitory Peptide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546653/ (accessed on 3 July 2025).
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes. Metab. 2021, 23 (Suppl. 3), 5–29. [Google Scholar] [CrossRef] [PubMed]
- Asmar, M.; Simonsen, L.; Madsbad, S.; Stallknecht, B.; Holst, J.J.; Bulow, J. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes 2010, 59, 2160–2163. [Google Scholar] [CrossRef]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Ryden, M.; Frisen, J.; Bernard, S.; Arner, P. Adipocyte turnover: Relevance to human adipose tissue morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Huh, J.Y.; Sohn, J.H.; Choe, S.S.; Lee, Y.S.; Lim, C.Y.; Jo, A.; Park, S.B.; Han, W.; Kim, J.B. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol. Cell. Biol. 2015, 35, 1686–1699. [Google Scholar] [CrossRef]
- Weyer, C.; Foley, J.E.; Bogardus, C.; Tataranni, P.A.; Pratley, R.E. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000, 43, 1498–1506. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Perry, R.J.; Camporez, J.G.; Kursawe, R.; Titchenell, P.M.; Zhang, D.; Perry, C.J.; Jurczak, M.J.; Abudukadier, A.; Han, M.S.; Zhang, X.M.; et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef]
- Lewis, G.F.; Vranic, M.; Harley, P.; Giacca, A. Fatty acids mediate the acute extrahepatic effects of insulin on hepatic glucose production in humans. Diabetes 1997, 46, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int. J. Clin. Pract. Suppl. 2004, 58, 9–21. [Google Scholar] [CrossRef]
- Zhang, A.M.Y.; Wellberg, E.A.; Kopp, J.L.; Johnson, J.D. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab. J. 2021, 45, 285–311, Erratum in Diabetes Metab. J. 2021, 45, 622. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Shankar, K.; Patel, S.; Gupta, A.; Varshney, S.; Gupta, S.; Rajan, S.; Srivastava, A.; Vishwakarma, A.L.; Gaikwad, A.N. Chronic hyperinsulinemia promotes meta-inflammation and extracellular matrix deposition in adipose tissue: Implications of nitric oxide. Mol. Cell. Endocrinol. 2018, 477, 15–28. [Google Scholar] [CrossRef]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.J.; Guilherme, A.; Danai, L.V.; Heyda, L.; Matevossian, A.; Cohen, J.; Nicoloro, S.M.; Straubhaar, J.; Noh, H.L.; Jung, D.; et al. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol. Metab. 2015, 4, 507–518. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Blaak, E.E.; van Loon, L.J.C. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes. Rev. 2019, 20, 1205–1217. [Google Scholar] [CrossRef]
- van Herpen, N.A.; Schrauwen-Hinderling, V.B. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol. Behav. 2008, 94, 231–241. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Elkanawati, R.Y.; Sumiwi, S.A.; Levita, J. Impact of Lipids on Insulin Resistance: Insights from Human and Animal Studies. Drug Des. Dev. Ther. 2024, 18, 3337–3360. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Leal Vde, O.; Mafra, D. Adipokines in obesity. Clin. Chim. Acta 2013, 419, 87–94. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Erion, D.M.; Shulman, G.I. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010, 16, 400–402. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Garcia, R.; Shulman, G.I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004, 350, 664–671. [Google Scholar] [CrossRef]
- Morino, K.; Petersen, K.F.; Dufour, S.; Befroy, D.; Frattini, J.; Shatzkes, N.; Neschen, S.; White, M.F.; Bilz, S.; Sono, S.; et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Investig. 2005, 115, 3587–3593. [Google Scholar] [CrossRef]
- Szendroedi, J.; Yoshimura, T.; Phielix, E.; Koliaki, C.; Marcucci, M.; Zhang, D.; Jelenik, T.; Muller, J.; Herder, C.; Nowotny, P.; et al. Role of diacylglycerol activation of PKCtheta in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 9597–9602. [Google Scholar] [CrossRef]
- Gaspar, R.C.; Lyu, K.; Hubbard, B.T.; Leitner, B.P.; Luukkonen, P.K.; Hirabara, S.M.; Sakuma, I.; Nasiri, A.; Zhang, D.; Kahn, M.; et al. Distinct subcellular localisation of intramyocellular lipids and reduced PKCepsilon/PKCtheta activity preserve muscle insulin sensitivity in exercise-trained mice. Diabetologia 2023, 66, 567–578. [Google Scholar] [CrossRef]
- Li, Y.; Soos, T.J.; Li, X.; Wu, J.; Degennaro, M.; Sun, X.; Littman, D.R.; Birnbaum, M.J.; Polakiewicz, R.D. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J. Biol. Chem. 2004, 279, 45304–45307. [Google Scholar] [CrossRef]
- Farese, R.V., Jr.; Zechner, R.; Newgard, C.B.; Walther, T.C. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab. 2012, 15, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Zhou, Y.; Sadevirta, S.; Leivonen, M.; Arola, J.; Oresic, M.; Hyotylainen, T.; Yki-Jarvinen, H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1167–1175. [Google Scholar] [CrossRef]
- Chavez, J.A.; Summers, S.A. A ceramide-centric view of insulin resistance. Cell Metab. 2012, 15, 585–594. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Scherer, P.E. Lowering ceramides to overcome diabetes. Science 2019, 365, 319–320. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol. Sci. 2017, 38, 649–665. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; Zhang, Y.; Zhang, D.; Kahn, M.; Ter Horst, K.W.; Rodrigues, M.R.S.; Gaspar, R.C.; Hirabara, S.M.; Luukkonen, P.K.; Lee, S.; et al. A Membrane-Bound Diacylglycerol Species Induces PKCϵ-Mediated Hepatic Insulin Resistance. Cell Metab. 2020, 32, 654–664.e5. [Google Scholar] [CrossRef] [PubMed]
- Cantley, J.L.; Yoshimura, T.; Camporez, J.P.; Zhang, D.; Jornayvaz, F.R.; Kumashiro, N.; Guebre-Egziabher, F.; Jurczak, M.J.; Kahn, M.; Guigni, B.A.; et al. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc. Natl. Acad. Sci. USA 2013, 110, 1869–1874. [Google Scholar] [CrossRef]
- Magkos, F.; Su, X.; Bradley, D.; Fabbrini, E.; Conte, C.; Eagon, J.C.; Varela, J.E.; Brunt, E.M.; Patterson, B.W.; Klein, S. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 2012, 142, 1444–1446.e2. [Google Scholar] [CrossRef] [PubMed]
- Pinnick, K.E.; Collins, S.C.; Londos, C.; Gauguier, D.; Clark, A.; Fielding, B.A. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity 2008, 16, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Hannaert, J.C.; Hoorens, A.; Eizirik, D.L.; Pipeleers, D.G. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes 2001, 50, 1771–1777. [Google Scholar] [CrossRef]
- Dobbins, R.L.; Chester, M.W.; Daniels, M.B.; McGarry, J.D.; Stein, D.T. Circulating fatty acids are essential for efficient glucose-stimulated insulin secretion after prolonged fasting in humans. Diabetes 1998, 47, 1613–1618. [Google Scholar] [CrossRef]
- Dubois, M.; Kerr-Conte, J.; Gmyr, V.; Bouckenooghe, T.; Muharram, G.; D’Herbomez, M.; Martin-Ponthieu, A.; Vantyghem, M.C.; Vandewalle, B.; Pattou, F. Non-esterified fatty acids are deleterious for human pancreatic islet function at physiological glucose concentration. Diabetologia 2004, 47, 463–469. [Google Scholar] [CrossRef][Green Version]
- Yu, T.Y.; Wang, C.Y. Impact of non-alcoholic fatty pancreas disease on glucose metabolism. J. Diabetes Investig. 2017, 8, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef]
- Sattar, N.; Gill, J.M. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014, 12, 123. [Google Scholar] [CrossRef]
- Tushuizen, M.E.; Bunck, M.C.; Pouwels, P.J.; Bontemps, S.; van Waesberghe, J.H.; Schindhelm, R.K.; Mari, A.; Heine, R.J.; Diamant, M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007, 30, 2916–2921. [Google Scholar] [CrossRef]
- Pories, W.J.; Swanson, M.S.; MacDonald, K.G.; Long, S.B.; Morris, P.G.; Brown, B.M.; Barakat, H.A.; deRamon, R.A.; Israel, G.; Dolezal, J.M.; et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann. Surg. 1995, 222, 339–350, discussion 350–332. [Google Scholar] [CrossRef]
- Kelly, C.T.; Mansoor, J.; Dohm, G.L.; Chapman, W.H., 3rd; Pender, J.R.t.; Pories, W.J. Hyperinsulinemic syndrome: The metabolic syndrome is broader than you think. Surgery 2014, 156, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Hollingsworth, K.G.; Small, P.K.; Woodcock, S.A.; Pucci, A.; Aribisala, B.; Al-Mrabeh, A.; Daly, A.K.; Batterham, R.L.; Taylor, R. Weight Loss Decreases Excess Pancreatic Triacylglycerol Specifically in Type 2 Diabetes. Diabetes Care 2016, 39, 158–165. [Google Scholar] [CrossRef]
- Sjostrom, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, A.; Bouchard, C.; Carlsson, B.; Karason, K.; Lonroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef]
- Sjostrom, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.; Peltonen, M.; Ahlin, S.; Anveden, A.; Bouchard, C.; Carlsson, B.; Jacobson, P.; Lonroth, H.; Maglio, C.; Naslund, I.; et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N. Engl. J. Med. 2012, 367, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Pathogenesis of type 2 diabetes: Tracing the reverse route from cure to cause. Diabetologia 2008, 51, 1781–1789. [Google Scholar] [CrossRef]
- Taylor, R.; Al-Mrabeh, A.; Zhyzhneuskaya, S.; Peters, C.; Barnes, A.C.; Aribisala, B.S.; Hollingsworth, K.G.; Mathers, J.C.; Sattar, N.; Lean, M.E.J. Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for beta Cell Recovery. Cell Metab. 2018, 28, 547–556.e3, Erratum in Cell Metab. 2018, 28, 667. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005, 54, 603–608. [Google Scholar] [CrossRef]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef]
- Zhyzhneuskaya, S.V.; Al-Mrabeh, A.; Peters, C.; Barnes, A.; Aribisala, B.; Hollingsworth, K.G.; McConnachie, A.; Sattar, N.; Lean, M.E.J.; Taylor, R. Time Course of Normalization of Functional beta-Cell Capacity in the Diabetes Remission Clinical Trial After Weight Loss in Type 2 Diabetes. Diabetes Care 2020, 43, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Jesuthasan, A.; Zhyzhneuskaya, S.; Peters, C.; Barnes, A.C.; Hollingsworth, K.G.; Sattar, N.; Lean, M.E.J.; Taylor, R.; Al-Mrabeh, A.H. Sex differences in intraorgan fat levels and hepatic lipid metabolism: Implications for cardiovascular health and remission of type 2 diabetes after dietary weight loss. Diabetologia 2022, 65, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Kelly, T.; Irvine, K.; Peters, C.; Zhyzhneuskaya, S.; et al. 5-year follow-up of the randomised Diabetes Remission Clinical Trial (DiRECT) of continued support for weight loss maintenance in the UK: An extension study. Lancet Diabetes Endocrinol. 2024, 12, 233–246, Erratum in Lancet Diabetes Endocrinol. 2024, 12, E17. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Cline, G.W.; Wang, Y.; Rabin-Court, A.; Song, J.D.; Zhang, D.; Zhang, X.M.; Nozaki, Y.; Dufour, S.; et al. Mechanisms by which a Very-Low-Calorie Diet Reverses Hyperglycemia in a Rat Model of Type 2 Diabetes. Cell Metab. 2018, 27, 210–217.e3. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssen, J.A.M.J.L. The Pivotal Role of the Western Diet, Hyperinsulinemia, Ectopic Fat, and Diacylglycerol-Mediated Insulin Resistance in Type 2 Diabetes. Int. J. Mol. Sci. 2025, 26, 9191. https://doi.org/10.3390/ijms26189191
Janssen JAMJL. The Pivotal Role of the Western Diet, Hyperinsulinemia, Ectopic Fat, and Diacylglycerol-Mediated Insulin Resistance in Type 2 Diabetes. International Journal of Molecular Sciences. 2025; 26(18):9191. https://doi.org/10.3390/ijms26189191
Chicago/Turabian StyleJanssen, Joseph A. M. J. L. 2025. "The Pivotal Role of the Western Diet, Hyperinsulinemia, Ectopic Fat, and Diacylglycerol-Mediated Insulin Resistance in Type 2 Diabetes" International Journal of Molecular Sciences 26, no. 18: 9191. https://doi.org/10.3390/ijms26189191
APA StyleJanssen, J. A. M. J. L. (2025). The Pivotal Role of the Western Diet, Hyperinsulinemia, Ectopic Fat, and Diacylglycerol-Mediated Insulin Resistance in Type 2 Diabetes. International Journal of Molecular Sciences, 26(18), 9191. https://doi.org/10.3390/ijms26189191






