The Localization of Cell Wall Components in the Whole-Mount Immunolabeled Nepenthes Digestive Glands
Abstract
1. Introduction
2. Results
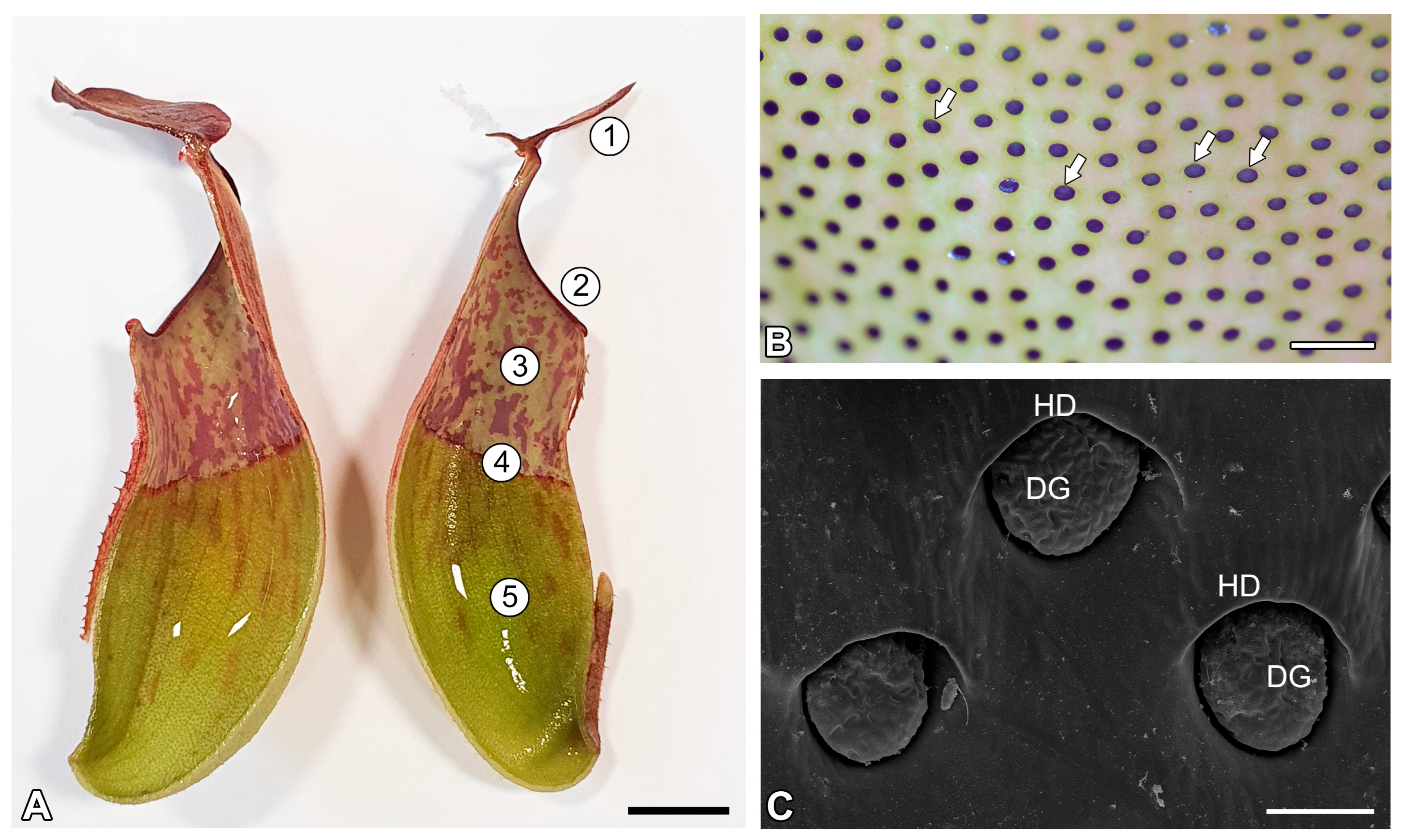
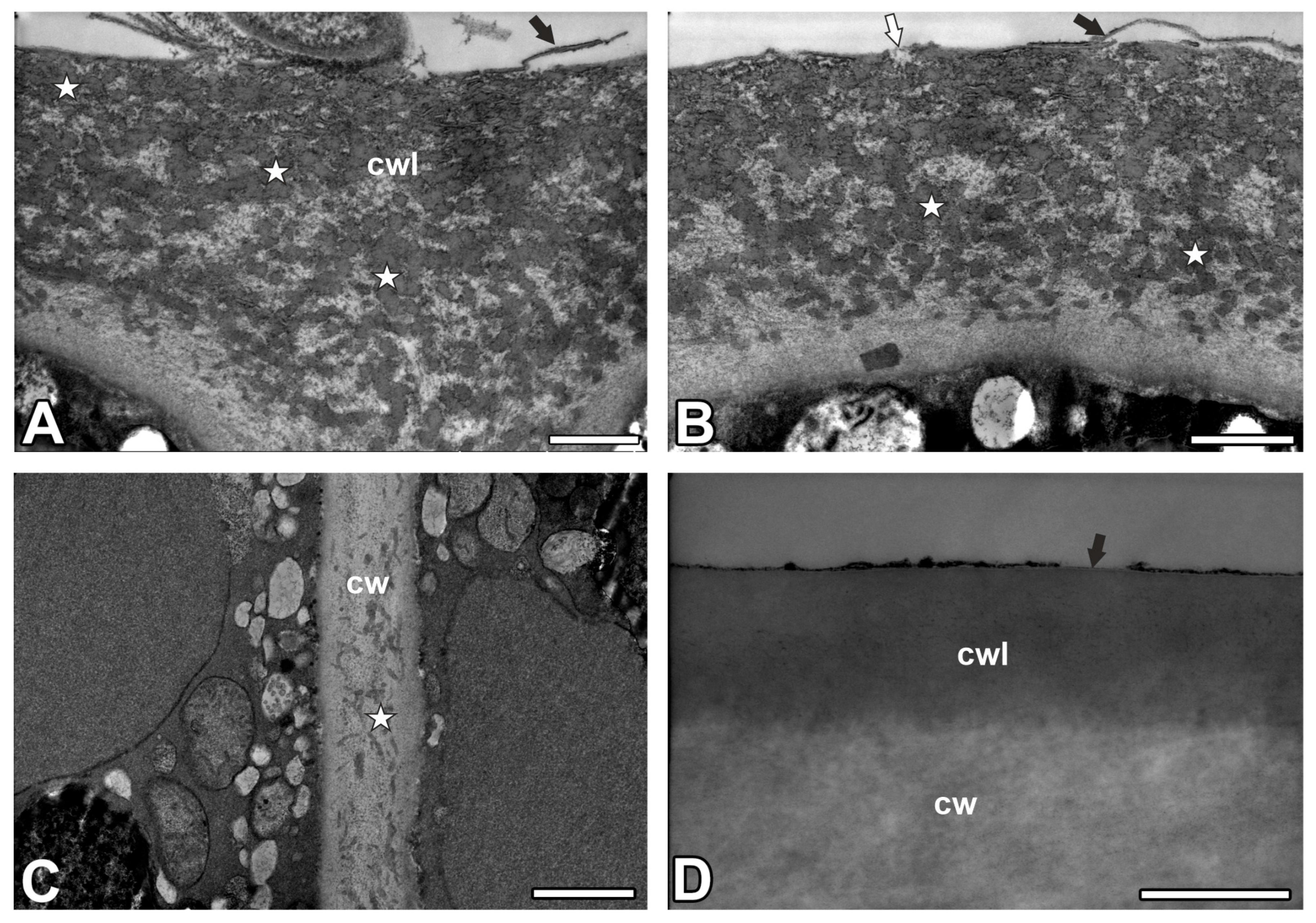
2.1. Cuticle and Cuticular Discontinuities
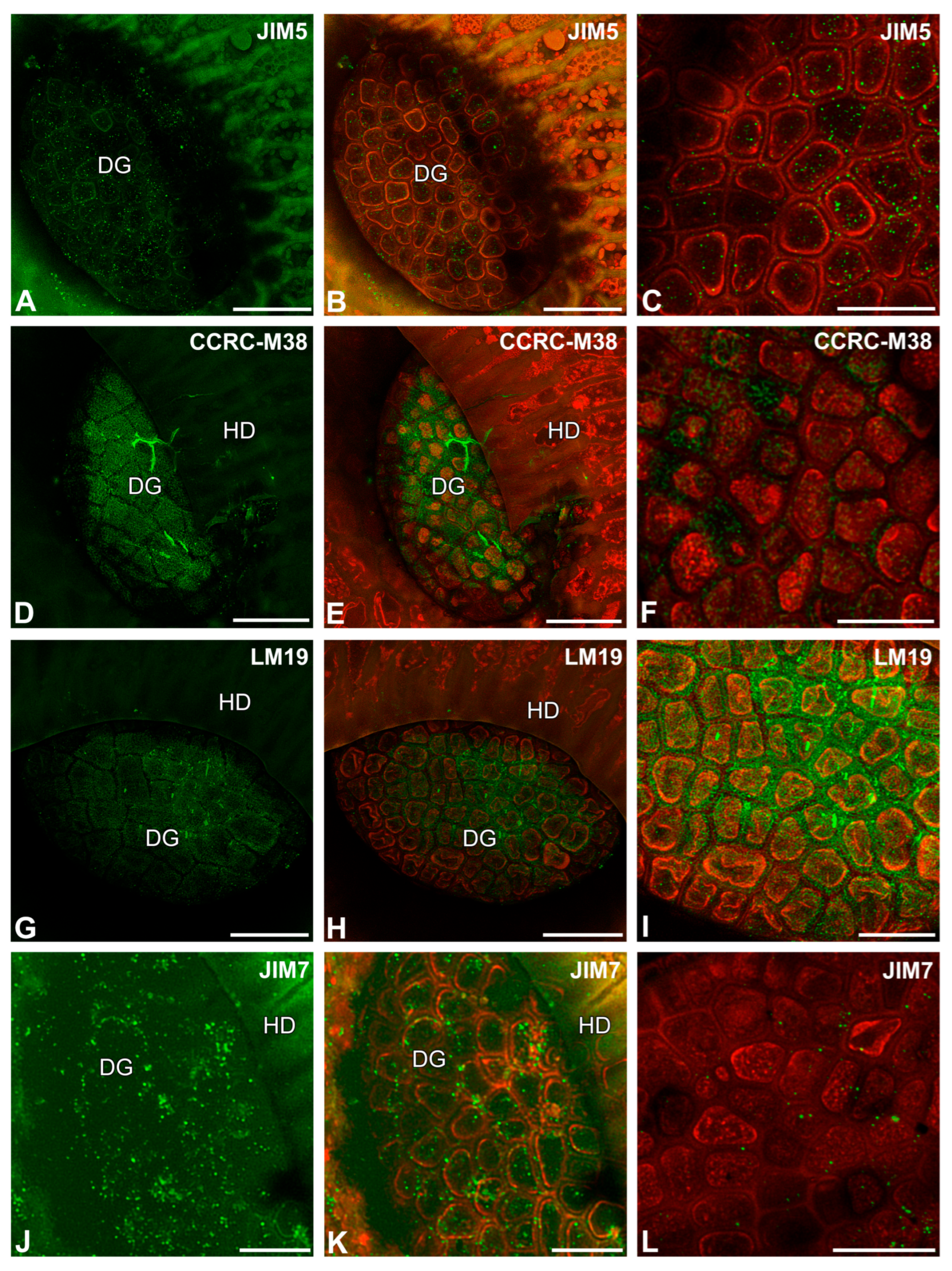
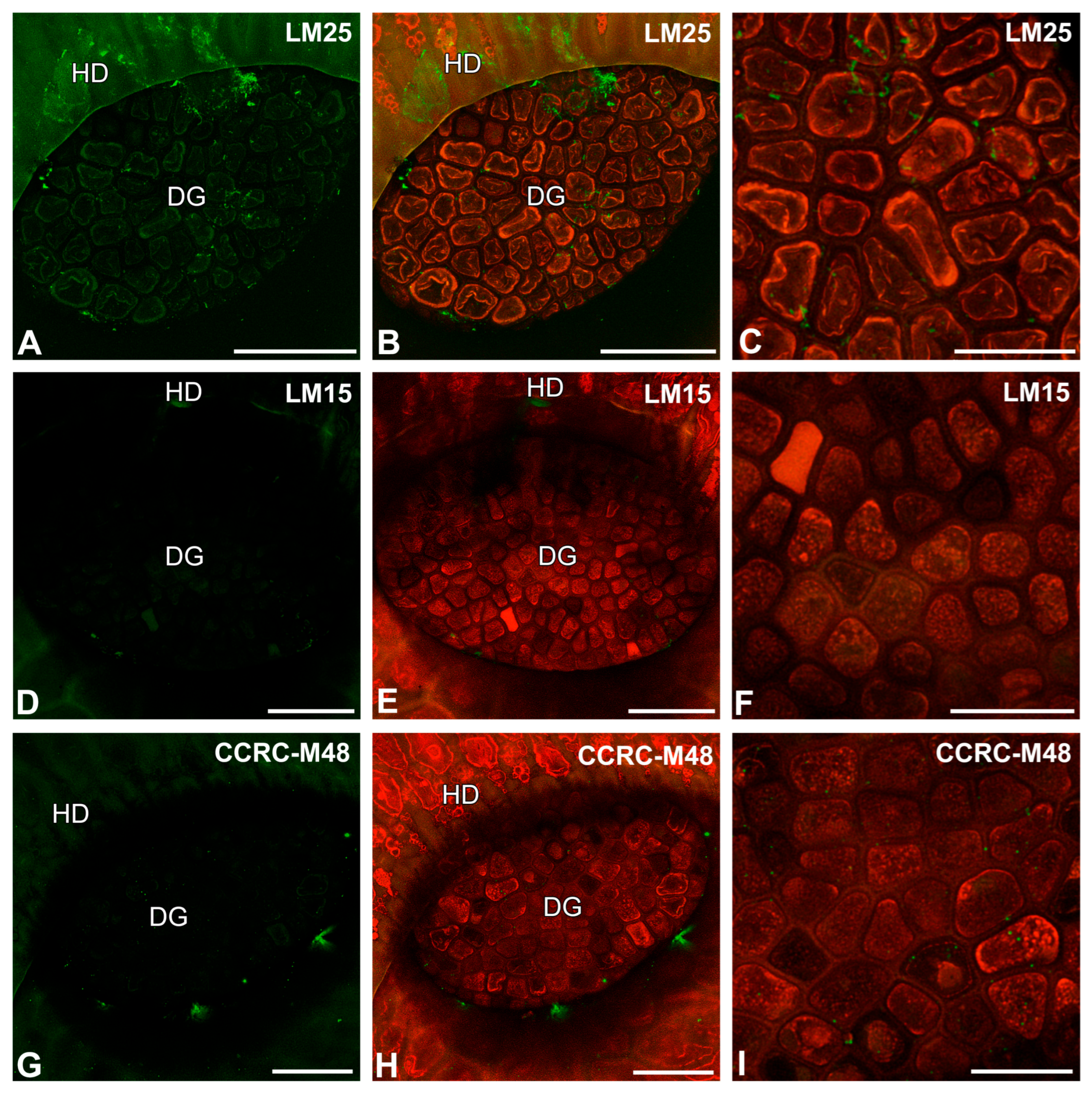
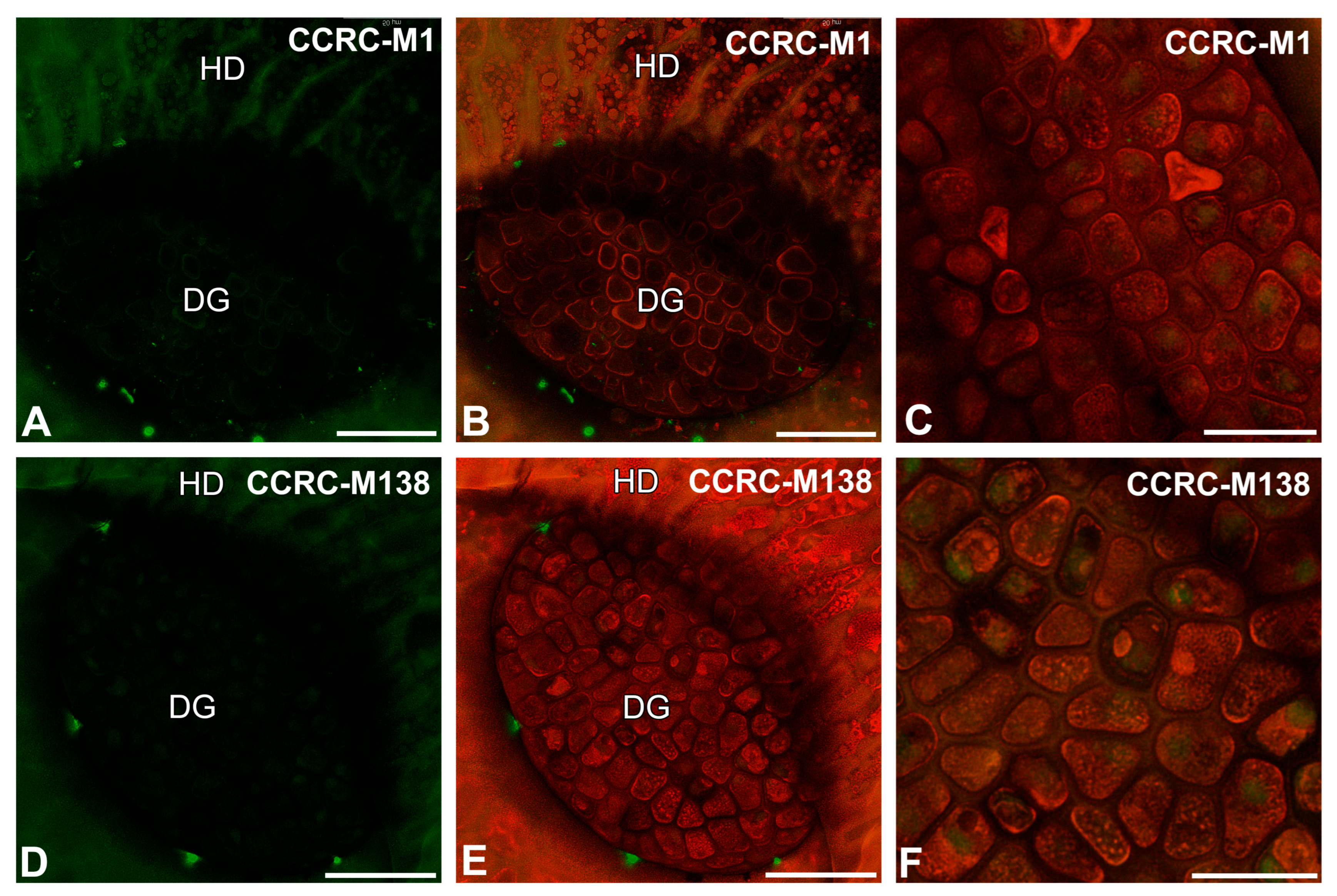
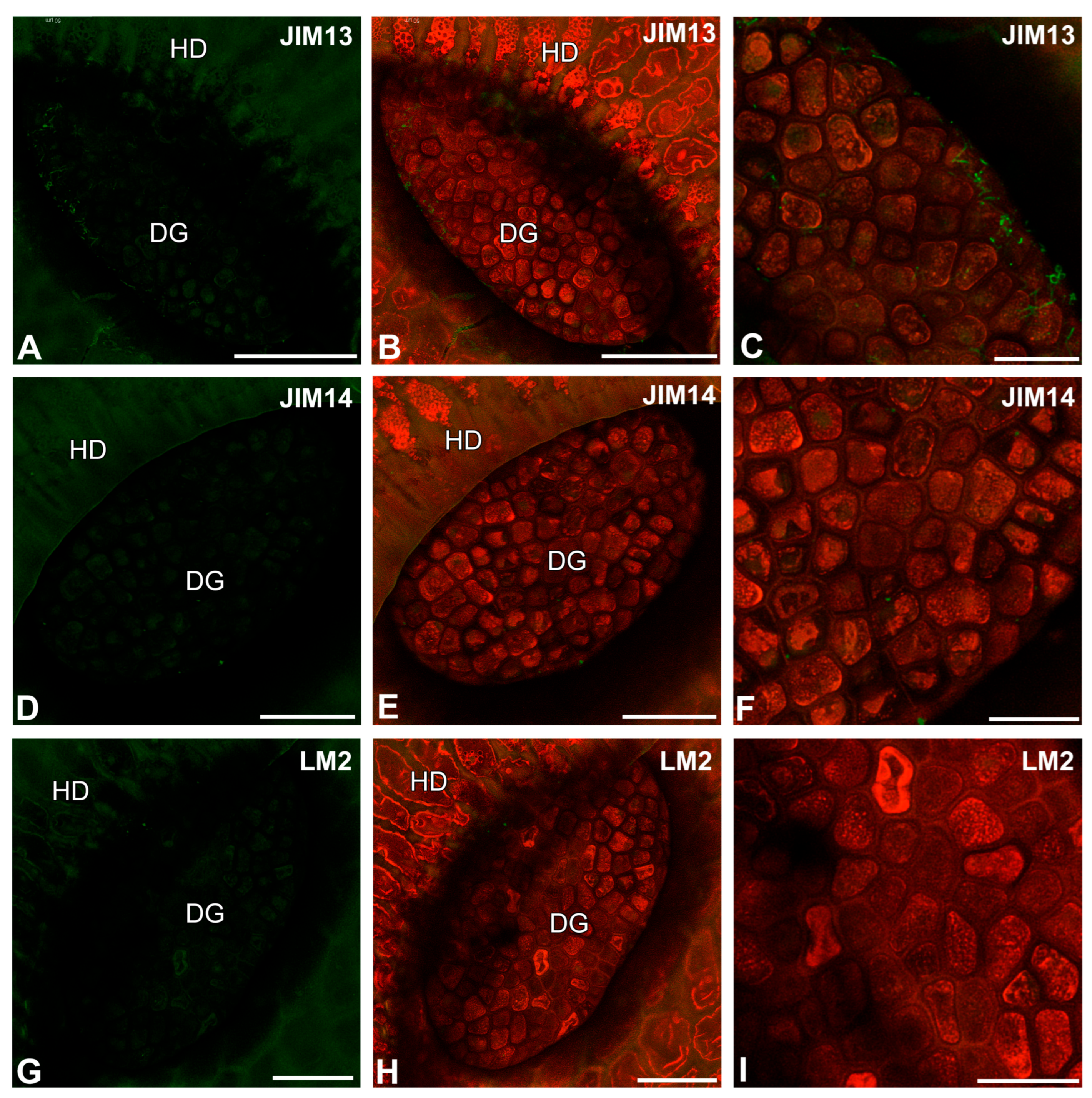
2.2. Pectic Homogalacturonan Distribution
2.3. Hemicellulose Distribution
2.4. The Arabinogalactan Protein (AGP) Distribution
3. Discussion
3.1. Cuticle Structure
3.2. Whole-Mount Immunolabeled Gland Technique
4. Materials and Methods
4.1. Plant Material
4.2. Immunochemical Analysis
4.3. Scanning Transmission Electron Microscopy
4.4. Scanning Electron Microscopy
4.5. Light Microscopy (LM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juniper, B.E.; Robbins, R.J.; Joel, D.M. The Carnivorous Plants; Academic Press: London, UK, 1989. [Google Scholar]
- Pavlovič, A.; Masarovičová, E.; Hudák, J. Carnivorous syndrome in Asian pitcher plants of the genus Nepenthes. Ann. Bot. 2007, 100, 527–536. [Google Scholar] [CrossRef]
- Król, E.; Płachno, B.J.; Adamec, L.; Stolarz, M.; Dziubińska, H.; Trębacz, K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann. Bot. 2012, 109, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.A.; Clarke, C.M. The carnivorous syndrome in Nepenthes pitcher plants: Current state of knowledge and potential future directions. Plant Signal. Behav. 2010, 5, 644–648. [Google Scholar] [CrossRef]
- Cheek, M.; Jebb, M.; Murphy, B. A classification of functional pitcher types in Nepenthes (Nepenthaceae). bioRxiv 2019. [Google Scholar] [CrossRef]
- Lloyd, F.E. The Carnivorous Plants; Ronald Press: New York, NY, USA, 1942. [Google Scholar]
- Gorb, E.; Kastner, V.; Peressadko, A.; Arzt, E.; Gaume, L.; Rowe, N.; Gorb, S. Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous plant Nepenthes ventrata and its role in insect trapping and retention. J. Exp. Biol. 2004, 207, 2947–2963. [Google Scholar] [CrossRef]
- Gorb, E.V.; Gorb, S.N. Physicochemical properties of functional surfaces in pitchers of the carnivorous plant Nepenthes alata Blanco (Nepenthaceae). Plant Biol. 2006, 8, 841–848. [Google Scholar] [CrossRef]
- Bauer, U.; Jetter, R.; Poppinga, S. Non-motile traps. In Carnivorous Plants: Physiology, Ecology, and Evolution; Ellison, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Lessware, O.C.; Mantell, J.M.; Bauer, U. Carnivorous Nepenthes pitcher plants combine common developmental processes to make a complex epidermal trapping surface. Ann. Bot. 2025, 135, 643–654. [Google Scholar] [CrossRef]
- Bauer, U.; Clemente, C.J.; Renner, T.; Federle, W. Form follows function: Morphological diversification and alternative trapping strategies in carnivorous Nepenthes pitcher plants. J. Evol. Biol. 2012, 25, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.K.; Bauer, U. Pitcher geometry facilitates extrinsically powered ‘springboard trapping’ in carnivorous Nepenthes gracilis pitcher plants. Biol. Lett. 2022, 18, 20220106. [Google Scholar] [CrossRef]
- Chomicki, G.; Burin, G.; Busta, L.; Gozdzik, J.; Jetter, R.; Mortimer, B.; Bauer, U. Convergence in carnivorous pitcher plants reveals a mechanism for composite trait evolution. Science 2024, 383, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Amagase, S. Digestive enzymes in insectivorous plants: III. Acid proteases in the genus Nepenthes and Drosera peltata. J. Bioch. 1972, 72, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tökés, Z.A.; Woon, W.C.; Chambers, S.M. Digestive enzymes secreted by the carnivorous plant Nepenthes macferlanei L. Planta 1974, 119, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hatano, N.; Hamada, T. Proteome analysis of pitcher fluid of the carnivorous plant Nepenthes alata. J. Prot. Res. 2008, 7, 809–816. [Google Scholar] [CrossRef]
- Buch, F.; Kaman, W.E.; Bikker, F.J.; Yilamujiang, A.; Mithöfer, A. Nepenthesin protease activity indicates digestive fluid dynamics in carnivorous Nepenthes plants. PLoS ONE 2015, 10, e0118853. [Google Scholar] [CrossRef] [PubMed]
- Saganová, M.; Bokor, B.; Stolárik, T.; Pavlovič, A. Regulation of enzyme activities in carnivorous pitcher plants of the genus Nepenthes. Planta 2018, 248, 451–464. [Google Scholar] [CrossRef]
- Heslop-Harrison, Y. Enzyme release in carnivorous plants. In Lysozymes in Biology and Pathology; Dingle, J.T., Dean, R.T., Eds.; North Holland Publishing Company: Amsterdam, The Netherlands, 1975; Volume 4, pp. 525–578. [Google Scholar]
- Parkes, D.M. Adaptive Mechanisms of Surface and Glands in Some Carnivorous Plants. Master’s Thesis, Monash University, Clayton, VIC, Australia, 1980. [Google Scholar]
- Płachno, B.J.; Adamec, L.; Lichtscheidl, I.K.; Peroutka, M.; Adlassnig, W.; Vrba, J. Fluorescence labelling of phosphatase activity in digestive glands of carnivorous plants. Plant Biol. 2006, 8, 813–820. [Google Scholar] [CrossRef]
- Thornhill, A.H.; Harper, I.S.; Hallam, N.D. The development of the digestive glands and enzymes in the pitchers of three Nepenthes species: N. alata, N. tobaica, and N. ventricosa (Nepenthaceae). Int. J. Plant. Sci. 2008, 169, 615–624. [Google Scholar] [CrossRef]
- Vassilyev, A.E.; Muravnik, L.E. The nectaries of the lid in closed pitchers of Nepenthes khasiana (Nepenthaceae) secrete a digestive fluid. Bot. Zhurnal 2007, 92, 1141–1144. [Google Scholar]
- Vassilyev, A.E. The nectaries of the peristome in the closed pitchers of Nepenthes khasiana (Nepenthaceae) secrete polysacharide slime. Bot. Zhurnal 2007, 92, 1554–1568. [Google Scholar]
- Owen, T.P.; Lennon, K.A.; Santo, M.J.; Anderson, A.Y. Pathways for nutrient transport in the pitchers of the carnivorous plant Nepenthes alata. Ann. Bot. 1999, 84, 459–466. [Google Scholar] [CrossRef]
- Fenner, C.A. Beitraege zur Kenntnis der Anatomie, Entwicklungsgeschichte und Biologie der Laubblaetter und Druesen einiger Insektivoren. Flora 1904, 93, 333–335. [Google Scholar]
- Schnepf, E. Die Morphologie der Sekretion in pflanzlichen Drüsen. Ber. Dtsch. Bot. Ges. 1965, 78, 478–483. [Google Scholar]
- Gorb, E.V.; Gorb, S.N. Functional Surfaces in the Pitcher of the Carnivorous Plant Nepenthes alata: A Cryo-Sem Approach. In Functional Surfaces in Biology; Gorb, S.N., Ed.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Ivesic, C.; Krammer, S.; Koller-Peroutka, M.; Laarouchi, A.; Gruber, D.; Lang, I.; Lichtscheidl, I.K.; Adlassnig, W. Quantification of Protein Uptake by Endocytosis in Carnivorous Nepenthales. Plants 2023, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Freund, M.; Graus, D.; Fleischmann, A.; Gilbert, K.J.; Lin, Q.; Renner, T.; Stigloher, C.; Albert, V.A.; Hedrich, R.; Fukushima, K. The digestive systems of carnivorous plants. Plant Physiol. 2022, 190, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; Pickard, B.G. Secretion, absorption and cuticular structure in Drosera tentacles. Plant Physiol. 1969, 44, 5. [Google Scholar]
- Ragetli, H.W.; Weintraub, L.O.E. Characteristics of Drosera tentacles. I. Anatomical and cytological details. Can. J. Bot. 1972, 50, 159–168. [Google Scholar] [CrossRef]
- Williams, S.E.; Pickard, B.G. Connections and barriers between cells of Drosera tentacles in relation to their electrophysiology. Planta 1974, 116, 1–16. [Google Scholar] [CrossRef]
- Płachno, B.J.; Faber, J.; Jankun, A. Cuticular discontinuities in glandular hairs of Genlisea St.-Hil. in relation to their functions. Acta Bot. Gall. 2005, 152, 125–130. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kozieradzka-Kiszkurno, M.; Świątek, P. Functional Ultrastructure of Genlisea (Lentibulariaceae) Digestive Hairs. Ann. Bot. 2007, 100, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Joel, D.M.; Juniper, B.E. Cuticular gaps in carnivorous plant glands. In The Plant Cuticle; Cutler, D.F., Alvin, K.L., Price, C.E., Eds.; Academic Press: London, UK, 1982; pp. 121–130. [Google Scholar]
- Joel, D.M.; Rea, P.A.; Juniper, B.E. The cuticle of Dionaea muscipula Ellis (Venus’s Flytrap) in relation to stimulation, secretion and absorption. Protoplasma 1983, 114, 44–51. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P. Do Cuticular Gaps Make It Possible to Study the Composition of the Cell Walls in the Glands of Drosophyllum lusitanicum? Int. J. Mol. Sci. 2024, 25, 1320. [Google Scholar] [CrossRef]
- Anderson, B. Adaptations to foliar absorption of faeces: A pathway in plant carnivory. Ann. Bot. 2005, 95, 757–761. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fineran, B.A.; Lee, M.S.L. Organization of quadrifid and bifid hairs in the trap of Utricularia monanthos. Protoplasma 1975, 84, 43–70. [Google Scholar] [CrossRef]
- Płachno, B.J.; Lancelle, S.; Świątek, P.; Hepler, P.K.; Weidinger, M.; Lichtscheidl, I. Cyto-architecture of Byblis glands and leaf cells based on freeze-substitution and conventional TEM. Ann. Bot. 2025, 135, 463–482. [Google Scholar] [CrossRef]
- Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Koshkin, E.; Lendl, T.; Lichtscheidl, I.K. Endocytotic uptake of nutrients in carnivorous plants. Plant J. 2012, 71, 303–313. [Google Scholar] [CrossRef]
- Bauer, U.; Grafe, T.U.; Federle, W. Evidence for alternative trapping strategies in two forms of the pitcher plant, Nepenthes rafflesiana. J. Exp. Bot. 2011, 62, 3683–3692. [Google Scholar] [CrossRef]
- Bauer, U.; Di Giusto, B.; Skepper, J.; Grafe, T.U.; Federle, W. With a flick of the lid: A novel trapping mechanism in Nepenthes gracilis pitcher plants. PLoS ONE 2012, 7, e38951. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S. Nepenthes: The Tropical Pitcher Plants; Redfern Natural History: Dorset, UK, 2023; Volume 1–3. [Google Scholar]
- Li, Y.Q.; Bruun, L.; Pierson, E.S.; Cresti, M. Periodic deposition of arabinogalactan epitopes in the cell wall of pollen tubes of Nicotiana tabacum L. Planta 1992, 188, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Chebli, Y.; Kaneda, M.; Zerzour, R.; Geitmann, A. The cell wall of the Arabidopsis pollen tube--spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol. 2012, 160, 1940–1955. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; McCartney, L.; Knox, J.P. In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 2001, 213, 37–44. [Google Scholar] [CrossRef]
- Larson, E.R.; Tierney, M.L.; Tinaz, B.; Domozych, D.S. Using monoclonal antibodies to label living root hairs: A novel tool for studying cell wall microarchitecture and dynamics in Arabidopsis. Plant Methods 2014, 10, 30. [Google Scholar] [CrossRef]
- Marzec, M.; Szarejko, I.; Melzer, M. Arabinogalactan proteins are involved in root hair development in barley. J. Exp. Bot. 2015, 5, 1245–1257. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M. The Localization of Cell Wall Components in the Quadrifids of Whole-Mount Immunolabeled Utricularia dichotoma Traps. Int. J. Mol. Sci. 2024, 25, 56. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Feldo, M.; Świątek, P. Do Arabinogalactan Proteins Occur in the Transfer Cells of Utricularia dichotoma? Int. J. Mol. Sci. 2024, 25, 6623. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Heubl, G.; Bringmann, G.; Meimberg, H. Molecular phylogeny and character evolution of carnivorous plant families in Caryophyllales—Revisited. Plant Biol. 2006, 8, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.F.; Yang, Y.; Moore, M.J.; Mikenas, J.; Timoneda, A.; Brockington, S.F.; Smith, S.A. Widespread paleopolyploidy, gene tree conflict, and recalcitrant relationships among the carnivorous Caryophyllales. Am. J. Bot. 2017, 104, 858–867. [Google Scholar] [CrossRef]
- Ridley, M.A.; O’Neill, D.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalac-turonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.; Höfte, H. Cell wall mechanics and growth control in plants: The role of pectins revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Feldo, M.; Stolarczyk, P.; Małota, K.; Banaś, K. External Glands of Nepenthes Traps: Structure and Potential Function. Int. J. Mol. Sci. 2025, 26, 7788. [Google Scholar] [CrossRef]
- Liners, F.; Letesson, J.J.; Didembourg, C.; Van Cutsem, P. Monoclonal Antibodies against Pectin: Recognition of a Conformation Induced by Calcium. Plant Physiol. 1989, 91, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Paul Knox, PhD, University of Leeds. Available online: https://www.kerafast.com/cat/799/paul-knox-phd (accessed on 13 November 2023).
- Knox, J.P.; Day, S.; Roberts, K. A set of cell surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development 1989, 106, 47–56. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 2009, 28, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Pattathil, S.; Avci, U.; Baldwin, D.; Swennes, A.G.; McGill, J.A.; Popper, Z.; Bootten, T.; Albert, A.; Davis, R.H.; Chennareddy, C.; et al. A comprehensive toolkit of plant cell wall gly-can-directed monoclonal antibodies. Plant Physiol. 2010, 153, 514–525. [Google Scholar] [CrossRef]
- McCartney, L.; Marcus, S.E.; Knox, J.P. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 2005, 53, 543–546. [Google Scholar] [CrossRef]
- Available online: https://www.kerafast.com/ (accessed on 13 November 2023).
- Płachno, B.J.; Świątek, P.; Jobson, R.W.; Małota, K.; Brutkowski, W. Serial block face SEM visualization of unusual plant nuclear tubular extensions in a carnivorous plant (Utricularia, Lentibulariaceae). Ann. Bot. 2017, 120, 673–680. [Google Scholar] [CrossRef]
- Lichtscheidl, I.; Lancelle, S.; Weidinger, M.; Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Krammer, S.; Hepler, P.K. Gland cell responses to feeding in Drosera capensis, a carnivorous plant. Protoplasma 2021, 258, 1291–1306. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płachno, B.J.; Kapusta, M.; Feldo, M.; Stolarczyk, P.; Świątek, P. The Localization of Cell Wall Components in the Whole-Mount Immunolabeled Nepenthes Digestive Glands. Int. J. Mol. Sci. 2025, 26, 9174. https://doi.org/10.3390/ijms26189174
Płachno BJ, Kapusta M, Feldo M, Stolarczyk P, Świątek P. The Localization of Cell Wall Components in the Whole-Mount Immunolabeled Nepenthes Digestive Glands. International Journal of Molecular Sciences. 2025; 26(18):9174. https://doi.org/10.3390/ijms26189174
Chicago/Turabian StylePłachno, Bartosz J., Małgorzata Kapusta, Marcin Feldo, Piotr Stolarczyk, and Piotr Świątek. 2025. "The Localization of Cell Wall Components in the Whole-Mount Immunolabeled Nepenthes Digestive Glands" International Journal of Molecular Sciences 26, no. 18: 9174. https://doi.org/10.3390/ijms26189174
APA StylePłachno, B. J., Kapusta, M., Feldo, M., Stolarczyk, P., & Świątek, P. (2025). The Localization of Cell Wall Components in the Whole-Mount Immunolabeled Nepenthes Digestive Glands. International Journal of Molecular Sciences, 26(18), 9174. https://doi.org/10.3390/ijms26189174






