Bitter Taste Receptors TAS2R8 and TAS2R10 Reduce Proton Secretion and Differentially Modulate Cadmium Uptake in Immortalized Human Gastric Cells
Abstract
1. Introduction
2. Results
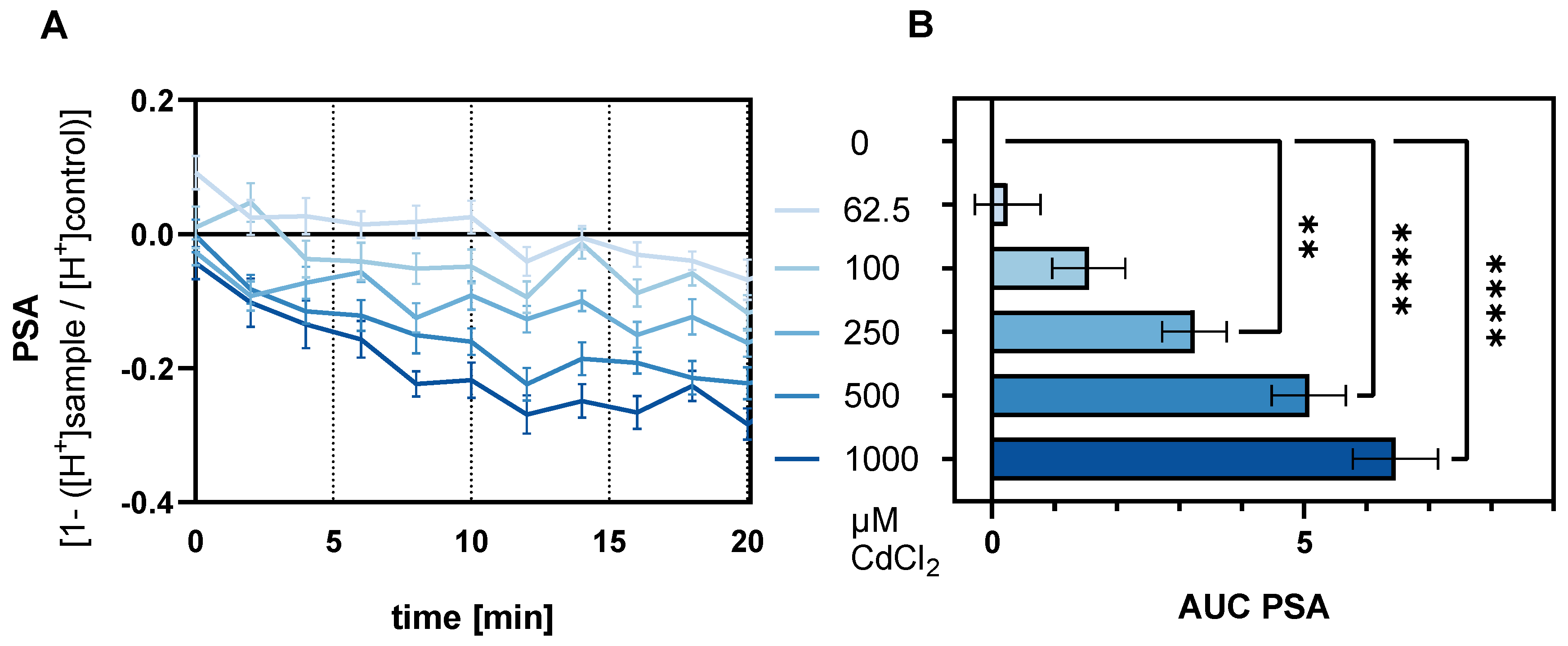
2.1. Cadmium-Induced Reduction of PSA in HGT-1 Cells Is Mediated by TAS2Rs
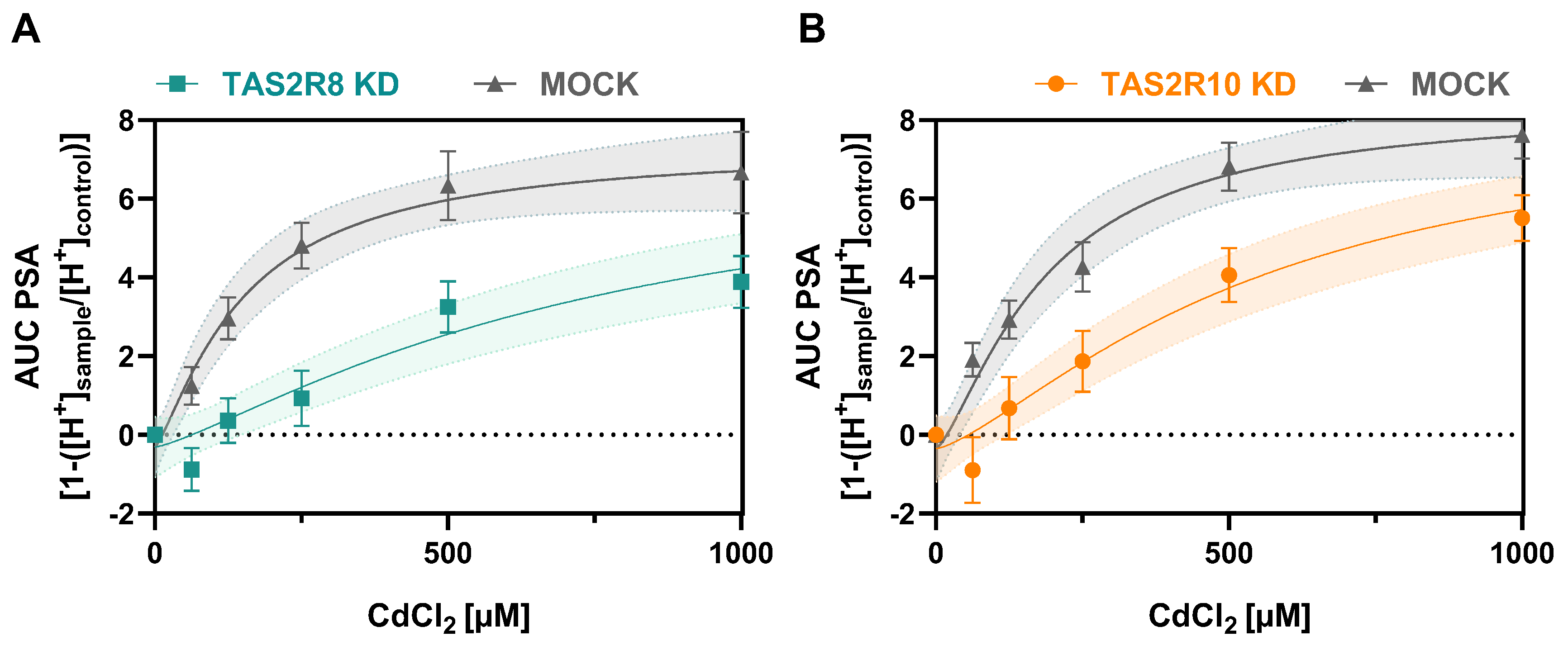
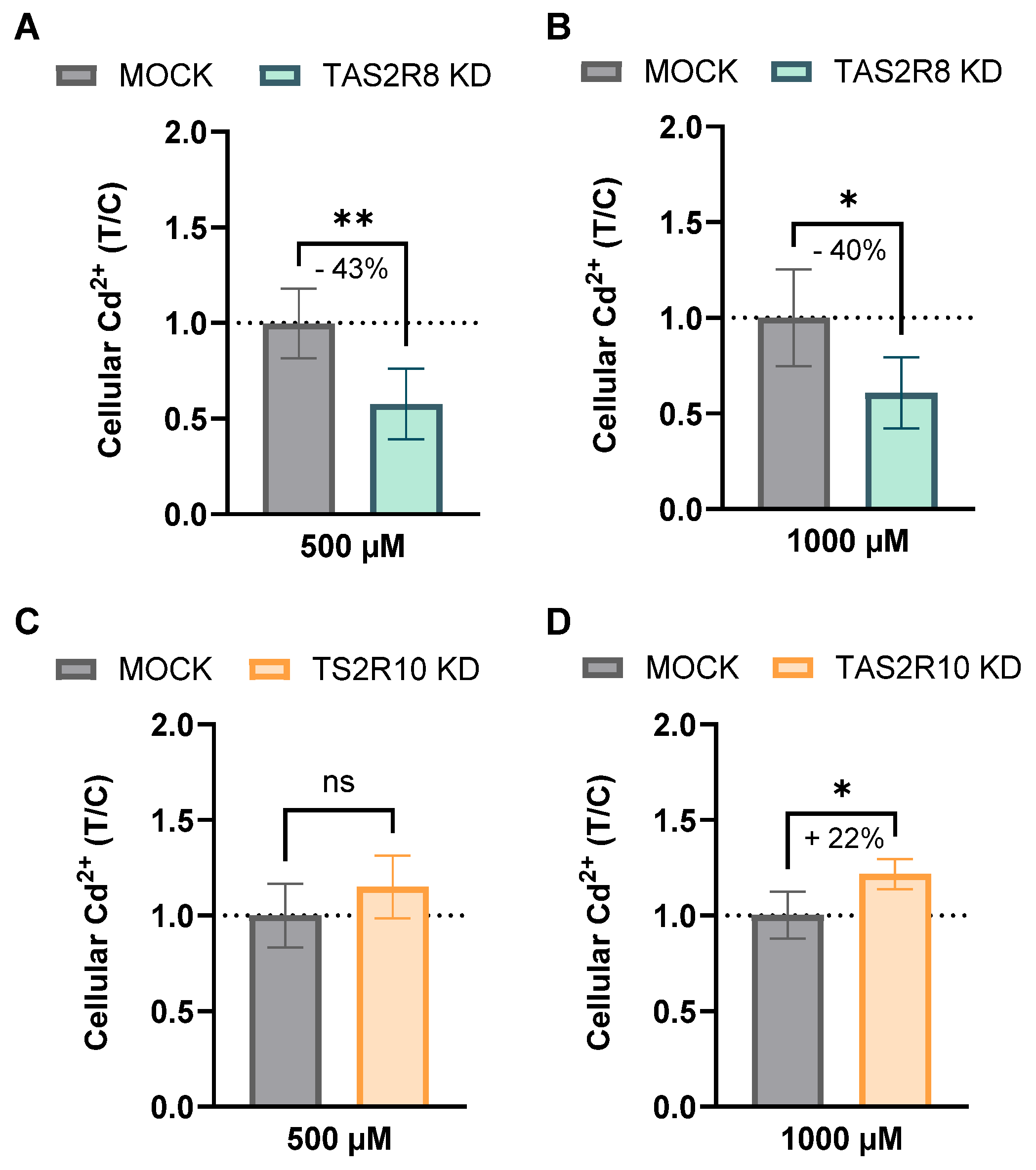
2.2. Functional Role of TAS2R8 and TAS2R10 in Cellular Cadmium Uptake
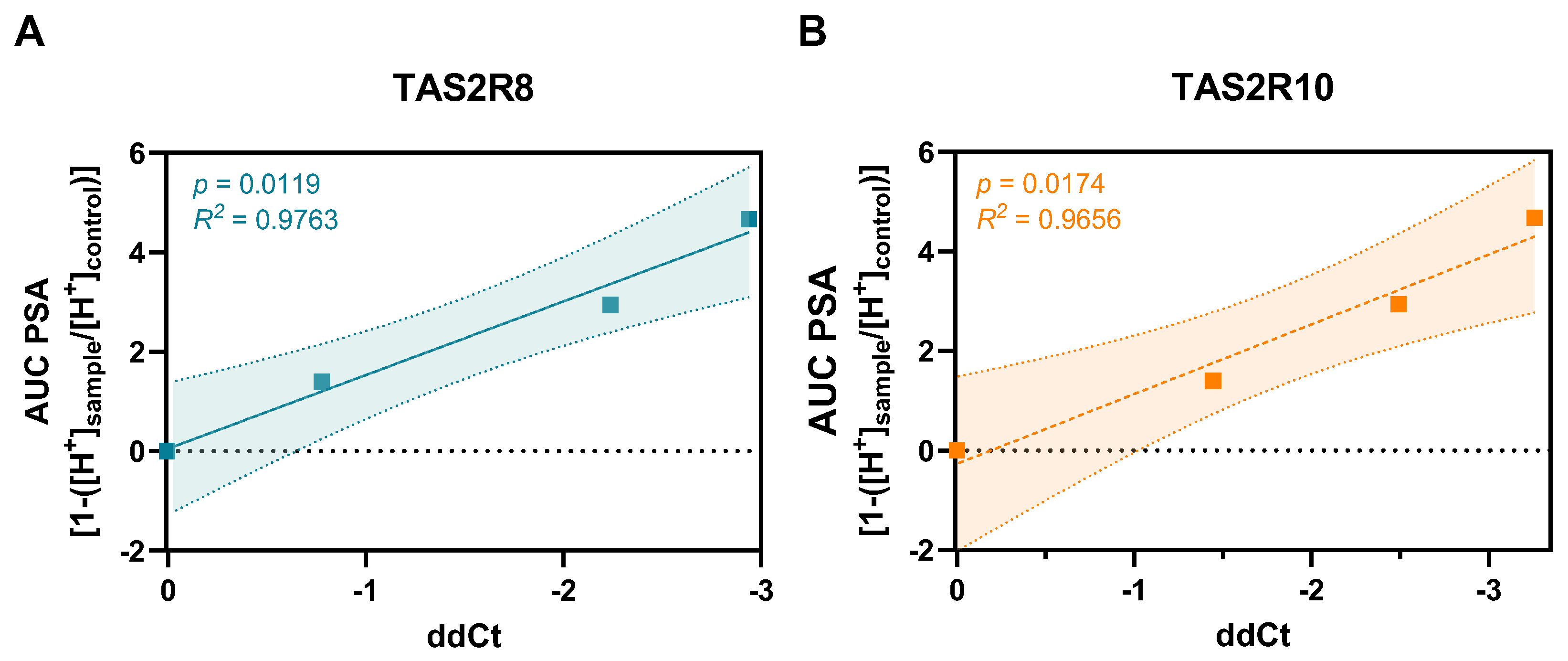
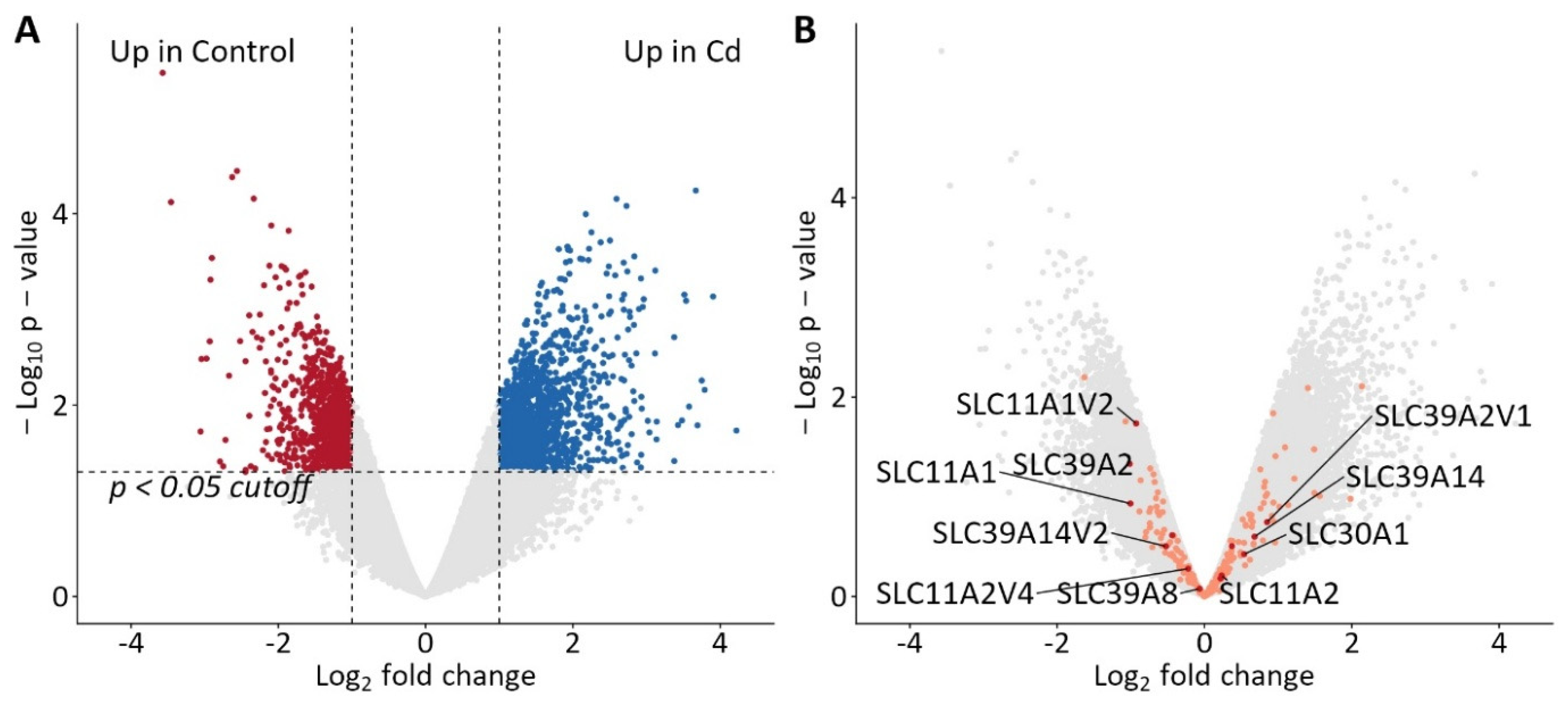
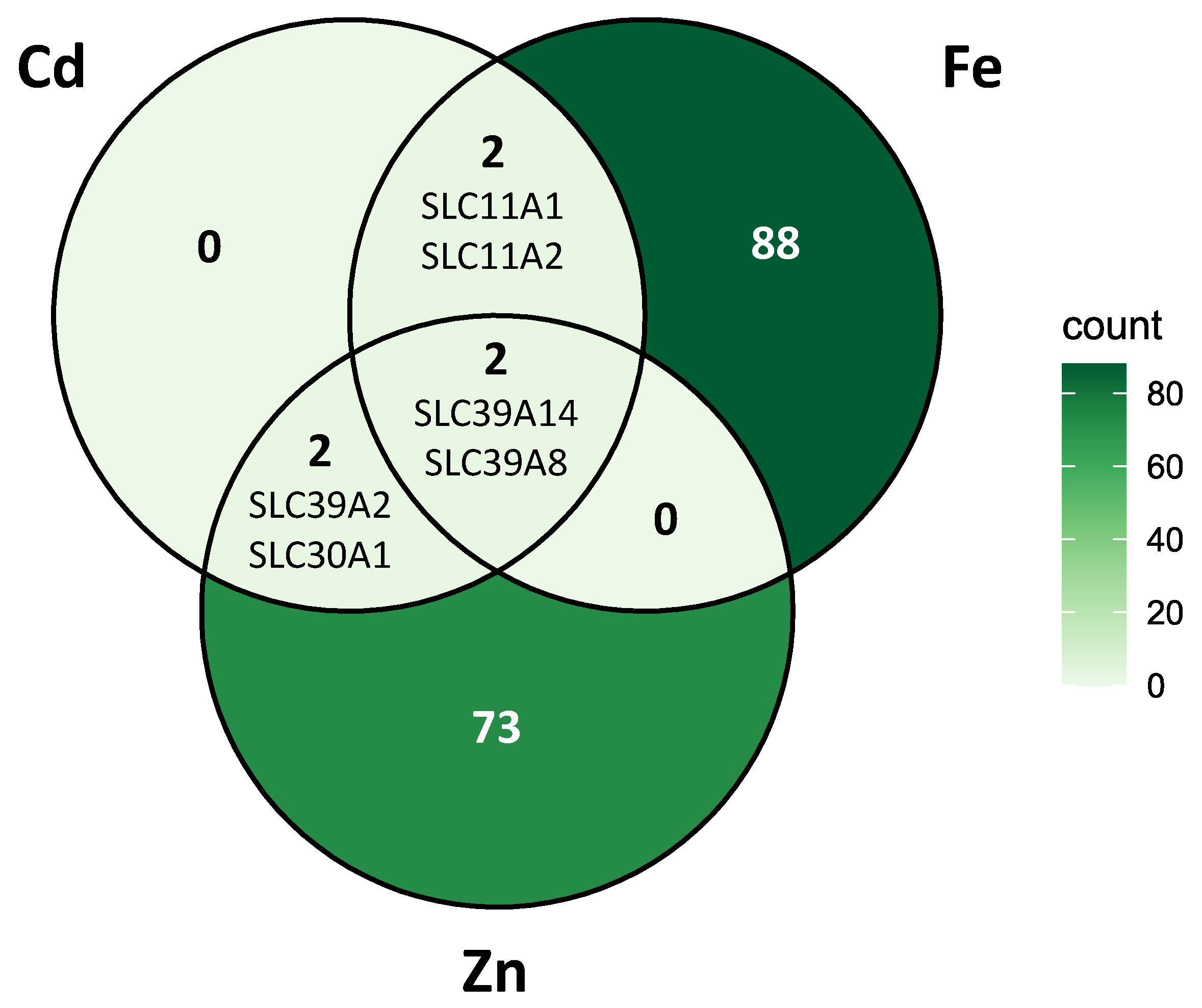
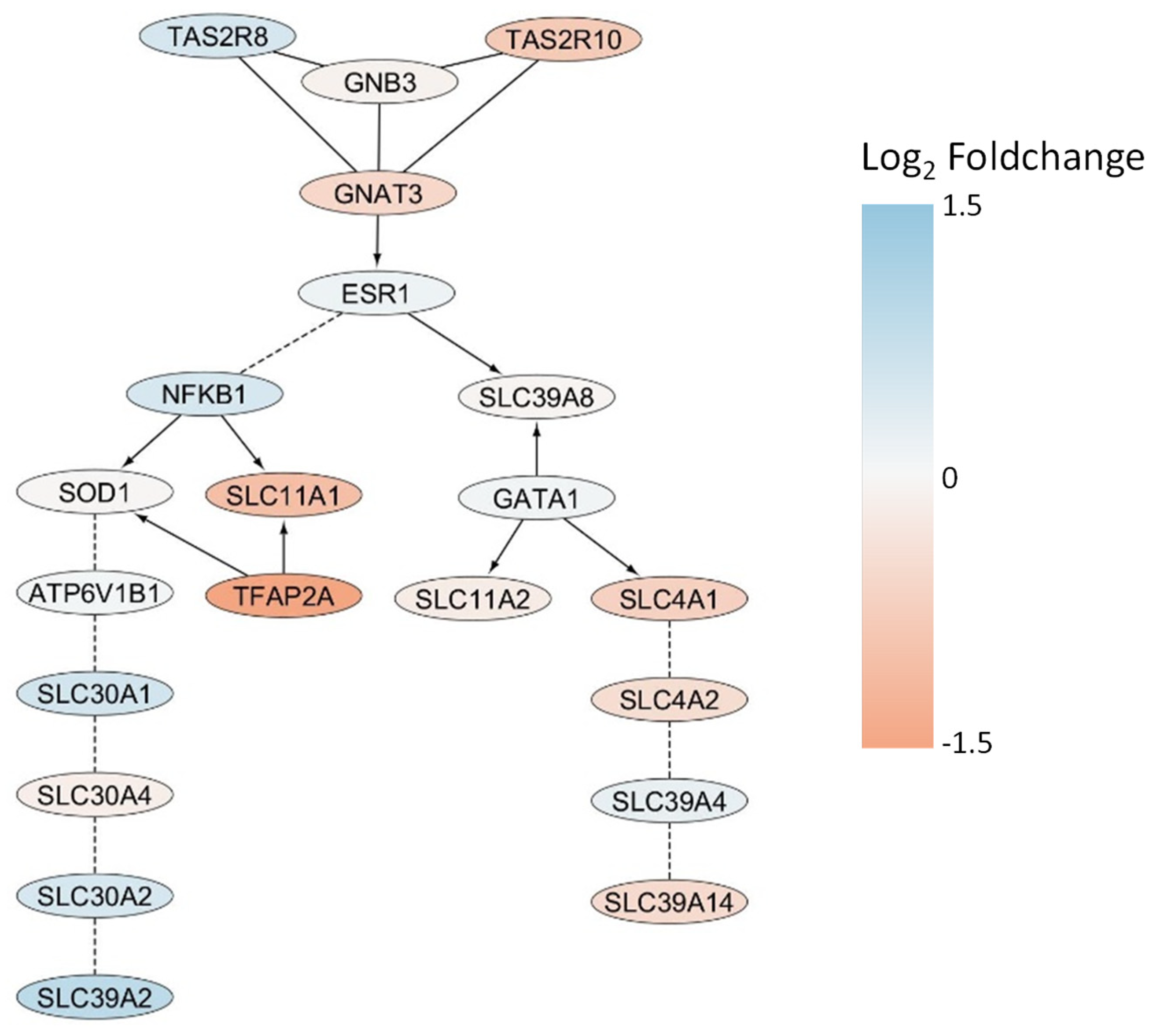
2.3. Genomic Targets of Cellular Cadmium Transport
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Proton Secretory Activity
4.5. Gene Expression
4.6. Intracellular Cadmium Concentration
4.7. Transient Knockdown of TAS2R8 and TAS2R10 Expression
4.8. cDNA Microarrays
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liszt, K.I.; Ley, J.P.; Lieder, B.; Behrens, M.; Stöger, V.; Reiner, A.; Hochkogler, C.M.; Köck, E.; Marchiori, A.; Hans, J.; et al. Caffeine Induces Gastric Acid Secretion via Bitter Taste Signaling in Gastric Parietal Cells. Proc. Natl. Acad. Sci. USA 2017, 114, E6260–E6269. [Google Scholar] [CrossRef] [PubMed]
- Richter, P.; Sebald, K.; Fischer, K.; Behrens, M.; Schnieke, A.; Somoza, V. Bitter Peptides YFYPEL, VAPFPEVF, and YQEPVLGPVRGPFPIIV, Released during Gastric Digestion of Casein, Stimulate Mechanisms of Gastric Acid Secretion via Bitter Taste Receptors TAS2R16 and TAS2R38. J. Agric. Food Chem. 2022, 70, 11591–11602. [Google Scholar] [CrossRef]
- Sterneder, S.; Stoeger, V.; Dugulin, C.A.; Liszt, K.I.; Di Pizio, A.; Korntheuer, K.; Dunkel, A.; Eder, R.; Ley, J.P.; Somoza, V. Astringent Gallic Acid in Red Wine Regulates Mechanisms of Gastric Acid Secretion via Activation of Bitter Taste Sensing Receptor TAS2R4. J. Agric. Food Chem. 2021, 69, 10550–10561. [Google Scholar] [CrossRef]
- Stoeger, V.; Holik, A.K.; Hölz, K.; Dingjan, T.; Hans, J.; Ley, J.P.; Krammer, G.E.; Niv, M.Y.; Somoza, M.M.; Somoza, V. Bitter-Tasting Amino Acids l-Arginine and l-Isoleucine Differentially Regulate Proton Secretion via T2R1 Signaling in Human Parietal Cells in Culture. J. Agric. Food Chem. 2020, 68, 3434–3444. [Google Scholar] [CrossRef]
- Behrens, M.; Redel, U.; Blank, K.; Meyerhof, W. The Human Bitter Taste Receptor TAS2R7 Facilitates the Detection of Bitter Salts. Biochem. Biophys. Res. Commun. 2019, 512, 877–881. [Google Scholar] [CrossRef]
- Wang, Y.; Zajac, A.L.; Lei, W.; Christensen, C.M.; Margolskee, R.F.; Bouysset, C.; Golebiowski, J.; Zhao, H.; Fiorucci, S.; Jiang, P. Metal Ions Activate the Human Taste Receptor TAS2R7. Chem. Senses 2019, 44, 339–347. [Google Scholar] [CrossRef]
- Orth, H.N.; Pirkwieser, P.; Benthin, J.; Koehler, M.; Sterneder, S.; Parlar, E.; Schaudy, E.; Lietard, J.; Michel, T.; Boger, V.; et al. Bitter Taste Receptor TAS2R43 Co-Regulates Mechanisms of Gastric Acid Secretion and Zinc Homeostasis. Int. J. Mol. Sci. 2025, 26, 6017. [Google Scholar] [CrossRef]
- Jin, T.; Kong, Q.; Ye, T.; Wu, X.; Nordberg, G.F. Renal Dysfunction of Cadmium-Exposed Workers Residing in a Cadmium-Polluted Environment. BioMetals 2004, 17, 513–518. [Google Scholar] [CrossRef]
- Koedrith, P.; Kim, H.L.; Weon, J., II; Seo, Y.R. Toxicogenomic Approaches for Understanding Molecular Mechanisms of Heavy Metal Mutagenicity and Carcinogenicity. Int. J. Hyg. Environ. Health 2013, 216, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Moulis, J.M. Cellular Mechanisms of Cadmium Toxicity Related to the Homeostasis of Essential Metals. BioMetals 2010, 23, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, A.; Cirovic, A.; Yimthiang, S.; Vesey, D.A.; Satarug, S. Modulation of Adverse Health Effects of Environmental Cadmium Exposure by Zinc and Its Transporters. Biomolecules 2024, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cadmium. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/1376 (accessed on 25 July 2025).
- Funk, A.E.; Day, F.A.; Brady, F.O. Displacement of Zinc and Copper from Copper-Induced Metallothionein by Cadmium and by Mercury: In Vivo and Ex Vivo Studies. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1987, 86, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, A.; Debellis, L.; Caroppo, R.; Curci, S.; Colella, M. Cadmium Inhibits Acid Secretion in Stimulated Frog Gastric Mucosa. Toxicol. Appl. Pharmacol. 2010, 245, 264–271. [Google Scholar] [CrossRef]
- Kirchhoff, P.; Socrates, T.; Sidani, S.; Duffy, A.; Breidthardt, T.; Grob, C.; Viehl, C.T.; Beglinger, C.; Oertli, D.; Geibel, J.P. Zinc Salts Provide a Novel, Prolonged and Rapid Inhibition of Gastric Acid Secretion. Am. J. Gastroenterol. 2011, 106, 62–70. [Google Scholar] [CrossRef]
- Asar, M.; Kayişli, Ü.A.; Izgüt-Uysal, V.N.; Öner, G.; Polat, S.; Kaya, M. Cadmium-Induced Changes in Parietal Cell Structure and Functions of Rats. Biol. Trace Elem. Res. 1999, 74, 153–170. [Google Scholar] [CrossRef]
- Giridhar, J.; Rathinavelu, A.; Isom, G.E. Interaction of Cadmium with Atrial Natriuretic Peptide Receptors: Implications for Toxicity. Toxicology 1992, 75, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.; Katzenellenbogen, B.S.; Martin, M. Activation of Estrogen Receptor-α by the Heavy Metal Cadmium. Mol. Endocrinol. 2000, 14, 545–553. [Google Scholar] [CrossRef]
- Thévenod, F. Cadmium and Cellular Signaling Cascades: To Be or Not to Be? Toxicol. Appl. Pharmacol. 2009, 238, 221–239. [Google Scholar] [CrossRef]
- Hongo, T.; Nojima, S.; Setaka, M. Purification and Characterization of (H++ K+)-ATPase from Hog Gastric Mucosa. Jpn. J. Pharmacol. 1990, 52, 295–305. [Google Scholar] [CrossRef]
- Kinne-Saffrran, E.; Hülseweh, M.; Pfaff, C.; Kinne, K.H. Inhibition of Na, K-ATPase by Cadmium; Different Mechanisms in Different Species. Toxicol. Appl. Pharmacol. 1993, 121, 22–29. [Google Scholar] [CrossRef]
- Herak-Kramberger, C.M.; Sabolic, I.; Blanusa, M.; Smith, P.J.S.; Brown, D.; Breton, S. Cadmium Inhibits Vacuolar H+ATPase-Mediated Acidification in the Rat Epididymis. Biol. Reprod. 2000, 63, 599–606. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Soleimani, M.; Girijashanker, K.; Reed, J.M.; He, L.; Dalton, T.P.; Nebert, D.W. Cd2+ versus Zn2+ Uptake by the ZIP8 HCO3-Dependent Symporter: Kinetics, Electrogenicity and Trafficking. Biochem. Biophys. Res. Commun. 2008, 365, 814–820. [Google Scholar] [CrossRef]
- Bressler, J.P.; Olivi, L.; Cheong, J.H.; Kim, Y.; Bannon, D. Divalent Metal Transporter 1 in Lead and Cadmium Transport. Ann. N. Y. Acad. Sci. 2004, 1012, 142–152. [Google Scholar] [CrossRef]
- Kwok, M.L.; Li, Z.P.; Law, T.Y.S.; Chan, K.M. Promotion of Cadmium Uptake and Cadmium-Induced Toxicity by the Copper Transporter CTR1 in HepG2 and ZFL Cells. Toxicol. Rep. 2020, 7, 1564–1570. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Yuce, K. Zinc Transporter Proteins. Neurochem. Res. 2018, 43, 517–530. [Google Scholar] [CrossRef]
- Dalton, T.P.; He, L.; Wang, B.; Miller, M.L.; Jin, L.; Stringer, K.F.; Chang, X.; Baxter, C.S.; Nebert, D.W. Identification of Mouse SLC39A8 as the Transporter Responsible for Cadmium-Induced Toxicity in the Testis. Proc. Natl. Acad. Sci. USA 2005, 102, 3401–3406. [Google Scholar] [CrossRef]
- Fujishiro, H.; Yano, Y.; Takada, Y.; Tanihara, M.; Himeno, S. Roles of ZIP8, ZIP14, and DMT1 in Transport of Cadmium and Manganese in Mouse Kidney Proximal Tubule Cells. Metallomics 2012, 4, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Jorge-Nebert, L.F.; Gálvez-Peralta, M.; Landero Figueroa, J.; Somarathna, M.; Hojyo, S.; Fukada, T.; Nebert, D.W. Comparing Gene Expression during Cadmium Uptake and Distribution: Untreated versus Oral Cd-Treated Wild-Type and ZIP14 Knockout Mice. Toxicol. Sci. 2015, 143, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, M.; Black, W.D.; Chan, D.Y.; Hale, B.A. The Effect of Pharmacologically Altered Gastric PH on Cadmium Absorption from the Diet and Its Accumulation in Murine Tissues. Food Chem. Toxicol. 2005, 43, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Iwata, N.; Tetsutikawahara, N.; Onosaka, S.; Tanaka, K. Effect of Hemolytic and Iron-Deficiency Anemia on Intestinal Absorption and Tissue Accumulation of Cadmium. Toxicol. Lett. 2008, 179, 48–52. [Google Scholar] [CrossRef]
- Berglund, M.; Akesson, A.; Nermell, B.; Vahter, M. Intestinal Absorption of Dietary Cadmium in Women Depends on Body Iron Stores and Fiber Intake. Environ. Health Perspect. 1994, 102, 1058–1066. [Google Scholar] [CrossRef]
- Goon, D.; Klaassen, C.D. Dosage-Dependent Absorption of Cadmium in the Rat Intestine Measured in Situ. Toxicol. Appl. Pharmacol. 1989, 100, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kimura, O.; Hatakeyama, M.; Takada, M.; Sakata, M. Effects of Zinc and Copper on Cadmium Uptake by Brush Border Membrane Vesicles. Toxicol. Lett. 1997, 91, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Muto, Y. Effects of Dietary Protein, Calcium, Phosphorus and Fiber on Renal Accumulation of Exogenous Cadmium in Young Rats. J. Nutr. Sci. Vitaminol. 1977, 23, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.M.; Zhu, L.F.; Zhong, R.; Grant, D.; Goyer, R.A.; Cherian, M.G. Nephrotoxicity in Rats Following Liver Transplantation from Cadmium-Exposed Rats. Toxicol. Appl. Pharmacol. 1993, 123, 89–96. [Google Scholar] [CrossRef]
- Nordberg, M.; Elinder, C.G.; Rahnster, B. Cadmium, Zinc and Copper in Horse Kidney Metallothionein. Environ. Res. 1979, 20, 341–350. [Google Scholar] [CrossRef]
- Chan, D.Y.; Fry, N.; Waisberg, M.; Black, W.D.; Hale, B.A. Accumulation of Dietary Cadmium (Cd) in Rabbit Tissues and Excretions: A Comparison of Lettuce Amended with Soluble Cd Salt and Lettuce with Plant-Incorporated Cd. J. Toxicol. Environ. Health-Part A 2004, 67, 397–411. [Google Scholar] [CrossRef]
- Duarte, A.C.; Rosado, T.; Costa, A.R.; Santos, J.; Gallardo, E.; Quintela, T.; Ishikawa, H.; Schwerk, C.; Schroten, H.; Gonçalves, I.; et al. The Bitter Taste Receptor TAS2R14 Regulates Resveratrol Transport across the Human Blood-Cerebrospinal Fluid Barrier. Biochem. Pharmacol. 2020, 177, 113953. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; Hill, D.P.; et al. The Gene Ontology Knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B.; et al. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Feng, X.; Stein, L. A Human Functional Protein Interaction Network and Its Application to Cancer Data Analysis. Genome Biol. 2010, 11. [Google Scholar] [CrossRef]
- Behrens, M. International Union of Basic and Clinical Pharmacology. CXVII: Taste 2 Receptors—Structures, Functions, Activators, and Blockers. Pharmacol. Rev. 2025, 77, 100001. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.P.; Shah, S.D.; Michael, J.V.; Deshpande, D.A. Bitter Taste Receptors for Asthma Therapeutics. Front. Physiol. 2019, 10, 884. [Google Scholar] [CrossRef]
- Kertesz, Z.; Harrington, E.O.; Braza, J.; Guarino, B.D.; Chichger, H. Agonists for Bitter Taste Receptors T2R10 and T2R38 Attenuate LPS-Induced Permeability of the Pulmonary Endothelium in Vitro. Front. Physiol. 2022, 13, 794370. [Google Scholar] [CrossRef]
- Fotsing, J.R.; Darmohusodo, V.; Patron, A.P.; Ching, B.W.; Brady, T.; Arellano, M.; Chen, Q.; Davis, T.J.; Liu, H.; Servant, G.; et al. Discovery and Development of S6821 and S7958 as Potent TAS2R8 Antagonists. J. Med. Chem. 2020, 63, 4957–4977. [Google Scholar] [CrossRef]
- Ferdigg, A.; Hopp, A.K.; Wolf, G.; Superti-Furga, G. Membrane Transporters Modulating the Toxicity of Arsenic, Cadmium, and Mercury in Human Cells. Life Sci. Alliance 2024, 8, e202402866. [Google Scholar] [CrossRef]
- Franz, M.C.; Pujol-Giménez, J.; Montalbetti, N.; Fernandez-Tenorio, M.; Degrado, T.R.; Niggli, E.; Romero, M.F.; Hediger, M.A. Reassessment of the Transport Mechanism of the Human Zinc Transporter SLC39A2. Biochemistry 2018, 57, 3976–3986. [Google Scholar] [CrossRef]
- McNeill, R.V.; Mason, A.S.; Hodson, M.E.; Catto, J.W.F.; Southgate, J. Specificity of the Metallothionein-1 Response by Cadmium-Exposed Normal Human Urothelial Cells. Int. J. Mol. Sci. 2019, 20, 1344. [Google Scholar] [CrossRef]
- Sun, S.; Zhuang, P.; Li, Z.; Mo, H. Transmembrane and Transcriptome Analysis Reveals the Mechanism of Dietary Supplements in Reducing Cadmium Absorption and Toxicity. Environ. Technol. Innov. 2024, 36, 103771. [Google Scholar] [CrossRef]
- Seier Poulsen, S.; Kirkegaard, P.; Skov Olsen, P.; Kürstein Jensen, K.; Christiansen, J. Role of Delayed Gastric Emptying in the Pathogenesis of Cysteamine-Induced Duodenal Ulcer in the Rat. Scand. J. Gastroenterol. 1982, 17, 325–330. [Google Scholar] [CrossRef]
- Laboisse, C.L.; Augeron, C.; Potet, F.; Couturier-Turpin, M.H.; Gespach, C.; Cheret, A.M. Characterization of a Newly Established Human Gastric Cancer Cell Line Hgt-1 Bearing Histamine H2-Receptors. Cancer Res. 1982, 42, 1541–1548. [Google Scholar]
- Weiss, C.; Rubach, M.; Lang, R.; Seebach, E.; Blumberg, S.; Frank, O.; Hofmann, T.; Somoza, V. Measurement of the Intracellular Ph in Human Stomach Cells: A Novel Approach to Evaluate the Gastric Acid Secretory Potential of Coffee Beverages. J. Agric. Food Chem. 2010, 58, 1976–1985. [Google Scholar] [CrossRef]
- Meyer, S.; López-Serrano, A.; Mitze, H.; Jakubowski, N.; Schwerdtle, T. Single-Cell Analysis by ICP-MS/MS as a Fast Tool for Cellular Bioavailability Studies of Arsenite. Metallomics 2018, 10, 73–76. [Google Scholar] [CrossRef]
- Richter, P.; Sebald, K.; Fischer, K.; Schnieke, A.; Jlilati, M.; Mittermeier-Klessinger, V.; Somoza, V. Gastric Digestion of the Sweet-Tasting Plant Protein Thaumatin Releases Bitter Peptides That Reduce H. Pylori Induced pro-Inflammatory IL-17A Release via the TAS2R16 Bitter Taste Receptor. Food Chem. 2024, 448, 139157. [Google Scholar] [CrossRef]
- Danzer, B.; Jukic, M.; Dunkel, A.; Andersen, G.; Lieder, B.; Schaudy, E.; Stadlmayr, S.; Lietard, J.; Michel, T.; Krautwurst, D.; et al. Impaired Metal Perception and Regulation of Associated Human Foliate Papillae Tongue Transcriptome in Long-COVID-19. Sci. Rep. 2024, 14, 15408. [Google Scholar] [CrossRef]
- Rohm, B.; Holik, A.K.; Somoza, M.M.; Pignitter, M.; Zaunschirm, M.; Ley, J.P.; Krammer, G.E.; Somoza, V. Nonivamide, a Capsaicin Analog, Increases Dopamine and Serotonin Release in SH-SY5Y Cells via a TRPV1-Independent Pathway. Mol. Nutr. Food Res. 2013, 57, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Holik, A.K.; Rohm, B.; Somoza, M.M.; Somoza, V. Nε-Carboxymethyllysine (CML), a Maillard Reaction Product, Stimulates Serotonin Release and Activates the Receptor for Advanced Glycation End Products (RAGE) in SH-SY5Y Cells. Food Funct. 2013, 4, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Michel, T.; Giridhar, M.; Santhosh, S.; Das, A.; Sabzalipoor, H.; Keki, T.; Parlar, E.; Ilić, I.; Ibáñez-Redín, G.; et al. An Open-Source Advanced Maskless Synthesizer for Light-Directed Chemical Synthesis of Large Nucleic Acid Libraries and Microarrays. ChemRxiv 2024, 1–20. [Google Scholar] [CrossRef]
- Holik, A.K.; Schweiger, K.; Stoeger, V.; Lieder, B.; Reiner, A.; Zopun, M.; Hoi, J.K.; Kretschy, N.; Somoza, M.M.; Kriwanek, S.; et al. Gastric Serotonin Biosynthesis and Its Functional Role in L-Arginine-Induced Gastric Proton Secretion. Int. J. Mol. Sci. 2021, 22, 5881. [Google Scholar] [CrossRef] [PubMed]
- Hölz, K.; Hoi, J.K.; Schaudy, E.; Somoza, V.; Lietard, J.; Somoza, M.M. High-Efficiency Reverse (5’→3’) Synthesis of Complex DNA Microarrays. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bolstad, B.M.; Irizarry, R.A.; Åstrand, M.; Speed, T.P. A Comparison of Normalization Methods for High Density Oligonucleotide Array Data Based on Variance and Bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Gao, C.; Dusa, A. GgVennDiagram: A “ggplot2” Implement of Venn Diagram 2025. Available online: https://cran.r-project.org/package=venn (accessed on 18 July 2025).
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping Identifiers for the Integration of Genomic Datasets with the R/Bioconductor Package BiomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orth, H.N.; Pirkwieser, P.; Giridhar, M.; Boger, V.; Somoza, M.M.; Dunkel, A.; Somoza, V. Bitter Taste Receptors TAS2R8 and TAS2R10 Reduce Proton Secretion and Differentially Modulate Cadmium Uptake in Immortalized Human Gastric Cells. Int. J. Mol. Sci. 2025, 26, 9166. https://doi.org/10.3390/ijms26189166
Orth HN, Pirkwieser P, Giridhar M, Boger V, Somoza MM, Dunkel A, Somoza V. Bitter Taste Receptors TAS2R8 and TAS2R10 Reduce Proton Secretion and Differentially Modulate Cadmium Uptake in Immortalized Human Gastric Cells. International Journal of Molecular Sciences. 2025; 26(18):9166. https://doi.org/10.3390/ijms26189166
Chicago/Turabian StyleOrth, H. Noreen, Philip Pirkwieser, Maya Giridhar, Valerie Boger, Mark M. Somoza, Andreas Dunkel, and Veronika Somoza. 2025. "Bitter Taste Receptors TAS2R8 and TAS2R10 Reduce Proton Secretion and Differentially Modulate Cadmium Uptake in Immortalized Human Gastric Cells" International Journal of Molecular Sciences 26, no. 18: 9166. https://doi.org/10.3390/ijms26189166
APA StyleOrth, H. N., Pirkwieser, P., Giridhar, M., Boger, V., Somoza, M. M., Dunkel, A., & Somoza, V. (2025). Bitter Taste Receptors TAS2R8 and TAS2R10 Reduce Proton Secretion and Differentially Modulate Cadmium Uptake in Immortalized Human Gastric Cells. International Journal of Molecular Sciences, 26(18), 9166. https://doi.org/10.3390/ijms26189166





