Exploring Cannabinoid Effects Using Zebrafish (Danio rerio) as an In Vivo Model: A Review of the Literature
Abstract
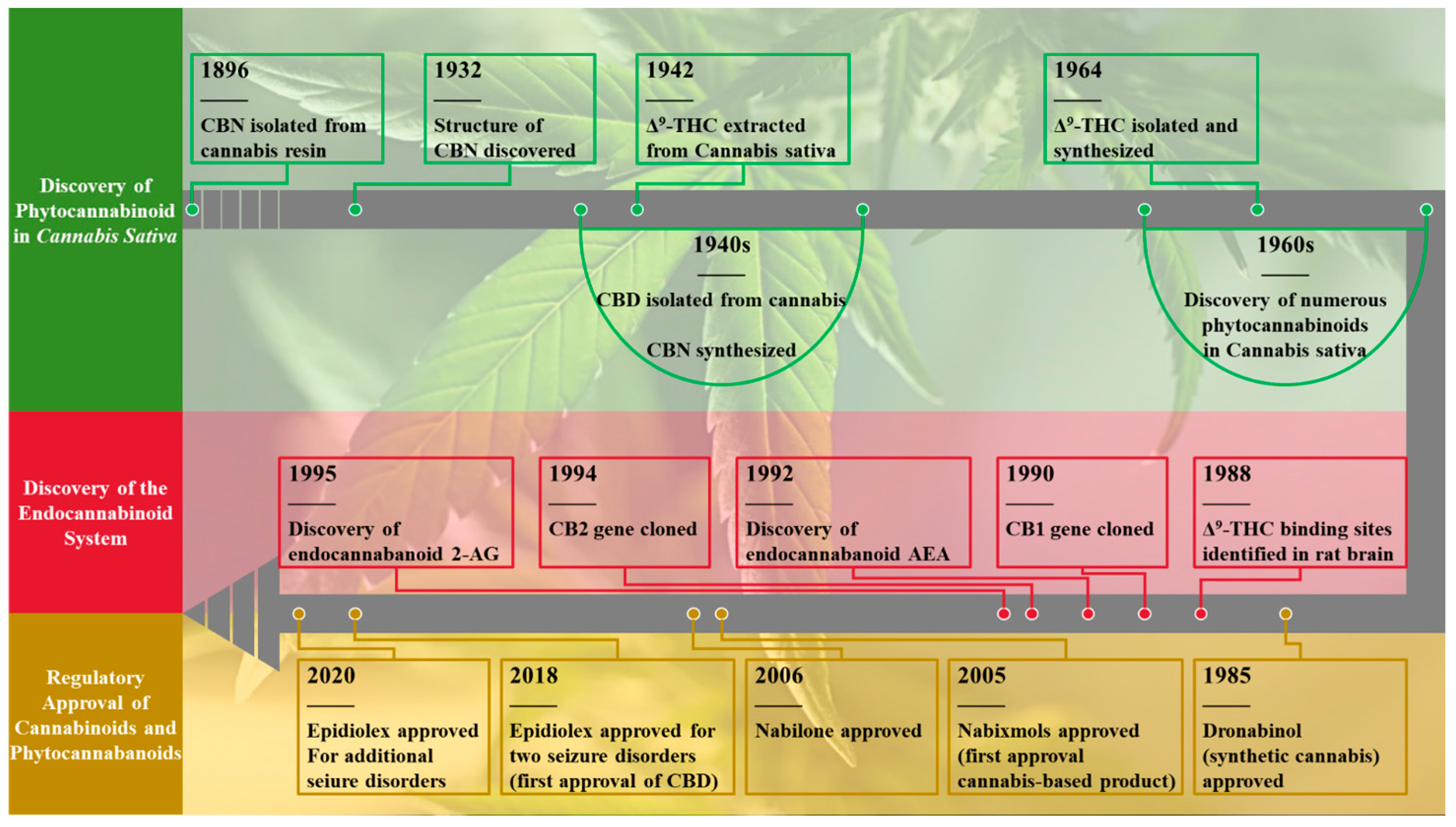
1. Introduction
2. Types of Cannabinoids
2.1. Phytocannabinoids (PCs)
2.2. Endogenous Cannabinoids or Endocannabinoids (eCB, or ECs)
2.3. Synthetic Cannabinoids (SCs)
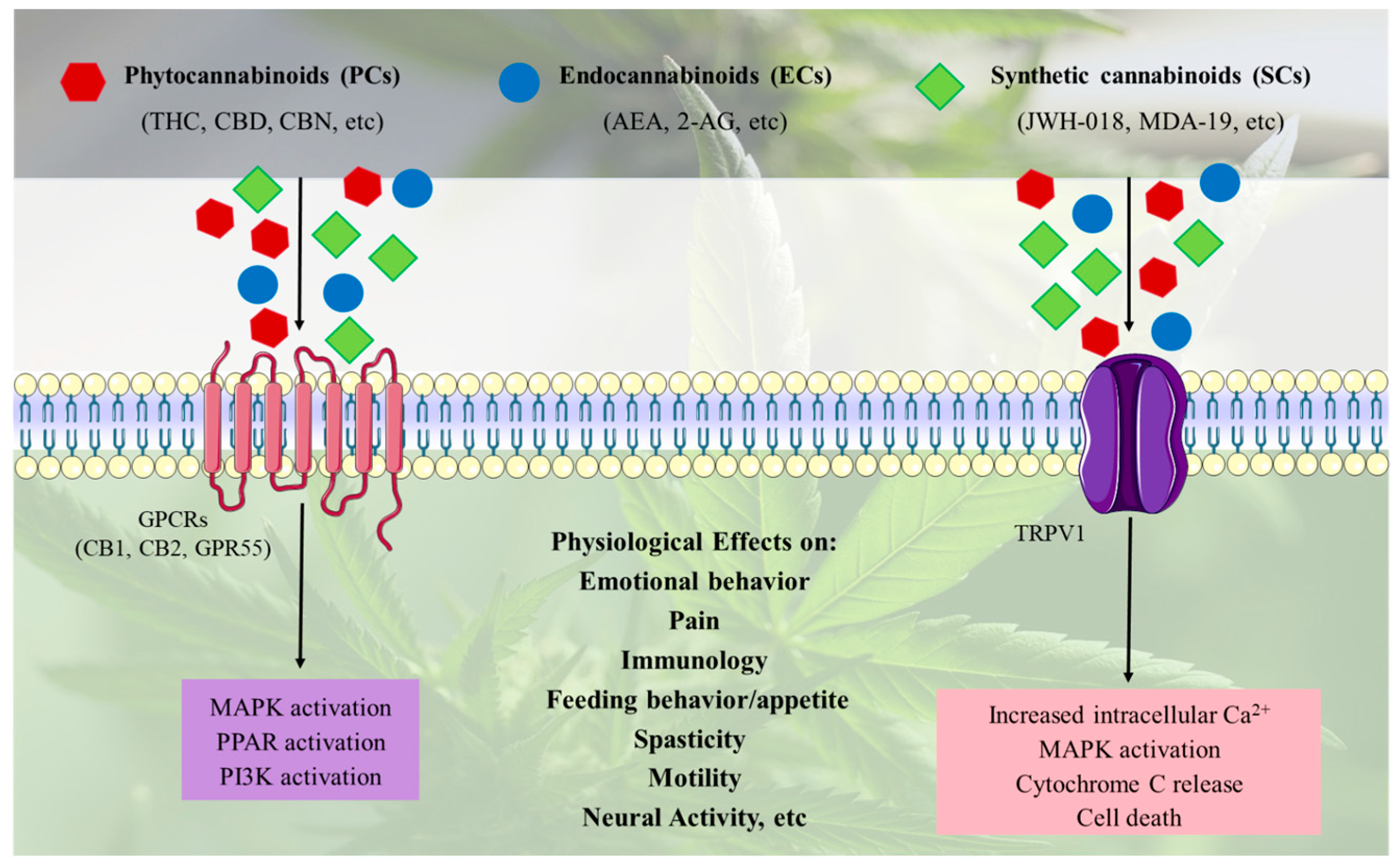
2.4. Cannabinoid Signaling Pathways and Effects
3. Zebrafish as a Translational Model for Cannabinoids-Induced Toxicological Profiling and Therapeutic Potential Evaluation
3.1. Human Cannabinoid Receptors
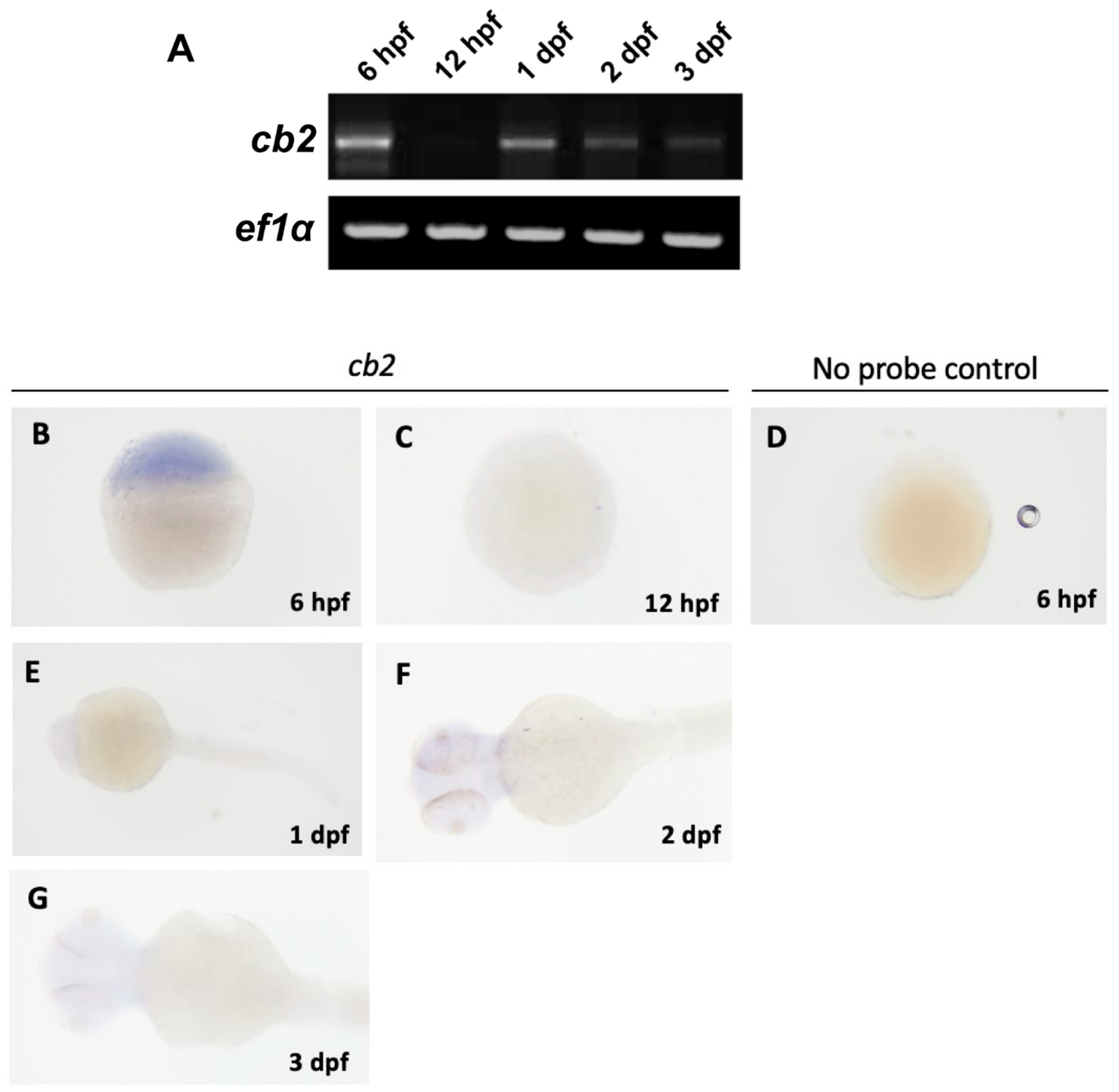
3.2. Zebrafish Cannabinoids Receptors
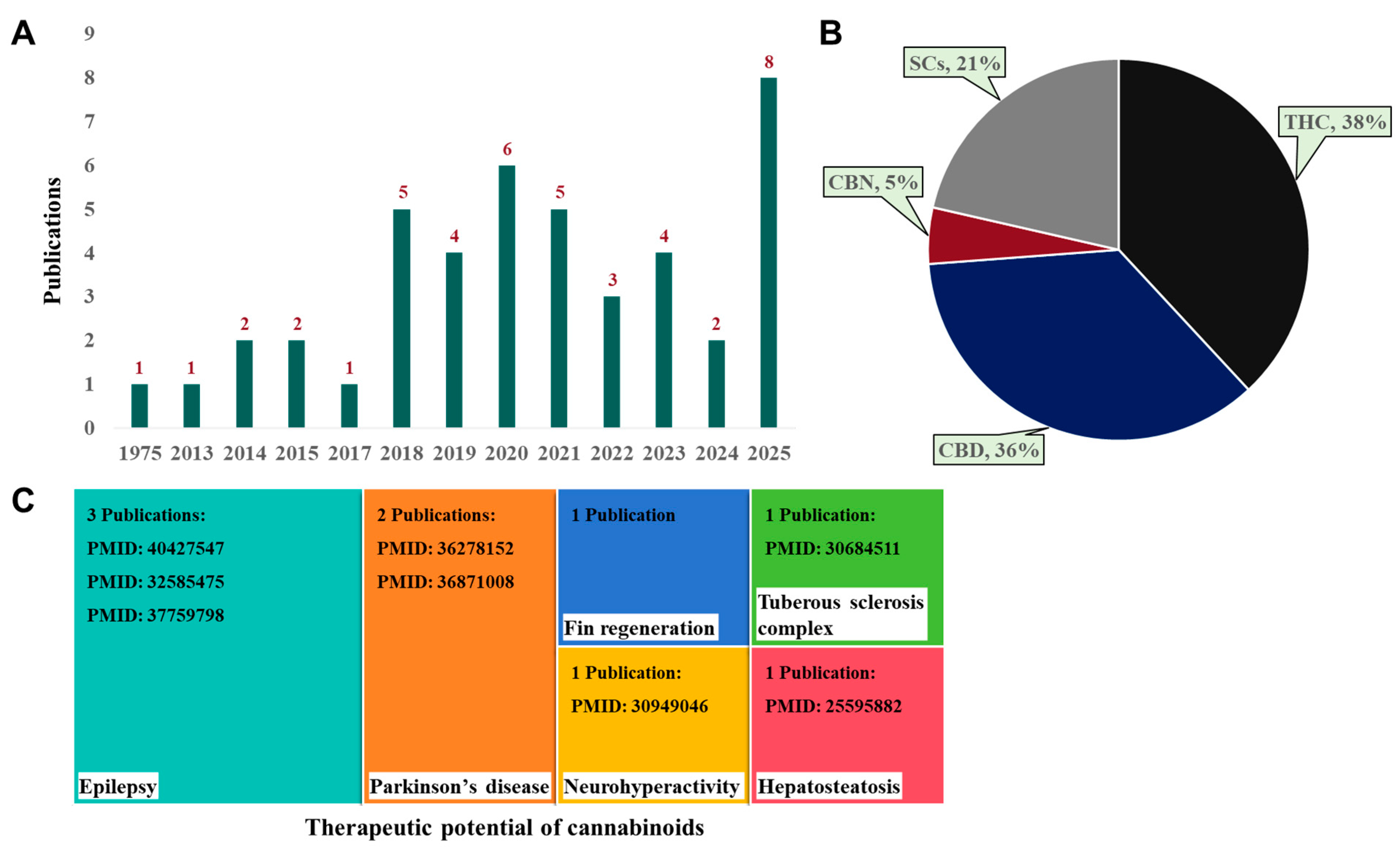
4. Searching Strategy
5. Effect of PCs Using Zebrafish as an In Vivo Model
5.1. Toxicity of THC
5.1.1. Developmental Toxicity of THC
5.1.2. Behavioral Effects and Neurotoxicity of THC
5.1.3. Multigenerational Effects of THC Exposure
5.2. Toxicity of CBD
5.2.1. Behavioral Effects and Neurotoxicity of CBD
5.2.2. Hepatotoxicity of CBD
5.2.3. Reproductive Toxicity of CBD
5.3. Toxicity of CBN
5.4. Comparative Toxicity of THC, CBD, and CBN
6. Effect of SCs Using Zebrafish as an In Vivo Model
7. Therapeutic Potential of Cannabinoids in Zebrafish Disease Models
7.1. Fin Regeneration and Anti-Apoptotic Effects
7.2. Parkinson’s Disease
7.3. Behavioral Hyperactivity and Neuroprotection
7.4. Tuberous Sclerosis Complex (TSC)
7.5. Antiseizure Activity in Epilepsy Models
8. Perspective
9. Conclusions
10. Limitations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borgan, F.; Beck, K.; Butler, E.; McCutcheon, R.; Veronese, M.; Vernon, A.; Howes, O.D. The effects of cannabinoid 1 receptor compounds on memory: A meta-analysis and systematic review across species. Psychopharmacology 2019, 236, 3257–3270. [Google Scholar] [CrossRef]
- Brill, H. Marihuana: The First Twelve Thousand Years. J. Psychoact. Drugs 1981, 13, 397–398. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The synthetic cannabinoids phenomenon: From structure to toxicological properties. A review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Sahu, P.K.; Behera, A.; Feehan, J.; Mishra, D.P.; Apostolopoulos, V. Cannabinoids and the male reproductive system: Implications of endocannabinoid signaling pathways. Maturitas 2025, 192, 108156. [Google Scholar] [CrossRef] [PubMed]
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef]
- Caprioglio, D.; Mattoteia, D.; Pollastro, F.; Negri, R.; Lopatriello, A.; Chianese, G.; Minassi, A.; Collado, J.A.; Munoz, E.; Taglialatela-Scafati, O.; et al. The Oxidation of Phytocannabinoids to Cannabinoquinoids. J. Nat. Prod. 2020, 83, 1711–1715. [Google Scholar] [CrossRef]
- Wollner, H.; Matchett, J.R.; Levine, J.; Loewe, S. Isolation of a physiologically active tetrahydrocannabinol from Cannabis sativa resin. J. Am. Chem. Soc. 1942, 64, 26–29. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Martinez, A.S.; Lanaridi, O.; Stagel, K.; Halbwirth, H.; Schnürch, M.; Bica-Schröder, K. Extraction techniques for bioactive compounds of cannabis. Nat. Prod. Rep. 2023, 40, 676–717. [Google Scholar] [CrossRef] [PubMed]
- Maurya, N.; Velmurugan, B.K. Therapeutic applications of cannabinoids. Chem. Biol. Interact. 2018, 293, 77–88. [Google Scholar] [CrossRef]
- Keating, G.M. Delta-9-Tetrahydrocannabinol/Cannabidiol Oromucosal Spray (Sativex(®)): A Review in Multiple Sclerosis-Related Spasticity. Drugs 2017, 77, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, D.; Langley, J.; Hartkopf, K.; Hawk, L.; Margolis, A.; Struck, A.; Felton, E.; Hsu, D.; Gidal, B.E. Real-world, long-term evaluation of the tolerability and therapy retention of Epidiolex® (cannabidiol) in patients with refractory epilepsy. Epilepsy Behav. 2023, 141, 109159. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System, Biological psychiatry. Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Devane, W.A.; Dysarz, F.A., 3rd; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar] [CrossRef]
- Teixeira, H.M. Phytocanabinoids and synthetic cannabinoids: From recreational consumption to potential therapeutic use—A review. Front. Toxicol. 2024, 6, 1495547. [Google Scholar] [CrossRef]
- Bajtel, Á.; Kiss, T.; Tóth, B.; Kiss, S.; Hegyi, P.; Vörhendi, N.; Csupor-Löffler, B.; Gede, N.; Hohmann, J.; Csupor, D. The Safety of Dronabinol and Nabilone: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmaceuticals 2022, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, H.N.; Leem, T.-S.; Jeon, J.-H.; Cho, S.; Lee, J. Baek, Identification of new synthetic cannabinoid analogue APINAC (adamantan-1-yl 1-pentyl-1H-indazole-3-carboxylate) with other synthetic cannabinoid MDMB (N)-Bz-F in illegal products. Forensic Toxicol. 2017, 35, 45–55. [Google Scholar] [CrossRef]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef]
- Bailone, R.L.; Fukushima, H.C.S.; de Aguiar, L.K.; Borra, R.C. The endocannabinoid system in zebrafish and its potential to study the effects of Cannabis in humans. Lab. Anim. Res. 2022, 38, 5. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Migliarini, B.; Carnevali, O. A novel role for the endocannabinoid system during zebrafish development. Mol. Cell. Endocrinol. 2009, 299, 172–177. [Google Scholar] [CrossRef]
- Son, H.-W. Characterizing Endocannabinoid System Development in Zebrafish and Investigating Cannabidiol-Mediated Downregulation of the Sonic Hedgehog Pathway. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2021. [Google Scholar]
- Son, H.W.; Ali, D.W. Endocannabinoid Receptor Expression in Early Zebrafish Development. Dev. Neurosci. 2022, 44, 142–152. [Google Scholar] [CrossRef]
- Oltrabella, F.; Melgoza, A.; Nguyen, B.; Guo, S. Role of the endocannabinoid system in vertebrates: Emphasis on the zebrafish model. Dev. Growth Differ. 2017, 59, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Rastegar, S.; Strähle, U. Distribution of cannabinoid receptor 1 in the CNS of zebrafish. Neuroscience 2006, 138, 83–95. [Google Scholar] [CrossRef]
- Thomas, R.J. The toxicologic and teratologic effects of delta-9-tetrahydrocannabinol in the zebrafish embryo. Toxicol. Appl. Pharmacol. 1975, 32, 184–190. [Google Scholar] [CrossRef]
- Carty, D.R.; Thornton, C.; Gledhill, J.H.; Willett, K.L. Developmental Effects of Cannabidiol and Δ9-Tetrahydrocannabinol in Zebrafish. Toxicol. Sci. 2018, 162, 137–145. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Ali, S.; Rashidi, H.; van der Kooy, F.; Verpoorte, R.; Richardson, M.K. Developmental effects of cannabinoids on zebrafish larvae. Zebrafish 2013, 10, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.T.; Mushtaq, M.Y.; Verpoorte, R.; Richardson, M.K.; Choi, Y.H. Metabolic effects of cannabinoids in zebrafish (Danio rerio) embryos determined by 1 H NMR. Metabolomics 2016, 12, 44. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Chousidis, I.; Leonardos, D.; Stalikas, C.; Leonardos, I. In the Swim of Cannabis: Developmental Toxicity and Metabolomic Pathway Alterations of Zebrafish Larvae Exposed to THC for the Assessment of Its Potential Environmental and Human Health Impact. Molecules 2022, 27, 5506. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, H.; Li, C.-Z.; Lai, P.-S.; Wang, G.; Chan, Y.S.; Cheng, S.H.; Chen, X. Cannabidiol promotes fin regeneration and reduces apoptosis in zebrafish embryos. J. Funct. Foods 2021, 86, 104694. [Google Scholar] [CrossRef]
- Li, L.; Fan, B.; Zhang, Y.; Zhao, M.; Kong, Z.; Wang, F.; Li, M. Cannabidiol exposure during embryonic period caused serious malformation in embryos and inhibited the development of reproductive system in adult zebrafish. Sci. Total Environ. 2024, 950, 175315. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, X.; Li, Y.; Guo, Y.; Zhang, S.; Jin, J.; Li, J.; Wu, D. Toxicological mechanism of cannabidiol (CBD) exposure on zebrafish embryonic development. Food Chem. Toxicol. 2024, 193, 114929. [Google Scholar] [CrossRef]
- Chousidis, I.; Chatzimitakos, T.; Leonardos, D.; Filiou, M.D.; Stalikas, C.D.; Leonardos, I.D. Cannabinol in the spotlight: Toxicometabolomic study and behavioral analysis of zebrafish embryos exposed to the unknown cannabinoid. Chemosphere 2020, 252, 126417. [Google Scholar] [CrossRef] [PubMed]
- Kullebi, B.; Alat, Ö.; Aksakal, Ö.; Yılmaztürk, D.; Lafzi, A.; Şişman, T. Embryotoxicity Evaluation of Novel Synthetic Cannabinoid 4F-MDMB-BUTICA Using Zebrafish Embryos. J. Appl. Toxicol. 2025, 45, 1314–1330. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, F.; Zhou, L.; Shen, Y.; Wang, A.; Qin, Y.; Wang, J.; Yao, W. ADB-FUBINACA-induced developmental toxicity; neurotoxicity, and cardiotoxicity in embryonic zebrafish (Danio rerio). Environ. Res. 2025, 276, 121517. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Godoi, A.B.; Fais, V.C.; Peterson, R.T.; Maurer-Morelli, C.V.; Costa, J.L. Zebrafish embryo-larval testing reveals differential toxicity of new psychoactive substances. Toxicol. Rep. 2025, 14, 102018. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, J.C.; Hill, J.; Hui, J.P.M.; Morash, M.G.; Berrue, F.; Ellis, L.D. Analysis of the Uptake, Metabolism, and Behavioral Effects of Cannabinoids on Zebrafish Larvae. Zebrafish 2018, 15, 349–360. [Google Scholar] [CrossRef]
- Pandelides, Z.; Thornton, C.; Lovitt, K.G.; Faruque, A.S.; Whitehead, A.P.; Willett, K.L.; Ashpole, N.M. Developmental exposure to Δ(9)-tetrahydrocannabinol (THC) causes biphasic effects on longevity. inflammation, and reproduction in aged zebrafish (Danio rerio). Geroscience 2020, 42, 923–936. [Google Scholar] [CrossRef]
- Carty, D.R.; Miller, Z.S.; Thornton, C.; Pandelides, Z.; Kutchma, M.L.; Willett, K.L. Multigenerational consequences of early-life cannabinoid exposure in zebrafish. Toxicol. Appl. Pharmacol. 2019, 364, 133–143. [Google Scholar] [CrossRef]
- Amin, M.R.; Ahmed, K.T.; Ali, D.W. Early Exposure to THC Alters M-Cell Development in Zebrafish Embryos. Biomedicines 2020, 8, 5. [Google Scholar] [CrossRef]
- Thornton, C.; Dickson, K.E.; Carty, D.R.; Ashpole, N.M.; Willett, K.L. Cannabis constituents reduce seizure behavior in chemically-induced and scn1a-mutant zebrafish. Epilepsy Behav. 2020, 110, 107152. [Google Scholar] [CrossRef]
- Kanyo, R.; Amin, M.R.; Locskai, L.F.; Bouvier, D.D.; Olthuis, A.M.; Allison, W.T.; Ali, D.W. Medium-throughput zebrafish optogenetic platform identifies deficits in subsequent neural activity following brief early exposure to cannabidiol and Δ(9)-tetrahydrocannabinol. Sci. Rep. 2021, 11, 11515. [Google Scholar]
- Razmara, P.; Zaveri, D.; Thannhauser, M.; Ali, D.W. Acute effect of Δ-9-tetrahydrocannabinol on neuromuscular transmission and locomotive behaviors in larval zebrafish. J. Neurophysiol. 2023, 129, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.T.; Amin, M.R.; Shah, P.; Ali, D.W. Motor neuron development in zebrafish is altered by brief (5-hr) exposures to THC (∆(9)-tetrahydrocannabinol) or CBD (cannabidiol) during gastrulation. Sci. Rep. 2018, 8, 10518. [Google Scholar] [CrossRef]
- Amin, M.R.; Khara, L.; Szaszkiewicz, J.; Kim, A.M.; Hamilton, T.J.; Ali, D.W. Brief exposure to (-) THC affects zebrafish embryonic locomotion with effects that persist into the next generation. Sci. Rep. 2025, 15, 2203. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, T.; Prinz, N.; Oellers, N.; Seidel, N.I.; Jonas, A.; Albayram, O.; Bilkei-Gorzo, A.; von der Emde, G. Acute administration of THC impairs spatial but not associative memory function in zebrafish. Psychopharmacology 2014, 231, 3829–3842. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, T.; Zeymer, M.; von der Emde, G. Cannabinoid modulation of zebrafish fear learning and its functional analysis investigated by c-Fos expression. Pharmacol. Biochem. Behav. 2017, 153, 18–31. [Google Scholar] [CrossRef]
- Stewart, A.M.; Kalueff, A.V. The behavioral effects of acute Δ9-tetrahydrocannabinol and heroin (diacetylmorphine) exposure in adult zebrafish. Brain Res. 2014, 1543, 109–119. [Google Scholar] [CrossRef]
- Dahlén, A.; Zarei, M.; Melgoza, A.; Wagle, M.; Guo, S. THC-induced behavioral stereotypy in zebrafish as a model of psychosis-like behavior. Sci. Rep. 2021, 11, 15693. [Google Scholar] [CrossRef]
- Brigante, T.A.V.; Abe, F.R.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.S.; de Oliveira, D.P. Cannabidiol did not induce teratogenicity or neurotoxicity in exposed zebrafish embryos. Chem. Biol. Interact. 2018, 291, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Hasumi, A.; Maeda, H.; Yoshida, K.I. Analyzing cannabinoid-induced abnormal behavior in a zebrafish model. PLoS ONE 2020, 15, e0236606. [Google Scholar] [CrossRef]
- Tomko, A.; O’Leary, L.; Trask, H.; Achenbach, J.C.; Hall, S.R.; Goralski, K.B.; Ellis, L.D.; Dupré, D.J. Antitumor Activity of Abnormal Cannabidiol and Its Analog O-1602 in Taxol-Resistant Preclinical Models of Breast Cancer. Front. Pharmacol. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Nazario, L.R.; Antonioli, R., Jr.; Capiotti, K.M.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Bonan, C.D.; da Silva, R.S. Caffeine protects against memory loss induced by high and non-anxiolytic dose of cannabidiol in adult zebrafish (Danio rerio). Pharmacol. Biochem. Behav. 2015, 135, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Razmara, P.; Son, H.-W.; Ali, D.W. Cannabidiol exerts teratogenic effects on developing zebrafish through the sonic hedgehog signaling pathway. Sci Rep. 2025, 15, 14533. [Google Scholar] [CrossRef]
- Kollipara, R.; Langille, E.; Tobin, C.; French, C.R. Phytocannabinoids Reduce Seizures in Larval Zebrafish and Affect Endocannabinoid Gene Expression. Biomolecules 2023, 13, 1398. [Google Scholar] [CrossRef]
- Jensen, H.M.; Korbut, R.; Kania, P.W.; Buchmann, K. Cannabidiol effects on behaviour and immune gene expression in zebrafish (Danio rerio). PLoS ONE 2018, 13, e0200016. [Google Scholar] [CrossRef]
- Li, L.; Fan, B.; Kong, Z.; Zhang, Y.; Zhao, M.; Simal-Gandara, J.; Wang, F.; Li, M. Short-term exposure of Cannabidiol on Zebrafish (Danio Rerio): Reproductive Toxicity. Environ. Sci. Pollut. Res. Int. 2023, 30, 75668–75680. [Google Scholar] [CrossRef]
- Amin, M.R.; Ahmed, K.T.; Ali, D.W. Cannabinoid receptor 2 (Cb2r) mediates cannabinol (CBN) induced developmental defects in zebrafish. Sci. Rep. 2022, 12, 20251. [Google Scholar] [CrossRef]
- Hutchings, D.E.; Martin, B.R.; Gamagaris, Z.; Miller, N.; Fico, T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989, 44, 697–701. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Wang, X.; Anderson, V.; Beck, O.; Minkoff, H.; Dow-Edwards, D. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol. Teratol. 2005, 27, 221–229. [Google Scholar] [CrossRef]
- Sainz-Cort, A.; Jimenez-Garrido, D.; Muñoz-Marron, E.; Viejo-Sobera, R.; Heeroma, J.; Bouso, J.C. Opposite Roles for Cannabidiol and δ-9-Tetrahydrocannabinol in Psychotomimetic Effects of Cannabis Extracts: A Naturalistic Controlled Study. J. Clin. Psychopharmacol. 2021, 41, 561–570. [Google Scholar] [CrossRef]
- Lo, L.A.; Christiansen, A.; Eadie, L.; Strickland, J.C.; Kim, D.D.; Boivin, M.; Barr, A.M.; MacCallum, C.A. Cannabidiol-associated hepatotoxicity: A systematic review and meta-analysis. J. Intern. Med. 2023, 293, 724–752. [Google Scholar] [CrossRef] [PubMed]
- Eadie, L.; Lo, L.A.; Boivin, M.; Deol, J.K.; MacCallum, C.A. Clinical guidance for cannabidiol-associated hepatotoxicity: A narrative review. J. Gastroenterol. Hepatol. 2024, 39, 2522–2532. [Google Scholar] [CrossRef]
- Somvanshi, R.K.; Zou, S.; Kadhim, S.; Padania, S.; Hsu, E.; Kumar, U. Cannabinol modulates neuroprotection and intraocular pressure: A potential multi-target therapeutic intervention for glaucoma. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166325. [Google Scholar] [CrossRef]
- Schubert, D.; Kepchia, D.; Liang, Z.; Dargusch, R.; Goldberg, J.; Maher, P. Efficacy of Cannabinoids in a Pre-Clinical Drug-Screening Platform for Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 7719–7730. [Google Scholar] [CrossRef]
- Di Meo, C.; Tortolani, D.; Standoli, S.; Ciaramellano, F.; Angelucci, B.C.; Tisi, A.; Kadhim, S.; Hsu, E.; Rapino, C.; Maccarrone, M. Cannabinol modulates the endocannabinoid system and shows TRPV1-mediated anti-inflammatory properties in human keratinocytes. BioFactors 2025, 51, e2122. [Google Scholar] [CrossRef] [PubMed]
- Pulver, B.; Fischmann, S.; Gallegos, A.; Christie, R. EMCDDA framework and practical guidance for naming synthetic cannabinoids. Drug Test. Anal. 2023, 15, 255–276. [Google Scholar] [CrossRef] [PubMed]
- García-González, J.; de Quadros, B.; Havelange, W.; Brock, A.J.; Brennan, C.H. Behavioral Effects of Developmental Exposure to JWH-018 in Wild-Type and Disrupted in Schizophrenia 1 (disc1) Mutant Zebrafish. Biomolecules 2021, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yan, J.; Zhou, Y.; Zhang, F.; Wang, B.; Wang, J.; Wu, Y.; Xu, Y. Effects of MDA-19 on Zebrafish Larval Behavior: Perspectives from Neurodevelopment, Oxidative Stress, and Metabolomics. J. Appl. Toxicol. 2025, 45, 440–451. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, J.; Fu, X.; Zhou, B.; Xu, Z.; Huang, H.; Han, S.; Li, X. Analysis of synthetic cannabinoids in wastewater of major cities in China. Sci. Total Environ. 2022, 827, 154267. [Google Scholar] [CrossRef]
- Lobato-Freitas, C.; Brito-da-Costa, A.M.; Dinis-Oliveira, R.J.; Carmo, H.; Carvalho, F.; Silva, J.P.; Dias-da-Silva, D. Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications. Pharmaceuticals 2021, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- De Morais, J.; Brandt, S.; Jorge, R.; Christie, R.; Gallegos, A.; Sedefov, R.; Evans-Brown, M. EMCDDA Technical Report on the New Psychoactive Substance Methyl 3, 3-dimethyl-2-{[1-(pent-4-en-1-yl)-1H-indazole-3-carbonyl] amino} Butanoate (MDMB-4en-PINACA). 2020. Available online: https://researchonline.ljmu.ac.uk/id/eprint/14252/ (accessed on 15 September 2025).
- Morbiato, E.; Bilel, S.; Tirri, M.; Arfè, R.; Fantinati, A.; Savchuk, S.; Appolonova, S.; Frisoni, P.; Tagliaro, F.; Neri, M.; et al. Potential of the zebrafish model for the forensic toxicology screening of NPS: A comparative study of the effects of APINAC and methiopropamine on the behavior of zebrafish larvae and mice. Neurotoxicology 2020, 78, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Markin, P.A.; Brito, A.; Moskaleva, N.E.; Tagliaro, F.; La Frano, M.R.; Savitskii, M.V.; Appolonova, S.A. Short- and long-term exposures of the synthetic cannabinoid 5F-APINAC induce metabolomic alterations associated with neurotransmitter systems and embryotoxicity confirmed by teratogenicity in zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 243, 109000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Shi, Y.; Tan, S.; Wang, X.; Yuan, W.; Tao, S.; Xiang, P.; Cong, B.; Ma, C.; Wen, D. Unveiling the toxicity of JWH-018 and JWH-019: Insights from behavioral and molecular studies in vivo and vitro. Ecotoxicol. Environ. Saf. 2025, 289, 117500. [Google Scholar] [CrossRef]
- Park, Y.M.; Dahlem, C.; Meyer, M.R.; Kiemer, A.K.; Müller, R.; Herrmann, J. Induction of Liver Size Reduction in Zebrafish Larvae by the Emerging Synthetic Cannabinoid 4F-MDMB-BINACA and Its Impact on Drug Metabolism. Molecules 2022, 27, 1290. [Google Scholar] [CrossRef]
- Salles, É.L.; Baban, B.; Qin, X.; Paffaro, V. Editorial: Therapeutic potential of cannabinoids: From health to disease. Front. Neurosci. 2025, 19, 1565790. [Google Scholar] [CrossRef]
- Serra, I.; Scheldeman, C.; Bazelot, M.; Whalley, B.J.; Dallas, M.L.; de Witte, P.A.M.; Williams, C.M. Cannabidiol modulates phosphorylated rpS6 signalling in a zebrafish model of Tuberous Sclerosis Complex. Behav. Brain Res. 2019, 363, 135–144. [Google Scholar] [CrossRef]
- Morash, M.G.; Nixon, J.; Shimoda, L.M.N.; Turner, H.; Stokes, A.J.; Small-Howard, A.L.; Ellis, L.D. Identification of minimum essential therapeutic mixtures from cannabis plant extracts by screening in cell and animal models of Parkinson’s disease. Front. Pharmacol. 2022, 13, 907579. [Google Scholar] [CrossRef]
- Hasumi, A.; Maeda, H. Cannabidiol improves haloperidol-induced motor dysfunction in zebrafish: A comparative study with a dopamine activating drug. J. Cannabis Res. 2023, 5, 6. [Google Scholar] [CrossRef]
- Samarut, É.; Nixon, J.; Kundap, U.P.; Drapeau, P.; Ellis, L.D. Single and Synergistic Effects of Cannabidiol and Δ-9-Tetrahydrocannabinol on Zebrafish Models of Neuro-Hyperactivity. Front. Pharmacol. 2019, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Shabat-Simon, M.; On, J.B.; Steckler, R.; Khatib, S.; Tamir, S.; Pitashny, P. Anticonvulsant Effects of Different Cannabis Extracts in a Zebrafish Model of Epilepsy. Biomolecules 2025, 15, 654. [Google Scholar] [CrossRef]
- Silvestri, C.; Paris, D.; Martella, A.; Melck, D.; Guadagnino, I.; Cawthorne, M.; Motta, A.; Di Marzo, V. Two non-psychoactive cannabinoids reduce intracellular lipid levels and inhibit hepatosteatosis. J. Hepatol. 2015, 62, 1382–1390. [Google Scholar] [CrossRef]
- Thiele, E.A.; Bebin, E.M.; Bhathal, H.; Jansen, F.E.; Kotulska, K.; Lawson, J.A.; O’Callaghan, F.J.; Wong, M.; Sahebkar, F.; Checketts, D.; et al. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurol. 2021, 78, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Jones, N.A.; Cunningham, M.O.; Jayasekera, B.A.P.; Devore, S.; Whalley, B.J. Cannabinoid treatments in epilepsy and seizure disorders. Physiol. Rev. 2024, 104, 591–649. [Google Scholar] [CrossRef] [PubMed]


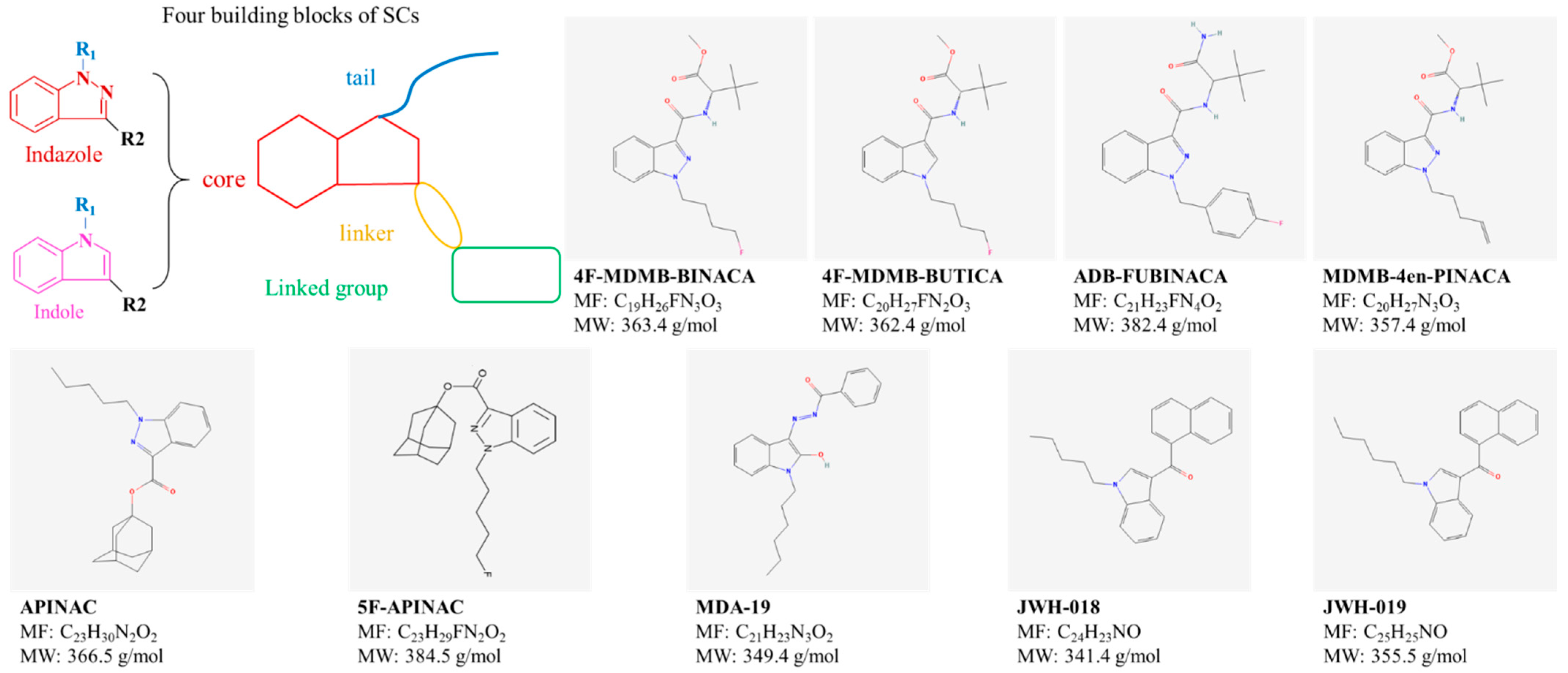
| Cannabinoids | Category | Select Compounds | CAS NO. * | Molecular Formula (MF) * | Molecular Weight (MW, g/mol) * |
|---|---|---|---|---|---|
| Phytocannabinoids | Psychoactive | delta-9-tetrahydrocannabinol (Δ9-THC) | 7663-50-5 | C21H30O2 | 314.4 |
| Cannabinol (CBN) | 521-35-7 | C21H26O2 | 310.4 | ||
| Non-psychoactive | Cannabidiol (CBD) | 13956-29-1 | C21H30O2 | 314.4 | |
| Cannabichromene (CBC) | 20675-51-8 | C21H30O2 | 314.4 | ||
| Cannabigerol (CBG) | 25654-31-3 | C21H32O2 | 316.5 | ||
| Cannabidivarin (CBDV) | 24274-48-4 | C19H26O2 | 286.4 | ||
| Endocannabinoids | Major | Arachidonylethanolamine (AEA) | 94421-68-8 | C22H37NO2 | 347.5 |
| 2-arachidonoyl glycerol (2-AG) | 53847-30-6 | C23H38O4 | 378.5 | ||
| Minor | Noladin ether | 222723-55-9 | C23H40O3 | 364.6 | |
| Virodhamine | 287937-12-6 | C22H37O2 | 347.5 | ||
| N-Arachidonyl dopamine (NADA) | 199875-69-9 | C28H41NO3 | 439.6 | ||
| Oleamide | 301-02-0 | C18H35NO | 281.5 | ||
| Synthetic cannabinoids | / | MDA-19 | 1048973-47-2 | C21H23N3O2 | 349.4 |
| JWH-018 | 209414-07-3 | C24H23NO | 341.4 | ||
| JWH-019 | 209414-08-4 | C25H25NO | 355.5 | ||
| APINAC | 2219331-93-6 | C23H30N2O2 | 366.5 | ||
| 5F-APINAC | 2365471-88-9 | C23H29FN2O2 | 384.5 | ||
| ADB-FUBINACA | 1445583-51-6 | C21H23FN4O2 | 382.4 | ||
| MDMB-4en-PINACA | 2504100-70-1 | C20H27N3O3 | 357.4 | ||
| 4F-MDMB-BINACA | 2390036-46-9 | C19H26FN3O3 | 363.4 | ||
| 4F-MDMB-BUTICA | 2682867-53-2 | C20H27FN2O3 | 362.4 |
| Drug | Active Pharmaceutical Ingredient | Medical Conditions | Company | Country and Year Approved |
|---|---|---|---|---|
| Epidiolex | Nabiximols (CBD) | Seizures associated with Lennox–Gastaut syndrome and Dravet syndrome | Greenwich Biosciences | USA 2018, EU 2019 |
| Sativex | 2.7 mg/mL of Δ9-THC and 2.5 mg/mL of CBD (approx. equal quantities of THC and CBD) | Neuropathic pain, spasticity, overactive bladder, and other symptoms of multiple sclerosis | GW Pharmaceuticals | Canada 2005, UK 2010, Spain 2010, Germany 2011, Denmark 2011, Sweden 2012, Australia 2012 |
| Marinol | Dronabinol (synthetic Δ9-THC) | HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting | Unimed Pharmaceuticals | USA 1985 |
| Cesamet | Nabilone (synthetic Δ9-THC) | Nausea, Mutiple sclerosis, Fibromyalgla | Valeant Pharmaceuticals | USA 1985/2006 |
| Syndros | Dronabinol (synthetic Δ9-THC in liquid formulation) | Nausea and vomiting caused by chemotherapy, loss of appetite | Insys Therapeutics | USA 2006 |
| Cannabinoid | Concentrations for LC50 Calculation | LC50 | Refs. |
|---|---|---|---|
| THC | 0.3, 0.6, 1.25, 2.5, 5 mg/L | 3.65 mg/L (11.61 µM) | [28] |
| 0.3–9.6 mg/L | 3.37 mg/L (10.72 µM) | [29] | |
| 0.3–9.6 mg/L | 3.4 mg/L (10.81 µM) | [30] | |
| 1, 1.25, 1.5, 2 mg/L | 1.54 mg/L (4.9 µM) | [31] | |
| CBD extract | 0.625, 1.25, 2.5, 5, 12.5, 25 mg/L | 48 hpf: 4.4 mg/L | [32] |
| 72 hpf: 3.7 mg/L | |||
| CBD | 0.07, 0.1, 0.3, 0.6, 1.25 mg/L | 793.28 µg/L (2.52 µM) | [33] |
| 0.07, 0.1, 0.3, 0.6, 1.25 mg/L | 0.53 mg/L (1.69 µM) | [28] | |
| 0.1, 0.5, 1, 5, 10, 25, 50 µM | 24 hpf: 49.33 µM (15.5 mg/L) | [34] | |
| 48 hpf: 32.25 µM (10.14 mg/L) | |||
| 72 hpf:16.98 µM (5.33 mg/L) | |||
| 96 hpf: 5.883 µM (1.85 mg/L) | |||
| CBN | 0.25–10 mg/L | 1.12 mg/L (3.61 µM) | [35] |
| 4F-MDMB-BUTICA | 0.15, 0.3, 0.6, 1.2, 2.4, 4.8 mg/L | 120 hpf: 1.932 mg/L (5.33 µM) | [36] |
| ADB-FUBINACA | 10, 20, 40, 50, 60, 80, 100 mg/L | 96 hpf: 47.72 mg/L (124.79 µM) | [37] |
| MDMB-4en-PINACA | 0.001–10 µM | 37.81 µM (13.51 mg/L) | [38] |
| Zebrafish Strain | Concentrations | Initial Exposure Stage | Exposure Duration | Phenotypes | Refs. |
|---|---|---|---|---|---|
| Not provided | 1, 2, 5, 10 mg/L | Blastula (4.5 hpf) | 4.5–24 hpf | Reduce spontaneous tail muscle twitch, curved trunks and bulbous-tipped tails | [27] |
| Not provided | 0.3, 0.6, 1.2, 2.4 mg/L | Larval (108 hpf) | 1, 4, 12 h | Dose-dependent dual-phase locomotor response, with activation at low doses and inhibition at high doses | [29] |
| 24 hpf | 96 h | Trigger habituation in basal/recovery phases across all concentrations, with only 1.2 mg/L stimulating activity | |||
| AB/TU hybrids | 0.1, 0.5, 1.5, 2 µM | Larval (5 dpf) | / | Distinct behavioral patterns and concentration response profiles | [39] |
| Tg (fli1:eGFP) | 0.3125, 0.625, 1.25, 2.5, 5 mg/L | Blastula (2 hpf) | 2–96 hpf | LC50: 3.65 mg/L; edemas, curved axis, eye/snout/jaw/trunk/fin deformities, swim bladder distention, behavioral abnormalities | [28] |
| 0.08, 0.4, 2 µM | Gastrula (6 hpf) | 6–14/24/48/72/96 hpf | Cause biphasic effects on longevity, inflammation, and reproduction in aged fish | [40] | |
| Tg (fli1: eGFP) | 0.024, 0.12, 0.6 mg/L | Gastrula (6 hpf) | 6–96 hpf | Reduce fecundity in adults. Did not cause notable morphological abnormalities in either F0 or F1 generations | [41] |
| TL | 6 mg/L | Gastrula (5.25 hpf) | 5.25–10.75 hpf | Reduce axonal diameter of Mauthner cells (M-cell), alters escape response properties | [42] |
| TL strain, scn1Lab−/−, scn1Lab+/− | 1, 4 µM | Larval (5 dpf) | 120–144 hpf | Reduce seizure behavior in chemically induced and scn1a-mutant zebrafish | [43] |
| CaMPARI transgenic/ Casper | 2, 3, 4, 6 mg/L | Zygote (0.5 hpf) | 0.5–10 hpf | Reduce neural activity and locomotion | [44] |
| TL | 10 mg/L | Larval (5 dpf) | 0.5 h | Alter motor neuron-muscle communication and motor behaviors | [45] |
| TL | 2, 4, 6, 8, 10 mg/L | Gastrula (5.25 hpf) | 5.25–10.75 hpf | Reduce heart rates, axial malformations and shorter trunks, alter synaptic activity at neuromuscular junctions, change in branching patterns and a reduction in the number of axonal branches in the trunk musculature | [46] |
| TL | 0.001, 0.01, 0.1, 0.5, 1, 10, 20 mg/L | Gastrula (5.25 hpf) | 5.25–10.75 hpf | Reduce spontaneous coiling of 1-dpf embryos, reduce swimming after touch-evoked responses and basal swimming in 5-dpf larvae. Reduce coiling activity of F1 embryos, reduce swimming after touch-evoked responses of 1-dpf F1 embryos. | [47] |
| Not provided | 100 µM | Adult | 1 h | Impairs spatial but not associative memory function, activation of extracellular signal-regulated kinases signaling in the lateral pallium | [48] |
| Not provided | 100 nM | Adult | 1 h | Inhibit acquisition of fear learning | [49] |
| Short-fin | 30, 50 mg/L | Adult | 20 min | Produce an anxiogenic-like reduction of top swimming, paralleled with a slower, continuous bottom swimming | [50] |
| EK-WT | 40 nM; 1, 2 µM | Adult | 20 min | Induce psychosis-like behavioral stereotypy | [51] |
| Zebrafish Strain | Concentrations | Initial Exposure Stage | Exposure Duration | Phenotypes | Refs. |
|---|---|---|---|---|---|
| CBD | |||||
| Not provided | 5, 20, 70, 150, 300 µg/L | Zygote | 96 h | No malformation, do not alter biochemical activity; increases the motor activity at 24 hpf, but not at 48 hpf. | [52] |
| 0.5, 1, 5, 10 mg/L | Larval (4~5 dpf) | 30 min | 0.5 and 10 mg/L reduce movement velocity and the total distance | [53] | |
| AB/TU | up to 3.14 mg/L | Larval (2 dpf) | 2–5 dpf | >2.5 µM led to higher levels of toxicity to the larvae | [54] |
| Tg (fli1:eGFP) | 0.075, 0.15, 0.3, 0.6, 1.2 mg/L | Blastula (2 hpf) | 2–96 hpf | LC50: 0.53 mg/L; Edemas, curved axis, eye/snout/jaw/trunk/fin deformities, swim bladder distention, behavioral abnormalities | [28] |
| TU | 0.1 0.5, 5.0, 10 mg/kg (i.p.) | Larval (3 dpf) | 1 h before analysis | Inverted U-shaped dose–response curve with 0.5 mg/kg reducing the anxiety. 5 mg/kg causes memory impairment. | [55] |
| CaMPARI transgenic/Casper | 1.5, 2, 3 mg/L | 0.5 hpf | 0.5–10 hpf | Dramatically reduce neural activity and locomotor activity. | [44] |
| Tg (fli1: eGFP) | 0.006, 0.03, 0.15 mg/L | Gastrula (6 hpf) | 6–96 hpf | Did not cause notable morphological abnormalities in either F0 or F1 generations | [41] |
| AB | 0.25, 0.5, 0.75, 1, 1.25, 1.5 mg/L for acute toxicity; 0.1, 0.2 mg/L for reproductive system development | Gastrula (4 hpf) | 4–7 dpf | LC50: 793.28 µg/L; developmental toxicity, lethal toxicity, and reproductive inhibition | [33] |
| TL | 1, 2, 3, 4 mg/L | Gastrula (5.25 hpf) | 5.25–10.75 hpf | Reduce heartbeat rates, axial malformations, shortened trunk length, and impair synaptic activity at neuromuscular junctions, along with altered axonal branching patterns and decrease axonal branches number in trunk musculature. | [46] |
| TL | 3 mg/L | Gastrula (5.25 hpf) | 5.25–10.75 hpf | Reduce hatching and survival rates and suppress Shh pathway activity, impair swimming activity. | [56] |
| Not provided | 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 25, 50 µM | Gastrula (6 hpf) | 6–96 hpf | Reduce heartbeat rates, induce pericardial edema, and reduce eye area, | [34] |
| AB | 0.1, 0.25, 0.5, 1.25 mg/L | Blastula (2 hpf) | 6, 48 h | Promote fin regeneration and inhibit neutrophil accumulation in a dose-dependent manner. | [32] |
| AB | 1, 2, 4 µM | Larval (6 dpf) | 30 min | Significantly inhibit PTZ-induced hyperactivity at 2 and 4 µM concentrations. | [57] |
| Not provided | 40 mg/L | Adult | 30 min | Decrease swimming speed and swimming distance, affect immune gene expression | [58] |
| AB | 500, 600, 700, 800, 900 µg/L | Adult | 96 h | Both sexes show lower gonadosomatic indices (GSI), higher hepatosomatic indices (HSI), immature gametes, and reduce vitellogenin (VTG) levels. E2/T ratio decreases in female while increases in males. Apoptosis-related genes are upregulated in the brain, gonad, and liver. | [59] |
| CBN | |||||
| TL | 0.01, 0.1, 0.5, 1, 2, 3, 4 mg/L | Gastrula (5.25 hpf) | 5.25–10.75 hpf | Causes dose-dependent malformations, higher mortality, reduced locomotion, and impaired motor neuron branching. It also disrupts hair cell development in the otic vesicles and lateral line, weakening sound response. | [60] |
| AB | 0.25, 0.75, 1.0, 1.125, 1.2, 1.25, 2.0 mg/L | Somite (26 hpf) | 24–120 hpf | Pericardial edema, yolk sac anomalies and tail bending, increases total movement distance and velocity | [35] |
| Type | Strain | Concentration | Initial Exposure Stage | Exposure Duration | Phenotypes | Refs. |
|---|---|---|---|---|---|---|
| 4F-MDMB-BINACA | AB | 25 µM | Larval (4 dpf) | 24 h | Impaired liver development | [78] |
| 4F-MDMB-BUTICA | AB | 0.15, 0.3, 0.6, 1.2, 2.4, 4.8 mg/L | Blastula (3 hpf) | 3–24 hpf (acute); 3–120 hpf (subacute) | Caused embryonic deformities, including spine formation, pericardial edema, impaired blood flow, yolk sac edema, delayed development. Induced hypoactivity in response to stimulus. altered the transcriptional expression levels of apoptosis, DNA repair, dopamine, serotonin, γ-aminobutyric, and behavior-related genes | [36] |
| ADB-FUBINACA | AB; Tg (Myl7:GFP), Tg (HuC:eGFP) | 10, 20, 30 mg/L | Blastula (4 hpf) | 4–96 hpf | Reduced heartbeat, shorter body length, spinal deformation, and pericardial edema, cardiac developmental defects, impaired motor activity, disrupted neuronal development, elevated ROS and MDA, dysregulated immune-related genes, disruptions in pathways related to alanine, purine, pyrimidine metabolism, arginine biosynthesis. | [37] |
| MDA-19 | AB; Tg (hb9: GFP) | 1, 10, 20 mg/L | / | 5 days | Accelerated hatching, reduced body length without affecting mortality or malformation, resulted in diminished swimming ability and reduced activity time, impaired development of spinal motor neurons, increased ROS, elevated SOD and CAT, affected energy metabolism | [71] |
| JWH-018 | Not provided | 3 µM | Pharyngula (28 hpf) | 1–6 dpf | Impaired locomotion during the forced light/dark test | [70] |
| AB | 0.01, 0.05, and 0.25 µg/g | Adult (intraperitoneally, i.p.) | / | Dose-dependent anxiogenic effects and lower aggression behavior, activated the CB1R-dependent extracellular signal-regulated kinase 1 and 2. Did not affect the vertical movement distance. | [77] | |
| JWH-019 | AB | 0.01, 0.05, and 0.25 µg/g | / | Did not change the movement trace line and vertical movement distance. | ||
| APINAC | Not provided | 0.001, 0.1, 1, 10 µM | Larval (6 dpf) | / | Reduced visual motor response, impairment of spontaneous motor and sensorimotor behavior | [75] |
| 5F-APINAC | Not provided | 0.001, 0.01, 0.1, 1.0 10 µM | Larval (6 dpf) | 4 h, 96 h | Morphological and developmental alterations, induced metabolomic alterations | [76] |
| MDMB-4en-PINACA | Not provided | 0.001, 0.01, 0.1, 1, 10 µM | Blastula (1.5 hpf) | 1.5–4 dpf | Positive correlation between exposure concentration and lethal effects, including lack of heartbeat, lack of somite formation, pericardial edema, and yolk edema. | [38] |
| Cannabinoids | Concentrations | Disease Models | Effects | Refs. |
|---|---|---|---|---|
| CBD crude extract | 0.1, 0.25, 0.5, 1.25 mg/L | Caudal fin amputation model (amputated at 3 dpf, observations at 48- and 72-h post-amputation) | Accelerated fin regeneration and suppressed post-amputation apoptosis | [32] |
| CBD | 1.25 µM | Tuberous sclerosis complex | Reduced anxiety-like behaviors without sedation | [80] |
| CBD, CBC, CBDV, CBG, CBN | CBD, CBN, and CBDV: 0.25–4 µM CBC: 0.1–3 µM CBG: 0.25–3 µM | 6-hydroxydopamine (OHDA)-induced Parkinson’s disease | Individual cannabinoids had no effect on OHDA-induced hypoactivity. However, three-component equimolar mixtures (e.g., CBD + CBDV + CBC, CBD + CBN + CBC, or CBD + CBDV + CBG) significantly attenuated OHDA-related motor symptoms | [81] |
| CBD | 1, 5, 10 mg/L | Haloperidol induced Parkinsonism model | CBD and ropinirole reversal haloperidol-induced motor dysfunction, CBD was more effective than ropinirole. | [82] |
| CBD, THC | CBD: 1, 1.5, 2 µM THC: 1.5, 2, 3 µM | PTZ-induced neurohyperactivity model; GABRA1−/− mutants model | CBD alleviated behavioral hyperactivity in two different zebrafish models. It not only calmed hyperactivity but also worked synergistically with THC to amplify therapeutic outcomes, surpassing the effectiveness of either compound used independently. | [83] |
| CBD, THC, CBVD, CBN, linalool (LN) | CBD: 0.3, 0.6, 1.0 µM THC: 1.0, 4.0 µM CBVD: 0.3, 0.6, 1.0 µM CBN: 0.3, 0.6, 1.0 µM LN: 0.3, 0.6, 1.0, 4.0 µM | (scn1Lab−/−) Dravet Syndrome model; PTZ-induced epilepsy model | CBD (0.6 µM), THC (1 µM), CBN (0.6, 1 µM), and LN (4 µM) significantly suppressed seizures, with CBN exhibiting maximal efficacy. Only CBD and THC attenuated PTZ-induced hyperactivity | [43] |
| CBD; Whole cannabis extracts | 6, 9, 12, 15, 18 µM | PTZ-induced epilepsy model | Pure CBD (5.7 µg/mL) and whole cannabis extracts (0.01 mg/mL) outperformed valproic acid (VPA, a commonly used antiepileptic drug) in seizure suppression, with extracts achieving comparable efficacy to CBD despite lower cannabinoid content | [84] |
| CBD, CBC, CBN | CBC: 1, 2, 4 µM CBN: 1, 2, 4 µM | PTZ-induced epilepsy model | CBD, CBC, and CBN effectively reduced PTZ-induced convulsions at low doses in zebrafish, with CBC showing the lowest tissue accumulation while maintaining efficacy. CBN inhibited PTZ-induced hyperactivity, with 2 and 4 µM doses being statistically significant. CBC inhibited PTZ-induced hyperactivity in a dose-dependent manner, with 1, 2, and 4 µM doses being statistically significant | [57] |
| THCV, CBD | THCV: 5 µM CBD: 5 µM | Naive larvae | CBD and THCV reduced AdipoRed staining of larvae yolk sacs within 24 h and 48 h respectively | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xie, H.; Shi, X.; Wu, K.; Huang, W. Exploring Cannabinoid Effects Using Zebrafish (Danio rerio) as an In Vivo Model: A Review of the Literature. Int. J. Mol. Sci. 2025, 26, 9165. https://doi.org/10.3390/ijms26189165
Wang X, Xie H, Shi X, Wu K, Huang W. Exploring Cannabinoid Effects Using Zebrafish (Danio rerio) as an In Vivo Model: A Review of the Literature. International Journal of Molecular Sciences. 2025; 26(18):9165. https://doi.org/10.3390/ijms26189165
Chicago/Turabian StyleWang, Xingbo, Han Xie, Xiaoling Shi, Kusheng Wu, and Wenlong Huang. 2025. "Exploring Cannabinoid Effects Using Zebrafish (Danio rerio) as an In Vivo Model: A Review of the Literature" International Journal of Molecular Sciences 26, no. 18: 9165. https://doi.org/10.3390/ijms26189165
APA StyleWang, X., Xie, H., Shi, X., Wu, K., & Huang, W. (2025). Exploring Cannabinoid Effects Using Zebrafish (Danio rerio) as an In Vivo Model: A Review of the Literature. International Journal of Molecular Sciences, 26(18), 9165. https://doi.org/10.3390/ijms26189165







