Prognostic Role of MMP2, MMP9, and IL-1β Markers in Cardiac Allograft Rejection After Transplantation
Abstract
1. Introduction
2. Results
2.1. Clinical and Pathological Characteristics of the Selected Cases
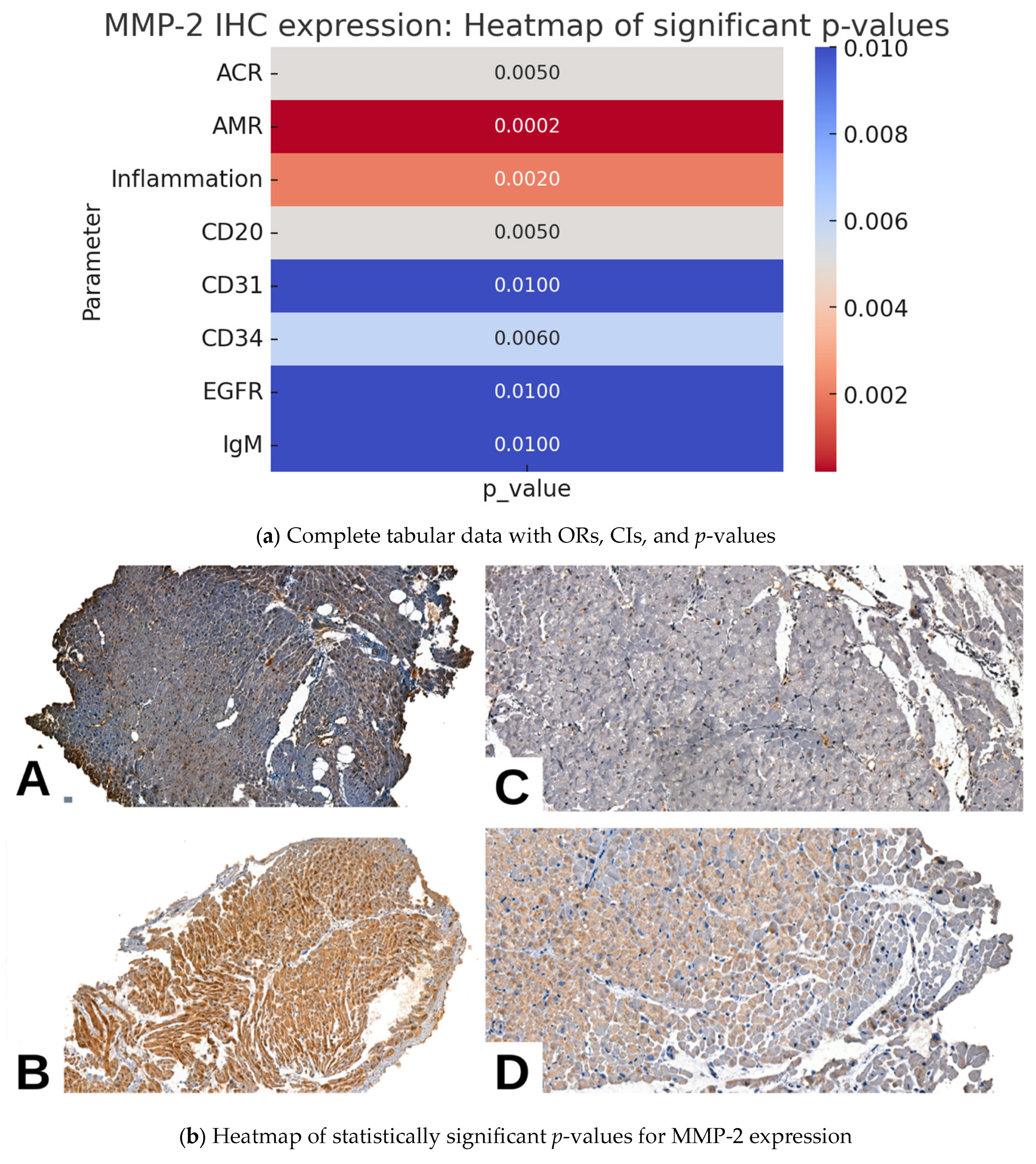
2.2. MMP2 IHC Expression and Pathological Parameters in Cardiac Allograft Rejection
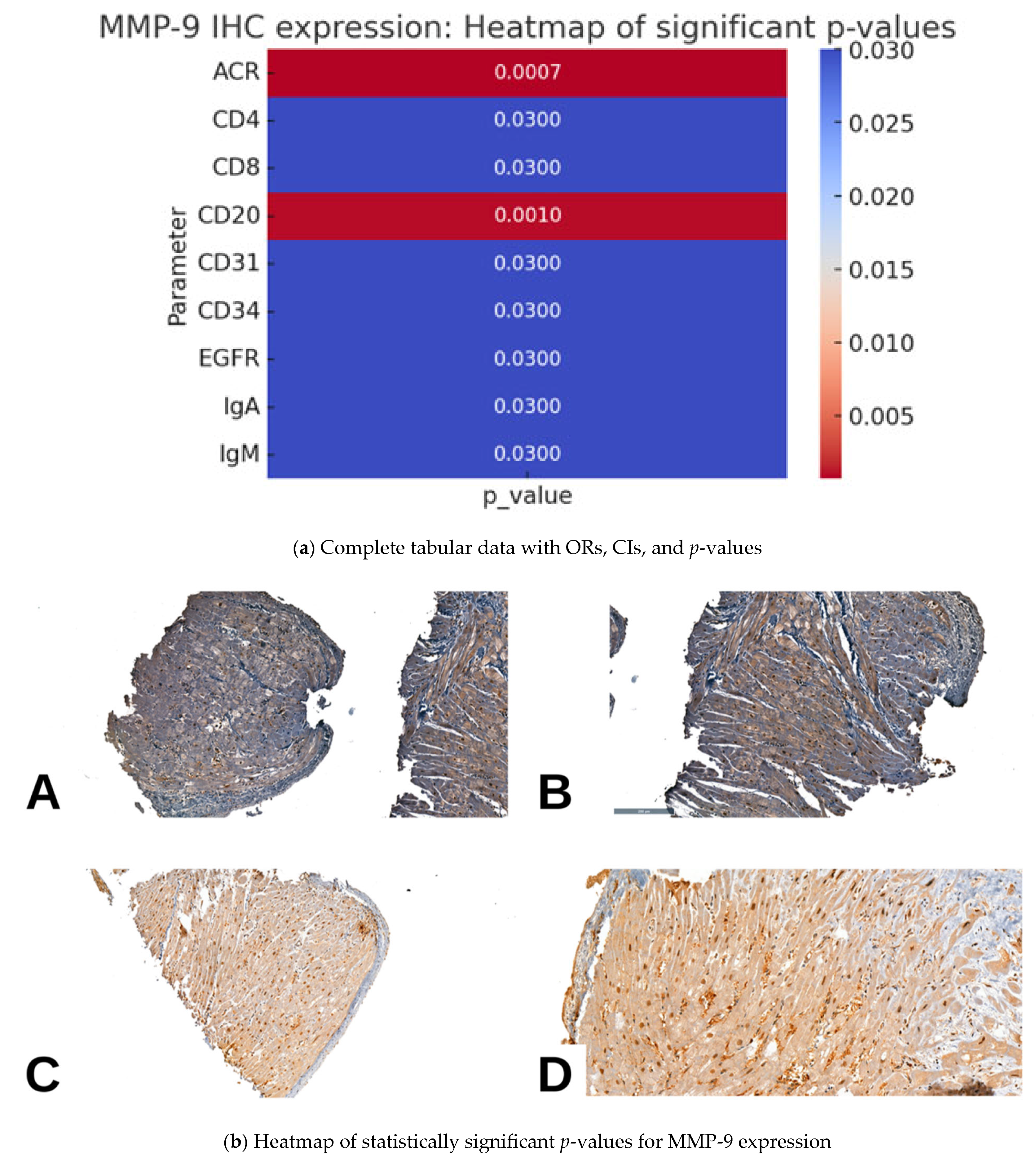
2.3. MMP9 IHC Expression and Pathological Parameters in Cardiac Allograft Rejection
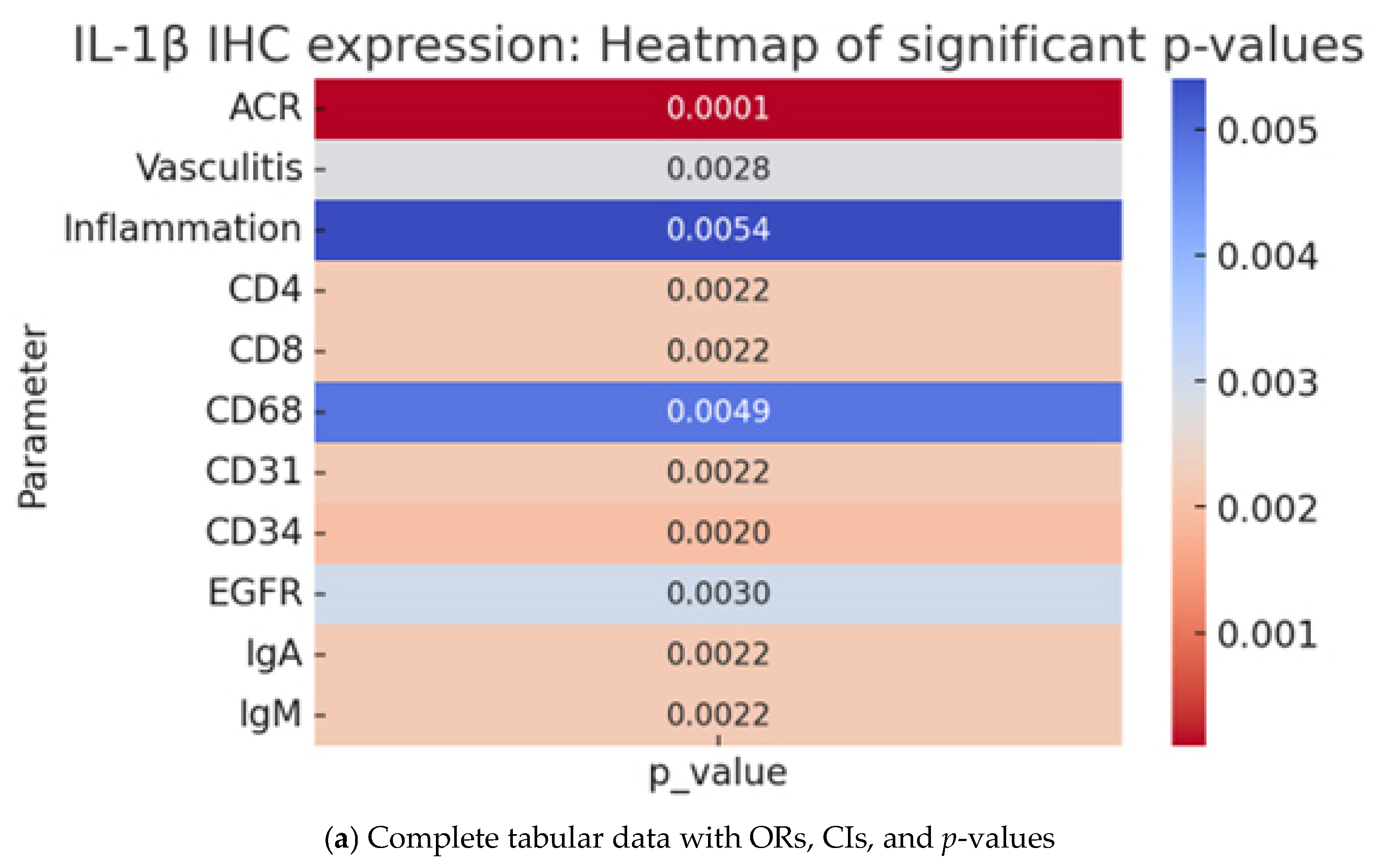
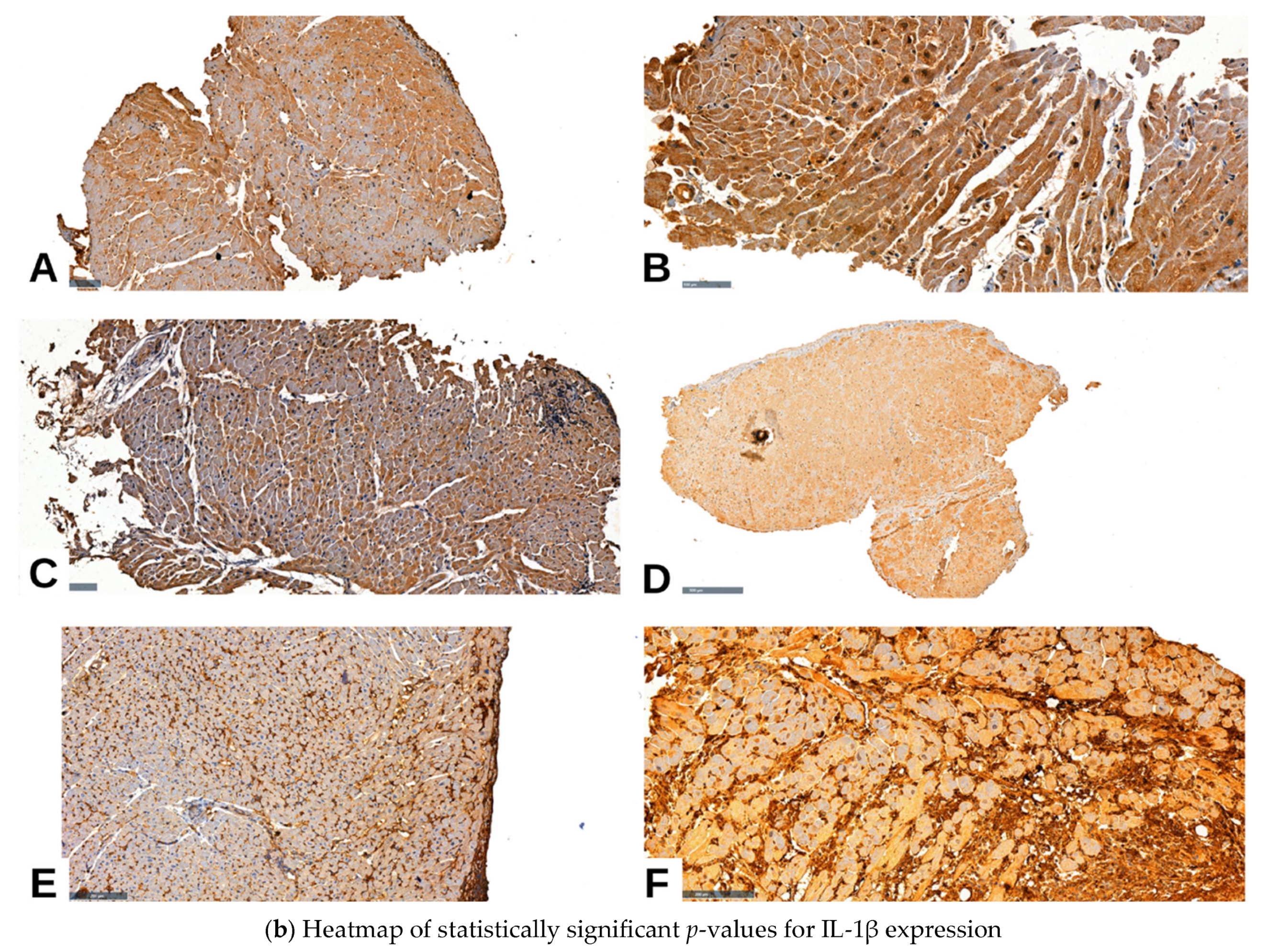
2.4. IL-1β IHC Expression and Pathological Parameters in Cardiac Allograft Rejection
3. Discussion
Future Directions
4. Materials and Methods
4.1. Study Participants
4.2. Immunohistochemical Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACR | Acute Cellular Rejection |
| AMR | Antibody-Mediated Rejection |
| IL-1β | Interleukin-1 beta |
| MMP | Matrix Metalloproteinase |
| MMP2 | Matrix Metalloproteinase 2 |
| MMP9 | Matrix Metalloproteinase 9 |
| IHC | Immunohistochemistry |
| ECM | Extracellular Matrix |
| ISHLT | International Society for Heart and Lung Transplantation |
| DSA | Donor-Specific Antibodies |
| EGFR | Epidermal Growth Factor Receptor |
| PECAM1 | Platelet and Endothelial Cell Adhesion Molecule 1 (CD31) |
| CD | Cluster of Differentiation |
| HE | Hematoxylin and Eosin |
| OR | Odds Ratio |
| CI | Confidence Interval |
References
- Christie, J.D.; Edwards, L.B.; Aurora, P.; Dobbels, F.; Kirk, R.; Rahmel, A.O.; Stehlik, J.; Taylor, D.O.; Kucheryavaya, A.Y.; Hertz, M.I. The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Lung and Heart-Lung Transplantation Report-2009. J. Heart Lung Transplant. 2009, 28, 1031–1049. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.S.; Veinot, J.P.; Butany, J. An approach to endomyocardial biopsy interpretation. J. Clin. Pathol. 2006, 59, 121–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porcari, A.; Baggio, C.; Fabris, E.; Merlo, M.; Bussani, R.; Perkan, A.; Sinagra, G. Endomyocardial biopsy in the clinical context: Current indications and challenging scenarios. Heart Fail. Rev. 2023, 28, 123–135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michaels, P.J.; Espejo, M.L.; Kobashigawa, J.; Alejos, J.C.; Burch, C.; Takemoto, S.; Reed, E.F.; Fishbein, M.C. Humoral rejection in cardiac transplantation: Risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J. Heart Lung Transplant. 2003, 22, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.K.; Kittleson, M.; Kobashigawa, J.A. Cardiac allograft rejection. Surgeon 2011, 9, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, D.; van Almen, G.C.; Van Aelst, L.N.; Van Cleemput, J.; Droogné, W.; Jin, Y.; Van de Werf, F.; Carmeliet, P.; Vanhaecke, J.; Papageorgiou, A.-P.; et al. Matricellular proteins and matrix metalloproteinases mark the inflammatory and fibrotic response in human cardiac allograft rejection. Eur. Heart J. 2013, 34, 1930–1941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stewart, S.; Winters, G.L.; Fishbein, M.C.; Tazelaar, H.D.; Kobashigawa, J.; Abrams, J.; Andersen, C.B.; Angelini, A.; Berry, G.J.; Burke, M.M.; et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J. Heart Lung Transplant. 2005, 24, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Reed, E.F.; Demetris, A.J.; Hammond, E.; Itescu, S.; Kobashigawa, J.A.; Reinsmoen, N.L.; Rodriguez, E.R.; Rose, M.; Stewart, S.; Suciu-Foca, N.; et al. Acute antibody-mediated rejection of cardiac transplants. J. Heart Lung Transplant. 2006, 25, 153–159. [Google Scholar] [CrossRef]
- Kobashigawa, J.; Crespo-Leiro, M.G.; Ensminger, S.M.; Reichenspurner, H.; Angelini, A.; Berry, G.; Burke, M.; Czer, L.; Hiemann, N.; Kfoury, A.G.; et al. Report from a consensus conference on antibody-mediated rejection in heart transplantation. J. Heart Lung Transplant. 2011, 30, 252–269. [Google Scholar] [CrossRef]
- Berry, G.J.; Angelini, A.; Burke, M.M.; Bruneval, P.; Fishbein, M.C.; Hammond, E.; Miller, D.; Neil, D.; Revelo, M.P.; Rodriguez, E.R.; et al. The ISHLT working formulation for pathologic diagnosis of antibody-mediated rejection in heart transplantation: Evolution and current status (2005–2011). J. Heart Lung Transplant. 2011, 30, 601–611. [Google Scholar] [CrossRef]
- Berry, G.J.; Burke, M.M.; Andersen, C.; Bruneval, P.; Fedrigo, M.; Fishbein, M.C.; Goddard, M.; Hammond, E.H.; Leone, O.; Marboe, C.; et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J. Heart Lung Transplant. 2013, 32, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Cazes, A.; Guillemain, R.; Amrein, C.; Hedjoudje, A.; Tible, M.; Pezzella, V.; Fabiani, J.N.; Suberbielle, C.; Nochy, D.; et al. Very late heart transplant rejection is associated with microvascular injury, complement deposition and progression to cardiac allograft vasculopathy. Am. J. Transplant. 2011, 11, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Fedrigo, M.; Gambino, A.; Tona, F.; Torregrossa, G.; Poli, F.; Benazzi, E.; Frigo, A.; Feltrin, G.; Toscano, G.; Caforio, A.P.; et al. Can C4d Immunostaining on Endomyocardial Biopsies Be Considered a Prognostic Biomarker in Heart Transplant Recipients? Transplantation 2010, 90, 791–798, Erratum in Transplantation 2011, 91, 135. https://doi.org/10.1097/TP.0b013e31820a3f22. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, Q.; Kong, W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018, 68–69, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Alandi, I.; Schafer, A.E.; Blaxall, B.C. Extracellular matrix-mediated cellular communication in the heart. J. Mol. Cell. Cardiol. 2016, 91, 228–237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patrichi, G.; Patrichi, A.; Satala, C.-B.; Sin, A.I. Matrix Metalloproteinases and Heart Transplantation—A Pathophysiological and Clinical View. Medicina 2023, 59, 1295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kidder, E.; Gangopadhyay, S.; Francis, S.; Alfaidi, M. How to Release or Not Release, That Is the Question. A Review of Interleukin-1 Cellular Release Mechanisms in Vascular Inflammation. J. Am. Heart Assoc. 2024, 13, e032987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simeoni, E.; Dudler, J.; Fleury, S.; Li, J.; Pagnotta, M.; Pascual, M.; von Segesser, L.K.; Vassalli, G. Gene transfer of a soluble IL-1 type 2 receptor-Ig fusion protein improves cardiac allograft survival in rats. Eur. J. Cardio-Thoracic Surg. 2007, 31, 222–228. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1. Cytokine Growth Factor Rev. 1997, 8, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.K.; Orloff, S.; Burg, J.M.; Haese, N.N.; Andoh, T.F.; Chambers, A.; Fei, S.S.; Gao, L.; Kreklywich, C.N.; Streblow, Z.J.; et al. Blocking the IL-1 receptor reduces cardiac transplant ischemia and reperfusion injury and mitigates CMV-accelerated chronic rejection. Am. J. Transplant. 2021, 21, 44–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rusai, K.; Huang, H.; Sayed, N.; Strobl, M.; Roos, M.; Schmaderer, C.; Heemann, U.; Lutz, J. Administration of interleukin-1 receptor antagonist ameliorates renal ischemia-reperfusion injury. Transpl. Int. 2008, 21, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Kontos, M.C.; Grizzard, J.D.; Biondi-Zoccai, G.G.; Van Tassell, B.W.; Robati, R.; Roach, L.M.; Arena, R.A.; Roberts, C.S.; Varma, A.; et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am. J. Cardiol. 2010, 105, 1371–1377.e1. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Chen, W.; Su, Y.; Rai, V.; Uche, O.U.; Li, N.; Frangogiannis, N.G. IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium. J. Immunol. 2013, 191, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.N.; Hartogensis, W.E.; Patten, M.; Fortuin, F.D.; Long, C.S. Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. J. Clin. Investig. 1995, 95, 2555–2564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Huyen, J.-P.D.; Fedrigo, M.; Fishbein, G.A.; Leone, O.; Neil, D.; Marboe, C.; Peyster, E.; von der Thüsen, J.; Loupy, A.; Mengel, M.; et al. The XVth Banff Conference on Allograft Pathology the Banff Workshop Heart Report: Improving the diagnostic yield from endomyocardial biopsies and Quilty effect revisited. Am. J. Transplant. 2020, 20, 3308–3318. [Google Scholar] [CrossRef]
- Ermolli, M.; Schumacher, M.; Lods, N.; Hammoud, M.; Marti, H.P. Differential expression of MMP-2/MMP-9 and potential benefit of an MMP inhibitor in experimental acute kidney allograft rejection. Am. J. Physiol. 2003, 284, F764–F773. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.T.; Pan, Y.; Liu, X.F.; Xu, J.W.; Cui, W.J.; Qiao, X.R.; Dong, L. Syndecan-1 Shedding by Matrix Metalloproteinase-9 Signaling Regulates Alveolar Epithelial Tight Junction in Lipopolysaccharide-Induced Early Acute Lung Injury. J. Inflamm. Res. 2021, 14, 5801–5816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cecrdlova, E.; Krupickova, L.; Fialova, M.; Novotny, M.; Tichanek, F.; Svachova, V.; Mezerova, K.; Viklicky, O.; Striz, I. Insights into IL-1 family cytokines in kidney allograft transplantation: IL-18BP and free IL-18 as emerging biomarkers. Cytokine 2024, 180, 156660. [Google Scholar] [CrossRef] [PubMed]
- Speck, N.E.; Schuurmans, M.M.; Benden, C.; Robinson, C.A.; Huber, L.C. Plasma and bronchoalveolar lavage samples in acute lung allograft rejection: The potential role of cytokines as diagnostic markers. Respir. Res. 2017, 18, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vázquez-Toledo, M.A.; Sánchez-Muñoz, F.; Zepeda-Quiroz, I.; Guzmán-Martín, C.A.; Osorio-Alonso, H.; Daniel, J.-V.; Soto-Abraham, M.V.; Moguel-González, B.; Chacón-Salinas, R.; Flores-Gama, C.; et al. Kidney Transplant Recipients with Acute Antibody-Mediated Rejection Show Altered Levels of Matrix Metalloproteinases and Their Inhibitors: Evaluation of Circulating MMP and TIMP Profiles. Int. J. Mol. Sci. 2025, 26, 6011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aleksova, A.; Beltrami, A.P.; Carriere, C.; Barbati, G.; Lesizza, P.; Perrieri-Montanino, M.; Isola, M.; Gentile, P.; Salvioni, E.; Not, T.; et al. Interleukin-1β levels predict long-term mortality and need for heart transplantation in ambulatory patients affected by idiopathic dilated cardiomyopathy. Oncotarget. 2017, 8, 25131–25140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Capozzi, A.; Riitano, G.; Recalchi, S.; Manganelli, V.; Costi, R.; Saccoliti, F.; Pulcinelli, F.; Garofalo, T.; Misasi, R.; Longo, A.; et al. Effect of heparanase inhibitor on tissue factor overexpression in platelets and endothelial cells induced by anti-β2-GPI antibodies. J. Thromb. Haemost. 2021, 19, 2302–2313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ludhwani, D.; Abraham, J.; Sharma, S.; Kanmanthareddy, A. Heart Transplantation Rejection. [Updated 11 August 2024]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537057/ (accessed on 15 July 2025).
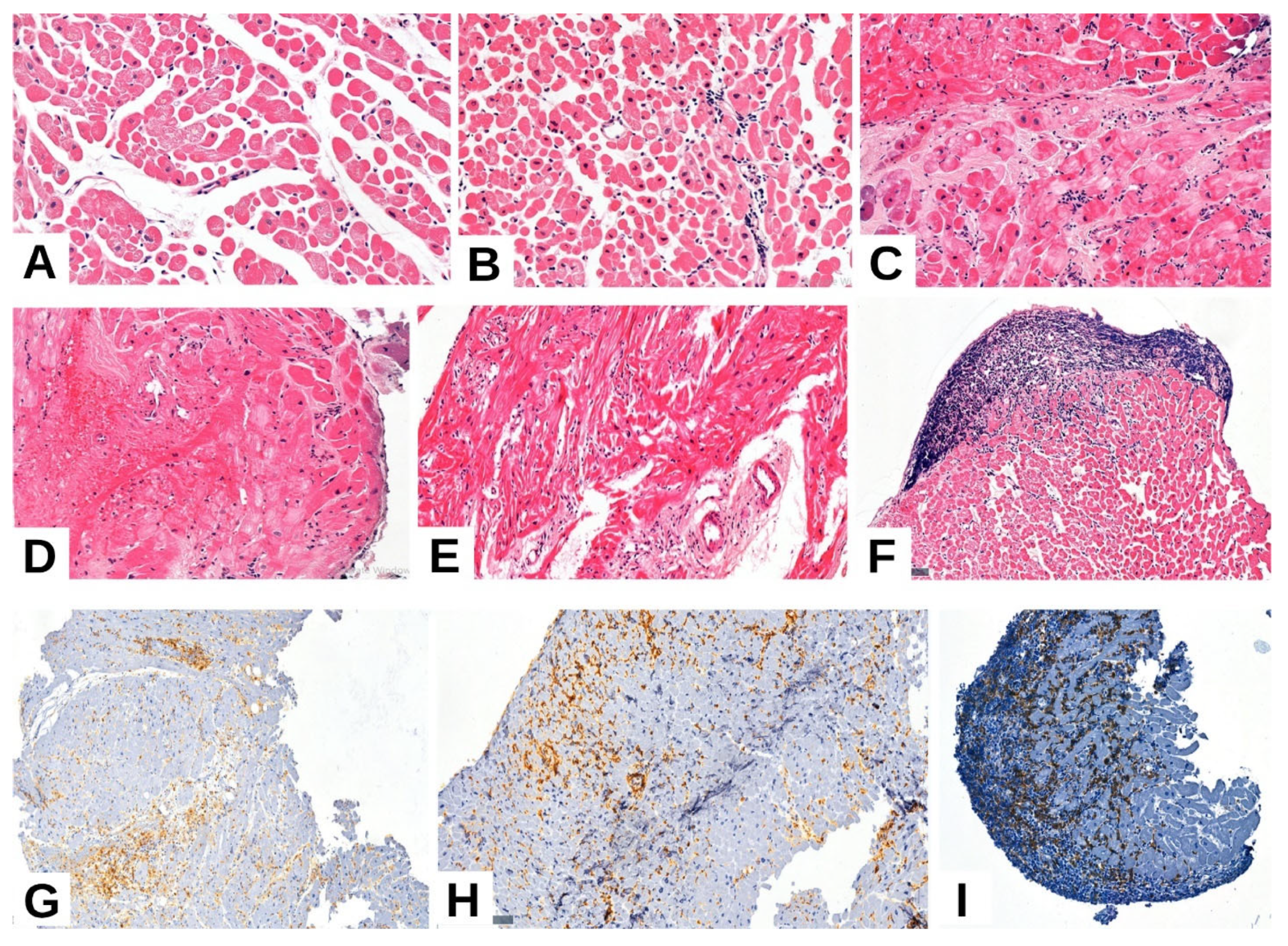
| Parameter | n = 59 | |
|---|---|---|
| Mean Age (Years) | 56 (±15.8) Years | |
| Gender | Males | 48 (81.4%) |
| Females | 11 (18.6%) | |
| Age (years) | <40 | 29 (49.2) |
| ≥40 | 30 (50.8%) | |
| Quilty effect | Absent | 52 (88.1%) |
| Subendocardial | 4 (6.8%) | |
| Endocardial | 3 (5.1%) | |
| Fibrosis | Absent | 13 (22.0%) |
| Mild | 25 (42.4%) | |
| Moderate | 7 (11.9%) | |
| Severe | 15 (25.4%) | |
| Vasculitis | Present | 16 (27.1%) |
| Absent | 43 (72.9%) | |
| Inflammation | Absent | 5 (8.5%) |
| Mild (1 focus) | 42 (71.2%) | |
| Moderate (2 foci) | 6 (10.2%) | |
| Severe (diffuse) | 5 (8.5%) | |
| Cardiomyocyte damage | Degenerative lesions | 14 (23.7%) |
| Premiocitolisis | 16 (27.1%) | |
| Myocite necrosis, coagulative necrosis | 29 (49.2%) | |
| Antibody mediated rejection (AMR) | Histologic and immunopathologic studies are both absent (pAMR0) | 49 (83.1%) |
| Histologic findings or immunopathologic findings are present (pAMR1) | 6 (10.2%) | |
| Histologic and immunopathologic findings are both present (pAMR2) | 3 (5.1%) | |
| Severe pathologic AMR (pAMR3) (interstitial hemorrhage, capillary fragmentation, mixed inflammatory infiltrates, pyknosis, karyorrhexis, edema) | 1 (1.7%) | |
| Acute cellular rejection (ACR) | Absent (0) | 48 (81.6%) |
| Mild (1R) | 8 (13.6%) | |
| Moderate (2R) | 2 (3.4%) | |
| Severe (3R) | 1 (1.7%) | |
| Parameter | MMP-2 IHC Expression (n = 59) | p Value | |||||
|---|---|---|---|---|---|---|---|
| Negative | Nuclear Positivity under 50% | Nuclear Positivity over 50% | Citoplasm Positivity under 50% | Citoplasm Positivity over 50% | |||
| Acute Cellular Rejection (ACR) | Absent | 16 (27.1%) | 0 | 4 (6.8%) | 3 (5.1%) | 1 (1.7%) | 0.005 |
| Mild | 10 (16.9%) | 0 | 1 (1.7%) | 13 (22.0%) | 9 (15.3%) | ||
| Moderate | 0 | 0 | 0 | 0 | 0 | ||
| Severe | 0 | 1 (1.7%) | 0 | 1 (1.7%) | 0 | ||
| OR vs. Negative CI | Ref | 4.71 | 0.40 | 7.47 | 14.4 | ||
| 0.18–126.91 | 0.04–4.11 | 1.71–32.68 | 1.58–135.52 | ||||
| Antibody-mediated Rejection (AMR) | Absent | 17 (28.8%) | 0 | 4 (6.8%) | 3 (5.1%) | 1 (1.7%) | 0.0002 |
| Mild | 10 (16.9%) | 0 | 1 (1.7%) | 13 (22.0%) | 9 (15.3%) | ||
| Moderate | 0 | 1 (1.7%) | 0 | 0 | 0 | ||
| Severe | 0 | 0 | 0 | 1 (1.7%) | 0 | ||
| OR vs. Negative CI | Ref | 4.71 | 0.4 | 7.47 | 14.4 | ||
| 0.18–126.91 | 0.04–4.11 | 1.71–32.68 | 1.58–131.52 | ||||
| Vasculitis | Absent | 14 (23.7%) | 0 | 3 (5.1%) | 5 (8.5%) | 1 (1.7%) | 0.06 |
| Present | 12 (20.3%) | 1 (1.7%) | 2 (3.4%) | 12 (20.3%) | 10 (16.9%) | ||
| OR vs. Negative CI | Ref | 3.48 | 0.78 | 2.82 | 11.67 | ||
| 0.13–93.31 | 0.11–5.46 | 0.77–10.25 | 1.30–104.82 | ||||
| Inflammation | Absent | 032w | 0 | 1 (1.7%) | 0 | 0 | 0.002 |
| Low | 21 (35.6%) | 0 | 3 (5.1%) | 7 (11.9%) | 6 (10.2%) | ||
| Moderate | 1 (1.7%) | 0 | 0 | 3 (5.1%) | 4 (6.8%) | ||
| Severe | 4 (6.8%) | 1 (1.7%) | 1 (1.7%) | 7 (11.9%) | 0 | ||
| OR vs. Negative CI | Ref | 0.23 | 0.08 | 2.08 | 1.62 | ||
| 0.0–17.06 | 0.0–2.99 | 0.04–116.87 | 0.03–91.82 | ||||
| Quilty Effect | Absent | 25 (42.4%) | 1 (1.7%) | 4 (6.8%) | 11 (18.6%) | 4 (6.8%) | 0.2299 |
| Subendocardial | 0 | 0 | 1 (1.7%) | 3 (5.1%) | 6 (10.2%) | ||
| Endocardial | 1 (1.7%) | 0 | 0 | 3 (5.1%) | 0 | ||
| OR vs. Negative CI | Ref | 5.67 | 6.25 | 13.64 | 37.5 | ||
| 0.15–207.27 | 0.32–121.34 | 1.46–127.15 | 3.52–399.38 | ||||
| CD4 | Negative | 12 (20.3%) | 0 | 2 (3.4%) | 2 (3.4%) | 1 (1.7%) | 0.07 |
| Positive | 14 (23.7%) | 0 | 2 (3.4%) | 2 (3.4%) | 1 (1.7%) | ||
| OR vs. Negative CI | Ref | 1.07 | 1.08 | 1.08 | 1.08 | ||
| 0.02–58.03 | 0.13–8.8 | 0.13–8.8 | 0.06–19.05 | ||||
| CD8 | Negative | 12 (20.3%) | 0 | 2 (3.4%) | 2 (3.4%) | 1 (1.7%) | 0.07 |
| Positive | 14 (23.7%) | 1 (1.7%) | 3 (5.1%) | 15 (25.4%) | 9 (15.3%) | ||
| OR vs. Negative CI | Ref | 6.33 | 3.23 | 16.15 | 19.38 | ||
| 0.24–165.89 | 0.48–21.74 | 3.21–81.25 | 2.22–169.47 | ||||
| CD68 | Negative | 13 (22.0%) | 0 | 2 (3.4%) | 5 (8.5%) | 1 (1.7%) | 0.19 |
| Positive | 13 (22.0%) | 1 (1.7%) | 3 (5.1%) | 12 (20.3%) | 9 (15.3%) | ||
| OR vs. Negative CI | Ref | 4.12 | 2.07 | 3.31 | 12.43 | ||
| 0.16–106.01 | 0.32–13.51 | 1.01–10.84 | 1.46–105.74 | ||||
| CD20 | Negative | 25 (42.4%) | 1 (1.7%) | 4 (6.8%) | 11 (18.6%) | 4 (6.8%) | 0.005 |
| Positive | 1 (1.7%) | 0 | 1 (1.7%) | 6 (10.2%) | 6 (10.2%) | ||
| OR vs. Negative CI | Ref | 0.12 | 0.09 | 0.19 | 0.51 | ||
| 0–3 | 0.01–0.82 | 0.06–0.59 | 0.13–2.06 | ||||
| CD31 | Negative | 14 (23.7%) | 0 | 2 (3.4%) | 2 (3.4%) | 1 (1.7%) | 0.01 |
| Positive | 12 (20.3%) | 1 (1.7%) | 3 (5.1%) | 15 (25.4%) | 9 (15.3%) | ||
| OR vs. Negative CI | Ref | 5.78 | 2.92 | 14.62 | 17.55 | ||
| 0.23–148.31 | 0.45–18.95 | 3.04–70.36 | 2.07–148.45 | ||||
| CD34 | Negative | 14 (23.7%) | 0 | 2 (3.4%) | 1 (1.7%) | 1 (1.7%) | 0.006 |
| Positive | 12 (20.3%) | 1 (1.7%) | 3 (5.1%) | 16 (27.1%) | 9 (15.3%) | ||
| OR vs. Negative CI | Ref | 6.73 | 3.42 | 36.44 | 20.5 | ||
| 0.26–173.08 | 0.52–22.24 | 4.49–296.11 | 2.41–174.07 | ||||
| EGFR | Negative | 18 (30.5%) | 0 | 2 (3.4%) | 6 (10.2%) | 1 (1.7%) | 0.01 |
| Positive | 8 (13.6%) | 1 (1.7%) | 3 (5.1%) | 11 (18.6%) | 9 (15.3%) | ||
| OR vs. Negative CI | Ref | 3.55 | 1.78 | 2.17 | 10.67 | ||
| 0.14–90.59 | 0.28–11.43 | 0.71–6.65 | 1.27–89.63 | ||||
| IgA | Negative | 12 (20.3%) | 0 | 2 (3.4%) | 2 (3.4%) | 1 (1.7%) | 0.07 |
| Positive | 14 (23.7%) | 1 (1.7%) | 3 (5.1%) | 15 (25.4%) | 9 (15.3%) | ||
| OR vs. Negative CI | Ref | 6.23 | 3.16 | 15.79 | 6.23 | ||
| 0.24–160.04 | 0.49–20.5 | 3.27–76.14 | 2.24–160.56 | ||||
| IgM | Negative | 14 (23.7%) | 0 | 2 (3.4%) | 2 (3.4%) | 1 (1.7%) | 0.01 |
| Positive | 12 (20.3%) | 1 (1.7%) | 3 (5.1%) | 15 (25.4%) | 9 (15.3%) | ||
| OR vs. Negative CI | Ref | 6.23 | 3.16 | 15.79 | 18.95 | ||
| 0.24–160.04 | 0.49–20.5 | 3.27–76.14 | 2.24–160.56 | ||||
| Parameter | MMP-9 IHC Expression (n = 59) | p Value | |||
|---|---|---|---|---|---|
| Negative | Nuclear Positivity under 50% | Nuclear Positivity over 50% | |||
| Acute Cellular Rejection (ACR) | Absent | 17 (28.8%) | 5 (8.5%) | 4 (6.8%) | 0.0007 |
| Mild | 14 (23.7%) | 7 (11.9%) | 0 | ||
| Moderate | 1 (1.7%) | 5 (8.5%) | 5 (8.5%) | ||
| Severe | 0 | 1 (1.7%) | 0 | ||
| OR vs. Negative CI | Ref | 2.95 | 1.42 | ||
| 0.85–10.22 | 0.32–6.27 | ||||
| Antibody-mediated Rejection (AMR) | Absent | 17 (28.8%) | 6 (10.2%) | 1 (1.7%) | 0.05 |
| Mild | 15 (25.4%) | 11 (18.6%) | 7 (11.9%) | ||
| Moderate | 0 | 0 | 0 | ||
| Severe | 0 | 1 (1.7%) | 1 (1.7%) | ||
| OR vs. Negative CI | Ref | 2.27 | 9.07 | ||
| 0.68–7.53 | 1.01–81.16 | ||||
| Vasculitis | Absent | 15 (25.4%) | 6 (10.2%) | 2 (3.4%) | 0.34 |
| Present | 17 (28.8%) | 12 (20.3%) | 7 (11.9%) | ||
| OR vs. Negative CI | Ref | 1.76 | 3.09 | ||
| 0.53–5.87 | 0.55–17.21 | ||||
| Inflammation | Absent | 0 | 1 (1.7%) | 0 | 0.12 |
| Low | 23 (39.0%) | 9 (15.3%) | 5 (8.5%) | ||
| Moderate | 1 (1.7%) | 4 (6.8%) | 3 (5.1%) | ||
| Severe | 8 (13.6%) | 4 (6.8%) | 1 (1.7%) | ||
| OR vs. Negative CI | Ref | 0.18 | 0.29 | ||
| 0.01–4.64 | 0.01–15.74 | ||||
| Quilty Effect | Absent | 30 (50.8%) | 11 (18.6%) | 4 (6.8%) | 0.21 |
| Subendocardial | 1 (1.7%) | 4 (6.8%) | 5 (8.5%) | ||
| Endocardial | 1 (1.7%) | 3 (5.1%) | 0 | ||
| OR vs. Negative CI | Ref | 9.55 | 18.75 | ||
| 1.72–53.13 | 2.68–130.95 | ||||
| CD4 | Negative | 15 (25.4%) | 3 (5.1%) | 1 (1.7%) | 0.03 |
| Positive | 17 (28.8%) | 15 (25.4%) | 8 (13.6%) | ||
| OR vs. Negative CI | Ref | 4.41 | 7.06 | ||
| 1.07–18.27 | 0.79–63.18 | ||||
| CD8 | Negative | 15 (25.4%) | 3 (5.1%) | 1 (1.7%) | 0.03 |
| Positive | 17 (28.8%) | 15 (25.4%) | 8 (13.6%) | ||
| OR vs. Negative CI | Ref | 4.41 | 7.06 | ||
| 1.07–18.27 | 0.79–63.18 | ||||
| CD68 | Negative | 14 (23.7%) | 6 (10.2%) | 1 (1.7%) | 0.19 |
| Positive | 18 (30.5%) | 12 (20.3%) | 8 (13.6%) | ||
| OR vs. Negative CI | Ref | 1.56 | 6.22 | ||
| 0.47–5.18 | 0.69–55.77 | ||||
| CD20 | Negative | 30 (50.8%) | 11 (18.6%) | 4 (6.8%) | 0.001 |
| Positive | 2 (3.4%) | 7 (11.9%) | 5 (8.5%) | ||
| OR vs. Negative CI | Ref | 9.55 | 18.78 | ||
| 1.72–53.13 | 2.68–130.95 | ||||
| CD31 | Negative | 15 (25.4%) | 3 (5.1%) | 1 (1.7%) | 0.03 |
| Positive | 17 (28.8%) | 15 (25.4%) | 8 (13.6%) | ||
| OR vs. Negative CI | Ref | 4.41 | 7.06 | ||
| 1.07–18.27 | 0.79–63.18 | ||||
| CD34 | Negative | 15 (25.4%) | 3 (5.1%) | 1 (1.7%) | 0.03 |
| Positive | 17 (28.8%) | 15 (25.4%) | 8 (13.6%) | ||
| OR vs. Negative CI | Ref | 4.41 | 7.06 | ||
| 1.07–18.27 | 0.79–63.18 | ||||
| EGFR | Negative | 19 (32.2%) | 8 (13.6%) | 1 (1.7%) | 0.03 |
| Positive | 13 (22.0%) | 10 (16.9%) | 8 (13.6%) | ||
| OR vs. Negative CI | Ref | 1.83 | 11.69 | ||
| 0.57–5.87 | 1.3–105.03 | ||||
| IgA | Negative | 15 (25.4%) | 3 (5.1%) | 1 (1.7%) | 0.03 |
| Positive | 17 (28.8%) | 15 (25.4%) | 8 (13.6%) | ||
| OR vs. Negative CI | Ref | 4.41 | 7.06 | ||
| 1.07–18.27 | 0.79–63.18 | ||||
| IgM | Negative | 15 (25.4%) | 3 (5.1%) | 1 (1.7%) | 0.03 |
| Positive | 17 (28.8%) | 15 (25.4%) | 8 (13.6%) | ||
| OR vs. Negative CI | Ref | 4.41 | 7.06 | ||
| 1.07–18.27 | 0.79–63.18 | ||||
| Parameter | IL-1 IHC Interpretation (n = 59) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Nuclear Positivity under 50% | Nuclear Positivity over 50% | Citoplasm Positivity under 50% | Citoplasm Positivity over 50% | Membranous Positivity under 50% | Membranous Positivity over 50% | |||
| Acute Cellular Rejection (ACR) | Absent | 14 (23.7%) | 3 (5.1%) | 4 (6.8%) | 3 (5.1%) | 0 | 2 (3.4%) | 0 | 0.0001 |
| Mild | 4 (6.8%) | 8 (13.6%) | 5 (8.5%) | 2 (3.4%) | 0 | 1 (1.7%) | 1 (1.7%) | ||
| Moderate | 2 (3.4%) | 4 (6.8%) | 2 (3.4%) | 0 | 0 | 2 (3.4%) | 1 (1.7%) | ||
| Severe | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | ||
| OR vs. Negative CI | Ref | 8.0 | 3.5 | 1.33 | 1.93 | 3.0 | 9.67 | ||
| 1.69–37.95 | 0.76–16.12 | 0.18–9.91 | 0.03–107.46 | 0.4–22.3 | 0.41–228.26 | ||||
| Antibody-mediated Rejection (AMR) | Absent | 13 (22.0%) | 3 (5.1%) | 3 (5.1%) | 3 (5.1%) | 0 | 1 (1.7%) | 1 (1.7%) | 0.371 |
| Mild | 7 (11.9%) | 11 (18.6%) | 8 (13.6%) | 2 (3.4%) | 0 | 4 (6.8%) | 1 (1.7%) | ||
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Severe | 1 (1.7%) | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | ||
| OR vs. Negative CI | Ref | 6.5 | 4.33 | 1.08 | 1.59 | 6.5 | 1.62 | ||
| 1.39–30.37 | 0.88–21.31 | 0.15–7.96 | 0.03–87.84 | 0.61–68.96 | 0.09–29.78 | ||||
| Vasculitis | Absent | 15 (25.4%) | 5 (8.5%) | 1 (1.7%) | 2 (3.4%) | 0 | 0 | 0 | 0.0028 |
| Present | 6 (10.2%) | 10 (16.9%) | 10 (16.9%) | 3 (5.1%) | 0 | 5 (8.5%) | 2 (3.4%) | ||
| OR vs. Negative CI | Ref | 5.0 | 25.0 | 3.75 | 2.5 | 25.0 | 10.0 | ||
| 1.19–20.92 | 2.6–240.34 | 0.5–28.39 | 0.04–141.02 | 1.18–531.79 | 0.39–255.43 | ||||
| Inflammation | Absent | 0 | 0 | 0 | 0 | 0 | 1 (1.7%) | 0 | 0.0054 |
| Low | 15 (25.4%) | 9 (15.3%) | 8 (13.6%) | 4 (6.8%) | 0 | 1 (1.7%) | 0 | ||
| Moderate | 3 (5.1%) | 1 (1.7%) | 0 | 0 | 0 | 2 (3.4%) | 2 (3.4%) | ||
| Severe | 3 (5.1%) | 5 (8.5%) | 3 (5.1%) | 1 (1.7%) | 0 | 1 (1.7%) | 0 | ||
| OR vs. Negative CI | Ref | 0.71 | 0.52 | 0.24 | 0.02 | 0.1 | 0.1 | ||
| 0.01–38.06 | 0.01–28.24 | 0–13.52 | 0–2.95 | 0–3.35 | 0–6.22 | ||||
| Quilty Effect | Absent | 18 (30.5%) | 12 (20.3%) | 6 (10.2%) | 5 (8.5%) | 0 | 3 (5.1%) | 1 (1.7%) | 0.3028 |
| Subendocardial | 2 (3.4%) | 2 (3.4%) | 4 (6.8%) | 0 | 0 | 2 (3.4%) | 0 | ||
| Endocardial | 1 (1.7%) | 1 (1.7%) | 1 (1.7%) | 0 | 0 | 0 | 1 (1.7%) | ||
| OR vs. Negative CI | Ref | 1.5 | 5.0 | 0.6 | 6.0 | 4.0 | 6.0 | ||
| 0.26–8.71 | 0.91–27.47 | 0.03–14.05 | 0.1–364.24 | 0.46–34.92 | 0.29–124.1 | ||||
| CD4 | Negative | 13 (22.0%) | 2 (3.4%) | 1 (1.7%) | 3 (5.1%) | 0 | 0 | 0 | 0.0022 |
| Positive | 8 (13.6%) | 13 (22.0%) | 10 (16.9%) | 2 (3.4%) | 0 | 5 (8.5%) | 2 (3.4%) | ||
| OR vs. Negative CI | Ref | 10.56 | 16.25 | 1.08 | 1.62 | 16.25 | 6.5 | ||
| 1.87–59.56 | 1.74–152.09 | 0.15–7.96 | 0.03–90.3 | 0.78–338.89 | 0.26–162.96 | ||||
| CD8 | Negative | 13 (22.0%) | 2 (3.4%) | 1 (1.7%) | 3 (5.1%) | 0 | 0 | 0 | 0.0022 |
| Positive | 8 (13.6%) | 13 (22.0%) | 10 (16.9%) | 2 (3.4%) | 0 | 5 (8.5%) | 2 (3.4%) | ||
| OR vs. Negative CI | Ref | 10.56 | 16.25 | 1.08 | 1.62 | 16.25 | 6.5 | ||
| 1.87–59.56 | 1.74–152.09 | 0.15–7.96 | 0.03–90.3 | 0.78–338.89 | 0.26–162.96 | ||||
| CD68 | Negative | 14 (23.7%) | 3 (5.1%) | 1 (1.7%) | 2 (3.4%) | 0 | 0 | 1 (1.7%) | 0.0049 |
| Positive | 7 (11.9%) | 12 (20.3%) | 10 (16.9%) | 3 (5.1%) | 0 | 5 (8.5%) | 1 (1.7%) | ||
| OR vs. Negative CI | Ref | 8.0 | 20.0 | 3.0 | 2.0 | 20.0 | 2.0 | ||
| 1.69–37.95 | 2.11–189.18 | 0.4–22.3 | 0.04–111.8 | 0.95–420.36 | 0.11–36.95 | ||||
| CD20 | Negative | 18 (30.5%) | 12 (20.3%) | 6 (10.2%) | 5 (8.5%) | 0 | 3 (5.1%) | 1 (1.7%) | 0.2156 |
| Positive | 3 (5.1%) | 3 (5.1%) | 5 (8.5%) | 0 | 0 | 2 (3.4%) | 1 (1.7%) | ||
| OR vs. Negative CI | Ref | 1.5 | 5.0 | 0.6 | 6.0 | 4.0 | 6.0 | ||
| 0.26–8.71 | 0.91–27.47 | 0.03–14.05 | 0.1–364.24 | 0.46–34.92 | 0.29–124.1 | ||||
| CD31 | Negative | 13 (22.0%) | 2 (3.4%) | 1 (1.7%) | 3 (5.1%) | 0 | 0 | 0 | 0.0022 |
| Positive | 8 (13.6%) | 13 (22.0%) | 10 (16.9%) | 2 (3.4%) | 0 | 5 (8.5%) | 2 (3.4%) | ||
| OR vs. Negative CI | Ref | 10.56 | 16.25 | 1.08 | 1.62 | 16.25 | 6.5 | ||
| 1.87–59.56 | 1.74–152.09 | 0.15–7.96 | 0.03–90.3 | 0.78–338.89 | 0.26–162.96 | ||||
| CD34 | Negative | 13 (22.0%) | 2 (3.4%) | 1 (1.7%) | 3 (5.1%) | 0 | 0 | 0 | 0.002 |
| Positive | 8 (13.6%) | 13 (22.0%) | 10 (16.9%) | 2 (3.4%) | 0 | 5 (8.5%) | 2 (3.4%) | ||
| OR vs. Negative CI | Ref | 10.56 | 16.25 | 1.08 | 1.62 | 16.25 | 6.5 | ||
| 1.87–59.56 | 1.74–152.09 | 0.15–7.96 | 0.03–90.3 | 0.78–338.89 | 0.26–162.96 | ||||
| EGFR | Negative | 15 (25.4%) | 7 (11.9%) | 1 (1.7%) | 4 (6.8%) | 0 | 0 | 1 (1.7%) | 0.003 |
| Positive | 6 (10.2%) | 8 (13.6%) | 10 (16.9%) | 1 (1.7%) | 0 | 5 (8.5%) | 1 (1.7%) | ||
| OR vs. Negative CI | Ref | 2.86 | 25.0 | 0.62 | 2.5 | 25.0 | 2.5 | ||
| 0.71–11.44 | 2.6–240.34 | 0.06–6.8 | 0.04–141.02 | 1.18–531.79 | 0.13–46.77 | ||||
| IgA | Negative | 13 (22.0%) | 2 (3.4%) | 1 (1.7%) | 3 (5.1%) | 0 | 0 | 0 | 0.0022 |
| Positive | 8 (13.6%) | 13 (22.0%) | 10 (16.9%) | 2 (3.4%) | 0 | 5 (8.5%) | 2 (3.4%) | ||
| OR vs. Negative CI | Ref | 10.56 | 16.25 | 1.08 | 1.62 | 16.25 | 6.5 | ||
| 1.87–59.56 | 1.74–152.09 | 0.15–7.96 | 0.03–90.3 | 0.78–338.89 | 0.26–162.96 | ||||
| IgM | Negative | 13 (22.0%) | 2 (3.4%) | 1 (1.7%) | 3 (5.1%) | 0 | 0 | 0 | 0.0022 |
| Positive | 8 (13.6%) | 13 (22.0%) | 10 (16.9%) | 2 (3.4%) | 0 | 5 (8.5%) | 2 (3.4%) | ||
| OR vs. Negative CI | Ref | 10.56 | 16.25 | 1.08 | 1.62 | 16.25 | 6.5 | ||
| 1.87–59.56 | 1.74–152.09 | 0.15–7.96 | 0.03–90.3 | 0.78–338.89 | 0.26–162.96 | ||||
| ACR | AMR | |
|---|---|---|
| Grade 0 | No rejection | Negative histologic and immunopathologic findings |
| Grade 1 | 1R, mild rejection: Interstitial and/or perivascular infiltrate with up to 1 focus of myocyte damage | Presence of positive histologic and immunopathologic findings |
| Grade 2 | 2 R, moderate rejection: 2 or more foci of infiltrates with associated myocyte damage | Presence of both histologic and immunopathologic findings |
| Grade 3 | 3R severe: Diffuse infiltrate with multifocal myocyte damage, with or without edema, hemorrhage, or vasculitis | Presence of severe histologic plus immunopathologic findings |
| Antibody (Clone) | Source | Manufacturer | Retrieval | Dilution |
|---|---|---|---|---|
| MMP2 | VMS Inc. | Fine Biotech (Finetest) | High pH | 1:50 |
| MMP9 | VMS Inc. | Fine Biotech (Finetest) | High pH | 1:100 |
| IL-1β | VMS Inc. | Fine Biotech (Finetest) | High pH | 1:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrichi, G.; Satala, C.-B.; Patrichi, A.I.; Tomut, A.-N.; Cotoi, O.S.; Suciu, H.; Sin, A.I. Prognostic Role of MMP2, MMP9, and IL-1β Markers in Cardiac Allograft Rejection After Transplantation. Int. J. Mol. Sci. 2025, 26, 9136. https://doi.org/10.3390/ijms26189136
Patrichi G, Satala C-B, Patrichi AI, Tomut A-N, Cotoi OS, Suciu H, Sin AI. Prognostic Role of MMP2, MMP9, and IL-1β Markers in Cardiac Allograft Rejection After Transplantation. International Journal of Molecular Sciences. 2025; 26(18):9136. https://doi.org/10.3390/ijms26189136
Chicago/Turabian StylePatrichi, Gabriela, Catalin-Bogdan Satala, Andrei Ionut Patrichi, Alexandru-Nicusor Tomut, Ovidiu Simion Cotoi, Horatiu Suciu, and Anca Ileana Sin. 2025. "Prognostic Role of MMP2, MMP9, and IL-1β Markers in Cardiac Allograft Rejection After Transplantation" International Journal of Molecular Sciences 26, no. 18: 9136. https://doi.org/10.3390/ijms26189136
APA StylePatrichi, G., Satala, C.-B., Patrichi, A. I., Tomut, A.-N., Cotoi, O. S., Suciu, H., & Sin, A. I. (2025). Prognostic Role of MMP2, MMP9, and IL-1β Markers in Cardiac Allograft Rejection After Transplantation. International Journal of Molecular Sciences, 26(18), 9136. https://doi.org/10.3390/ijms26189136







