N-Acetylchitooligosaccharides Alleviate Pulmonary Inflammation and Modulate Glycerophospholipid Metabolism in Murine Acute Lung Injury
Abstract
1. Introduction
2. Results
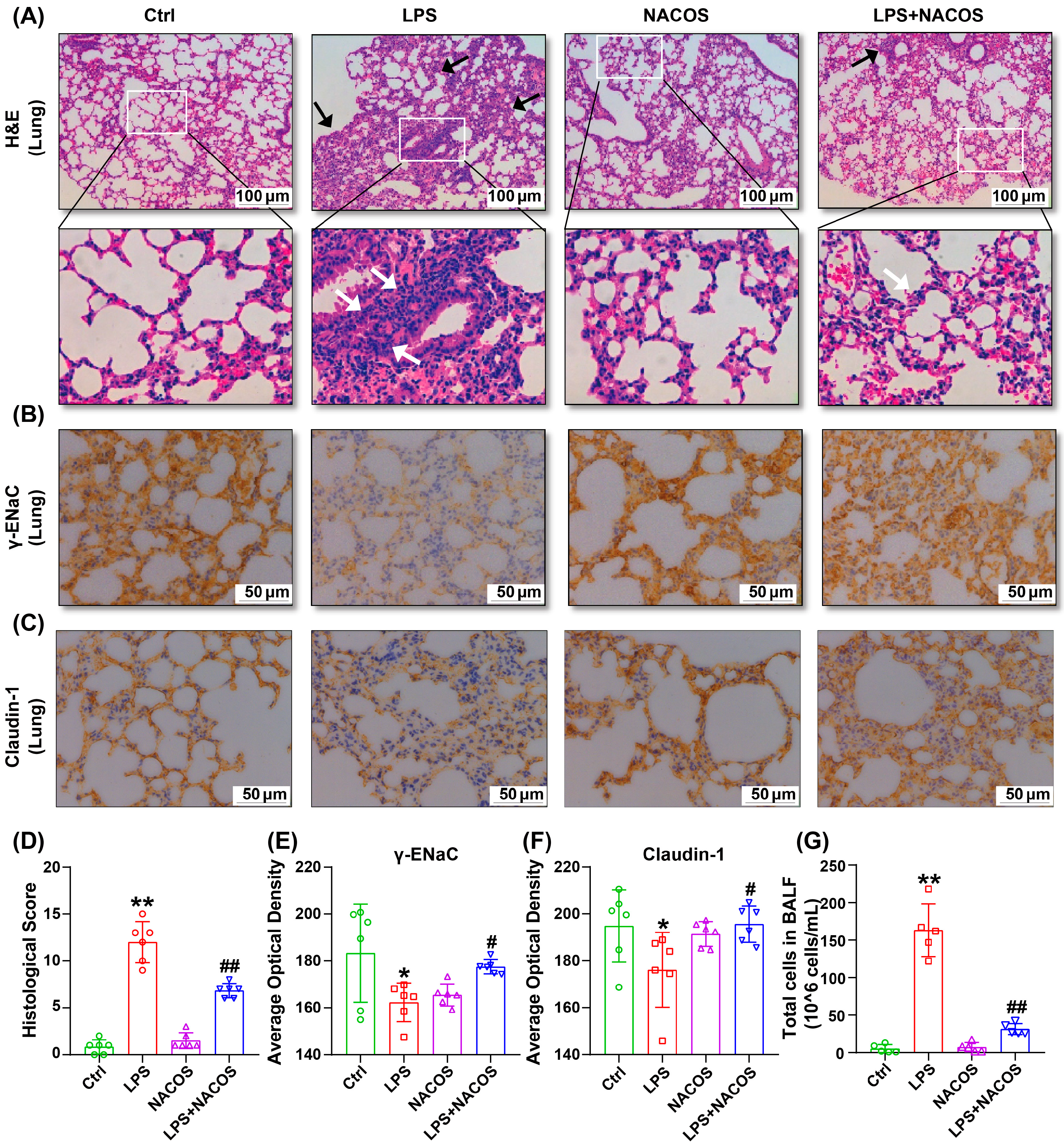
2.1. Effect of NACOS on LPS-Induce ALI in Mice
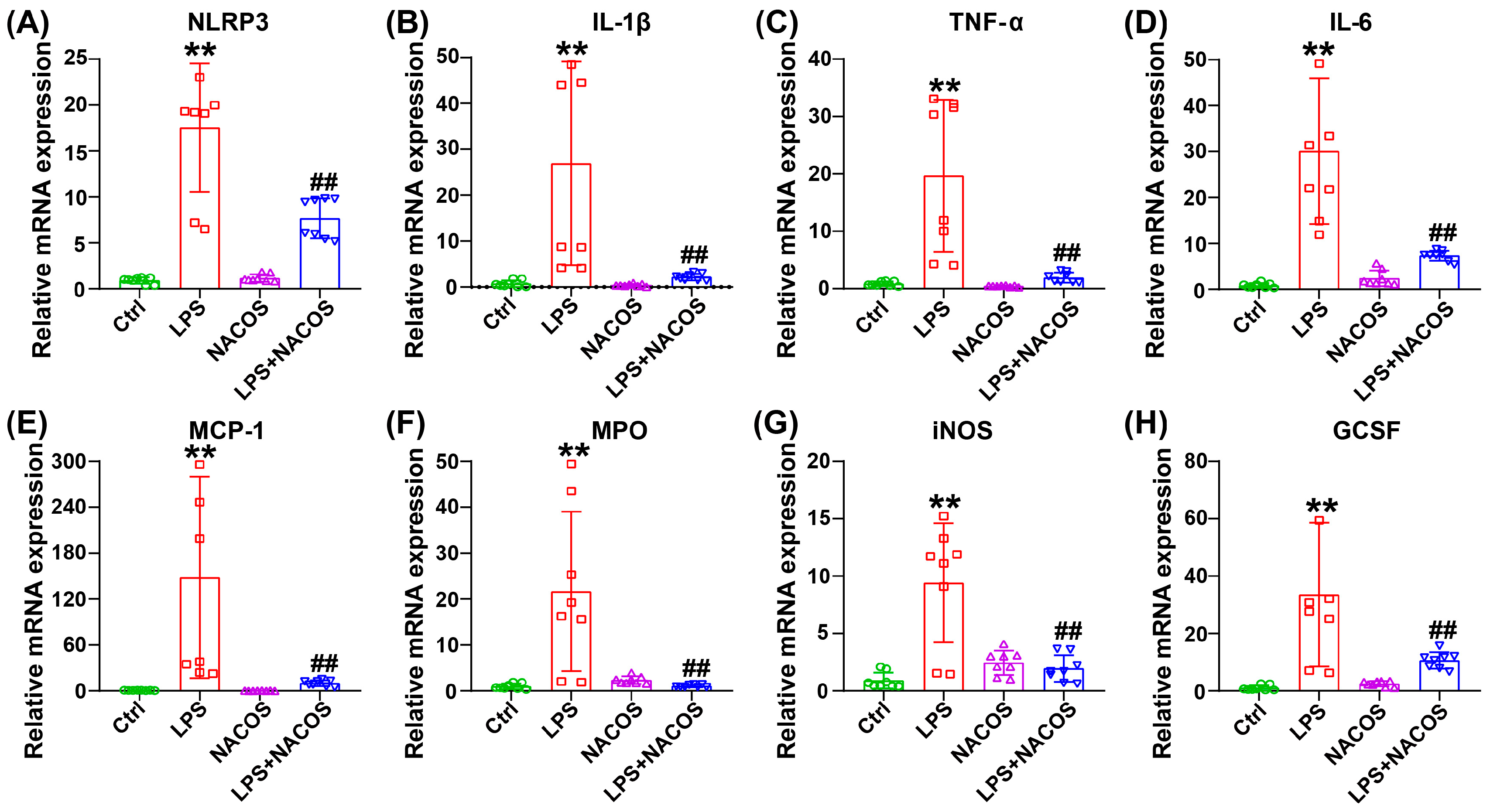
2.2. Effect of NACOS Treatment on Inflammatory Responses in Lung Tissues of ALI Mice
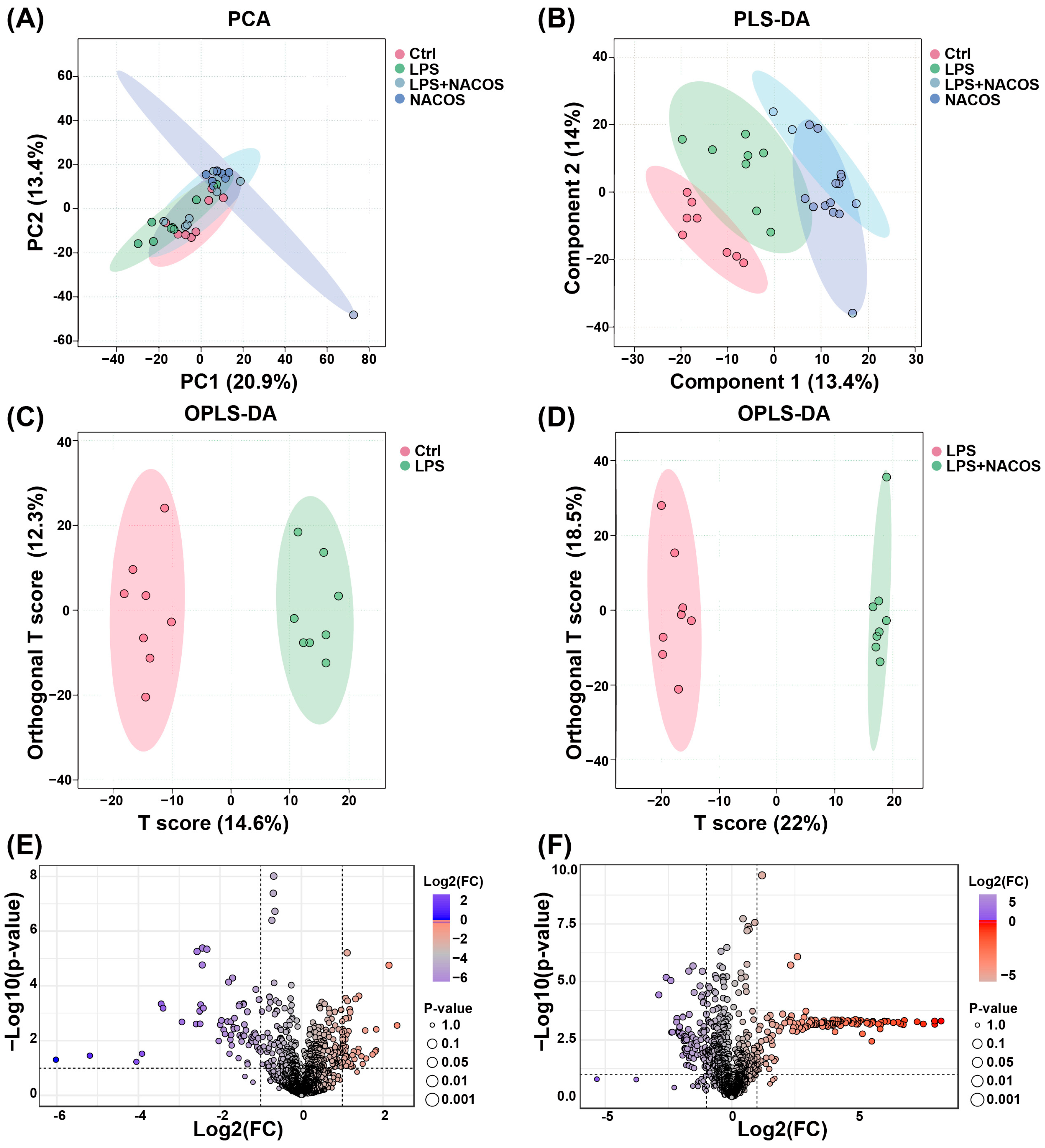
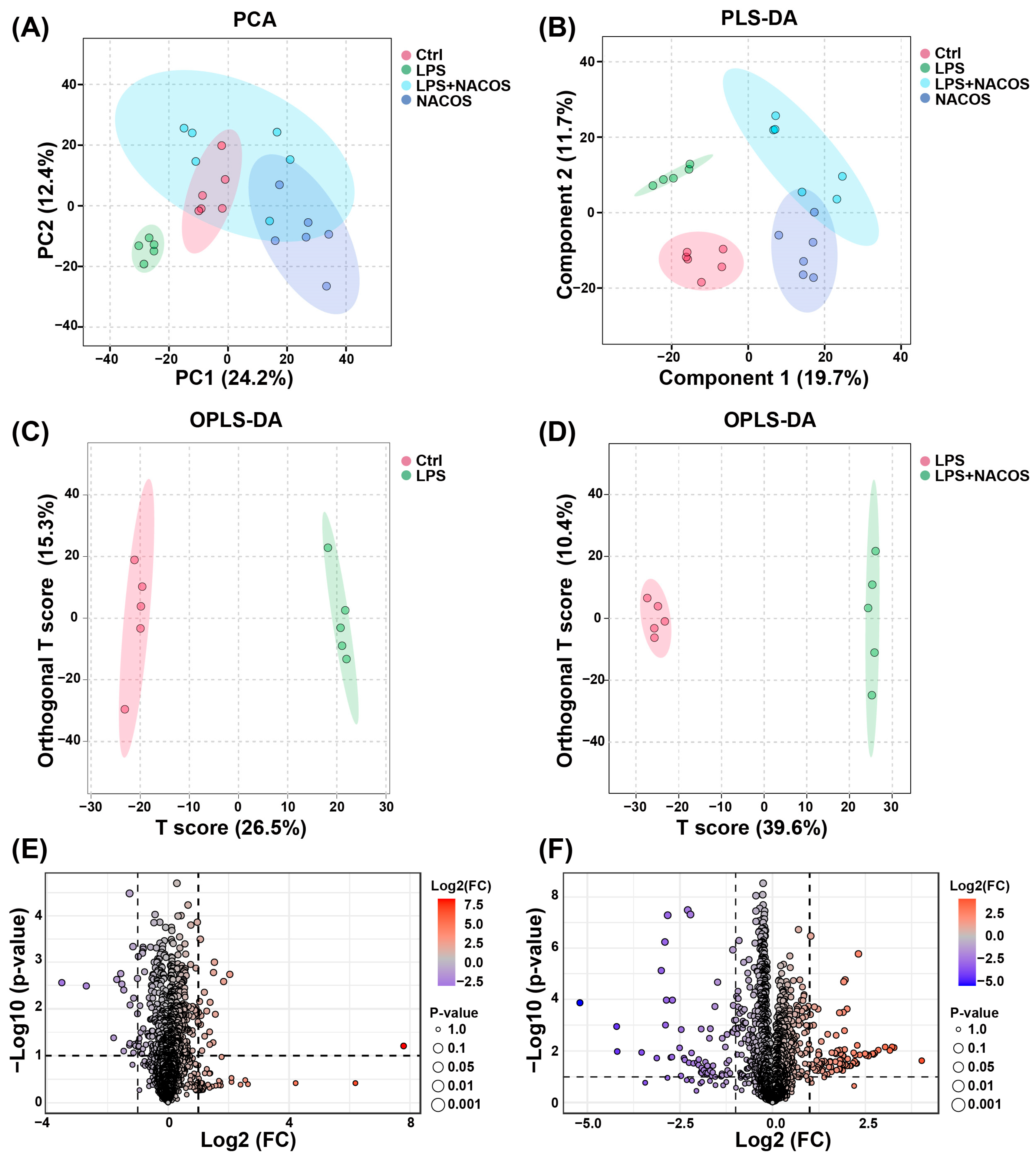
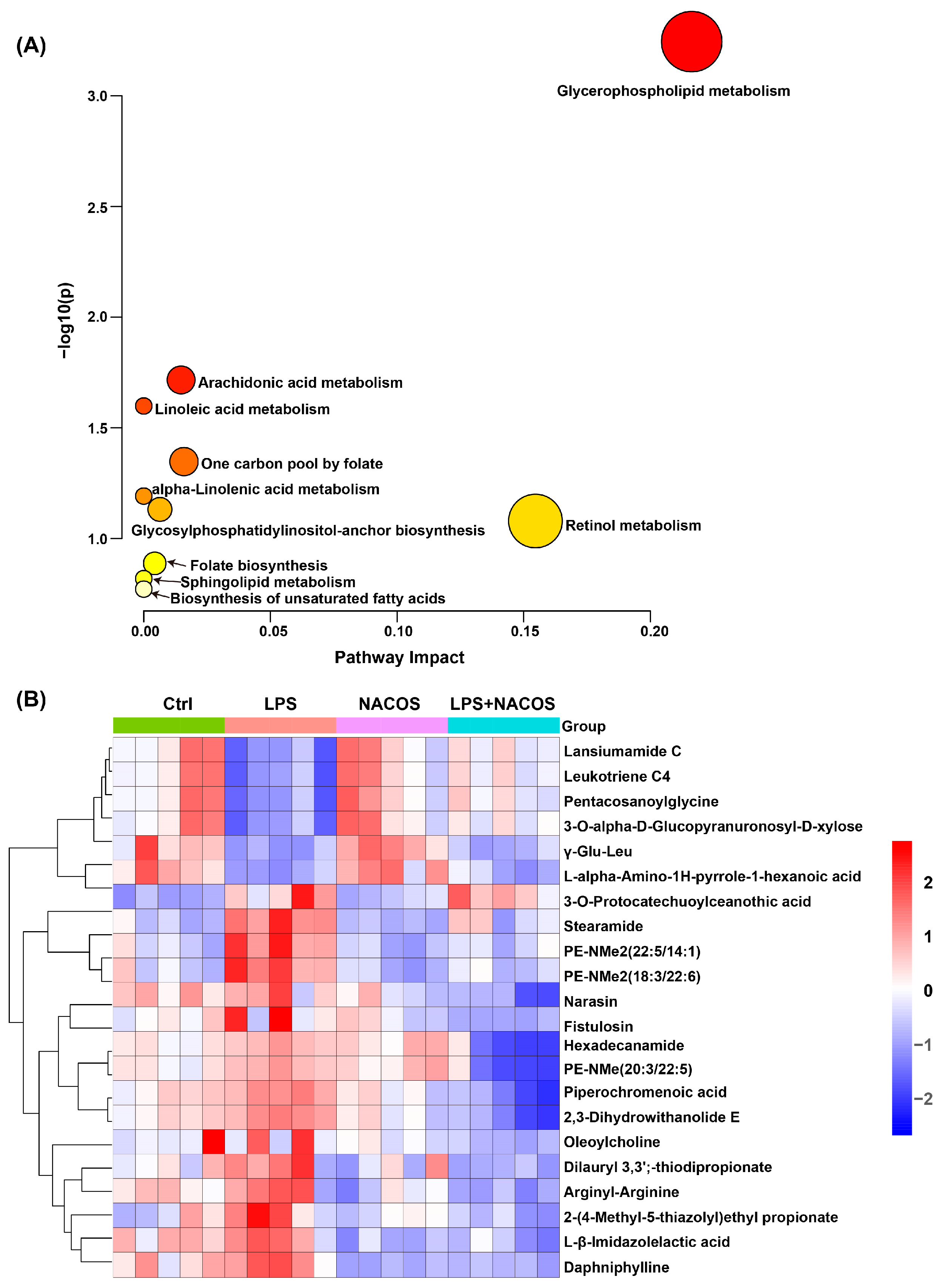
2.3. Multivariate Statistical Analysis of Plasma Metabolomic Profiles
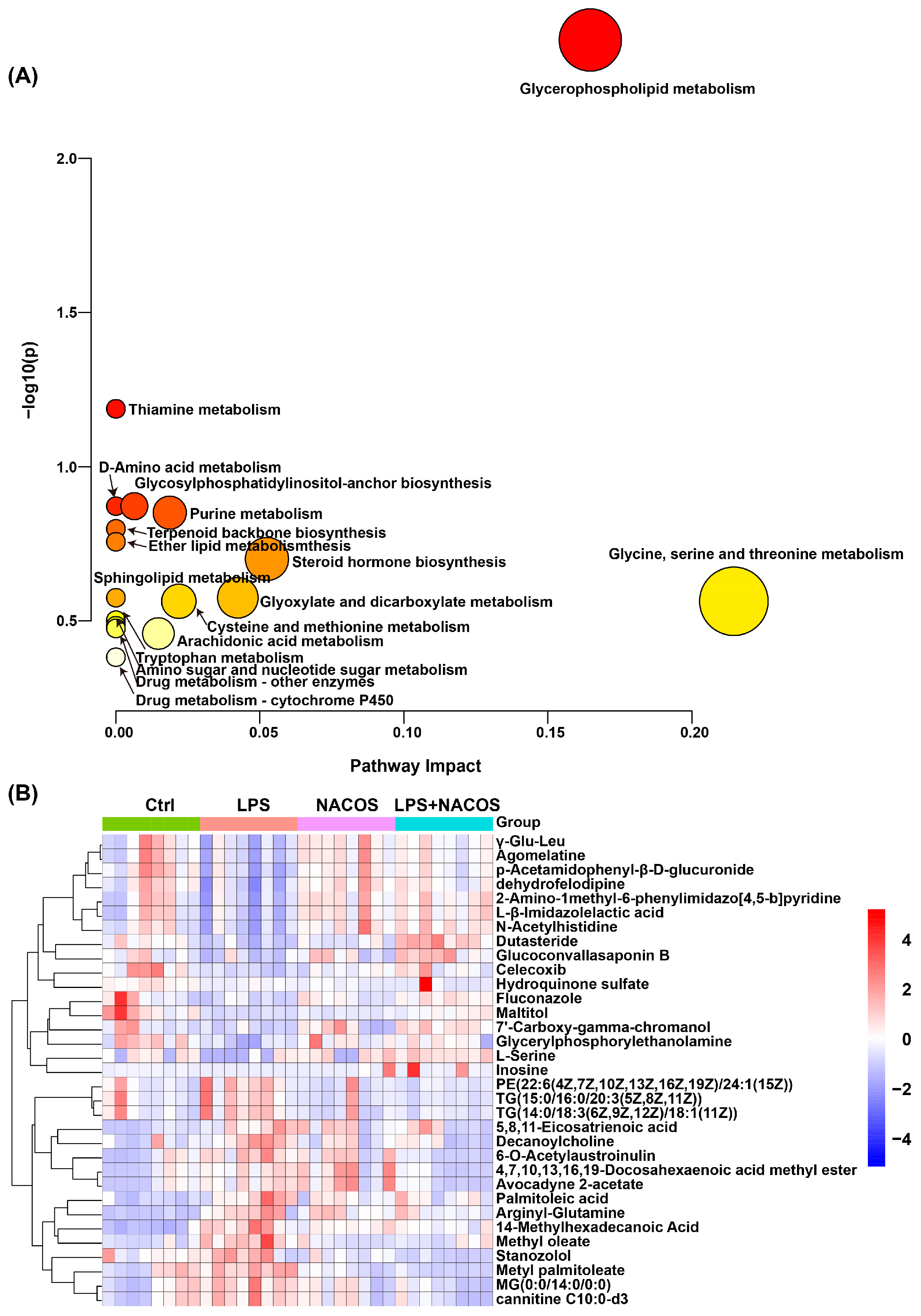
2.4. Heatmap Analysis of Plasma Metabolic Profiles Across Experimental Groups
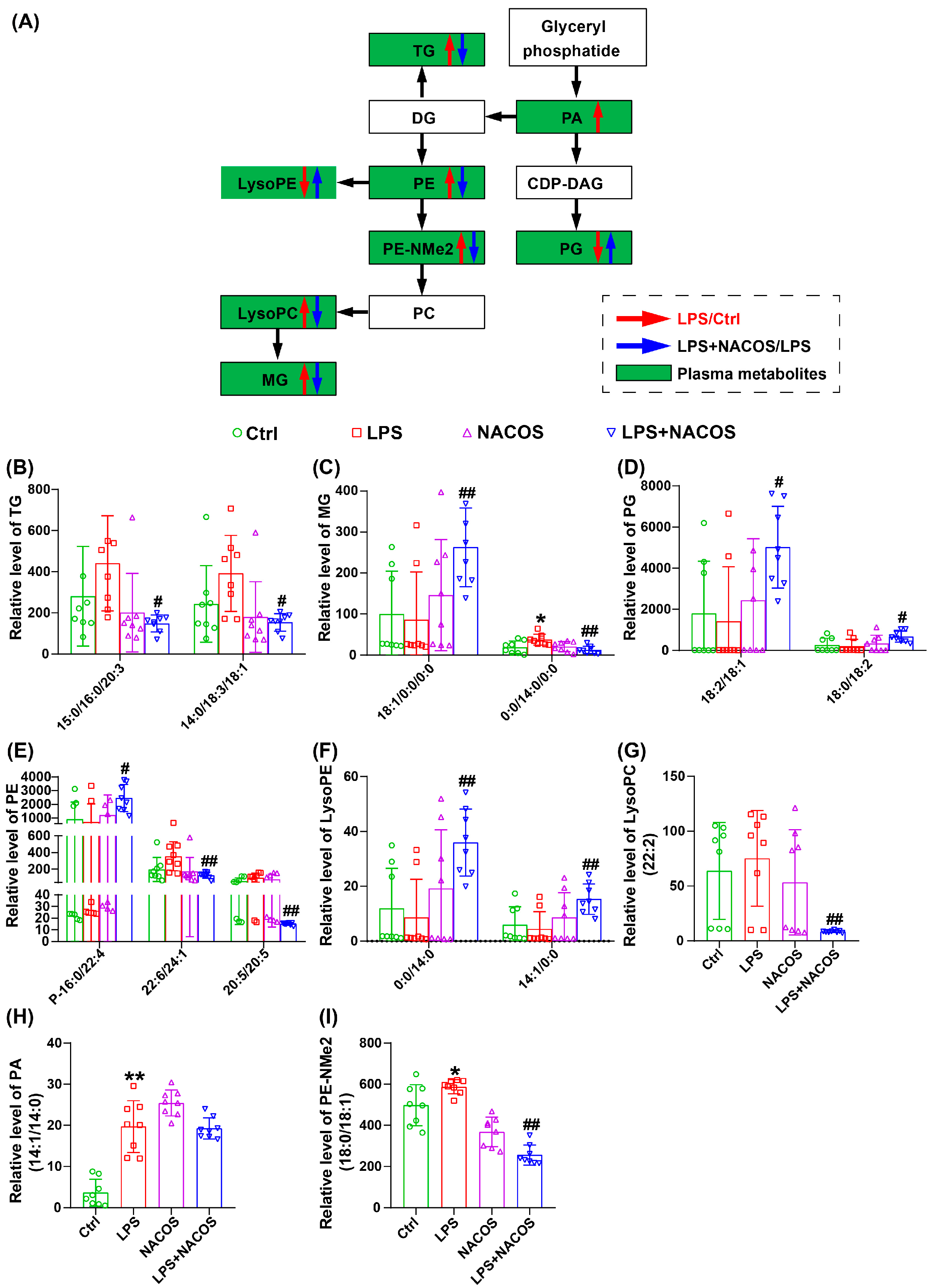
2.5. NACOS Reversed Glycerophospholipid Metabolism in ALI Mice
2.6. Multivariate Statistical Analysis of Metabolites in Lung Tissue
2.7. Heatmap Analysis of Metabolic Profiles in Lung Tissues of Experimental Groups
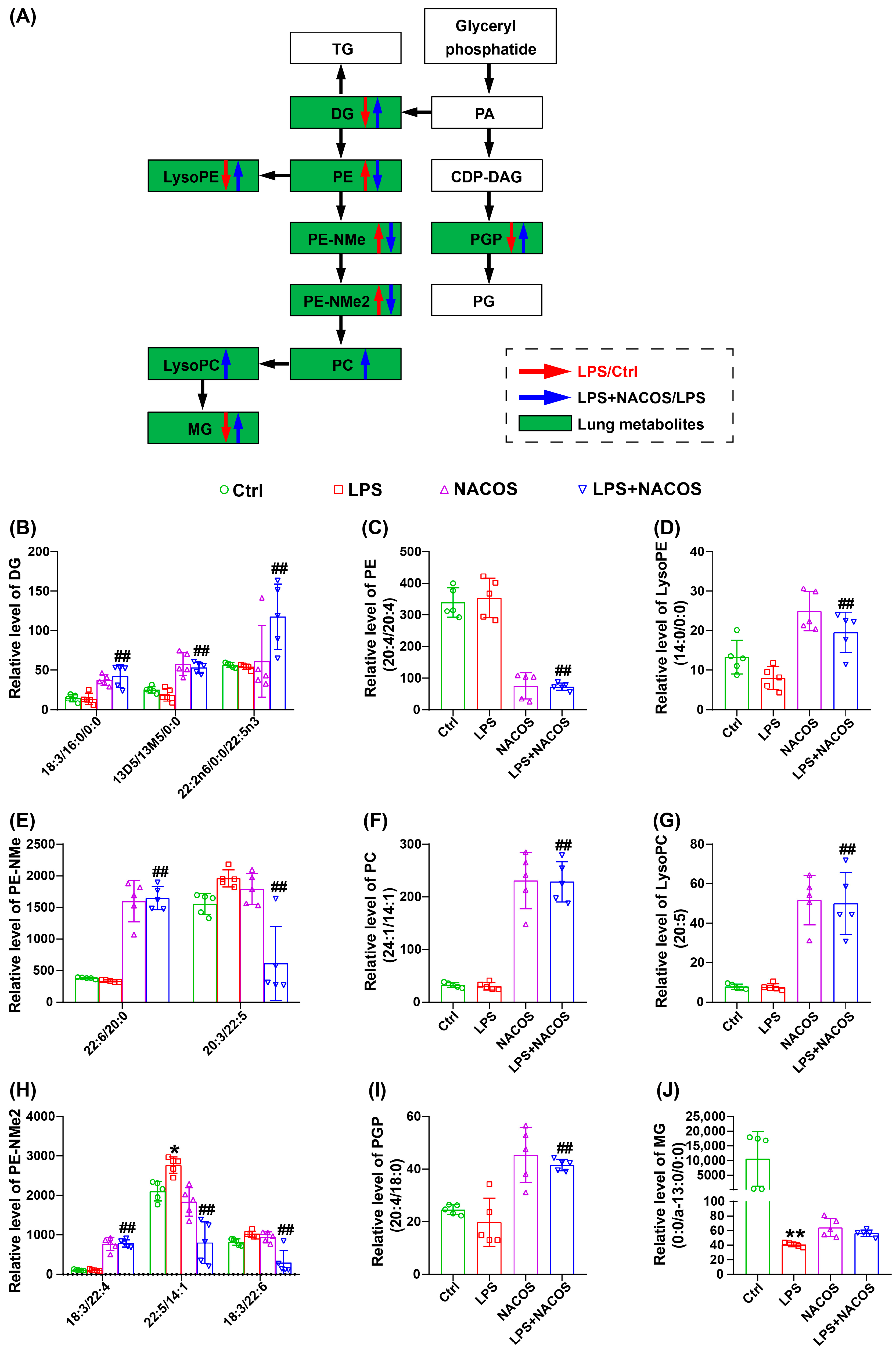
2.8. NACOS Reversed Glycerophospholipid Metabolism in Lung Tissues of ALI Mice
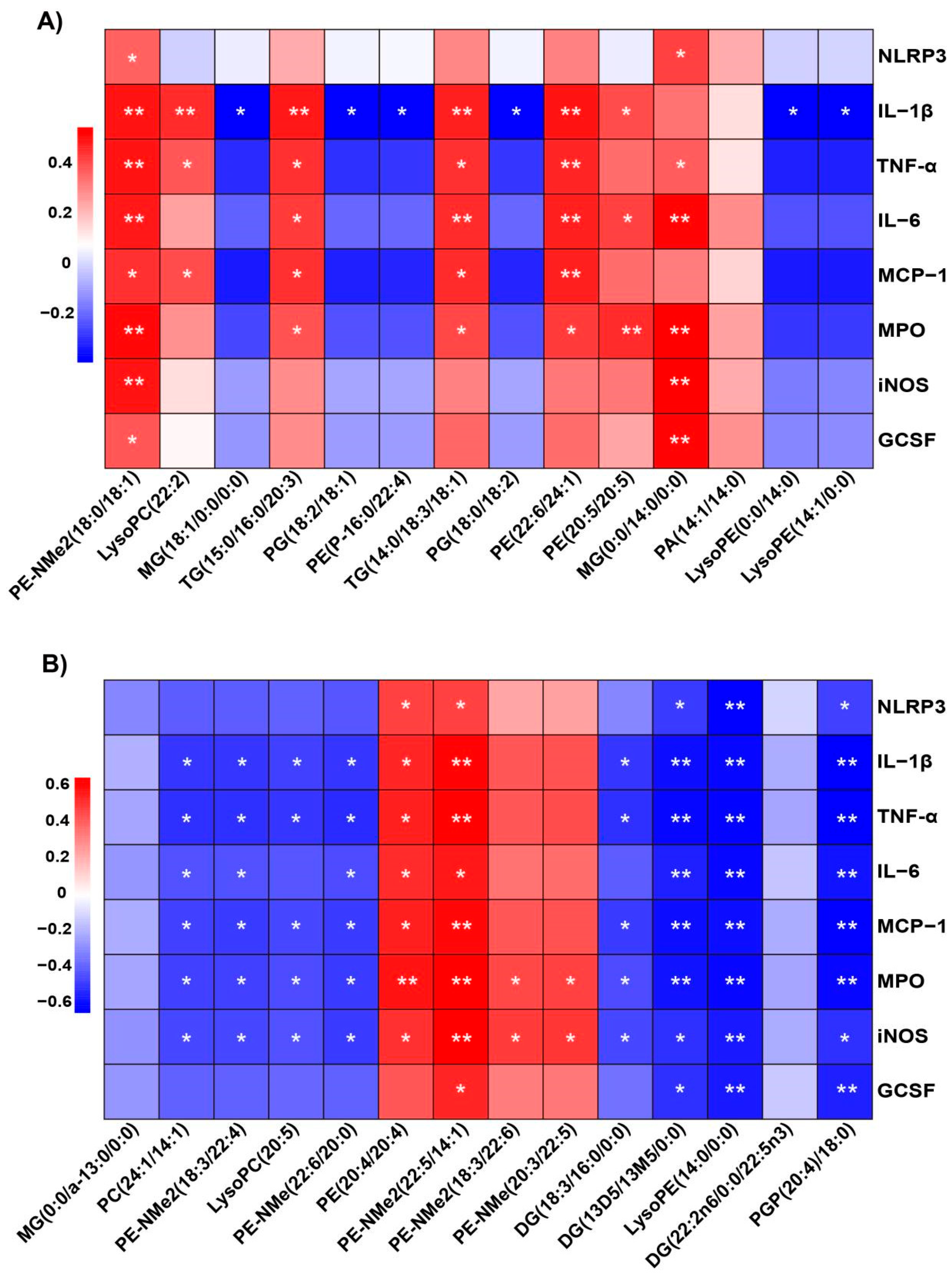
2.9. Correlation Between Plasma and Lung Tissue Metabolites and Inflammatory Markers
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animal Experiment
4.3. Histopathology Analysis of Lung Tissues
4.4. Bronchoalveolar Lavage Fluid (BALF) Collection and Cell Counting
4.5. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALI | Acute lung injury |
| ARDS | Acute respiratory distress syndrome |
| BALF | Bronchoalveolar lavage fluid |
| FC | Fold change |
| GCSF | Granulocyte Colony-Stimulating Factor |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| LC-MS | Liquid chromatography–mass spectrometry |
| MCP-1 | Monocyte chemotactic protein-1 |
| MPO | Myeloperoxidase |
| NACOS | N-acetylchitooligosaccharides |
| NLRP3 | Nod-like receptor pyrin domain containing 3 |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| PCA | Principal component analysis |
| PLS-DA | Partial least squares-discriminant analysis |
| qRT-PCR | Quantitative real-time PCR |
| TNF-α | Tumor necrosis factor-α |
| VIP | Variable importance in projection |
References
- Li, J.; Deng, S.H.; Li, J.; Li, L.; Zhang, F.; Zou, Y.; Wu, D.M.; Xu, Y. Obacunone alleviates ferroptosis during lipopolysaccharide-induced acute lung injury by upregulating Nrf2-dependent antioxidant responses. Cell Mol. Biol. Lett. 2022, 27, 29. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xia, Y.; Jin, S.; Xue, C.; Wang, Y.; Hu, R.; Jiang, H. Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11. Cell Death Dis. 2021, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Cao, L.; Sun, X.; Zhou, S.; Zhu, T.; Zheng, J.; Liu, S.; Liu, H. Metabolomic profile of plasma approach to investigate the mechanism of Poria cocos oligosaccharides attenuated LPS-induced acute lung injury in mice. J. Pharm. Biomed. Anal. 2024, 247, 116262. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, M.; Li, W.; Zhao, M.; Cao, X.; Li, C.; Zhang, H.; Yang, M.; Liang, L.; Yue, Y.; et al. Inhibition of inflammasome activation via sphingolipid pathway in acute lung injury by Huanglian Jiedu decoction: An integrative pharmacology approach. Phytomedicine 2022, 107, 154469. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, Y.; Huang, M.; Chen, J.; Zhang, Z.; Li, J.; Yang, R.; Liu, Y.; Cai, S. Fuzhengjiedu formula exerts protective effect against LPS-induced acute lung injury via gut-lung axis. Phytomedicine 2024, 123, 155190. [Google Scholar] [CrossRef]
- Zhong, W.J.; Liu, T.; Yang, H.H.; Duan, J.X.; Yang, J.T.; Guan, X.X.; Xiong, J.B.; Zhang, Y.F.; Zhang, C.Y.; Zhou, Y.; et al. TREM-1 governs NLRP3 inflammasome activation of macrophages by firing up glycolysis in acute lung injury. Int. J. Biol. Sci. 2023, 19, 242–257. [Google Scholar] [CrossRef]
- Li, D.; Yang, L.; Wang, W.; Song, C.; Xiong, R.; Pan, S.; Li, N.; Geng, Q. Eriocitrin attenuates sepsis-induced acute lung injury in mice by regulating MKP1/MAPK pathway mediated-glycolysis. Int. Immunopharmacol. 2023, 118, 110021. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Liu, Y.; Luo, G.; Ding, S.; Zhang, T.; Li, N.; Geng, Q. The role of fatty acid metabolism in acute lung injury: A special focus on immunometabolism. Cell Mol. Life Sci. 2024, 81, 120. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Feng, L.; Shao, H.; Zhou, Y.; Shan, J.; Lin, L.; Ye, J.; Wang, S. Integrated network pharmacology, molecular docking, and lipidomics to reveal the regulatory effect of Qingxuan Zhike granules on lipid metabolism in lipopolysaccharide-induced acute lung injury. Biomed. Chromatogr. 2024, 38, e5853. [Google Scholar] [CrossRef]
- DeBose-Boyd, R.A. Significance and regulation of lipid metabolism. Semin. Cell Dev. Biol. 2018, 81, 97. [Google Scholar] [CrossRef]
- Liu, G.; Summer, R. Cellular Metabolism in Lung Health and Disease. Annu. Rev. Physiol. 2019, 81, 403–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, K.; Yang, L.; Liu, R.; Chu, Y.; Qin, X.; Yang, P.; Yu, H. Lipid metabolism in inflammation-related diseases. Analyst 2018, 143, 4526–4536. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Chang, C.C.; Mau, W.J.; Yen, L.S. Evaluation of N-acetylchitooligosaccharides as the main carbon sources for the growth of intestinal bacteria. FEMS Microbiol. Lett. 2002, 209, 53–56. [Google Scholar] [CrossRef][Green Version]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef]
- Zhou, W.; Hong, J.; Liu, T.; Li, M.; Jin, H.; Wang, X. Polygonatum Polysaccharide Regulates Macrophage Polarization and Improves LPS-Induced Acute Lung Injury through TLR4-MAPK/NF-κB Pathway. Can. Respir. J. 2022, 2022, 2686992. [Google Scholar] [CrossRef]
- Yu, P.J.; Wan, L.M.; Wan, S.H.; Chen, W.Y.; Xie, H.; Meng, D.M.; Zhang, J.J.; Xiao, X.L. Standardized myrtol attenuates lipopolysaccharide induced acute lung injury in mice. Pharm. Biol. 2016, 54, 3211–3216. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bai, C.; Wang, X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert. Rev. Respir. Med. 2010, 4, 773–783. [Google Scholar] [CrossRef]
- Qi, W.; Li, H.; Cai, X.H.; Gu, J.Q.; Meng, J.; Xie, H.Q.; Zhang, J.L.; Chen, J.; Jin, X.G.; Tang, Q.; et al. Lipoxin A4 activates alveolar epithelial sodium channel gamma via the microRNA-21/PTEN/AKT pathway in lipopolysaccharide-induced inflammatory lung injury. Lab. Investig. 2015, 95, 1258–1268. [Google Scholar] [CrossRef]
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflug. Arch. 2017, 469, 135–147. [Google Scholar] [CrossRef]
- Li, X.; Jamal, M.; Guo, P.; Jin, Z.; Zheng, F.; Song, X.; Zhan, J.; Wu, H. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed. Pharmacother. 2019, 118, 109363. [Google Scholar] [CrossRef]
- Mokra, D.; Kosutova, P. Biomarkers in acute lung injury. Respir. Physiol. Neurobiol. 2015, 209, 52–58. [Google Scholar] [CrossRef]
- Fung, Y.L.; Silliman, C.C. The role of neutrophils in the pathogenesis of transfusion-related acute lung injury. Transfus. Med. Rev. 2009, 23, 266–283. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101 Pt B, 107598. [Google Scholar] [CrossRef]
- Gomez-Mejiba, S.E.; Zhai, Z.; Gimenez, M.S.; Ashby, M.T.; Chilakapati, J.; Kitchin, K.; Mason, R.P.; Ramirez, D.C. Myeloperoxidase-induced genomic DNA-centered radicals. J. Biol. Chem. 2010, 285, 20062–20071. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, T.; Zhao, J.; Gao, H.; Xie, W. Depletion of iNOS-positive inflammatory cells decelerates neuronal degeneration and alleviates cerebral ischemic damage by suppressing the inflammatory response. Free Radic. Biol. Med. 2022, 181, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, N.; Zafar, I.; Robichaud, A.; Wei, C.C.; Shakil, S.; Ahmad, A.; Goymer, H.M.; Abdelsalam, A.; Kashyap, M.P.; Foote, J.B.; et al. Pulmonary pathogenesis in a murine model of inhaled arsenical exposure. Arch. Toxicol. 2023, 97, 1847–1858. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kanazawa, K.; Kanai, R.; Tomita, H.; Saito, M.; Watanabe, N.; Morimoto, J.; Umeda, T.; Kawamata, T.; Rikimaru, M.; et al. A case of primary lung squamous cell carcinoma mimicking malignant mesothelioma producing granulocyte colony stimulating factor with chemotherapy (cisplatin and gemcitabine)-associated thrombotic thrombocytopenic purpura (TTP); An autopsy case report. Lung Cancer 2019, 136, 105–108. [Google Scholar] [CrossRef]
- Pietzner, M.; Stewart, I.D.; Raffler, J.; Khaw, K.T.; Michelotti, G.A.; Kastenmuller, G.; Wareham, N.J.; Langenberg, C. Plasma metabolites to profile pathways in noncommunicable disease multimorbidity. Nat. Med. 2021, 27, 471–479. [Google Scholar] [CrossRef]
- Karki, P.; Birukov, K.G. Oxidized Phospholipids in Healthy and Diseased Lung Endothelium. Cells 2020, 9, 981. [Google Scholar] [CrossRef]
- Hsing, V.; Zhao, H.Q.; Post, M.; Devine, D.; McVey, M.J. Preservation of recipient plasma sphingosine-1-phosphate levels reduces transfusion-related acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 326, L589–L595. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y. Lysophospholipids in Lung Inflammatory Diseases. Adv. Exp. Med. Biol. 2021, 1303, 373–391. [Google Scholar] [CrossRef]
- Wang, M.Y.; Cui, P.; Xin, H.M. Research advances of the roles of sphingosine-1-phosphate in acute lung injury. Chin. J. Burns Wounds 2022, 38, 496–500. [Google Scholar] [CrossRef]
- Karki, P.; Birukov, K.G. Oxidized Phospholipids in Control of Endothelial Barrier Function: Mechanisms and Implication in Lung Injury. Front. Endocrinol. 2021, 12, 794437. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, B.; Guo, Y.; Yang, H.; Zheng, J.; Yao, X.; Hu, H.; Liu, H. Effect of Chitosan oligosaccharides on ischemic symptom and gut microbiota disbalance in mice with hindlimb ischemia. Carbohydr. Polym. 2020, 240, 116271. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Han, Y.; Zhang, W.; Wu, L.; Zhou, A.; Shi, L.; Zhang, J. Glaesserella parasuis infection triggers endoplasmic reticulum stress-mediated pyroptosis via PERK/eIF2α/ATF4 axis and metabolic reprogramming in porcine alveolar macrophages. Vet. Res. 2025, 56, 150. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.H.; Mei, D.; Chua, G.L.; Galam, D.L.; Wenk, M.R.; Torta, F.; Silver, D.L. The lipid transporter Mfsd2a maintains pulmonary surfactant homeostasis. J. Biol. Chem. 2022, 298, 101709. [Google Scholar] [CrossRef]
- Andersen, C.J. Lipid Metabolism in Inflammation and Immune Function. Nutrients 2022, 14, 1414. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Gilroy, D.W. Lipid Mediators in Inflammation. Microbiol. Spectr. 2016, 4, 6. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, M.; Xie, D.; Wu, X.; Hong, C.; Fu, J.; Fan, L.; Wang, S.; Han, S. Glycinergic Signaling in Macrophages and Its Application in Macrophage-Associated Diseases. Front. Immunol. 2021, 12, 762564. [Google Scholar] [CrossRef]
- Gauthier, T.; Chen, W. Modulation of Macrophage Immunometabolism: A New Approach to Fight Infections. Front. Immunol. 2022, 13, 780839. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Mills, E.L. Redox regulation of macrophages. Redox Biol. 2024, 72, 103123. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Mazzon, E.; Sautebin, L.; Serraino, I.; Dugo, L.; Calabró, G.; Caputi, A.P.; Maggi, A. The protective role of endogenous estrogens in carrageenan-induced lung injury in the rat. Mol. Med. 2001, 7, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, G.; Li, Q.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitin Oligosaccharide Modulates Gut Microbiota and Attenuates High-Fat-Diet-Induced Metabolic Syndrome in Mice. Mar. Drugs 2018, 16, 66. [Google Scholar] [CrossRef]
| Gene | Primer Name | Sequence (5′→3′) |
|---|---|---|
| NLRP3 | NLRP3-F | ATTACCCGCCCGAGAAAGG |
| NLRP3-R | TCGCAGCAAAGATCCACACAG | |
| IL-1β | IL-1β-F | GGCTGGACTGTTTCTAATGC |
| IL-1β-R | ATGGTTTCTTGTGACCCTGA | |
| TNF-α | TNF-α-F | GGGTGTTCATCCATTCTC |
| TNF-α-R | GGAAAGCCCATTTGAGT | |
| IL-6 | IL-6-F | GAAACCGCTATGAAGTTCCTCTCTG |
| IL-6-R | TGTTGGGAGTGGTATCCTCTGTGA | |
| MCP-1 | MCP-1-F | TGACCCCAAGAAGGAATGGG |
| MCP-1-R | ACCTTAGGGCAGATGCAGTT | |
| MPO | MPO-F | GCCCACCGAATGACAAG |
| MPO-R | GAAGCCATTGCGATTGA | |
| iNOS | iNOS-F | TTCAGTATCACAACCTCAGCAAG |
| iNOS-R | TGGACCTGCAAGTTAAAATCCC | |
| GCSF | GCSF-F | ATGGCTCAACTTTCTGCCCAG |
| GCSF-R | CTGACAGTGACCAGGGGAAC | |
| β-actin | β-actin-F | CGTGAAAAGATGACCCAGA |
| β-actin-R | GTCCATCACAATGCCTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Liu, F.; Hu, B.; Zhu, L.; Yang, H.; Zheng, J.; Hu, H.; Liu, H. N-Acetylchitooligosaccharides Alleviate Pulmonary Inflammation and Modulate Glycerophospholipid Metabolism in Murine Acute Lung Injury. Int. J. Mol. Sci. 2025, 26, 9128. https://doi.org/10.3390/ijms26189128
Sun X, Liu F, Hu B, Zhu L, Yang H, Zheng J, Hu H, Liu H. N-Acetylchitooligosaccharides Alleviate Pulmonary Inflammation and Modulate Glycerophospholipid Metabolism in Murine Acute Lung Injury. International Journal of Molecular Sciences. 2025; 26(18):9128. https://doi.org/10.3390/ijms26189128
Chicago/Turabian StyleSun, Xiongjie, Fengnan Liu, Baifei Hu, Lin Zhu, Huabing Yang, Junping Zheng, Haiming Hu, and Hongtao Liu. 2025. "N-Acetylchitooligosaccharides Alleviate Pulmonary Inflammation and Modulate Glycerophospholipid Metabolism in Murine Acute Lung Injury" International Journal of Molecular Sciences 26, no. 18: 9128. https://doi.org/10.3390/ijms26189128
APA StyleSun, X., Liu, F., Hu, B., Zhu, L., Yang, H., Zheng, J., Hu, H., & Liu, H. (2025). N-Acetylchitooligosaccharides Alleviate Pulmonary Inflammation and Modulate Glycerophospholipid Metabolism in Murine Acute Lung Injury. International Journal of Molecular Sciences, 26(18), 9128. https://doi.org/10.3390/ijms26189128






