SERCA Silencing Alleviates Aß(1-42)-Induced Toxicity in a C. elegans Model
Abstract
1. Introduction
2. Results
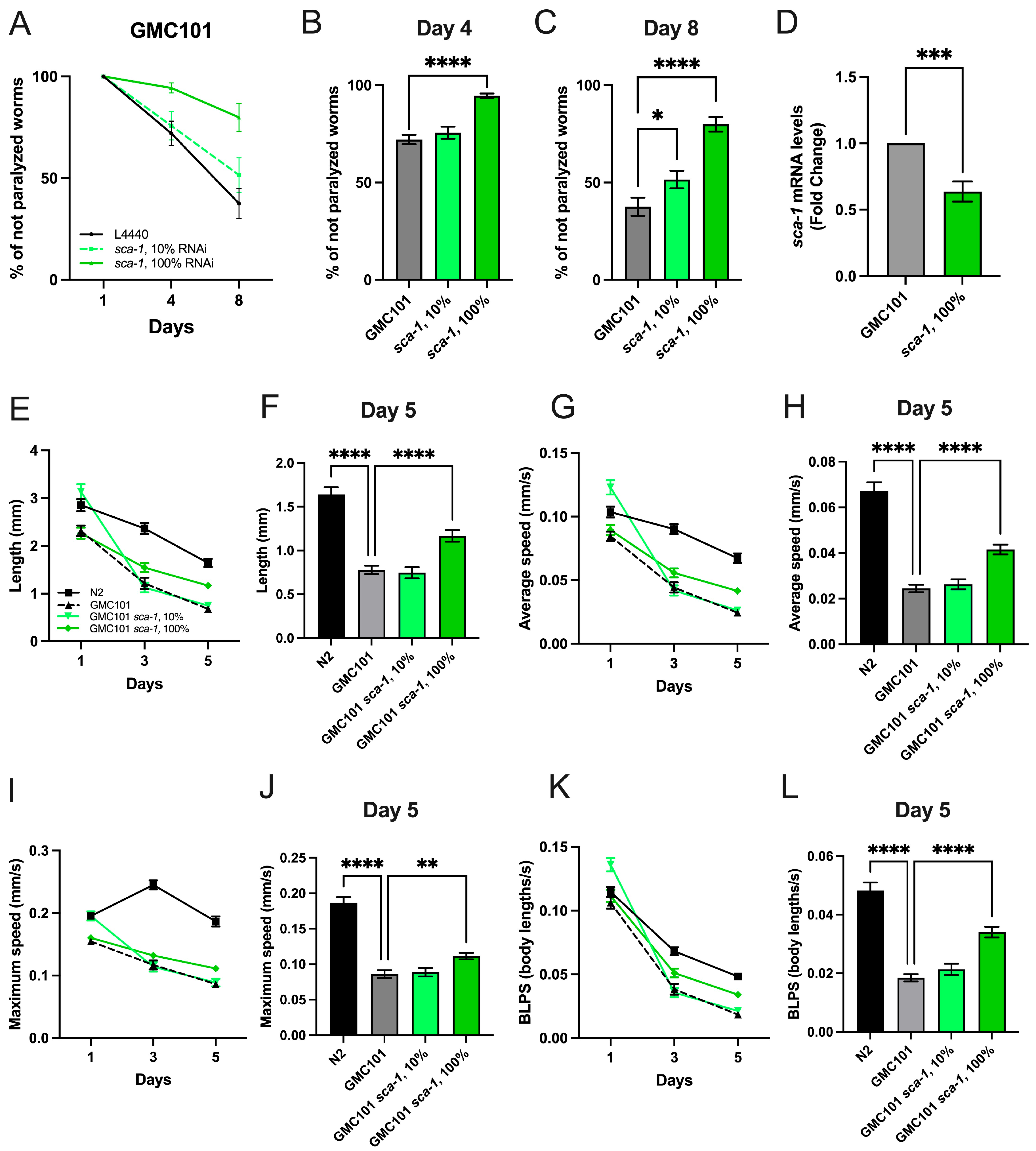
2.1. Sca-1 Silencing Prevents Paralysis and Enhances Mobility in Aβ(1-42)-Overexpressing Worms
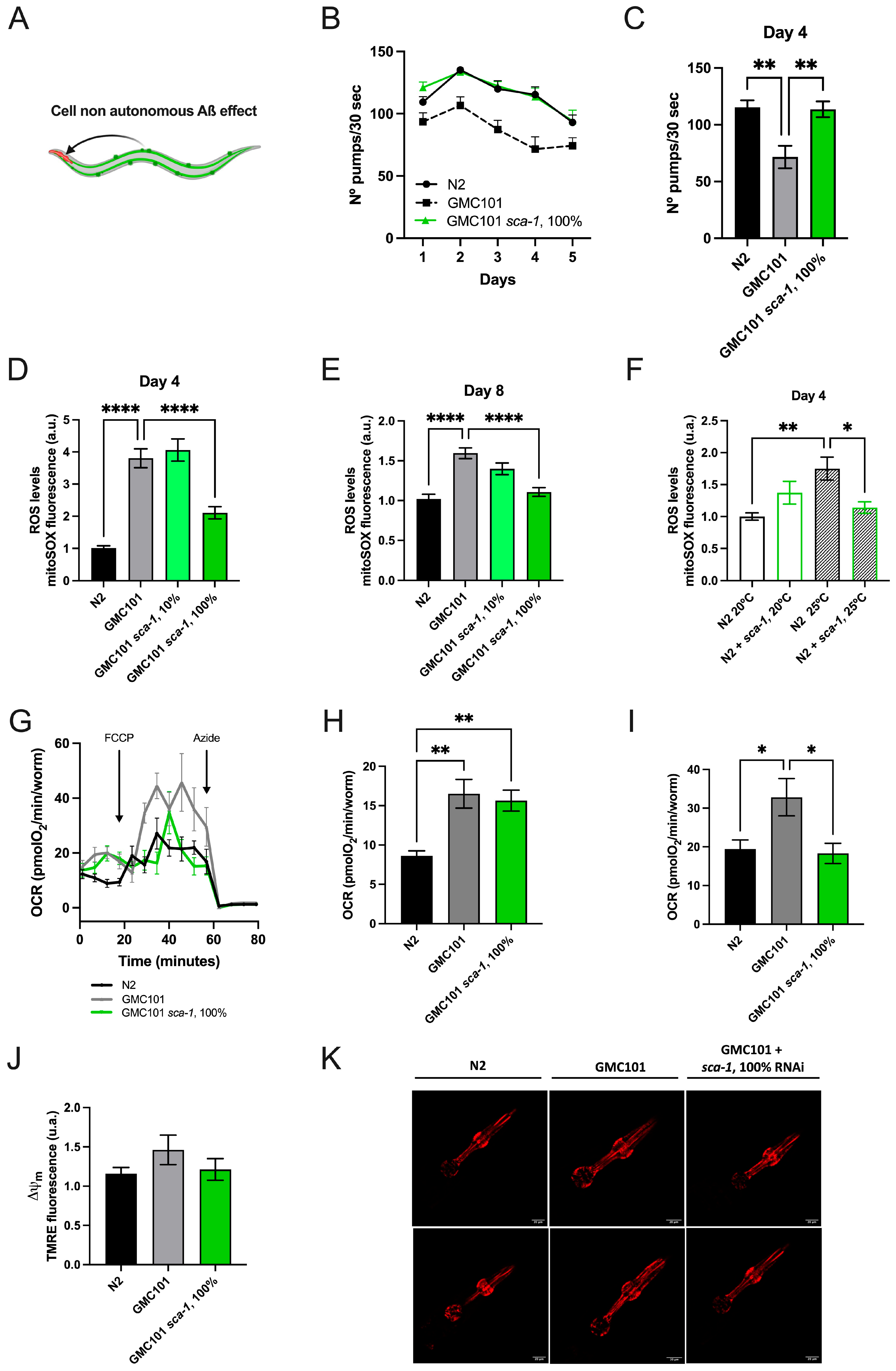
2.2. Sca-1 Silencing Prevents the Development of Early Markers of Aβ(1-42)-Driven Toxicity
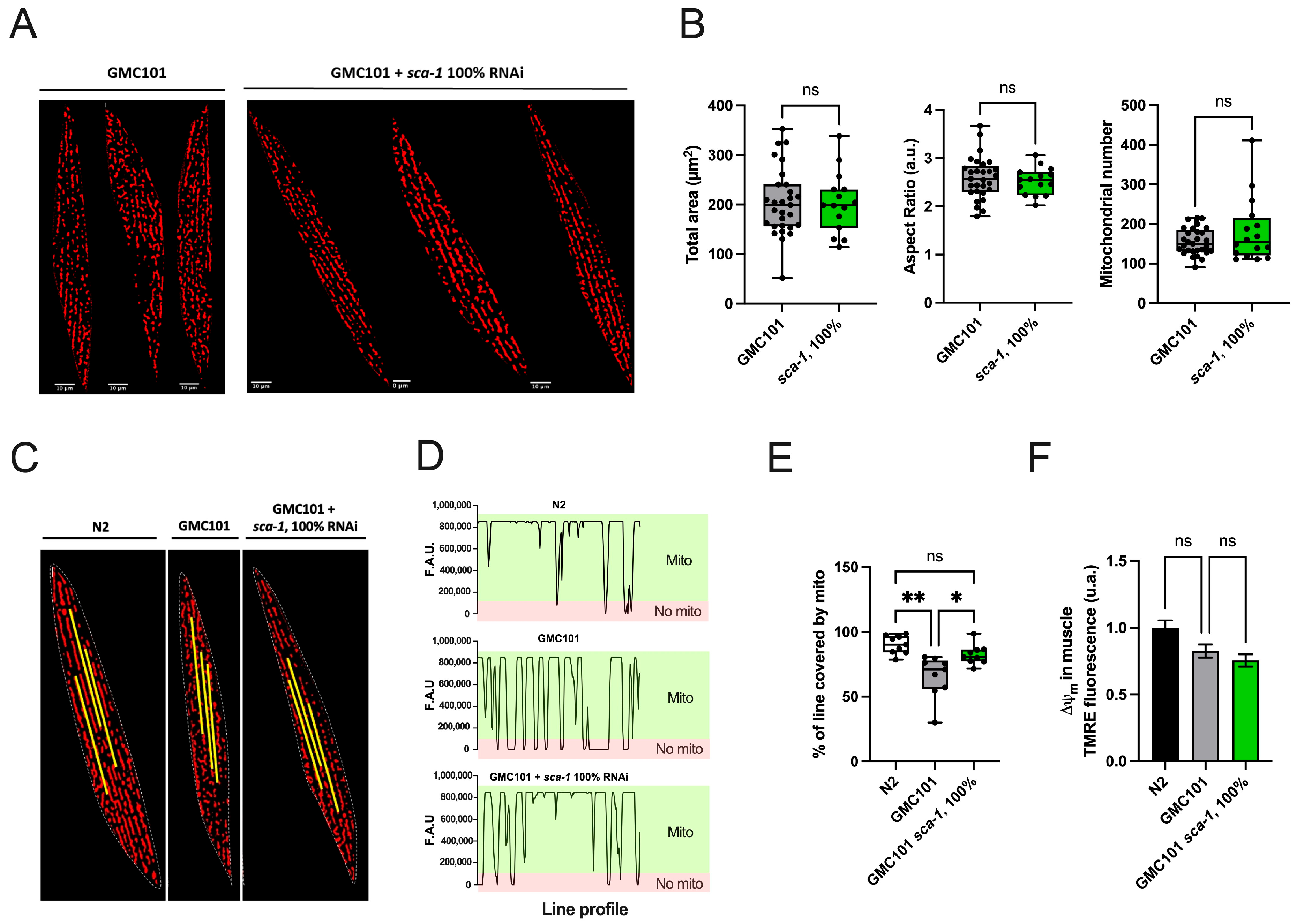
2.3. Sca-1 Silencing Restores the Mitochondrial Network Organization in the Body-Wall Muscle
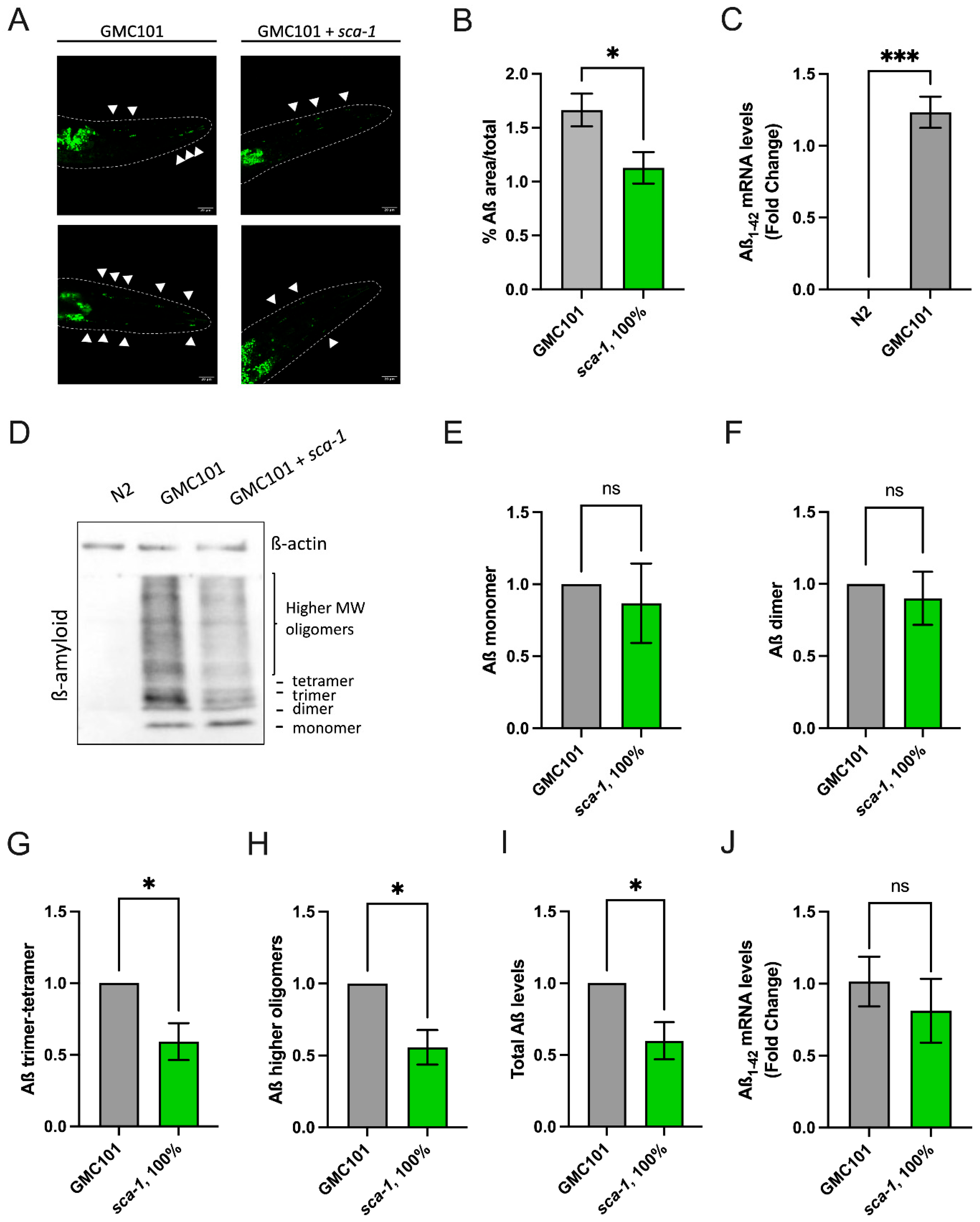
2.4. Sca-1 Knockdown Reduces Aß(1-42) Aggregation and Oligomerization in Body Wall Muscle Cells
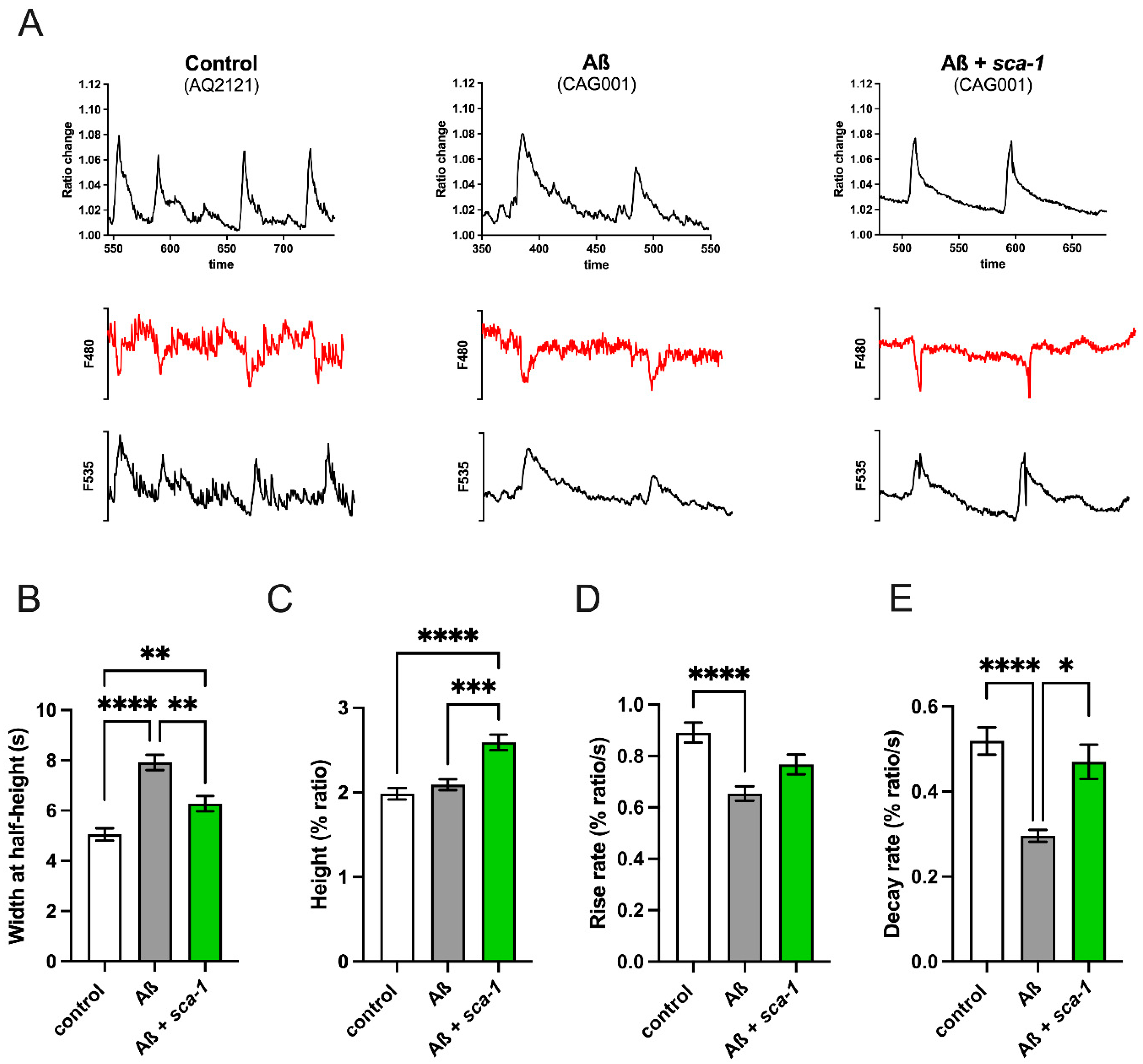
2.5. Sca-1 Silencing Partially Restores Aß(1-42)-Induced Remodeling of Cytosolic Ca2+ Dynamics in Muscle Cells
3. Discussion
4. Materials and Methods
4.1. C. elegans Strains and Maintenance
4.2. RNAi Feeding Worms and Experimental Specifications
4.3. RNA Extraction
4.4. cDNA Synthesis and qRT-PCR
4.5. Paralysis Assays
4.6. Tracking Assays
4.7. Superoxide Measurements
4.8. Mitochondrial Membrane Potential Measurements
4.9. Body-Wall Muscle Mitochondrial Organization
4.10. Oxygen-Consumption Assays
4.11. Calcium Measurements
4.12. Aß In Vivo Aggregate Staining
4.13. Protein Extraction and Wester Blotting
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drouin, E.; Drouin, G. The First Report of Alzheimer’s Disease. Available online: https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(17)30258-2/fulltext (accessed on 8 July 2025).
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat. Rev. Neurosci. 2002, 3, 862–872. [Google Scholar] [CrossRef]
- Yoo, A.S.; Cheng, I.; Chung, S.; Grenfell, T.Z.; Lee, H.; Pack-Chung, E.; Handler, M.; Shen, J.; Xia, W.; Tesco, G.; et al. Presenilin-Mediated Modulation of Capacitative Calcium Entry. Neuron 2000, 27, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z.; Wang, B.; Justice, N.J.; Zheng, H. Amyloid Precursor Protein Regulates Cav1.2 L-type Calcium Channel Levels and Function to Influence GABAergic Short-Term Plasticity. J. Neurosci. 2009, 29, 15660–15668. [Google Scholar] [CrossRef] [PubMed]
- Leissring, M.A.; Murphy, M.P.; Mead, T.R.; Akbari, Y.; Sugarman, M.C.; Jannatipour, M.; Anliker, B.; Müller, U.; Saftig, P.; De Strooper, B.; et al. A physiologic signaling role for the γ-secretase-derived intracellular fragment of APP. Proc. Natl. Acad. Sci. USA 2002, 99, 4697–4702. [Google Scholar] [CrossRef]
- Green, K.N.; Demuro, A.; Akbari, Y.; Hitt, B.D.; Smith, I.F.; Parker, I.; LaFerla, F.M. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J. Cell Biol. 2008, 181, 1107–1116. [Google Scholar] [CrossRef]
- Jin, H.; Sanjo, N.; Uchihara, T.; Watabe, K.; George-Hyslop, P.S.; Fraser, P.E.; Mizusawa, H. Presenilin-1 Holoprotein is an Interacting Partner of Sarco Endoplasmic Reticulum Calcium-ATPase and Confers Resistance to Endoplasmic Reticulum Stress. J. Alzheimer’s Dis. 2010, 20, 261–273. [Google Scholar] [CrossRef]
- Greotti, E.; Capitanio, P.; Wong, A.; Pozzan, T.; Pizzo, P.; Pendin, D. Familial Alzheimer’s disease-linked presenilin mutants and intracellular Ca2+ handling: A single-organelle, FRET-based analysis. Cell Calcium 2019, 79, 44–56. [Google Scholar] [CrossRef]
- Querfurth, H.W.; Selkoe, D.J. Calcium Ionophore Increases Amyloid. beta. Peptide Production by Cultured Cells. Biochemistry 1994, 33, 4550–4561. [Google Scholar] [CrossRef] [PubMed]
- Krajnak, K.; Dahl, R. A new target for Alzheimer’s disease: A small molecule SERCA activator is neuroprotective in vitro and improves memory and cognition in APP/PS1 mice. Bioorg. Med. Chem. Lett. 2018, 28, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.; Moore, A.C.; Knight, C.; Mauger, C.; Zhang, H.; Schiltz, G.E.; Koss, W.A.; Bezprozvanny, I. Positive Allosteric Modulator of SERCA Pump NDC-1173 Exerts Beneficial Effects in Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 11057. [Google Scholar] [CrossRef] [PubMed]
- Rakovskaya, A.; Erofeev, A.; Vinokurov, E.; Pchitskaya, E.; Dahl, R.; Bezprozvanny, I. Positive Allosteric Modulators of SERCA Pump Restore Dendritic Spines and Rescue Long-Term Potentiation Defects in Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2023, 24, 13973. [Google Scholar] [CrossRef]
- Alvarez, J.; Alvarez-Illera, P.; García-Casas, P.; Fonteriz, R.I.; Montero, M. The Role of Ca2+ Signaling in Aging and Neurodegeneration: Insights from Caenorhabditis elegans Models. Cells 2020, 9, 204. [Google Scholar] [CrossRef]
- Alvarez, J.; Alvarez-Illera, P.; Santo-Domingo, J.; Fonteriz, R.I.; Montero, M. Modeling Alzheimer’s Disease in Caenorhabditis elegans. Biomedicines 2022, 10, 288. [Google Scholar] [CrossRef]
- Zwaal, R.R.; Van Baelen, K.; Groenen, J.T.M.; van Geel, A.; Rottiers, V.; Kaletta, T.; Dode, L.; Raeymaekers, L.; Wuytack, F.; Bogaert, T. The Sarco-Endoplasmic Reticulum Ca2+ ATPase Is Required for Development and Muscle Function in Caenorhabditis elegans. J. Biol. Chem. 2001, 276, 43557–43563. [Google Scholar] [CrossRef]
- García-Casas, P.; Arias-Del-Val, J.; Alvarez-Illera, P.; Fonteriz, R.I.; Montero, M.; Alvarez, J. Inhibition of Sarco-Endoplasmic Reticulum Ca2+ ATPase Extends the Lifespan in C. elegans Worms. Front. Pharmacol. 2018, 9, 669. [Google Scholar] [CrossRef]
- García-Casas, P.; Alvarez-Illera, P.; Fonteriz, R.I.; Montero, M.; Alvarez, J. Mechanism of the lifespan extension induced by submaximal SERCA inhibition in C. elegans. Mech. Ageing Dev. 2021, 196, 111474. [Google Scholar] [CrossRef]
- Xu, K.; Tavernarakis, N.; Driscoll, M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron 2001, 31, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Aggad, D.; Vérièpe, J.; Tauffenberger, A.; Parker, J.A. TDP-43 Toxicity Proceeds via Calcium Dysregulation and Necrosis in Aging Caenorhabditis elegans Motor Neurons. J. Neurosci. 2014, 34, 12093–12103. [Google Scholar] [CrossRef]
- Betzer, C.; Lassen, L.B.; Olsen, A.; Kofoed, R.H.; Reimer, L.; Gregersen, E.; Zheng, J.; Calì, T.; Gai, W.; Chen, T.; et al. Alpha-synuclein aggregates activate calcium pump SERCA leading to calcium dysregulation. EMBO Rep. 2018, 19, e44617. [Google Scholar] [CrossRef]
- Griffin, E.F.; Scopel, S.E.; Stephen, C.A.; Holzhauer, A.C.; Vaji, M.A.; Tuckey, R.A.; Berkowitz, L.A.; Caldwell, K.A.; Caldwell, G.A. ApoE-associated modulation of neuroprotection from Aβ-mediated neurodegeneration in transgenic Caenorhabditis elegans. Dis. Model. Mech. 2019, 12, dmm037218. [Google Scholar] [CrossRef]
- McColl, G.; Roberts, B.R.; Pukala, T.L.; Kenche, V.B.; Roberts, C.M.; Link, C.D.; Ryan, T.M.; Masters, C.L.; Barnham, K.J.; Bush, A.I.; et al. Utility of an improved model of amyloid-beta (Aβ1-42) toxicity in Caenorhabditis elegans for drug screening for Alzheimer’s disease. Mol. Neurodegener. 2012, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Caldero-Escudero, E.; Romero-Sanz, S.; Álvarez-Illera, P.; De la Fuente, S.; García-Casas, P.; Fonteriz, R.I.; Montero, M.; Álvarez, J.; Santo-Domingo, J. Calreticulin (crt-1) silencing reduces Aβ1-42-induced toxicity and restores muscle function in C. elegans. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2025, 1871, 167946. [Google Scholar] [CrossRef] [PubMed]
- Weeks, J.C.; Robinson, K.J.; Wanjeri, B.; Copenhaver, P.F.; Roberts, W.M. Microfluidic EPG recordings show striking pharyngeal pumping phenotype in a C. elegans Alzheimer’s disease model. MicroPubl. Biol. 2016, 10-17912. [Google Scholar] [CrossRef]
- Romani, M.; Sorrentino, V.; Oh, C.-M.; Li, H.; de Lima, T.I.; Zhang, H.; Shong, M.; Auwerx, J. NAD+ boosting reduces age-associated amyloidosis and restores mitochondrial homeostasis in muscle. Cell Rep. 2021, 34, 108660. [Google Scholar] [CrossRef]
- Ng, L.F.; Gruber, J.; Cheah, I.K.; Goo, C.K.; Cheong, W.F.; Shui, G.; Sit, K.P.; Wenk, M.R.; Halliwell, B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic. Biol. Med. 2014, 71, 390–401. [Google Scholar] [CrossRef]
- Alvarez-Illera, P.; Sanchez-Blanco, A.; Lopez-Burillo, S.; Fonteriz, R.I.; Alvarez, J.; Montero, M. Long-term monitoring of Ca2+ dynamics in C. elegans pharynx: An in vivo energy balance sensor. Oncotarget 2016, 7, 67732. [Google Scholar] [CrossRef]
- Gieseler, K.; Qadota, H.; Benian, G.M. Development, structure, and maintenance of C. elegans body wall muscle. In The Online Review of C. elegans Biology; WormBook: Pasadena, CA, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK426064/ (accessed on 11 July 2025).
- Etheridge, T.; Rahman, M.; Gaffney, C.J.; Shaw, D.; Shephard, F.; Magudia, J.; Solomon, D.E.; Milne, T.; Blawzdziewicz, J.; Constantin-Teodosiu, D.; et al. The integrin-adhesome is required to maintain muscle structure, mitochondrial ATP production, and movement forces in Caenorhabditis elegans. FASEB J. 2015, 29, 1235–1246. [Google Scholar] [CrossRef]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.-S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Sinha, S.; Elbaz-Alon, Y.; Avinoam, O. Ca2+ as a coordinator of skeletal muscle differentiation, fusion and contraction. FEBS J. 2022, 289, 6531–6542. [Google Scholar] [CrossRef]
- Burr, A.R.; Molkentin, J.D. Genetic evidence in the mouse solidifies the calcium hypothesis of myofiber death in muscular dystrophy. Cell Death Differ. 2015, 22, 1402–1412. [Google Scholar] [CrossRef]
- Ciechanover, A.; Kwon, Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015, 47, e147. [Google Scholar] [CrossRef]
- Kapulkin, V.; Hiester, B.G.; Link, C.D. Compensatory regulation among ER chaperones in C. elegans. FEBS Lett. 2005, 579, 3063–3068. [Google Scholar] [CrossRef]
- Dai, L.-L.; Gao, J.-X.; Zou, C.-G.; Ma, Y.-C.; Zhang, K.-Q. mir-233 Modulates the Unfolded Protein Response in C. elegans during Pseudomonas aeruginosa Infection. PLoS Pathog. 2015, 11, e1004606. [Google Scholar] [CrossRef]
- Howard, A.C.; Rollins, J.; Snow, S.; Castor, S.; Rogers, A.N. Reducing translation through eIF4G/IFG-1 improves survival under ER stress that depends on heat shock factor HSF-1 in Caenorhabditis elegans. Aging Cell 2016, 15, 1027–1038. [Google Scholar] [CrossRef]
- Lehmann, S.; Shephard, F.; Jacobson, L.A.; Szewczyk, N.J. Integrated Control of Protein Degradation in C. elegans Muscle. In Worm; Taylor & Francis: Abingdon, UK, 2012. Available online: https://pubmed.ncbi.nlm.nih.gov/23457662/ (accessed on 12 May 2025).
- Florez-McClure, M.L.; Hohsfield, L.A.; Fonte, G.; Bealor, M.T.; Link, C.D. Decreased insulin-receptor signaling promotes the autophagic degradation of beta-amyloid peptide in C. elegans. Autophagy 2007, 3, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Ishiwata, M.; Weitemier, A.Z.; Shoji, H.; Monai, H.; Miyamoto, H.; Kato, T. Brain-specific heterozygous loss-of-function of ATP2A2, endoplasmic reticulum Ca2+ pump responsible for Darier’s disease, causes behavioral abnormalities and a hyper-dopaminergic state. Hum. Mol. Genet. 2021, 30, 1762–1772, Correction in Hum. Mol. Genet. 2023, 32, 3204. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidou, E.; Gioran, A.; Bano, D.; Chondrogianni, N. Neuron-specific proteasome activation exerts cell non-autonomous protection against amyloid-beta (Aβ) proteotoxicity in Caenorhabditis elegans. Redox Biol. 2023, 65, 102817. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.; Shi, R.; Luciani, D.S. A pipeline for multidimensional confocal analysis of mitochondrial morphology, function, and dynamics in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E87–E101. [Google Scholar] [CrossRef] [PubMed]
- Koopman, M.; Michels, H.; Dancy, B.M.; Kamble, R.; Mouchiroud, L.; Auwerx, J.; Houtkooper, R.H. A screening-based platform for the assessment of cellular respiration in Caenorhabditis elegans. Nat. Protoc. 2016, 11, 1798–1816. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldero-Escudero, E.; Romero-Sanz, S.; Álvarez-Illera, P.; Fernandez-Martinez, S.; De la Fuente, S.; García-Casas, P.; Fonteriz, R.I.; Montero, M.; Alvarez, J.; Santo-Domingo, J. SERCA Silencing Alleviates Aß(1-42)-Induced Toxicity in a C. elegans Model. Int. J. Mol. Sci. 2025, 26, 9126. https://doi.org/10.3390/ijms26189126
Caldero-Escudero E, Romero-Sanz S, Álvarez-Illera P, Fernandez-Martinez S, De la Fuente S, García-Casas P, Fonteriz RI, Montero M, Alvarez J, Santo-Domingo J. SERCA Silencing Alleviates Aß(1-42)-Induced Toxicity in a C. elegans Model. International Journal of Molecular Sciences. 2025; 26(18):9126. https://doi.org/10.3390/ijms26189126
Chicago/Turabian StyleCaldero-Escudero, Elena, Silvia Romero-Sanz, Pilar Álvarez-Illera, Silvia Fernandez-Martinez, Sergio De la Fuente, Paloma García-Casas, Rosalba I. Fonteriz, Mayte Montero, Javier Alvarez, and Jaime Santo-Domingo. 2025. "SERCA Silencing Alleviates Aß(1-42)-Induced Toxicity in a C. elegans Model" International Journal of Molecular Sciences 26, no. 18: 9126. https://doi.org/10.3390/ijms26189126
APA StyleCaldero-Escudero, E., Romero-Sanz, S., Álvarez-Illera, P., Fernandez-Martinez, S., De la Fuente, S., García-Casas, P., Fonteriz, R. I., Montero, M., Alvarez, J., & Santo-Domingo, J. (2025). SERCA Silencing Alleviates Aß(1-42)-Induced Toxicity in a C. elegans Model. International Journal of Molecular Sciences, 26(18), 9126. https://doi.org/10.3390/ijms26189126










