Estrogens and Antioxidants Prevent the Formation of Tubular Aggregates in Aging Male Mice
Abstract
1. Introduction
2. Results
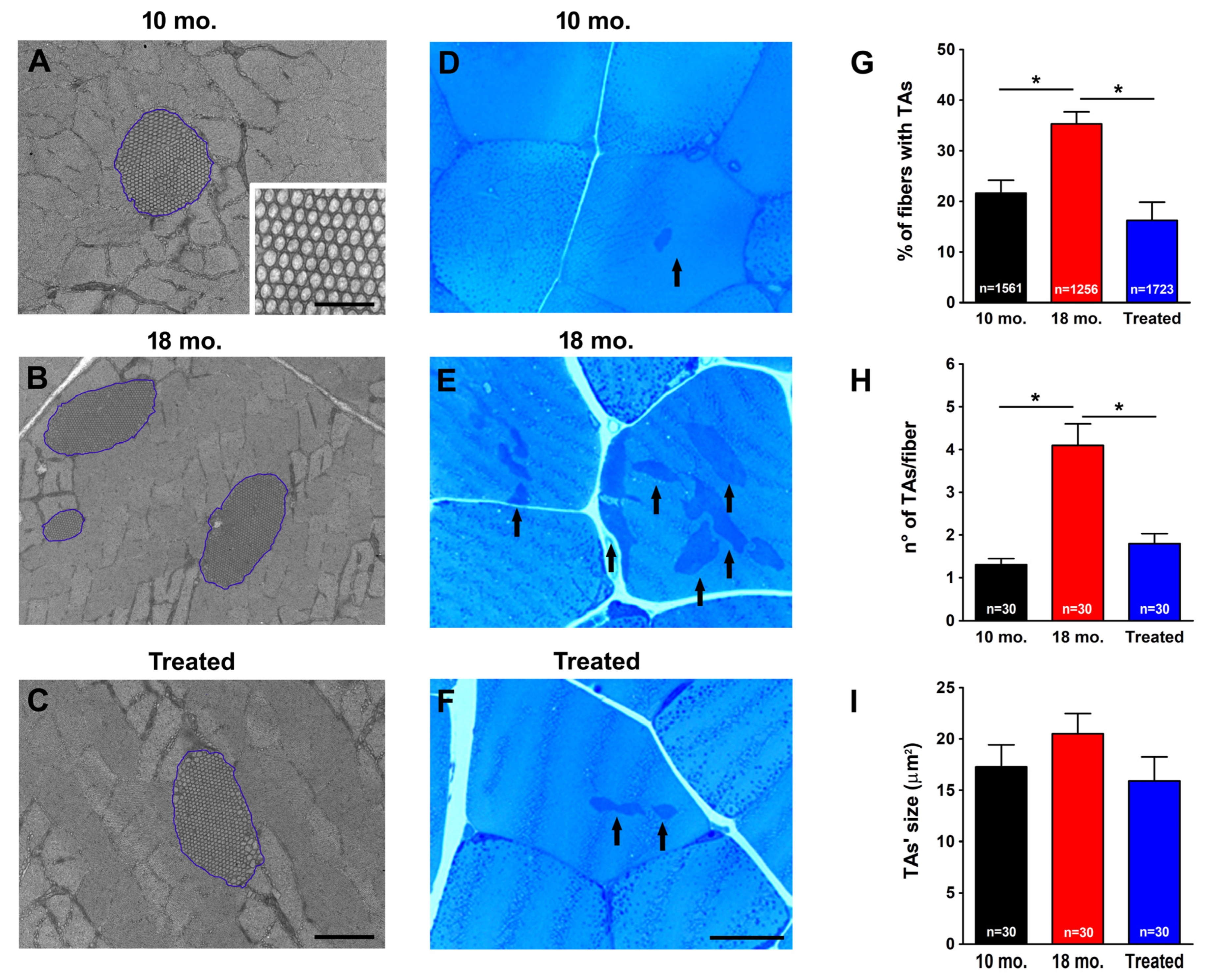
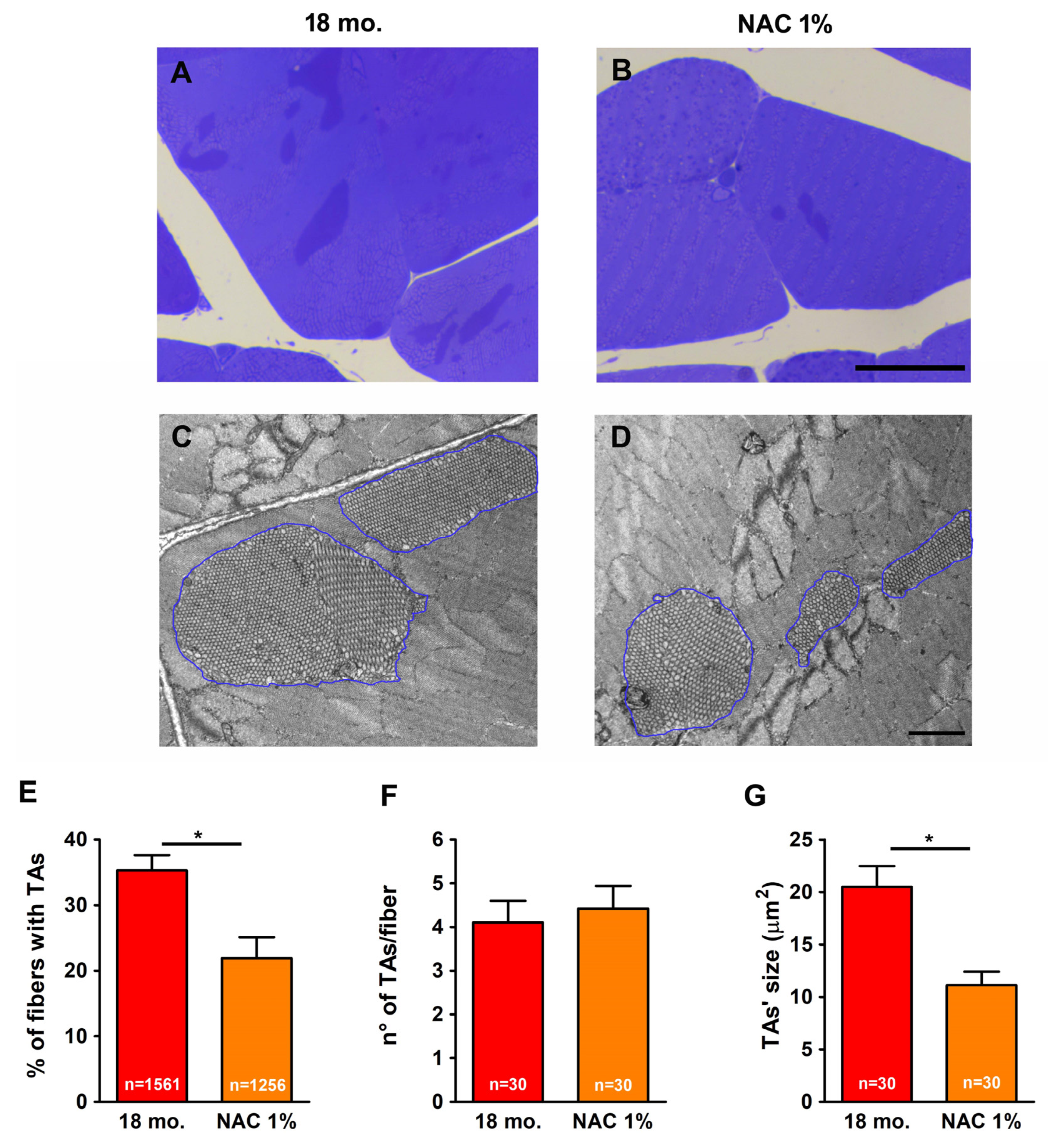
2.1. Estrogen Prevented Formation of TAs
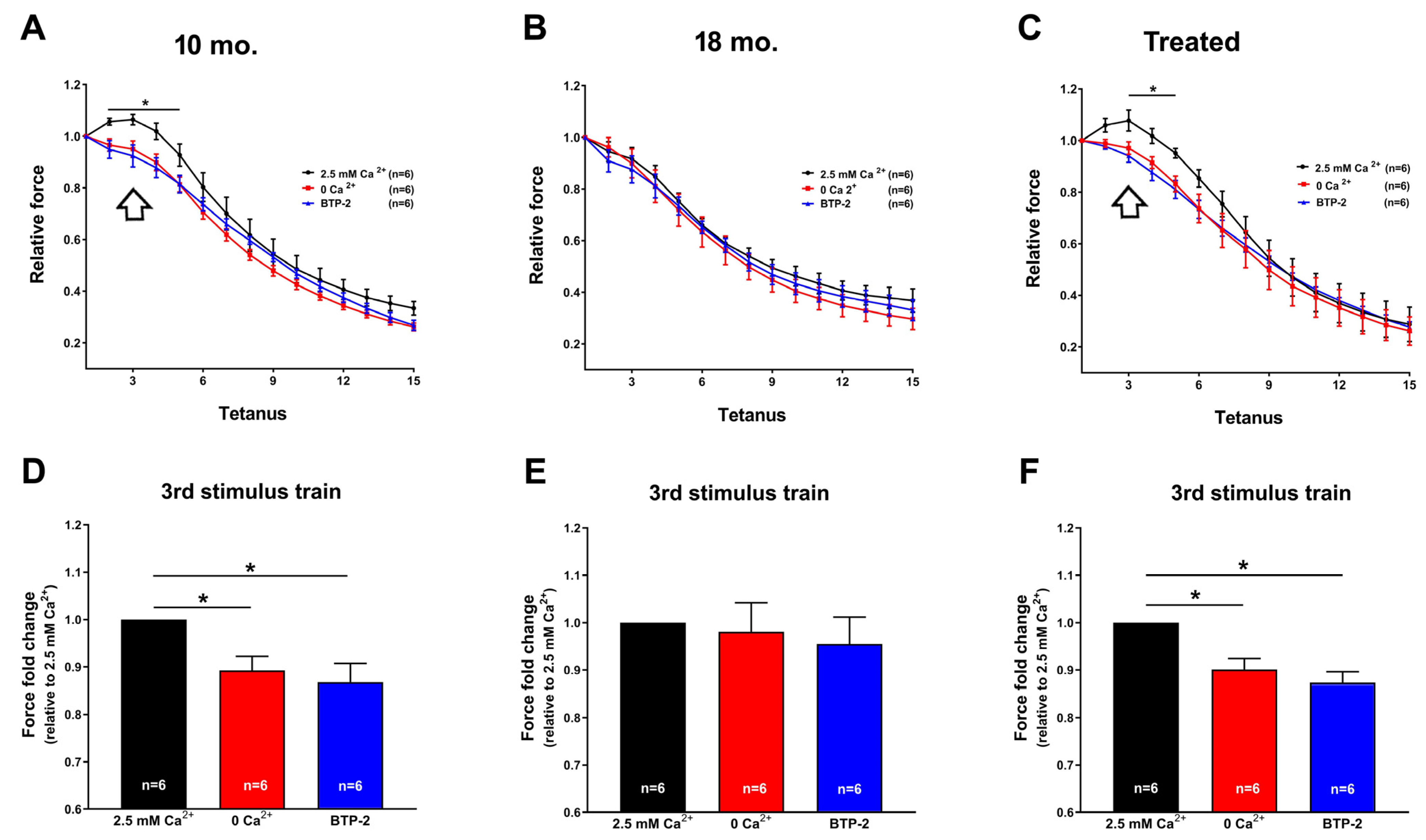
2.2. Estrogen Maintained Extracellular Ca2+ Dependence and Cross-Sectional Area (CSA) of Muscle Fibers
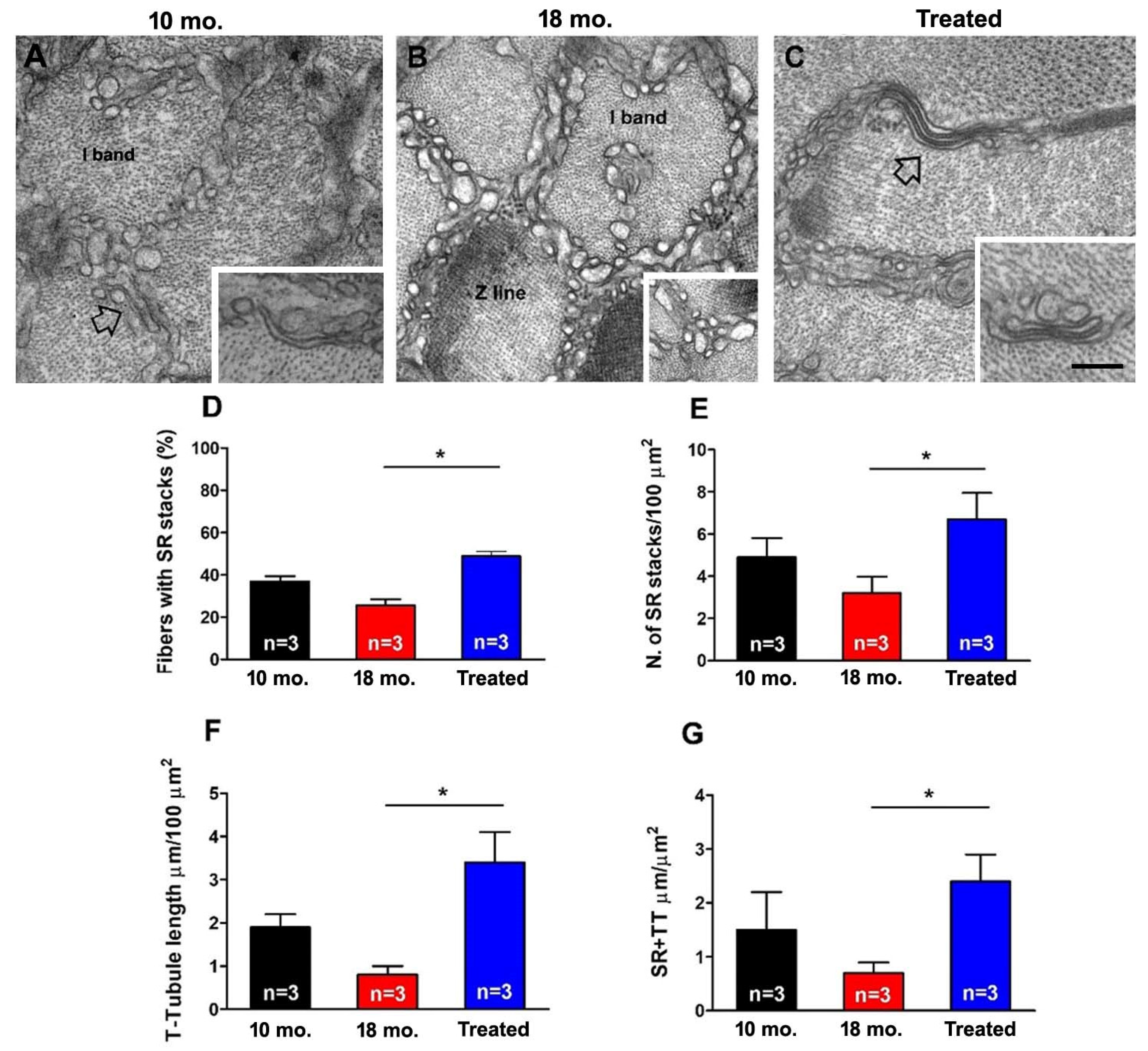
2.3. Estrogen Treatment Counteracted the Age-Related Decline in Structural Components of Ca2+ Entry Units (CEUs)
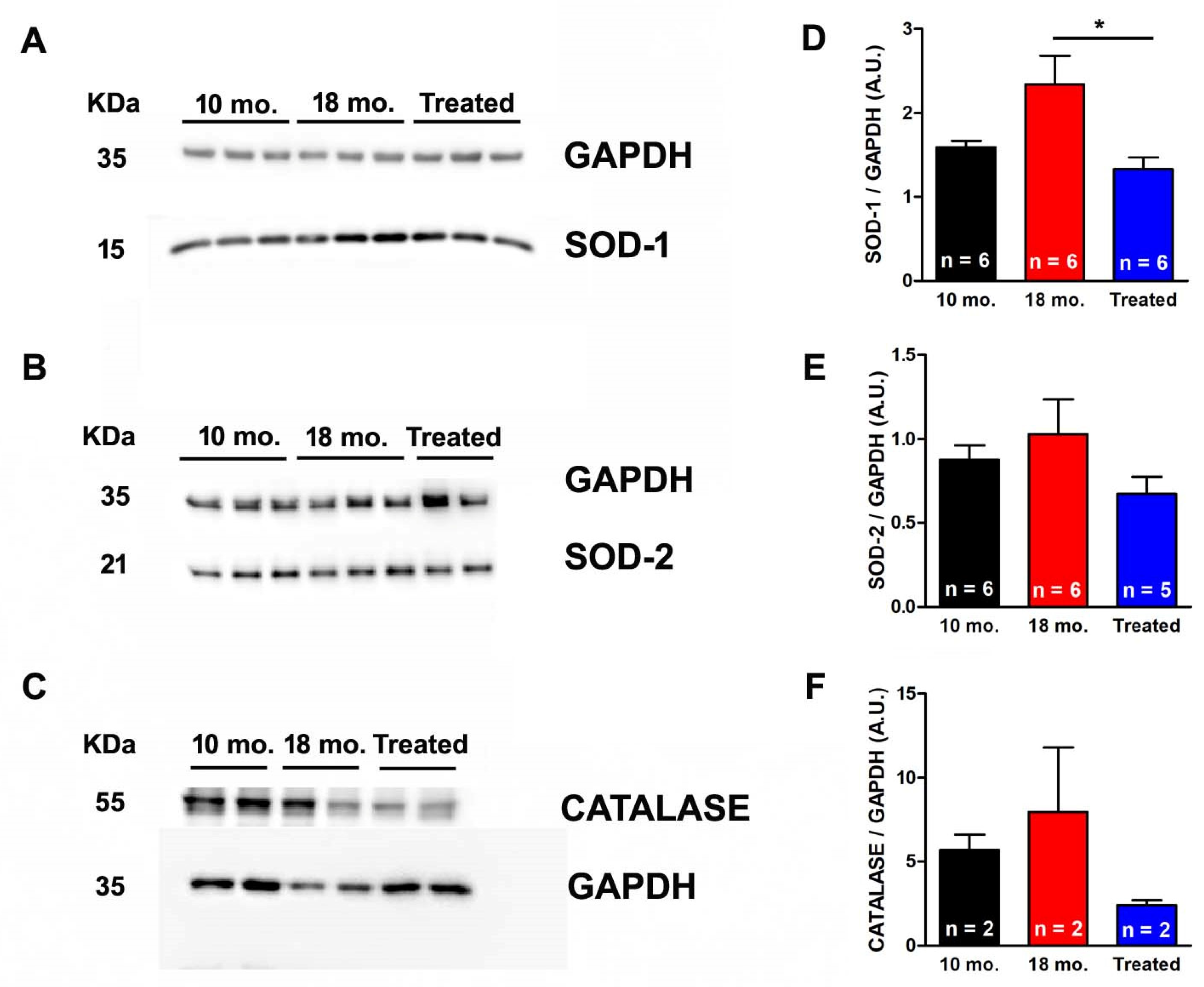
2.4. Estrogen Reduced Expression Levels of SOD1, SOD2, and Catalase
3. Discussion
3.1. The State of the Art
3.2. Main Findings
3.3. Effect of Estrogen Administration on Membrane Remodeling and SOCE Function
3.4. Effect of Estrogen Administration on Oxidative Stress
3.5. Final Remarks
4. Materials and Methods
4.1. Animals
4.2. Pharmacological Treatment of Mice
- (a)
- Treatment with 17-β-Estradiol (referred to as treated). 17-β-Estradiol was administered in drinking water ad libitum to male WT mice from 10 to 18 months of age. Because of estrogen’s low solubility in water, it was initially dissolved in 95% ethanol (5 mg/mL). Solubilized estrogen was subsequently administered to WT male mice in drinking water at a final dose of 440 ng/mL H2O, and it was changed twice a week. After 8 months of treatment (i.e., at 18 months of age), mice were killed by cervical dislocation as approved by the D. lgs n.26/2014, and EDL muscles were dissected for ex vivo analysis.
- (b)
- Treatment with N-acetylcysteine (NAC 1%). The NAC group of male WT animals received ad libitum drinking water containing 1% weight/volume (1% w/v) of NAC from 10 to 18 months of age. This dose of NAC for 8 months did not cause any noticeable negative side effects to WT mice. After 8 months of treatment (i.e., at 18 months of age), mice were killed by cervical dislocation as approved by the D. lgs n.26/2014, and EDL muscles were dissected for ex vivo analysis.
4.3. Preparation of Samples for Histology and EM
4.4. Quantitative Analysis
4.5. Ex Vivo Fatigue Protocol
4.6. Measurements of Oxidative Stress
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEU | Ca2+ entry unit |
| EDL | Extensor digitorum longus |
| EM | Electron microscopy |
| LM | Light microscopy |
| NAC | N-acetylcysteine |
| SR | Sarcoplasmic reticulum |
| SOCE | Store-operated Ca2+ entry |
| STIM1 | Stromal interaction molecule-1 |
| TA | Tubular aggregate |
| TAM | TA myopathy |
| TT | Transverse tubule |
| WT | Wild type |
References
- Lacruz, R.S.; Feske, S. Diseases Caused by Mutations in ORAI1 and STIM1. Ann. N. Y. Acad. Sci. 2015, 1356, 45–79. [Google Scholar] [CrossRef]
- Feske, S. CRAC Channels and Disease–From Human CRAC Channelopathies and Animal Models to Novel Drugs. Cell Calcium 2019, 80, 112–116. [Google Scholar] [CrossRef]
- Morin, G.; Biancalana, V.; Echaniz-Laguna, A.; Noury, J.B.; Lornage, X.; Moggio, M.; Ripolone, M.; Violano, R.; Marcorelles, P.; Marechal, D.; et al. Tubular Aggregate Myopathy and Stormorken Syndrome: Mutation Spectrum and Genotype/Phenotype Correlation. Hum. Mutat. 2020, 41, 17–37. [Google Scholar] [CrossRef]
- Stormorken, H.; Sjaastad, O.; Langslet, A.; Sulg, I.; Egge, K.; Diderichsen, J. A New Syndrome: Thrombocytopathia, Muscle Fatigue, Asplenia, Miosis, Migraine, Dyslexia and Ichthyosis. Clin. Genet. 1985, 28, 367–374. [Google Scholar] [CrossRef]
- Bohm, J.; Chevessier, F.; Koch, C.; Peche, G.A.; Mora, M.; Morandi, L.; Pasanisi, B.; Moroni, I.; Tasca, G.; Fattori, F.; et al. Clinical, Histological and Genetic Characterisation of Patients with Tubular Aggregate Myopathy Caused by Mutations in STIM1. J. Med. Genet. 2014, 51, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Bohm, J.; Bulla, M.; Urquhart, J.E.; Malfatti, E.; Williams, S.G.; O’Sullivan, J.; Szlauer, A.; Koch, C.; Baranello, G.; Mora, M.; et al. ORAI1 Mutations with Distinct Channel Gating Defects in Tubular Aggregate Myopathy. Hum. Mutat. 2017, 38, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Bohm, J.; Laporte, J. Gain-of-Function Mutations in STIM1 and ORAI1 Causing Tubular Aggregate Myopathy and Stormorken Syndrome. Cell Calcium 2018, 76, 1–9. [Google Scholar] [CrossRef]
- Chevessier, F.; Bauché-Godard, S.; Leroy, J.P.; Koenig, J.; Paturneau-Jouas, M.; Eymard, B.; Hantaï, D.; Verdière-Sahuqué, M. The Origin of Tubular Aggregates in Human Myopathies. J. Pathol. 2005, 207, 313–323. [Google Scholar] [CrossRef]
- Hedberg, C.; Niceta, M.; Fattori, F.; Lindvall, B.; Ciolfi, A.; D’Amico, A.; Tasca, G.; Petrini, S.; Tulinius, M.; Tartaglia, M.; et al. Childhood Onset Tubular Aggregate Myopathy Associated with de Novo STIM1 Mutations. J. Neurol. 2014, 261, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Silva-Rojas, R.; Laporte, J.; Bohm, J. STIM1/ORAI1 Loss-of-Function and Gain-of-Function Mutations Inversely Impact on SOCE and Calcium Homeostasis and Cause Multi-Systemic Mirror Diseases. Front. Physiol. 2020, 11, 604941. [Google Scholar] [CrossRef]
- Barone, V.; Re, V.D.; Gamberucci, A.; Polverino, V.; Galli, L.; Rossi, D.; Costanzi, E.; Toniolo, L.; Berti, G.; Malandrini, A.; et al. Identification and Characterization of Three Novel Mutations in the CASQ1 Gene in Four Patients with Tubular Aggregate Myopathy. Hum. Mutat. 2017, 38, 1761–1773. [Google Scholar] [CrossRef]
- Gamberucci, A.; Nanni, C.; Pierantozzi, E.; Serano, M.; Protasi, F.; Rossi, D.; Sorrentino, V. TAM-Associated CASQ1 Mutants Diminish Intracellular Ca2+ Content and Interfere with Regulation of SOCE. J. Muscle Res. Cell Motil. 2024, 45, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Vattemi, G.N.A.; Rossi, D.; Galli, L.; Catallo, M.R.; Pancheri, E.; Marchetto, G.; Cisterna, B.; Malatesta, M.; Pierantozzi, E.; Tonin, P.; et al. Ryanodine Receptor 1 (RYR1) Mutations in Two Patients with Tubular Aggregate Myopathy. Eur. J. Neurosci. 2022, 56, 4214–4223. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Pierantozzi, E.; Amadsun, D.O.; Buonocore, S.; Rubino, E.M.; Sorrentino, V. The Sarcoplasmic Reticulum of Skeletal Muscle Cells: A Labyrinth of Membrane Contact Sites. Biomolecules 2022, 12, 488. [Google Scholar] [CrossRef]
- Weber, A. Energized Calcium Transport and Relaxing Factors. Curr. Top. Bioenerg. 1966, 1, 203–254. [Google Scholar]
- Böhm, J.; Chevessier, F.; De Paula, A.M.; Koch, C.; Attarian, S.; Feger, C.; Hantaï, D.; Laforêt, P.; Ghorab, K.; Vallat, J.M.; et al. Constitutive Activation of the Calcium Sensor STIM1 Causes Tubular-Aggregate Myopathy. Am. J. Hum. Genet. 2013, 92, 271–278. [Google Scholar] [CrossRef]
- Misceo, D.; Holmgren, A.; Louch, W.E.; Holme, P.A.; Mizobuchi, M.; Morales, R.J.; Paula, A.M.D.; Stray-Pedersen, A.; Lyle, R.; Dalhus, B.; et al. A Dominant STIM1 Mutation Causes Stormorken Syndrome. Hum. Mutat. 2014, 35, 556–564. [Google Scholar] [CrossRef]
- Nesin, V.; Wiley, G.; Kousi, M.; Ong, E.C.; Lehmann, T.; Nicholl, D.J.; Suri, M.; Shahrizaila, N.; Katsanis, N.; Gaffney, P.M.; et al. Activating Mutations in STIM1 and ORAI1 Cause Overlapping Syndromes of Tubular Myopathy and Congenital Miosis. Proc. Natl. Acad. Sci. USA 2014, 111, 4197–4202. [Google Scholar] [CrossRef]
- Picard, C.; McCarl, C.A.; Papolos, A.; Khalil, S.; Luthy, K.; Hivroz, C.; LeDeist, F.; Rieux-Laucat, F.; Rechavi, G.; Rao, A.; et al. STIM1 Mutation Associated with a Syndrome of Immunodeficiency and Autoimmunity. N. Engl. J. Med. 2009, 360, 1971–1980. [Google Scholar] [CrossRef]
- Prakriya, M.; Feske, S.; Gwack, Y.; Srikanth, S.; Rao, A.; Hogan, P.G. Orai1 Is an Essential Pore Subunit of the CRAC Channel. Nature 2006, 443, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, N.; Ogawa, Y. Depletion of Ca2+ in the Sarcoplasmic Reticulum Stimulates Ca2+ Entry into Mouse Skeletal Muscle Fibres. J. Physiol. 2001, 533, 230–233. [Google Scholar] [CrossRef]
- Launikonis, B.S.; Barnes, M.; Stephenson, D.G. Identification of the Coupling between Skeletal Muscle Store-Operated Ca2+ Entry and the Inositol Trisphosphate Receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 2941–2944. [Google Scholar] [CrossRef]
- Launikonis, B.S.; Rios, E. Store-Operated Ca2+ Entry during Intracellular Ca2+ Release in Mammalian Skeletal Muscle. J. Physiol. 2007, 583, 81–97. [Google Scholar] [CrossRef]
- Edwards, J.N.; Blackmore, D.G.; Gilbert, D.F.; Murphy, R.M.; Launikonis, B.S. Store-Operated Calcium Entry Remains Fully Functional in Aged Mouse Skeletal Muscle despite a Decline in STIM1 Protein Expression. Aging Cell 2011, 10, 675–685. [Google Scholar] [CrossRef]
- Lyfenko, A.D.; Dirksen, R.T. Differential Dependence of Store-Operated and Excitation-Coupled Ca2+ Entry in Skeletal Muscle on STIM1 and Orai1. J. Physiol. 2008, 586, 4815–4824. [Google Scholar] [CrossRef]
- Michelucci, A.; García-Castañeda, M.; Boncompagni, S.; Dirksen, R.T. Role of STIM1/ORAI1-Mediated Store-Operated Ca2+ Entry in Skeletal Muscle Physiology and Disease. Cell Calcium 2018, 76, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Avila-Medina, J.; Mayoral-Gonzalez, I.; Dominguez-Rodriguez, A.; Gallardo-Castillo, I.; Ribas, J.; Ordoñez, A.; Rosado, J.A.; Smani, T. The Complex Role of Store Operated Calcium Entry Pathways and Related Proteins in the Function of Cardiac, Skeletal and Vascular Smooth Muscle Cells. Front. Physiol. 2018, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Weisleder, N.; Thornton, A.; Oppong, Y.; Campbell, R.; Ma, J.; Brotto, M. Compromised Store-Operated Ca2+ Entry in Aged Skeletal Muscle. Aging Cell 2008, 7, 561–568. [Google Scholar] [CrossRef]
- Carrell, E.M.; Coppola, A.R.; McBride, H.J.; Dirksen, R.T. Orai1 Enhances Muscle Endurance by Promoting Fatigue-Resistant Type I Fiber Content but Not through Acute Store-Operated Ca2+ Entry. FASEB J. 2016, 30, 4109. [Google Scholar] [CrossRef] [PubMed]
- Stiber, J.; Hawkins, A.; Zhang, Z.S.; Wang, S.; Burch, J.; Graham, V.; Ward, C.C.; Seth, M.; Finch, E.; Malouf, N.; et al. STIM1 Signalling Controls Store-Operated Calcium Entry Required for Development and Contractile Function in Skeletal Muscle. Nat. Cell Biol. 2008, 10, 688–697. [Google Scholar] [CrossRef]
- Goonasekera, S.A.; Davis, J.; Kwong, J.Q.; Accornero, F.; Wei-LaPierre, L.; Sargent, M.A.; Dirksen, R.T.; Molkentin, J.D. Enhanced Ca(2)(+) Influx from STIM1-Orai1 Induces Muscle Pathology in Mouse Models of Muscular Dystrophy. Hum. Mol. Genet. 2014, 23, 3706–3715. [Google Scholar] [CrossRef]
- Boncompagni, S.; Michelucci, A.; Pietrangelo, L.; Dirksen, R.T.; Protasi, F. Addendum: Exercise-Dependent Formation of New Junctions That Promote STIM1-Orai1 Assembly in Skeletal Muscle. Sci. Rep. 2017, 7, 14286, Erratum in Sci. Rep. 2018, 8, 17463. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; García-Castañeda, M.; Takano, T.; Malik, S.; Dirksen, R.T.; Protasi, F. Transverse Tubule Remodeling Enhances Orai1-Dependent Ca2+ Entry in Skeletal Muscle. eLife 2019, 8, 47576. [Google Scholar] [CrossRef]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; Takano, T.; Protasi, F.; Dirksen, R.T. Pre-Assembled Ca2+ Entry Units and Constitutively Active Ca2+ Entry in Skeletal Muscle of Calsequestrin-1 Knockout Mice. J. Gen. Physiol. 2020, 152, e202012617. [Google Scholar] [CrossRef] [PubMed]
- Girolami, B.; Serano, M.; Di Fonso, A.; Paolini, C.; Pietrangelo, L.; Protasi, F. Searching for Mechanisms Underlying the Assembly of Calcium Entry Units: The Role of Temperature and PH. Int. J. Mol. Sci. 2023, 24, 5328. [Google Scholar] [CrossRef]
- Boncompagni, S.; Protasi, F.; Franzini-Armstrong, C. Sequential Stages in the Age-Dependent Gradual Formation and Accumulation of Tubular Aggregates in Fast Twitch Muscle Fibers: SERCA and Calsequestrin Involvement. Age 2012, 34, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Chevessier, F.; Marty, I.; Paturneau-Jouas, M.; Hantaï, D.; Verdière-Sahuqué, M. Tubular Aggregates Are from Whole Sarcoplasmic Reticulum Origin: Alterations in Calcium Binding Protein Expression in Mouse Skeletal Muscle during Aging. Neuromuscul. Disord. 2004, 14, 208–216. [Google Scholar] [CrossRef]
- MacLennan, D.H.; de Leon, S. Biosynthesis of Sarcoplasmic Reticulum Proteins. Methods Enzymol. 1983, 96, 570–579. [Google Scholar] [CrossRef]
- Beard, N.A.; Sakowska, M.M.; Dulhunty, A.F.; Laver, D.R. Calsequestrin Is an Inhibitor of Skeletal Muscle Ryanodine Receptor Calcium Release Channels. Biophys. J. 2002, 82, 310–320. [Google Scholar] [CrossRef]
- Beard, N.A.; Casarotto, M.G.; Wei, L.; Varsanyi, M.; Laver, D.R.; Dulhunty, A.F. Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation. Biophys. J. 2005, 88, 3444–3454. [Google Scholar] [CrossRef]
- Dulhunty, A.F.; Beard, N.A.; Pouliquin, P.; Kimura, T. Novel Regulators of RyR Ca2+ Release Channels: Insight into Molecular Changes in Genetically-Linked Myopathies. J. Muscle Res. Cell Motil. 2006, 27, 351–365. [Google Scholar] [CrossRef]
- Gilchrist, J.S.; Belcastro, A.N.; Katz, S. Intraluminal Ca2+ Dependence of Ca2+ and Ryanodine-Mediated Regulation of Skeletal Muscle Sarcoplasmic Reticulum Ca2+ Release. J. Biol. Chem. 1992, 267, 20850–20856. [Google Scholar] [CrossRef]
- Ikemoto, N.; Ronjat, M.; Meszaros, L.G.; Koshita, M. Postulated Role of Calsequestrin in the Regulation of Calcium Release from Sarcoplasmic Reticulum. Biochemistry 1989, 28, 6764–6771. [Google Scholar] [CrossRef]
- Shin, D.W.; Pan, Z.; Kim, E.K.; Lee, J.M.; Bhat, M.B.; Parness, J.; Kim, D.H.; Ma, J. A Retrograde Signal from Calsequestrin for the Regulation of Store-Operated Ca2+ Entry in Skeletal Muscle. J. Bio. Chem. 2003, 278, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Li, S.; Zheng, Y.; Yan, X.; Chen, M.; Wang, H.; Putney, J.W.; Luo, D. Retrograde Regulation of STIM1-Orai1 Interaction and Store-Operated Ca2+ Entry by Calsequestrin. Sci. Rep. 2015, 5, 11349. [Google Scholar] [CrossRef]
- Boncompagni, S.; Pecorai, C.; Michelucci, A.; Pietrangelo, L.; Protasi, F. Long-Term Exercise Reduces Formation of Tubular Aggregates and Promotes Maintenance of Ca2+ Entry Units in Aged Muscle. Front. Physiol. 2021, 11, 601057. [Google Scholar] [CrossRef]
- Agbulut, O.; Destombes, J.; Thiesson, D.; Butler-Browne, G. Age-Related Appearance of Tubular Aggregates in the Skeletal Muscle of Almost All Male Inbred Mice. Histochem. Cell Biol. 2000, 114, 477–481. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide Anion Radical (O2-.), Superoxide Dismutases, and Related Matters. J. Biol. Chem. 1997, 272, 18515–18517. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide Dismutase. An Enzymic Function for Erythrocuprein (Hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Aracena-Parks, P.; Long, C.; Rossi, A.E.; Goonasekera, S.A.; Boncompagni, S.; Galvan, D.L.; Gilman, C.P.; Baker, M.R.; Shirokova, N.; et al. RyR1 S-Nitrosylation Underlies Environmental Heat Stroke and Sudden Death in Y522S RyR1 Knockin Mice. Cell 2008, 133, 53–65. [Google Scholar] [CrossRef]
- Paolini, C.; Quarta, M.; Wei-LaPierre, L.; Michelucci, A.; Nori, A.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Oxidative Stress, Mitochondrial Damage, and Cores in Muscle from Calsequestrin-1 Knockout Mice. Skelet. Muscle 2015, 5, 10. [Google Scholar] [CrossRef]
- Michelucci, A.; Paolini, C.; Canato, M.; Wei-Lapierre, L.; Pietrangelo, L.; Marco, A.D.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Antioxidants Protect Calsequestrin-1 Knockout Mice from Halothane- and Heat-Induced Sudden Death. Anesthesiology 2015, 123, 603–617. [Google Scholar] [CrossRef]
- Endo, Y.; Noguchi, S.; Hara, Y.; Hayashi, Y.K.; Motomura, K.; Miyatake, S.; Murakami, N.; Tanaka, S.; Yamashita, S.; Kizu, R.; et al. Dominant Mutations in ORAI1 Cause Tubular Aggregate Myopathy with Hypocalcemia via Constitutive Activation of Store-Operated Ca(2)(+) Channels. Hum. Mol. Genet. 2015, 24, 637–648. [Google Scholar] [CrossRef]
- Garibaldi, M.; Fattori, F.; Riva, B.; Labasse, C.; Brochier, G.; Ottaviani, P.; Sacconi, S.; Vizzaccaro, E.; Laschena, F.; Romero, N.B.; et al. A Novel Gain-of-Function Mutation in ORAI1 Causes Late-Onset Tubular Aggregate Myopathy and Congenital Miosis. Clin. Genet. 2017, 91, 780–786. [Google Scholar] [CrossRef]
- Harris, E.; Burki, U.; Marini-Bettolo, C.; Neri, M.; Scotton, C.; Hudson, J.; Bertoli, M.; Evangelista, T.; Vroling, B.; Polvikoski, T.; et al. Complex Phenotypes Associated with STIM1 Mutations in Both Coiled Coil and EF-Hand Domains. Neuromuscul. Disord. 2017, 27, 861–872. [Google Scholar] [CrossRef]
- Jain, D.; Sharma, M.C.; Sarkar, C.; Suri, V.; Sharma, S.K.; Singh, S.; Das, T.K. Tubular Aggregate Myopathy: A Rare Form of Myopathy. J. Clin. Neurosci. 2008, 15, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Pierobon-Bormioli, S.; Armani, M.; Ringel, S.P.; Angelini, C.; Vergani, L.; Betto, R.; Salviati, G. Familial Neuromuscular Disease with Tubular Aggregates. Muscle Nerve 1985, 8, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Salviati, G.; Pierobon-Bormioli, S.; Betto, R.; Damiani, E.; Angelini, C.; Ringel, S.P.; Salvatori, S.; Margreth, A. Tubular Aggregates: Sarcoplasmic Reticulum Origin, Calcium Storage Ability, and Functional Implications. Muscle Nerve 1985, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Horie-Inoue, K.; Inoue, S. Functions of Estrogen and Estrogen Receptor Signaling on Skeletal Muscle. JSBMB 2019, 191, 105375. [Google Scholar] [CrossRef]
- Muramatsu, M.; Inoue, S. Estrogen Receptors: How Do They Control Reproductive and Nonreproductive Functions? Biochem Biophys. Res. Commun. 2000, 270, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Gustafsson, J.Å. Estrogen Receptors: Therapies Targeted to Receptor Subtypes. Clin. Pharmacol. Ther. 2011, 89, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Strehlow, K.; Rotter, S.; Wassmann, S.; Adam, O.; Grohé, C.; Laufs, K.; Böhm, M.; Nickenig, G. Modulation of Antioxidant Enzyme Expression and Function by Estrogen. Circ. Res. 2003, 93, 170–177. [Google Scholar] [CrossRef]
- Subbiah, M.T.R.; Kessel, B.; Agrawal, M.; Rajan, R.; Abplanalp, W.; Rymaszewski, Z. Antioxidant Potential of Specific Estrogens on Lipid Peroxidation. J. Clin. Endocrinol. Metab. 1993, 77, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Sugioka, K.; Shimosegawa, Y.; Nakano, M. Estrogens as Natural Antioxidants of Membrane Phospholipid Peroxidation. FEBS Lett. 1987, 210, 37–39. [Google Scholar] [CrossRef]
- Tiidus, P.M. Can Estrogens Diminished Exercise Induced Muscle Damage? Can. J. Appl. Physiol. 1995, 20, 26–38. [Google Scholar] [CrossRef]
- Whiting, K.P.; Restall, C.J.; Brain, P.F. Steroid Hormone-Induced Effects on Membrane Fluidity and Their Potential Roles in Non-Genomic Mechanisms. Life Sci. 2000, 67, 743–757. [Google Scholar] [CrossRef]
- Enns, D.L.; Iqbal, S.; Tiidus, P.M. Oestrogen Receptors Mediate Oestrogen-Induced Increases in Post-Exercise Rat Skeletal Muscle Satellite Cells. Acta Physiol. 2008, 194, 81–93. [Google Scholar] [CrossRef]
- Kendall, B.; Eston, R. Exercise-Induced Muscle Damage and the Potential Protective Role of Estrogen. Sports Med. 2002, 32, 77. [Google Scholar] [CrossRef]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef]
- Pietrangelo, L.; D’Incecco, A.; Ainbinder, A.; Michelucci, A.; Kern, H.; Dirksen, R.T.; Boncompagni, S.; Protasi, F. Age-Dependent Uncoupling of Mitochondria from Ca2+ Release Units in Skeletal Muscle. Oncotarget 2015, 6, 35358–35371. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H.; Laher, I.; Knechtle, B.; Zouhal, H. Exercise and Mitochondrial Mechanisms in Patients with Sarcopenia. Front. Physiol. 2022, 13, 1040381. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; Tano, G.D.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fano, G. The Contribution of Reactive Oxygen Species to Sarcopenia and Muscle Ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef]
- Miao, Y.; Xie, L.; Song, J.; Cai, X.; Yang, J.; Ma, X.; Chen, S.; Xie, P. Unraveling the Causes of Sarcopenia: Roles of Neuromuscular Junction Impairment and Mitochondrial Dysfunction. Physiol. Rep. 2024, 12, e15917. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative Damage and Mitochondrial Decay in Aging. Proc. Natl. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, S.; Pietrangelo, L.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Pond, A.; Grim-Stieger, M.; Cvecka, J.; Sedliak, M.; et al. Lifelong Physical Exercise Delays Age-Associated Skeletal Muscle Decline. J. GerontolSeries A Biol. Scie Med. Sci. 2015, 70, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, L.; Michelucci, A.; Ambrogini, P.; Sartini, S.; Guarnier, F.A.; Fusella, A.; Zamparo, I.; Mammucari, C.; Protasi, F.; Boncompagni, S. Muscle Activity Prevents the Uncoupling of Mitochondria from Ca 2+ Release Units Induced by Ageing and Disuse. Arch. Biochem. Biophys. 2019, 663, 22–33. [Google Scholar] [CrossRef]
- Dainese, M.; Quarta, M.; Lyfenko, A.D.; Paolini, C.; Canato, M.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Anesthetic- and Heat-Induced Sudden Death in Calsequestrin-1-Knockout Mice. FASEB J. 2009, 23, 1710–1720. [Google Scholar] [CrossRef]
- Michelucci, A.; Boncompagni, S.; Canato, M.; Reggiani, C.; Protasi, F. Estrogens Protect Calsequestrin-1 Knockout Mice from Lethal Hyperthermic Episodes by Reducing Oxidative Stress in Muscle. Oxid. Med. Cell Longev. 2017, 2017, 6936897. [Google Scholar] [CrossRef] [PubMed]
- Guarnier, F.A.; Michelucci, A.; Serano, M.; Pietrangelo, L.; Pecorai, C.; Boncompagni, S.; Protasi, F. Aerobic Training Prevents Heatstrokes in Calsequestrin-1 Knockout Mice by Reducing Oxidative Stress. Oxid. Med. Cell Longev. 2018, 2018, 4652480. [Google Scholar] [CrossRef] [PubMed]
- Mulnard, R.A.; Cotman, C.W.; Kawas, C.; van Dyck, C.H.; Sano, M.; Doody, R.; Koss, E.; Pfeiffer, E.; Jin, S.; Gamst, A.; et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: A randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA 2000, 283, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Zitt, C.; Strauss, B.; Schwarz, E.C.; Spaeth, N.; Rast, G.; Hatzelmann, A.; Hoth, M. Potent Inhibition of Ca2+ Release-Activated Ca2+ Channels and T-Lymphocyte Activation by the Pyrazole Derivative BTP2. J. Biol. Chem. 2004, 279, 12427–12437. [Google Scholar] [CrossRef]
- Hakim, C.H.; Grange, R.W.; Duan, D. The Passive Mechanical Properties of the Extensor Digitorum Longus Muscle Are Compromised in 2- to 20-Mo-Old Mdx Mice. J. Appl. Physiol. 2011, 110, 1656–1663. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastelli, G.; Serano, M.; Girolami, B.; Brasile, A.; Sorrentino, V.; Pietrangelo, L.; Protasi, F. Estrogens and Antioxidants Prevent the Formation of Tubular Aggregates in Aging Male Mice. Int. J. Mol. Sci. 2025, 26, 9122. https://doi.org/10.3390/ijms26189122
Rastelli G, Serano M, Girolami B, Brasile A, Sorrentino V, Pietrangelo L, Protasi F. Estrogens and Antioxidants Prevent the Formation of Tubular Aggregates in Aging Male Mice. International Journal of Molecular Sciences. 2025; 26(18):9122. https://doi.org/10.3390/ijms26189122
Chicago/Turabian StyleRastelli, Giorgia, Matteo Serano, Barbara Girolami, Alice Brasile, Vincenzo Sorrentino, Laura Pietrangelo, and Feliciano Protasi. 2025. "Estrogens and Antioxidants Prevent the Formation of Tubular Aggregates in Aging Male Mice" International Journal of Molecular Sciences 26, no. 18: 9122. https://doi.org/10.3390/ijms26189122
APA StyleRastelli, G., Serano, M., Girolami, B., Brasile, A., Sorrentino, V., Pietrangelo, L., & Protasi, F. (2025). Estrogens and Antioxidants Prevent the Formation of Tubular Aggregates in Aging Male Mice. International Journal of Molecular Sciences, 26(18), 9122. https://doi.org/10.3390/ijms26189122






