Potential of Natural Products in Hangeshashinto Water Extract on the Direct Suppression of Stomatitis Induced by Intra-/Extracellular Advanced Glycation End-Products
Abstract
1. Introduction
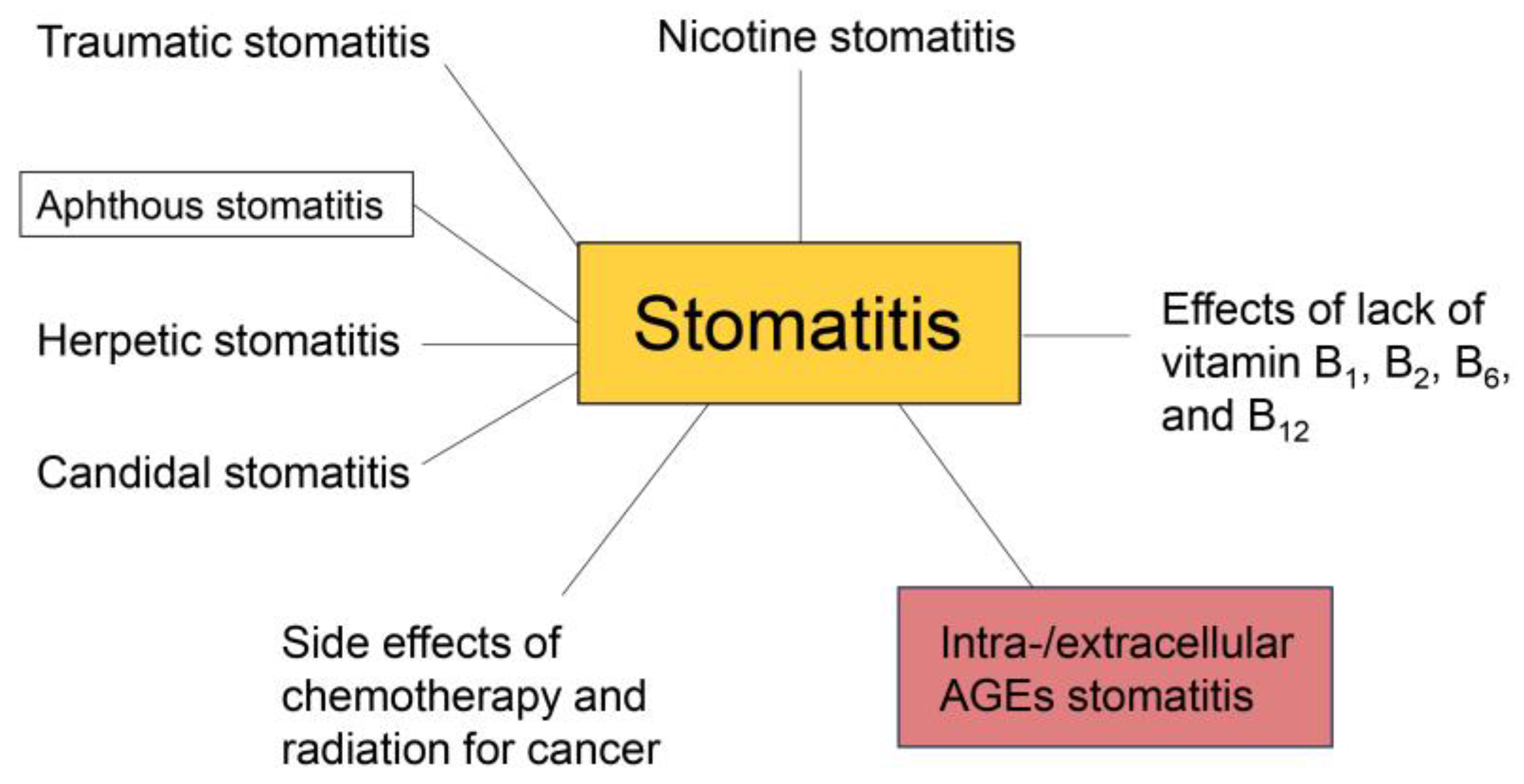
2. Various Types of Stomatitis
3. Treatment of Stomatitis with Hangeshashinto and Predicted Mechanisms
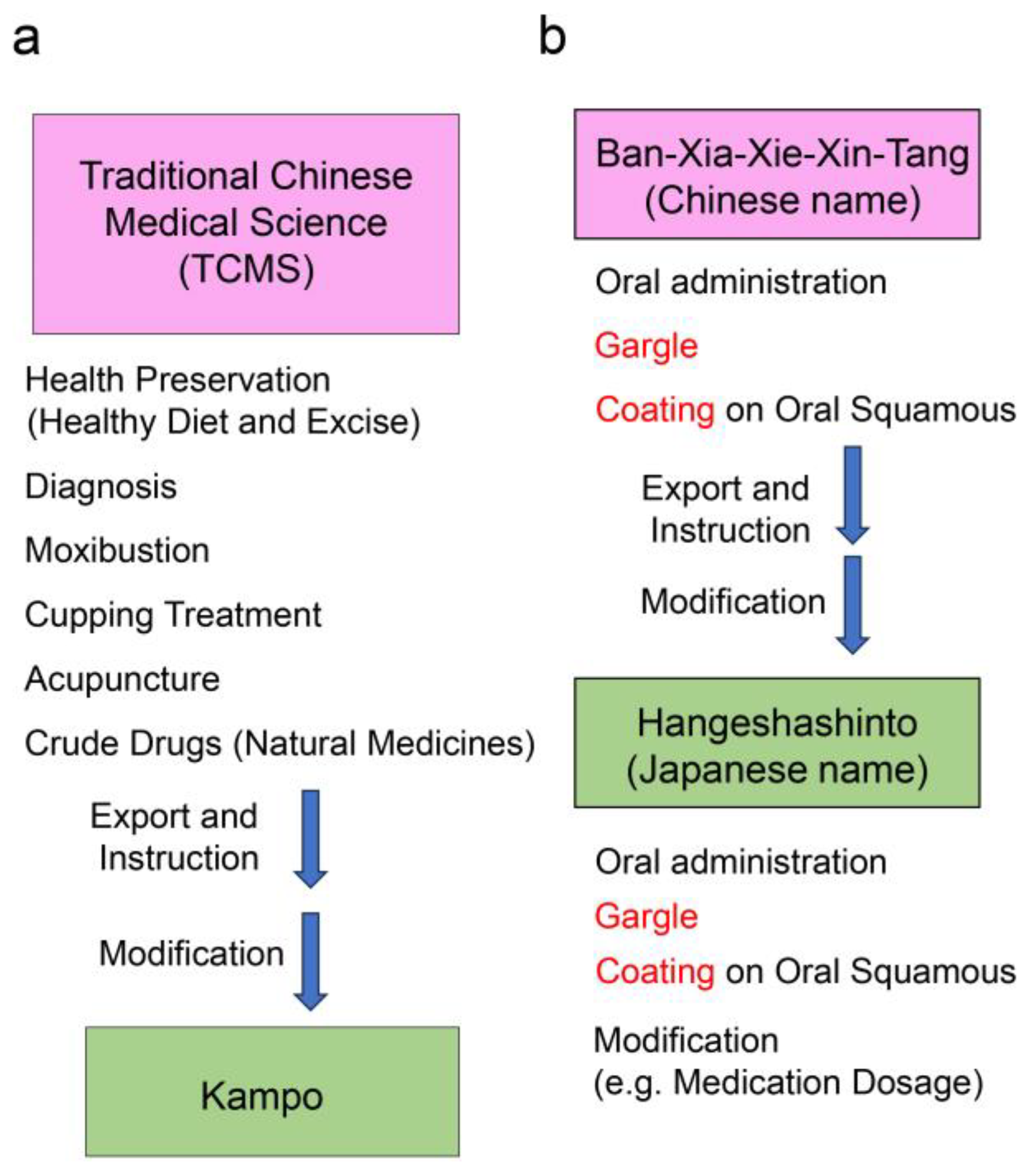
3.1. Traditional Chinese and Kampo Medicines
3.2. Hangeshashinto (Ban-Xia-Xie-Xin-Tang) Treatment Effects and Mechanisms
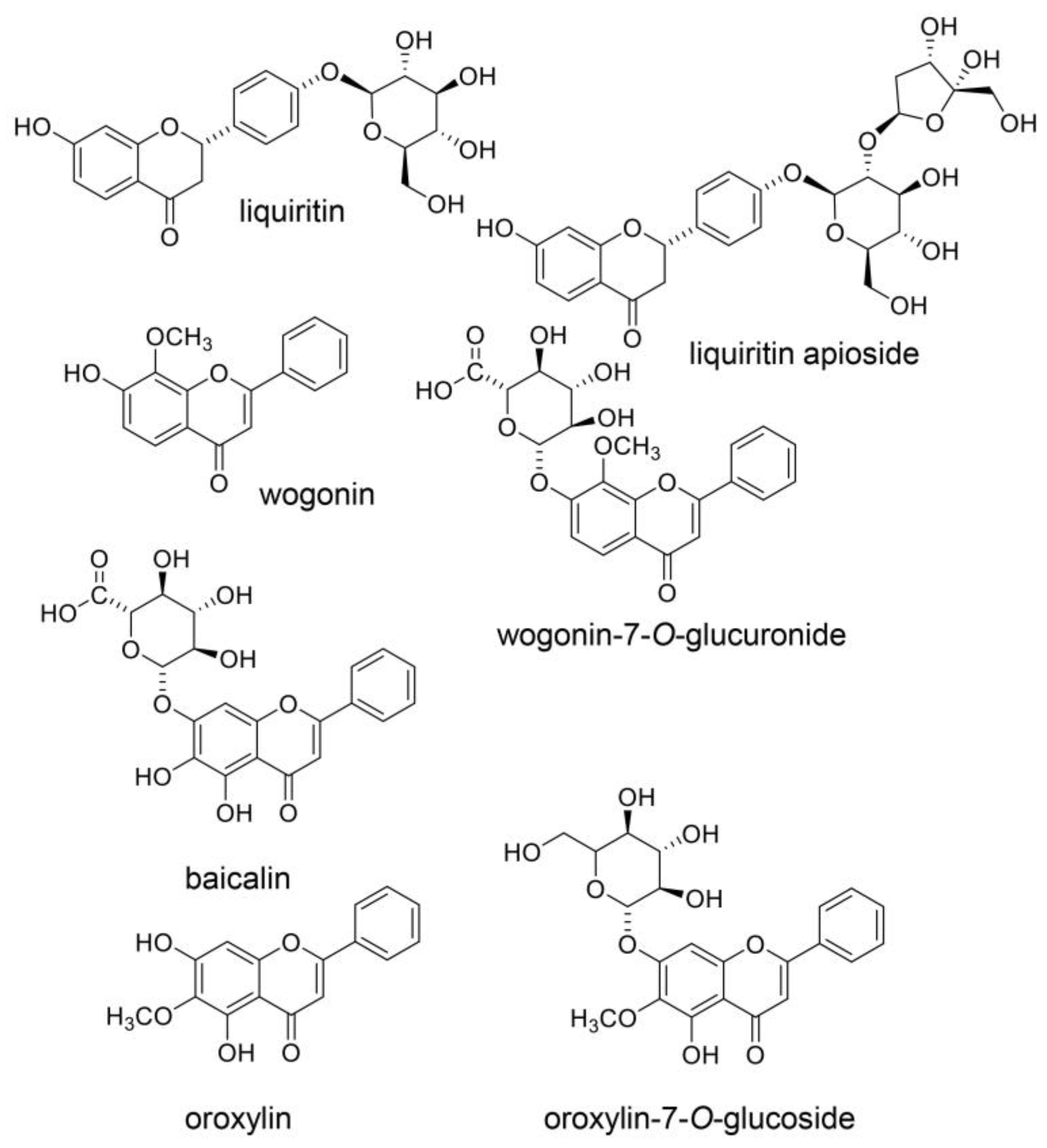
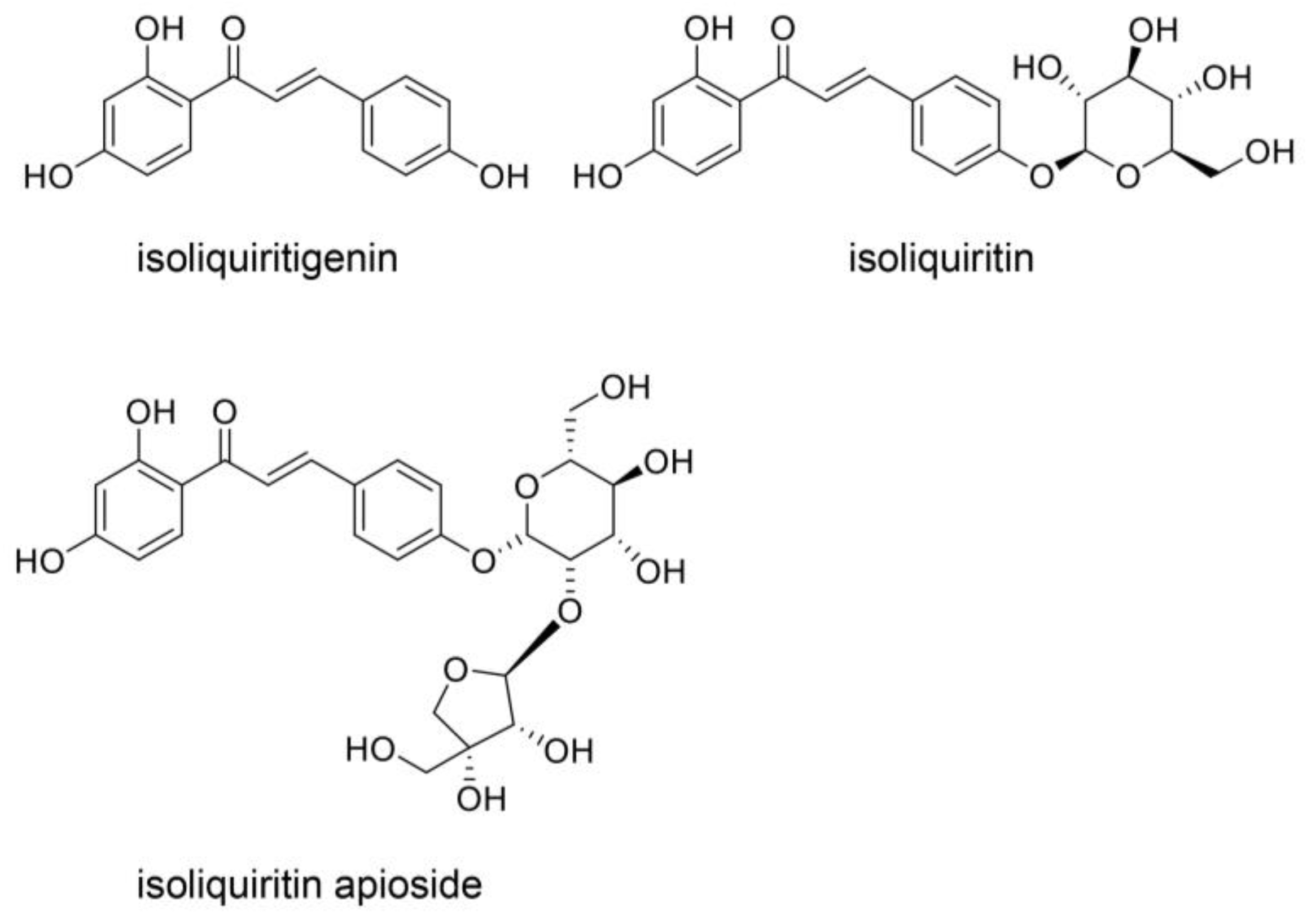
3.3. Natural Products in Hangeshashinto and Their Anti-Inflammation Effects
4. AGEs
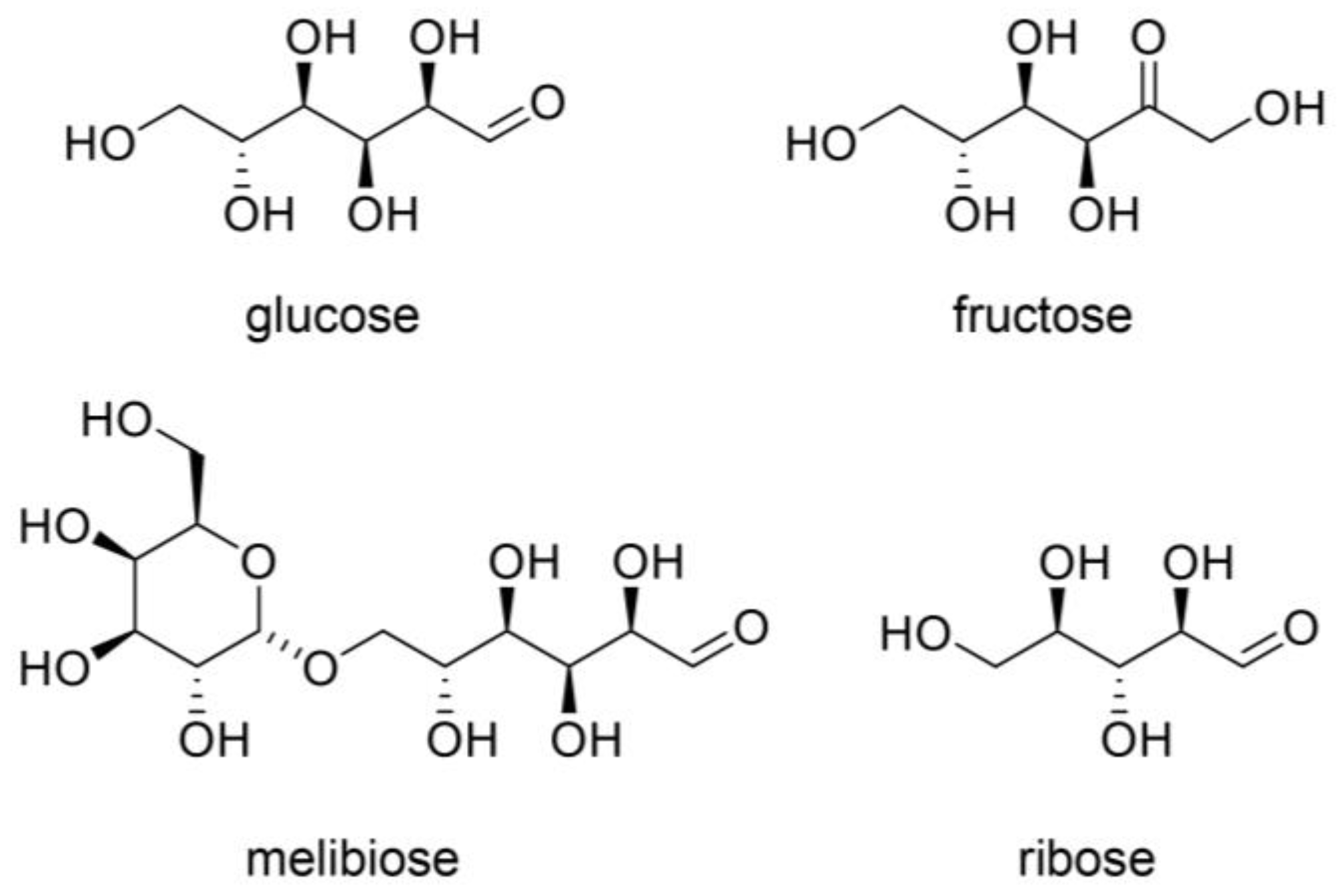
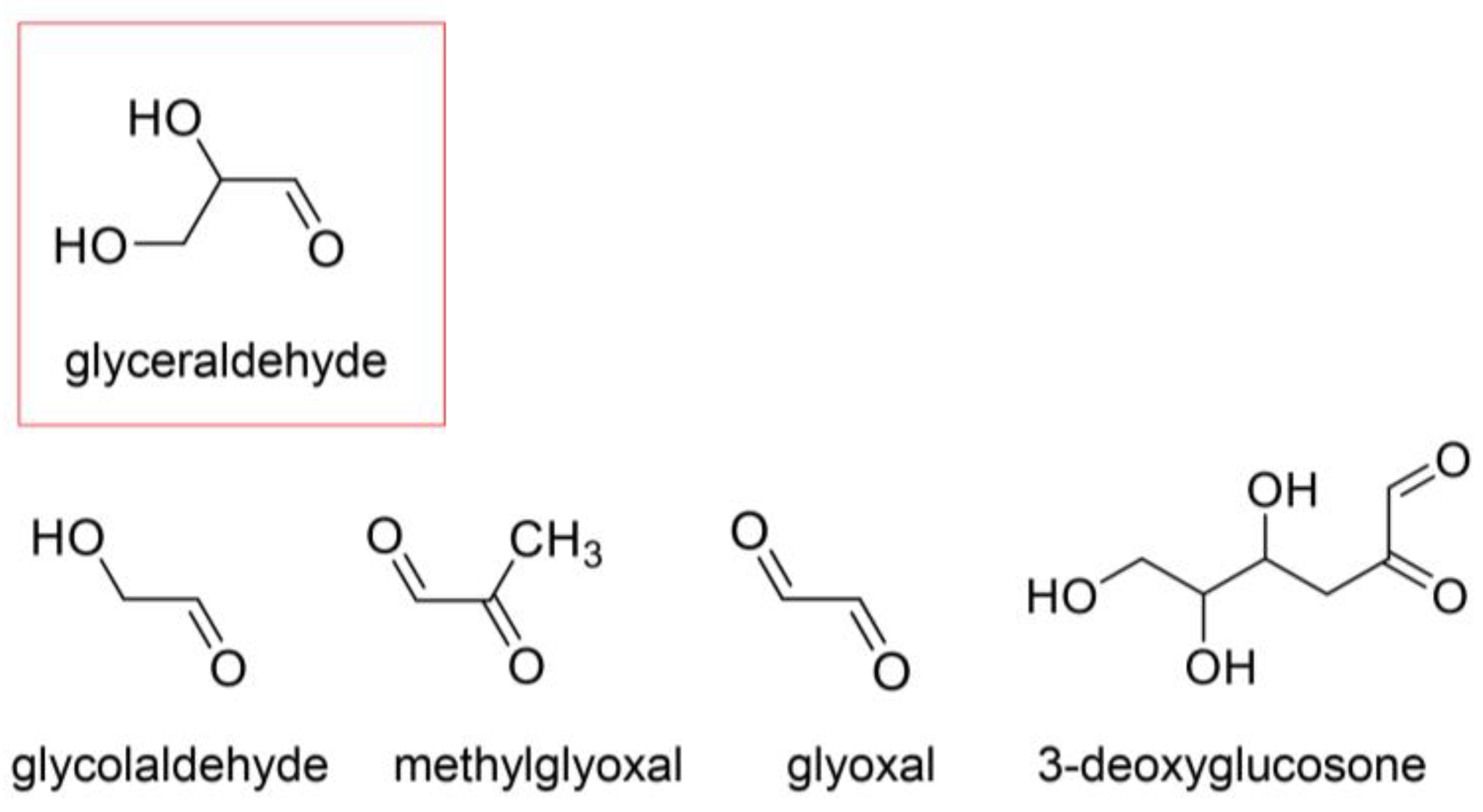
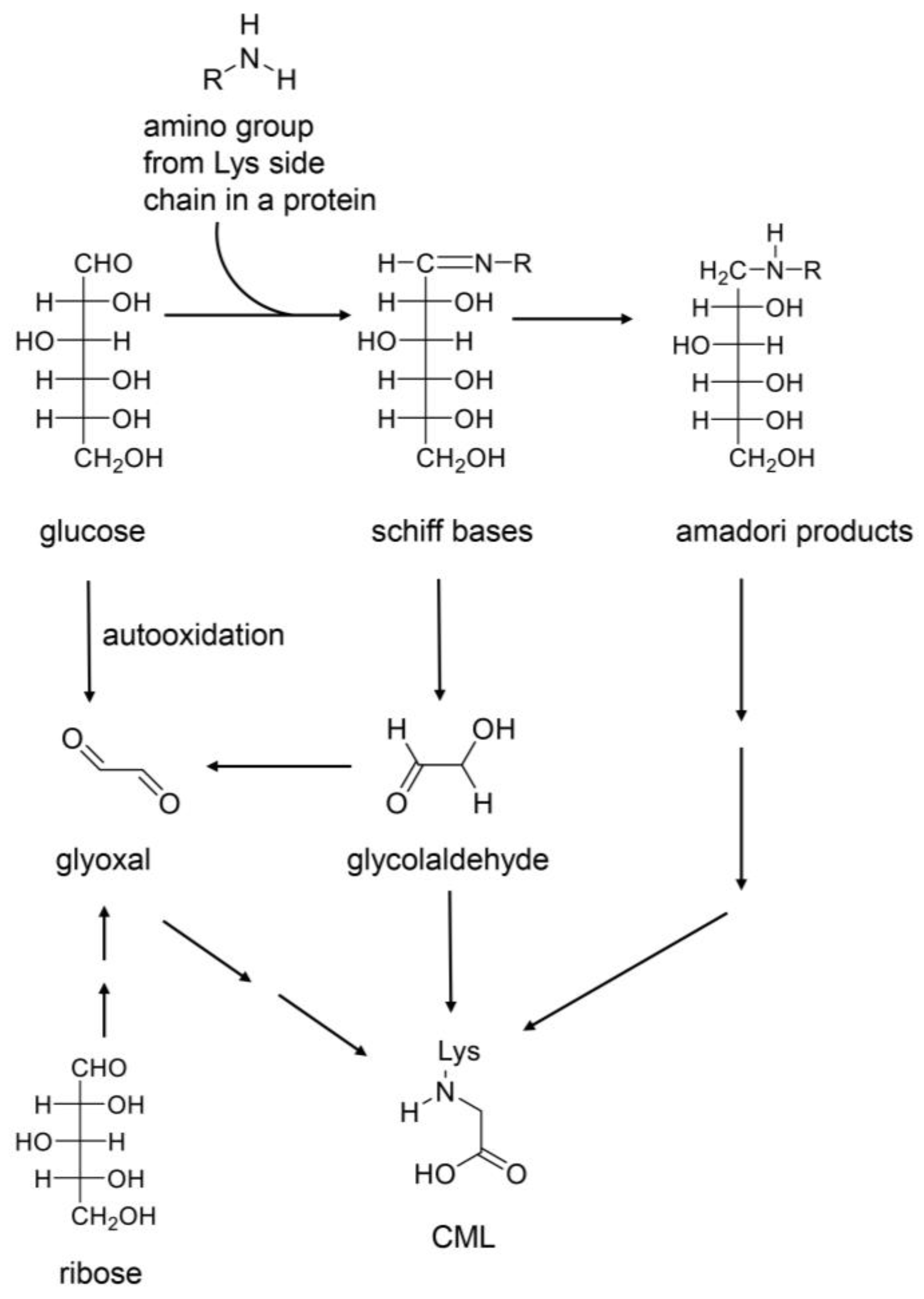
4.1. AGE Origins
4.2. Free AGEs
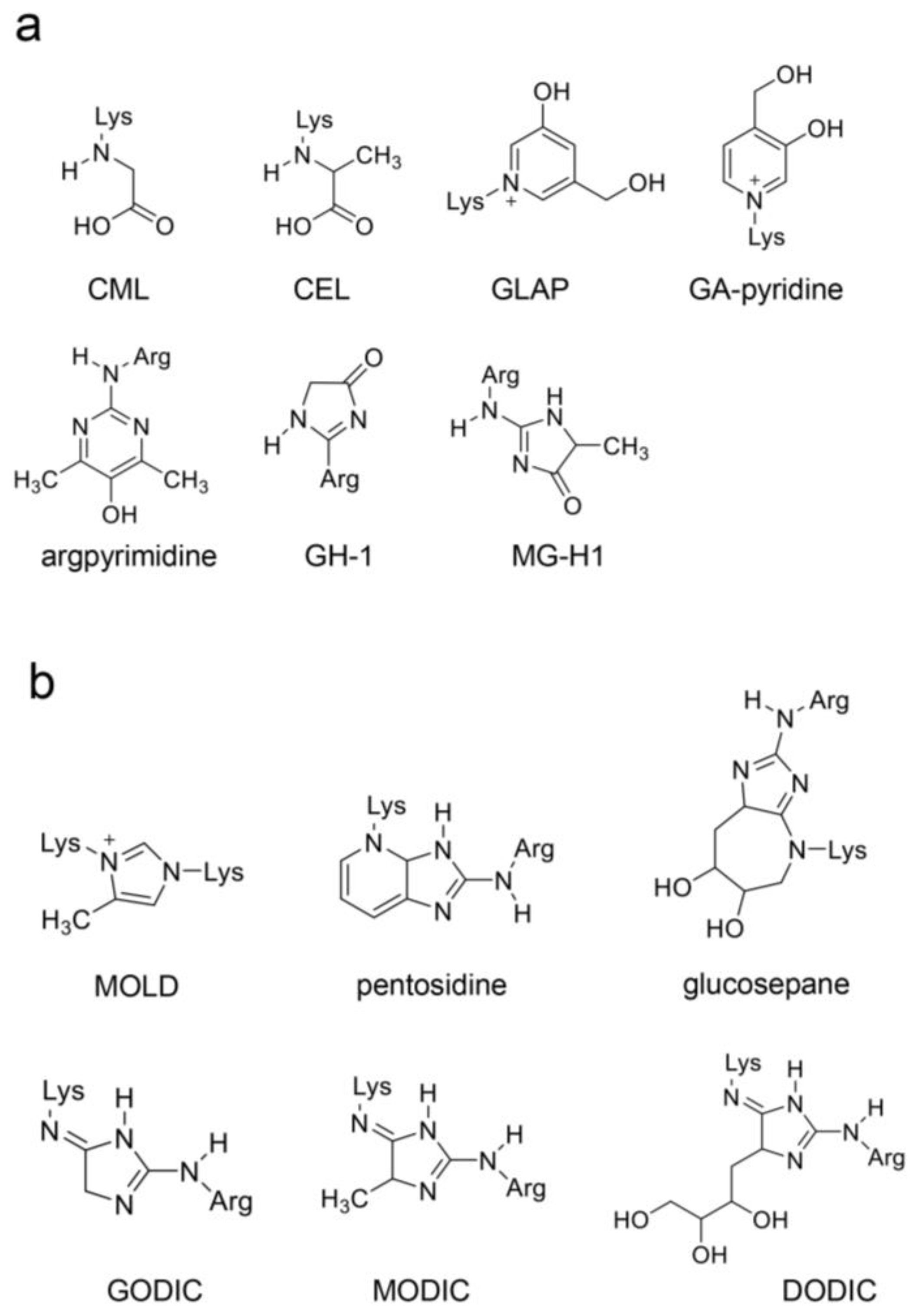
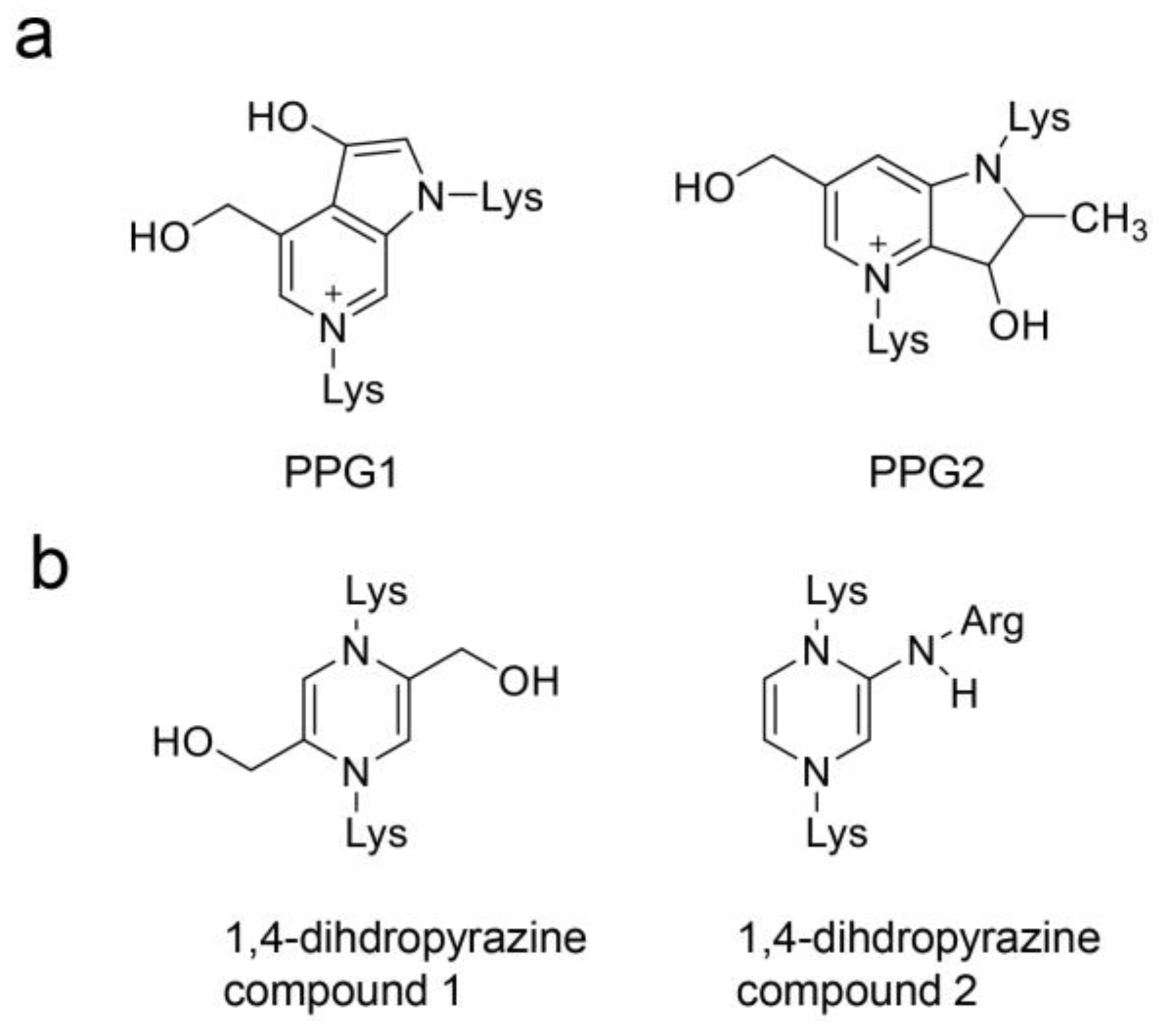
4.3. Previous AGE Categories and Potential Improvements
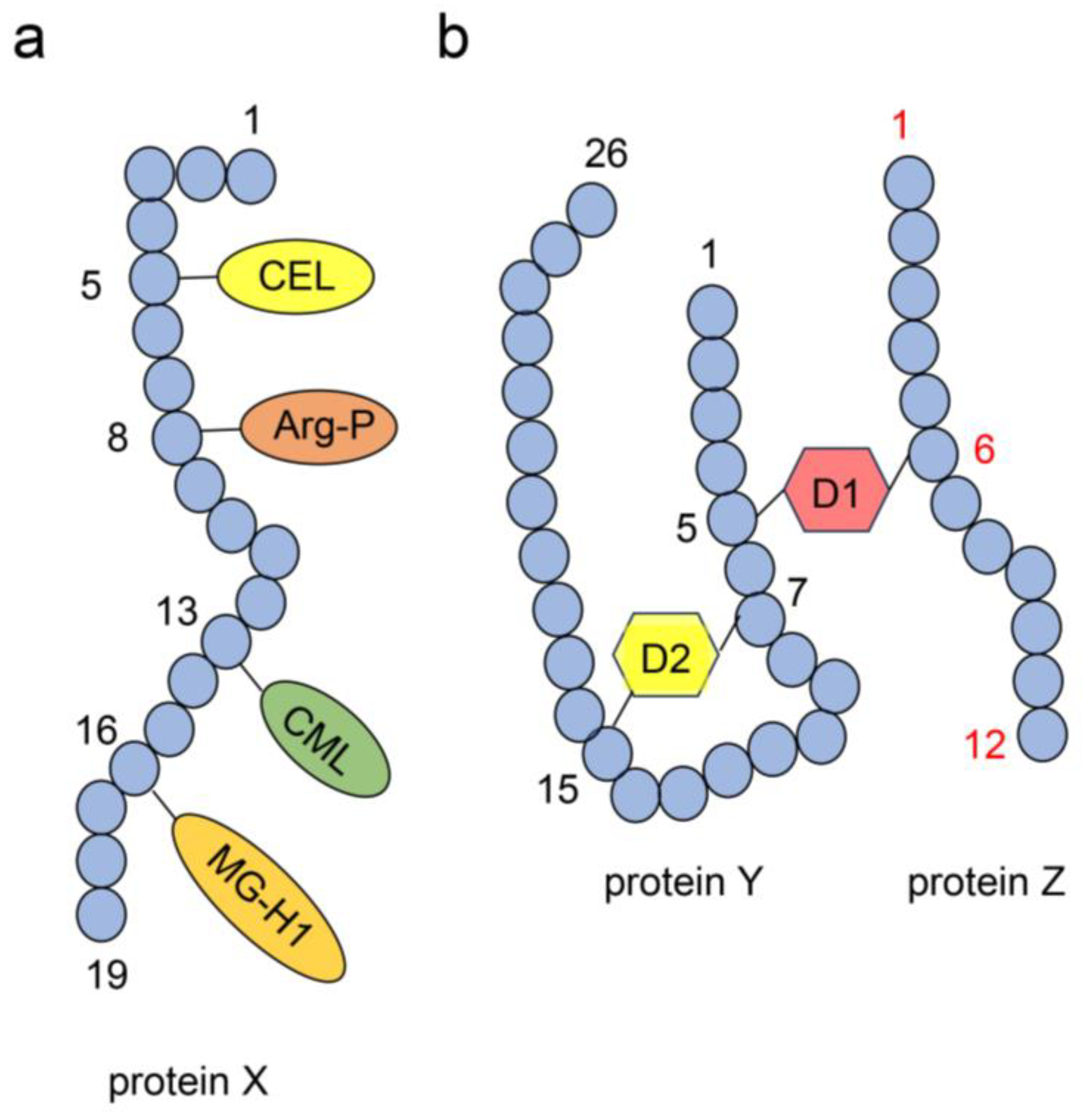
4.4. Structure of AGE-Modified Proteins
4.5. Intra-/Extracellular AGEs and LSRDs
4.5.1. Intracellular AGEs and LSRDs
4.5.2. AGEs in the Extracellular Matrix and LSRDs
4.5.3. AGEs in the Blood, Urine, Saliva, and LSRDs
4.5.4. Dietary AGEs and LSRDs
4.5.5. AGEs in the Body Fluid as a Biomarker for LSRDs and Dietary Lifestyle
4.6. Identification and Quantification of AGEs
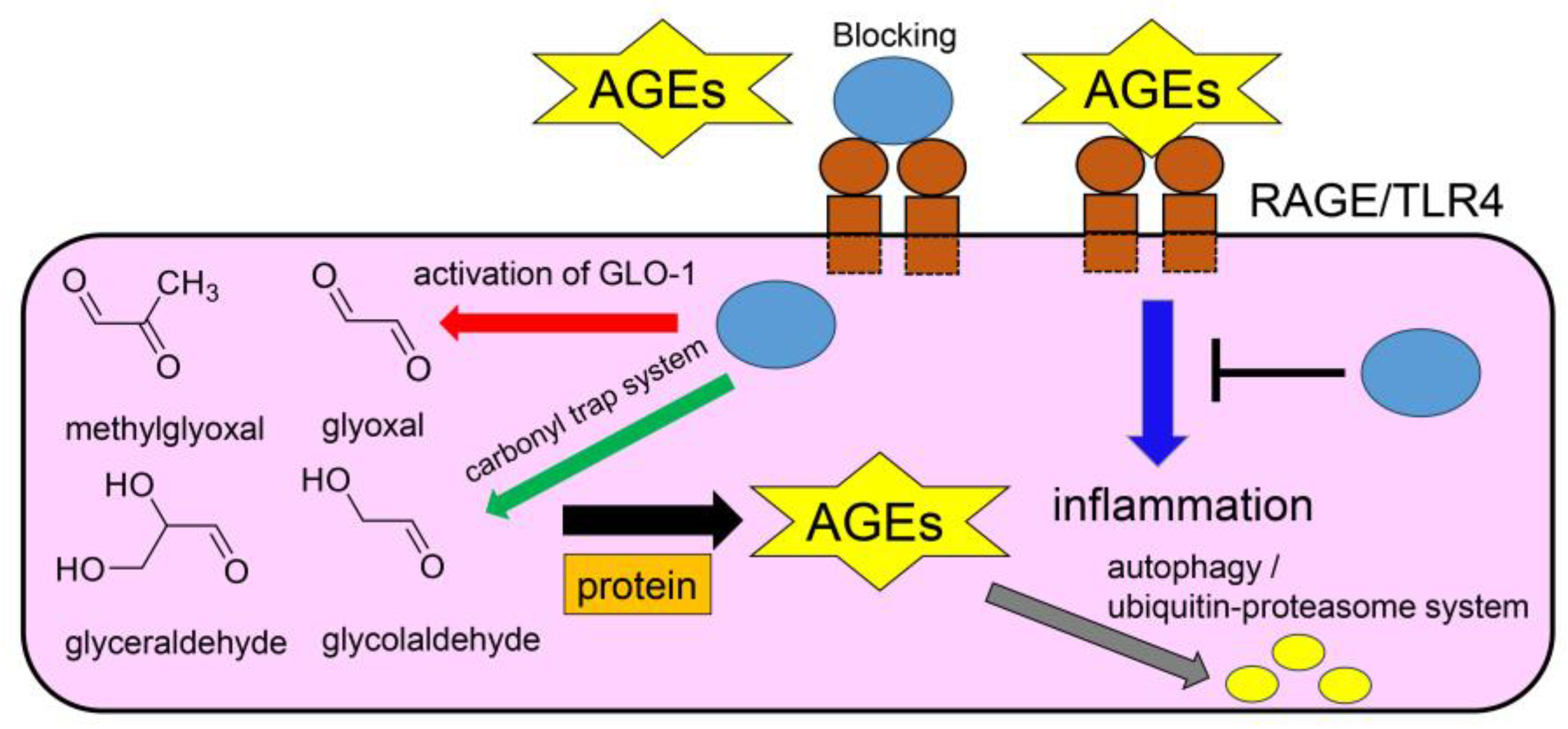
5. Mechanisms of Inhibition of Intra-/Extracellular AGE-Induced Cytotoxicity by Anti-AGE Compounds
6. Potential of AGE-Induced Cytotoxicity and Dysfunction for Oral Squamous Cells
6.1. Potential of Intracellular AGE-Induced Cytotoxicity and Dysfunction for Oral Epithelial Cells
6.2. Potential of Extracellular AGE-Induced Cytotoxicity and Dysfunction for Oral Epithelial Cells
7. Natural Products in Hangeshashinto Water Extract Inhibit the Generation of Intracellular AGEs in Stomatitis
8. Natural Products Obtained from Hangeshashinto Water Extract Suppress Extracellular AGEs-RAGE/TLR4 Signaling in Stomatitis
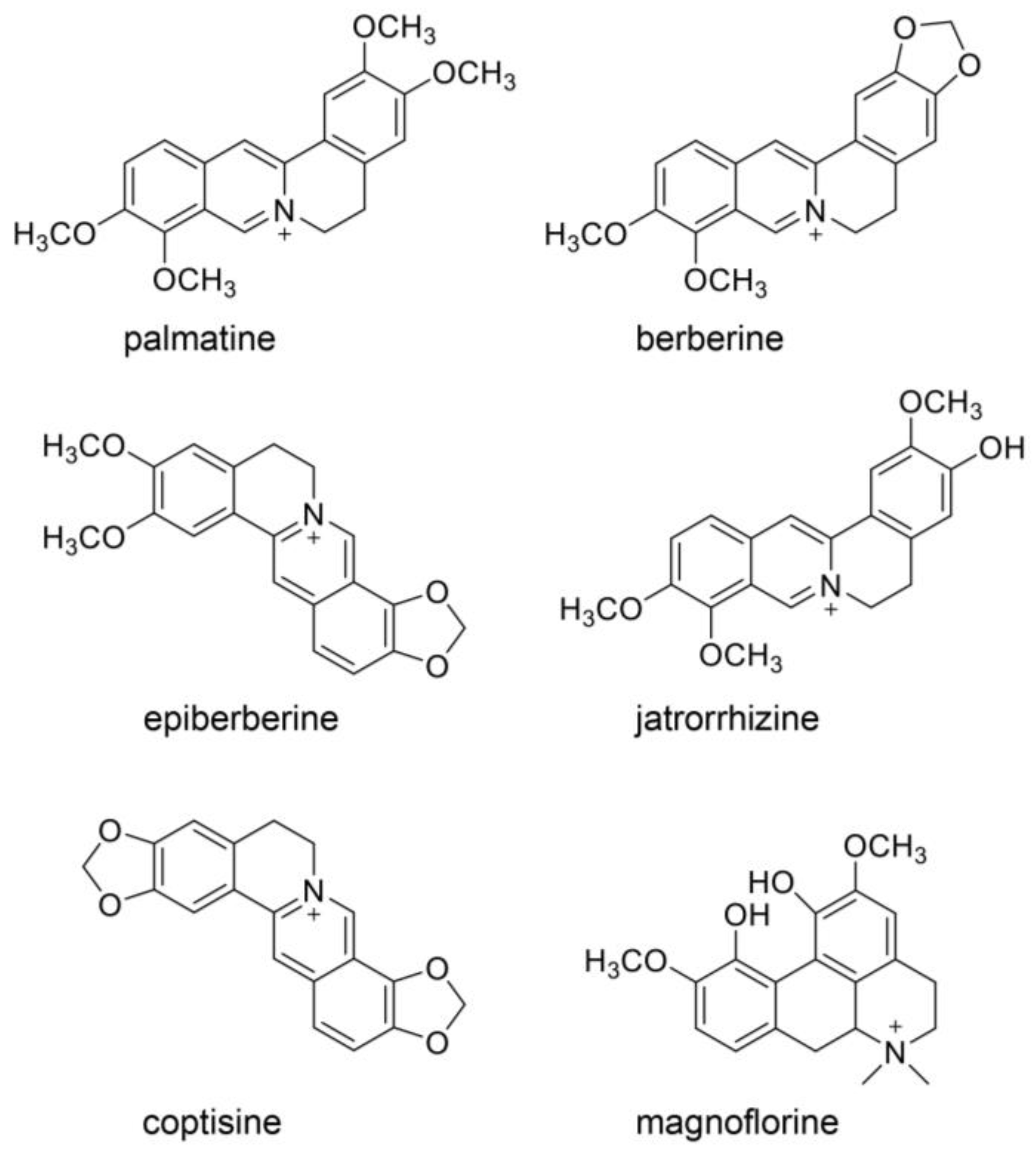
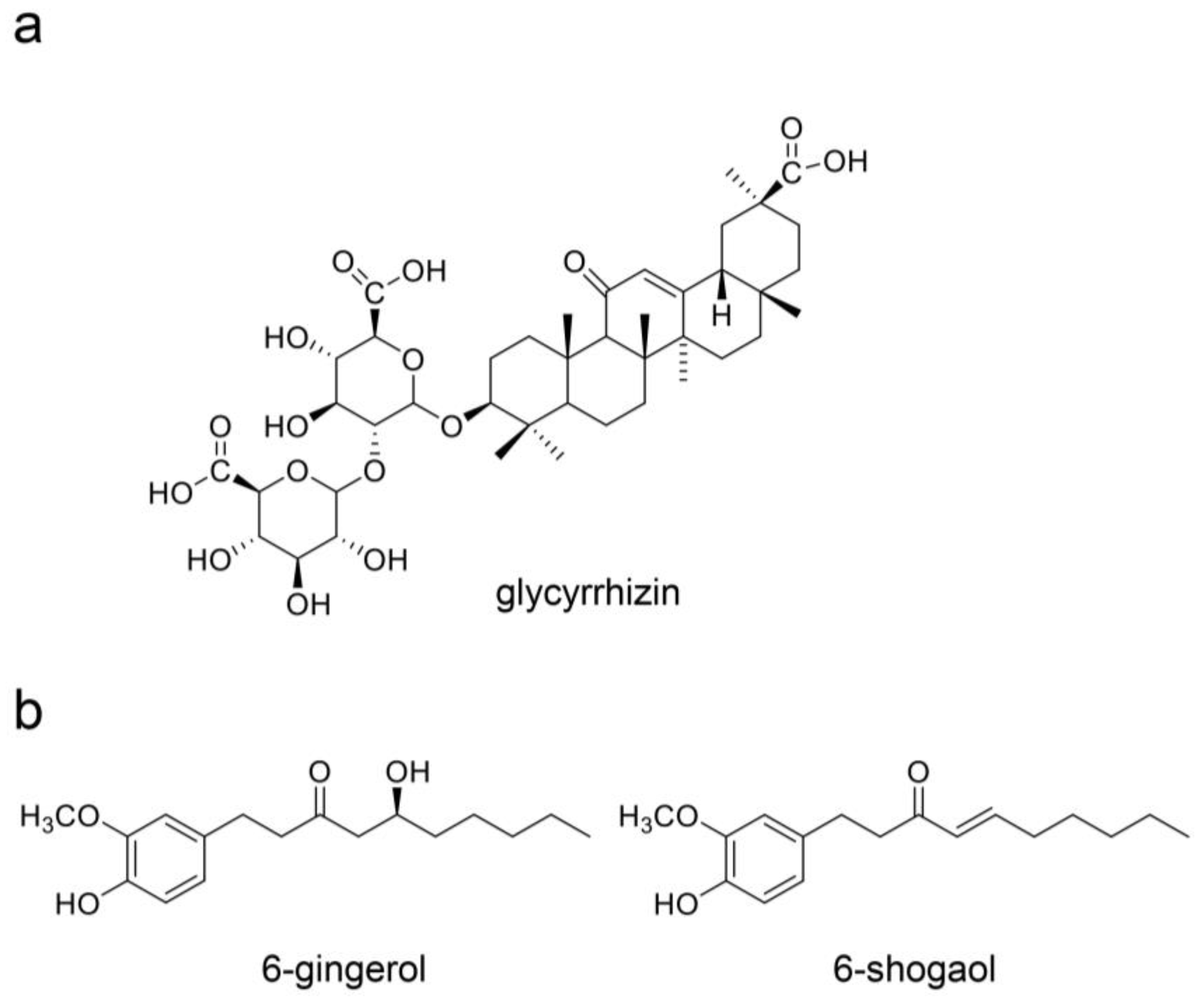
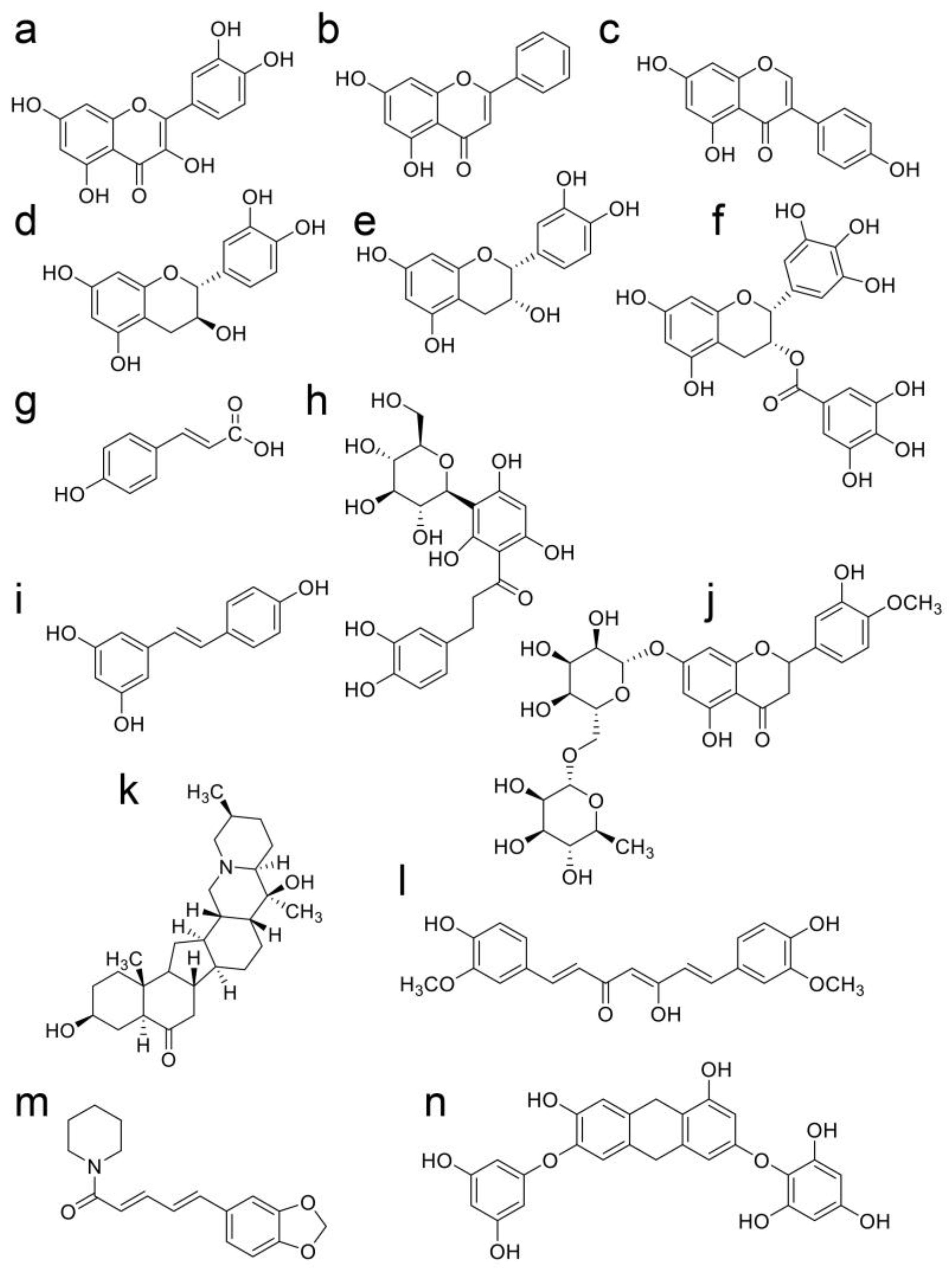
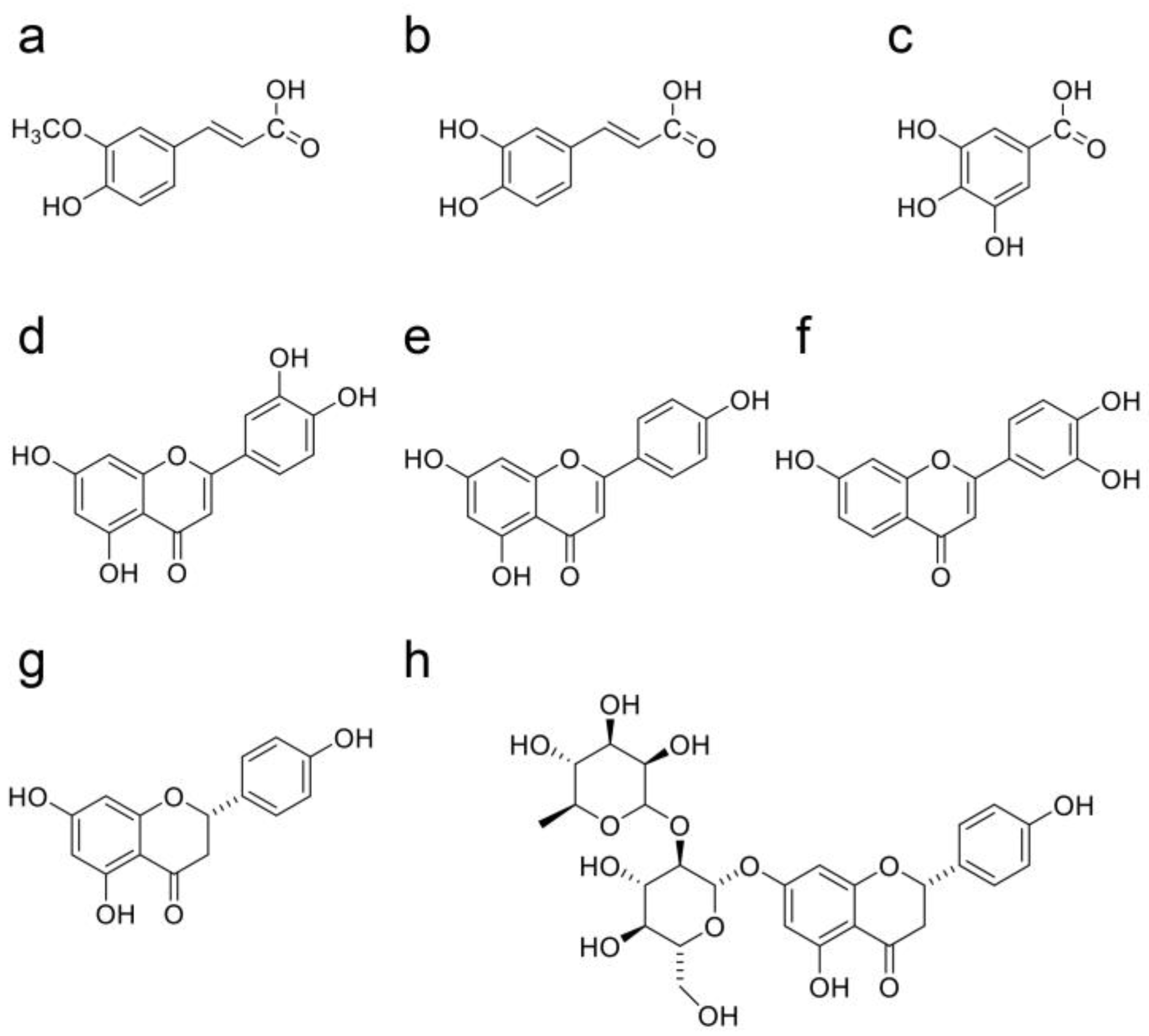
9. Beneficial Natural Products in Seven Crude Drugs Obtained from Hangeshashinto
10. Natural Products in the Crude Drugs Obtained from Hangeshashinto That Inhibit the Generation of Intracellular AGEs
11. Natural Products in Crude Drugs from Hangeshashinto Suppress Extracellular AGEs-RAGE/TLR4 Signaling
12. Limitations
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Advanced glycation end-product |
| ALE | Advanced lipoxidation end-product |
| BSA | Bovine serum albumin |
| CEL | Nε-carboxyethyl–lysine |
| CML | Nε-carboxymethyl–lysine |
| COX | Cyclooxygenase |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | C-X-C chemokine receptor 4 |
| CVD | Cardiovascular disease |
| DODIC | 3-Deoxyglucosone-derived imidazolium cross-link |
| 3D-HPLC | Three-dimensional high-performance liquid chromatography |
| DM | Diabetes mellitus |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK | Extracellular signal-regulated kinase |
| ESI | Electrospray ionization |
| ESI-MS | Electrospray ionization–mass spectrometry |
| GA | Glyceraldehyde |
| GC | Gas chromatography |
| GC–MS | Gas chromatography–mass spectrometry |
| G-H1 | Glyoxal-hydro-imidazolone |
| GLAP | Glyceraldehyde-derived pyridinium |
| GODIC | Glyoxal-derived imidazolium cross-link |
| HAS | Human serum albumin |
| HDL-C | High-density lipoprotein cholesterol |
| HILIC | Hydrophilic interaction liquid chromatography |
| HMGB1 | High-mobility group box-1 |
| HPLC | High-performance liquid chromatography |
| HSP90 | Heat shock protein 90 |
| IL | Interleukin |
| LDL-C | Low-density lipoprotein cholesterol |
| LSRD | Lifestyle-related disease |
| MAGE | Melibiose-derived advanced glycation end-product |
| MALDI | Matrix-assisted laser desorption/ionization |
| MG-H1 | Methylglyoxal-hydro-imidazolone |
| MODIC | Methylglyoxal-derived imidazolium cross-link |
| MOLD | Methylglyoxal–lysine dimer |
| MS | Mass spectrometry |
| MyD88 | Myeloid differentiation factor |
| NASH | Non-alcoholic heptosteatosis |
| NF-κβ | Nuclear factor-κβ |
| NMR | Nuclear magnetic resonance |
| PGE2 | Prostaglandin E2 |
| PPG | Pyrrolopyridinium–lysine dimer-derived glyceraldehyde |
| PVDF | Polyvinylidene fluoride |
| RAGE | Receptor for advanced glycation end-product |
| ROS | Reactive oxygen species |
| sRAGE | Soluble receptor for advanced glycation end-product |
| SGLT1 | Sodium-glucose cotransporter 1 |
| TAGE | Toxic advanced glycation end-product |
| TCM | Traditional Chinese medicine |
| TLR4 | Toll-like receptor 4 |
| TCMS | Traditional Chinese medical science |
| TNF-α | Tumor necrosis factor alpha |
References
- Horvat, A.L.; Prpić, J.; Muhvić, U.M.; Pezelj-Ribarić, S.; Ivančić-Jokić, N.; Peršić, B.R.; Aleksijević, M.; Glažar, I. Oral Mucosal Lesions in Childhood. Dent. J. 2022, 10, 214. [Google Scholar] [CrossRef]
- Ashari, K.A.; Parvaneh, N.; Mirnia, K.; Ayati, M.; Saeedi, M.; Salehzaden, F.; Shahrooei, M.; Sangsari, R.; Rohani, P.; Ziaee, V. Three cases of autoinflammatory disease with novel NLRC4 mutations, and the first mutation reported in the CARD domain of NLRC4 associated with autoinflammatory infantile enterocolitis (AIFEC). Pediatr. Rheumatol. 2024, 22, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Pan, W.; Ming, X.; Wu, J.; Zhang, X.; Miao, J.; Cui, W. The effect of probiotics on severe oral mucositis in cancer patients undergoing chemotherapy and/or radiotherapy: A meta-analysis. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101983. [Google Scholar] [CrossRef] [PubMed]
- Mahakunakorn, P.; Sangchart, P.; Panyatip, P.; Ratha, J.; Damrongrungruang, T.; Priprem, A.; Puthongking, P. In vitro cytoprotective and in vivo anti-oral mucositis effects of melatonin and its derivatives. PeerJ. 2024, 12, e17608. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, M.; Yamaguchi, K.; Tsukada, M.; Ebihara, N.; Ikemoto, H.; Hisamitsu, T. Kampo (Traditional Japanese Herbal) Formulae for Treatment of Stomatitis and Oral Mucositis. Medicines 2018, 10, 130. [Google Scholar] [CrossRef]
- Oh, H.; Makita, Y.; Masuno, K.; Imamura, Y. Hangeshashinto Inhibits Porphyromonas gingivalis Pathogen-Associated Molecular Patterns-Mediated IL-6 and IL-8 Production through Toll-Like Receptors in CAL27 Cells. Evid.-Based Complement. Altern. Med. 2024, 2024, 9866670. [Google Scholar] [CrossRef]
- Hato, H.; Kaneko, A.; Maeda, C.; Sakata, K.; Ono, Y.; Mizukami, Y.; Kono, T.; Kitagawa, Y. Comparison between hangeshashinto and dexamethasone for IL-1α and β-defensin 1 production by human oral keratinocytes. J. Oral Biosci. 2024, 66, 188–195. [Google Scholar] [CrossRef]
- Chan, W.J.; Adiwidjaja, J.; Mclachlen, A.; Boddy, A.V.; Haenett, J.E. Interactions between natural products and cancer treatments: Underlying mechanisms and clinical importance. Cancer Chemother. Pharmacol. 2023, 91, 103–119. [Google Scholar] [CrossRef]
- Nishimura, M.; Taniguchi, S.; Tamaoki, S.; Fujita, T. Inhibition of compound action potentials in the frog sciatic nerve by inchinkoto, a traditional Japanese medicine used for oral mucositis. J. Oral. Biosci. 2024, 66, 420–429. [Google Scholar] [CrossRef]
- Takata, T.; Motoo, Y. Novel In Vitro Assay of the Effects of Kampo Medicines against Intra/Extracellular Advanced Glycation End-Products in Oral, Esophageal, and Gastric Epithelial Cells. Metabolites 2023, 13, 878. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.Y.; Kwon, O.; Jung, S.Y.; Joung, J.; Yang, C.S.; Lee, J.; Cho, J.; Son, C. Efficacy of a Traditional Herbal Formula, Banha-Sasim-Tang in Functional Dyspepsia Classified as Excess Pattern. Front. Pharmacol. 2021, 12, 698887. [Google Scholar] [CrossRef]
- Nishiwaki, R.; Inoue, Y.; Sugao, M.; Sugimasa, N.; Hamaguchi, T.; Noji, M.; Takeuchi, K.; Ito, Y.; Kato, T.; Yasuma, T.; et al. Hangeshashinto-Associated Mesenteric Phlebosclerosis and Highly Atypical Adenoma Requiring Laparoscopic Right Hemicolectomy. Diagnostics 2024, 14, 565. [Google Scholar] [CrossRef]
- Murai, T.; Matsuo, M.; Tanaka, H.; Manabe, Y.; Takaoka, T.; Hachiya, K.; Yamaguchi, T.; Otsuka, S.; Shibamoto, Y. Efficacy of herbal medicine TJ-14 for acute radiation-induced enteritis: A multi-institutional prospective Phase II trial. J. Radiat. Res. 2020, 61, 140–145. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Xiao, C.; Liu, H.; Fu, X.; You, F. Hangeshashinto for preventing oral mucositis in patients receiving cancer treatment: Protocol for a systematic review and meta-analysis. BMJ Open 2021, 11, e047627. [Google Scholar] [CrossRef]
- Kato, T.; Sakaguchi, H. Efficacy of Cryotherapy and Hangeshashinto for Radiation-induced Oral Stomatitis: Preliminary Study. In Vivo 2023, 37, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Kaneko, A.; Matsumoto, C.; Miyagi, C.; Ohbuchi, K.; Mozuhara, Y.; Miyano, K.; Uezono, Y. Multitargeted effects of hangeshashinto for treatment of chemotherapy-induced oral mucositis on inducible prostaglandin E2 production in human oral keratinocytes. Integra. Cancer Ther. 2014, 13, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Fukamichi, H.; Matsumoto, C.; Omiya, Y.; Arimoto, T.; Morisaki, H.; Kataoka, H.; Kadena, M.; Funatsu, T.; Fukutake, M.; Kase, Y.; et al. Effects of Hangeshashinto on Growth of Oral Microorganisms. Evid.-Based Complement. Alternat. Med. 2015, 2015, 512947. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, N.; Onda, T.; Hayashi, K.; Honda, G.; Shibahara, T. Effects of Topical Hangeshashinto (TJ-14) on Chemotherapy-Induced Oral Mucositis. Cancer Manag. Res. 2020, 12, 1069–1078. [Google Scholar] [CrossRef]
- Ogihara, T.; Kagawa, M.; Yamanaka, R.; Imai, S.; Itohara, K.; Hira, D.; Nakagawa, S.; Yonezawa, A.; Ito, M.; Nakagawa, T.; et al. Preparation and pharmaceutical properties of Hangeshashinto oral ointment and its safety and efficacy in Syrian hamsters with 5-fluorouracil-induced oral mucositis. J. Nat. Med. 2023, 77, 53–63, Erratum in J. Nat. Med. 2024, 78, 803. [Google Scholar] [CrossRef]
- Endo, M.; Oikawa, T.; Tonooka, M.; Hanawa, T.; Odaguchi, H.; Hori, M. Hangekobokuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus through its anti-inflammatory action. J. Smooth Muscle Res. 2022, 58, 78–88. [Google Scholar] [CrossRef]
- Tu, C.; Ma, Y.; Song, M.; Yan, J.; Xiao, Y.; Wu, H. Liquiritigenin inhibits IL-1β-induced inflammation and cartilage matrix degradation in rat chondrocytes. Eur. J. Pharmacol. 2019, 858, 172445. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, G.; Si, Z.; Yu, L.; Hou, L. Glycyrrhizin, an HMGB1 inhibitor, Suppresses Interleukin-1β-Induced Inflammatory Responses in Chondrocytes from Patients with Osteoarthritis. Cartilage 2020, 13, 947S–955S. [Google Scholar] [CrossRef]
- Li, B.; Wang, M.; Chen, S.; Li, M.; Zeng, J.; Wu, S.; Tu, Y.; Li, Y.; Zhang, R.; Huang, F.; et al. Baicalin Mitigates the Neuroinflammation through the TLR4/MyD88/NF-κB and MAPK Pathways in LPS-Stimulated BV-2 Microglia. Biomed. Res. Int. 2022, 2022, 3263446. [Google Scholar] [CrossRef]
- Eren, D.; Betul, Y.M. Revealing the effect of 6-gingerol, 6-shogaol and curcumin on mPGES-1, GSK-3β and β-catenin pathway in A549 cell line. Chem. Biol. Interact. 2016, 258, 257–265. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, Y.; Yang, X.; Wei, Q.; Wang, H. 6-Shogaol reduces progression of experimental endometriosis in vivo and in vitro via regulation of VGEF and inhibition of COX-2 and PGE2-mediated inflammatory responses. Korean J. Physiol. Pharmacol. 2018, 22, 627–636. [Google Scholar] [PubMed]
- Takata, T. Is the Novel Slot Blot a Useful Method for Quantification of Intracellular Advanced Glycation End-Products? Metabolites 2023, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Msauji, T.; Motoo, Y. Analysis of Crude, Diverse, and Multiple Advanced Glycation End-Product Patterns May Be Important and Beneficial. Metabolites 2024, 14, 3. [Google Scholar]
- Takata, T.; Inoue, S.; Masauji, T.; Miyazawa, T.; Motoo, Y. Generation and Accumulation of Various Advanced Glycation End-Products in Cardiomyocytes May Induce Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 7319. [Google Scholar] [CrossRef]
- Takata, T.; Inoue, S.; Kunii, K.; Masauji, T.; Miyazawa, K. Slot Blot- and Electrospray Ionization-Mass Spectrometry/Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry-Based Novel Analysis Methods for the Identification and Quantification of Advanced Glycation End-Products in the Urine. Int. J. Mol. Sci. 2024, 25, 9632. [Google Scholar] [CrossRef]
- Oya-Ito, T.; Naito, Y.; Takagi, T.; Handa, O.; Matsui, H.; Yamada, M.; Shima, K.; Yoshikawa, T. Heat-shock protein 27 (Hsp27) as a target of methylglyoxal in gastrointestinal cancer. Biochem. Biophys. Acta. 2011, 1812, 769–781. [Google Scholar]
- Chen, P.; Gregersen, H.; Zhao, J. Advanced glycation end-product expression is upregulated in the gastrointestinal tract of type 2 diabetic rats. World J. Diabetes 2015, 6, 662–672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.; Fang, H.; Xie, C. Advanced glycation end products in gastric cancer: A promising future. World J. Clin. Oncol. 2024, 15, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Mark, R.; Bermejo, J.L.; Bierhaus, A.; Plinkert, P.K.; Angel, P.; Hess, J. The receptor for advanced glycation end products is dispensable in a mouse model of oral and esophageal carcinogenesis. Histol. Histopathol. 2013, 28, 1585–1594. [Google Scholar] [PubMed]
- Tancharoen, S.; Gando, S.; Binita, S.; Nagasato, T.; Kikuchi, K.; Nawa, Y.; Dararat, P.; Yamamoto, M.; Narkpinit, S.; Maruyama, I. HMGB1 Promotes Intraoral Palatal Wound Healing through RAGE-Dependent Mechanisms. Int. J. Mol. Sci. 2016, 17, 1961. [Google Scholar] [CrossRef]
- Sinduja, P.; Ramani, P.; Sekaran, S. Quantitative Assessment of Receptors of Advanced Glycation End Products Expression in Tissue Samples from Patients with oral Submucous Fibrosis, Leukoplakia, and Oral Squamous Cell Carcinoma. Contemp. Clin. Dent. 2024, 15, 71–76. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, H.; Deng, P.; He, P.; Chen, Q.; Wang, Z.; Qui, F.; Oz, F.; Chen, E.; Zeng, M. Investigation on the simultaneous inhibition of advanced glycation end products, 4-methylimidazole and hydroxymethylfurfural in thermal reaction meat flavorings by liquiritigenin, liquiritin and glycyrrhizic acid and possible pathways. Food Res. Int. 2023, 173, 113414. [Google Scholar] [CrossRef]
- Alvi, S.S.; Nabi, R.; Khan, M.S.; Akhter, F.; Ahmad, S.; Khan, M.S. Glycyrrhizic Acid Scavenges Reactive Carbonyl Species and Attenuates Glycation-Induced Multiple Protein Modification: An In Vitro and In Silico Study. Oxid. Med. Cell. Longev. 2021, 2021, 7086951. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, X.; Wu, Q.; Jin, X.; Li, H.; Roy, R. Pharmacological Action of Baicalin on Gestational Diabetes Mellitus in Pregnant Animals Induced by Streptozotocin via AGE-RAGE Signaling Pathway. Appl. Biochem. Biotechnol. 2024, 196, 1636–1651. [Google Scholar] [CrossRef]
- Fu, S.; Zhao, W.; Xiog, C.; Guo, L.; Guo, J.; Qiu, Y.; Hu, C.A.; Ye, C.; Liu, Y.; Wu, Z.; et al. Baicalin modulates apoptosis via RAGE, MAPK, and AP-1 in vascular endothelial cells during Haemophilus parasuis invasion. Innate Immun. 2019, 25, 420–432. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.; Wang, P.; Ahmedna, M.; Sang, S. Bioactive ginger constituents alleviate protein glycation by trapping methylglyoxal. Chem. Res. Toxicol. 2015, 28, 1842–1849. [Google Scholar] [CrossRef]
- Sampath, C.; Zhu, Y.; Sang, S.; Ahmedna, M. Bioactive compounds isolated from apple, tea, and ginger protect against dicarbonyl induced stress in cultured human retinal epithelial cells. Phytomedicine 2016, 23, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, K.; Bando, M.; Sakamoto, E.; Inagaki, Y.; Naruishi, K.; Yumoto, H.; Kido, J. 6-Shogaol Inhibits Advanced Glycation End-Products-Induced IL-6 and ICAM-1 Expression by Regulating Oxidative Responses in Human Gingival Fibroblasts. Molecules 2019, 24, 3705. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, C.; Guo, Y.; Wang, H. Screening potential treatments for mpox from Traditional Chinese Medicine by using a data-driven approach. Medicine 2023, 102, e35116. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Zhao, F.; Yu, Z.; Zhu, X.; Li, C.G. Interactions Between Natural Products and Tamoxifen in Breast Cancer: A Comprehensive Literature Review. Front. Pharmacol. 2022, 13, 847113. [Google Scholar] [CrossRef]
- Akash, S.; Bibi, S.; Biswas, P.; Mukerjee, N.; Khan, D.A.; Hasan, M.N.; Sultana, N.A.; Hosen, M.E.; Jardan, Y.A.B.; Nafidi, H.; et al. Revolutionizing anti-cancer drug discovery against breast cancer and lung cancer by modification of natural genistein: An advanced computational and drug design approach. Front. Oncol. 2023, 13, 1228865. [Google Scholar] [CrossRef]
- Duan, X.; Chen, L.; Liu, Y.; Chen, H.; Wang, F.; Hu, Y. Integrated physicochemical, hormonal, and transcriptomic analysis reveals the underlying mechanism of callus formation in Pinellia ternata hydroponic cuttings. Front. Plant Sci. 2023, 14, 1189499. [Google Scholar] [CrossRef]
- Song, Y.; Hing, H.; Son, J.S.; Kwon, Y.O.; Lee, H.H.; Kim, H.J.; Park, J.H.; Son, M.J.; Oh, J.; Yoon, M. Investigation of Ginsenosides and Antioxidant Activities in the Roots, Leaves, and Stems of Hydroponic-Cultured Ginseng (Panax ginseng Meyer). Prev. Nutr. Food Sci. 2019, 24, 283–292. [Google Scholar] [CrossRef]
- Xu, Y.; Woo, S.B.; Treister, N.S. Thalidomide for management of refractory oral mucosal diseases. Oral Surg. Oral Med. Pathol. Oral Radiol. 2024, 137, 372–378. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Yuan, C.; Cheng, Z. Recurrent aphthous stomatitis and neoplasms of the mouth and pharynx: A two-sample Mendelian randomization study. BMC Cancer 2024, 24, 1372. [Google Scholar] [CrossRef]
- Coppola, N.; Cantile, T.; Adamo, D.; Canfora, F.; Baidares, S.; Riccitiello, F.; Musella, G.; Mignogna, M.D.D.; Leuci, S. Supportive care and antiviral treatments in primary herpetic gingivostomatitis: A systematic review. Clin. Oral. Investig. 2023, 27, 6333–6344. [Google Scholar] [CrossRef]
- Uesugi, A.; Tsushima, F.; Miyamoto, Y.; Harada, H. Pollen Food Allergy Syndrome Caused by Japanese Radish: A Case Report. Indian J. Dermatol. 2023, 68, 123. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Y.; Chen, D.; Liao, B.; Wang, J.; Shen, J.; Gou, L.; Zhou, Y.; Zhou, X.; Liao, G.; et al. Moxidectin elevates Candida albicans ergosterol levels to synergize with polyenes against oral candidiasis. Appl. Microbiol. Biotechnol. 2024, 108, 509. [Google Scholar] [CrossRef]
- Pezzotti, G.; Adachi, T.; Imamura, H.; Ikegami, S.; Kitahara, R.; Yamamoto, T.; Kanakura, N.; Zhu, W.; Ishibashi, K.; Okumura, K.; et al. Raman Spectroscopic Algorithms for Assessing Virulence in Oral Candidiasis: The Fight-or-Flight Response. Int. J. Mol. Sci. 2024, 25, 11410. [Google Scholar] [CrossRef]
- Manpreet, K.; Ajmal, M.B.; Raheel, S.A.; Saleem, M.C.; Mubeen, K.; Gaballah, K.; Faden, A.; Kujan, O. Oral health status among transgender young adults: A cross-sectional study. BMC Oral Health 2021, 21, 575. [Google Scholar] [CrossRef]
- Dashti, H.; Sundaram, D. The association between nicotine stomatitis and waterpipe smoking. Tob. Induc. Dis. 2024, 22, 118. [Google Scholar] [CrossRef]
- Nolan, A.; Mclntosh, W.B.; Allam, B.F.; Lamey, P.J. Recurrent aphthous ulceration: Vitamin B1, B2 and B6 status and response to replacement therapy. J. Oeal. Pathol. Med. 1991, 20, 389–391. [Google Scholar] [CrossRef]
- Lakshmi, A.V.; Ramalakshmi, B.A. Effect of pyridoxine or riboflavin supplementation on plasma homocysteine levels in women with oral lesions. Natl. Med. J. India 1998, 11, 171–172. [Google Scholar] [PubMed]
- Hus, P.; Chen, J.; Kuo, S.; Wang, W.; Jan, F.; Yang, S.; Yang, C. San-Zhong-Kui-Jian-Tang Exerts Antitumor Effects Associated With Decreased Cell Proliferation and Metastasis by Targeting ERK and the Epithelial-Mesenchymal Transition Pathway in Oral Cavity Squamous Cell Carcinoma. Intrgr. Cancer Ther. 2022, 21, 15347354221134921. [Google Scholar]
- Shirahata, T.; Kanzawa, A.; Uematsu, M.; Fuchino, H.; Kawano, N.; Kawahara, N.; Yoshimatsu, K.; Hanawa, T.; Odaguchi, H.; Kobayashi, Y. Near-Infrared Metabolic Profiling for Discrimination of Apricot and Peach Kernels. Chem. Pharm. Bull. 2022, 70, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Motoo, Y.; Cameron, S. Kampo medicines for supportive care of patients with cancer: A brief review. Integr. Med. Res. 2022, 11, 100839. [Google Scholar] [CrossRef]
- Motoo, Y.; Yasui, H. Analysis on Kampo case reports from the viewpoint of “Yasui Classification”. Tradit. Kampo Med. 2024, 11, 60–64. [Google Scholar] [CrossRef]
- Chung, H.; Moroi, M.; Hojo, Y.; Chen, F.; Yukawa, K.; Motoo, Y.; Arai, I. The Status of Nationwide Implementation of Integrative Medicine Programs by Japanese Local Government from a “Social Model” Viewpoint. Prespect. Integr. Med. 2024, 3, 98–105. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.; Zhou, S.; Chi, K.; Wu, J.; Hung, F. Multiple tophi deposits in the spine: A case report. World J. Clin. Case 2022, 10, 10647–10654. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, X.; Du, P.; Wang, J.; Du, Y.; Li, B.; Xue, Y.; Jiang, J.; Cai, Y.; Yang, Q. Mechanistic insights into the ameliorative effects of Xianglianhuazhuo formula on chronic atrophic gastritis through ferroptosis mediated by YY1/miR-320a/TFRC signal pathway. J. Ethnopharmacol. 2024, 323, 117608. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Z.; Cheng, N.; Jia, X.; Wang, J.; Ma, H.; Zhao, R.; Li, B.; Xue, Y.; Cai, Y.; et al. High-throughput sequencing analysis of differential microRNA expression in the process of blocking the progression of chronic atrophic gastritis to gastric cancer by Xianglian Huazhuo formula. J. Tradit. Chin. Med. 2024, 44, 703–712. [Google Scholar]
- Gong, C.; Li, H.; Li, H.; Li, Q.; Gu, P.; Xiao, Q.; Jia, Y.; Xiao, Q.; Mi, Y.; Wei, S.; et al. Efficacy and mechanism of long-snake moxibustion for treating insomnia in breast cancer survivors: Study protocol for a randomized controlled trial. Front. Neurol. 2025, 16, 1524412. [Google Scholar] [CrossRef]
- Samadi, M.; Huang, L.; Mo, P.; Hernandez, M.; Hunh, I.Y.; Jan, Y. Effects of negative pressure of four-cup cupping therapy on hemodynamic responses of the gastrocnemius. J. Bodyw. Mov. Ther. 2025, 42, 446–451. [Google Scholar] [CrossRef]
- Kashkash, F.; Swed, S.; Mustafa, A.; Azizi, S.; Bakkour, A.; Abdalla, A.; Alkammar, M.; Hafez, W. Acinetobacter-Induced Endocarditis Post Cupping Therapy: Case Report. Clin. Case Rep. 2025, 13, e70490. [Google Scholar] [CrossRef]
- Meng, X.; Guo, X.; Zhang, J.; Moriya, J.; Kobayashi, J.; Yamaguchi, R.; Yamada, S. Acupuncture on ST36, CV4 and KI1 Suppresses the Progression of Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in Mice. Metabolites 2019, 9, 299. [Google Scholar] [CrossRef]
- Han, J.; Guo, X.; Meng, X.; Zhang, J.; Yamaguchi, R.; Motoo, Y.; Yamada, S. Acupuncture improved lipid metabolism by regulating intestinal absorption in mice. World J. Gatsroenterol. 2020, 26, 5118–5129. [Google Scholar] [CrossRef]
- Li, H.; Wang, D. Acupuncture, a Promising Therapy for Insulin Resistance and Non-Alcoholic Fatty Liver Disease. Int. J. Gen. Med. 2024, 17, 4917–4928. [Google Scholar] [CrossRef] [PubMed]
- Motoo, Y.; Arai, I.; Tsutani, K. Use of Kampo Diagnosis in Randomized Controlled Trials of Kampo Products in Japan: A Systematic Review. PLoS ONE 2014, 9, e104422. [Google Scholar] [CrossRef] [PubMed]
- Uema, M.; Hyuga, M.; Yonemitsu, K.; Hyuga, S.; Amakura, Y.; Uchiyama, N.; Mizoguchi, K.; Odaguchi, H.; Goda, Y. Antiviral Effect of Ephedrine Alkaloids-Free Ephedra Herb Extract against SARS-CoV-2 In Vitro. Microorganisms 2023, 11, 534. [Google Scholar] [PubMed]
- Huang, X.; Hyuga, S.; Amakura, Y.; Hyuga, M.; Uchiyama, N.; Hakamatsuka, T.; Goda, Y.; Odaguchi, H.; Hanawa, T.; Kobayashi, Y. Overlooked switch from transient sedation to sustained excitement in the Biphasic effects of Ephedra Herb extract administered orally to mice. J. Ethnopharmacol. 2023, 301, 115827. [Google Scholar]
- Arai, I. Clinical studies of traditional Japanese herbal medicines (Kampo): Need for evidence by the modern scientific methodology. Integr. Med. Res. 2021, 10, 100722. [Google Scholar] [CrossRef]
- Arai, I.; Kawahara, N. Kampo pharmaceutical products in the Japanese health-care system: Legal status and quality assurance. Tradit. Kampo Med. 2019, 6, 3–11. [Google Scholar]
- Miyano, K.; Hasegawa, S.; Asai, N.; Uzu, M.; Yatsuoka, W.; Ueno, T.; Nonaka, M.; Fujii, H.; Uezono, Y. The Japanese Herbal Medicine Hangeshashinto Induces Oral Keratinocyte Migration by Mediating the Expression of CXCL12 Through the Activation of Extracellular Signal-Regulated Kinase. Front. Pharmacol. 2022, 12, 695039. [Google Scholar] [CrossRef]
- Zhu, Y.; Duan, A.; Yu, Q.; Tian, S.; Zhou, Z.; Li, P.; Pan, D.; Tao, H.; Zhu, Q. Screening bioactive compounds from Fangji Huangqi decoction for treating rheumatoid arthritis via COX-2 magnetic ligand fishing combined with in vivo validation. J. Ethnopharmacol. 2025, 337, 11875. [Google Scholar] [CrossRef]
- Park, E.; Lee, M.; Jeon, W.; Seo, C.; You, S.; Shin, H. Paljung-San, a traditional herbal medicine, attenuates benign prostatic hyperplasia in vitro and in vivo. J. Ethnopharmacol. 2018, 218, 109–115. [Google Scholar] [CrossRef]
- Ji, Y.; Han, J.; Lee, N.; Yoon, J.; Youn, K.; Ha, H.J.; Yoon, E.; Kim, D.H.; Jun, M. Neuroprotective Effects of Baicalein, Wogonin, and Oroxylin A on Amyloid Beta-Induced Toxicity via NF-κB/MAPK Pathway Modulation. Molecules 2020, 25, 5087. [Google Scholar] [CrossRef]
- Li, C.; Fong, S.Y.K.; Mei, Q.; Lin, G.; Zuo, Z. Influence of mefenamic acid on the intestinal absorption and metabolism of three bioactive flavones in Radix Scutellariae and potential pharmacological impact. Pharm. Biol. 2014, 52, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.J.; Yun, K.; Cho, Y.; Park, H.; Lee, K. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages. Eur. J. Pharmacol. 2008, 584, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Wang, D.; Dong, S.; Cheng, Z.; Na, L.; Sang, M.; Yang, H.; Yang, Z.; Zhang, S.; Yan, Z. Palmatine inhibits TRIF-dependent NF-κB pathway against inflammation induced by LPS in goat endometrial epithelial cells. Int. Immunopharmacol. 2017, 45, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, M.P.; Kraus, S.I.; Melzer, T.M.; Bustos, P.S.; Ortega, M.G. Oral treatment with Berberine reduces peripheral nociception: Possible interaction with different nociceptive pathways activated by different allogeneic substances. J. Ethnopharmacol. 2024, 321, 117564. [Google Scholar] [CrossRef]
- Ma, S.; Feng, C.; Dai, G.; Song, Y.; Zhou, G.; Zhang, X.; Miao, C.; Yu, H.; Ju, W. In silico target fishing for the potential bioactive components contained in Huanglian Jiedu Tang (HLJDD) and elucidating molecular mechanisms for the treatment of sepsis. Chin. J. Nat. Med. 2015, 13, 30–40. [Google Scholar] [CrossRef]
- Luo, C.; Chen, H.; Wang, Y.; Lin, G.; Li, C.; Tan, L.; Su, Z.; Lai, X.; Xie, J.; Zeng, H. Protective effect of coptisine free base on indomethacin-induced gastric ulcers in rats: Characterization of potential molecular mechanisms. Life Sci. 2018, 193, 47–56. [Google Scholar] [CrossRef]
- Haque, M.A.; Jantan, I.; Harikrishnan, H.; Wahab, S.M.A. Magnoflorine Enhances LPS-Activated Pro-Inflammatory Responses via MyD88-Dependent Pathways in U937 Macrophages. Planta Med. 2018, 84, 1255–1264. [Google Scholar] [CrossRef]
- Hwang, Y.; Kim, T.; Kim, R.; Ha, H. The Natural Product 6-Gingerol Inhibits Inflammation-Associated Osteoclast Differentiation via Reduction of Prostaglandin E2 Levels. Int. J. Mol. Sci. 2018, 19, 2068. [Google Scholar] [CrossRef]
- Sharma, C.; Kaur, A.; Thind, S.S.; Singh, B.; Raina, S. Advanced glycation End-products (AGEs): An emerging concern for processed food industries. J. Food Sci. Technol. 2015, 52, 7561–7676. [Google Scholar] [CrossRef]
- Plemmenos, G.; Piperi, C. Pathogenic Molecular Mechanisms in Periodontitis and Peri-Implantitis: Role of Advanced Glycation End Products. Life 2022, 12, 218. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, Z.; Cai, H.; Li, Z.; Zhu, J.; Wu, D.; Xu, W.; Qiu, H.; Zhang, N.; Li, G.; et al. WormCNN-Assisted Establishment and Analysis of Glycation Stress Models in C. elegans: Insights into Disease and Healthy Aging. Int. J. Mol. Sci. 2024, 25, 9675. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.G.D.; Barbosa, F.C.; Santos, H.H.; Granero, F.O.; Figueiredo, C.C.M.; Granero, F.O.; Figueiredo, C.C.M.; Nicolau-Junior, N.; Hamaguchi, A.; Silva, L.P. Antioxidant and anti-glycation activities of Mandevilla velutina extract and effect on parasitemia levels in Trypanosoma cruzi experimental infection: In vivo, in vitro and in silico approaches. J. Ethnopharmacol. 2025, 337, 118994. [Google Scholar] [CrossRef] [PubMed]
- Ohno, R.; Ichimaru, K.; Tanaka, S.; Sugawa, H.; Katsuta, N.; Sakae, S.; Tominaga, Y.; Ban, I.; Shirakawa, J.; Yamaguchi, Y.; et al. Glucoselysine is derived from fructose and accumulates in the eye lens of diabetic rats. J. Biol. Chem. 2019, 294, 17326–17338. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Matsumura, T.; Sugawa, H.; Niimi, N.; Sango, K.; Nagai, R. Glucoselysine, a unique advanced glycation end-product of the polyol pathway and its association with vascular complications in type 2 diabetes. J. Biol. Chem. 2024, 300, 107479. [Google Scholar] [CrossRef]
- Litwinowicz, K.; Waszczuk, E.; Kuzan, A.; Bronowicka-Szydełko, A.; Gostomska-Pampuch, K.; Naporowski, P.; Gamian, A. Alcoholic Liver Disease Is Associated with Elevated Plasma Levels of Novel Advanced Glycation End-Products: A Preliminary Study. Nutrients 2022, 14, 5266. [Google Scholar] [CrossRef]
- Sugawa, H.; Ikeda, T.; Tominaga, Y.; Katsuta, N.; Nagai, R. Rapid formation of N ε-(carboxymethyl)lysine (CML) from ribose depends on glyoxal production by oxidation. RSC Chem. Biol. 2024, 5, 1140–1146. [Google Scholar] [CrossRef]
- Ban, I.; Sugawa, H.; Nagai, R. Protein Modification with Ribose Generates Nδ-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine. Int. J. Mol. Sci. 2022, 23, 1224. [Google Scholar] [CrossRef]
- Melvin, M.; Avery, K.C.; Ballentine, R.M.; Flora, J.W.; Gardner, W.; Karles, G.D.; Pithawalla, Y.B.; Smith, D.C.; Ehman, K.D.; Wagner, K.A. Formation of Diacetyl and Other α-Dicarbonyl Compounds during the Generation of E-Vapor Product Aerosols. ACS Omega 2020, 5, 17565–17575. [Google Scholar] [CrossRef]
- Sugiura, K.; Koike, S.; Suzuki, T.; Ogasawara, Y. Oxidative Formation of Methylglyoxal in Glycerol Preparations during Storage. Biol. Pharm. Bull. 2020, 43, 879–883. [Google Scholar] [CrossRef]
- LeWinter, M.M.; Taatjes, D.; Ashikaga, T.; Palmer, B.; Bishop, N.; VanBuren, P.; Bell, S.; Donaldson, C.; Meyer, M.; Margulies, K.B.; et al. Abundance, localization, and functional correlates of the advanced glycation end-product carboxymethyl lysine in human myocardium. Physol. Rep. 2017, 5, e13462. [Google Scholar]
- Jung, W.K.; Park, S.; Kim, H.R.; Ryu, H.Y.; Kim, Y.H.; Kim, J. Advanced Glycation End Products Increase Salivary Gland Hypofunction in d-Galactose-Induced Aging Rats and Its Prevention by Physical Exercise. Curr. Issues Mol. Biol. 2021, 43, 2059–2067. [Google Scholar] [CrossRef]
- Matsui, T.; Joo, H.D.; Lee, J.M.; Ju, S.M.; Tao, W.H.; Higashimoto, Y.; Fukami, K.; Yamagishi, S. Development of a monoclonal antibody-based ELISA system for glyceraldehyde-derived advanced glycation end products. Immunol. Lett. 2015, 167, 141–146. [Google Scholar] [CrossRef]
- Kehm, R.; Rückriemen, J.; Weber, D.; Deubel, S.; Grune, T.; Höhn, A. Endogenous advanced glycation end products in pancreatic islets after short-term carbohydrate intervention in obese, diabetes-prone mice. Nutr. Diabetes 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Shigeta, T.; Sasamoto, K.; Yamamoto, T. Glyceraldehyde-derived advanced glycation end-products having pyrrolopyridinium-based crosslinks. Biochem. Biophys. Rep. 2021, 26, 100963. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Suzuki, H.; Takeda, K.; Sakai-Sakasai, A. Toxic advanced glycation end-products (TAGE) are major structures of cytotoxic AGEs derived from glyceraldehyde. Med. Hypotheses 2024, 183, 111248. [Google Scholar] [CrossRef]
- Simkova, P.; Capcarova, M. The influence of toxic advanced glycation end-products (TAGEs) on the development of diabetic nephropathy. Arch. Ecotoxicol. 2024, 6, 13–16. [Google Scholar] [CrossRef]
- Shen, C.; Lu, C.; Cheng, C.; Li, K.; Kuo, Y.; Wu, C.; Liu, C.; Hsien, S.; Tsai, C.; Yu, C. Advanced Glycation End-Products Acting as Immunomodulators for Chronic Inflammation, Inflammaging and Carcinogenesis in Patients with Diabetes and Immune-Related Diseases. Biomedicines 2024, 12, 1699. [Google Scholar] [CrossRef]
- Lee, H.; Gu, M.; Lee, J.; Kim, Y. Methylglyoxal-Lysine Dimer, an Advanced Glycation End Product, Induces Inflammation via Interaction with RAGE in Mesangial Cells. Mol. Nutr. Food Res. 2021, 65, e200799. [Google Scholar]
- Papadaki, M.; Holewinski, R.J.; Prevus, S.B.; Martin, T.G.; Stachowski, M.J.; Li, A.; Blair, C.A.; Moravec, C.S.; Van Eyk, J.E.; Campbell, K.S.; et al. Diabetes with heart failure increases methylglyoxal modifications in the sarcomere, which inhibit function. JCI. Insight 2018, 3, e121264. [Google Scholar] [CrossRef]
- Ruiz-Meana, M.; Minguet, M.; Bou-Teen, D.; Miro-Casas, E.; Castans, C.; Castellano, J.; Bonzon-Kulichenko, E.; Igual, A.; Rodriguez-Lecoq, R.; Vázquez, J.; et al. Ryanodine Receptor Glycation Favors Mitochondrial Damage in the Senescent Heart. Circulation 2019, 139, 949–964. [Google Scholar] [CrossRef]
- Papadaki, M.; Kampaengsri, T.; Barrick, S.K.; Campbell, S.G.; von Lewiski, D.; Rainer, P.P.; Harris, S.P.; Greenberg, M.J.; Kirk, J.A. Myofilament glycation in diabetes reduces contractility by inhibiting tropomyosin movement, is rescued by cMyBPC domains. J. Mol. Cell. Cardiol. 2022, 162, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Morioka, Y.; Teshigawara, K.; Tomono, Y.; Wang, D.; Izushi, Y.; Wake, H.; Liu, K.; Takahashi, H.K.; Mori, S.; Nishibori, M. The specific localization of advanced glycation end-products (AGEs) in rat pancreatic islets. J. Pharmacol. Sci. 2017, 134, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Indyk, D.; Bronowicka-Szydełko, A.; Gamian, A.; Kuzan, A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci. Rep. 2021, 11, 13264. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, N.; Nagai, M.; Saruwatari, K.; Nakamura, M.; Nagai, R. Mitochondrial stress and glycoxidation increase with decreased kidney function. J. Clin. Biochem. Nutr. 2023, 72, 147–156. [Google Scholar] [CrossRef]
- Kato, S.; Matsumura, T.; Sugawa, H.; Nagai, R. Correlation between serum advanced glycation end-products and vascular complications in patient with type 2 diabetes. Sci. Rep. 2024, 14, 18722. [Google Scholar] [CrossRef]
- Senavirathna, L.; Ma, C.; Chen, R.; Pan, S. Proteomic Investigation of Glyceraldehyde-Derived Intracellular AGEs and Their Potential Influence on Pancreatic Ductal Cells. Cells 2021, 10, 1005. [Google Scholar] [CrossRef]
- Kinoshita, S.; Mera, K.; Ichikawa, H.; Shimasaki, S.; Nagai, M.; Taga, Y.; Iijima, K.; Hattori, S.; Fujiwara, Y.; Shirakawa, J.; et al. Nω-(Carboxymethyl)arginine Is One of the Dominant Advanced Glycation End Products in Glycated Collagens and Mouse Tissues. Oxidative Med. Cell. Longev. 2019, 2019, 9073451. [Google Scholar] [CrossRef]
- Takata, T.; Inoue, S.; Kunii, K.; Masauji, T.; Moriya, J.; Motoo, Y.; Miyazawa, K. Advanced Glycation End-Product-Modified Heat Shock Protein 90 May Be Associated with Urinary Stones. Diseases 2025, 13, 7. [Google Scholar] [CrossRef]
- Takahashi, A.; Takabatake, Y.; Kimura, T.; Maejima, I.; Namba, T.; Yamamoro, T.; Matsuda, J.; Minami, S.; Kaimori, J.; Matsui, I.; et al. Autophagy Inhibits the Accumulation of Advanced Glycation End Products by Promoting Lysosomal Biogenesis and Function in the Kidney Proximal Tubules. Diabetes 2017, 66, 1359–1372. [Google Scholar] [CrossRef]
- Mastrocola, R.; Collino, M.; Nigro, D.; Chiazza, F.; D’Antona, G.; Aragno, M.; Minetto, M.A. Accumulation of advanced glycation end-products and activation of the SCAP/SREBP Lipogenetic pathway occur in diet-induced obese mouse skeletal muscle. PLoS ONE 2015, 10, e0119587. [Google Scholar] [CrossRef]
- Añazco, C.; Riedelsberger, J.; Vega-Montoto, L.; Rojas, A. Exploring the Interplay between Polyphenols and Lysyl Oxidase Enzymes for Maintaining Extracellular Matrix Homeostasis. Int. J. Mol. Sci. 2023, 24, 10985. [Google Scholar] [CrossRef]
- Quansah, E.; Shaik, T.A.; Çevik, E.; Wang, X.; Höppener, C.; Meyer-Zedler, T.; Deckert, V.; Schmitt, M.; Popp, J.; Krafft, C. Investigating biochemical and structural changes of glycated collagen using multimodal multiphoton imaging, Raman spectroscopy, and atomic force microscopy. Anal. Bioanal. Chem. 2023, 415, 6257–6267. [Google Scholar] [CrossRef]
- Nomi, Y.; Kudo, H.; Miyamoto, K.; Okura, T.; Yamamoto, K.; Shimohiro, H.; Kitao, S.; Ito, Y.; Egawa, S.; Kawahara, K.; et al. Free advanced glycation end product distribution in blood components and the effect of genetic polymorphisms. Biochemie 2020, 179, 69–76. [Google Scholar] [CrossRef]
- Okura, T.; Ueta, E.; Nakmura, R.; Fujioka, Y.; Sumi, K.; Matsumoto, K.; Shoji, K.; Matsuzawa, K.; Izawa, S.; Nomi, Y.; et al. High Serum Advanced Glycation End Products Are Associated with Decreased Insulin Secretion in Patients with Type 2 Diabetes: A Brief Report. J. Diabetes Res. 2017, 2017, 5139750. [Google Scholar] [CrossRef]
- Damasiewicz-Bodzek, A.; Łabuz-Roszak, B.; Kumaszka, B.; Tadeusiak, B.; Tyrpień-Golder, K. The Assessment of Serum Concentrations of AGEs and Their Soluble Receptor (sRAGE) in Multiple Sclerosis Patients. Brain Sci. 2021, 11, 1021. [Google Scholar] [CrossRef]
- Kashiwabara, S.; Hosoe, H.; Ohno, R.; Nagai, R.; Shiraki, M. Development and Evaluation of Novel ELISA for Determination of Urinary Pentosidine. J. Nutr. Sci. Vitaminol. 2019, 65, 526–533. [Google Scholar] [CrossRef]
- Martin-Morales, A.; Arakawa, T.; Sato, M.; Matsumura, Y.; Mano-Usui, F.; Ikeda, K.; Inagaki, N.; Sato, K. Development of a Method for Quantitation of Glyceraldehyde in Various Body Compartments of Rodents and Humans. J. Agric. Food Chem. 2021, 69, 13246–13254. [Google Scholar] [CrossRef] [PubMed]
- Hassel, B.; Sørnes, K.; Elsais, A.; Cordero, P.R.; Frøland, A.S.; Rise, F. Glyceraldehyde metabolism in mouse brain and the entry of blood-borne glyceraldehyde into the brain. J. Neurochem. 2024, 168, 2868–2879. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, B.; Hedin, U.; Caidahl, K. Glycolaldehyde and maleyl conjugated human serum albumin as potential macrophage-targeting carriers for molecular imaging purposes. Contrast Media Mol. Imaging 2015, 10, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Kandhro, A.J.; Memon, S.Q.; Khuhawar, M.Y.; Arain, R. Determination of glyoxal and methylglyoxal in the serum of diabetic patients by MEKC using stilbenediamine as derivatizing reagent. Electrophoresis 2007, 28, 3940–3947. [Google Scholar] [CrossRef]
- Kido, R.; Hiroshima, Y.; Kido, J.; Ikuta, T.; Sakamoto, E.; Inagaki, Y.; Naruishi, K.; Yumoto, H. Advanced glycation end-products increase lipocalin 2 expression in human oral epithelial cells. J. Periodontal Res. 2020, 55, 539–550. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Liu, L.; Li, W.; Li, Y.; Li, B. ENO1 Promotes OSCC Migration and Invasion by Orchestrating IL-6 Secretion from Macrophages via a Positive Feedback Loop. Int. J. Mol. Sci. 2023, 24, 737. [Google Scholar] [CrossRef]
- Phuong-Nguyen, K.; Mcneill, B.A.; Aston-Mourney, K.; Rivera, L.R. Advanced Glycation End-Products and Their Effects on Gut Health. Nutrients 2023, 15, 405. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Sato, K.; Ochi, S.; Kawano, S.; Munesue, S.; Harashima, A.; Oshima, Y.; Kyoi, T.; Yamamoto, Y. Inhibitory Effects of Saururus chinensis Extract on Receptor for Advanced Glycation End-Products-Dependent Inflammation and Diabetes-Induced Dysregulation of Vasodilation. Int. J. Mol. Sci. 2022, 23, 5757. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, Z.; Zhang, Y.; Qiu, C.; Zhu, L.; Zhao, N.; Liu, Z. Matrine alleviates AGEs- induced cardiac dysfunctions by attenuating calcium overload via reducing ryanodine receptor 2 activity. Eur. J. Pharmacol. 2019, 842, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Gu, M.J.; Kim, Y.; Lee, J.; Lee, S.; Choi, I.; Ha, S.K. Glyoxal-Lysine Dimer, an Advanced Glycation End Product, Induces Oxidative Damage and Inflammatory Response by Interacting with RAGE. Antioxidants 2021, 10, 1486. [Google Scholar] [CrossRef]
- Wada, K.; Nakashima, Y.; Yamakawa, M.; Hori, A.; Seishima, M.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; Nagata, C. Dietary advanced glycation end products and cancer risk in Japan: From the Takayama study. Cancer Sci. 2022, 113, 2839–2848. [Google Scholar] [CrossRef]
- Chen, J.; Radjabzadeh, D.; Medina-Gomez, C.; Voortman, T.; van Meurs, J.B.J.; Ikram, M.A.; Uitterlinden, A.G.; Kraaij, R.; Zillikens, M.C. Advanced Glycation End Products (AGEs) in Diet and Skin in Relation to Stool Microbiota: The Rotterdam Study. Nutrients 2023, 15, 2567. [Google Scholar] [CrossRef]
- Takata, T.; Taniguchi, S.; Mae, Y.; Kageyama, K.; Fujino, Y.; Iyama, T.; Hikita, K.; Sugihara, T.; Isomoto, H. Comparative assessment of the effects of dotinurad and febuxostat on the renal function in chronic kidney disease patients with hyperuricemia. Sci. Rep. 2025, 15, 8990. [Google Scholar] [CrossRef]
- Shakthiya, T.; Chand, L.; Annamalai, R. A Recent Update on Candidate Biomarkers in the Pathogenesis of Diabetic Retinopathy. Open Biomark. J. 2025, 15, e18753183372247. [Google Scholar] [CrossRef]
- Kato, A.; Sugawa, H.; Tabe, K.; Ito, K.; Nakashima, H.; Nagai, R. Rapid pretreatment for multi-sample analysis of advanced glycation end products and their role in nephropathy. J. Clin. Biochem. Nutr. 2022, 70, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, W.; Zhang, Z.; He, M.; Zhang, Y.; Song, B.; Liu, J.; Zhang, H. FPS-ZM1 attenuates the deposition of lipid in the liver of diabetic mice by sterol regulatory element binding protein-1c. BMC Endocr. Disord. 2024, 24, 164. [Google Scholar] [CrossRef] [PubMed]
- Ameera, K.; Mukunda, D.C.; Basha, S.; Rodrigues, J.; Biswas, S.; Mazumder, N.; Prabhu, V.; Prabhu, M.M.; Mahato, K.K. Fluorescence in probing the biochemical and conformational changes in non-enzymatically glycated proteins. Appl. Spectrosc. Rev. 2025, 60, 638–670. [Google Scholar] [CrossRef]
- Pinto, R.S.; Ferreira, G.S.; Silvestre, G.C.R.; Santana, M.F.M.; Nunes, V.S.; Ledesma, L.; Pinto, P.R.; de Assis, S.I.S.; Machado, U.F.; de Silva, E.S.; et al. Plasma advanced glycation end products and soluble receptor for advanced glycation end products as indicators of sterol content in human carotid atherosclerotic plaques. Diabetes Vasc. Dis. Res. 2022, 19, 14791641221085269. [Google Scholar] [CrossRef]
- Yan, Y.; Hemmler, D.; Schmitt-Kopplin, P. HILIC-MS for Untargeted Profiling of the Free Glycation Product Diversity. Metabolites 2022, 12, 1179. [Google Scholar] [CrossRef]
- Takata, T.; Murayama, H.; Masauji, T. Slot Blot Analysis of Intracellular Glyceraldehyde-Derived Advanced Glycation End Products Using a Novel Lysis Buffer and Polyvinylidene Difluoride Membrane. Bio-Protoc. 2024, 14, e5038. [Google Scholar] [CrossRef]
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Intracellular Toxic Advanced Glycation End-Products May Induce Cell Death and Suppress Cardiac Fibroblasts. Metabolites 2022, 12, 615. [Google Scholar] [CrossRef]
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Intracellular Toxic Advanced Glycation End-Products in 1.4E7 Cell Line Induce Death with Reduction of Microtubule-Associated Protein 1 Light Chain 3 and p62. Nutrients 2022, 14, 332. [Google Scholar] [CrossRef]
- Miyazaki, S.; Takino, J.; Nagamine, K.; Takeuchi, M.; Hori, T. RasGRP2 Attenuates TAGE Modification of eNOS in Vascular Endothelial Cells. Biol. Pharm. Bull. 2025, 48, 262–266. [Google Scholar] [CrossRef]
- Tominaga, Y.; Sugawa, H.; Hirabayashi, K.; Ikeda, T.; Hoshi, Y.; Nagai, R. Drosera tokaiensis extract containing multiple phenolic compounds inhibits the formation of advanced glycation end-products. Arch. Biochem. Biophys. 2020, 693, 108586. [Google Scholar] [CrossRef]
- Sembiring, E.R.; Fuad, A.M.; Suryadi, H. Expression and purification of recombinant human granulocyte colony-stimulating factor (rG-CSF) from Pichia pastoris. Indones. J. Biotechnol. 2024, 29, 205–212. [Google Scholar] [CrossRef]
- Baskal, S.; Tsikas, D. Free L-Lysine and Its Methyl Ester React with Glyoxal and Methylglyoxal in Phosphate Buffer (100 mM, pH 7.4) to Form Nε-Carboxymethyl-Lysine, Nε-Carboxyethyl-Lysine and Nε-Hydroxymethyl-Lysine. Int. J. Mol. Sci. 2022, 23, 3446. [Google Scholar] [CrossRef] [PubMed]
- Baskal, S.; Kaiser, A.; Meis, C.; Kruger, R.; Tsikas, D. Specific and sensitive GC-MS analysis of hypusine, Nε-(4-amino-2-hydroxybutyl)lysine, a biomarker of hypusinated eukaryotic initiation factor eIF5A, and its application to the bi-ethnic ASOS study. Amino Acids 2022, 54, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Baskal, S.; Bollenbach, A.; Mels, C.; Kruger, R.; Tsikas, D. Development, validation of a GC-MS method for the simultaneous measurement of amino acids, their PTM metabolites and AGEs in human urine, and application to the bi-ethnic ASOS study with special emphasis to lysine. Amino Acids 2022, 54, 615–641. [Google Scholar] [CrossRef] [PubMed]
- Baskal, S.; Büttner, P.; Werner, S.; Besler, C.; Lurz, P.; Thiele, H.; Tsikas, D. Profile of urinary amino acids and their post-translational modifications (PTM) including advanced glycation end-products (AGEs) of lysine, arginine and cysteine in lean and obese ZSF1 rats. Amino Acids 2022, 54, 643–652. [Google Scholar] [CrossRef]
- Katsuta, N.; Takahashi, H.; Nagai, M.; Sugawa, H.; Nagai, R. Changes in S-(2-succinyl)cysteine and advanced glycation end-products levels in mouse tissues associated with aging. Amino Acids 2022, 54, 653–661. [Google Scholar] [CrossRef]
- Suzuki, R.; Fijiwara, Y.; Saito, M.; Arakawa, S.; Shirakawa, J.; Yamanaka, M.; Komohara, Y.; Marumo, K.; Nagai, R. Intracellular Accumulation of Advanced Glycation End Products Induces Osteoblast Apoptosis Via Endoplasmic Reticulum Stress. J. Bone Miner. Res. 2020, 35, 1992–2003. [Google Scholar] [CrossRef]
- Singh, V.; Singh, A.K. Oral mucositis. Natl. J. Maxillofac. Surg. 2020, 11, 159–168. [Google Scholar] [CrossRef]
- Yokoyama, A.; Watanabe, K.; Inoue, Y.; Hirano, T.; Tamaoki, M.; Hirohashi, K.; Kawaguchi, S.; Ishida, Y.; Takeuchi, Y.; Kishimoto, Y.; et al. Somatic mosaicism in the buccal mucosa reflects lifestyle and germline risk factors for esophageal squamous cell carcinoma. Sci. Transl. Med. 2025, 17, eadq6740. [Google Scholar] [CrossRef]
- Yadav, N.; Palkhede, J.D.; Kim, S. Anti-Glucotoxicity Effect of Phytoconstituents via Inhibiting MGO-AGEs Formation and Breaking MGO-AGEs. Int. J. Mol. Sci. 2023, 24, 7672. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I.; Scheijen, J.L.J.M.; Ahles, S.; Vangrieken, P.; Schalkwijk, C.G. A Citrus and Pomegranate Complex Reduces Methylglyoxal in Healthy Elderly Subjects: Secondary Analysis of a Double-Blind Randomized Cross-Over Clinical Trial. Int. J. Mol. Sci. 2023, 24, 13168. [Google Scholar] [CrossRef]
- Schmidt, B.; Ferreira, C.; Alves Passos, C.L.; Silva, J.L.; Fialho, E. Resveratrol, Curcumin and Piperine Alter Human Glyoxalase 1 in MCF-7 Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 5244. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Sakai-Sakasai, A.; Kajinami, K.; Takeuchi, M. A Novel Approach: Investigating the Intracellular Clearance Mechanism of Glyceraldehyde-Derived Advanced Glycation End-Products Using the Artificial Checkpoint Kinase 1 d270KD Mutant as a Substrate Model. Cells 2023, 12, 2838. [Google Scholar] [CrossRef] [PubMed]
- Senavirathna, L.; Hasapes, C.; Chen, R.; Pan, S. Accumulation of Intracellular Protein Advanced Glycation End Products Alters Cellular Homeostasis for Protein Clearance in Pancreatic Ductal Epithelial Cells. Biochemistry 2024, 63, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Sekar, P.; Hsiao, G.; Hsu, S.; Huang, D.; Lin, W.; Chan, C. Metformin inhibits methylglyoxal-induced retinal pigment epithelial cell death and retinopathy via AMPK-dependent mechanisms: Reversing mitochondrial dysfunction and upregulating glyoxalase 1. Redox Biol. 2023, 64, 102786. [Google Scholar] [CrossRef]
- Prisco, S.Z.; Hartweck, L.; Keen, J.L.; Vogel, N.; Kazmirczak, F.; Eklund, M.; Hemnes, A.R.; Brittain, E.L.; Prins, K.W. Glyoxylase-1 combats dicarbonyl stress and right ventricular dysfunction in rodent pulmonary arterial hypertension. Front. Cardiovasc. Med. 2022, 9, 940932. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Z.; Sheng, Z. Ability of resveratrol to inhibit advanced glycation end product formation and carbohydrate-hydrolyzing enzyme activity, and to conjugate methylglyoxal. Food Chem. 2017, 216, 153–160. [Google Scholar] [CrossRef]
- Lee, S.M.; Zheng, L.W.; Jung, Y.; Hwang, G.; Kim, Y. Effects of hydroxycinnamic acids on the reduction of furan and α-dicarbonyl compounds. Food Chem. 2019, 312, 126085. [Google Scholar] [CrossRef]
- Froldi, G.; Djeujo, F.M.; Bulf, N.; Caparelli, E.; Ragazzi, E. Comparative Evaluation of the Antiglycation and Anti-α-Glucosidase Activities of Baicalein, Baicalin (Baicalein 7- O-Glucuronide) and the Antidiabetic Drug Metformin. Pharmaceutics 2022, 14, 2141. [Google Scholar] [CrossRef]
- Carnovali, M.; Luzi, L.; Terruzzi, L.; Banfi, G.; Mariotti, M. Liquiritigenin Reduces Blood Glucose Level and Bone Adverse Effects in Hyperglycemic Adult Zebrafish. Nutrients 2019, 11, 1042. [Google Scholar] [CrossRef]
- Ahmad, Y.; Nabi, F.; Siddiqui, S.; Khan, R.H.; Habib, S.; Moin, S. Assessing the role of Berberine as an inhibitor of advanced glycation end products (AGEs) formation using in vitro and molecular interaction studies. Arch. Biochem. Biophys. 2025, 766, 110292. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, M.N.C.; Vergoten, G.; Bailly, C. Mechanism of action of glycyrrhizin against Plasmodium falciparum. Mem. Inst. Oswaldo Cruz 2021, 116, e210084. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Siddiquei, M.M.; Nawaz, M.I.; Geboes, K.; Mohammad, G. The proinflammatory cytokine high-mobility group box-1 mediates retinal neuropathy induced by diabetes. Mediat. Inflamm. 2014, 2014, 746415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hsieh, C.; Liu, W.; Yin, M. Glycyrrhizic acid attenuated glycative stress in kidney of diabetic mice through enhancing glyoxalase pathway. Mol. Nutr. Food. Res. 2014, 58, 1426–1435. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, P.; Zhu, Y.; Lv, L.; Sang, S. Additive Capacity of [6]-Shogaol and Epicatechin To Trap Methylglyoxal. J. Agric. Food. Chem. 2017, 65, 8356–8362. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Han, X.; Feng, L.; Wang, R.; Zhang, M.; Zhu, M.; Jia, X.; Hu, S. Liquiritin attenuates advanced glycation end products-induced endothelial dysfunction via RAGE/NF-κB pathway in human umbilical vein endothelial cells. Mol. Cell Biochem. 2013, 374, 191–201. [Google Scholar] [CrossRef]
- Dai, X.; Liu, Y.; Liu, T.; Zhang, Y.; Wang, S.; Xu, T.; Yin, J.; Shi, H.; Ye, Z.; Zhu, R.; et al. SiJunZi decoction ameliorates bone quality and redox homeostasis and regulates advanced glycation end products/receptor for advanced glycation end products and WNT/β-catenin signaling pathways in diabetic mice. J. Ethnopharmacol. 2024, 219, 117167. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Z.; Chen, S.; Wang, X.; Wang, D.; Nie, X.; Yao, Y. Ge-Gen-Qin-Lian decoction alleviates the symptoms of type 2 diabetes mellitus with inflammatory bowel disease via regulating the AGE-RAGE pathway. BMC Complement. Med. Ther. 2024, 24, 225. [Google Scholar] [CrossRef]
- Lin, C.; Lin, Y.; Paul, C.R.; Hsieh, D.J.; Day, C.H.; Chen, R.; Kuo, C.; Ho, T.; Shibu, M.A.; Kai, C.; et al. Isoliquiritigenin ameliorates advanced glycation end-products toxicity on renal proximal tubular epithelial cells. Environ. Toxicol. 2022, 37, 2096–2102. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, T.; Hou, W.; Zhou, Y.; Deng, S.; Song, Y. Isoliquiritigenin Protects Against Diabetic Nephropathy in db/db Mice by Inhibiting Advanced Glycation End Product-Receptor for Advanced Glycation End Product Axis. Drug. Dev. Res. 2025, 86, e70051. [Google Scholar] [CrossRef]
- Goto, K.; Fujiwara- Tani, R.; Nukaga, S.; Miyagawa, Y.; Kawahara, I.; Nishida, R.; Ikemoto, A.; Sasaki, R.; Ogata, R.; Kishi, S.; et al. Berberine Improves Cancer-Derived Myocardial Impairment in Experimental Cachexia Models by Targeting High-Mobility Group Box-1. Int. J. Mol. Sci. 2024, 25, 4735. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lao, G.; Ran, J.; Zhu, P. Berberine alleviates AGEs-induced ferroptosis by activating NRF2 in the skin of diabetic mice. Exp. Biol. Med. 2024, 249, 10280. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Gao, M.; Tang, X.; Jiao, M.; Wang, C.; Wei, Y.; Gong, Q.; Zhong, J. HMGB1/RAGE axis in tumor development: Unraveling its significance. Front. Oncol. 2024, 14, 1336191. [Google Scholar] [CrossRef]
- Takata, T.; Masauji, T.; Motoo, Y. Potential of the Novel Slot Blot Method with a PVDF Membrane for Protein Identification and Quantification in Kampo Medicines. Membranes 2023, 13, 896. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Gołąb, K.; Gburek, J.; Wysokińska, H.; Matkowski, A. Inhibition of Advanced Glycation End-Product Formation and Antioxidant Activity by Extracts and Polyphenols from Scutellaria alpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef]
- Motoo, Y.; Hakamatsuka, T.; Kawahara, N.; Arai, I.; Tsutani, K. Standards of Reporting Kampo Products (STORK) in research articles. J. Integr. Med. 2017, 15, 182–185. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.; Mitsuhashi, S.; Sugetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef]
- Long, P.; Xia, Y.; Yang, Y.; Cao, J. Network-based pharmacology and molecular docking exploring the “Bupleuri Radix-Scutellariae Radix” mechanism of action in the viral hepatitis B treatment. Medicine 2022, 101, e31835. [Google Scholar] [CrossRef]
- Wei, M.; Li, H.; Li, Q.; Qiao, Y.; Ma, Q.; Xie, R.; Wang, R.; Liu, Y.; Wei, C.; Li, B.; et al. Based on Network Pharmacology to Explore the Molecular Targets and Mechanisms of Gegen Qinlian Decoction for the Treatment of Ulcerative Colitis. Biomed. Res. Int. 2020, 2020, 5217405. [Google Scholar] [CrossRef]
- Shi, X.; Guo, J.; Saravelos, S.; Hung, X.; Xia, E.; Feng, L.; Li, T. The use of intrauterine balloon therapy in reproductive medicine and surgery: A guidance for practice. Hum. Fertil. 2023, 26, 742–756. [Google Scholar] [CrossRef]
- Chawes, B.L.; Rechnitzer, C.; Schmiegelow, K.; Tvede, M. [Procalcitonin for early diagnosis of bacteraemia in children with cancer]. Ugeskr. Laeger 2007, 169, 138–142. [Google Scholar] [PubMed]
- Yimam, M.; Brownell, L.; Pantier, M.; Jia, Q. UP446, analgesic and anti-inflammatory botanical composition. Pharmacogn. Res. 2013, 5, 139–145. [Google Scholar] [CrossRef]
- Yimam, M.; Brownell, L.; Hodges, M.; Jia, Q. Analgesic effects of a standardized bioflavonoid composition from Scutellaria baicalensis and Acacia catechu. J. Diet. Suppl. 2012, 9, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Eliaz, I.; Sliva, D. Suppression of growth and invasive behavior of human prostate cancer cells by ProstaCaid™: Mechanism of activity. Int. J. Oncol. 2011, 38, 1675–1682. [Google Scholar] [PubMed]
- Lee, C.J.; Lee, J.H.; Seok, J.H.; Hur, G.M.; Park, Y.C.; Seol, I.C.; Kim, Y.H. Effects of baicalein, berberine, curcumin and hesperidin on mucin release from airway goblet cells. Planta Med. 2003, 69, 523–526. [Google Scholar]
- Yao, H.; Li, S.; Hu, J.; Chen, Y.; Huang, L.; Lin, J.; Li, G.; Lin, X. Chromatographic fingerprint and quantitative analysis of seven bioactive compounds of Scutellaria barbata. Planta Med. 2011, 77, 388–393. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, N.; Shukla, S.; Behl, T.; Gupta, S.; Anwer, M.K.; Vargas-De-La-Cruz, C.; Bungau, S.G. Understanding the Potential Role of Nanotechnology in Liver Fibrosis: A Paradigm in Therapeutics. Molecules 2023, 28, 2811. [Google Scholar] [CrossRef]
- Duan, L.; Jin, D.; An, X.; Zhang, Y.; Zhao, S.; Zhou, R.; Duan, Y.; Zhang, Y.; Liu, X.; Lian, F. The Potential Effect of Rhizoma coptidis on Polycystic Ovary Syndrome Based on Network Pharmacology and Molecular Docking. Evid.-Based Complement. Altern. Med. 2021, 2021, 5577610. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Yu, H.; Yuan, G.; Luo, L.; Xu, X.; Xu, Y.; Sui, X.; Leung, E.L.; Wu, Q. The Role and Mechanisms of Action of Natural Compounds in the Prevention and Treatment of Cancer and Cancer Metastasis. Front. Biosci. 2022, 27, 192. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, N.; Peng, X.; Ma, W.; Qin, Y.; Yao, Z.; Huang, C.; Zhang, X. Network pharmacology analysis and clinical verification of Jishe Qushi capsules in rheumatoid arthritis treatment. Medicine 2023, 102, e34883. [Google Scholar] [CrossRef]
- Kim, J. Investigation of Phenolic, Flavonoid, and Vitamin Contents in Different Parts of Korean Ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Z.; Dong, K.; Li, G.; Zhu, H. Yizhijiannao Granule and a combination of its effective monomers, icariin and Panax notoginseng saponins, inhibit early PC12 cell apoptosis induced by beta-amyloid (25-35). Neural Regen. Res. 2012, 7, 1845–1850. [Google Scholar] [PubMed]
- Du, G.; Wang, C.; Qi, L.; Zhang, Z.; Calway, T.; He, T.; Du, W.; Yuan, C. The synergistic apoptotic interaction of panaxadiol and epigallocatechin gallate in human colorectal cancer cells. Phytother. Res. 2013, 27, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, H.; An, J.; Zhang, R.; Chen, B.; Hao, D. Therapeutic Effects of Traditional Chinese Medicine on Spinal Cord Injury: A Promising Supplementary Treatment in Future. Evid.-Based Complement. Altern. Med. 2016, 2016, 89587211. [Google Scholar] [CrossRef]
- Liu, J.; Xu, X.; Zhou, J.; Sun, G.; Li, Z.; Zhai, L.; Wang, J.; Ma, R.; Zhao, D.; Jiang, R.; et al. Phenolic acids in Panax ginseng inhibit melanin production through bidirectional regulation of melanin synthase transcription via different signaling pathways. J. Ginseng Res. 2023, 47, 714–725. [Google Scholar] [CrossRef]
- Moon, P.; Han, N.; Lee, J.S.; Kim, H.; Jeong, H. p-coumaric acid, an active ingredient of Panax ginseng, ameliolates atopic dermatitis-like skin lesions through inhibition of thymic stromal lymphopoietin in mice. J. Ginseng Res. 2021, 45, 176–182. [Google Scholar] [CrossRef]
- Aiexander, C.; Parsaee, A.; Vasefi, M. Polyherbal and Multimodal Treatments: Kaempferol- and Quercetin-Rich Herbs Alleviate Symptoms of Alzheimer’s Disease. Biology 2023, 12, 1453. [Google Scholar]
- Kim, J.; Shin, H.; Seo, C. Chemical interaction between Paeonia lactiflora and Glycyrrhiza uralensis, the components of Jakyakgamcho-tang, using a validated high-performance liquid chromatography method: Herbal combination and chemical interaction in a decoction. J. Sep. Sci. 2014, 37, 2704–2715. [Google Scholar] [CrossRef]
- Rapaport, J.; Sadgrove, N.J.; Arruda, S.; Swearingen, A.; Abidi, Z.; Sadick, N. Real-World, Open-Label Study of the Efficacy and Safety of a Novel Serum in Androgenetic Alopecia. J. Drugs Dermatol. 2023, 22, 559–564. [Google Scholar] [CrossRef]
- Chociej, P.; Foss, K.; Jabłońska, M.; Ustarbowska, M.; Sawicki, T. The Profile and Content of Polyphenolic Compounds and Antioxidant and Anti-Glycation Properties of Root Extracts of Selected Medicinal Herbs. Plant Food Hum. Nutr. 2024, 79, 468–473. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, J.; Jang, C.H.; Lim, J.S.; Lee, J.S.; Kim, J. In Vivo Anti-inflammatory Potential of Viscozyme®-Treated Jujube Fruit. Foods 2020, 9, 1033. [Google Scholar] [CrossRef]
- Yan, M.; Wang, Y.; Watharkar, R.B.; Pu, Y.; Wu, C.; Lin, M.; Lu, D.; Liu, M.; Bao, J.; Xia, Y. Physicochemical and antioxidant activity of fruit harvested from eight jujube (Ziziphus jujuba Mill.) cultivars at different development stages. Sci. Rep. 2022, 12, 2272. [Google Scholar] [PubMed]
- Yuan, S.; Li, W.; Li, Q.; Wang, L.; Cao, J.; Jiang, W. Defense Responses, Induced by p-Coumaric Acid and Methyl p-Coumarate, of Jujube (Ziziphus jujuba Mill.) Fruit against Black Spot Rot Caused by Alternaria alternata. J. Agric. Food Chem. 2019, 67, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Bekheit, S.O.; Kolieb, E.; El-Awady, E.E.; Alwaili, M.A.; Alharthi, A.; Khodeer, D.M. Cardioprotective Effects of Ferulic Acid Through Inhibition of Advanced Glycation End Products in Diabetic Rats with Isoproterenol-Induced Myocardial Infarction. Pharmaceuticals 2025, 18, 319. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Stancu, C.S.; Sima, A.V. Caffeic acid attenuates the inflammatory stress induced by glycated LDL in human endothelial cells by mechanisms involving inhibition of AGE-receptor, oxidative, and endoplasmic reticulum stress. BioFactors 2017, 43, 685–697. [Google Scholar] [CrossRef]
- Umadevi, S.; Gopi, V.; Elangovan, V. Regulatory mechanism of gallic acid against advanced glycation end products induced cardiac remodeling in experimental rats. Chem.-Biol. Interact. 2014, 208, 28–36. [Google Scholar] [CrossRef]
- Umadevi, S.; Gopi, V.; Vellaichamy, E. Inhibitory effect of gallic acid on advanced glycation end products induced up-regulation of inflammatory cytokines and matrix proteins in H9C2 (2-1) cells. Cardiovasc. Toxicol. 2013, 13, 396–405. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, P.; Liang, X.; Sun, J.; Liu, Q.; Guan, S.; Wang, Q. Luteolin targets the AGE-RAGE signaling to mitigate inflammation and ferroptosis in chronic atrophic gastritis. Aging 2024, 16, 10918–10930. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, K.; Gong, J.; Li, E.T.S.; Wang, M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem. Pharmacol. 2019, 166, 231–241. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Z.; Zhang, Y.; Luo, C.; Huang, P.; Tong, J.; Ding, H.; Liu, H. Apigenin and baicalein ameliorate thoracic aortic structural deterioration and cognitive deficit via inhibiting AGEs/RAGE/NF-κB pathway in D-galactose-induced aging rats. Eur. J. Pharmacol. 2024, 976, 176660. [Google Scholar] [CrossRef]
- Garg, S.; Malhotra, R.K.; Khan, S.I.; Sarkar, S.; Susrutha, P.N.; Singh, V.; Goyal, S.; Nag, T.C.; Ray, R.; Bhatia, J.; et al. Fisetin attenuates isoproterenol-induced cardiac ischemic injury in vivo by suppressing RAGE/NF-κB mediated oxidative stress, apoptosis and inflammation. Phytomedicine 2019, 56, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xu, H.; Zhao, Y.; Liu, B.; Cheng, K.; Xhen, F.; Wang, M. 6-C-(E-Phenylethenyl)-naringenin, a Styryl Flavonoid, Inhibits Advanced Glycation End Product-Induced Inflammation by Upregulation of Nrf2. J. Agric. Food Chem. 2022, 70, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.A.; Reza, M.I.; Shafiq, M.; Kumariya, S.; Singh, P.; Husain, A.; Hanif, K.; Gayen, J.R. Naringin ameliorates type 2 diabetes mellitus-induced steatohepatitis by inhibiting RAGE/NF-κB mediated mitochondrial apoptosis. Life Sci. 2020, 257, 118118. [Google Scholar] [CrossRef]
- Zu, G.; Sun, K.; Li, L.; Zu, X.; Han, T.; Huang, H. Mechanism of quercetin therapeutic targets for Alzheimer disease and type 2 diabetes mellitus. Sci. Rep. 2021, 11, 22959. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hou, J.; Liu, Y.; Liu, P.; Zhao, T.; Wang, X. Using Network Pharmacology to Explore the Mechanism of Panax notoginseng in the Treatment of Myocardial Fibrosis. J. Diabetes Res. 2022, 2022, 8895950. [Google Scholar] [CrossRef]
- Kang, C.; Li, X.; Liu, P.; Liu, Y.; Niu, Y.; Zeng, X.; Liu, J.; Zhao, H.; Qiu, S. Quercetin inhibits the activity and function of dendritic cells through the TLR4/IRAK4/NF-κB signalling pathway. Contemp. Oncol. 2023, 27, 182–189. [Google Scholar]
- Gong, X.; Huang, Y.; Ma, Q.; Jing, M.; Zhan, K.; Zhao, G. Quercetin Alleviates Lipopolysaccharide-Induced Cell Damage and Inflammation via Regulation of the TLR4/NF-κB Pathway in Bovine Intestinal Epithelial Cells. Curr. Issues Mol. Biol. 2022, 44, 5234–5246. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Liu, Z.; Wang, J. Quercetin effectively improves LPS-induced intestinal inflammation, pyroptosis, and disruption of the barrier function through the TLR4/NF-κB/NLRP3 signaling pathway in vivo and in vitro. Food Nutr. Res. 2022, 66, 8948. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, K.; Huang, Y.; Zhang, X.; Yang, T.; Zhan, K.; Zhao, G. Quercetin Alleviates Lipopolysaccharide-Induced Cell Oxidative Stress and Inflammatory Responses via Regulation of the TLR4-NF-κB Signaling Pathway in Bovine Rumen Epithelial Cells. Toxins 2023, 15, 512. [Google Scholar] [CrossRef]
- Shams, S.G.E.; Eissa, R.G. Amelioration of ethanol-induced gastric ulcer in rats by quercetin: Implication of Nrf2/HO1 and HMGB1/TLR4/NF-κB pathways. Heliyon 2022, 8, e11159. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, D.; Xiao, C.; Zhou, Y.; Li, X.; Wang, L.; He, Z.; Reilly, J.; Xiao, Z.; Shu, X. P-Coumaric Acid Reverses Depression-Like Behavior and Memory Deficit Via Inhibiting AGE-RAGE-Mediated Neuroinflammation. Cells 2022, 11, 1594. [Google Scholar] [CrossRef]
- Wu, Q.; Kong, Y.; Liang, Y.; Niu, M.; Feng, N.; Zhang, C.; Qi, Y.; Guo, Z.; Xiao, J.; Zhou, M.; et al. Protective mechanism of fruit vinegar polyphenols against AGEs-induced Caco-2 cell damage. Food Chem. X 2023, 19, 100736. [Google Scholar] [CrossRef]
- Zabad, O.M.; Samra, Y.A.; Eissa, L.A. P-Coumaric acid alleviates experimental diabetic nephropathy through modulation of Toll like receptor-4 in rats. Life Sci. 2019, 238, 116965. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.M.T.; Seo, S.H.; Chung, S.; Kang, I. Attenuation of hepatic fibrosis by p-Coumaric acid via modulation of NLRP3 inflammasome activation in C57BL/6 mice. J. Nutr. Biochem. 2023, 112, 109204. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zhang, M.; Xu, Q.; Song, F.; Wang, L.; Gai, S.; Tang, H.; Wang, S.; Zhou, L.; Li, H. Exploration of molecular targets and mechanisms of Chinese medicinal formula Acacia Catechu -Scutellariae Radix in the treatment of COVID-19 by a systems pharmacology strategy. Phytother. Res. 2022, 36, 4210–4229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Fu, J.; Hu, J.; Zhang, H.; Ye, M.; Yang, X.; Yu, H.; Xu, H.; Lu, J.; et al. Dissecting the antitumor effects of Scutellaria barbata: Initial insights into the metabolism of scutellarin and luteolin by gut microbiota. J. Pharm. Biomed. Anal. 2024, 248, 116325, Correction in J. Pharm. Biomed. Anal. 2024, 249, 116393. [Google Scholar] [CrossRef]
- Li, H.; Luo, D.; Wei, R.; Sun, M.; Zhang, X.; Deng, H.; Bian, W.; Wei, H.; Huang, Y. Investigating the Mechanism of Rhizoma Coptidis-Eupatorium fortunei Medicine in the Treatment of Type 2 Diabetes Based on Network Pharmacology and Molecular Docking. Biomed. Res. Int. 2022, 2022, 7978258. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Liu, Y.; Ji, X.; Li, X.; Wei, Y.; Zi, F.; Tan, Y. Differences in autotoxic substances and microbial community in the root space of Panax notoginseng coinducing the occurrence of root rot. Appl. Environ. Microbiol. 2024, 90, e0228723. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Zhao, D.; Jiang, R.; Sun, L. Panax ginseng Meyer cv. Silvatica phenolic acids protect DNA from oxidative damage by activating Nrf2 to protect HFF-1 cells from UVA-induced photoaging. J. Ethnopharmacol. 2023, 302, 115883. [Google Scholar]
- Park, W.H.; Chang, M.S.; Yang, W.M.; Bae, H.; Kim, N.I.; Park, S.K. Cytoprotective effect of Panax ginseng on gallic acid-induced toxicity in TM3 mouse Leydig cells. Fitoterapia 2007, 78, 577–579. [Google Scholar] [CrossRef]
- Umukoro, S.; Kalejaye, H.A.; Ben-Azu, B.; Ajayi, A.M. Naringenin attenuates behavioral derangements induced by social defeat stress in mice via inhibition of acetylcholinesterase activity, oxidative stress and release of pro-inflammatory cytokines. Biomed. Pharmacother. 2018, 105, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, W.; Huang, Y.; Zeng, X. Licochalcone B, a chalcone derivative from Glycyrrhiza inflata, as a multifunctional agent for the treatment of Alzheimer’s disease. Nat. Prod. Res. 2020, 34, 736–739. [Google Scholar] [CrossRef]
- Dey, S.; Deepak, M.; Setty, M.; D’Souza, P.; Agarwl, A.; Sangli, G.K. Bioactive caffeic acid esters from Glycyrrhiza glabra. Nat. Prod. Res. 2009, 23, 1657–1663. [Google Scholar] [CrossRef]
- Niu, W.; Wu, F.; Cui, H.; Cao, W.; Xhao, Y.; Wu, Z.; Fan, M.; Liang, C. Network Pharmacology Analysis to Identify Phytochemicals in Traditional Chinese Medicines That May Regulate ACE2 for the Treatment of COVID-19. Evid.-Based Complement. Altern. Med. 2020, 2020, 7493281. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Wang, Y.; Asada, Y.; Koike, K. Prenylflavonoids from Glycyrrhiza uralensis and their protein tyrosine phosphatase-1B inhibitory activities. Bioorganic Med. Chem. Lett. 2010, 20, 5398–5401. [Google Scholar] [CrossRef]
- Yamagishi, N.; Yamamoto, Y.; Noda, C.; Hatayama, T. Naringenin inhibits the aggregation of expanded polyglutamine tract-containing protein through the induction of endoplasmic reticulum chaperone GRP78. Biol. Pharm. Bull. 2012, 35, 1836–1840. [Google Scholar] [CrossRef]
- Khamseekaew, J.; Iampanichakul, M.; Potue, P.; Maneesai, P.; Tangsucharit, P.; Rattanakanokchai, S.; Pakdeechote, P. The Alleviative Effect of Naringin Against Cardiovascular Dysfunction and Remodeling in Hypertensive Rats by Suppressing the Angiotensin II Pathway. Food Sci. Nutr. 2025, 13, e70484. [Google Scholar] [CrossRef] [PubMed]
- Alifarsangi, A.; Esmaeili-Mahani, S.; Salarkia, E.; Alizadeh, E.; Abbasnejad, M. Investigation of the Inhibitory Effect of Naringin on the Development of Morphine Physical Dependency in Male Rats. Addict. Health 2025, 17, 1543. [Google Scholar] [CrossRef] [PubMed]
- Eltahir, A.O.E.; Omoruyi, S.I.; Augustine, T.N.; Luckay, R.C.; Hussein, A.A. Neuroprotective Effects of Glycyrrhiza glabra Total Extract and Isolated Compounds. Pharmaceuticals 2024, 17, 852. [Google Scholar] [CrossRef]
| No. | Japanese Name | English Description | Description of Latin Name |
|---|---|---|---|
| 1 | Hange | Pinellia tuber | Tuber of Pinellia ternate Breitenbach, Araceae |
| 2 | Ogon | Scutellaria root | Root of Scutellaria baicalensis Georgi, Labiatae |
| 3 | Oren | Coptidis rhizome | Rhizome of Coptis japonica Makino, Ranueculaceae |
| 4 | Kankyo | Ginger rhizome | Steamed rhizome of Zingiber officinale Roscoe, Zingiberaceae |
| 5 | Ninjin | Ginseng root | Root of Panax ginseng C.A. Meyer, Araliaceae |
| 6 | Kanzo | Glycyrrhiza root | Root or stolon of Glycyrrhiza uralensis Fischer, Laguminosae |
| 7 | Taiso | Jujube fruit | Fruit of Zizpbus jujura Miller var. inermis Pehder, Rhamnaceae |
| No. | Japanese Name | Description of Latin Name | Natural Product | Reference |
|---|---|---|---|---|
| 1 | Hange | Tuber of Pinellia ternate Breitenbach, Araceae | No information | No information |
| 2 | Ogon | Root of Scutellaria baicalensis Georgi, Labiatae | quercetin | [188,189] |
| genistein | [190,191] | |||
| (+)-catechin | [192,193] | |||
| epigallocatechin-3-gallate | [194] | |||
| hesperidin | [195] | |||
| p-coumaric acid | [196] | |||
| curcumin | [195,197] | |||
| 3 | Oren | Rhizome of Coptis japonica Makino, Ranueculaceae | quercetin | [198] |
| 4 | Kankyo | Steamed rhizome of Zingiber officinale Roscoe, Zingiberaceae | No information | No information |
| 5 | Ninjin | Root of Panax ginseng C.A. Meyer, Araliaceae | quercetin | [199,200] |
| (+)-catechin | [201,202] | |||
| epigallocatechin-3-gallate | [203,204] | |||
| p-coumaric acid | [205,206] | |||
| 6 | Kanzo | Root or stolon of Glycyrrhiza uralensis Fischer, Laguminosae | quercetin | [43,207] |
| genistein | [45] | |||
| (+)-catechin | [208] | |||
| epigallocatechin-3-gallate | [44,209] | |||
| p-coumaric acid | [210] | |||
| 7 | Taiso | Fruit of Zizpbus jujura Miller var. inermis Pehder, Rhamnaceae | quercetin | [211] |
| (+)-catechin | [212] | |||
| (−)-epicatechin | [212] | |||
| p-coumaric acid | [213] |
| No. | Japanese Name | Description of Latin Name | Natural Product | Reference |
|---|---|---|---|---|
| 1 | Hange | Tuber of Pinellia ternate Breitenbach, Araceae | No information | No information |
| 2 | Ogon | Root of Scutellaria baicalensis Georgi, Labiatae | fisetin | [235] |
| luteolin | [236] | |||
| quercetin | [188,189] | |||
| p-coumaric acid | [196] | |||
| 3 | Oren | Rhizome of Coptis japonica Makino, Ranueculaceae | luteolin | [237] |
| quercetin | [198] | |||
| 4 | Kankyo | Steamed rhizome of Zingiber officinale Roscoe, Zingiberaceae | No information | No information |
| 5 | Ninjin | Root of Panax ginseng C.A. Meyer, Araliaceae | ferulic acid | [238] |
| caffeic acid | [205,239] | |||
| gallic acid | [47,240] | |||
| naringenin | [241] | |||
| quercetin | [199,200] | |||
| p-coumaric acid | [205,208] | |||
| 6 | Kanzo | Root or stolon of Glycyrrhiza uralensis Fischer, Laguminosae | caffeic acid | [242,243] |
| gallic acid | [244] | |||
| apigenin | [245] | |||
| naringenin | [246] | |||
| quercetin | [43,207] | |||
| p-coumaric acid | [210] | |||
| 7 | Taiso | Fruit of Zizpbus jujura Miller var. inermis Pehder, Rhamnaceae | quercetin | [211] |
| p-coumaric acid | [212] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takata, T.; Moriya, J.; Miyazawa, K.; Inoue, S.; Yamada, S.; Han, J.; Yang, Q.; Guo, X.; Mizuta, S.; Nakahashi, T.; et al. Potential of Natural Products in Hangeshashinto Water Extract on the Direct Suppression of Stomatitis Induced by Intra-/Extracellular Advanced Glycation End-Products. Int. J. Mol. Sci. 2025, 26, 9118. https://doi.org/10.3390/ijms26189118
Takata T, Moriya J, Miyazawa K, Inoue S, Yamada S, Han J, Yang Q, Guo X, Mizuta S, Nakahashi T, et al. Potential of Natural Products in Hangeshashinto Water Extract on the Direct Suppression of Stomatitis Induced by Intra-/Extracellular Advanced Glycation End-Products. International Journal of Molecular Sciences. 2025; 26(18):9118. https://doi.org/10.3390/ijms26189118
Chicago/Turabian StyleTakata, Takanobu, Junji Moriya, Katsuhito Miyazawa, Shinya Inoue, Sohsuke Yamada, Jia Han, Qian Yang, Xin Guo, Shuichi Mizuta, Takeshi Nakahashi, and et al. 2025. "Potential of Natural Products in Hangeshashinto Water Extract on the Direct Suppression of Stomatitis Induced by Intra-/Extracellular Advanced Glycation End-Products" International Journal of Molecular Sciences 26, no. 18: 9118. https://doi.org/10.3390/ijms26189118
APA StyleTakata, T., Moriya, J., Miyazawa, K., Inoue, S., Yamada, S., Han, J., Yang, Q., Guo, X., Mizuta, S., Nakahashi, T., Onai, N., Nakano, H., Masauji, T., & Motoo, Y. (2025). Potential of Natural Products in Hangeshashinto Water Extract on the Direct Suppression of Stomatitis Induced by Intra-/Extracellular Advanced Glycation End-Products. International Journal of Molecular Sciences, 26(18), 9118. https://doi.org/10.3390/ijms26189118







