Development of a Raman-Based Method for the Diagnosis of People with Obstructive Sleep Apnea Syndrome: The Role of Lactic Acid
Abstract
1. Introduction
2. Results
2.1. Participants Characterization
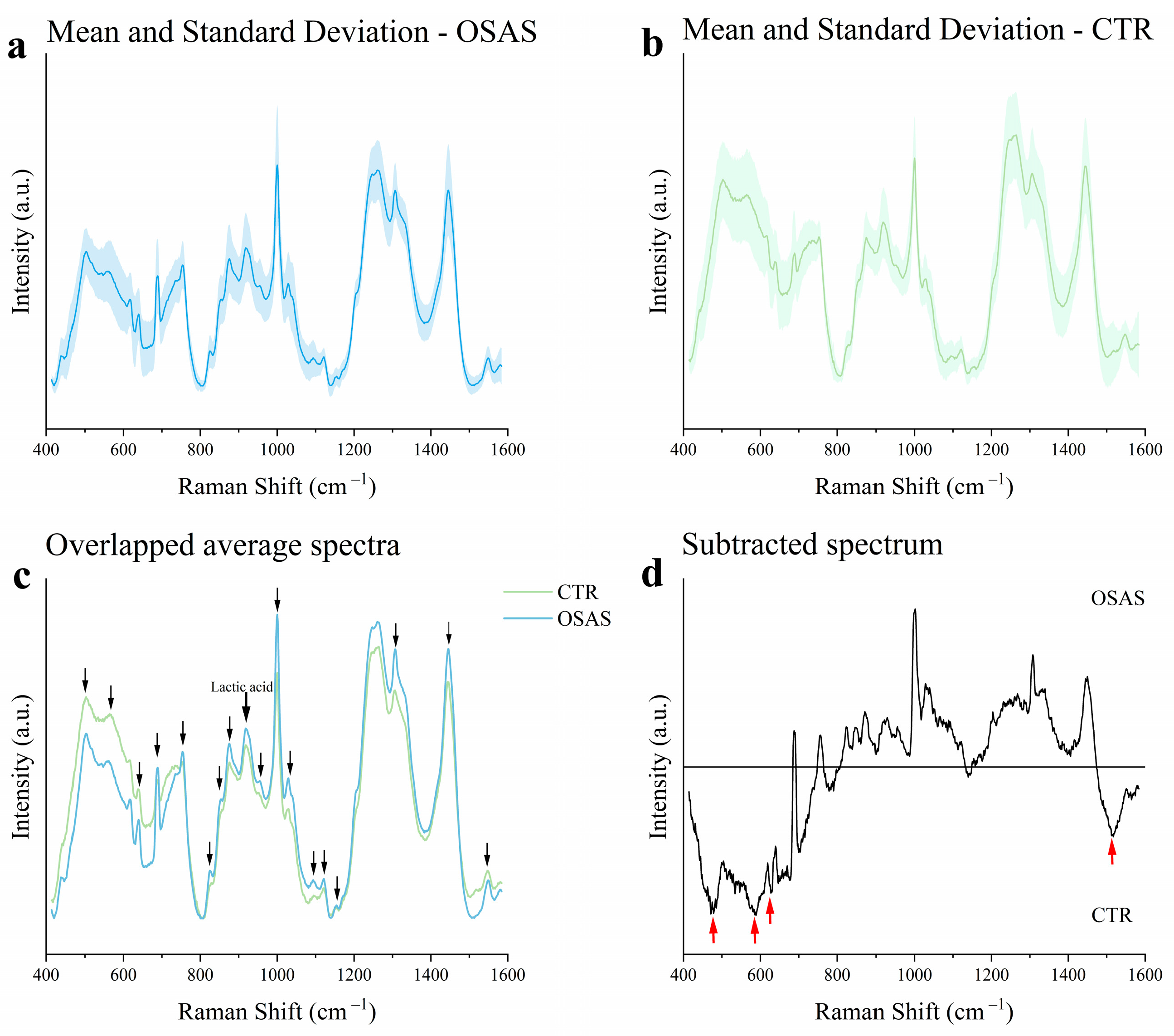
2.2. Raman Fingerprint of Salivary Samples
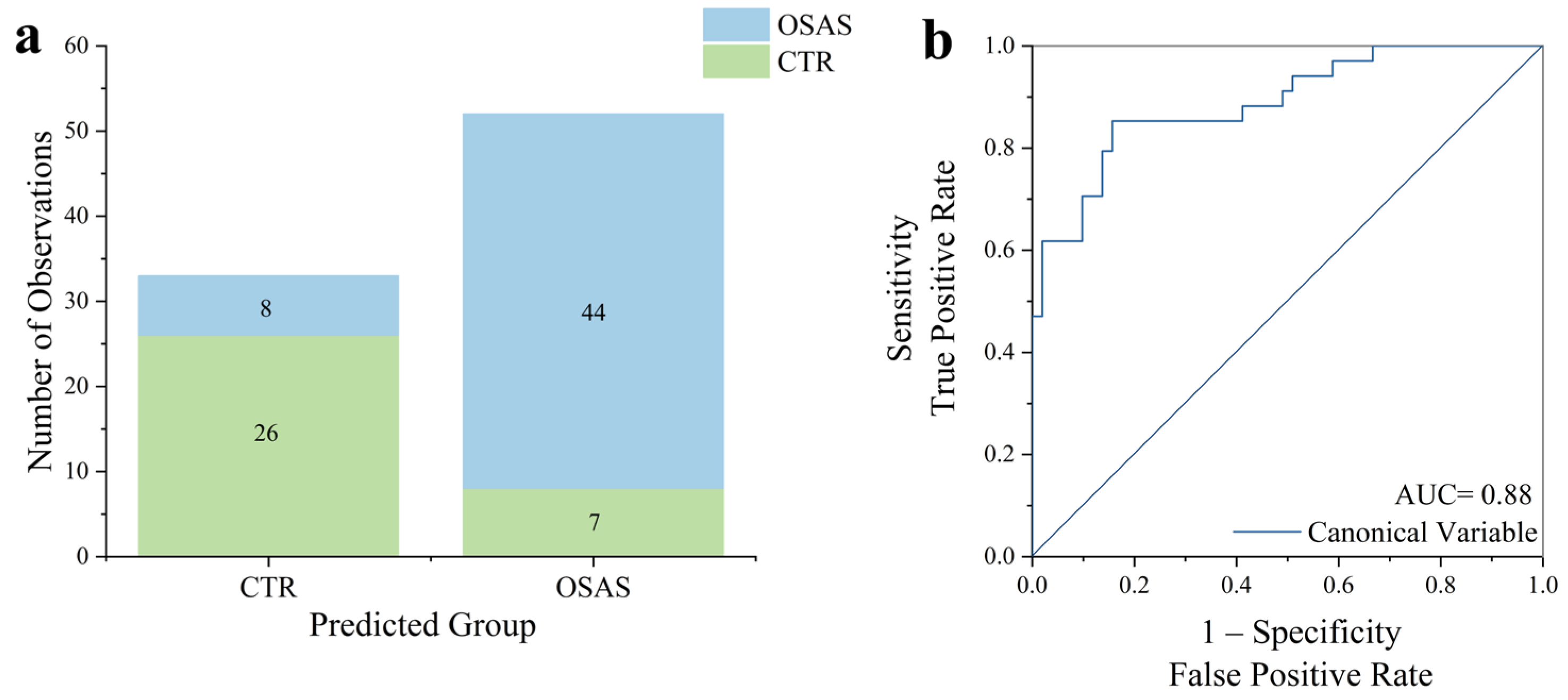
2.3. Multivariate Statistical Analysis
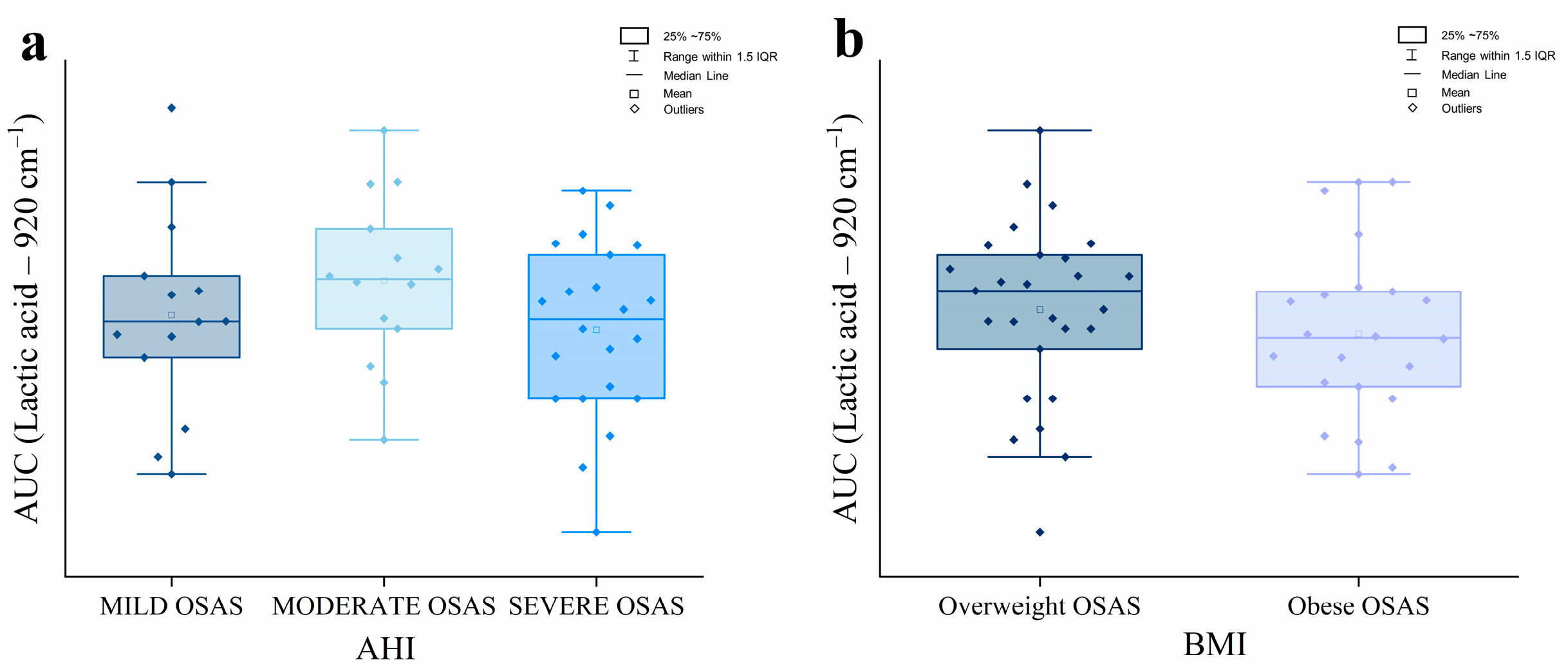
2.4. Analysis of the Lactic Acid Raman Peak
2.5. Quantification of Target Molecules in Salivary Samples
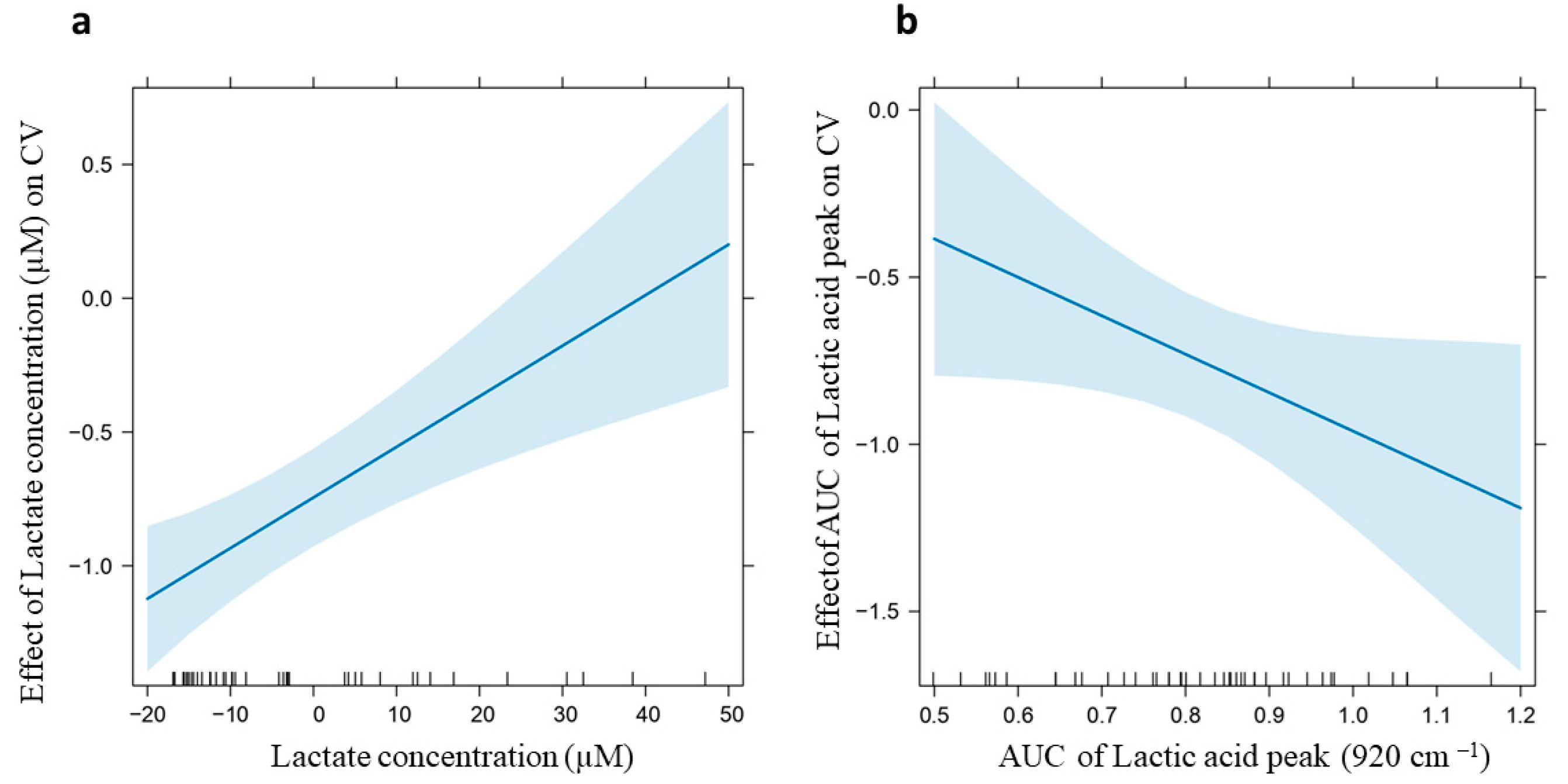
2.6. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Saliva Collection and Processing
4.3. Raman Analysis
4.4. ELISA and Colorimetric Assays
4.5. Statistical Analysis
4.6. Correlation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHI | Apnea and Hypopnea Index |
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| CPAP | Continuous Positive Airway Pressure |
| CTR | Healthy controls |
| CV | Canonical Variable |
| ELISA | Enzyme-Linked ImmunoSorbent Assay |
| ESS | Epworth Sleepiness Scale |
| HPAa | Hypothalamic–Pituitary–Adrenocortical axis |
| HSAT | Home Sleep Apnea Test |
| LDA | Linear Discriminant Analysis |
| LM | Linear Model |
| LOOVC | Leave One Out Cross Validation |
| OSAS | Obstructive sleep apnea syndrome |
| PCA | Principal Component Analysis |
| PSG | Polysomnography |
| RS | Raman spectroscopy |
| SOD3 | Superoxide Dismutase 3 |
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the Global Prevalence and Burden of Obstructive Sleep Apnoea: A Literature-Based Analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological Mechanisms and Therapeutic Approaches in Obstructive Sleep Apnea Syndrome. Sig. Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef]
- Iannella, G.; Pace, A.; Bellizzi, M.G.; Magliulo, G.; Greco, A.; De Virgilio, A.; Croce, E.; Gioacchini, F.M.; Re, M.; Costantino, A.; et al. The Global Burden of Obstructive Sleep Apnea. Diagnostics 2025, 15, 1088. [Google Scholar] [CrossRef]
- Theorell-Haglöw, J.; Miller, C.B.; Bartlett, D.J.; Yee, B.J.; Openshaw, H.D.; Grunstein, R.R. Gender Differences in Obstructive Sleep Apnoea, Insomnia and Restless Legs Syndrome in Adults—What Do We Know? A Clinical Update. Sleep Med. Rev. 2018, 38, 28–38. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of Sleep Apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Strohl, K.P. Adult Obstructive Sleep Apnea/Hypopnea Syndrome: Definitions, Risk Factors, and Pathogenesis. Clin. Chest Med. 2010, 31, 179–186. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389. [Google Scholar] [CrossRef] [PubMed]
- Jean-Louis, G.; Zizi, F.; Clark, L.T.; Brown, C.D.; McFarlane, S.I. Obstructive Sleep Apnea and Cardiovascular Disease: Role of the Metabolic Syndrome and Its Components. J. Clin. Sleep Med. 2008, 4, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Yeh, N.-C.; Tien, K.-J.; Yang, C.-M.; Wang, J.-J.; Weng, S.-F. Increased Risk of Parkinson’s Disease in Patients With Obstructive Sleep Apnea: A Population-Based, Propensity Score-Matched, Longitudinal Follow-Up Study. Medicine 2016, 95, e2293. [Google Scholar] [CrossRef]
- Muraki, I.; Wada, H.; Tanigawa, T. Sleep Apnea and Type 2 Diabetes. J. Diabetes. Investig. 2018, 9, 991–997. [Google Scholar] [CrossRef]
- Cheng, L.; Guo, H.; Zhang, Z.; Yao, Y.; Yao, Q. Obstructive Sleep Apnea and Incidence of Malignant Tumors: A Meta-Analysis. Sleep Med. 2021, 84, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Accattoli, M.P.; Muzi, G.; dell’Omo, M.; Mazzoli, M.; Genovese, V.; Palumbo, G.; Abbritti, G. [Occupational accidents, work performance and obstructive sleep apnea syndrome (OSAS)]. G. Ital. Med. Lav. Ergon. 2008, 30, 297–303. [Google Scholar]
- Udholm, N.; Rex, C.E.; Fuglsang, M.; Lundbye-Christensen, S.; Bille, J.; Udholm, S. Obstructive Sleep Apnea and Road Traffic Accidents: A Danish Nationwide Cohort Study. Sleep Med. 2022, 96, 64–69. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Lee, J.J.; Sundar, K.M. Evaluation and Management of Adults with Obstructive Sleep Apnea Syndrome. Lung 2021, 199, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Ish, P.; Rathi, V.; Kumawat, S.K.; Chakrabarti, S.; Suri, J. Rehabilitation in Obstructive Sleep Apnea: An Ignored Treatment Adjunct. Monaldi Arch. Chest Dis. 2024, 95, 3014. [Google Scholar] [CrossRef]
- Shen, H.; Xu, Y.; Zhang, Y.; Ren, L.; Chen, R. Efficacy of Pulmonary Rehabilitation in Patients with Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea; a Randomized Controlled Trial. J. Rehabil. Med. 2024, 56, jrm23757. [Google Scholar] [CrossRef]
- Toraldo, D.M.; Passali, D.; Sanna, A.; De Nuccio, F.; Conte, L.; De Benedetto, M. Cost-Effectiveness Strategies in OSAS Management: A Short Review. Acta Otorhinolaryngol. Ital. 2017, 37, 447–453. [Google Scholar] [CrossRef]
- Gaspar, L.S.; Santos-Carvalho, A.; Santos, B.; Carvalhas-Almeida, C.; Barros-Viegas, A.T.; Oliveiros, B.; Donato, H.; Santos, C.; Moita, J.; Cavadas, C.; et al. Peripheral Biomarkers to Diagnose Obstructive Sleep Apnea in Adults: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2022, 64, 101659. [Google Scholar] [CrossRef] [PubMed]
- Fiedorczuk, P.; Polecka, A.; Walasek, M.; Olszewska, E. Potential Diagnostic and Monitoring Biomarkers of Obstructive Sleep Apnea–Umbrella Review of Meta-Analyses. J. Clin. Med. 2022, 12, 60. [Google Scholar] [CrossRef]
- Dodo, K.; Fujita, K.; Sodeoka, M. Raman Spectroscopy for Chemical Biology Research. J. Am. Chem. Soc. 2022, 144, 19651–19667. [Google Scholar] [CrossRef]
- Neugebauer, U.; Rösch, P.; Popp, J. Raman Spectroscopy towards Clinical Application: Drug Monitoring and Pathogen Identification. Int. J. Antimicrob. Agents 2015, 46, S35–S39. [Google Scholar] [CrossRef]
- Krafft, C.; Popp, J. The Many Facets of Raman Spectroscopy for Biomedical Analysis. Anal. Bioanal. Chem. 2015, 407, 699–717. [Google Scholar] [CrossRef]
- Cialla-May, D.; Krafft, C.; Rösch, P.; Deckert-Gaudig, T.; Frosch, T.; Jahn, I.J.; Pahlow, S.; Stiebing, C.; Meyer-Zedler, T.; Bocklitz, T.; et al. Raman Spectroscopy and Imaging in Bioanalytics. Anal. Chem. 2022, 94, 86–119. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Allakhverdiev, E.S.; Kossalbayev, B.D.; Sadvakasova, A.K.; Bauenova, M.O.; Belkozhayev, A.M.; Rodnenkov, O.V.; Martynyuk, T.V.; Maksimov, G.V.; Allakhverdiev, S.I. Spectral Insights: Navigating the Frontiers of Biomedical and Microbiological Exploration with Raman Spectroscopy. J. Photochem. Photobiol. B Biol. 2024, 252, 112870. [Google Scholar] [CrossRef]
- Carlomagno, C.; Bertazioli, D.; Gualerzi, A.; Picciolini, S.; Banfi, P.I.; Lax, A.; Messina, E.; Navarro, J.; Bianchi, L.; Caronni, A.; et al. COVID-19 Salivary Raman Fingerprint: Innovative Approach for the Detection of Current and Past SARS-CoV-2 Infections. Sci. Rep. 2021, 11, 4943. [Google Scholar] [CrossRef]
- Laxton, C.S.; Peno, C.; Hahn, A.M.; Allicock, O.M.; Perniciaro, S.; Wyllie, A.L. The Potential of Saliva as an Accessible and Sensitive Sample Type for the Detection of Respiratory Pathogens and Host Immunity. Lancet Microbe 2023, 4, e837–e850. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, F.; Wang, K.; Zhang, W.; Li, Y.; Sun, Y.; Sun, X.; Li, C.; Dong, B.; Wang, L.; et al. Smart Biosensors and Intelligent Devices for Salivary Biomarker Detection. TrAC Trends Anal. Chem. 2021, 140, 116281. [Google Scholar] [CrossRef]
- Bonne, N.J.; Wong, D.T. Salivary Biomarker Development Using Genomic, Proteomic and Metabolomic Approaches. Genome Med. 2012, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-S.; Guilleminault, C.; Hwang, S.-H.; Jeong, J.-H.; Park, D.-S.; Maeng, J.-H. Correlation of Salivary Cortisol Level with Obstructive Sleep Apnea Syndrome in Pediatric Subjects. Sleep Med. 2013, 14, 978–984. [Google Scholar] [CrossRef]
- Tóthová, L.; Hodosy, J.; Mucska, I.; Celec, P. Salivary Markers of Oxidative Stress in Patients with Obstructive Sleep Apnea Treated with Continuous Positive Airway Pressure. Sleep Breath. 2014, 18, 563–570. [Google Scholar] [CrossRef]
- Cassanas, G.; Morssli, M.; Fabrègue, E.; Bardet, L. Vibrational Spectra of Lactic Acid and Lactates. J. Raman Spectrosc. 1991, 22, 409–413. [Google Scholar] [CrossRef]
- Carlomagno, C.; Banfi, P.I.; Gualerzi, A.; Picciolini, S.; Volpato, E.; Meloni, M.; Lax, A.; Colombo, E.; Ticozzi, N.; Verde, F.; et al. Human Salivary Raman Fingerprint as Biomarker for the Diagnosis of Amyotrophic Lateral Sclerosis. Sci. Rep. 2020, 10, 10175. [Google Scholar] [CrossRef]
- Carlomagno, C.; Bertazioli, D.; Gualerzi, A.; Picciolini, S.; Andrico, M.; Rodà, F.; Meloni, M.; Banfi, P.I.; Verde, F.; Ticozzi, N.; et al. Identification of the Raman Salivary Fingerprint of Parkinson’s Disease Through the Spectroscopic– Computational Combinatory Approach. Front. Neurosci. 2021, 15, 704963. [Google Scholar] [CrossRef]
- Falamas, A.; Faur, C.I.; Ciupe, S.; Chirila, M.; Rotaru, H.; Hedesiu, M.; Cinta Pinzaru, S. Rapid and Noninvasive Diagnosis of Oral and Oropharyngeal Cancer Based on Micro-Raman and FT-IR Spectra of Saliva. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119477. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, B.; Murray, J.; Haverstick, J.; Chen, X.; Tripp, R.A.; Zhao, Y. Rapid and Quantitative Detection of Respiratory Viruses Using Surface-Enhanced Raman Spectroscopy and Machine Learning. Biosens. Bioelectron. 2022, 217, 114721. [Google Scholar] [CrossRef]
- Chen, C.; Qi, J.; Li, Y.; Li, D.; Wu, L.; Li, R.; Chen, Q.; Sun, N. Applications of Raman Spectroscopy in the Diagnosis and Monitoring of Neurodegenerative Diseases. Front. Neurosci. 2024, 18, 1301107. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Mishra, S.V.; Joshi, A.; Sarkar, D.; Hole, A.; Mishra, R.; Dutt, S.; Chilakapati, M.K.; Gupta, S.; Dutt, A. Raman Spectroscopy-based Detection of RNA Viruses in Saliva: A Preliminary Report. J. Biophotonics 2020, 13, e202000189. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, C.; Gualerzi, A.; Picciolini, S.; Rodà, F.; Banfi, P.I.; Lax, A.; Bedoni, M. Characterization of the COPD Salivary Fingerprint through Surface Enhanced Raman Spectroscopy: A Pilot Study. Diagnostics 2021, 11, 508. [Google Scholar] [CrossRef]
- Ember, K.; Daoust, F.; Mahfoud, M.; Dallaire, F.; Ahmad, E.Z.; Tran, T.; Plante, A.; Diop, M.-K.; Nguyen, T.; St-Georges-Robillard, A.; et al. Saliva-Based Detection of COVID-19 Infection in a Real-World Setting Using Reagent-Free Raman Spectroscopy and Machine Learning. J. Biomed. Opt. 2022, 27, 025002. [Google Scholar] [CrossRef]
- Ucar, Z.Z.; Taymaz, Z.; Erbaycu, A.E.; Kirakli, C.; Tuksavul, F.; Guclu, S.Z. Nocturnal Hypoxia and Arterial Lactate Levels in Sleep-Related Breathing Disorders. South. Med. J. 2009, 102, 693–700. [Google Scholar] [CrossRef]
- Buchan, E.; Kelleher, L.; Clancy, M.; Stanley Rickard, J.J.; Oppenheimer, P.G. Spectroscopic Molecular-Fingerprint Profiling of Saliva. Anal. Chim. Acta 2021, 1185, 339074. [Google Scholar] [CrossRef]
- Muro, C.K.; De Souza Fernandes, L.; Lednev, I.K. Sex Determination Based on Raman Spectroscopy of Saliva Traces for Forensic Purposes. Anal. Chem. 2016, 88, 12489–12493. [Google Scholar] [CrossRef]
- Gonchukov, S.; Sukhinina, A.; Bakhmutov, D.; Minaeva, S. Raman Spectroscopy of Saliva as a Perspective Method for Periodontitis Diagnostics. Laser Phys. Lett. 2012, 9, 73–77. [Google Scholar] [CrossRef]
- Song, M.; Bai, H.; Zhang, P.; Zhou, X.; Ying, B. Promising Applications of Human-Derived Saliva Biomarker Testing in Clinical Diagnostics. Int. J. Oral Sci. 2023, 15, 2. [Google Scholar] [CrossRef]
- Alkhuder, K. Raman Scattering-Based Optical Sensing of Chronic Liver Diseases. Photodiagn. Photodyn. Ther. 2023, 42, 103505. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, L.; Li, H.; Chen, F.; Chen, C.; Gao, R.; Lv, X.; Tang, J. Raman Spectroscopy Combined with Multiple Algorithms for Analysis and Rapid Screening of Chronic Renal Failure. Photodiagn. Photodyn. Ther. 2020, 30, 101792. [Google Scholar] [CrossRef] [PubMed]
- Rimai, L.; Heyde, M.E.; Gill, D. Vibrational Spectra of Some Carotenoids and Related Linear Polyenes. Raman Spectroscopic Study. J. Am. Chem. Soc. 1973, 95, 4493–4501. [Google Scholar] [CrossRef]
- Cutshaw, G.; Uthaman, S.; Hassan, N.; Kothadiya, S.; Wen, X.; Bardhan, R. The Emerging Role of Raman Spectroscopy as an Omics Approach for Metabolic Profiling and Biomarker Detection toward Precision Medicine. Chem. Rev. 2023, 123, 8297–8346. [Google Scholar] [CrossRef] [PubMed]
- Kinnula, V.L.; Crapo, J.D. Superoxide Dismutases in the Lung and Human Lung Diseases. Am. J. Respir. Crit. Care Med. 2003, 167, 1600–1619. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Sanjabi, A.; Brand, S.; Brühl, A.; Sadeghi Bahmani, D. Associations Between Morning Salivary and Blood Cortisol Concentrations in Individuals With Obstructive Sleep Apnea Syndrome: A Meta-Analysis. Front. Endocrinol. 2021, 11, 568823. [Google Scholar] [CrossRef]
- Qin, W.; Yeping, B.; Fuchao, Y.; Qiang, Z.; Guanghao, Z.; Yang, L.; Songsong, S.; Xiaomei, R.; Jiayi, T. Chronic Intermittent Hypoxia Induces Cardiac Inflammation Anddysfunction in a Rat Obstructive Sleep Apnea Model. J. Biomed. Res. 2016, 30, 490. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhang, X.; Wu, X.; Zhao, Y.; Li, Y.; Chen, Z.; Hu, Y.; Cao, X. Association between Non-High-Density Lipoprotein Cholesterol to High-Density Lipoprotein Cholesterol Ratio and Obstructive Sleep Apnea: A Cross-Sectional Study from NHANES. Lipids Health Dis. 2024, 23, 209. [Google Scholar] [CrossRef]
- Hira, H.S.; Shukla, A.; Kaur, A.; Kapoor, S. Serum Uric Acid and Lactate Levels among Patients with Obstructive Sleep Apnea Syndrome: Which Is a Better Marker of Hypoxemia? Ann. Saudi Med. 2012, 32, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Malhotra, A. Pathophysiology of Adult Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef]
- Olsen, T.; Øvrebø, B.; Turner, C.; Bastani, N.E.; Refsum, H.; Vinknes, K.J. Effects of Short-Term Methionine and Cysteine Restriction and Enrichment with Polyunsaturated Fatty Acids on Oral Glucose Tolerance, Plasma Amino Acids, Fatty Acids, Lactate and Pyruvate: Results from a Pilot Study. BMC Res. Notes 2021, 14, 43. [Google Scholar] [CrossRef]
- Nikolaidis, S.; Kosmidis, I.; Koulidou, T.; Panagakis, S.; Tsalis, G.; Loupos, D.; Mougios, V. Improved Reliability of the Urine Lactate Concentration under Controlled Hydration after Maximal Exercise. Biomarkers 2016, 22, 614–620. [Google Scholar] [CrossRef]
- Jamieson, L.E.; Li, A.; Faulds, K.; Graham, D. Ratiometric Analysis Using Raman Spectroscopy as a Powerful Predictor of Structural Properties of Fatty Acids. R. Soc. Open sci. 2018, 5, 181483. [Google Scholar] [CrossRef]
- Hardy, M.; Kelleher, L.; De Carvalho Gomes, P.; Buchan, E.; Chu, H.O.M.; Goldberg Oppenheimer, P. Methods in Raman Spectroscopy for Saliva Studies—A Review. Appl. Spectrosc. Rev. 2022, 57, 177–233. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, Y.; Guo, J.; Guo, J. Single-Molecule Immunoassay Technology: Recent Advances. Talanta 2023, 265, 124903. [Google Scholar] [CrossRef]
- Ahmed, T.; Powner, M.B.; Qassem, M.; Kyriacou, P.A. Rapid Optical Determination of Salivary Cortisol Responses in Individuals Undergoing Physiological and Psychological Stress. Sci. Rep. 2024, 14, 31578. [Google Scholar] [CrossRef] [PubMed]
- Rattu, G.; Khansili, N.; Maurya, V.K.; Krishna, P.M. Lactate Detection Sensors for Food, Clinical and Biological Applications: A Review. Environ. Chem. Lett. 2021, 19, 1135–1152. [Google Scholar] [CrossRef]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of Multidimensional Data Processing Approaches for Raman and Infrared Spectroscopy. EPJ Tech. Instrum. 2015, 2, 8. [Google Scholar] [CrossRef]


| AGE | SEX | AHI (events/h) | ||||
|---|---|---|---|---|---|---|
| Median (IQR) | M (%) | F (%) | 5–15 (%) | 15–30 (%) | >30 (%) | |
| OSAS (n = 51) | 66 (14) | 29 (57%) | 22 (43%) | 14 (27%) | 14 (27%) | 23 (45%) |
| CTR (n = 34) | 61.5 (21) | 15 (44%) | 19 (56%) | |||
| p-value | 0.58 | 0.43 | ||||
| Raman Shift (cm−1) | Attribution | ||||
|---|---|---|---|---|---|
| Nucleotides | Proteins | Lipids | Carbohydrates | Pigments | |
| 505 | Methoxy group | ||||
| 589 | Glycerol | ||||
| 618 | C-C twisting of proteins | ||||
| 630 | Glycerol | ||||
| 640 | C-S stretching and C-C twisting of Tyrosine | ||||
| 755 | Tryptophan | ||||
| 825 | Phosphodiester bond | ||||
| 853 | Tyrosine and Proline | Glycogen | |||
| 875 | Phospholipids (Phosphatidylcholine, sphingomyelin) | ||||
| 920 | C-C stretch of proline ring | Glucose/ Lactic acid | |||
| 957 | Carotenoids | ||||
| 1003 | Phenylalanine | ||||
| 1030 | Phenylalanine of collagen | ||||
| 1095 | Phosphodioxy group | C-N | |||
| 1120 | The strong C-O band of ribose | ||||
| 1153 | Carbohydrates peak | ||||
| 1250 | Aluminum substrate band | ||||
| 1308 | C-N asymmetric stretching in asymmetric aromatic amines | ||||
| 1444 | Cholesterol band, fatty acids | ||||
| 1548 | Tryptophan | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forleo, L.; Picciolini, S.; Gualerzi, A.; Battaglia, E.; Compalati, E.; Banfi, P.I.; Bedoni, M. Development of a Raman-Based Method for the Diagnosis of People with Obstructive Sleep Apnea Syndrome: The Role of Lactic Acid. Int. J. Mol. Sci. 2025, 26, 9095. https://doi.org/10.3390/ijms26189095
Forleo L, Picciolini S, Gualerzi A, Battaglia E, Compalati E, Banfi PI, Bedoni M. Development of a Raman-Based Method for the Diagnosis of People with Obstructive Sleep Apnea Syndrome: The Role of Lactic Acid. International Journal of Molecular Sciences. 2025; 26(18):9095. https://doi.org/10.3390/ijms26189095
Chicago/Turabian StyleForleo, Luana, Silvia Picciolini, Alice Gualerzi, Elvia Battaglia, Elena Compalati, Paolo I. Banfi, and Marzia Bedoni. 2025. "Development of a Raman-Based Method for the Diagnosis of People with Obstructive Sleep Apnea Syndrome: The Role of Lactic Acid" International Journal of Molecular Sciences 26, no. 18: 9095. https://doi.org/10.3390/ijms26189095
APA StyleForleo, L., Picciolini, S., Gualerzi, A., Battaglia, E., Compalati, E., Banfi, P. I., & Bedoni, M. (2025). Development of a Raman-Based Method for the Diagnosis of People with Obstructive Sleep Apnea Syndrome: The Role of Lactic Acid. International Journal of Molecular Sciences, 26(18), 9095. https://doi.org/10.3390/ijms26189095







