Long COVID and Type I IFN Signature in Working-Age Adults: A Cross-Sectional Study
Abstract
1. Introduction
2. Results
2.1. Clinical and Demographic Characteristics of Study Population
2.2. IFN-I and ISGs Expression Among Previously SARS-CoV-2 Infected Individuals According to the Development of LC
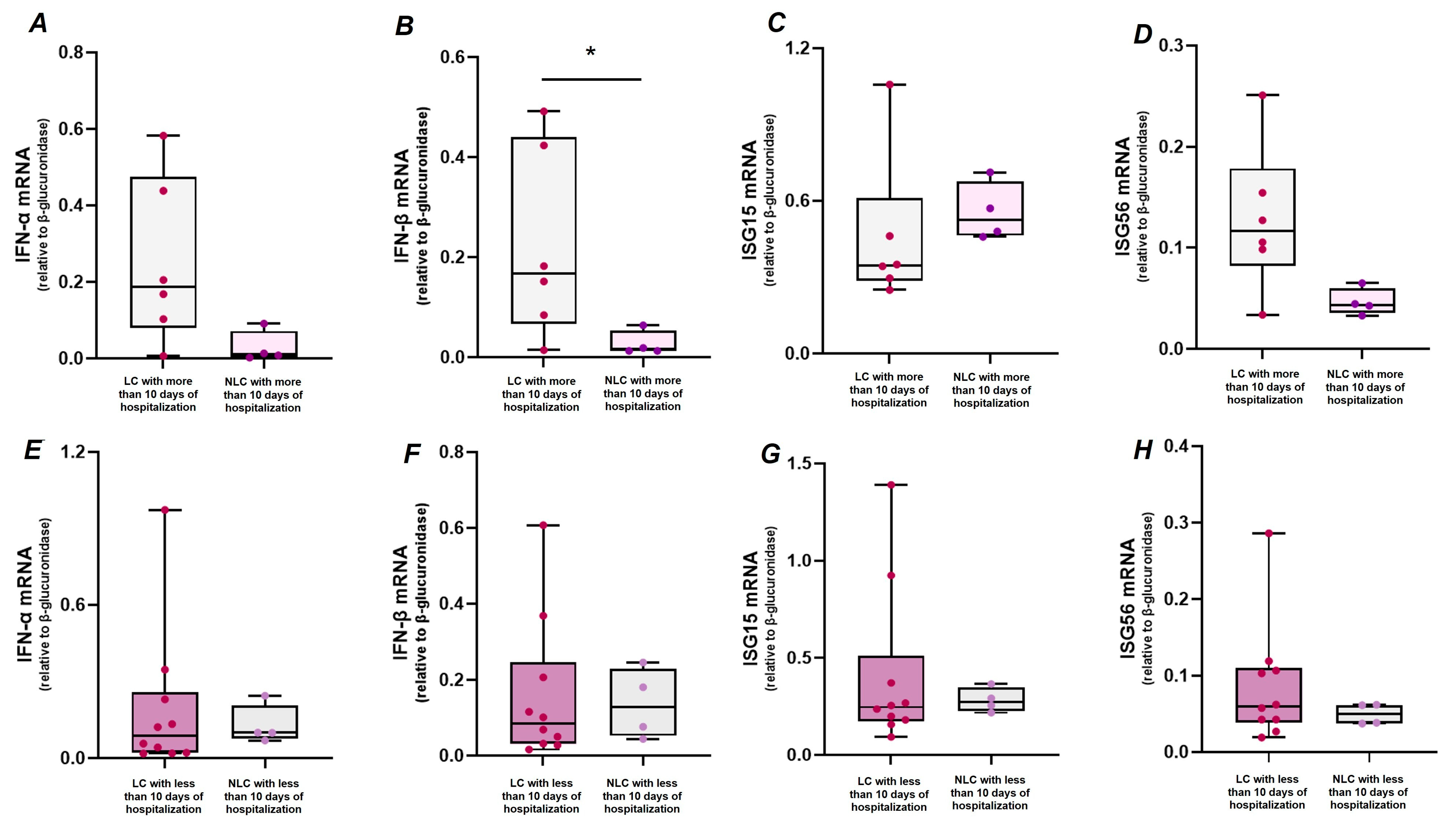
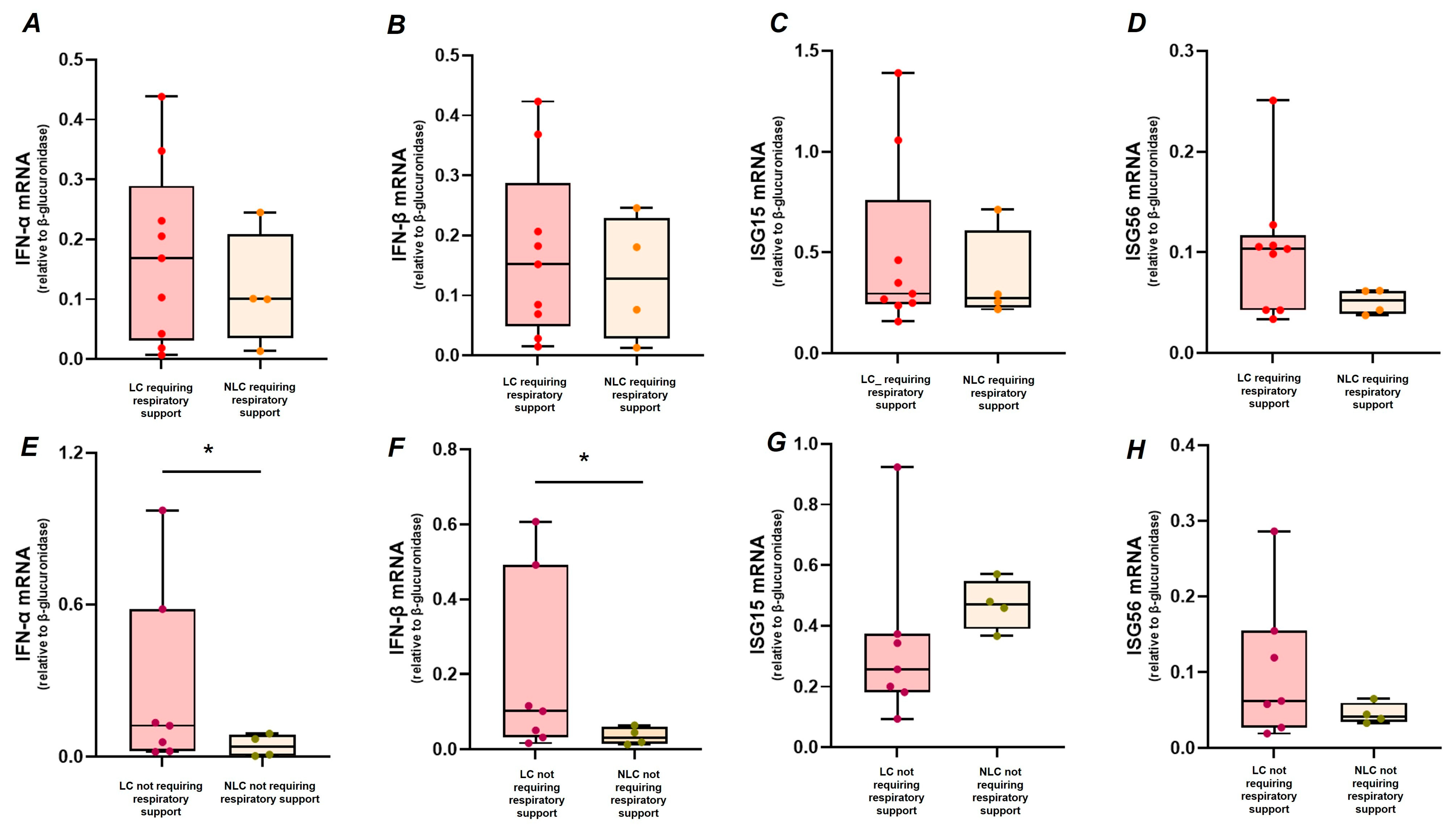
2.3. Association Between Clinical Features of Acute Infection, LC, and IFN-I Response
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Diagnosis of Long COVID
4.3. Anti-IFN-I Autoantibodies Detection
4.4. Peripheral Blood Mononuclear Cells Isolation and Real-Time PCR Assay
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631, Erratum in Nat. Med. 2021, 27, 1116. https://doi.org/10.1038/s41591-021-01361-2. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 399, 747–758, Erratum in Lancet 2022, 399, 1778. https://doi.org/10.1016/S0140-6736(22)00795-4. [Google Scholar] [CrossRef]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Iwasaki, A. Type I and Type III Interferons-Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe. 2020, 27, 870–878. [Google Scholar] [CrossRef]
- Lei, X.; Dong, X.; Ma, R.; Wang, W.; Xiao, X.; Tian, Z.; Wang, C.; Wang, Y.; Li, L.; Ren, L.; et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020, 11, 3810. [Google Scholar] [CrossRef]
- Song, L.; Wang, D.; Abbas, G.; Li, M.; Cui, M.; Wang, J.; Lin, Z.; Zhang, X.E. The main protease of SARS-CoV-2 cleaves histone deacetylases and DCP1A, attenuating the immune defense of the interferon-stimulated genes. J. Biol. Chem. 2023, 299, 102990. [Google Scholar] [CrossRef]
- Frasca, F.; Scordio, M.; Santinelli, L.; Gabriele, L.; Gandini, O.; Criniti, A.; Pierangeli, A.; Angeloni, A.; Mastroianni, C.M.; d’Ettorre, G.; et al. Anti-IFN-α/-ω neutralizing antibodies from COVID-19 patients correlate with downregulation of IFN response and laboratory biomarkers of disease severity. Eur. J. Immunol. 2022, 52, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.K.; Guevara-Coto, J.; Yogendra, R.; Francisco, E.B.; Long, E.; Pise, A.; Rodrigues, H.; Parikh, P.; Mora, J.; Mora-Rodríguez, R.A. Immune-Based Prediction of COVID-19 Severity and Chronicity Decoded Using Machine Learning. Front. Immunol. 2021, 12, 700782. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Hattori, F.; Nishiyama, J.; Hasuo, H. Correlation of interferons and autoimmune aspects in long COVID-19 patients. Int. Immunol. 2025, 37, 355–363, Erratum in Int. Immunol. 2025, 37, 655–658. https://doi.org/10.1093/intimm/dxaf044. [Google Scholar] [CrossRef] [PubMed]
- Deer, R.R.; Rock, M.A.; Vasilevsky, N.; Carmody, L.; Rando, H.; Anzalone, A.J.; Basson, M.D.; Bennett, T.D.; Bergquist, T.; Boudreau, E.A.; et al. Characterizing Long COVID: Deep Phenotype of a Complex Condition. EBioMedicine 2021, 74, 103722. [Google Scholar] [CrossRef]
- Van Herck, M.; Goërtz, Y.M.; Houben-Wilke, S.; Machado, F.V.; Meys, R.; Delbressine, J.M.; Vaes, A.W.; Burtin, C.; Posthuma, R.; Franssen, F.M.; et al. Severe Fatigue in Long COVID: Web-Based Quantitative Follow-up Study in Members of Online Long COVID Support Groups. J. Med. Internet. Res. 2021, 23, e30274. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Zaccardi, F.; Pouwels, K.B.; Walker, A.S.; Houston, D.; Alwan, N.A.; Martin, J.; Khunti, K.; Nafilyan, V. Employment outcomes of people with Long Covid symptoms: Community-based cohort study. Eur. J. Public Health 2024, 34, 489–496. [Google Scholar] [CrossRef]
- Mortaz, E.; Tabarsi, P.; Varahram, M.; Folkerts, G.; Adcock, I.M. The Immune Response and Immunopathology of COVID-19. Front. Immunol. 2020, 11, 2037. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146, Erratum in Nat. Rev. Microbiol. 2023, 21, 408. https://doi.org/10.1038/s41579-023-00896-0. [Google Scholar] [CrossRef]
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.; Silverwood, R.J.; Di Gessa, G.; et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022, 13, 3528. [Google Scholar] [CrossRef]
- Rahmati, M.; Udeh, R.; Kang, J.; Dolja-Gore, X.; McEvoy, M.; Kazemi, A.; Soysal, P.; Smith, L.; Kenna, T.; Fond, G.; et al. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of Symptoms 3 Years Post-SARS-CoV-2 Infection. J. Med. Virol. 2025, 97, e70429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Aly, Z. SARS-CoV-2 antivirals and post-COVID-19 condition. Lancet Infect. Dis. 2025, 25, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Lukhele, S.; Boukhaled, G.M.; Brooks, D.G. Type I interferon signaling, regulation and gene stimulation in chronic virus infection. Semin. Immunol. 2019, 43, 101277. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Maddaloni, L.; Santinelli, L.; Bugani, G.; Cacciola, E.G.; Lazzaro, A.; Lofaro, C.M.; Caiazzo, S.; Frasca, F.; Fracella, M.; Ajassa, C.; et al. Differential expression of Type I interferon and inflammatory genes in SARS-CoV-2-infected patients treated with monoclonal antibodies. Immun. Inflamm. Dis. 2023, 11, e968. [Google Scholar] [CrossRef]
- Yang, A.C.; Kern, F.; Losada, P.M.; Agam, M.R.; Maat, C.A.; Schmartz, G.P.; Fehlmann, T.; Stein, J.A.; Schaum, N.; Lee, D.P.; et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 2021, 595, 565–571, Erratum in Nature 2021, 598, E4. https://doi.org/10.1038/s41586-021-04080-3. [Google Scholar] [CrossRef]
- Fracella, M.; Mancino, E.; Nenna, R.; Virgillito, C.; Frasca, F.; D’Auria, A.; Sorrentino, L.; Petrarca, L.; La Regina, D.; Matera, L.; et al. Age-related transcript changes in type I interferon signaling in children and adolescents with long COVID. Eur. J. Immunol. 2024, 54, e2350682. [Google Scholar] [CrossRef]
- Xu, D.; Qin, X. Type I Interferonopathy among Non-Elderly Female Patients with Post-Acute Sequelae of COVID-19. Viruses 2024, 16, 1369. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement. Int. J. Mol. Sci. 2024, 25, 6389. [Google Scholar] [CrossRef]
- Cervia-Hasler, C.; Brüningk, S.C.; Hoch, T.; Fan, B.; Muzio, G.; Thompson, R.C.; Ceglarek, L.; Meledin, R.; Westermann, P.; Emmenegger, M.; et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science 2024, 383, eadg7942. [Google Scholar] [CrossRef]
- Tripathi, A.; Whitehead, C.; Surrao, K.; Pillai, A.; Madeshiya, A.; Li, Y.; Khodadadi, H.; Ahmed, A.O.; Turecki, G.; Baban, B.; et al. Type 1 interferon mediates chronic stress-induced neuroinflammation and behavioral deficits via complement component 3-dependent pathway. Mol. Psychiatry 2021, 26, 3043–3059. [Google Scholar] [CrossRef]
- Jodele, S.; Medvedovic, M.; Luebbering, N.; Chen, J.; Dandoy, C.E.; Laskin, B.L.; Davies, S.M. Interferon-complement loop in transplant-associated thrombotic microangiopathy. Blood Adv. 2020, 4, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, L.; Liu, G.; Gack, M.U. ISG15: Its roles in SARS-CoV-2 and other viral infections. Trends Microbiol. 2023, 31, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 2023, 29, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Pavli, A.; Theodoridou, M.; Maltezou, H.C. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch. Med. Res. 2021, 52, 575–581. [Google Scholar] [CrossRef]
- Evans, R.A.; McAuley, H.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; Lone, N.I.; Leavy, O.C.; et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir. Med. 2021, 9, 1275–1287, Erratum in Lancet Respir. Med. 2022, 10, e9. https://doi.org/10.1016/S2213-2600(21)00540-3. Erratum in Lancet Respir. Med. 2024, 12, e41. https://doi.org/10.1016/S2213-2600(24)00142-5. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Pierangeli, A.; Gentile, M.; Oliveto, G.; Frasca, F.; Sorrentino, L.; Matera, L.; Nenna, R.; Viscido, A.; Fracella, M.; Petrarca, L.; et al. Comparison by Age of the Local Interferon Response to SARS-CoV-2 Suggests a Role for IFN-ε and -ω. Front. Immunol. 2022, 13, 873232. [Google Scholar] [CrossRef]
- Sweis, J.J.G.; Alnaimat, F.; Esparza, V.; Prasad, S.; Azam, A.; Modi, Z.; Al-Awqati, M.; Jetanalin, P.; Sweis, N.J.; Ascoli, C.; et al. From Acute Infection to Prolonged Health Consequences: Understanding Health Disparities and Economic Implications in Long COVID Worldwide. Int. J. Environ. Res. Public Health 2024, 21, 325. [Google Scholar] [CrossRef] [PubMed]
- De Paul University. Available online: https://condor.depaul.edu/ljason/cfs/measures.html (accessed on 10 November 2021).
- Jason, L.A.; Evans, M.; Porter, N.; Brown, M.; Brown, A.; Hunnell, J.; Anderson, V.; Lerch, A.; De Meirleir, K.; Friedberg, F. The Development of a Revised Canadian Myalgic Encephalomyelitis Chronic Fatigue Syndrome Case Definition. Am. J. Biochem. Biotechnol. 2010, 6, 120–135. [Google Scholar] [CrossRef]
- Babiloni, C.; Cacciola, E.G.; Tucci, F.; Vassalini, P.; Chilovi, A.; Jakhar, D.; Musat, A.M.; Salvatore, M.; Soricelli, A.; Stocchi, F.; et al. Resting-state EEG rhythms are abnormal in post COVID-19 patients with brain fog without cognitive and affective disorders. Clin. Neurophysiol. 2024, 161, 159–172. [Google Scholar] [CrossRef]
- Santinelli, L.; De Girolamo, G.; Borrazzo, C.; Vassalini, P.; Pinacchio, C.; Cavallari, E.N.; Statzu, M.; Frasca, F.; Scordio, M.; Bitossi, C.; et al. Alteration of type I interferon response is associated with subclinical atherosclerosis in virologically suppressed HIV-1-infected male patients. J. Med. Virol. 2021, 93, 4930–4938. [Google Scholar] [CrossRef]

| Parameters * | Previous SARS-CoV-2 Infection (n = 34) ** | LC (n = 26) (A) | NLC (n = 8) (B) | p-Value A vs. B |
|---|---|---|---|---|
| Age (years) | 59 (±6) | 58 (±5) | 60 (±6) | 0.395 |
| Sex assigned at birth (male/female) | 20/14 | 15/11 | 6/2 | NA |
| Days between last SARS-CoV-2 swab and blood sampling (days) | 481 (413–551) | 483 (379–547) | 486 (472–590) | 0.262 |
| Comorbidities (n, %) *** | 9 (26.5%) | 7 (27%) | 2 (25%) | 0.912 |
| Length of hospitalization (days) | 10 (6–17) | 10 (7–18) | 8 (5–14) | 0.788 |
| ICU admission [n (%)] | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| C-reactive protein (mg/dL) | 2.8 (0.99–5.25) | 2.69 (1.2–5.41) | 2.49 (0.76–7.78) | 0.917 |
| Respiratory support during hospitalization | ||||
| Venti-mask [n (%)] | 14 (41%) | 12 (46%) | 2 (25%) | 0.298 |
| HFNC [n (%)] | 5 (14.7%) | 3 (11.5%) | 2 (25%) | 0.352 |
| CPAP [n (%)] | 9 (26%) | 6 (23%) | 3 (37.5%) | 0.422 |
| Symptoms * | LC n (%) | NLC n (%) |
|---|---|---|
| Fatigue ** | 8 (50%) | 1 (12.5) |
| Sleep disorders *** | 14 (87.5) | 1 (12.5) |
| Musculoskeletal symptoms **** | 3 (18.8) | 0 (0) |
| Neurocognitive manifestations ***** | 14 (87.5) | 3 (37.5) |
| IFN-α mRNA * | IFN-β mRNA | ISG56 mRNA * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| aβ | 95% CI | p-Value | aβ | 95% CI | p-Value | aβ | 95% CI | p-Value | |
| Long COVID (yes) | 0.89 | −0.34 to 2.13 | 0.148 | 0.10 | −0.02 to 0.21 | 0.090 | 0.55 | 0.11 to 0.99 | 0.016 |
| CCI (≥2) | −1.54 | −2.80 to −0.27 | 0.020 | −0.18 | −0.29 to −0.07 | 0.002 | −0.62 | −1.18 to −0.05 | 0.034 |
| Days of hospitalization (≥10) | −0.08 | −1.44 to 1.28 | 0.903 | 0.06 | −0.07 to 0.19 | 0.360 | 0.51 | 0.08 to 0.94 | 0.022 |
| Respiratory support (yes) | 0.25 | −4.39 to −1.25 | 0.698 | −0.03 | −0.18 to 0.12 | 0.677 | −0.16 | −0.67 to 0.35 | 0.522 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santinelli, L.; Gentilini Cacciola, E.; Bortolani, L.; Ridolfi, M.; Maddaloni, L.; Frasca, F.; Fracella, M.; Bugani, G.; d’Ettorre, G.; Mastroianni, C.M.; et al. Long COVID and Type I IFN Signature in Working-Age Adults: A Cross-Sectional Study. Int. J. Mol. Sci. 2025, 26, 9089. https://doi.org/10.3390/ijms26189089
Santinelli L, Gentilini Cacciola E, Bortolani L, Ridolfi M, Maddaloni L, Frasca F, Fracella M, Bugani G, d’Ettorre G, Mastroianni CM, et al. Long COVID and Type I IFN Signature in Working-Age Adults: A Cross-Sectional Study. International Journal of Molecular Sciences. 2025; 26(18):9089. https://doi.org/10.3390/ijms26189089
Chicago/Turabian StyleSantinelli, Letizia, Elio Gentilini Cacciola, Luca Bortolani, Marco Ridolfi, Luca Maddaloni, Federica Frasca, Matteo Fracella, Ginevra Bugani, Gabriella d’Ettorre, Claudio M. Mastroianni, and et al. 2025. "Long COVID and Type I IFN Signature in Working-Age Adults: A Cross-Sectional Study" International Journal of Molecular Sciences 26, no. 18: 9089. https://doi.org/10.3390/ijms26189089
APA StyleSantinelli, L., Gentilini Cacciola, E., Bortolani, L., Ridolfi, M., Maddaloni, L., Frasca, F., Fracella, M., Bugani, G., d’Ettorre, G., Mastroianni, C. M., Ceccarelli, G., & d’Ettorre, G. (2025). Long COVID and Type I IFN Signature in Working-Age Adults: A Cross-Sectional Study. International Journal of Molecular Sciences, 26(18), 9089. https://doi.org/10.3390/ijms26189089










