Trivalent and Pentavalent Antimonials Impair Cardiac Mitochondrial Function in Mice
Abstract
1. Introduction
2. Results
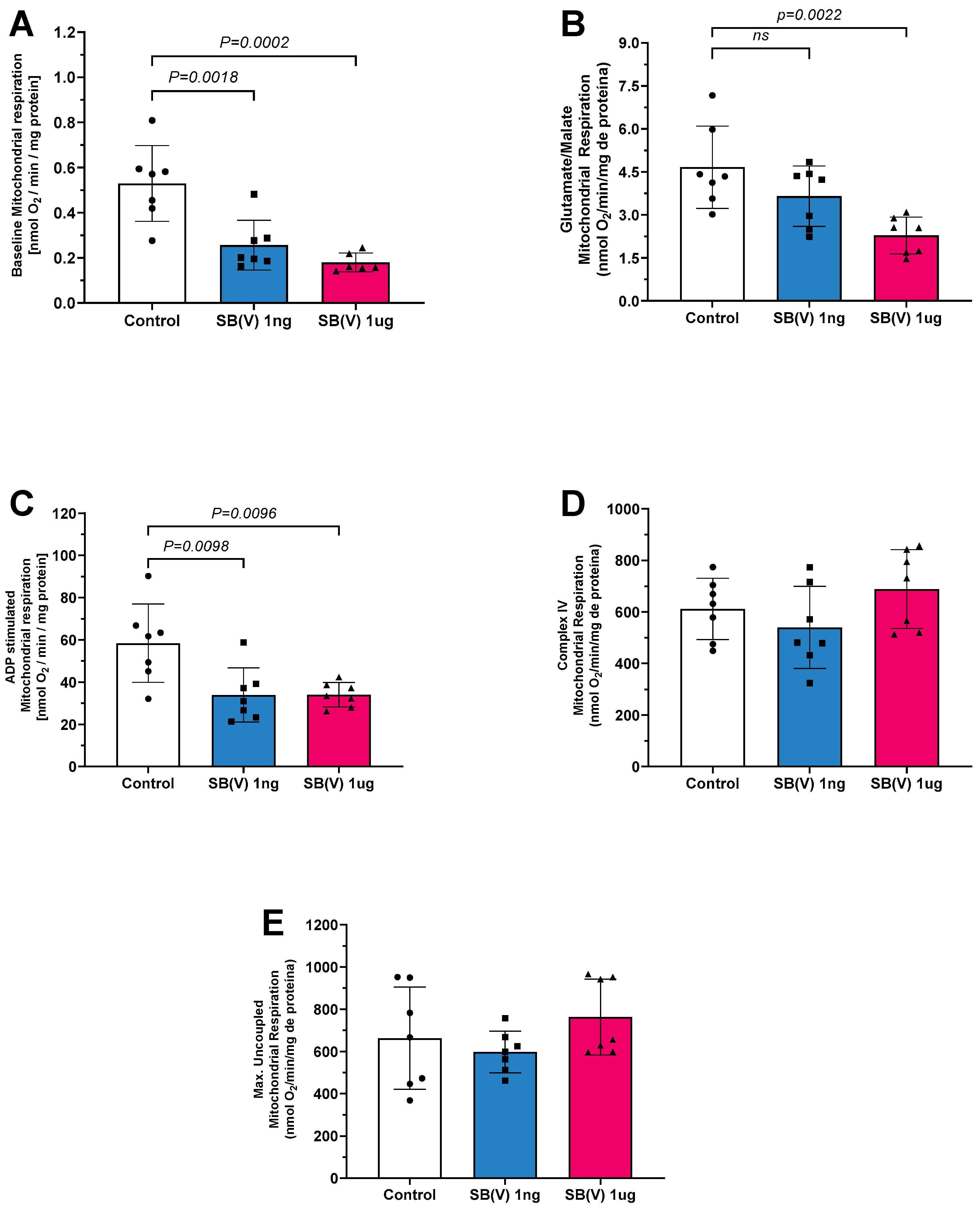
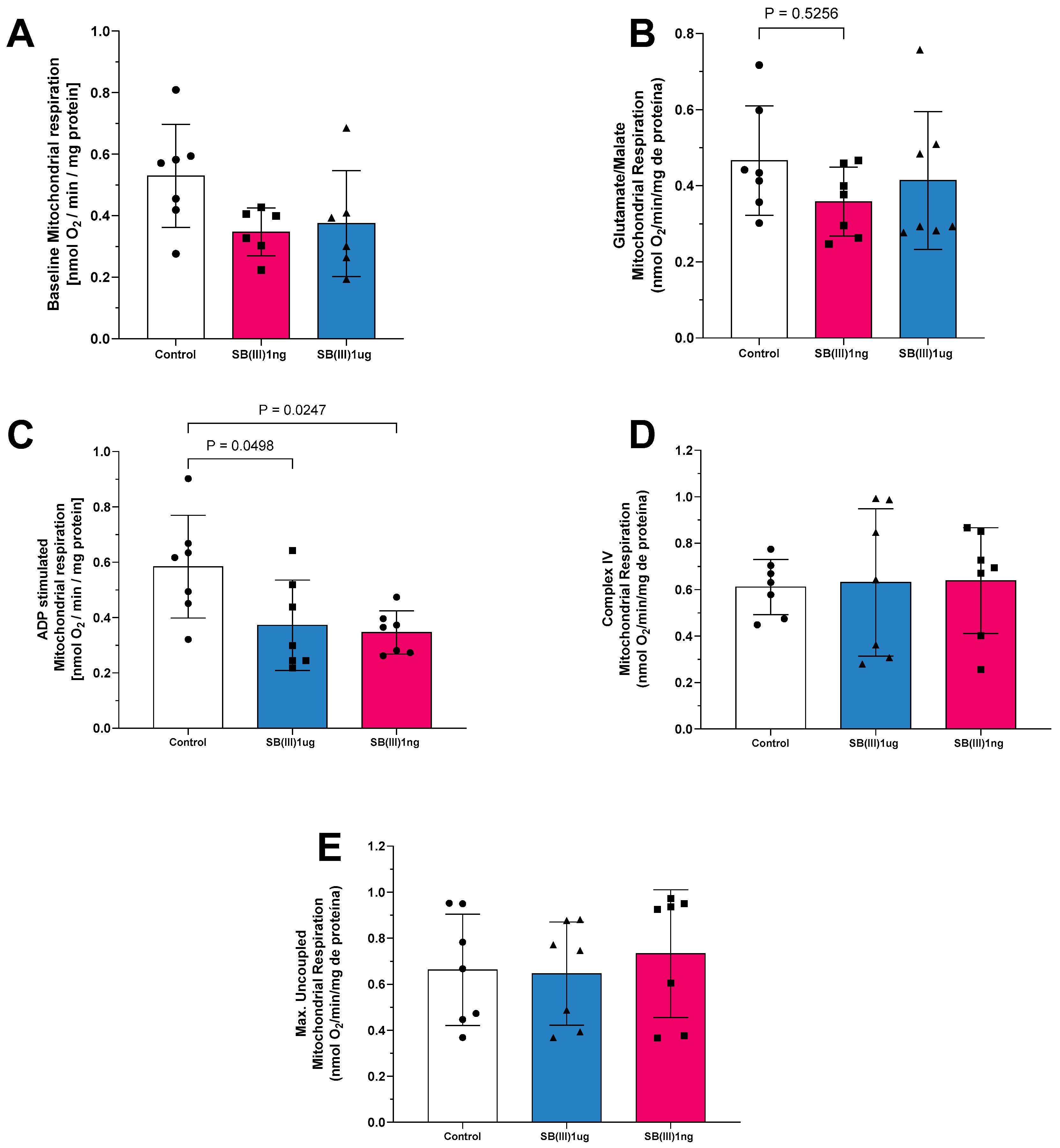
2.1. Mitochondrial Oxygen Consumption
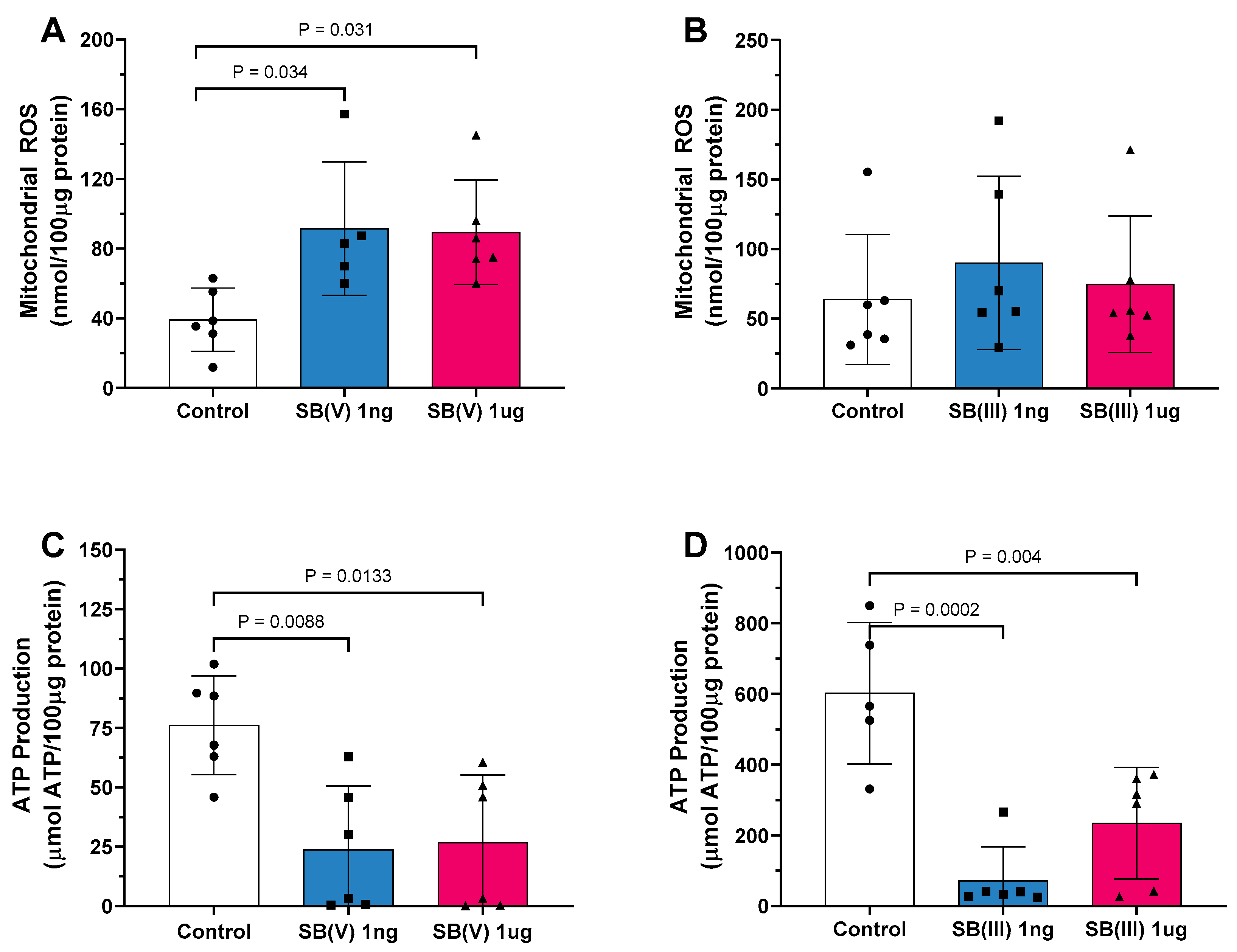
2.2. Mitochondrial ROS Production and ATP Production
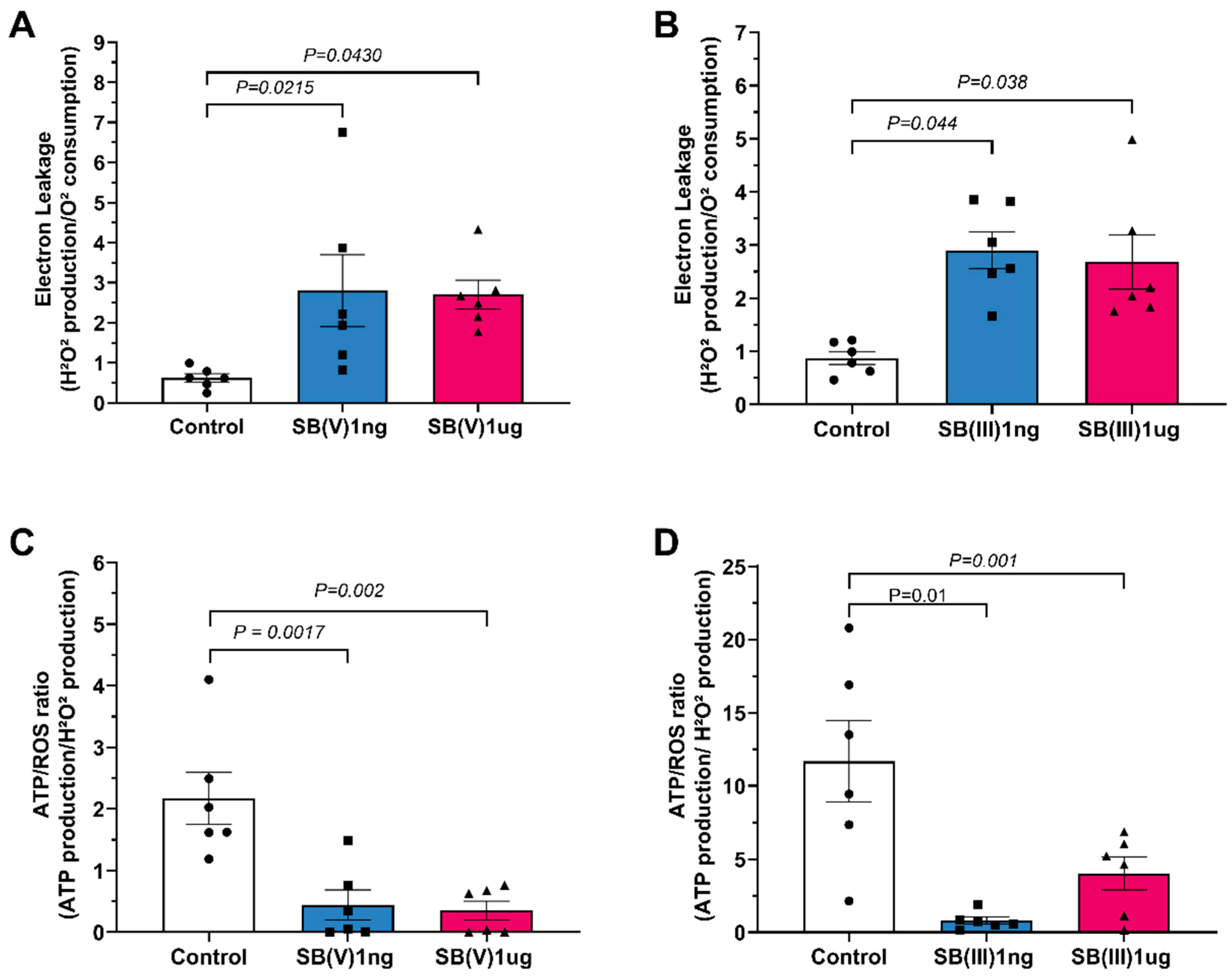
2.3. Mitochondrial Electron Leakage and ATP/ROS Ratio
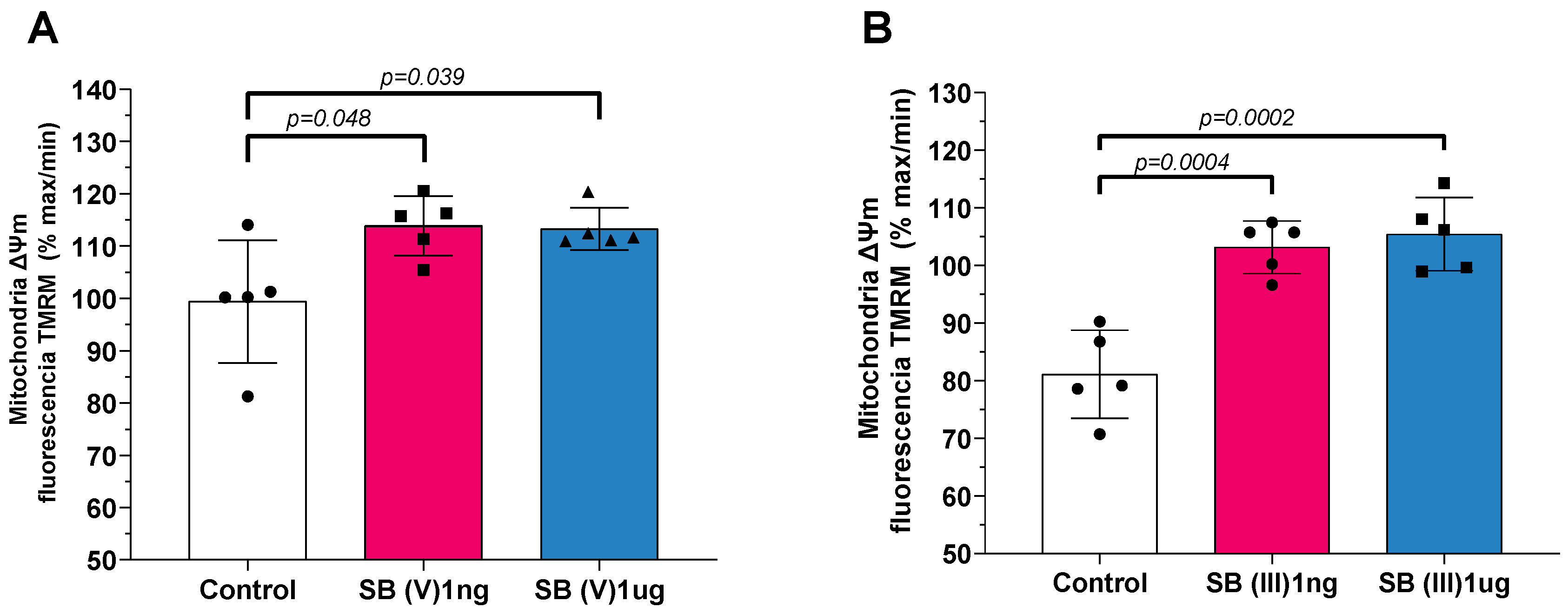
2.4. Mitochondrial Transmembrane Potential (Δψm)
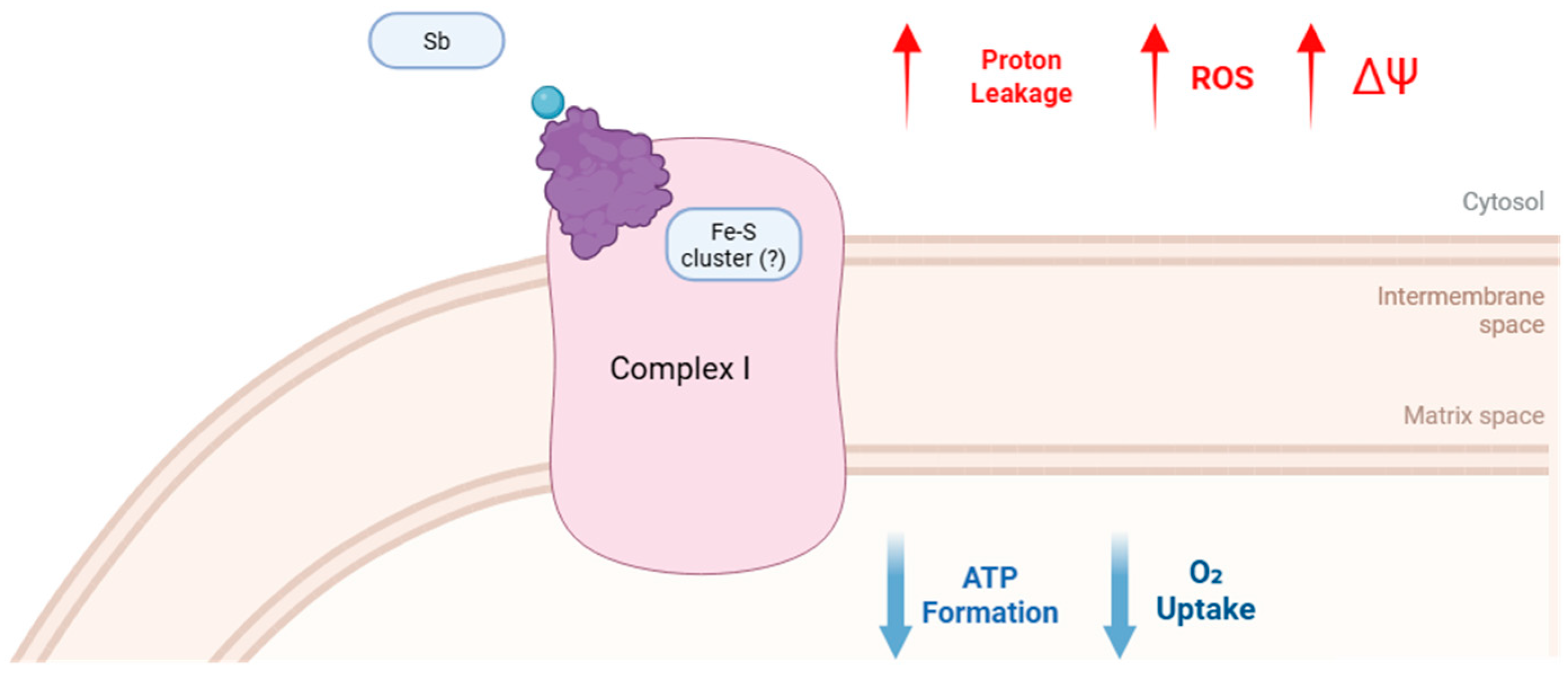
3. Discussion
Study Limitations
4. Materials and Method
4.1. Materials
4.2. Animals and Ethical Approval
4.3. Mitochondria Isolation
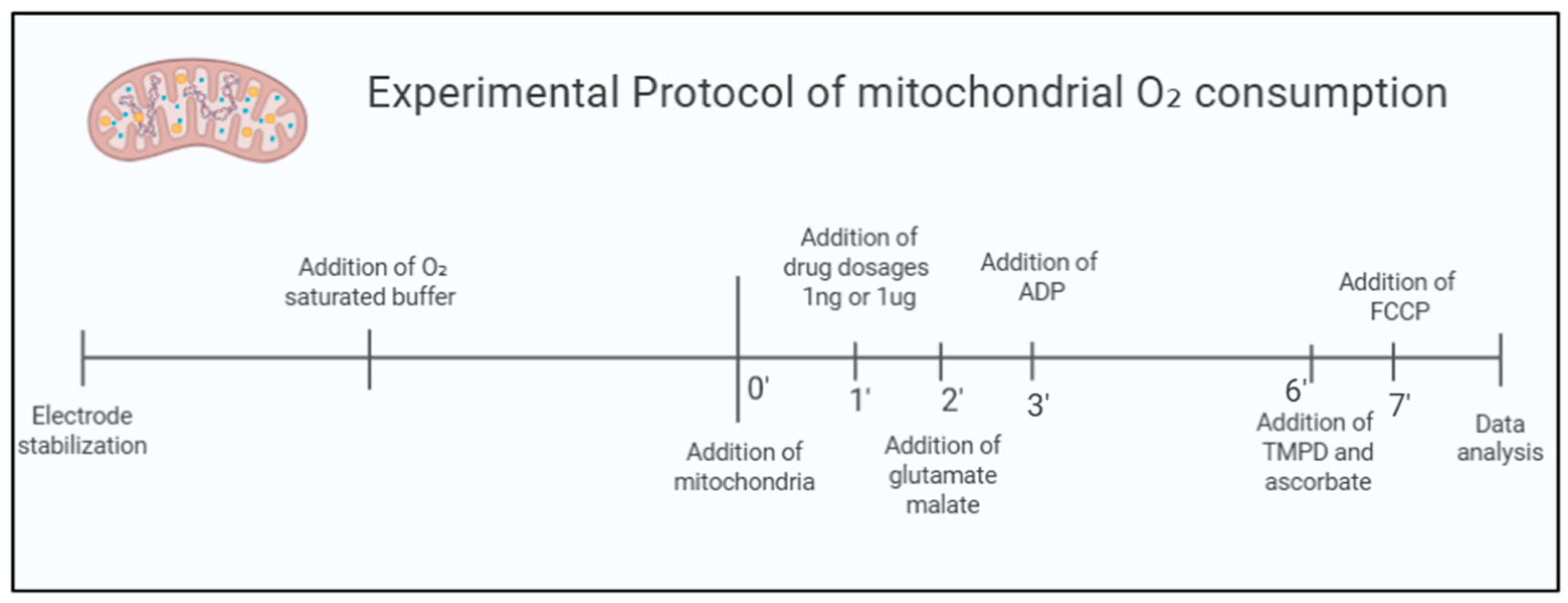
4.4. Mitochondrial Oxygen Consumption Protocol
4.5. Mitochondrial ATP Production
4.6. Mitochondrial ROS Concentration
4.7. Mitochondrial Transmembrane Potential
4.8. Electron Leakage and ATP/ROS Production Ratio
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loeuillet, C.; Bañuls, A.L.; Hide, M. Study of Leishmania pathogenesis in mice: Experimental considerations. Parasit. Vectors 2016, 9, 144. [Google Scholar] [CrossRef]
- Lopes, E.A.O.; Florencio-Henschel, P.; Jordão, F.T.; Sperança, M.A.; Martins, L.P.A.; Suzuki, R.B. Leishmania infantum (syn. Leishmania chagasi) detection in blood donors living in an endemic area. Parasitol. Res. 2023, 122, 671–674. [Google Scholar] [CrossRef]
- Mathison, B.A.; Bradley, B.T. Review of the Clinical Presentation, Pathology, Diagnosis, and Treatment of Leishmaniasis. Lab. Med. 2023, 54, 363–371. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host-pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Demicheli, C. New delivery strategies for the old pentavalent antimonial drugs. Expert Opin. Drug Deliv. 2010, 7, 1343–1358. [Google Scholar] [CrossRef]
- Mokni, M. Leishmanioses cutanées [Cutaneous leishmaniasis]. Ann. Dermatol. Venereol 2019, 146, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Berbert, T.R.N.; de Mello, T.F.P.; Wolf Nassif, P.; Mota, C.A.; Silveira, A.V.; Duarte, G.C.; Demarchi, I.G.; Aristides, S.M.A.; Lonardoni, M.V.C.; Vieira Teixeira, J.J.; et al. Pentavalent Antimonials Combined with Other Therapeutic Alternatives for the Treatment of Cutaneous and Mucocutaneous Leishmaniasis: A Systematic Review. Dermatol. Res. Pract. 2018, 2018, 9014726. [Google Scholar] [CrossRef]
- Azim, M.; Khan, S.A.; Ullah, S.; Ullah, S.; Anjum, S.I. Therapeutic advances in the topical treatment of cutaneous leishmaniasis: A review. PLoS Negl. Trop. Dis. 2021, 15, e0009099. [Google Scholar] [CrossRef]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent antimonials: New perspectives for old drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef]
- Denton, H.; Mcgregor Joanne, C.; Coombs Graham, H. Reduction of anti-leishmanial pentavalent antimonial drugs by a parasite-specific thiol-dependent reductase, TDR1. Biochem. J. 2004, 381 Pt 2, 405–412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Messier, N.; Ouellette, M.; Rosen, B.P.; Mukhopadhyay, R. Leishmania Major LmACR2 Is a Pentavalent Antimony Reductase That Confers Sensitivity to the Drug Pentostam. J. Biol. Chem. 2004, 279, 37445–37451. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Cerami, A. Metabolism and Functions of Trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 1992, 46, 695–729. [Google Scholar] [CrossRef]
- Sun, H.; Yan, S.C.; Cheng, W.S. Interaction of Antimony Tartrate with the Tripeptide Glutathione. Eur. J. Biochem. 2000, 267, 5450–5457. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Demicheli, C.; Claúdio, S.F.; Michelle, A.P.C. Glutathione-induced Conversion of Pentavalent Antimony to Trivalent Antimony in Meglumine Antimoniate. Antimicrob. Agents Chemother. 2001, 45, 913–916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wyllie, S.; Cunningham, M.L.; Fairlamb, A.H. Dual Action of Antimonial Drugs on Thiol Redox Metabolism in the Human Pathogen Leishmania donovani. J. Biol. Chem. 2004, 279, 39925–39932. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.L.; Berman, J.D.; Rainey, P.M. In vitro antileishmanial properties of tri- and pentavalent antimonial preparations. Antimicrob. Agents Chemother. 1995, 39, 1234–1239. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Dey, S.; Xu, N.; Gage, D.; Lightbody, J.; Ouellette, M.; Rosen, B.P. Trypanothione Overproduction and Resistance to Antimonials and Arsenicals in Leishmania. Proc. Natl. Acad. Sci. USA 1996, 93, 10383–10387. [Google Scholar] [CrossRef]
- Ouellette, M.; Drummelsmith, J.; Papadopoulou, B. Leishmaniasis: Drugs in the clinic, Resistance and New Developments. Drug Resist. Updates 2004, 7, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.C.; Spickett, C.; Pereira, O.C.; Mullen, A.B. The In Vivo Susceptibility of Leishmania donovani to Sodium Stibogluconate Is Drug Specific and Can Be Reversed by Inhibiting Glutathione Biosynthesis. Antimicrob. Agents Chemother. 2003, 47, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Yardley, V.; Khan, A.A.; Martin, M.B.; Slifer, T.R.; Araujo, F.G.; Moreno, S.N.; Docampo, R.; Croft, S.L.; Oldfield, E. In vivo activities of farnesyl pyrophosphate synthase inhibitors against Leishmania donovani and Toxoplasma gondii. Antimicrob. Agents Chemother. 2002, 46, 929–931. [Google Scholar] [CrossRef]
- Singh, S.; Sivakumar, R. Challenges and new discoveries in the treatment of leishmaniasis. J. Infect. Chemother. 2004, 10, 307–315. [Google Scholar] [CrossRef]
- Chulay, J.D.; Spencer, H.C.; Mugambi, M. Electrocardiographic changes during treatment of leishmaniasis with pentavalent antimony (sodium stibogluconate). Am. J. Trop. Med. Hyg. 1985, 34, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, N.C.; Nolan, J.; Fenn, L.; Herd, R.M.; Neilson, J.M.; Sutherland, G.R.; Fox, K.A. Cardiac effects of sodium stibogluconate: Myocardial, electrophysiological and biochemical studies. QJM 1994, 87, 465–472. [Google Scholar]
- Tirmenstein, M.A.; Plews, P.I.; Walker, C.V.; Woolery, M.D.; Wey, H.E.; Toraason, M.A. Antimony-induced oxidative stress and toxicity in cultured cardiac myocytes. Toxicol. Appl. Pharmacol. 1995, 130, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Adam-Vizi, V.; Chinopoulos, C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 2006, 27, 639–645. [Google Scholar] [CrossRef]
- Dykens, J.A.; Will, Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov. Today 2007, 12, 777–785. [Google Scholar] [CrossRef]
- Lößler, S.; Schlief, S.; Kneifel, C.; Thiel, E.; Schrezenmeier, H.; Rojewski, M.T. Antimony-trioxide- and arsenic-trioxide-induced apoptosis in myelogenic and lymphatic cell lines, recruitment of caspases, and loss of mitochondrial membrane potential are enhanced by modulators of the cellular glutathione redox system. Ann. Hematol. 2009, 88, 1047–1058. [Google Scholar] [CrossRef]
- Verdugo, M.; Ogra, Y.; Quiroz, W. Mechanisms underlying the toxic effects of antimony species in human embryonic kidney cells (HEK-293) and their comparison with arsenic species. J. Toxicol. Sci. 2016, 41, 783–792. [Google Scholar] [CrossRef]
- Lou, C.; Ma, C.; Liu, Z.; Shi, J.; Zheng, G.; Zhang, C.; Zhang, Z. Antimony exposure promotes bladder tumor cell growth by inhibiting PINK1-Parkin-mediated mitophagy. Ecotoxicol. Environ. Saf. 2021, 221, 112420. [Google Scholar] [CrossRef]
- Volpato, H.; Desoti, V.C.; Cogo, J.; Panice, M.R.; Sarragiotto, M.H.; Silva Sde, O.; Ueda-Nakamura, T.; Nakamura, C.V. The Effects of N-Butyl-1-(4-dimethylamino)phenyl-1,2,3,4-tetrahydro- β -carboline-3-carboxamide against Leishmania amazonensis Are Mediated by Mitochondrial Dysfunction. Evid Based Complement. Altern. Med. 2013, 2013, 874367. [Google Scholar] [CrossRef]
- Berman, J.D. Human leishmaniasis: Clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 1997, 24, 684–703. [Google Scholar] [CrossRef]
- Dröse, S.; Brandt, U.; Wittig, I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim. Biophys. Acta 2014, 1844, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Hantson, P. Mechanisms of toxic cardiomyopathy. Clin. Toxicol. 2019, 57, 1–9. [Google Scholar] [CrossRef]
- Bento, D.B.; de Souza, B.; Steckert, A.V.; Dias, R.O.; Leffa, D.D.; Moreno, S.E.; Petronilho, F.; de Andrade, V.M.; Dal-Pizzol, F.; Romão, P.R. Oxidative stress in mice treated with antileishmanial meglumine antimoniate. Res. Vet. Sci. 2013, 95, 1134–1141. [Google Scholar] [CrossRef]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Müller, M.; Donhauser, E.; Maske, T.; Bischof, C.; Dumitrescu, D.; Rudolph, V.; Klinke, A. Mitochondrial Integrity Is Critical in Right Heart Failure Development. Int. J. Mol. Sci. 2023, 24, 11108. [Google Scholar] [CrossRef]
- Nicholls, D.G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur. J. Biochem. 1974, 50, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Divakaruni, A.S.; Brand, M.D. The regulation and physiology of mitochondrial proton leak. Physiology 2011, 26, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Nadanaciva, S.; Will, Y. New insights in drug-induced mitochondrial toxicity. Curr. Pharm. Des. 2011, 17, 2100–2112. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Ardalan, A.; Smith, M.D.; Jelokhani-Niaraki, M. Uncoupling Proteins and Regulated Proton Leak in Mitochondria. Int. J. Mol. Sci. 2022, 23, 1528. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, D.A.F.; de Oliveira, D.F.; Cavalcanti-de-Albuquerque, J.P.; Nascimento, J.H.M.; Zin, W.A.; Maciel, L. Isolation of Mitochondria From Fresh Mice Lung Tissue. Front. Physiol. 2021, 12, 748261. [Google Scholar] [CrossRef] [PubMed]
- Maciel, L.; de Oliveira, D.F.; Monnerat, G.; Campos de Carvalho, A.C.; Nascimento, J.H.M. Exogenous 10 kDa-Heat Shock Protein Preserves Mitochondrial Function After Hypoxia/Reoxygenation. Front. Pharmacol. 2020, 11, 545. [Google Scholar] [CrossRef]
- Gedik, N.; Maciel, L.; Schulte, C.; Skyschally, A.; Heusch, G.; Kleinbongard, P. Cardiomyocyte mitochondria as targets of humoral factors released by remote ischemic preconditioning. Arch. Med. Sci. 2017, 13, 448–458. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, I.I.A.d.; Pavarino, M.E.M.F.; Rosa, C.F.M.d.; Marinho, L.E.; Moraes, C.d.S.; Nascimento, J.H.M.; Carvalho, A.C.C.d.; Maciel, L. Trivalent and Pentavalent Antimonials Impair Cardiac Mitochondrial Function in Mice. Int. J. Mol. Sci. 2025, 26, 9073. https://doi.org/10.3390/ijms26189073
Souza IIAd, Pavarino MEMF, Rosa CFMd, Marinho LE, Moraes CdS, Nascimento JHM, Carvalho ACCd, Maciel L. Trivalent and Pentavalent Antimonials Impair Cardiac Mitochondrial Function in Mice. International Journal of Molecular Sciences. 2025; 26(18):9073. https://doi.org/10.3390/ijms26189073
Chicago/Turabian StyleSouza, Itanna Isis Araujo de, Maria Eduarda Maciel Fernandes Pavarino, César Francisco Maricato da Rosa, Laís Eduardo Marinho, Caroline da Silva Moraes, José Hamilton Matheus Nascimento, Antonio Carlos Campos de Carvalho, and Leonardo Maciel. 2025. "Trivalent and Pentavalent Antimonials Impair Cardiac Mitochondrial Function in Mice" International Journal of Molecular Sciences 26, no. 18: 9073. https://doi.org/10.3390/ijms26189073
APA StyleSouza, I. I. A. d., Pavarino, M. E. M. F., Rosa, C. F. M. d., Marinho, L. E., Moraes, C. d. S., Nascimento, J. H. M., Carvalho, A. C. C. d., & Maciel, L. (2025). Trivalent and Pentavalent Antimonials Impair Cardiac Mitochondrial Function in Mice. International Journal of Molecular Sciences, 26(18), 9073. https://doi.org/10.3390/ijms26189073








