Reply to Markaryan et al. Comment on “Aji et al. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421”
1. Introduction
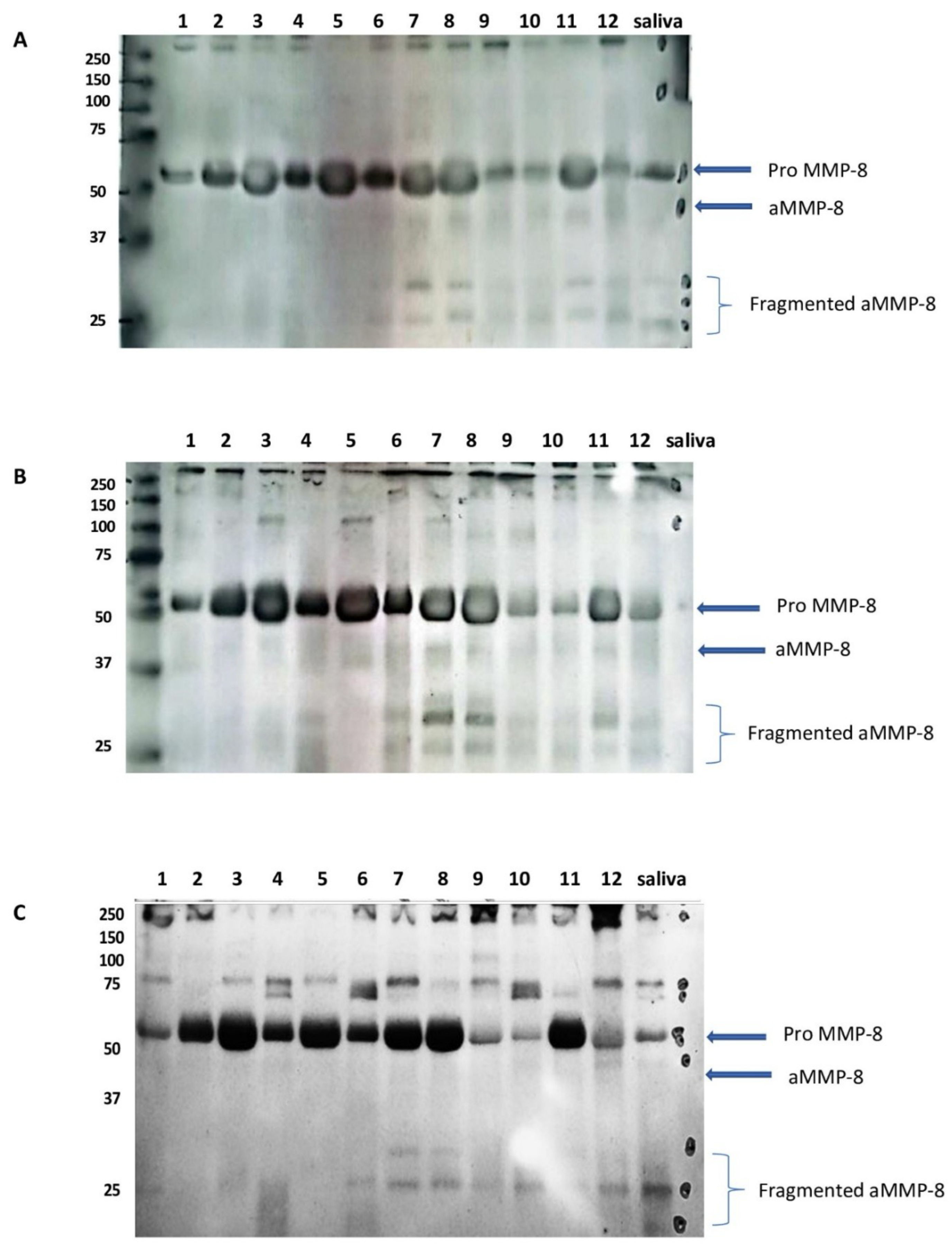
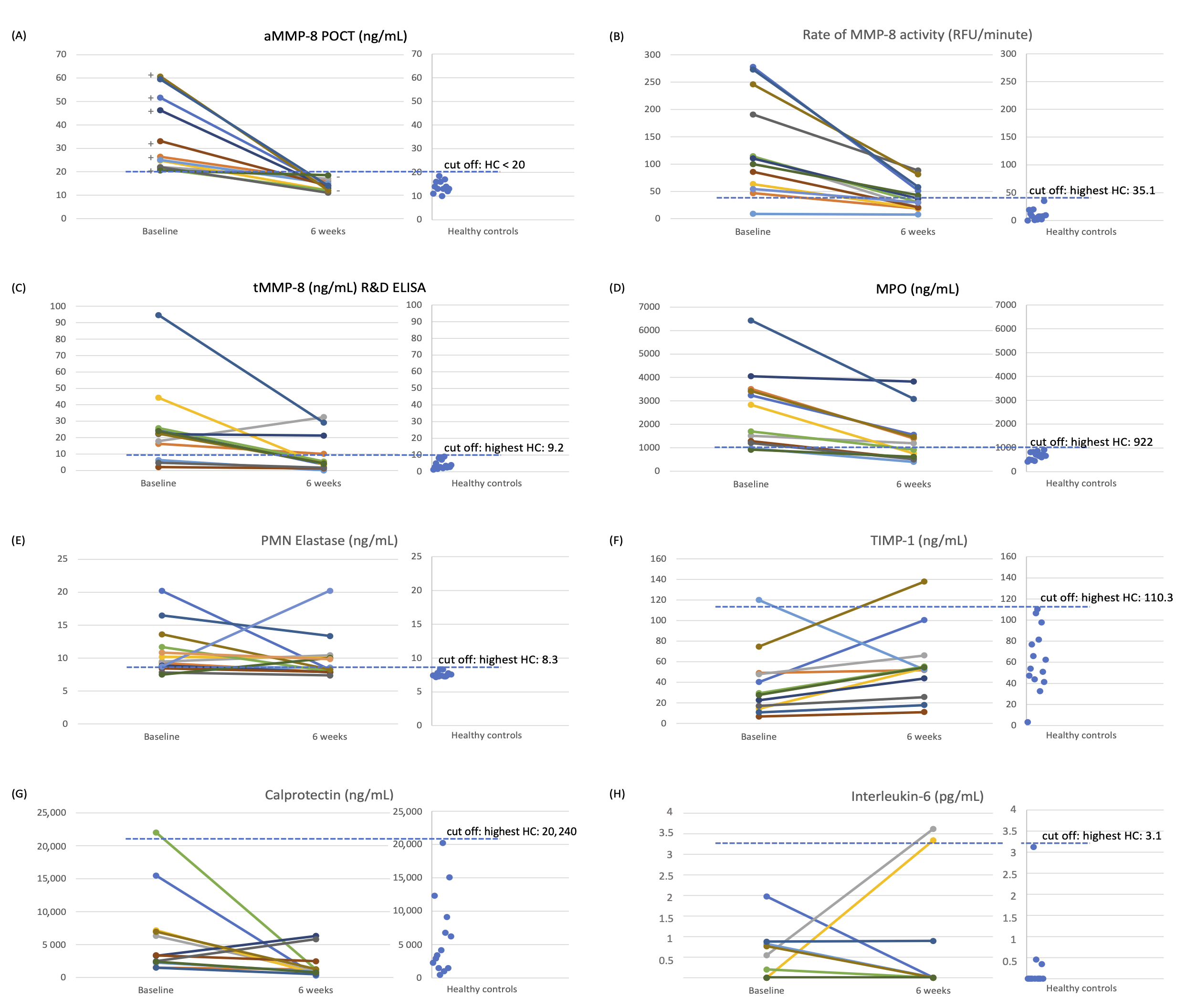
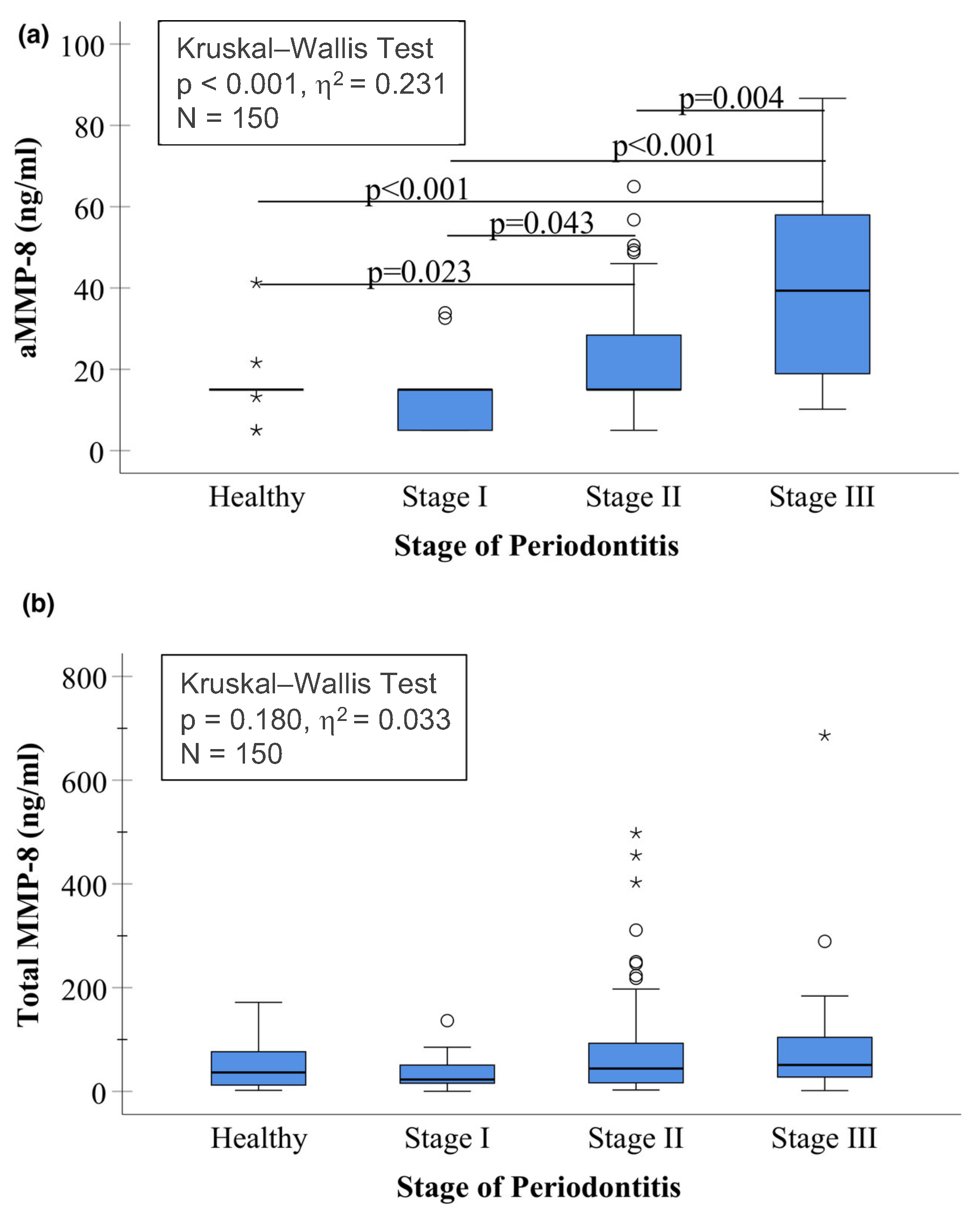
2. Comments and Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Markaryan, A.; Gellibolian, R.; Miller, C.S.; Ebersole, J.L.; Kantarci, A. Comment on Aji et al. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421. Int. J. Mol. Sci. 2025, 26, 9072. [Google Scholar] [CrossRef]
- Chen, J.; Qin, S.; Liu, S.; Zhong, K.; Jing, Y.; Wu, X.; Peng, F.; Li, D.; Peng, C. Correction in Targeting matrix metalloproteases in diabetic wound healing. Front. Immunol. 2023, 14, 1089001. [Google Scholar]
- Holopainen, J.M.; Moilanen, J.A.; Sorsa, T.; Kivelä-Rajamäki, M.; Tervahartiala, T.; Vesaluoma, M.H.; Tervo, T.M. Activation of matrix metalloproteinase-8 by membrane type 1-MMP and their expression in human tears after photorefractive keratectomy. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2550–2556. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar]
- Zalewska, E.A.; Ławicka, R.; Grygorczuk, P.; Nowosielska, M.; Kicman, A.; Ławicki, S. Importance of Metalloproteinase 8 (MMP-8) in the Diagnosis of Periodontitis. Int. J. Mol. Sci. 2024, 25, 2721. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Gangbar, S.; Overall, C.M.; McCulloch, C.A.; Sodek, J. Identification of polymorphonuclear leukocyte collagenase and gelatinase activities in mouthrinse samples: Correlation with periodontal disease activity in adult and juvenile periodontitis. J. Periodontal. Res. 1990, 25, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Aitken, S.; Sodek, J.; McCulloch, C.A. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: Role of active enzyme in human periodontitis. J. Periodontal. Res. 1995, 30, 23–33. [Google Scholar] [CrossRef]
- Romanelli, R.; Mancini, S.; Laschinger, C.; Overall, C.M.; Sodek, J.; McCulloch, C.A. Activation of neutrophil collagenase in periodontitis. Infect. Immun. 1999, 67, 2319–2326. [Google Scholar] [CrossRef]
- Mancini, S.; Romanelli, R.; Laschinger, C.A.; Overall, C.M.; Sodek, J.; McCulloch, C.A. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal diseases. J. Periodontol. 1999, 70, 1292–1302. [Google Scholar] [CrossRef]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Balsa-Castro, C.; Nibali, L.; Donos, N.; Tomás, I. Accuracy of single molecular biomarkers in gingival crevicular fluid for the diagnosis of periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 1166–1182. [Google Scholar] [CrossRef]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Balsa-Castro, C.; Nibali, L.; Donos, N.; Tomás, I. Accuracy of single molecular biomarkers in saliva for the diagnosis of periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 2–18. [Google Scholar] [CrossRef]
- Kc, S.; Wang, X.Z.; Gallagher, J.E. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: Systematic review. J. Clin. Periodontol. 2019, 47, 289–308. [Google Scholar] [CrossRef]
- Blanco-Pintos, T.; Regueira-Iglesias, A.; Seijo-Porto, I.; Balsa-Castro, C.; Castelo-Baz, P.; Nibali, L.; Tomás, I. Accuracy of periodontitis diagnosis obtained using multiple molecular biomarkers in oral fluids: A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50, 1420–1443. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, C.; Liang, M.; Shao, W.; Wang, P.; Yang, Y.; Guo, K. Diagnostic Accuracy of Matrix Metalloproteinase-8 for Detecting Periodontal Disease: A Meta-Analysis. Oral. Dis. 2025. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Hernández, M.; Leppilahti, J.; Munjal, S.; Netuschil, L.; Mäntylä, P. Detection of gingival crevicular fluid MMP-8 levels with different laboratory and chair-side methods. Oral. Dis. 2010, 16, 39–45. [Google Scholar] [CrossRef]
- Mäntylä, P.; Stenman, M.; Kinane, D.; Salo, T.; Suomalainen, K.; Tikanoja, S.; Sorsa, T. Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. J. Periodontal. Res. 2006, 41, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Penttala, M.; Sorsa, T.; Thomas, J.T.; Grigoriadis, A.; Sakellari, D.; Gupta, S.; Pärnänen, P.; Pätilä, T.; Räisänen, I.T. Periodontitis Home Screening with Mouth Rinse Cut-Off 20 Ng/mL aMMP-8 Test and Mobile Application. Diagnostics 2025, 15, 296. [Google Scholar] [CrossRef]
- Deng, K.; Pelekos, G.; Jin, L.; Tonetti, M.S. Diagnostic accuracy of a point-of-care aMMP-8 test in the discrimination of periodontal health and disease. J. Clin. Periodontol. 2021, 48, 1051–1065. [Google Scholar] [CrossRef]
- Lorenz, K.; Keller, T.; Noack, B.; Freitag, A.; Netuschil, L.; Hoffmann, T. Evaluation of a novel point-of-care test for active matrix metalloproteinase-8: Agreement between qualitative and quantitative measurements and relation to periodontal inflammation. J. Periodontal. Res. 2017, 52, 277–284. [Google Scholar] [CrossRef]
- Izadi Borujeni, S.; Mayer, M.; Eickholz, P. Activated matrix metalloproteinase-8 in saliva as diagnostic test for periodontal disease? A case-control study. Med. Microbiol. Immunol. 2015, 204, 665–672. [Google Scholar]
- Aji, N.R.A.S.; Räisänen, I.T.; Rathnayake, N.; Lundy, F.T.; Mc Crudden, M.T.C.; Goyal, L.; Sorsa, T.; Gupta, S. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421. [Google Scholar]
- Aji, N.R.A.S.; Sahni, V.; Penttala, M.T.; Sakellari, D.; Grigoriadis, A.; Pätilä, T.; Pärnänen, P.; Neefs, D.; Pfützner, A.; Gupta, S.; et al. Oral Medicine and Oral Clinical Chemistry Game Changers for Future Plaque Control and Maintenance: PerioSafe® aMMP-8 POCT, Lumoral® 2× PDT- and Lingora® Fermented Lingonberry Oral Rinse-Treatments. Dent. J. 2025, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Hasty, K.A.; Hibbs, M.S.; Kang, A.H.; Mainardi, C.L. Secreted forms of human neutrophil collagenase. J. Biol. Chem. 1986, 261, 5645–5650. [Google Scholar] [CrossRef] [PubMed]
- Knäuper, V.; Krämer, S.; Reinke, H.; Tschesche, H. Characterization and activation of procollagenase from human polymorpho-nuclear leucocytes. N-terminal sequence determination of the proenzyme and various proteolytically activated forms. Eur. J. Biochem. 1990, 189, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. S1), S1–S8. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S313–S318. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Guarnieri, R.; Reda, R.; Di Nardo, D.; Miccoli, G.; Pagnoni, F.; Zanza, A.; Testarelli, L. Expression of IL-1β, IL-6, TNF-α, and a-MMP-8 in sites with healthy conditions and with periodontal and peri-implant diseases: A case-control study. J. Dent. Res. Dent. Clin. Dent. Prospects. 2024, 18, 135–142. [Google Scholar] [CrossRef]
- Alassy, H.; Parachuru, P.; Wolff, L. Peri-Implantitis Diagnosis and Prognosis Using Biomarkers in Peri-Implant Crevicular Fluid: A Narrative Review. Diagnostics 2019, 9, 214. [Google Scholar] [CrossRef]
- Boelen, G.J.; Boute, L.; d’Hoop, J.; EzEldeen, M.; Lambrichts, I.; Opdenakker, G. Matrix metalloproteinases and inhibitors in dentistry. Clin. Oral. Investig. 2019, 23, 2823–2835. [Google Scholar] [CrossRef]
- Janska, E.; Mohr, B.; Wahl, G. Correlation between peri-implant sulcular fluid rate and expression of collagenase2 (MMP8). Clin. Oral. Investig. 2016, 20, 261–266. [Google Scholar] [CrossRef]
- Gul, S.S.; Abdulkareem, A.A.; Sha, A.M.; Rawlinson, A. Diagnostic Accuracy of Oral Fluids Biomarker Profile to Determine the Current and Future Status of Periodontal and Peri-Implant Diseases. Diagnostics 2020, 10, 838. [Google Scholar] [CrossRef]
- Gupta, S.; Sahni, V.; Räisänen, I.T.; Grigoriadis, A.; Sakellari, D.; Gieselmann, D.R.; Sorsa, T. Linking oral microbial proteolysis to aMMP-8 PoC diagnostics along with the stage and grade of periodontitis: A cross-sectional study. Oral. Dis. 2023, 29, 285–289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aji, N.R.A.S.; Thomas, J.T.; Rathnayake, N.; Lundy, F.T.; Mc Crudden, M.T.C.; Goyal, L.; Sahni, V.; Penttala, M.T.; Grigoriadis, A.; Pätilä, T.; et al. Reply to Markaryan et al. Comment on “Aji et al. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421”. Int. J. Mol. Sci. 2025, 26, 9074. https://doi.org/10.3390/ijms26189074
Aji NRAS, Thomas JT, Rathnayake N, Lundy FT, Mc Crudden MTC, Goyal L, Sahni V, Penttala MT, Grigoriadis A, Pätilä T, et al. Reply to Markaryan et al. Comment on “Aji et al. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421”. International Journal of Molecular Sciences. 2025; 26(18):9074. https://doi.org/10.3390/ijms26189074
Chicago/Turabian StyleAji, Nur Rahman Ahmad Seno, Julie Toby Thomas, Nilminie Rathnayake, Fionnuala T. Lundy, Maelíosa T. C. Mc Crudden, Lata Goyal, Vaibhav Sahni, Miika T. Penttala, Andreas Grigoriadis, Tommi Pätilä, and et al. 2025. "Reply to Markaryan et al. Comment on “Aji et al. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421”" International Journal of Molecular Sciences 26, no. 18: 9074. https://doi.org/10.3390/ijms26189074
APA StyleAji, N. R. A. S., Thomas, J. T., Rathnayake, N., Lundy, F. T., Mc Crudden, M. T. C., Goyal, L., Sahni, V., Penttala, M. T., Grigoriadis, A., Pätilä, T., Pärnänen, P., Sakellari, D., Neefs, D., Pfützner, A., Gupta, S., Sorsa, T., & Räisänen, I. T. (2025). Reply to Markaryan et al. Comment on “Aji et al. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421”. International Journal of Molecular Sciences, 26(18), 9074. https://doi.org/10.3390/ijms26189074




