Microglial Dysfunction and Amyloid-Beta Pathology in Alzheimer’s Disease and HIV-Associated Neurocognitive Disorders
Abstract
1. Introduction
2. Microglia: History, Function, and Role in CNS Homeostasis
2.1. Microglia Discovery and Historical Perspective
2.2. Homeostatic Functions and Dynamic Surveillance
2.3. Specialized Immune Functions and Transition to Neuroinflammation
3. Microglial Role in Aβ Clearance
3.1. Phagocytosis and Degradation of Aβ
3.2. Microglia Surface Receptors and Their Response to Aβ
3.2.1. TREM2
Overview of TREM2 in the CNS
Mechanisms and Functional Significance of TREM2 in Aβ and Tau Clearance
TREM2 Regulates Microglial Metabolism
3.2.2. Toll-like Receptors
Pathological Relevance
Evidence from Human and Animal Models
3.2.3. Scavenger Receptors (SRs)
Class A Scavenger Receptors (SR-AI/II)
Class B Scavenger Receptors (CD36 and SR-BI)
| Receptor | Aβ Role | Mechanism of Action | Human Evidence | Animal Evidence | References |
|---|---|---|---|---|---|
| SR-AI/II (SCARA1/MSR1) | Aβ binding, uptake, degradation | Clathrin-independent macropinocytosis; Aβ targeting to lysosomes; activates PI3K/NF-κB, JNK, and p38 MAPK; suppresses TLR4 signaling | High expression in early-stage microglia; decreased in advanced AD with plaque burden | SR-A knockout in PS1/APP mice leads to reduced Aβ uptake, increased plaques, impaired migration | [155,156,157,160,161] |
| CD36 (SCARB2) | Aβ recognition, uptake, inflammation | Binds fibrillar Aβ; forms CD36–TLR4–TLR6 complex; activates MAPK, NF-κB, and NLRP3 inflammasome; promotes ROS, TNF-α, and IL-1β production | Upregulated in plaque-associated microglia; absent in non-AD controls; declines with disease progression | CD36–/– mice show reduced cytokines and microglial recruitment to Aβ; reduced ROS and proinflammatory output | [167,171,172,176,177] |
| SR-BI (SCARB1) | Potential role in Aβ transport | Binds fibrillar Aβ; may mediate transcytosis across the BBB; limited microglial expression | Expressed on brain endothelial cells; involvement in Aβ efflux from brain to periphery hypothesized | Reduced SCARB1 leads to worsened cognitive outcomes in AD mice; no change in microglial clustering. | [168] |
| CD163 | Unknown role in Aβ clearance; immune modulation | Hemoglobin–haptoglobin scavenger; anti-inflammatory via IL-10, HO-1 signaling; possible role in resolution of neuroinflammation | Detected in microglia of people with HAND; not well studied in AD | Upregulated in neuroinflammation; may reflect anti-inflammatory activation | [186,187] |
3.2.4. Receptor for Advanced Glycation End Products (RAGE)
3.2.5. Low-Density Lipoprotein Receptor-Protein 1 (LRP1)
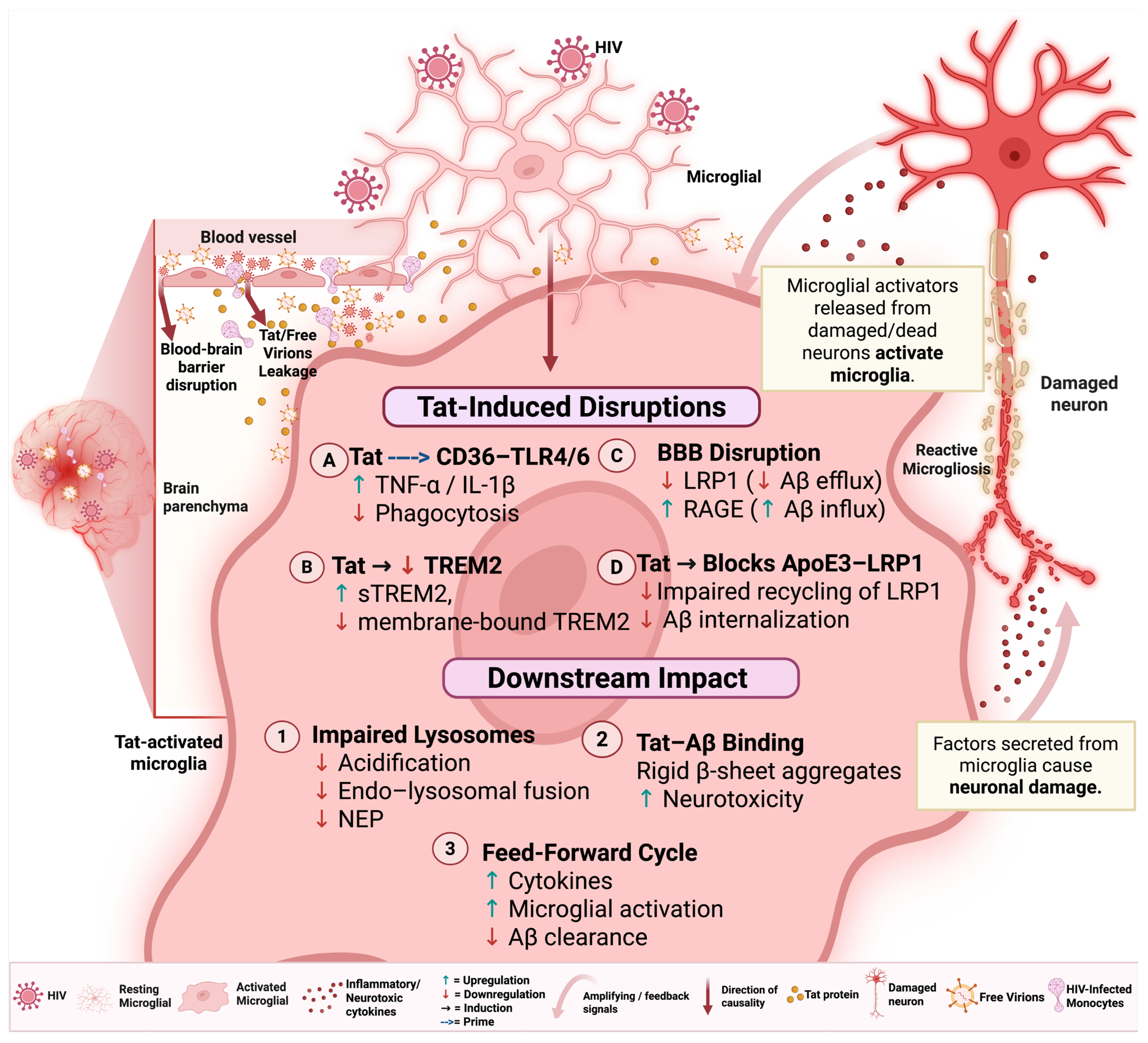
4. Microglial Activation and Dysfunction in HIV Infection
| HIV Protein | Reported Microglial Effects | Potential Impact on Aβ Handling | References |
|---|---|---|---|
| Tat | Alters receptor expression (TREM2, LRP1, SCARB1), impairs lysosomes, increases inflammatory cytokines | Reduced phagocytosis, enhanced extracellular Aβ | [207,209,210] |
| gp120 | Activates CXCR4/CCR5, induces Ca2+ influx and excitotoxicity, increases BACE1 expression | Promotes amyloidogenic APP cleavage, neurotoxicity | [212,213] |
| Nef | Alters lipid rafts, disrupts cholesterol trafficking, modulates exosome release | May influence APP processing and Aβ aggregation | [217,226,227,228,229] |
| Vpr | Causes mitochondrial dysfunction, oxidative stress, cell-cycle arrest in glia | Energy deficits impair phagocytosis, promote inflammation | [230,231] |
| Vpu | Modulates ion channels and tetherin, alters membrane trafficking | Potential effects on cytokine release and receptor dynamics | [219] |
5. HIV-Associated Disruption of Microglial Aβ Clearance
5.1. Microglial Aβ Binding, Uptake, and Phagocytosis
5.2. Aβ Transport and Degradation
6. Detection of Aβ Fibrils and Pathology
7. Therapeutic Strategies in AD and HAND
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Liu, W.; Deng, W.; Gong, X.; Ou, J.; Yu, S.; Chen, S. Global Burden of Alzheimer’s Disease and Other Dementias in Adults Aged 65 Years and over, and Health Inequality Related to SDI, 1990–2021: Analysis of Data from GBD 2021. BMC Public Health 2025, 25, 1256. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, J.; Lai, X.; Wang, X.; Deng, J.; Long, Y.; Li, B. Global Burden of Alzheimer’s Disease and Other Dementias in Adults Aged 65 Years and Older, 1991–2021: Population-Based Study. Front. Public Health 2025, 13, 1585711. [Google Scholar] [CrossRef]
- Li, M.; Ye, X.; Huang, Z.; Ye, L.; Chen, C. Global Burden of Parkinson’s Disease from 1990 to 2021: A Population-Based Study. BMJ Open 2025, 15, e095610. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef]
- Hattori, Y. The Multifaceted Roles of Embryonic Microglia in the Developing Brain. Front. Cell. Neurosci. 2023, 17, 988952. [Google Scholar] [CrossRef]
- Casali, B.T.; Reed-Geaghan, E.G. Microglial Function and Regulation during Development, Homeostasis and Alzheimer’s Disease. Cells 2021, 10, 957. [Google Scholar] [CrossRef] [PubMed]
- Ris, L. Origin, Diversity, and Roles of Microglia. In Neuroimmune Diseases; Springer: Cham, Switzerland, 2024; pp. 1–33. ISBN 978-3-031-24297-7. [Google Scholar]
- Del Rio-Hortega, P. El Tercer Elemento de Los Centros Nerviosos. I. La Microglia En Estado Normal. II. Intervencíon de La Microglia En Los Procesos Patológicos. III. Naturaleza Probable de La Microglia. Bol. Soc. Esp. Biol. 1919, 9, 69. Available online: https://cir.nii.ac.jp/crid/1370009142679807360 (accessed on 11 September 2025).
- Luo, Y.; Wang, Z. The Impact of Microglia on Neurodevelopment and Brain Function in Autism. Biomedicines 2024, 12, 210. [Google Scholar] [CrossRef]
- Pallarés-Moratalla, C.; Bergers, G. The Ins and Outs of Microglial Cells in Brain Health and Disease. Front. Immunol. 2024, 15, 1305087. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J. Neuroinflammation 2021, 18, 258. [Google Scholar] [CrossRef]
- Valiukas, Z.; Tangalakis, K.; Apostolopoulos, V.; Feehan, J. Microglial Activation States and Their Implications for Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2025, 12, 100013. [Google Scholar] [CrossRef] [PubMed]
- Borrajo López, A.; Penedo, M.A.; Rivera-Baltanas, T.; Pérez-Rodríguez, D.; Alonso-Crespo, D.; Fernández-Pereira, C.; Olivares, J.M.; Agís-Balboa, R.C. Microglia: The Real Foe in HIV-1-Associated Neurocognitive Disorders? Biomedicines 2021, 9, 925. [Google Scholar] [CrossRef]
- Butler, C.A.; Popescu, A.S.; Kitchener, E.J.A.; Allendorf, D.H.; Puigdellívol, M.; Brown, G.C. Microglial Phagocytosis of Neurons in Neurodegeneration, and Its Regulation. J. Neurochem. 2021, 158, 621–639. [Google Scholar] [CrossRef]
- Pansambal, P.S.; Kurle, S.N. Unveiling the Role of Microglia in HIV-Associated Neurodegeneration: Current Insights and Future Directions. Fortune J. Health Sci. 2024, 7, 408–417. [Google Scholar]
- Sian-Hulsmann, J.; Riederer, P. Virus-Induced Brain Pathology and the Neuroinflammation-Inflammation Continuum: The Neurochemists View. J. Neural Transm. 2024, 131, 1429–1453. [Google Scholar] [CrossRef]
- Metschnikoff, E. Lecture on Phagocytosis and Immunity. Br. Med. J. 1891, 1, 213–217. [Google Scholar] [CrossRef]
- Tremblay, M.-È.; Lecours, C.; Samson, L.; Sánchez-Zafra, V.; Sierra, A. From the Cajal Alumni Achúcarro and Río-Hortega to the Rediscovery of Never-Resting Microglia. Front. Neuroanat. 2015, 9, 45. [Google Scholar] [CrossRef]
- Cajal, S.R. Histologie Du Systeme Nerveux de L’homme et Des Vertebres II; Maloine: Paris, France, 1909. [Google Scholar]
- Sierra, A.; Paolicelli, R.C.; Kettenmann, H. Cien Años de Microglía: Milestones in a Century of Microglial Research. Trends Neurosci. 2019, 42, 778–792. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia States and Nomenclature: A Field at Its Crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Harry, G.J. Developmental Associations between Neurovascularization and Microglia Colonization. Int. J. Mol. Sci. 2024, 25, 1281. [Google Scholar] [CrossRef]
- Hattori, Y. The Behavior and Functions of Embryonic Microglia. Anat. Sci. Int. 2022, 97, 1–14. [Google Scholar] [CrossRef]
- Bernier, L.-P.; York, E.M.; Kamyabi, A.; Choi, H.B.; Weilinger, N.L.; MacVicar, B.A. Microglial Metabolic Flexibility Supports Immune Surveillance of the Brain Parenchyma. Nat. Commun. 2020, 11, 1559. [Google Scholar] [CrossRef] [PubMed]
- Petry, P.; Oschwald, A.; Kierdorf, K. Microglial Tissue Surveillance: The Never-Resting Gardener in the Developing and Adult CNS. Eur. J. Immunol. 2023, 53, 2250232. [Google Scholar] [CrossRef] [PubMed]
- Franco-Bocanegra, D.K.; McAuley, C.; Nicoll, J.A.R.; Boche, D. Molecular Mechanisms of Microglial Motility: Changes in Ageing and Alzheimer’s Disease. Cells 2019, 8, 639. [Google Scholar] [CrossRef]
- Guedes, J.R.; Ferreira, P.A.; Costa, J.M.; Cardoso, A.L.; Peça, J. Microglia-dependent Remodeling of Neuronal Circuits. J. Neurochem. 2022, 163, 74–93. [Google Scholar] [CrossRef]
- Naffaa, M.M. Mechanisms of Astrocytic and Microglial Purinergic Signaling in Homeostatic Regulation and Implications for Neurological Disease. Explor. Neurosci. 2025, 4, 100676. [Google Scholar] [CrossRef]
- Cui, Y.; Rolova, T.; Fagerholm, S.C. The Role of Integrins in Brain Health and Neurodegenerative Diseases. Eur. J. Cell Biol. 2024, 103, 151441. [Google Scholar] [CrossRef]
- Guan, F.; Wang, R.; Yi, Z.; Luo, P.; Liu, W.; Xie, Y.; Liu, Z.; Xia, Z.; Zhang, H.; Cheng, Q. Tissue Macrophages: Origin, Heterogenity, Biological Functions, Diseases and Therapeutic Targets. Signal Transduct. Target. Ther. 2025, 10, 93. [Google Scholar] [CrossRef]
- Rodriguez, D.; Church, K.A.; Pietramale, A.N.; Cardona, S.M.; Vanegas, D.; Rorex, C.; Leary, M.C.; Muzzio, I.A.; Nash, K.R.; Cardona, A.E. Fractalkine Isoforms Differentially Regulate Microglia-Mediated Inflammation and Enhance Visual Function in the Diabetic Retina. J. Neuroinflammation 2024, 21, 42. [Google Scholar] [CrossRef]
- Benmamar-Badel, A.; Owens, T.; Wlodarczyk, A. Protective Microglial Subset in Development, Aging, and Disease: Lessons From Transcriptomic Studies. Front. Immunol. 2020, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-L.; Yuan, Y.; Tian, L. Microglial Regional Heterogeneity and Its Role in the Brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Kenkhuis, B.; Somarakis, A.; Kleindouwel, L.R.; van Roon-Mom, W.M.C.; Höllt, T.; van der Weerd, L. Co-Expression Patterns of Microglia Markers Iba1, TMEM119 and P2RY12 in Alzheimer’s Disease. Neurobiol. Dis. 2022, 167, 105684. [Google Scholar] [CrossRef]
- Walker, D.G. Defining Activation States of Microglia in Human Brain Tissue: An Unresolved Issue for Alzheimer’s Disease. Neuroimmunol. Neuroinflammation 2020, 7, 194–214. [Google Scholar] [CrossRef]
- Abe, N.; Nishihara, T.; Yorozuya, T.; Tanaka, J. Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems. Cells 2020, 9, 2132. [Google Scholar] [CrossRef]
- De Schepper, S.; Crowley, G.; Hong, S. Understanding Microglial Diversity and Implications for Neuronal Function in Health and Disease. Dev. Neurobiol. 2021, 81, 507–523. [Google Scholar] [CrossRef]
- Li, N.; Deng, M.; Hu, G.; Li, N.; Yuan, H.; Zhou, Y. New Insights into Microglial Mechanisms of Memory Impairment in Alzheimer’s Disease. Biomolecules 2022, 12, 1722. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Qin, J.; Jiao, J.; Ji, F. The Roles of Immune Factors in Neurodevelopment. Front. Cell. Neurosci. 2025, 19, 1451889. [Google Scholar] [CrossRef]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The Role of Neuroinflammation in Neurodegenerative Diseases: Current Understanding and Future Therapeutic Targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef]
- Grubman, A.; Choo, X.Y.; Chew, G.; Ouyang, J.F.; Sun, G.; Croft, N.P.; Rossello, F.J.; Simmons, R.; Buckberry, S.; Landin, D.V.; et al. Transcriptional Signature in Microglia Associated with Aβ Plaque Phagocytosis. Nat. Commun. 2021, 12, 3015. [Google Scholar] [CrossRef]
- Smit, T.; Ormel, P.R.; Sluijs, J.A.; Hulshof, L.A.; Middeldorp, J.; de Witte, L.D.; Hol, E.M.; Donega, V. Transcriptomic and Functional Analysis of Aβ1-42 Oligomer-Stimulated Human Monocyte-Derived Microglia-like Cells. Brain. Behav. Immun. 2022, 100, 219–230. [Google Scholar] [CrossRef]
- Gauthier, A.E.; Rotjan, R.D.; Kagan, J.C. Lipopolysaccharide Detection by the Innate Immune System May Be an Uncommon Defence Strategy Used in Nature. Open Biol. 2022, 12, 220146. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Cicchinelli, S.; Pignataro, G.; Gemma, S.; Piccioni, A.; Picozzi, D.; Ojetti, V.; Franceschi, F.; Candelli, M. PAMPs and DAMPs in Sepsis: A Review of Their Molecular Features and Potential Clinical Implications. Int. J. Mol. Sci. 2024, 25, 962. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Chen, O.; Ji, R.-R. How Do Sensory Neurons Sense Danger Signals? Trends Neurosci. 2020, 43, 822–838. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release Mechanisms of Major DAMPs. Apoptosis Int. J. Program. Cell Death 2021, 26, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, J.; Wang, B.; Sun, M.; Yang, H. Microglia in the Neuroinflammatory Pathogenesis of Alzheimer’s Disease and Related Therapeutic Targets. Front. Immunol. 2022, 13, 856376. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Li, L.; Sun, B.; Harris, O.A.; Luo, J. TGF-β Signaling in Microglia: A Key Regulator of Development, Homeostasis and Reactivity. Biomedicines 2024, 12, 2468. [Google Scholar] [CrossRef]
- Yu, S.P.; Jiang, M.Q.; Shim, S.S.; Pourkhodadad, S.; Wei, L. Extrasynaptic NMDA Receptors in Acute and Chronic Excitotoxicity: Implications for Preventive Treatments of Ischemic Stroke and Late-Onset Alzheimer’s Disease. Mol. Neurodegener. 2023, 18, 43. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, Calcium and Mitochondria: A Triad in Synaptic Neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef]
- Hur, J.-Y. γ-Secretase in Alzheimer’s Disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Carapeto, A.P.; Marcuello, C.; Faísca, P.F.N.; Rodrigues, M.S. Morphological and Biophysical Study of S100A9 Protein Fibrils by Atomic Force Microscopy Imaging and Nanomechanical Analysis. Biomolecules 2024, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Ziaunys, M.; Sakalauskas, A.; Mikalauskaite, K.; Smirnovas, V. Polymorphism of Alpha-Synuclein Amyloid Fibrils Depends on Ionic Strength and Protein Concentration. Int. J. Mol. Sci. 2021, 22, 12382. [Google Scholar] [CrossRef]
- Chemparthy, D.T.; Kannan, M.; Gordon, L.; Buch, S.; Sil, S. Alzheimer’s-Like Pathology at the Crossroads of HIV-Associated Neurological Disorders. Vaccines 2021, 9, 930. [Google Scholar] [CrossRef]
- Jha, N.K.; Sharma, A.; Jha, S.K.; Ojha, S.; Chellappan, D.K.; Gupta, G.; Kesari, K.K.; Bhardwaj, S.; Shukla, S.D.; Tambuwala, M.M.; et al. Alzheimer’s Disease-like Perturbations in HIV-Mediated Neuronal Dysfunctions: Understanding Mechanisms and Developing Therapeutic Strategies. Open Biol. 2020, 10, 200286. [Google Scholar] [CrossRef]
- Mustafa, M.; Musselman, D.; Jayaweera, D.; da Fonseca Ferreira, A.; Marzouka, G.; Dong, C. HIV-Associated Neurocognitive Disorder (HAND) and Alzheimer’s Disease Pathogenesis: Future Directions for Diagnosis and Treatment. Int. J. Mol. Sci. 2024, 25, 11170. [Google Scholar] [CrossRef]
- Alzheimer’s Disease Fact Sheet. Available online: https://www.nia.nih.gov/health/alzheimers-and-dementia/alzheimers-disease-fact-sheet (accessed on 4 June 2025).
- Kamatham, P.T.; Shukla, R.; Khatri, D.K.; Vora, L.K. Pathogenesis, Diagnostics, and Therapeutics for Alzheimer’s Disease: Breaking the Memory Barrier. Ageing Res. Rev. 2024, 101, 102481. [Google Scholar] [CrossRef]
- Li, J.; Haj Ebrahimi, A.; Ali, A.B. Advances in Therapeutics to Alleviate Cognitive Decline and Neuropsychiatric Symptoms of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5169. [Google Scholar] [CrossRef]
- Soni, U.; Singh, K.; Jain, D.; Pujari, R. Exploring Alzheimer’s Disease Treatment: Established Therapies and Novel Strategies for Future Care. Eur. J. Pharmacol. 2025, 998, 177520. [Google Scholar] [CrossRef]
- Kamila, P.; Kar, K.; Chowdhury, S.; Chakraborty, P.; Dutta, R.; Sowmiya, S.; Singh S, A.; Prajapati, B.G. Effect of Neuroinflammation on the Progression of Alzheimer’s Disease and Its Significant Ramifications for Novel Anti-Inflammatory Treatments. IBRO Neurosci. Rep. 2025, 18, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Wong-Guerra, M.; Calfio, C.; Maccioni, R.B.; Rojo, L.E. Revisiting the Neuroinflammation Hypothesis in Alzheimer’s Disease: A Focus on the Druggability of Current Targets. Front. Pharmacol. 2023, 14, 1161850. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, M.C.B.; Kanaan, S.; Geller, M.; Praticò, D.; Daher, J.P.L. Mitochondrial Dysfunction in Alzheimer’s Disease. Ageing Res. Rev. 2025, 107, 102713. [Google Scholar] [CrossRef]
- Kazemeini, S.; Nadeem-Tariq, A.; Shih, R.; Rafanan, J.; Ghani, N.; Vida, T.A. From Plaques to Pathways in Alzheimer’s Disease: The Mitochondrial-Neurovascular-Metabolic Hypothesis. Int. J. Mol. Sci. 2024, 25, 11720. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Si, X.; Li, J.; Wu, G.; Wang, M. The Role of Mitochondrial Dysfunction in the Pathogenesis of Alzheimer’s Disease and Future Strategies for Targeted Therapy. Eur. J. Med. Res. 2025, 30, 434. [Google Scholar] [CrossRef]
- Peggion, C.; Calì, T.; Brini, M. Mitochondria Dysfunction and Neuroinflammation in Neurodegeneration: Who Comes First? Antioxidants 2024, 13, 240. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Liang, J.; Huang, J.; Wu, T.; Chen, N. Calcium Signaling Hypothesis: A Non-Negligible Pathogenesis in Alzheimer’s Disease. J. Adv. Res. 2025, in press. [CrossRef]
- Cáceres, C.; Heusser, B.; Garnham, A.; Moczko, E. The Major Hypotheses of Alzheimer’s Disease: Related Nanotechnology-Based Approaches for Its Diagnosis and Treatment. Cells 2023, 12, 2669. [Google Scholar] [CrossRef]
- Fleming, A.; Bourdenx, M.; Fujimaki, M.; Karabiyik, C.; Krause, G.J.; Lopez, A.; Martín-Segura, A.; Puri, C.; Scrivo, A.; Skidmore, J.; et al. The Different Autophagy Degradation Pathways and Neurodegeneration. Neuron 2022, 110, 935–966. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, X. Alzheimer’s Disease: Insights into Pathology, Molecular Mechanisms, and Therapy. Protein Cell 2025, 16, 83–120. [Google Scholar] [CrossRef]
- de Oliveira, J.; Kucharska, E.; Garcez, M.L.; Rodrigues, M.S.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells 2021, 10, 2581. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y. Tau and Neuroinflammation in Alzheimer’s Disease: Interplay Mechanisms and Clinical Translation. J. Neuroinflammation 2023, 20, 165. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Valdez-Gaxiola, C.A.; Rosales-Leycegui, F.; Gaxiola-Rubio, A.; Moreno-Ortiz, J.M.; Figuera, L.E. Early- and Late-Onset Alzheimer’s Disease: Two Sides of the Same Coin? Diseases 2024, 12, 110. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S.; Müller, V. From Homeostasis to Neuroinflammation: Insights into Cellular and Molecular Interactions and Network Dynamics. Cells 2025, 14, 54. [Google Scholar] [CrossRef]
- Divecha, Y.A.; Rampes, S.; Tromp, S.; Boyanova, S.T.; Fleckney, A.; Fidanboylu, M.; Thomas, S.A. The Microcirculation, the Blood-Brain Barrier, and the Neurovascular Unit in Health and Alzheimer Disease: The Aberrant Pericyte Is a Central Player. Pharmacol. Rev. 2025, 77, 100052. [Google Scholar] [CrossRef]
- Orobets, K.S.; Karamyshev, A.L. Amyloid Precursor Protein and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14794. [Google Scholar] [CrossRef]
- Ullah, R.; Lee, E.J. Advances in Amyloid-β Clearance in the Brain and Periphery: Implications for Neurodegenerative Diseases. Exp. Neurobiol. 2023, 32, 216–246. [Google Scholar] [CrossRef] [PubMed]
- Takatori, S.; Kondo, M.; Tomita, T. Unraveling the Complex Role of Microglia in Alzheimer’s Disease: Amyloid β Metabolism and Plaque Formation. Inflamm. Regen. 2025, 45, 16. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, C.; Parker, E.; Liu, T.C.-Y.; Duan, R.; Yang, L. Microglia and Astrocytes in Alzheimer’s Disease: Significance and Summary of Recent Advances. Aging Dis. 2024, 15, 1537–1564. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Baligács, N.; Albertini, G.; Borrie, S.C.; Serneels, L.; Pridans, C.; Balusu, S.; Strooper, B.D. Microglia Initially Seed and Later Reshape Amyloid Plaques in Alzheimer’s Disease. bioRxiv 2024. bioRxiv:2024.08.06.606783. [Google Scholar] [CrossRef]
- Dias, D.; Socodato, R. Beyond Amyloid and Tau: The Critical Role of Microglia in Alzheimer’s Disease Therapeutics. Biomedicines 2025, 13, 279. [Google Scholar] [CrossRef]
- Choi, I.; Wang, M.; Yoo, S.; Xu, P.; Seegobin, S.P.; Li, X.; Han, X.; Wang, Q.; Peng, J.; Zhang, B.; et al. Autophagy Enables Microglia to Engage Amyloid Plaques and Prevents Microglial Senescence. Nat. Cell Biol. 2023, 25, 963–974. [Google Scholar] [CrossRef]
- Fu, J.; Wang, R.; He, J.; Liu, X.; Wang, X.; Yao, J.; Liu, Y.; Ran, C.; Ye, Q.; He, Y. Pathogenesis and Therapeutic Applications of Microglia Receptors in Alzheimer’s Disease. Front. Immunol. 2025, 16, 1508023. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Peripheral Blood Amyloid-β Involved in the Pathogenesis of Alzheimer’s Disease via Impacting on Peripheral Innate Immune Cells. J. Neuroinflammation 2024, 21, 5. [Google Scholar] [CrossRef]
- Taylor, X.; Clark, I.M.; Fitzgerald, G.J.; Oluoch, H.; Hole, J.T.; DeMattos, R.B.; Wang, Y.; Pan, F. Amyloid-β (Aβ) Immunotherapy Induced Microhemorrhages Are Associated with Activated Perivascular Macrophages and Peripheral Monocyte Recruitment in Alzheimer’s Disease Mice. Mol. Neurodegener. 2023, 18, 59. [Google Scholar] [CrossRef]
- Wu, X.; Miller, J.A.; Lee, B.T.K.; Wang, Y.; Ruedl, C. Reducing Microglial Lipid Load Enhances β Amyloid Phagocytosis in an Alzheimer’s Disease Mouse Model. Sci. Adv. 2025, 11, eadq6038. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our Current Understanding of a Universal Biological Process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhang, L.; Guo, C.; Xin, Q.; Gu, X.; Jiang, C.; Wu, J. “Find Me” and “Eat Me” Signals: Tools to Drive Phagocytic Processes for Modulating Antitumor Immunity. Cancer Commun. 2024, 44, 791–832. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, M.; Li, Z.; Li, B.; Wang, J.; Zhang, K. Systematic Review of Amyloid-Beta Clearance Proteins from the Brain to the Periphery: Implications for Alzheimer’s Disease Diagnosis and Therapeutic Targets. Neural Regen. Res. 2025, 20, 3574–3590. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Read, M.; Barreto, G.E.; Ávila-Rodriguez, M.; Gheibi-Hayat, S.M.; Sahebkar, A. Apoptotic Neurons and Amyloid-Beta Clearance by Phagocytosis in Alzheimer’s Disease: Pathological Mechanisms and Therapeutic Outlooks. Eur. J. Pharmacol. 2021, 895, 173873. [Google Scholar] [CrossRef]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s Disease: Pathogenesis, Mechanisms, and Therapeutic Potentials. Front. Aging Neurosci. 2023, 15, 1201982. [Google Scholar] [CrossRef]
- Sahoo, B.R.; Panda, P.K.; Liang, W.; Tang, W.-J.; Ahuja, R.; Ramamoorthy, A. Degradation of Alzheimer’s Amyloid-β by a Catalytically Inactive Insulin-Degrading Enzyme. J. Mol. Biol. 2021, 433, 166993. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, X.; Li, Y.; Bu, G.; Chen, X.-F. TREM2 and sTREM2 in Alzheimer’s Disease: From Mechanisms to Therapies. Mol. Neurodegener. 2025, 20, 43. [Google Scholar] [CrossRef]
- Colonna, M. The Biology of TREM Receptors. Nat. Rev. Immunol. 2023, 23, 580–594. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Li, X.; Jiang, L.-L.; Gui, X.; Liu, Y.; Sun, Y.; Zhu, B.; Piña-Crespo, J.C.; Zhang, M.; et al. TREM2 Is a Receptor for β-Amyloid That Mediates Microglial Function. Neuron 2018, 97, 1023–1031.e7. [Google Scholar] [CrossRef]
- Stefansson, H.; Walters, G.B.; Sveinbjornsson, G.; Tragante, V.; Einarsson, G.; Helgason, H.; Sigurðsson, A.; Beyter, D.; Snaebjarnarson, A.S.; Ivarsdottir, E.V.; et al. Homozygosity for R47H in TREM2 and the Risk of Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 2217–2219. [Google Scholar] [CrossRef]
- Wu, M.; Liao, M.; Huang, R.; Chen, C.; Tian, T.; Wang, H.; Li, J.; Li, J.; Sun, Y.; Wu, C.; et al. Hippocampal Overexpression of TREM2 Ameliorates High Fat Diet Induced Cognitive Impairment and Modulates Phenotypic Polarization of the Microglia. Genes Dis. 2022, 9, 401–414. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin Inhibits LPS-Induced Neuroinflammation by Promoting Microglial M2 Polarization via TREM2/ TLR4/ NF-κB Pathways in BV2 Cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Chen, K.; Li, F.; Zhang, S.; Chen, Y.; Ikezu, T.C.; Li, Z.; Martens, Y.A.; Qiao, W.; Meneses, A.; Zhu, Y.; et al. Enhancing TREM2 Expression Activates Microglia and Modestly Mitigates Tau Pathology and Neurodegeneration. J. Neuroinflammation 2025, 22, 93. [Google Scholar] [CrossRef]
- Greven, J.A.; Wydra, J.R.; Greer, R.A.; Zhi, C.; Price, D.A.; Svoboda, J.D.; Camitta, C.L.M.; Washington, M.; Leung, D.W.; Song, Y.; et al. Biophysical Mapping of TREM2-Ligand Interactions Reveals Shared Surfaces for Engagement of Multiple Alzheimer’s Disease Ligands. Mol. Neurodegener. 2025, 20, 3. [Google Scholar] [CrossRef]
- Wang, S.; Sudan, R.; Peng, V.; Zhou, Y.; Du, S.; Yuede, C.M.; Lei, T.; Hou, J.; Cai, Z.; Cella, M.; et al. TREM2 Drives Microglia Response to Amyloid-β via SYK-Dependent and -Independent Pathways. Cell 2022, 185, 4153–4169.e19. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, Y.; Yang, S.; Gong, E.J.; Kim, J.; Ha, N.-C.; Jo, D.-G.; Mattson, M.P.; Lee, J. Akt-Activated GSK3β Inhibitory Peptide Effectively Blocks Tau Hyperphosphorylation. Arch. Pharm. Res. 2024, 47, 812–828. [Google Scholar] [CrossRef]
- Peng, X.; Guo, H.; Zhang, X.; Yang, Z.; Ruganzu, J.B.; Yang, Z.; Wu, X.; Bi, W.; Ji, S.; Yang, W. TREM2 Inhibits Tau Hyperphosphorylation and Neuronal Apoptosis via the PI3K/Akt/GSK-3β Signaling Pathway In Vivo and In Vitro. Mol. Neurobiol. 2023, 60, 2470–2485. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, L.; Fan, Y.; Ji, W. The Pathogenesis in Alzheimer’s Disease: TREM2 as a Potential Target. J. Integr. Neurosci. 2023, 22, 150. [Google Scholar] [CrossRef]
- Shi, Q.; Gutierrez, R.A.; Bhat, M.A. Microglia, Trem2, and Neurodegeneration. Neuroscientist 2025, 31, 159–176. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Y.; Grajales-Reyes, G.; Colonna, M. TREM2 Dependent and Independent Functions of Microglia in Alzheimer’s Disease. Mol. Neurodegener. 2022, 17, 84. [Google Scholar] [CrossRef]
- Meilandt, W.J.; Ngu, H.; Gogineni, A.; Lalehzadeh, G.; Lee, S.-H.; Srinivasan, K.; Imperio, J.; Wu, T.; Weber, M.; Kruse, A.J.; et al. Trem2 Deletion Reduces Late-Stage Amyloid Plaque Accumulation, Elevates the Aβ42:Aβ40 Ratio, and Exacerbates Axonal Dystrophy and Dendritic Spine Loss in the PS2APP Alzheimer’s Mouse Model. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 1956–1974. [Google Scholar] [CrossRef]
- Lee, S.-H.; Meilandt, W.J.; Xie, L.; Gandham, V.D.; Ngu, H.; Barck, K.H.; Rezzonico, M.G.; Imperio, J.; Lalehzadeh, G.; Huntley, M.A.; et al. Trem2 Restrains the Enhancement of Tau Accumulation and Neurodegeneration by β-Amyloid Pathology. Neuron 2021, 109, 1283–1301.e6. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Y.; Hwang, S.; Archuleta, K.; Huang, H.; Campos, A.; Murad, R.; Piña-Crespo, J.; Xu, H.; Huang, T.Y. Trem2 Deletion Enhances Tau Dispersion and Pathology through Microglia Exosomes. Mol. Neurodegener. 2022, 17, 58. [Google Scholar] [CrossRef]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheimer’s Disease Model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef]
- Jay, T.R.; Miller, C.M.; Cheng, P.J.; Graham, L.C.; Bemiller, S.; Broihier, M.L.; Xu, G.; Margevicius, D.; Karlo, J.C.; Sousa, G.L.; et al. TREM2 Deficiency Eliminates TREM2+ Inflammatory Macrophages and Ameliorates Pathology in Alzheimer’s Disease Mouse Models. J. Exp. Med. 2015, 212, 287–295. [Google Scholar] [CrossRef]
- Jay, T.R.; Hirsch, A.M.; Broihier, M.L.; Miller, C.M.; Neilson, L.E.; Ransohoff, R.M.; Lamb, B.T.; Landreth, G.E. Disease Progression-Dependent Effects of TREM2 Deficiency in a Mouse Model of Alzheimer’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 637–647. [Google Scholar] [CrossRef]
- Lee, C.Y.D.; Daggett, A.; Gu, X.; Jiang, L.-L.; Langfelder, P.; Li, X.; Wang, N.; Zhao, Y.; Park, C.S.; Cooper, Y.; et al. Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer’s Disease Models. Neuron 2018, 97, 1032–1048.e5. [Google Scholar] [CrossRef]
- Li, R.-Y.; Qin, Q.; Yang, H.-C.; Wang, Y.-Y.; Mi, Y.-X.; Yin, Y.-S.; Wang, M.; Yu, C.-J.; Tang, Y. TREM2 in the Pathogenesis of AD: A Lipid Metabolism Regulator and Potential Metabolic Therapeutic Target. Mol. Neurodegener. 2022, 17, 40. [Google Scholar] [CrossRef]
- Lin, M.; Yu, J.-X.; Zhang, W.-X.; Lao, F.-X.; Huang, H.-C. Roles of TREM2 in the Pathological Mechanism and the Therapeutic Strategies of Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2024, 11, 1682–1695. [Google Scholar] [CrossRef]
- Xue, F.; Du, H. TREM2 Mediates Microglial Anti-Inflammatory Activations in Alzheimer’s Disease: Lessons Learned from Transcriptomics. Cells 2021, 10, 321. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, H.; Li, R.; Wu, G.; Qin, Q.; Tang, Y. A Systematic Review of the Role of TREM2 in Alzheimer’s Disease. Chin. Med. J. (Engl.) 2024, 137, 1684–1694. [Google Scholar] [CrossRef]
- Shi, Q.; Chang, C.; Saliba, A.; Bhat, M.A. Microglial mTOR Activation Upregulates Trem2 and Enhances β-Amyloid Plaque Clearance in the 5XFAD Alzheimer’s Disease Model. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 5294–5313. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Qin, S.; Zhang, T.; Yao, J.; Yi, Y.; Deng, L. Mechanistic Target of Rapamycin Complex 1: From a Nutrient Sensor to a Key Regulator of Metabolism and Health. Adv. Nutr. 2022, 13, 1882–1900. [Google Scholar] [CrossRef]
- Lepiarz-Raba, I.; Gbadamosi, I.; Florea, R.; Paolicelli, R.C.; Jawaid, A. Metabolic Regulation of Microglial Phagocytosis: Implications for Alzheimer’s Disease Therapeutics. Transl. Neurodegener. 2023, 12, 48. [Google Scholar] [CrossRef]
- Ulland, T.K.; Song, W.M.; Huang, S.C.-C.; Ulrich, J.D.; Sergushichev, A.; Beatty, W.L.; Loboda, A.A.; Zhou, Y.; Cairns, N.J.; Kambal, A.; et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 2017, 170, 649–663.e13. [Google Scholar] [CrossRef]
- Carroll, S.L.; Pasare, C.; Barton, G.M. Control of Adaptive Immunity by Pattern Recognition Receptors. Immunity 2024, 57, 632–648. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Abarca-Merlin, D.M.; Martínez-Durán, J.A.; Medina-Pérez, J.D.; Rodríguez-Santos, G.; Alvarez-Arellano, L. From Immunity to Neurogenesis: Toll-like Receptors as Versatile Regulators in the Nervous System. Int. J. Mol. Sci. 2024, 25, 5711. [Google Scholar] [CrossRef]
- Frederiksen, H.R.; Haukedal, H.; Freude, K. Cell Type Specific Expression of Toll-Like Receptors in Human Brains and Implications in Alzheimer’s Disease. BioMed Res. Int. 2019, 2019, 7420189. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Han, J.; Li, S.; Gao, X.; Wang, M.; Zhu, J.; Jin, T. Role of Toll-Like Receptors in Neuroimmune Diseases: Therapeutic Targets and Problems. Front. Immunol. 2021, 12, 777606. [Google Scholar] [CrossRef]
- Sanchez-Varo, R.; Mejias-Ortega, M.; Fernandez-Valenzuela, J.J.; Nuñez-Diaz, C.; Caceres-Palomo, L.; Vegas-Gomez, L.; Sanchez-Mejias, E.; Trujillo-Estrada, L.; Garcia-Leon, J.A.; Moreno-Gonzalez, I.; et al. Transgenic Mouse Models of Alzheimer’s Disease: An Integrative Analysis. Int. J. Mol. Sci. 2022, 23, 5404. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2020, 78, 1233–1261. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, H.; Lee, J.-H.; Hwangbo, C. Toll-like Receptor 4 (TLR4): New Insight Immune and Aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Duggan, M.R.; Morgan, D.G.; Price, B.R.; Rajbanshi, B.; Martin-Peña, A.; Tansey, M.G.; Walker, K.A. Immune Modulation to Treat Alzheimer’s Disease. Mol. Neurodegener. 2025, 20, 39. [Google Scholar] [CrossRef]
- Dallas, M.L.; Widera, D. TLR2 and TLR4-Mediated Inflammation in Alzheimer’s Disease: Self-Defense or Sabotage? Neural Regen. Res. 2021, 16, 1552–1553. [Google Scholar] [CrossRef]
- Frank, S.; Copanaki, E.; Burbach, G.J.; Müller, U.C.; Deller, T. Differential Regulation of Toll-like Receptor mRNAs in Amyloid Plaque-Associated Brain Tissue of Aged APP23 Transgenic Mice. Neurosci. Lett. 2009, 453, 41–44. [Google Scholar] [CrossRef]
- Song, M.; Jin, J.; Lim, J.-E.; Kou, J.; Pattanayak, A.; Rehman, J.A.; Kim, H.-D.; Tahara, K.; Lalonde, R.; Fukuchi, K. TLR4 Mutation Reduces Microglial Activation, Increases Aβ Deposits and Exacerbates Cognitive Deficits in a Mouse Model of Alzheimer’s Disease. J. Neuroinflammation 2011, 8, 92. [Google Scholar] [CrossRef]
- Tahara, K.; Kim, H.-D.; Jin, J.-J.; Maxwell, J.A.; Li, L.; Fukuchi, K. Role of Toll-like Receptor Signalling in Abeta Uptake and Clearance. Brain J. Neurol. 2006, 129, 3006–3019. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Hao, W.; Wolf, L.; Kiliaan, A.J.; Penke, B.; Rübe, C.E.; Walter, J.; Heneka, M.T.; Hartmann, T.; et al. TLR2 Is a Primary Receptor for Alzheimer’s Amyloid β Peptide to Trigger Neuroinflammatory Activation. J. Immunol. 2012, 188, 1098–1107. [Google Scholar] [CrossRef]
- Richard, K.L.; Filali, M.; Préfontaine, P.; Rivest, S. Toll-like Receptor 2 Acts as a Natural Innate Immune Receptor to Clear Amyloid Beta 1-42 and Delay the Cognitive Decline in a Mouse Model of Alzheimer’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 5784–5793. [Google Scholar] [CrossRef]
- Chen, K.; Iribarren, P.; Hu, J.; Chen, J.; Gong, W.; Cho, E.H.; Lockett, S.; Dunlop, N.M.; Wang, J.M. Activation of Toll-like Receptor 2 on Microglia Promotes Cell Uptake of Alzheimer Disease-Associated Amyloid Beta Peptide. J. Biol. Chem. 2006, 281, 3651–3659. [Google Scholar] [CrossRef]
- Reed-Geaghan, E.G.; Savage, J.C.; Hise, A.G.; Landreth, G.E. CD14 and Toll-like Receptors 2 and 4 Are Required for Fibrillar A{beta}-Stimulated Microglial Activation. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 11982–11992. [Google Scholar] [CrossRef] [PubMed]
- Scholtzova, H.; Kascsak, R.J.; Bates, K.A.; Boutajangout, A.; Kerr, D.J.; Meeker, H.C.; Mehta, P.D.; Spinner, D.S.; Wisniewski, T. Induction of Toll-like Receptor 9 Signaling as a Method for Ameliorating Alzheimer’s Disease-Related Pathology. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Colleselli, K.; Stierschneider, A.; Wiesner, C. An Update on Toll-like Receptor 2, Its Function and Dimerization in Pro- and Anti-Inflammatory Processes. Int. J. Mol. Sci. 2023, 24, 12464. [Google Scholar] [CrossRef] [PubMed]
- Alquraini, A.; El Khoury, J. Scavenger Receptors. Curr. Biol. CB 2020, 30, R790–R795. [Google Scholar] [CrossRef]
- Taban, Q.; Mumtaz, P.T.; Masoodi, K.Z.; Haq, E.; Ahmad, S.M. Scavenger Receptors in Host Defense: From Functional Aspects to Mode of Action. Cell Commun. Signal. 2022, 20, 2. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Hu, H.; Zhao, M.; Sun, L. Microglia in Alzheimer’s Disease: A Target for Therapeutic Intervention. Front. Cell. Neurosci. 2021, 15, 749587. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Zhou, W.; Wang, S.; Xu, P.; Yu, X.; Liu, R. Activated Scavenger Receptor A Promotes Glial Internalization of Aβ. PLoS ONE 2014, 9, e94197. [Google Scholar] [CrossRef]
- Doens, D.; Fernández, P.L. Microglia Receptors and Their Implications in the Response to Amyloid β for Alzheimer’s Disease Pathogenesis. J. Neuroinflammation 2014, 11, 48. [Google Scholar] [CrossRef]
- Wilkinson, K.; El Khoury, J. Microglial Scavenger Receptors and Their Roles in the Pathogenesis of Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2012, 2012, 489456. [Google Scholar] [CrossRef]
- Jaye, S.; Sandau, U.S.; Saugstad, J.A. Clathrin Mediated Endocytosis in Alzheimer’s Disease: Cell Type Specific Involvement in Amyloid Beta Pathology. Front. Aging Neurosci. 2024, 16, 1378576. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, D.; Wilkinson, K.; Zhao, L.; Hickman, S.E.; Means, T.K.; Puckett, L.; Farfara, D.; Kingery, N.D.; Weiner, H.L.; El Khoury, J. Scara1 Deficiency Impairs Clearance of Soluble Amyloid-β by Mononuclear Phagocytes and Accelerates Alzheimer’s-like Disease Progression. Nat. Commun. 2013, 4, 2030. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Paterniti, I.; Siracusa, R.; Filippone, A.; Esposito, E.; Cuzzocrea, S. TLR4 Absence Reduces Neuroinflammation and Inflammasome Activation in Parkinson’s Diseases in Vivo Model. Brain. Behav. Immun. 2019, 76, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ye, R.D. Microglial Aβ Receptors in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2015, 35, 71–83. [Google Scholar] [CrossRef]
- Bornemann, K.D.; Wiederhold, K.-H.; Pauli, C.; Ermini, F.; Stalder, M.; Schnell, L.; Sommer, B.; Jucker, M.; Staufenbiel, M. Aβ-Induced Inflammatory Processes in Microglia Cells of APP23 Transgenic Mice. Am. J. Pathol. 2001, 158, 63–73. [Google Scholar] [CrossRef]
- Chung, H.; Brazil, M.I.; Irizarry, M.C.; Hyman, B.T.; Maxfield, F.R. Uptake of Fibrillar β-Amyloid by Microglia Isolated from MSR-A (Type I and Type II) Knockout Mice. NeuroReport 2001, 12, 1151–1154. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial Dysfunction and Defective β-Amyloid Clearance Pathways in Aging Alzheimer’s Disease Mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Cornejo, F.; Vruwink, M.; Metz, C.; Muñoz, P.; Salgado, N.; Poblete, J.; Andrés, M.E.; Eugenín, J.; von Bernhardi, R. Scavenger Receptor-A Deficiency Impairs Immune Response of Microglia and Astrocytes Potentiating Alzheimer’s Disease Pathophysiology. Brain. Behav. Immun. 2018, 69, 336–350. [Google Scholar] [CrossRef]
- Powers, H.R.; Sahoo, D. SR-B1’s Next Top Model: Structural Perspectives on the Functions of the HDL Receptor. Curr. Atheroscler. Rep. 2022, 24, 277–288. [Google Scholar] [CrossRef]
- Shen, W.-J.; Asthana, S.; Kraemer, F.B.; Azhar, S. Scavenger Receptor B Type 1: Expression, Molecular Regulation, and Cholesterol Transport Function. J. Lipid Res. 2018, 59, 1114–1131. [Google Scholar] [CrossRef]
- Wang, W.; Yan, Z.; Hu, J.; Shen, W.-J.; Azhar, S.; Kraemer, F.B. Scavenger Receptor Class B, Type 1 Facilitates Cellular Fatty Acid Uptake. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158554. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, M.; Adlimoghaddam, A.; Mahmoodzadeh, A.; Sharifzad, F.; Yasavoli-Sharahi, H.; Lorzadeh, S.; Albensi, B.C.; Ghavami, S. Alzheimer’s Disease Pathogenesis: Role of Autophagy and Mitophagy Focusing in Microglia. Int. J. Mol. Sci. 2021, 22, 3330. [Google Scholar] [CrossRef] [PubMed]
- Asch, A.S.; Barnwell, J.; Silverstein, R.L.; Nachman, R.L. Isolation of the Thrombospondin Membrane Receptor. J. Clin. Investig. 1987, 79, 1054–1061. [Google Scholar] [CrossRef]
- Baranova, I.N.; Bocharov, A.V.; Vishnyakova, T.G.; Chen, Z.; Ke, Y.; Birukova, A.A.; Yuen, P.S.T.; Tsuji, T.; Star, R.A.; Birukov, K.G.; et al. Class B Scavenger Receptor CD36 as a Potential Therapeutic Target in Inflammation Induced by Danger-Associated Molecular Patterns. Cells 2024, 13, 1992. [Google Scholar] [CrossRef]
- Feng, M.; Zhou, Q.; Xie, H.; Liu, C.; Zheng, M.; Zhang, S.; Zhou, S.; Zhao, J. Role of CD36 in Central Nervous System Diseases. Neural Regen. Res. 2024, 19, 512–518. [Google Scholar] [CrossRef]
- Dobri, A.-M.; Dudău, M.; Enciu, A.-M.; Hinescu, M.E. CD36 in Alzheimer’s Disease: An Overview of Molecular Mechanisms and Therapeutic Targeting. Neuroscience 2021, 453, 301–311. [Google Scholar] [CrossRef]
- Ioghen, O.; Chițoiu, L.; Gherghiceanu, M.; Ceafalan, L.C.; Hinescu, M.E. CD36—A Novel Molecular Target in the Neurovascular Unit. Eur. J. Neurosci. 2021, 53, 2500–2510. [Google Scholar] [CrossRef]
- Kagan, J.C.; Horng, T. NLRP3 Inflammasome Activation: CD36 Serves Double Duty. Nat. Immunol. 2013, 14, 772–774. [Google Scholar] [CrossRef]
- El Khoury, J.B.; Moore, K.J.; Means, T.K.; Leung, J.; Terada, K.; Toft, M.; Freeman, M.W.; Luster, A.D. CD36 Mediates the Innate Host Response to Beta-Amyloid. J. Exp. Med. 2003, 197, 1657–1666. [Google Scholar] [CrossRef]
- Gadagkar, S.G.; Lalancette-Hébert, M.; Thammisetty, S.S.; Vexler, Z.S.; Kriz, J. CD36 Neutralisation Blunts TLR2-IRF7 but Not IRF3 Pathway in Neonatal Mouse Brain and Immature Human Microglia Following Innate Immune Challenge. Sci. Rep. 2023, 13, 2304. [Google Scholar] [CrossRef]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 Ligands Promote Sterile Inflammation through Assembly of a Toll-like Receptor 4 and 6 Heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef]
- Oury, C. CD36: Linking Lipids to the NLRP3 Inflammasome, Atherogenesis and Atherothrombosis. Cell. Mol. Immunol. 2014, 11, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Coraci, I.S.; Husemann, J.; Berman, J.W.; Hulette, C.; Dufour, J.H.; Campanella, G.K.; Luster, A.D.; Silverstein, S.C.; El-Khoury, J.B. CD36, a Class B Scavenger Receptor, Is Expressed on Microglia in Alzheimer’s Disease Brains and Can Mediate Production of Reactive Oxygen Species in Response to Beta-Amyloid Fibrils. Am. J. Pathol. 2002, 160, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; D’Abramo, C.; Zingg, J.-M.; Giliberto, L.; Markesbery, W.; Azzi, A.; Marinari, U.M.; Pronzato, M.A.; Tabaton, M. CD36 Overexpression in Human Brain Correlates with Beta-Amyloid Deposition but Not with Alzheimer’s Disease. Free Radic. Biol. Med. 2004, 36, 1018–1024. [Google Scholar] [CrossRef]
- Grajchen, E.; Wouters, E.; van de Haterd, B.; Haidar, M.; Hardonnière, K.; Dierckx, T.; Van Broeckhoven, J.; Erens, C.; Hendrix, S.; Kerdine-Römer, S.; et al. CD36-Mediated Uptake of Myelin Debris by Macrophages and Microglia Reduces Neuroinflammation. J. Neuroinflammation 2020, 17, 224. [Google Scholar] [CrossRef]
- Robert, J.; Osto, E.; von Eckardstein, A. The Endothelium Is Both a Target and a Barrier of HDL’s Protective Functions. Cells 2021, 10, 1041. [Google Scholar] [CrossRef]
- Alarcón, R.; Fuenzalida, C.; Santibáñez, M.; von Bernhardi, R. Expression of Scavenger Receptors in Glial Cells: Comparing the Adhesion of Astrocytes and Microglia from Neonatal Rats to Surface-Bound β-Amyloid. J. Biol. Chem. 2005, 280, 30406–30415. [Google Scholar] [CrossRef]
- Thanopoulou, K.; Fragkouli, A.; Stylianopoulou, F.; Georgopoulos, S. Scavenger Receptor Class B Type I (SR-BI) Regulates Perivascular Macrophages and Modifies Amyloid Pathology in an Alzheimer Mouse Model. Proc. Natl. Acad. Sci. USA 2010, 107, 20816–20821. [Google Scholar] [CrossRef]
- Yu, L.; Dai, Y.; Mineo, C. Novel Functions of Endothelial Scavenger Receptor Class B Type I. Curr. Atheroscler. Rep. 2021, 23, 6. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship Between Amyloid-β Deposition and Blood–Brain Barrier Dysfunction in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef]
- Etzerodt, A.; Moestrup, S.K. CD163 and Inflammation: Biological, Diagnostic, and Therapeutic Aspects. Antioxid. Redox Signal. 2013, 18, 2352. [Google Scholar] [CrossRef]
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases. Int. J. Mol. Sci. 2020, 21, 5497. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Cheng, J.; Tang, Y. Brain Perivascular Macrophages: Current Understanding and Future Prospects. Brain 2024, 147, 39–55. [Google Scholar] [CrossRef]
- Fabriek, B.O.; van Bruggen, R.; Deng, D.M.; Ligtenberg, A.J.M.; Nazmi, K.; Schornagel, K.; Vloet, R.P.M.; Dijkstra, C.D.; van den Berg, T.K. The Macrophage Scavenger Receptor CD163 Functions as an Innate Immune Sensor for Bacteria. Blood 2009, 113, 887–892. [Google Scholar] [CrossRef]
- Cross, K.; Vetter, S.W.; Alam, Y.; Hasan, M.Z.; Nath, A.D.; Leclerc, E. Role of the Receptor for Advanced Glycation End Products (RAGE) and Its Ligands in Inflammatory Responses. Biomolecules 2024, 14, 1550. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in Inflammatory Diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Odum, J.; Shunnarah, J.G.; Austin, N.; Kaddoumi, A. Blood–Brain Barrier Breakdown in Alzheimer’s Disease: Mechanisms and Targeted Strategies. Int. J. Mol. Sci. 2023, 24, 16288. [Google Scholar] [CrossRef]
- Li, W.; Chen, Q.; Peng, C.; Yang, D.; Liu, S.; Lv, Y.; Jiang, L.; Xu, S.; Huang, L. Roles of the Receptor for Advanced Glycation End Products and Its Ligands in the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 403. [Google Scholar] [CrossRef]
- Du, H.; Li, P.; Wang, J.; Qing, X.; Li, W. The Interaction of Amyloid β and the Receptor for Advanced Glycation Endproducts Induces Matrix Metalloproteinase-2 Expression in Brain Endothelial Cells. Cell. Mol. Neurobiol. 2012, 32, 141–147. [Google Scholar] [CrossRef]
- Fang, F.; Lue, L.-F.; Yan, S.; Xu, H.; Luddy, J.S.; Chen, D.; Walker, D.G.; Stern, D.M.; Yan, S.; Schmidt, A.M.; et al. RAGE-Dependent Signaling in Microglia Contributes to Neuroinflammation, Abeta Accumulation, and Impaired Learning/Memory in a Mouse Model of Alzheimer’s Disease. FASEB J. 2010, 24, 1043–1055. [Google Scholar] [CrossRef]
- Chakraborty, A.; Sami, S.A.; Marma, K.K.S. A Comprehensive Review on RAGE-Facilitated Pathological Pathways Connecting Alzheimer’s Disease, Diabetes Mellitus, and Cardiovascular Diseases. Egypt. J. Intern. Med. 2021, 33, 47. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, C.; Zhou, Y.; Qi, J.; Zhang, C.; Sun, B.; Wang, J.; Guan, Y. Progress of RAGE Molecular Imaging in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, G.; Wu, J.; Tang, Z.; Luo, M. The Molecular Mechanism of LRP1 in Physiological Vascular Homeostasis and Signal Transduction Pathways. Biomed. Pharmacother. 2021, 139, 111667. [Google Scholar] [CrossRef]
- Na, H.; Yang, J.B.; Zhang, Z.; Gan, Q.; Tian, H.; Rajab, I.M.; Potempa, L.A.; Tao, Q.; Qiu, W.Q. Peripheral Apolipoprotein E Proteins and Their Binding to LRP1 Antagonize Alzheimer’s Disease Pathogenesis in the Brain during Peripheral Chronic Inflammation. Neurobiol. Aging 2023, 127, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Belaidi, A.A.; Bush, A.I.; Ayton, S. Apolipoprotein E in Alzheimer’s Disease: Molecular Insights and Therapeutic Opportunities. Mol. Neurodegener. 2025, 20, 47. [Google Scholar] [CrossRef]
- Raulin, A.-C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.-C. ApoE in Alzheimer’s Disease: Pathophysiology and Therapeutic Strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef]
- N’Songo, A.; Kanekiyo, T.; Bu, G. LRP1 Plays a Major Role in the Amyloid-β Clearance in Microglia. Mol. Neurodegener. 2013, 8, P33. [Google Scholar] [CrossRef][Green Version]
- He, Y.; Ruganzu, J.B.; Jin, H.; Peng, X.; Ji, S.; Ma, Y.; Zheng, L.; Yang, W. LRP1 Knockdown Aggravates Aβ1-42-Stimulated Microglial and Astrocytic Neuroinflammatory Responses by Modulating TLR4/NF-κB/MAPKs Signaling Pathways. Exp. Cell Res. 2020, 394, 112166. [Google Scholar] [CrossRef]
- Yang, L.; Liu, C.-C.; Zheng, H.; Kanekiyo, T.; Atagi, Y.; Jia, L.; Wang, D.; N’songo, A.; Can, D.; Xu, H.; et al. LRP1 Modulates the Microglial Immune Response via Regulation of JNK and NF-κB Signaling Pathways. J. Neuroinflammation 2016, 13, 304. [Google Scholar] [CrossRef]
- Tang, Y.; Chaillon, A.; Gianella, S.; Wong, L.M.; Li, D.; Simermeyer, T.L.; Porrachia, M.; Ignacio, C.; Woodworth, B.; Zhong, D.; et al. Brain Microglia Serve as a Persistent HIV Reservoir despite Durable Antiretroviral Therapy. J. Clin. Investig. 2023, 133, e167417. [Google Scholar] [CrossRef]
- Lazar, M.; Moroti, R.; Barbu, E.C.; Chitu-Tisu, C.E.; Tiliscan, C.; Erculescu, T.M.; Rosca, R.R.; Frasila, S.; Schmilevschi, E.T.; Simion, V.; et al. The Impact of HIV on Early Brain Aging—A Pathophysiological (Re)View. J. Clin. Med. 2024, 13, 7031. [Google Scholar] [CrossRef]
- Sil, S.; Periyasamy, P.; Thangaraj, A.; Niu, F.; Chemparathy, D.T.; Buch, S. Advances in the Experimental Models of HIV-Associated Neurological Disorders. Curr. HIV/AIDS Rep. 2021, 18, 459–474. [Google Scholar] [CrossRef]
- Williams, M.E.; Zulu, S.S.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. Signatures of HIV-1 Subtype B and C Tat Proteins and Their Effects in the Neuropathogenesis of HIV-Associated Neurocognitive Impairments. Neurobiol. Dis. 2020, 136, 104701. [Google Scholar] [CrossRef]
- Chen, X.; Hui, L.; Geiger, N.H.; Haughey, N.J.; Geiger, J.D. Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol. Aging 2013, 34, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoon, J.-H.; Kim, Y.-S. HIV-1 Tat Interacts with and Regulates the Localization and Processing of Amyloid Precursor Protein. PLoS ONE 2013, 8, e77972. [Google Scholar] [CrossRef] [PubMed]
- Hategan, A.; Masliah, E.; Nath, A. HIV and Alzheimer’s Disease: Complex Interactions of HIV-Tat with Amyloid β Peptide and Tau Protein. J. Neurovirol. 2019, 25, 648–660. [Google Scholar] [CrossRef]
- Kodidela, S.; Gerth, K.; Haque, S.; Gong, Y.; Ismael, S.; Singh, A.; Ishrat, T.; Kumar, S. Extracellular Vesicles: A Possible Link between HIV and Alzheimer’s Disease-Like Pathology in HIV Subjects? Cells 2019, 8, 968. [Google Scholar] [CrossRef]
- Fields, J.; Dumaop, W.; Elueteri, S.; Campos, S.; Serger, E.; Trejo, M.; Kosberg, K.; Adame, A.; Spencer, B.; Rockenstein, E.; et al. HIV-1 Tat Alters Neuronal Autophagy by Modulating Autophagosome Fusion to the Lysosome: Implications for HIV-Associated Neurocognitive Disorders. J. Neurosci. 2015, 35, 1921–1938, Erratum in J. Neurosci. 2015, 35, 8376. [Google Scholar] [CrossRef]
- Datta, G.; Miller, N.M.; Afghah, Z.; Geiger, J.D.; Chen, X. HIV-1 Gp120 Promotes Lysosomal Exocytosis in Human Schwann Cells. Front. Cell. Neurosci. 2019, 13, 329. [Google Scholar] [CrossRef]
- Halcrow, P.W.; Lakpa, K.L.; Khan, N.; Afghah, Z.; Miller, N.; Datta, G.; Chen, X.; Geiger, J.D. HIV-1 Gp120-Induced Endolysosome de-Acidification Leads to Efflux of Endolysosome Iron, and Increases in Mitochondrial Iron and Reactive Oxygen Species. J. Neuroimmune Pharmacol. 2022, 17, 181–194. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Fujinaga, K.; Gross, J.D.; Frankel, A.D. Enhanced NF-κB Activation via HIV-1 Tat-TRAF6 Cross-Talk. Sci. Adv. 2024, 10, eadi4162. [Google Scholar] [CrossRef]
- Muvenda, T.; Williams, A.A.; Williams, M.E. Transactivator of Transcription (Tat)-Induced Neuroinflammation as a Key Pathway in Neuronal Dysfunction: A Scoping Review. Mol. Neurobiol. 2024, 61, 9320–9346. [Google Scholar] [CrossRef] [PubMed]
- Griciuc, A.; Patel, S.; Federico, A.N.; Choi, S.H.; Innes, B.J.; Oram, M.K.; Cereghetti, G.; McGinty, D.; Anselmo, A.; Sadreyev, R.I.; et al. TREM2 Acts Downstream of CD33 in Modulating Microglial Pathology in Alzheimer’s Disease. Neuron 2019, 103, 820–835.e7. [Google Scholar] [CrossRef] [PubMed]
- Ditiatkovski, M.; Mukhamedova, N.; Dragoljevic, D.; Hoang, A.; Low, H.; Pushkarsky, T.; Fu, Y.; Carmichael, I.; Hill, A.F.; Murphy, A.J.; et al. Modification of Lipid Rafts by Extracellular Vesicles Carrying HIV-1 Protein Nef Induces Redistribution of Amyloid Precursor Protein and Tau, Causing Neuronal Dysfunction. J. Biol. Chem. 2020, 295, 13377–13392. [Google Scholar] [CrossRef]
- Wang, Y.; Santerre, M.; Tempera, I.; Martin, K.; Mukerjee, R.; Sawaya, B.E. HIV-1 Vpr Disrupts Mitochondria Axonal Transport and Accelerates Neuronal Aging. Neuropharmacology 2017, 117, 364–375. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Li, H.; Zhang, H.; Guo, H.; Zheng, X.; Yu, X.-F.; Wei, W. HIV-1 Vpu Induces Neurotoxicity by Promoting Caspase 3-Dependent Cleavage of TDP-43. EMBO Rep. 2024, 25, 4337–4357. [Google Scholar] [CrossRef]
- Borrajo, A.; Spuch, C.; Penedo, M.A.; Olivares, J.M.; Agís-Balboa, R.C. Important Role of Microglia in HIV-1 Associated Neurocognitive Disorders and the Molecular Pathways Implicated in Its Pathogenesis. Ann. Med. 2020, 53, 43–69. [Google Scholar] [CrossRef]
- Anderson, F.L.; Biggs, K.E.; Rankin, B.E.; Havrda, M.C. NLRP3 Inflammasome in Neurodegenerative Disease. Transl. Res. J. Lab. Clin. Med. 2023, 252, 21–33. [Google Scholar] [CrossRef]
- Chivero, E.T.; Guo, M.-L.; Periyasamy, P.; Liao, K.; Callen, S.E.; Buch, S. HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 3599–3609. [Google Scholar] [CrossRef]
- Rubin, L.H.; Sundermann, E.E.; Moore, D.J. The Current Understanding of Overlap between Characteristics of HIV-Associated Neurocognitive Disorders and Alzheimer’s Disease. J. Neurovirol. 2019, 25, 661–672. [Google Scholar] [CrossRef]
- Green, D.A.; Masliah, E.; Vinters, H.V.; Beizai, P.; Moore, D.J.; Achim, C.L. Brain Deposition of Beta-Amyloid Is a Common Pathologic Feature in HIV Positive Patients. AIDS 2005, 19, 407–411. [Google Scholar] [CrossRef]
- Turner, R.S.; Chadwick, M.; Horton, W.A.; Simon, G.L.; Jiang, X.; Esposito, G. An Individual with Human Immunodeficiency Virus, Dementia, and Central Nervous System Amyloid Deposition. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2016, 4, 1–5. [Google Scholar] [CrossRef]
- Schenck, J.K.; Karl, M.T.; Clarkson-Paredes, C.; Bastin, A.; Pushkarsky, T.; Brichacek, B.; Miller, R.H.; Bukrinsky, M.I. Extracellular Vesicles Produced by HIV-1 Nef-Expressing Cells Induce Myelin Impairment and Oligodendrocyte Damage in the Mouse Central Nervous System. J. Neuroinflammation 2024, 21, 127. [Google Scholar] [CrossRef]
- Gagliardi, S.; Hotchkin, T.; Hillmer, G.; Engelbride, M.; Diggs, A.; Tibebe, H.; Izumi, C.; Sullivan, C.; Cropp, C.; Lantz, O.; et al. Oxidative Stress in HIV-Associated Neurodegeneration: Mechanisms of Pathogenesis and Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 6724. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Wang, Y.; Du, W.; Yang, R.; Wu, J.; Li, Y. Lipid Droplet Accumulation in Microglia and Their Potential Roles. Lipids Health Dis. 2025, 24, 215, Erratum in Lipids Health Dis. 2025, 24, 247. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.L.; Grant, A.; Mukhamedova, N.; Pushkarsky, T.; Jennelle, L.; Dubrovsky, L.; Gaus, K.; Fitzgerald, M.L.; Sviridov, D.; Bukrinsky, M. HIV-1 Nef Mobilizes Lipid Rafts in Macrophages through a Pathway That Competes with ABCA1-Dependent Cholesterol Efflux. J. Lipid Res. 2012, 53, 696–708. [Google Scholar] [CrossRef]
- Kitayama, H.; Miura, Y.; Ando, Y.; Hoshino, S.; Ishizaka, Y.; Koyanagi, Y. Human Immunodeficiency Virus Type 1 Vpr Inhibits Axonal Outgrowth through Induction of Mitochondrial Dysfunction. J. Virol. 2008, 82, 2528–2542. [Google Scholar] [CrossRef]
- Williams, M.E.; Williams, A.A.; Naudé, P.J.W. Viral Protein R (Vpr)-Induced Neuroinflammation and Its Potential Contribution to Neuronal Dysfunction: A Scoping Review. BMC Infect. Dis. 2023, 23, 512. [Google Scholar] [CrossRef]
- Giunta, B.; Zhou, Y.; Hou, H.; Rrapo, E.; Fernandez, F.; Tan, J. HIV-1 TAT Inhibits Microglial Phagocytosis of Aβ Peptide. Int. J. Clin. Exp. Pathol. 2008, 1, 260–275. [Google Scholar]
- Bai, R.; Song, C.; Lv, S.; Chang, L.; Hua, W.; Weng, W.; Wu, H.; Dai, L. Role of Microglia in HIV-1 Infection. AIDS Res. Ther. 2023, 20, 16. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; Jiang, W.; Wu, X.; Ye, B.; Zhou, X. HIV-1 Tat Regulates Occludin and Aβ Transfer Receptor Expression in Brain Endothelial Cells via Rho/ROCK Signaling Pathway. Oxid. Med. Cell. Longev. 2016, 2016, 4196572. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, Y.; Yu, Y.; Wei, J.; Huang, W. Rho-Kinase Inhibitor Hydroxyfasudil Protects against HIV-1 Tat-Induced Dysfunction of Tight Junction and Neprilysin/Aβ Transfer Receptor Expression in Mouse Brain Microvessels. Mol. Cell. Biochem. 2021, 476, 2159–2170. [Google Scholar] [CrossRef]
- András, I.E.; Eum, S.Y.; Huang, W.; Zhong, Y.; Hennig, B.; Toborek, M. HIV-1-Induced Amyloid Beta Accumulation in Brain Endothelial Cells Is Attenuated by Simvastatin. Mol. Cell. Neurosci. 2010, 43, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Rempel, H.C.; Pulliam, L. HIV-1 Tat Inhibits Neprilysin and Elevates Amyloid Beta. AIDS 2005, 19, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Xu, J.; Kiyota, T.; Peng, H.; Zheng, J.C.; Ikezu, T. HIV-1 Reduces Aβ-Degrading Enzymatic Activities in Primary Human Mononuclear Phagocytes. J. Immunol. 2011, 186, 6925–6932. [Google Scholar] [CrossRef]
- Hategan, A.; Bianchet, M.A.; Steiner, J.; Karnaukhova, E.; Masliah, E.; Fields, A.; Lee, M.-H.; Dickens, A.M.; Haughey, N.; Dimitriadis, E.K.; et al. HIV-Tat Protein and Amyloid β Peptide Form Multifibrillar Structures That Cause Neurotoxicity. Nat. Struct. Mol. Biol. 2017, 24, 379–386. [Google Scholar] [CrossRef]
- Chang, N.P.; DaPrano, E.M.; Lindman, M.; Estevez, I.; Chou, T.-W.; Evans, W.R.; Nissenbaum, M.; McCourt, M.; Alzate, D.; Atkins, C.; et al. Neuronal DAMPs Exacerbate Neurodegeneration via Astrocytic RIPK3 Signaling. JCI Insight 2024, 9, e177002. [Google Scholar] [CrossRef]
- Kim, S.-M.; Mun, B.-R.; Lee, S.-J.; Joh, Y.; Lee, H.-Y.; Ji, K.-Y.; Choi, H.-R.; Lee, E.-H.; Kim, E.-M.; Jang, J.-H.; et al. TREM2 Promotes Aβ Phagocytosis by Upregulating C/EBPα-Dependent CD36 Expression in Microglia. Sci. Rep. 2017, 7, 11118. [Google Scholar] [CrossRef]
- Fields, J.A.; Spencer, B.; Swinton, M.; Qvale, E.M.; Marquine, M.J.; Alexeeva, A.; Gough, S.; Soontornniyomkij, B.; Valera, E.; Masliah, E.; et al. Alterations in Brain TREM2 and Amyloid-β Levels Are Associated with Neurocognitive Impairment in HIV-Infected Persons on Antiretroviral Therapy. J. Neurochem. 2018, 147, 784–802. [Google Scholar] [CrossRef]
- Avalos, B.; Kulbe, J.; Ford, M.; Laird, A.; Walter, K.; Mante, M.; Florio, J.; Boustani, A.; Chaillon, A.; Schlachetzki, J.; et al. HIV-Induced Modulation of TREM2 Expression Is Reversed by Cannabidiol: Implications for Age-Related Neuropathogenesis in People with HIV. Preprints 2024. [Google Scholar] [CrossRef]
- Jana, A.K.; Keskin, R.; Yaşar, F. Molecular Insight into the Effect of HIV-TAT Protein on Amyloid-β Peptides. ACS Omega 2024, 9, 27480–27491. [Google Scholar] [CrossRef]
- Lenahan, C.; Huang, L.; Travis, Z.D.; Zhang, J.H. Scavenger Receptor Class B Type 1 (SR-B1) and the Modifiable Risk Factors of Stroke. Chin. Neurosurg. J. 2019, 5, 30. [Google Scholar] [CrossRef]
- Li, B.; Chen, M.; Aguzzi, A.; Zhu, C. The Role of Macrophage Scavenger Receptor 1 (Msr1) in Prion Pathogenesis. J. Mol. Med. 2021, 99, 877–887. [Google Scholar] [CrossRef]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of Cerebral Aβ in Alzheimer’s Disease: Reassessing the Role of Microglia and Monocytes. Cell. Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Ansari, S.; Maurya, V.K.; Kumar, S.; Sharma, D.; Malhotra, H.S.; Tiwari, S.; Srivastava, C.; Paweska, J.T.; Abdel-Moneim, A.S.; et al. Neprilysin-Mediated Amyloid Beta Clearance and Its Therapeutic Implications in Neurodegenerative Disorders. ACS Pharmacol. Transl. Sci. 2024, 7, 3645–3657. [Google Scholar] [CrossRef]
- Miners, J.S.; Barua, N.; Kehoe, P.G.; Gill, S.; Love, S. Aβ-Degrading Enzymes: Potential for Treatment of Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2011, 70, 944–959. [Google Scholar] [CrossRef]
- Giunta, B.; Hou, H.; Zhu, Y.; Rrapo, E.; Tian, J.; Takashi, M.; Commins, D.; Singer, E.; He, J.; Fernandez, F.; et al. HIV-1 Tat Contributes to Alzheimer’s Disease-like Pathology in PSAPP Mice. Int. J. Clin. Exp. Pathol. 2009, 2, 433–443. [Google Scholar]
- Maguire, E.; Connor-Robson, N.; Shaw, B.; O’Donoghue, R.; Stöberl, N.; Hall-Roberts, H. Assaying Microglia Functions In Vitro. Cells 2022, 11, 3414. [Google Scholar] [CrossRef]
- Fukase, K.; Iida-Adachi, A.; Nabika, H. Spectral Heterogeneity of Thioflavin T Binding to Aβ42:Aβ40 Mixed Fibrils: Implications for Alzheimer’s Disease Screening. ACS Omega 2025, 10, 17043–17050. [Google Scholar] [CrossRef]
- Prakash, P.; Jethava, K.P.; Korte, N.; Izquierdo, P.; Favuzzi, E.; Rose, I.V.L.; Guttenplan, K.A.; Manchanda, P.; Dutta, S.; Rochet, J.-C.; et al. Monitoring Phagocytic Uptake of Amyloid β into Glial Cell Lysosomes in Real Time. Chem. Sci. 2021, 12, 10901–10918. [Google Scholar] [CrossRef]
- Wisch, J.K.; Gordon, B.A.; Boerwinkle, A.H.; Luckett, P.H.; Bollinger, J.G.; Ovod, V.; Li, Y.; Henson, R.L.; West, T.; Meyer, M.R.; et al. Predicting Continuous Amyloid PET Values with CSF and Plasma Aβ42/Aβ40. Alzheimers Dement. Amst. Neth. 2023, 15, e12405. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Gong, K.; Zhao, B.; Ning, Y.; Chen, M.; Li, Y.; Ali, M.; Timsina, J.; Liu, M.; et al. Cerebrospinal Fluid Proteomics Identification of Biomarkers for Amyloid and Tau PET Stages. Cell Rep. Med. 2025, 6, 102031. [Google Scholar] [CrossRef]
- Ellis, R.J.; Chenna, A.; Petropoulos, C.J.; Lie, Y.; Curanovic, D.; Crescini, M.; Winslow, J.; Sundermann, E.; Tang, B.; Letendre, S.L. Higher Cerebrospinal Fluid Biomarkers of Neuronal Injury in HIV-Associated Neurocognitive Impairment. J. Neurovirol. 2022, 28, 438–445. [Google Scholar] [CrossRef]
- Waite, L.M. New and Emerging Drug Therapies for Alzheimer Disease. Aust. Prescr. 2024, 47, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Vu, H.T.; Nguyen, H.T.; Nguyen, A.T. Effectiveness of Non-Pharmacological Interventions for Dementia among the Elderly: A Randomized Controlled Trial. Geriatrics 2024, 9, 52. [Google Scholar] [CrossRef]
- Elendu, C.; Aguocha, C.M.; Okeke, C.V.; Okoro, C.B.; Peterson, J.C. HIV-Related Neurocognitive Disorders: Diagnosis, Treatment, and Mental Health Implications: A Review. Medicine 2023, 102, e35652. [Google Scholar] [CrossRef]
- Zondo, S. The Cognitive Remediation of Attention in HIV-Associated Neurocognitive Disorders (HAND): A Meta-Analysis and Systematic Review. F1000Research 2023, 12, 1133. [Google Scholar] [CrossRef]
- Nightingale, S.; Ances, B.; Cinque, P.; Dravid, A.; Dreyer, A.J.; Gisslén, M.; Joska, J.A.; Kwasa, J.; Meyer, A.-C.; Mpongo, N.; et al. Cognitive Impairment in People Living with HIV: Consensus Recommendations for a New Approach. Nat. Rev. Neurol. 2023, 19, 424–433. [Google Scholar] [CrossRef]
- Jäntti, H.; Sitnikova, V.; Ishchenko, Y.; Shakirzyanova, A.; Giudice, L.; Ugidos, I.F.; Gómez-Budia, M.; Korvenlaita, N.; Ohtonen, S.; Belaya, I.; et al. Microglial Amyloid Beta Clearance Is Driven by PIEZO1 Channels. J. Neuroinflammation 2022, 19, 147. [Google Scholar] [CrossRef]
- Omeragic, A.; Kara-Yacoubian, N.; Kelschenbach, J.; Sahin, C.; Cummins, C.L.; Volsky, D.J.; Bendayan, R. Peroxisome Proliferator-Activated Receptor-Gamma Agonists Exhibit Anti-Inflammatory and Antiviral Effects in an EcoHIV Mouse Model. Sci. Rep. 2019, 9, 9428. [Google Scholar] [CrossRef] [PubMed]
- Potula, R.; Ramirez, S.H.; Knipe, B.; Leibhart, J.; Schall, K.; Heilman, D.; Morsey, B.; Mercer, A.; Papugani, A.; Dou, H.; et al. Peroxisome proliferator-activated receptor-gamma activation suppresses HIV-1 replication in an animal model of encephalitis. AIDS 2008, 22, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Meleady, L.; Towriss, M.; Kim, J.; Bacarac, V.; Dang, V.; Rowland, M.E.; Ciernia, A.V. Histone Deacetylase 3 Regulates Microglial Function through Histone Deacetylation. Epigenetics 2023, 18, 2241008. [Google Scholar] [CrossRef]
- Bhargavan, B.; Woollard, S.M.; McMillan, J.E.; Kanmogne, G.D. CCR5 Antagonist Reduces HIV-Induced Amyloidogenesis, Tau Pathology, Neurodegeneration, and Blood-Brain Barrier Alterations in HIV-Infected Hu-PBL-NSG Mice. Mol. Neurodegener. 2021, 16, 78. [Google Scholar] [CrossRef]
- Madhu, L.N.; Kodali, M.; Upadhya, R.; Rao, S.; Somayaji, Y.; Attaluri, S.; Shuai, B.; Kirmani, M.; Gupta, S.; Maness, N.; et al. Extracellular Vesicles from Human-induced Pluripotent Stem Cell-derived Neural Stem Cells Alleviate Proinflammatory Cascades within Disease-associated Microglia in Alzheimer’s Disease. J. Extracell. Vesicles 2024, 13, e12519. [Google Scholar] [CrossRef]
- Matt, S.M.; Nickoloff-Bybel, E.A.; Rong, Y.; Runner, K.; Johnson, H.; O’Connor, M.H.; Haddad, E.K.; Gaskill, P.J. Dopamine Levels Induced by Substance Abuse Alter Efficacy of Maraviroc and Expression of CCR5 Conformations on Myeloid Cells: Implications for NeuroHIV. Front. Immunol. 2021, 12, 663061. [Google Scholar] [CrossRef]
- Shikuma, C.M.; Wojna, V.; De Gruttola, V.; Siriwardhana, C.; Souza, S.A.; Rodríguez-Benítez, R.J.; Turner, E.H.; Kallianpur, K.; Bolzenius, J.; Chow, D.; et al. Impact of antiretroviral therapy intensification with C-C motif chemokine receptor 5 antagonist maraviroc on HIV-associated neurocognitive impairment. AIDS 2023, 37, 1987–1995. [Google Scholar] [CrossRef]
- Canet, G.; Dias, C.; Gabelle, A.; Simonin, Y.; Gosselet, F.; Marchi, N.; Makinson, A.; Tuaillon, E.; Van de Perre, P.; Givalois, L.; et al. HIV Neuroinfection and Alzheimer’s Disease: Similarities and Potential Links? Front. Cell. Neurosci. 2018, 12, 307. [Google Scholar] [CrossRef]
- Van Acker, Z.P.; Bretou, M.; Annaert, W. Endo-Lysosomal Dysregulations and Late-Onset Alzheimer’s Disease: Impact of Genetic Risk Factors. Mol. Neurodegener. 2019, 14, 20. [Google Scholar] [CrossRef]
- Filipello, F.; You, S.-F.; Mirfakhar, F.S.; Mahali, S.; Bollman, B.; Acquarone, M.; Korvatska, O.; Marsh, J.A.; Sivaraman, A.; Martinez, R.; et al. Defects in Lysosomal Function and Lipid Metabolism in Human Microglia Harboring a TREM2 Loss of Function Mutation. Acta Neuropathol. 2023, 145, 749–772. [Google Scholar] [CrossRef]
- Said, N.; Venketaraman, V. Neuroinflammation, Blood–Brain Barrier, and HIV Reservoirs in the CNS: An In-Depth Exploration of Latency Mechanisms and Emerging Therapeutic Strategies. Viruses 2025, 17, 572. [Google Scholar] [CrossRef]
| TLR | Aβ Role | Mechanism of Action | Human Evidence | Animal Evidence | References |
|---|---|---|---|---|---|
| TLR2 | Recognizes Aβ; skews microglia to inflammatory state | Interacts with CD14; activates MyD88–NF-κB pathway; ↓ phagocytosis, ↑ proinflammatory cytokines (TNF-α, IL-1β, IL-6) | Co-localized with CD14+ microglia near plaques in human AD tissues | TLR2–/– mice exhibit ↓ cytokines and ↑ Aβ clearance; anti-TLR2 antibody ↓ ROS in vitro | [142,143,145,147] |
| TLR4 | Recognition of fibrillar Aβ triggers inflammation and clearance | Binds Aβ via CD14–MD2 co-receptor complex; activates MyD88-dependent NF-κB and MAPK pathways ↑ TNF-α, IL-1β, ROS, iNOS | Upregulated in plaque-associated microglia in AD brains | TLR4–/– mice show impaired Aβ clearance, ↑ plaque burden, cognitive decline | [140,141,145] |
| TLR9 | Peripheral modulation of Aβ burden | Endosomal TLR; activated by CpG ODNs, enhances peripheral monocyte activation and recruitment into the CNS; does not engage Aβ directly. | Not upregulated in microglia in AD brains | Systemic CpG ODN injection ↓ cortical Aβ by 66% and improves memory in AD mice | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njoku, G.C.; Kanmogne, G.D. Microglial Dysfunction and Amyloid-Beta Pathology in Alzheimer’s Disease and HIV-Associated Neurocognitive Disorders. Int. J. Mol. Sci. 2025, 26, 9069. https://doi.org/10.3390/ijms26189069
Njoku GC, Kanmogne GD. Microglial Dysfunction and Amyloid-Beta Pathology in Alzheimer’s Disease and HIV-Associated Neurocognitive Disorders. International Journal of Molecular Sciences. 2025; 26(18):9069. https://doi.org/10.3390/ijms26189069
Chicago/Turabian StyleNjoku, George Chigozie, and Georgette Djuidje Kanmogne. 2025. "Microglial Dysfunction and Amyloid-Beta Pathology in Alzheimer’s Disease and HIV-Associated Neurocognitive Disorders" International Journal of Molecular Sciences 26, no. 18: 9069. https://doi.org/10.3390/ijms26189069
APA StyleNjoku, G. C., & Kanmogne, G. D. (2025). Microglial Dysfunction and Amyloid-Beta Pathology in Alzheimer’s Disease and HIV-Associated Neurocognitive Disorders. International Journal of Molecular Sciences, 26(18), 9069. https://doi.org/10.3390/ijms26189069







