Why Are Internal Mammary (Thoracic) Arteries Less Prone to Developing Atherosclerosis Compared to Coronary Arteries? Do Gut Microbiota Play a Role? A Narrative Review
Abstract
1. Introduction
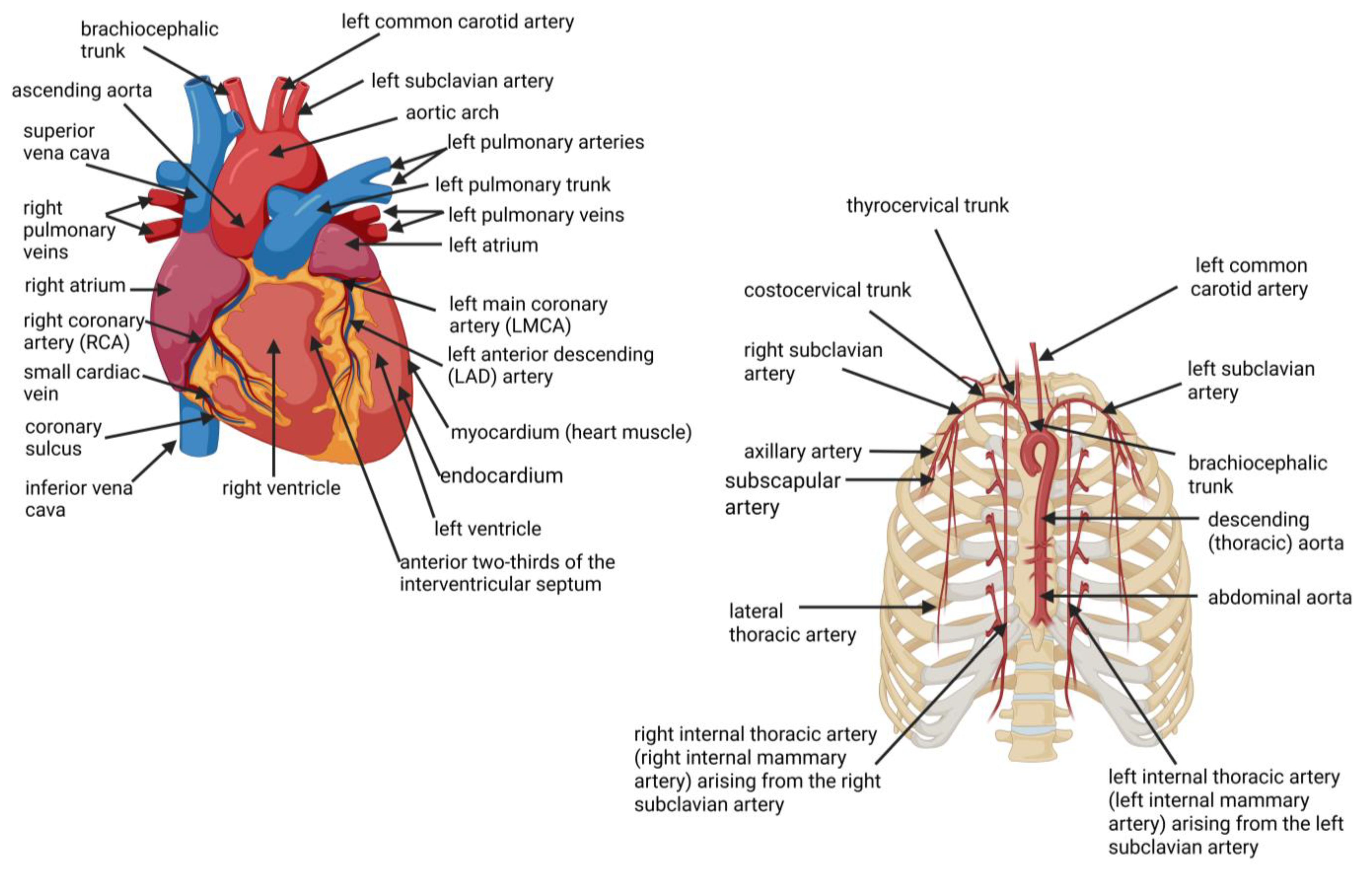
2. Location of Coronary Arteries and Internal Mammary Arteries (IMAs)
3. Anatomy of Coronary Arteries and Internal Mammary Arteries
4. Physiological Differences Between Coronary and Internal Mammary Arteries
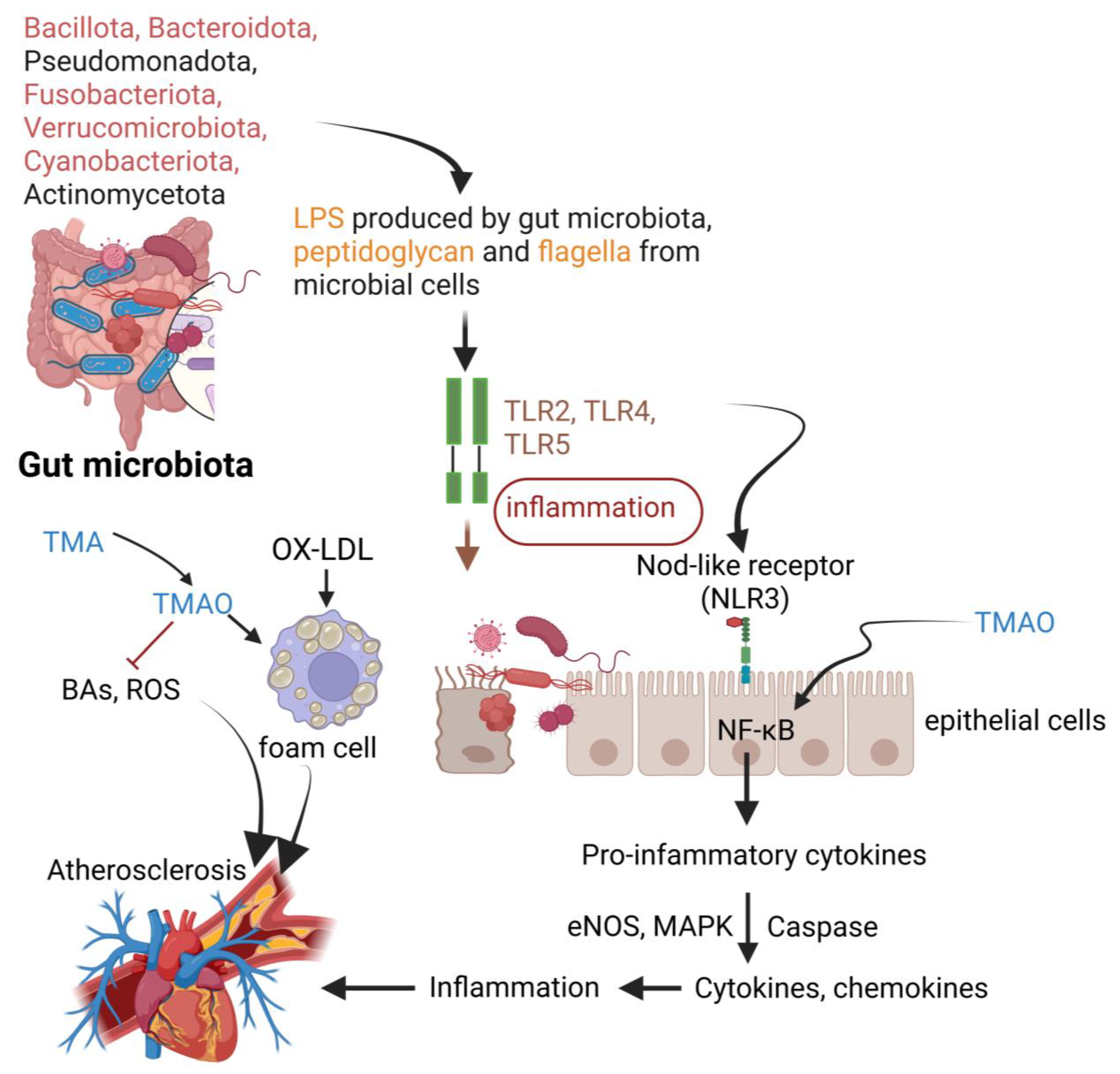
5. The Role of Gut Microbiota in Atherosclerosis (AS)
6. Aging Arteries and Changes in the Gut Microbiome Accelerates Atherosclerosis (AS)
7. Conclusions
Funding
Conflicts of Interest
References
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Williams, K.J.; Boren, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.D.; Arora, Y.; Mahajan, K. Anatomy, Blood Vessels. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470401/ (accessed on 1 August 2025).
- Holmstedt, C.A.; Turan, T.N.; Chimowitz, M.I. Atherosclerotic intracranial arterial stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol. 2013, 12, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.V.; Stancu, C.S.; Simionescu, M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009, 335, 191–203. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Tabas, I.; Garcia-Cardena, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Traghella, I.; Mastorci, F.; Alessia, P.; Pingitore, A.; Vassalle, C. Nontraditional cardiovascular biomarkers and risk factors: Rationale and future perspectives. Biomolecules 2018, 8, 40, Erratum in Biomolecules 2018, 8, E168. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoǧlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M. Circulating isoprostanes: Gatekeepers in the route from oxidative stress to vascular dysfunction. Circ. Res. 2008, 103, 907–909. [Google Scholar] [CrossRef]
- Björkegren, J.L.M.; Lusis, A. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Harber, K.J.; de Goede, K.E.; Verberk, S.G.S.; Meinster, E.; de Vries, H.E.; van Weeghel, M.; de Winther, M.P.J.; Van den Bossche, J. Succinate is an inflammation-induced immunoregulatory metabolite in macrophages. Metabolites 2020, 10, 372. [Google Scholar] [CrossRef]
- Zecchini, V.; Paupe, V.; Herranz-Montoya, I.; Janssen, J.; Wortel, I.M.N.; Morris, J.L.; Ferguson, A.; Chowdury, S.R.; Segarra-Mondejar, M.; Costa, A.S.H.; et al. Fumarate induces vesicular release of mtDNA to drive innate immunity. Nature 2023, 615, 499–506. [Google Scholar] [CrossRef]
- Ye, J.; Keller, J.N. Regulation of energy metabolism by inflammation: A feedback response in obesity and calorie restriction. Aging 2010, 2, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Dąbek, J.; Kułach, A.; Gąsior, Z. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB): A new potential therapeutic target in atherosclerosis. Pharmacol. Rep. 2010, 62, 778–783. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Sukhorukov, V.N.; Kalmykov, V.A.; Grechko, A.V.; Shakhpazyan, N.K.; Orekhov, A.N. The role of KLF2 768 in the regulation of atherosclerosis development and potential use of KLF2-targeted therapy. Biomedicines 2022, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Guo, L.; Zhang, X.; Yu, Q.; Yang, Q.; Zhang, Y.; Zeng, W.; Jiang, X.; Guo, M. Anti-inflammatory therapy of atherosclerosis: Focusing on IKKβ. J. Inflamm. 2023, 20, 8. [Google Scholar] [CrossRef]
- Wilson, S.H.; Caplice, N.M.; Simari, R.D.; Holmes Jr., D.R.; Carlson, P.J.; Lerman, A. Activated nuclear factor-kappaB is present in the coronary vasculature in experimental hypercholesterolemia. Atherosclerosis 2000, 148, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Cardiovascular disease may be triggered by gut microbiota, microbial metabolites, gut wall reactions, and inflammation. Int. J. Mol. Sci. 2024, 25, 10634. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Ding, L.; Chang, M.; Guo, Y.; Zhang, L.; Xue, C.; Yanagita, T.; Zhang, T.; Wang, Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018, 17, 286. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L.; Febbraio, M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef] [PubMed]
- Tandon, N.N.; Kralisz, U.; Jamieson, G.A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J. Biol. Chem. 1989, 264, 7576–7583. [Google Scholar] [CrossRef]
- Feingold, K.R. Introduction to lipids and lipoproteins. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000; [Updated 14 January 2024]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK305896/ (accessed on 1 August 2025).
- Almeida, C.; Barata, P.; Fernandes, R. The influence of gut microbiota in cardiovascular diseases—A brief review. Porto Biomed. J. 2021, 6, e106. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, P.G. Desialylation in physiological and pathological processes: New target for diagnostic and therapeutic development. Prog. Mol. Biol. Transl. Sci. 2019, 162, 25–57. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate byproduct and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides- dominated gut microbiota. Sci. Rep. 2017, 7, 2597. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean diet as an antioxidant: The impact on metabolic health and overall wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y.; et al. American gut: An open platform for citizen science microbiome research. mSystems 2018, 3, 744. [Google Scholar] [CrossRef] [PubMed]
- Amiri, P.; Hosseini, S.A.; Ghaffari, S.; Tutunchi, H.; Ghaffari, S.; Mosharkesh, E.; Asghari, S.; Roshanravan, N. Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: A comprehensive narrative review. Front. Pharmacol. 2022, 12, 837509. [Google Scholar] [CrossRef] [PubMed]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef]
- Ktsoyan, Z.A.; Mkrtchyan, M.S.; Zakharyan, M.K.; Mnatsakanyan, A.A.; Arakelova, K.A.; Gevorgyan, Z.U.; Sedrakyan, A.M.; Hovhannisyan, A.I.; Arakelyan, A.A.; Aminov, R.I. Systemic concentrations of short chain fatty acids are elevated in salmonellosis and exacerbation of familial Mediterranean fever. Front. Microbiol. 2016, 7, 776. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Y.; Huang, R.; Wang, F.; Xie, J.; Liu, H.; Wang, Y.; Wang, Y.; Luo, S.; Hu, D. The role of short-chain fatty acids in myocardial ischemia-reperfusion injury. Curr. Nutr. Rep. 2024, 13, 701–708. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Z.; Huang, W.; Chen, X.; Shan, P.; Zhong, P.; Khan, Z.; Wang, J.; Fang, Q.; Liang, G.; et al. Inhibition of epidermal growth factor receptor attenuates atherosclerosis via decreasing inflammation and oxidative stress. Sci. Rep. 2017, 8, 45917. [Google Scholar] [CrossRef]
- Zou, Z.G.; Rios, F.J.; Neves, K.B.; Alves-Lopes, R.; Ling, J.; Baillie, G.S.; Gao, X.; Fuller, W.; Camargo, L.L.; Gudermann, T.; et al. Epidermal growth factor signaling through transient receptor potential melastatin cation channel regulates vascular smooth muscle cell function. Clin. Sci. 2020, 134, 2019–2035. [Google Scholar] [CrossRef]
- Wang, T.; Baron, M.; Trump, D. An overview of Notch3 function in vascular smooth muscle cells. Prog. Biophys. Mol. Biol. 2008, 96, 499–509. [Google Scholar] [CrossRef]
- Davis-Knowlton, J.; Turner, J.E.; Turner, A.; Damian-Loring, S.; Hagler, N.; Henderson, T.; Emery, I.F.; Bond, K.; Duarte, C.W.; Vary, C.P.H.; et al. Characterization of smooth muscle cells from human atherosclerotic lesions and their responses to Notch signaling. Lab. Investig. 2019, 99, 290–304. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Sig. Transduct. Target Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Scirpo, R.; Fiorotto, R.; Villani, A.; Amenduni, M.; Spirli, C.; Strazzabosco, M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 2015, 62, 1551–1562. [Google Scholar] [CrossRef]
- Hou, Y.; Moreau, F.; Chadee, K. PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nat. Commun. 2012, 3, 1300. [Google Scholar] [CrossRef]
- Stump, M.; Mukohda, M.; Hu, C.; Sigmund, C.D. PPARγ regulation in hypertension and metabolic syndrome. Curr. Hypertens. Rep. 2015, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Campbell, J.I.; King, T.P.; Grant, G.; Jansson, E.A.; Coutts, A.G.P.; Pettersson, S.; Conway, S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat. Immunol. 2004, 5, 104–112. [Google Scholar] [CrossRef]
- Aguilar, E.C.; Da Silva, J.F.; Navia-Pelaez, J.M.; Leonel, A.J.; Lopes, L.G.; Menezes-Garcia, Z.; Ferreira, A.V.M.; Capettini, L.d.S.A.; Teixeira, L.G.; Lemos, V.S.; et al. Sodium butyrate modulates adipocyte expansion, adipogenesis, and insulin receptor signaling by upregulation of PPAR-γ in obese Apo E knockout mice. Nutrition 2018, 47, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Kang, J.; Liu, S.; Xu, Y.; Shao, B. The protective effects of peroxisome proliferator-activated receptor gamma in cerebral ischemia-reperfusion injury. Front. Neurol. 2020, 11, 588516. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, E.-J.; Lee, J.-C.; Kim, W.-K.; Kim, H.-S. Anti-inflammatory effects of short chain fatty acids in IFN-γ-stimulated RAW 264.7 murine macrophage cells: Involvement of NF-κB and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef]
- Aguilar, E.C.; Leonel, V.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.N.; Capettini, L.S.A.; Lemos, V.S.; Santos, R.A.S.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef] [PubMed]
- He, G.-W. Arterial grafts for coronary artery bypass grafting: Biological characteristics, functional classification, and clinical choice. Ann. Thorac. Surg. 1999, 67, 277–284. [Google Scholar] [CrossRef]
- Goetz, R.H.; Rohman, M.; Haller, J.D.; Dee, R.; Rosenak, S.S. Internal mammary-coronary artery anastomosis. A nonsuture method employing tantalum rings. J. Thorac. Cardiovasc. Surg. 1961, 41, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, R.G. Bilateral internal mammary artery implants. Operative technique. A preliminary report. Clev. Clin. Quart. 1967, 34, 61. [Google Scholar] [CrossRef] [PubMed]
- Squiers, J.J.; Mack, M.J. Coronary artery bypass grafting—Fifty years of quality initiatives since Favaloro. Ann. Cardiothorac. Surg. 2018, 7, 516–520. [Google Scholar] [CrossRef]
- Khot, U.N.; Friedman, D.T.; Pettersson, G.; Smedira, N.G.; Li, J.; Ellis, S.G. Radial artery bypass grafts have an increased occurrence of angiographically severe stenosis and occlusion compared with left internal mammary arteries and saphenous vein grafts. Circulation. 2004, 109, 2086–2091. [Google Scholar] [CrossRef]
- Etienne, P.Y.; D’Hoore, W.; Papadatos, S.; Mairy, Y.; El Khoury, G.; Noirhomme, P.; Hanet, C.; Glineur, D. Five-year follow-up of drug-eluting stents implantation vs minimally invasive direct coronary artery bypass for left anterior descending artery disease: A propensity score analysis. Eur. J. Cardiothorac. Surg. 2013, 44, 884–890. [Google Scholar] [CrossRef]
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J.; et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of Veterans Affairs Cooperative Study. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef]
- Kitamura, S.; Seki, T.; Kawachi, K.; Morita, R.; Kawata, T.; Mizuguchi, K.; Kobayashi, S.; Fukutomi, M.; Nishii, T.; Kobayashi, H.; et al. Excellent patency and growth potential of internal mammary artery grafts in pediatric coronary artery bypass surgery. New evidence for a “live” conduit. Circulation 1988, 78 Pt 2, I129–I139. [Google Scholar]
- Ahmed, I.; Yandrapalli, S. Internal mammary artery bypass. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; [Updated 30 July 2023]. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK560835/ (accessed on 1 August 2025).
- Shahoud, J.S.; Kerndt, C.C.; Burns, B. Anatomy, thorax, internal mammary (internal thoracic) arteries. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537337/ (accessed on 1 August 2025).
- Uflacker, A.; Guimaraes, M. Uflacker’s Atlas of Vascular Anatomy: An Angiographic Approach, 3rd ed.; Lippincott Williams & Wilkins (LWW): Ambler, PA, USA, 2020. [Google Scholar]
- Otsuka, F.; Yahagi, K.; Sakakura, K.; Virmani, R. Why is the mammary artery so special and what protects it from atherosclerosis? Ann. Cardiothorac. Surg. 2013, 2, 519–526. [Google Scholar] [PubMed]
- Skålén, K.; Gustafsson, M.; Rydberg, E.K.; Hultén, L.M.; Wiklund, O.; Innerarity, T.L.; Borén, J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002, 417, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, G.; Di Taranto, M.D.; Bracale, U.M.; Del Guercio, L.; Carbone, F.; Mazzaccara, C.; Morgante, A.; D’Armiento, F.P.; D’Armiento, M.; Porcellini, M.; et al. Decreased paraoxonase-2 expression in human carotids during the progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 594–600. [Google Scholar] [CrossRef]
- van Son, J.A.; Smedts, F.; de Wilde, P.C.; Pijls, N.H.; Wong-Alcala, L.; Kubat, K.; Tavilla, G.; Lacquet, L.K. Histological study of the internal mammary artery with emphasis on its suitability as a coronary artery bypass graft. Ann. Thorac. Surg. 1993, 55, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Talusan, P.; Bedri, S.; Yang, S.; Kattapuram, T.; Silva, N.; Roughley, P.J.; Stone, J.R. Analysis of intimal proteoglycans in atherosclerosis-prone and atherosclerosis-resistant human arteries by mass spectrometry. Mol. Cell. Proteom. 2005, 4, 1350–1357. [Google Scholar] [CrossRef]
- Svendsen, E.; Dregelid, E.; Eide, G.E. Internal elastic membrane in the internal mammary and left anterior descending coronary arteries and its relationship to intimal thickening. Atherosclerosis 1990, 83, 239–248. [Google Scholar] [CrossRef]
- Shimizu, S.; Hiroi, T.; Ishii, M.; Hagiwara, T.; Wajima, T.; Miyazaki, A.; Kiuchi, Y. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through activation of the Jak2 tyrosine kinase pathway in vascular endothelial cells. Int. J. Biochem. Cell Biol. 2008, 40, 755–765. [Google Scholar] [CrossRef]
- Pan, S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid. Redox Signal. 2009, 11, 1669–1682. [Google Scholar] [CrossRef]
- Kraler, S.; Libby, P.; Evans, P.C.; Akhmedov, A.; Schmiady, M.O.; Reinehr, M.; Camici, G.G.; Lüscher, T.F. Resilience of the internal mammary artery to atherogenesis: Shifting from risk to resistance to address unmet needs. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2237–2251. [Google Scholar] [CrossRef]
- Foglieni, C.; Maisano, F.; Dreas, L.; Giazzon, A.; Ruotolo, G.; Ferrero, E.; Li Volsi, L.; Coli, S.; Sinagra, G.; Zingone, B.; et al. Mild inflammatory activation of mammary arteries in patients with acute coronary syndromes. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2831–H2837. [Google Scholar] [CrossRef]
- Khattib, A.; Musa, S.; Halabi, M.; Hayek, T.; Khatib, S. Lyso-DGTS lipid derivatives enhance PON1 activities and prevent oxidation of LDL: A structure-activity relationship study. Antioxidants 2022, 11, 2058. [Google Scholar] [CrossRef]
- Mulligan-Kehoe, M.J.; Simons, M. Vasa vasorum in normal and diseased arteries. Circulation 2014, 129, 2557–2566. [Google Scholar] [CrossRef]
- Clancy, R.M.; Leszczynska-Piziak, J.; Abramson, S.B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J. Clin. Investig. 1992, 90, 1116–1121. [Google Scholar] [CrossRef]
- Gopal, D.; Singh, N.G.; Jagadeesh, A.M.; Ture, A.; Thimmarayappa, A. Comparison of left internal mammary artery diameter before and after left stellate ganglion block. Ann Card. Anaesth. 2013, 16, 238–242. [Google Scholar] [CrossRef]
- Cuminetti, G.; Gelsomino, S.; Curello, S.; Lorusso, R.; Maessen, J.G.; Hoorntje, J.C. Contemporary use of arterial and venous conduits in coronary artery bypass grafting: Anatomical, functional and clinical aspects. Neth. Heart J. 2017, 25, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S. Nitric oxide deficiency is a primary driver of hypertension. Biochem. Pharmacol. 2022, 206, 115325. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Qin, L.; Baeyens, N. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Investig. 2015, 125, 4514–4528. [Google Scholar] [CrossRef]
- Bouly, M.; Bourguignon, M.P.; Roesch, S.; Rigouin, P.; Gosgnach, W.; Bossard, E.; Royere, E.; Diguet, N.; Sansilvestri-Morel, P.; Bonnin, A.; et al. Aging increases circulating BH2 without modifying BH4 levels and impairs peripheral vascular function in healthy adults. Transl. Res. 2021, 238, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef] [PubMed]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Mügge, A.; Barton, M.R.; Cremer, J.; Frombach, R.; Lichtlen, P.R. Different vascular reactivity of human internal mammary and inferior epigastric arteries in vitro. Ann. Thorac. Surg. 1993, 56, 1085–1089. [Google Scholar] [CrossRef]
- Lau, K.E.; Lui, F. Physiology, Prostaglandin I2. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; p. 637. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562273/ (accessed on 1 August 2025).
- Ancion, A.; Tridetti, J.; Nguyen Trung, M.L.; Oury, C.; Lancellotti, P. A Review of the role of bradykinin and nitric oxide in the cardioprotective action of angiotensin-converting enzyme inhibitors: Focus on Perindopril. Cardiol. Ther. 2019, 8, 179–191. [Google Scholar] [CrossRef]
- Sutherland, A.J.; Nataatmadja, M.I.; Walker, P.J.; Cuttle, L.; Garlick, R.B.; West, M.J. Vascular remodeling in the internal mammary artery graft and association with in situ endothelin-1 and receptor expression. Circulation 2006, 113, 1180–1188. [Google Scholar] [CrossRef][Green Version]
- Shao, H.; Scott, S.G.; Nakata, C.; Hamad, A.R.; Chakravarti, S. Extracellular matrix protein lumican promotes clearance and resolution of Pseudomonas aeruginosa keratitis in a mouse model. PLoS ONE 2013, 8, e54765. [Google Scholar] [CrossRef]
- Vij, N.; Roberts, L.; Joyce, S.; Chakravarti, S. Lumican suppresses cell proliferation and aids Fas-Fas ligand-mediated apoptosis: Implications in the cornea. Exp. Eye Res. 2004, 78, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Kay, H.R.; Korns, M.E.; Flemma, R.J.; Tector, A.J.; Lepley, D., Jr. Atherosclerosis of the internal mammary artery. Ann. Thorac. Surg. 1976, 21, 504–507. [Google Scholar] [CrossRef]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. SR-B1: A unique multifunctional receptor for cholesterol influx and efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Wirka, R.C.; Wagh, D.; Paik, D.T.; Pjanic, M.; Nguyen, T.; Miller, C.L.; Kundu, R.; Nagao, M.; Coller, J.; Koyano, T.K.; et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 2019, 25, 1280–1289. [Google Scholar] [CrossRef]
- Yang, Z.; Oemar, B.S.; Carrel, T.; Kipfer, B.; Julmy, F.; Lüscher, T.F. Different proliferative properties of smooth muscle cells of human arterial and venous bypass vessels: Role of PDGF receptors, mitogen-activated protein kinase, and cyclin-dependent kinase inhibitors. Circulation 1998, 97, 181–187. [Google Scholar] [CrossRef]
- Yang, Z.; Ruschitzka, F.; Rabelink, T.J.; Noll, G.; Julmy, F.; Joch, H.; Gafner, V.; Aleksic, I.; Althaus, U.; Lüscher, T.F. Different effects of thrombin receptor activation on endothelium and smooth muscle cells of human coronary bypass vessels. Implications for venous bypass graft failure. Circulation 1997, 95, 1870–1876. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef]
- Mahadevan, V.S.; Campbell, M.; McKeown, P.P.; Bayraktutan, U. Internal mammary artery smooth muscle cells resist migration and possess high antioxidant capacity. Cardiovasc. Res. 2006, 72, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Jawien, A.; Bowen-Pope, D.F.; Lindner, V.; Schwartz, S.M.; Clowes, A.W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J. Clin. Investig. 1992, 89, 507–511. [Google Scholar] [CrossRef]
- Mendivil, C.O.; Zheng, C.; Furtado, J.; Lel, J.; Sacks, F.M. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler. Thromb. Vasc. Biol. 2020, 30, 239–245, Erratum in Arterioscler. Thromb. Vasc. Biol. 2010, 30, e171. [Google Scholar] [CrossRef] [PubMed]
- Ruengsakulrach, P.; Sinclair, R.; Komeda, M.; Raman, J.; Gordon, I.; Buxton, B. Comparative histopathology of radial artery versus internal thoracic artery and risk factors for development of intimal hyperplasia and atherosclerosis. Circulation. 1999, 100 (Suppl. 19), II139–II144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devarajan, A.; Bourquard, N.; Hama, S.; Navab, M.; Grijalva, V.R.; Morvardi, S.; Clarke, C.F.; Vergnes, L.; Reue, K.; Teiber, J.F.; et al. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxid. Redox Signal. 2011, 14, 341–351. [Google Scholar] [CrossRef]
- Shih, D.M.; Lusis, A.J. The roles of PON1 and PON2 in cardiovascular disease and innate immunity. Curr. Opin. Lipidol. 2009, 20, 288–292. [Google Scholar] [CrossRef]
- Altenhöfer, S.; Witte, I.; Teiber, J.F.; Wilgenbus, P.; Pautz, A.; Li, H.; Daiber, A.; Witan, H.; Clement, A.M.; Förstermann, U.; et al. One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J. Biol. Chem. 2010, 285, 24398–24403. [Google Scholar] [CrossRef]
- Wagner, C.; Zimmermann, S.; Brenner-Weiss, G.; Hug, F.; Prior, B.; Obst, U.; Hänsch, G.M. The quorum-sensing molecule N-3-oxododecanoyl homoserine lactone (3OC12-HSL) enhances the host defence by activating human polymorphonuclear neutrophils (PMN). Anal. Bioanal. Chem. 2007, 387, 481–487. [Google Scholar] [CrossRef]
- Trøseid, M.; Nestvold, T.K.; Rudi, K.; Thoresen, H.; Nielsen, E.W.; Lappegård, K.T. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity. Evidence from bariatric surgery. Diabetes Care 2013, 36, 3627–3632. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Zhang, Q.; He, C.; Fu, C.; Wei, Q. The role of the gut microbiota in health and cardiovascular diseases. Mol. Biomed. 2022, 3, 30. [Google Scholar] [CrossRef]
- An, H.; Qian, C.; Cao, X. Regulation of Toll-like receptor signaling in the innate immunity. Sci. China Life Sci. 2010, 53, 34–43. [Google Scholar] [CrossRef]
- Liu, H.-Q.; Zhang, X.-Y.; Edfeldt, K.; Nijhuis, M.O.; Idborg, H.; Bäck, M.; Roy, J.; Hedin, U.; Jakobsson, P.-J.; Laman, J.D.; et al. NOD2-mediated innate immune signaling regulates the eicosanoids in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.; Gatheral, T. Therapeutic targeting of NOD1 receptors. Br. J. Pharmacol. 2013, 170, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, S.; Goepp, M.; Pollock, J.; Robb, C.T.; Smyth, D.J.; Zhou, Y.; Andrews, R.; Tyrrell, V.; Gkikas, K.; Adima, A.; et al. Prostaglandin E2 promotes intestinal inflammation via inhibiting microbiota-dependent regulatory T cells. Sci. Adv. 2021, 7, eabd7954. [Google Scholar] [CrossRef]
- Santaolalla, R.; Fukata, M.; Abreu, M.T. Innate immunity in the small intestine. Curr. Opin. Gastroenterol. 2011, 27, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Izhar, U.; Hasdai, D.; Richardson, D.M.; Cohen, P.; Lerman, A. Insulin and insulin-like growth factor-I cause vasorelaxation in human vessels in vitro. Coron. Artery Dis. 2000, 11, 69–76. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Liu, Y.; Xiao, N.; Suo, H.; Xie, K.; Yang, C.; Wu, C. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation 2012, 35, 1676–1684. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Cheng, D.; Xu, J.-H.; Li, J.-Y.; Wang, S.-Y.; Wu, T.-F.; Chen, Q.-K.; Yu, T. Butyrate ameliorated-NLRC3 protects the intestinal barrier in a GPR43-dependent manner. Exp. Cell Res. 2018, 368, 101–110. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Jafarabadi, M.A.; Hedayati, M.; Ghavami, A.; Alipour, S.; Alamdari, N.M.; Barati, M.; Ostadrahimi, A. Effect of butyrate and inulin supplementation on glycemic status, lipid profile and glucagon-like peptide 1 level in patients with type 2 diabetes: A randomized double-blind, placebo-controlled trial. Horm. Metab. Res. 2017, 49, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 2016, 68, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, X.; Su, C.; Xi, M.; Zhang, X.; Jiang, Z.; Wang, L.; Hong, B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br. J. Pharmacol. 2020, 177, 1754–1772. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Yang, M.; Zhang, M.; Xiao, M.; Li, X. Butyrate mitigates TNF-a-induced attachment of monocytes to endothelial cells. J. Bioenerg. Biomembr. 2020, 52, 247–256. [Google Scholar] [CrossRef]
- Guizoni, D.M.; Vettorazzi, J.F.; Carneiro, E.M.; Davel, A.P. Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide 2020, 94, 48–53. [Google Scholar] [CrossRef]
- Pols, T.W. TGR5 in inflammation and cardiovascular disease. Biochem. Soc. Trans. 2014, 42, 244–249. [Google Scholar] [CrossRef]
- Pols, T.W.; Nomura, M.; Harach, T.; Sasso, G.L.; Oosterveer, M.H.; Thomas, C.; Rizzo, G.; Gioiello, A.; Adorini, L.; Pellicciari, R.; et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011, 14, 747–757. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Ma, G.; Pan, B.; Chen, Y.; Guo, C.; Zhao, M.; Zheng, L.; Chen, B. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37, BSR20160244. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Butyrate produced by gut microbiota regulates atherosclerosis: A narrative review of the latest findings. Int. J. Mol. Sci. 2025, 26, 6744. [Google Scholar] [CrossRef]
- Jeffery, C.J. Intracellular/surface moonlighting proteins that aid in the attachment of gut microbiota to the host. AIMS Microbiol. 2019, 5, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, A.; Cetrullo, D.; Polishuck, R.; Alberta, M.M.; Calafiore, A.; Pellegrini, G.; Vitacolonna, E.; Capani, F.; Consoli, A. Plasminogen activator inhibitor type 1 is increased in the arterial wall of type II diabetic subjects. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1378–1382. [Google Scholar] [CrossRef]
- Candela, M.; Miccoli, G.; Bergmann, S.; Turroni, S.; Vitali, B.; Hammerschmidt, S.; Brigidi, P. Plasminogen-dependent proteolytic activity in Bifidobacterium lactis. Microbiology 2008, 154, 2457–2462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Satala, D.; Bednarek, A.; Kozik, A.; Rapala-Kozik, M.; Karkowska-Kuleta, J. The recruitment and activation of plasminogen by bacteria—The involvement in chronic infection development. Int. J. Mol. Sci. 2023, 24, 10436. [Google Scholar] [CrossRef]
- Candela, M.; Bergmann, S.; Vici, M.; Vitali, B.; Turroni, S.; Eikmanns, B.J.; Hammerschmidt, S.; Brigidi, P. Binding of human plasminogen to Bifidobacterium. J. Bacteriol. 2007, 189, 5929–5936. [Google Scholar] [CrossRef]
- Hamsten, A.; Wiman, B.; De Faire, U.; Blombäck, M. Increased plasma levels of a rapid inhibitor of tissue plasminogen activator in young survivors of myocardial infarction. N. Engl. J. Med. 1985, 313, 1557–1563. [Google Scholar] [CrossRef]
- Tofler, G.; Massaro, J.; O’Donnell, C.; Wilson, P.; Vasan, R.; Sutherland, P.; Meigs, J.; Levy, D.; D’Agostino, R. Plasminogen activator inhibitor and the risk of cardiovascular disease: The Framingham Heart Study. Thromb. Res. 2016, 140, 30–35. [Google Scholar] [CrossRef]
- Jung, R.G.; Motazedian, P.; Ramirez, F.D.; Simard, T.; Di Santo, P.; Visintini, S.; Faraz, M.A.; Labinaz, A.; Jung, Y.; Hibbert, B. Association between plasminogen activator inhibitor-1 and cardiovascular events: A systematic review and meta-analysis. Thromb. J. 2018, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Burgess, S.; Eicher, J.D.; O’Donnell, C.J.; Johnson, A.D.; Huang, J.; Sabater-Lleal, M.; Asselbergs, F.W.; Tregouet, D.; Shin, S.; et al. Causal effect of plasminogen activator inhibitor type 1 on coronary heart disease. J. Am. Heart Assoc. 2017, 6, e004918. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, M.E.; Lisman, T.; De Groot, P.G.; Meijers, J.C.M.; Le Cessie, S.; Doggen, C.J.M.; Rosendaal, F.R. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood 2010, 116, 113–121. [Google Scholar] [CrossRef]
- Chomiki, N.; Henry, M.; Alessi, M.C.; Anfosso, F.; Juhan-Vague, I. Plasminogen activator inhibitor-1 expression in human liver and healthy or atherosclerotic vessel walls. Thromb. Haemost. 1994, 72, 44–53. [Google Scholar] [CrossRef]
- Rath, E.; Moschetta, A.; Haller, D. Mitochondrial function—Gatekeeper of intestinal epithelial cell homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 158, 497–516. [Google Scholar] [CrossRef]
- Tang, W.W.; Wang, Z.; Shrestha, K.; Borowski, A.G.; Wu, Y.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Intestinal microbiota dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card. Fail. 2015, 21, 91–96. [Google Scholar] [CrossRef]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Effects of Cytokines (or Activating Factors) on Arterial Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 8142. [Google Scholar] [CrossRef] [PubMed]
- Auguet, T.; Aragonès, G.; Guiu-Jurado, E.; Berlanga, A.; Curriu, M.; Martinez, S.; Alibalic, A.; Aguilar, C.; Camara, M.-L.; Hernández, E.; et al. Adipo/cytokines in atherosclerotic secretomes: Increased visfatin levels in unstable carotid plaque. BMC Cardiovasc. Disord 2016, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Wijdeveld, M.; Wortelboer, K.; Rampanelli, E.; Levels, J.H.M.; Collard, D.; Cammenga, M.; Nageswaran, V.; Haghikia, A.; Landmesser, U.; et al. Effects of oral butyrate on blood pressure in patients with hypertension: A randomized, placebo-controlled trial. Hypertension 2024, 81, 2124–2136. [Google Scholar] [CrossRef]
- Gee, T.; Farrar, E.; Wang, Y.; Wu, B.; Hsu, K.; Zhou, B.A.; Butcher, J. NFκB (nuclear factor κ-light-chain enhancer of activated B cells) activity regulates cell-type-specific and context-specific susceptibility to calcification in the aortic valve. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 638–655. [Google Scholar] [CrossRef]
- Chen, M.L.; Zhu, X.H.; Ran, L.; Lang, H.D.; Yi, L.; Mi, M.T. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the sirt3-sod2-mtros signaling pathway. J. Am. Heart Assoc. 2017, 6, e003698. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Are an Aging Gut and a Decrease in Butyrate Production the Reasons for Atherosclerosis? Int. J. Mol. Sci. 2025, 26, 8276. [Google Scholar] [CrossRef]
- Puspitasari, Y.M.; Ministrini, S.; Schwarz, L.; Karch, C.; Liberale, L.; Camici, G.G. Modern concepts in cardiovascular disease: Inflamm-Aging. Front. Cell Dev. Biol. 2022, 10, 882211. [Google Scholar] [CrossRef]
- Picos, A.; Seoane, N.; Campos-Toimil, M.; Viña, D. Vascular senescence and aging: Mechanisms, clinical implications, and ther-apeutic prospects. Biogerontology 2025, 26, 118. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Liu, T.; Zhu, X.; Pan, X. The multifaceted role of the SASP in atherosclerosis: From mechanisms to therapeutic opportunities. Cell Biosci. 2022, 12, 74. [Google Scholar] [CrossRef]
- Vellasamy, D.M.; Lee, S.J.; Goh, K.W.; Goh, B.H.; Tang, Y.Q.; Ming, L.C.; Yap, W.H. Targeting immune senescence in atheroscle-rosis. Int. J. Mol. Sci. 2022, 23, 13059. [Google Scholar] [CrossRef]
- Cobo, I.; Tanaka, T.N.; Mangalhara, K.C.; Lana, A.; Yeang, C.; Han, C.; Schlachetzki, J.; Challcombe, J.; Fixsen, B.R.; Sakai, M.; et al. DNA methyltransferase 3 alpha and TET methylcytosine dioxygenase 2 restrain mitochondrial DNA-mediated interferon signaling in macrophages. Immunity 2022, 55, 1386–1401. [Google Scholar] [CrossRef]
- Choi, E.L.; Taheri, N.; Tan, E.; Matsumoto, K.; Hayashi, Y. The crucial role of the interstitial cells of Cajal in neurointestinal diseases. Biomolecules 2023, 13, 1358. [Google Scholar] [CrossRef] [PubMed]
- Broad, J.; Kung, V.W.S.; Palmer, A.; Elahi, S.; Karami, A.; Darreh-Shori, T.; Ahmed, S.; Thaha, M.A.; Carroll, R.; Chin-Aleong, J.; et al. Changes in neuromuscular structure and functions of human colon during ageing are region-dependent. Gut 2019, 68, 1210–1223. [Google Scholar] [CrossRef]
- Baidoo, N.; Sanger, G.J.; Belai, A. Effect of old age on the subpopulations of enteric glial cells in human descending colon. Glia 2023, 71, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.; Epton, S.; Crawley, E.; Straface, M.; Gammon, L.; Edgar, M.M.; Xu, Y.; Elahi, S.; Chin-Aleong, J.; Martin, J.E.; et al. Expression of p16 within myenteric neurons of the aged Colon: A potential marker of declining function. Front. Neurosci. 2021, 15, 747067. [Google Scholar] [CrossRef]
- Baidoo, N.; Crawley, E.; Knowles, C.H.; Sanger, G.J.; Belai, A. Total collagen content and distribution is increased in human colon during advancing age. PLoS ONE 2022, 17, e0269689. [Google Scholar] [CrossRef]
- Ho, Y.-H.; Del Toro, R.; Rivera-Torres, J.; Rak, J.; Korn, C.; Garcia-Garcia, A.; Macías, D.; González-Gómez, C.; del Monte, A.; Wittner, M.; et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during prema-ture or physiological aging. Cell Stem Cell 2019, 25, 407–418.e6. [Google Scholar] [CrossRef]
- Linton, P.J.; Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004, 5, 133–139. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, F.; Casula, M.; Olmastrini, E. Apolipoprotein B compared with low-density lipoprotein cholesterol in the athero-sclerotic cardiovascular diseases risk assessment. Pharmacol. Res. 2023, 95, 106873. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Coronary Arteries | Internal Mammary Arteries (IMAs) | References |
|---|---|---|---|
| Location | Branches directly from the ascending aorta and run along the surface of the heart muscle (myocardium), with smaller penetrating branches | Ascend from the left and right proximal subclavian arteries and are located on the inside of the rib cage, one on each side of the sternum. | [57,66] |
| Main function | Supplies oxygen-rich blood to the myocardium | Supplies blood to the anterior chest wall, including the sternum and breasts. | [57,67] |
| Blood flow | Rhythmic, with most blood flow during diastole (relaxation phase) | Steady, but blood flow can alter in response to demand. | [57,68] |
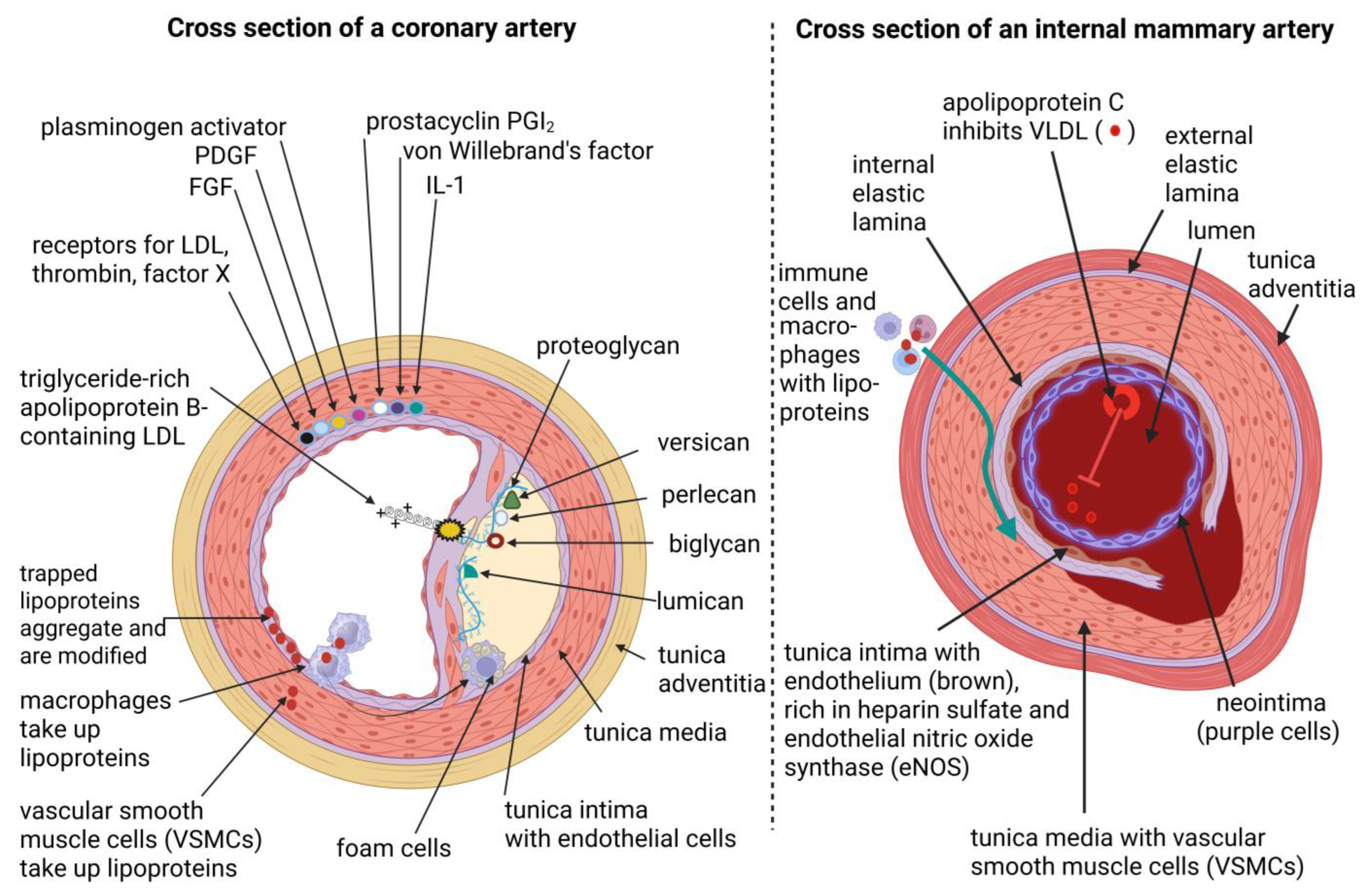
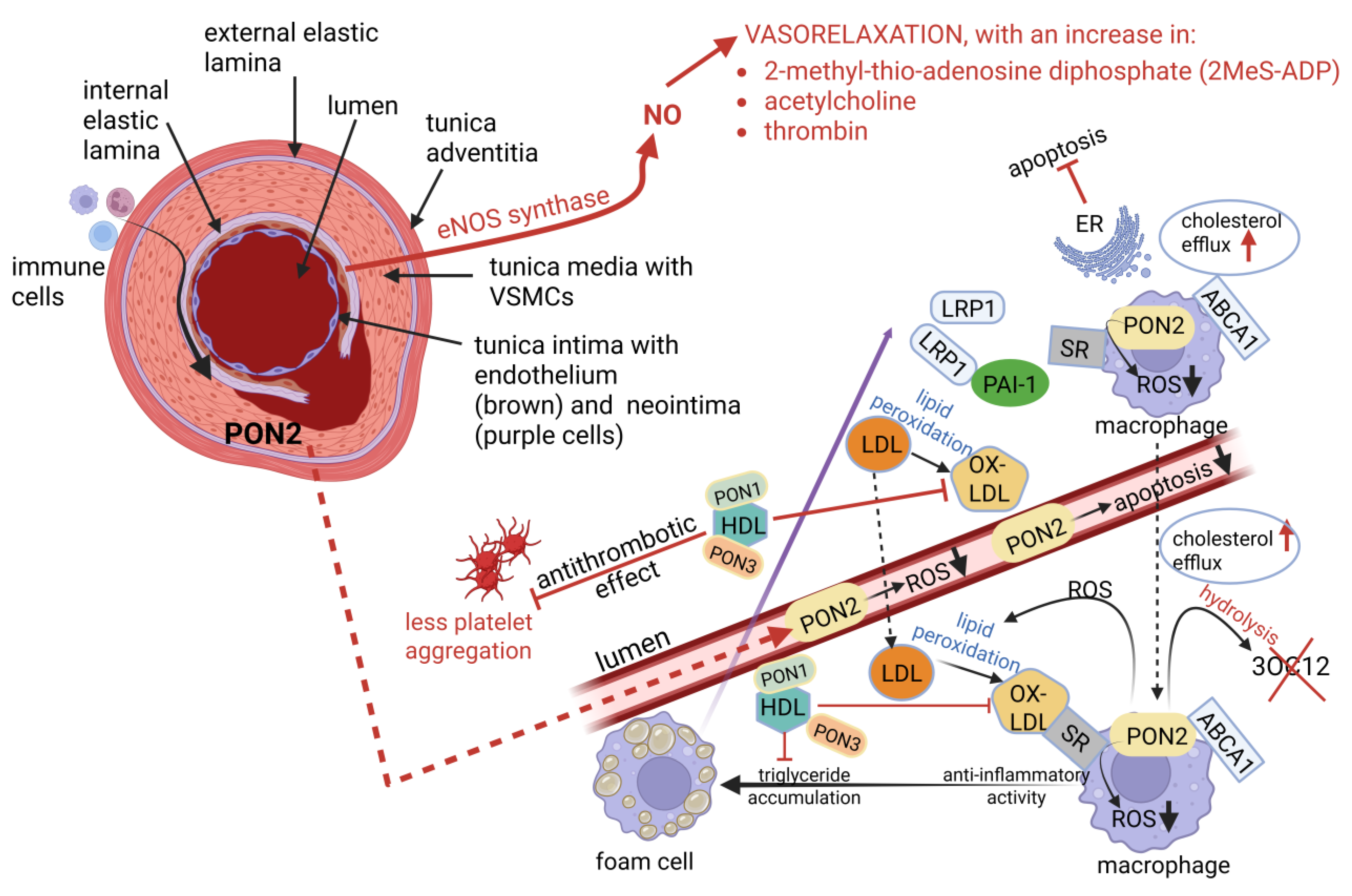
| Atherosclerosis (AS) | Plaque buildup due to the deposit of oxidized low-density lipoproteins (oxLDL), calcium, fibrin, and cellular debris onto the inner lining (tunica intima) of arterial walls, leading to hypertension and coronary artery disease (CAD) | Highly resistant to plaque buildup and AS, due to unique cellular and structural properties, and increased production of apolipoprotein C-III (apo-CIII), which inhibits the buildup of LDL and plaque formation. Paraoxonase 2 (PON2) expressed in VSMCs lowers the production of reactive oxygen species (ROS). PON1 regulates reverse cholesterol transport, is antioxidative, anti-inflammatory, anti-apoptotic, vasodilative, and antithrombotic. | [69,70] |
| Internal elastic lamina | Fenestrated, and supports the migration of vascular smooth muscle cells (VSMCs), and intimal hyperplasia | Non-fenestrated and forms a barrier preventing intimal hyperplasia and AS. | [71] |
| Tunica intima | Composed of smooth, longitudinally orientated endothelial cells (ECs), connective tissue, and an internal elastic lamina. Thickness increases with age. Prone to the formation of lesions | Similar in composition, but thinner and lacks a prominent internal elastic lamina. The neointima is separated from the tunica media by an internal non-fragmented (and less defined) internal elastic lamina. Rich in heparin sulfate and endothelial nitric oxide synthase (eNOS). The increased production of nitric oxide (NO) relaxes VSMCs and suppresses GTP cyclohydrolase (GTPCH), thereby lowering blood pressure and reducing plaque formation. Higher levels of prostacyclin (PG12) inhibit platelet aggregation. | [72,73,74] |
| Arterial blockage | Prone to becoming blocked in CAD | More resistant to blockage and used as grafts in coronary arterial bypass surgery | [57,58] |
| Clinical significance | Ischemic heart disease, leading to angina and myocardial infarction (MI) | Surgical replacement of blocked coronary arteries with IMAs lowers the risk of developing angina and MI and has superior long-term patency. | [57,58] |
| Characteristic | Coronary Arteries | Internal Mammary Arteries (IMAs) | References |
|---|---|---|---|
| Hemodynamic stress | Withstand extreme hemodynamic stress, high pressures, and shear forces. | Less resilient to hemodynamic stress than the coronary arteries. | [75] |
| Endothelial function | Impaired in atherosclerotic coronary artery disease (ACAD). | Robust endothelial function and are generally more resistant to pathological changes. | [76] |
| Nitric oxide (NO) production | Basal NO production due to reduced endothelial nitric oxide synthase (eNOS) expression. More susceptible to atherosclerosis (AS) compared to IMAs. | Abundant production of NO, which contributes to its vasodilatory properties and long-term patency. | [72,73,74,77,78] |
| Vasa vasorum (VV) density | High VV density, but it varies among individual coronary artery branches. The right coronary artery has a significantly higher VV density compared to the left anterior descending coronary branch. The high VV density is crucial for supplying blood to the thickened walls of the coronary arteries. | Lower VV density compared to the coronary arteries. This contributes to the protection of IMAs to AS. | [71,79] |
| Sympathetic innervation | Dense adrenergic innervation in the media and adventitia, primarily associated with the epicardial arteries and larger vessels. | Sympathetic fibers in the adventitia, though perhaps with lower density. IMAs have less direct sympathetic innervation than the coronary arteries, suggesting they are less subject to neural regulation. | [71] |
| Exposure to circulating lipids | Directly exposed to high concentrations of circulating lipids, such as cholesterol and triglycerides, making them vulnerable to plaque buildup. | Protected from AS, despite being exposed to the same circulating lipids. This is due to unique biological properties that protect them from plaque buildup. | [69,70,80] |
| Mechanism | Products Produced by Gut Microbiota and Their Effect on Epithelial and Endothelial Cells | Impact on AS | References |
|---|---|---|---|
| Metabolites produced | Short-chain fatty acids (SCFAs) such as butyrate, acetate, and propionate, reactive oxygen species (ROS), lipopolysaccharides (LPS), trimethylamine-N-oxide (TMAO), and bile acids (BAs) | SCFAs alter the vascular tone of coronary arteries and internal mammary arteries (IMAs), promote the production of inflammatory cytokines, suppress the production of pro-inflammatory cytokines, prevent platelet membrane glycoprotein 4 (CD36) to take up oxidized low-density lipoprotein (ox-LDL), suppress plaque formation, lower diastolic blood pressure (DPB), and prevent the adhesion of monocytes to injured endothelia. SCFAs also increase the production of insulin-like growth factor 1 (IGF-1) ROS leads to the formation of ox-LDL, which is a potent inducer of macrophage foam cells LPS induces inflammation and immune responses, leading to drastic changes in intestinal permeability. TMAO triggers platelet formation, inhibits the synthesis of bile acids (BAs), accelerates the formation of aortic lesions, and upregulates the expression of CD36 on the surface of platelets and epithelial cells. TMAO stimulates the development of macrophage foam cells, increasing the risk of thrombosis. Bile acids affect lipid metabolism and may promote reverse cholesterol transport. Binding of BAs to Farnesoid receptors (FXRs) or G protein receptors (GPRs) reduces the risk of AS. | [15,109,110,113,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129] |
| Gut barrier integrity | Dysbiosis alters the integrity of the gut barrier by weakening the tight junction proteins. | Increases intestinal permeability, allowing microbial components such as LPS to enter the systemic circulation, which triggers systemic inflammation and AS. | [21,130] |
| Systemic inflammation | Microbial products activate Toll-like receptor (TLR) signaling in immune cells, promoting the production of inflammatory cytokines. | Systemic inflammation is a key driver of AS, leading to the proliferation of VSMCs, migration of monocytes and T lymphocytes, and the development of lipid-rich macrophages (foam cells). Activation of the Toll-like receptor 4 (TLR4) pathway leads to an increase in endothelial nitric oxide synthase (eNOS) and mitogen-activated protein kinase (MAPK)/nuclear factor kappa B (NF-κB) pathways, which lead to an increase in the expression of cytokines and chemokines. | [27,111,112,113] |
| Platelet function and thrombosis | Microbial metabolites such as phenylacetylglutamine (PAGln) and plasmin increase the risk of cardiovascular events and heart failure. | PAGln binds to receptors on platelets, increasing the risk of blood clotting. Pathogenic bacteria convert plasminogen to plasmin, a protease that degrades extracellular matrix components and fibrinogen. This stimulates plaque formation, bacterial colonization, and inflammation, contributing to AS. | [131,132] |
| Immunomodulation | Influences immune responses, particularly via B cell activation through TLR signaling | Activated B cells increase circulating antibodies such as immunoglobulin G (IgG). The differentiation of T-regulatory (Treg) cells and prostaglandin production is regulated by specific microbial components that induce the production of type I interferon (IFN) in macrophages and dendritic cells. The regulation of Treg cells by gut microbiota maintains intestinal immune homeostasis and prevents inflammatory diseases. | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicks, L.M.T. Why Are Internal Mammary (Thoracic) Arteries Less Prone to Developing Atherosclerosis Compared to Coronary Arteries? Do Gut Microbiota Play a Role? A Narrative Review. Int. J. Mol. Sci. 2025, 26, 9052. https://doi.org/10.3390/ijms26189052
Dicks LMT. Why Are Internal Mammary (Thoracic) Arteries Less Prone to Developing Atherosclerosis Compared to Coronary Arteries? Do Gut Microbiota Play a Role? A Narrative Review. International Journal of Molecular Sciences. 2025; 26(18):9052. https://doi.org/10.3390/ijms26189052
Chicago/Turabian StyleDicks, Leon M. T. 2025. "Why Are Internal Mammary (Thoracic) Arteries Less Prone to Developing Atherosclerosis Compared to Coronary Arteries? Do Gut Microbiota Play a Role? A Narrative Review" International Journal of Molecular Sciences 26, no. 18: 9052. https://doi.org/10.3390/ijms26189052
APA StyleDicks, L. M. T. (2025). Why Are Internal Mammary (Thoracic) Arteries Less Prone to Developing Atherosclerosis Compared to Coronary Arteries? Do Gut Microbiota Play a Role? A Narrative Review. International Journal of Molecular Sciences, 26(18), 9052. https://doi.org/10.3390/ijms26189052






