Mentha rotundifolia, a Source of Amoebicidal Agents Against Naegleria fowleri
Abstract
1. Introduction
2. Results
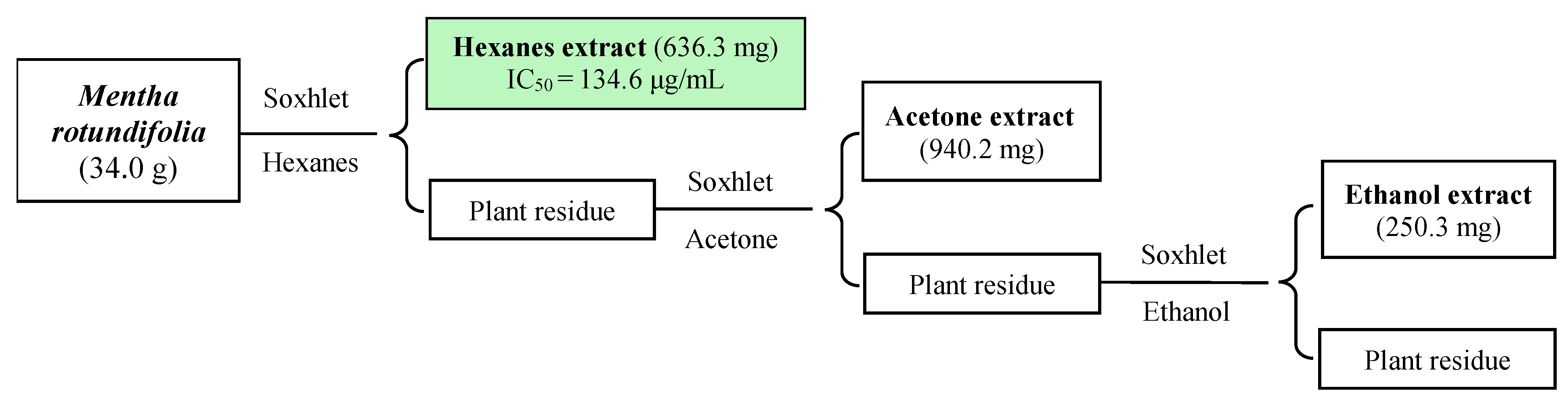
2.1. Bioassay-Guided Isolation
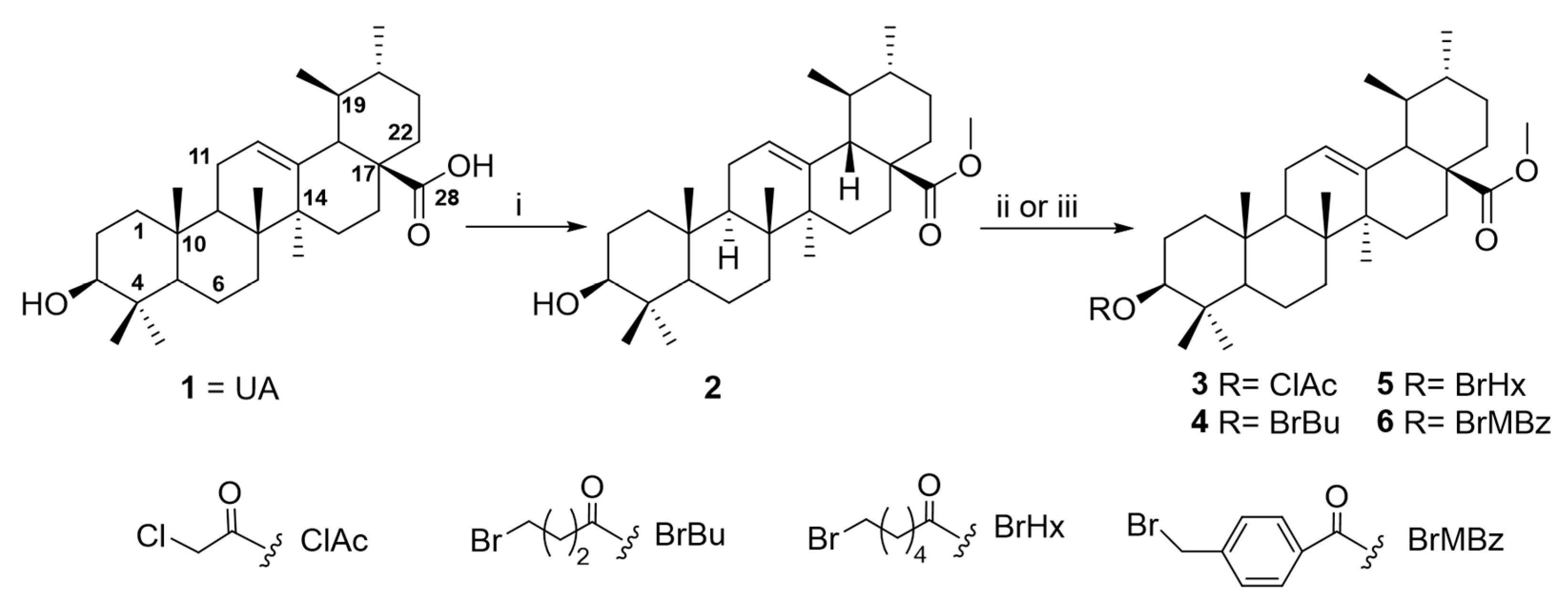
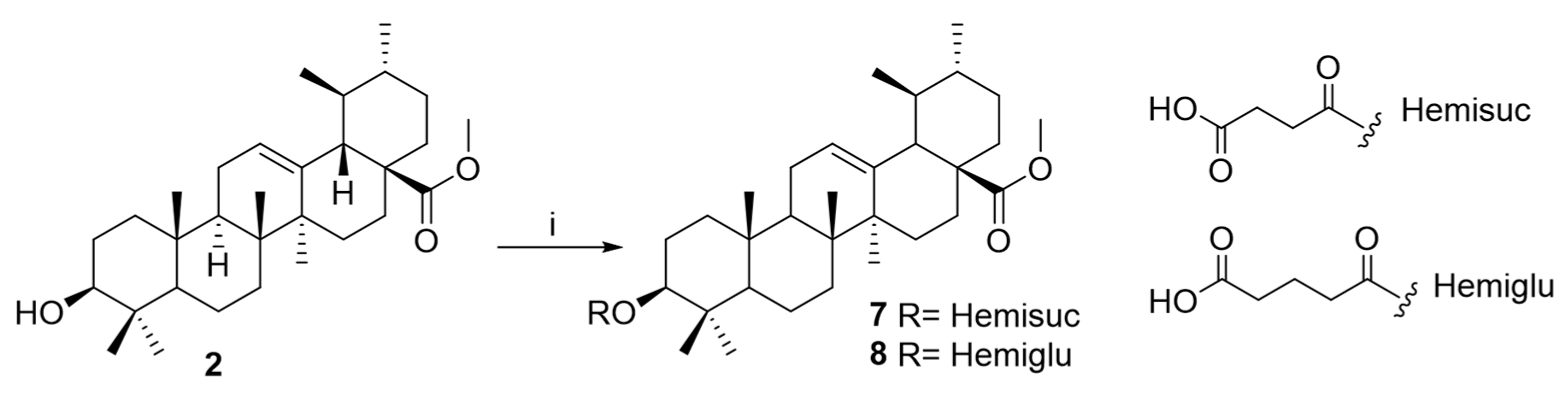
2.2. Chemical Modification
2.3. In Vitro Amoebicidal Activity and Cytotoxicity of Ursolic Acid Derivatives
2.4. Structure–Amoebicidal Activity Relationship Study
2.5. In Vitro Cysticidal Activity on Naegleria fowleri
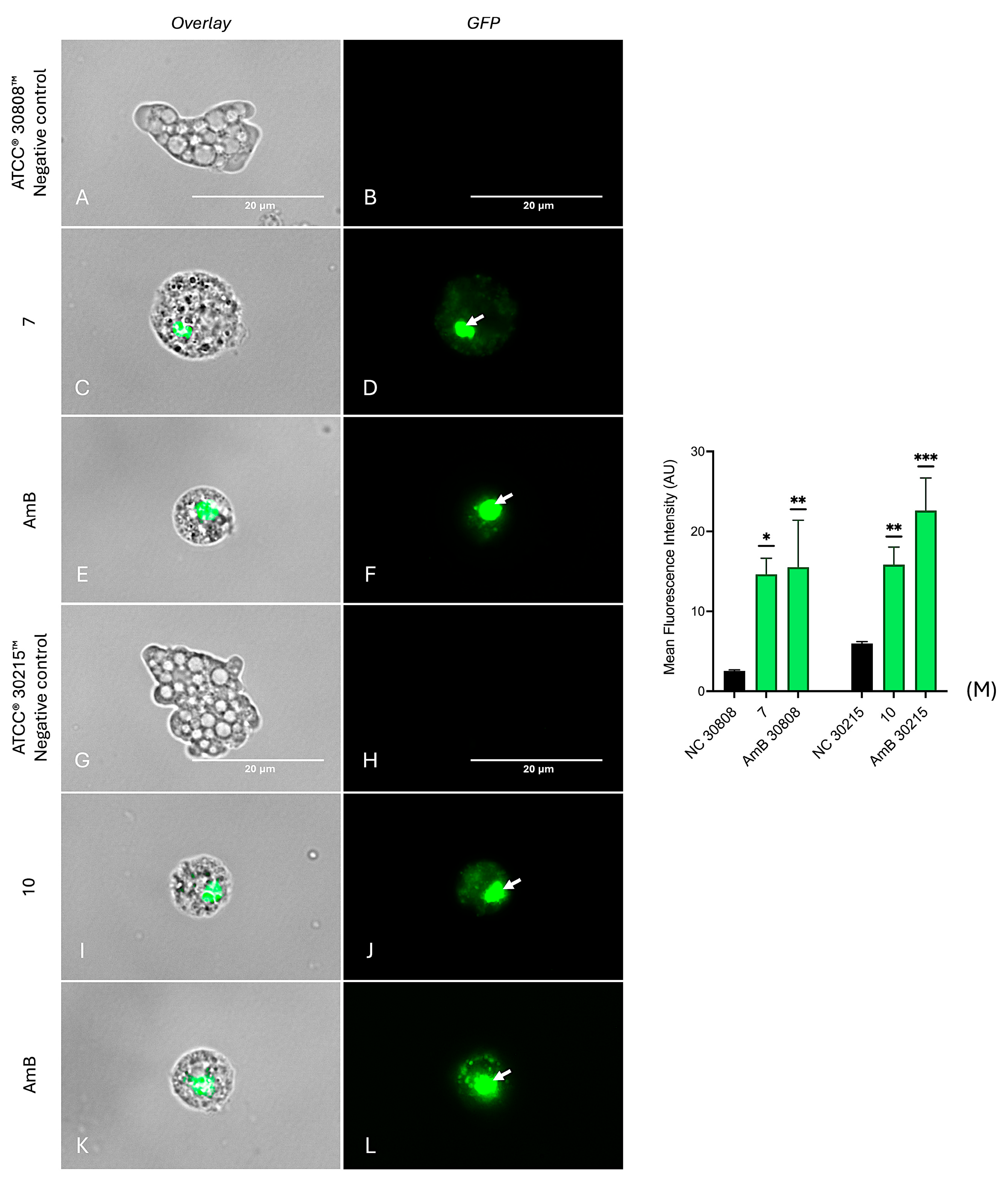
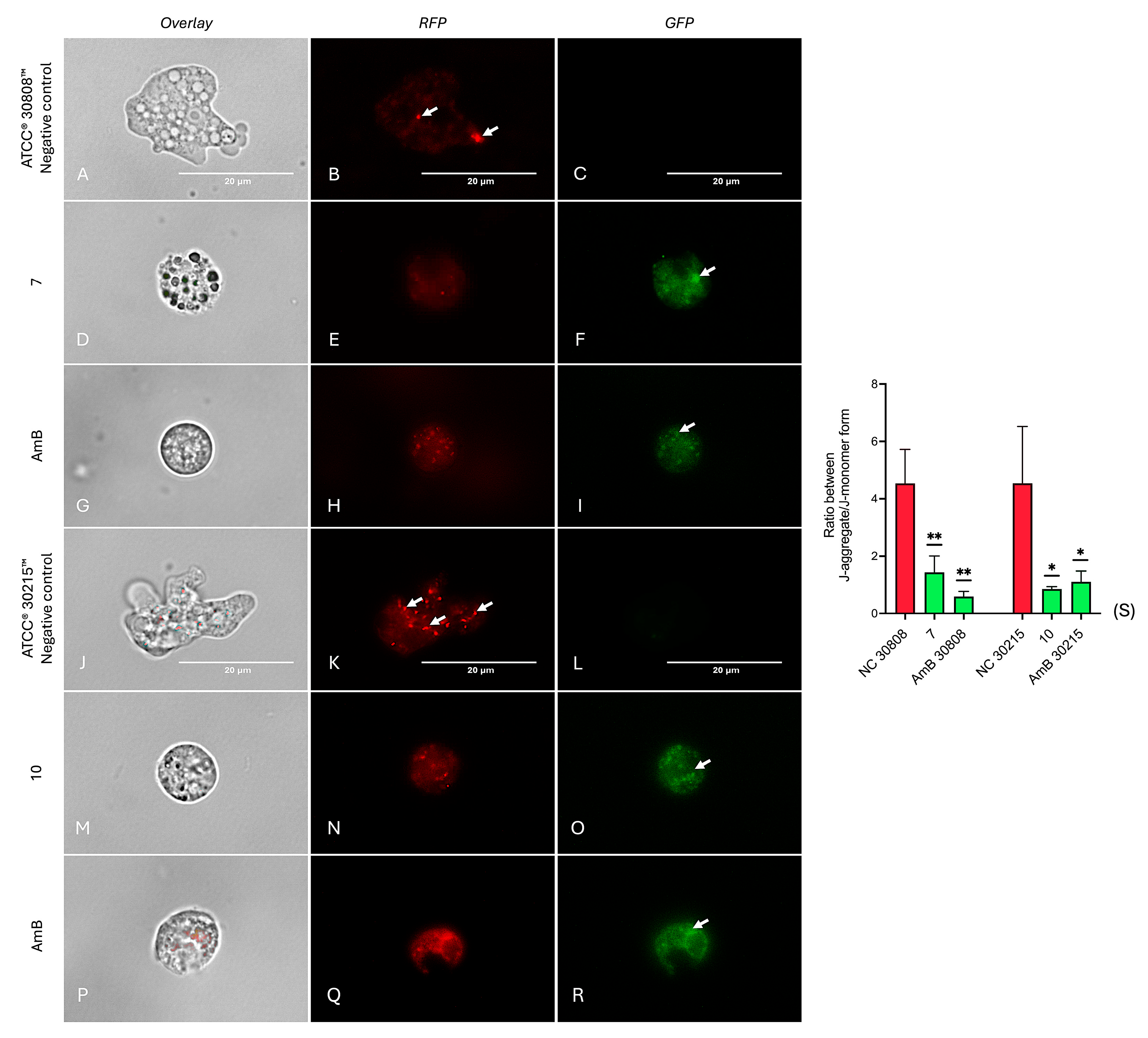
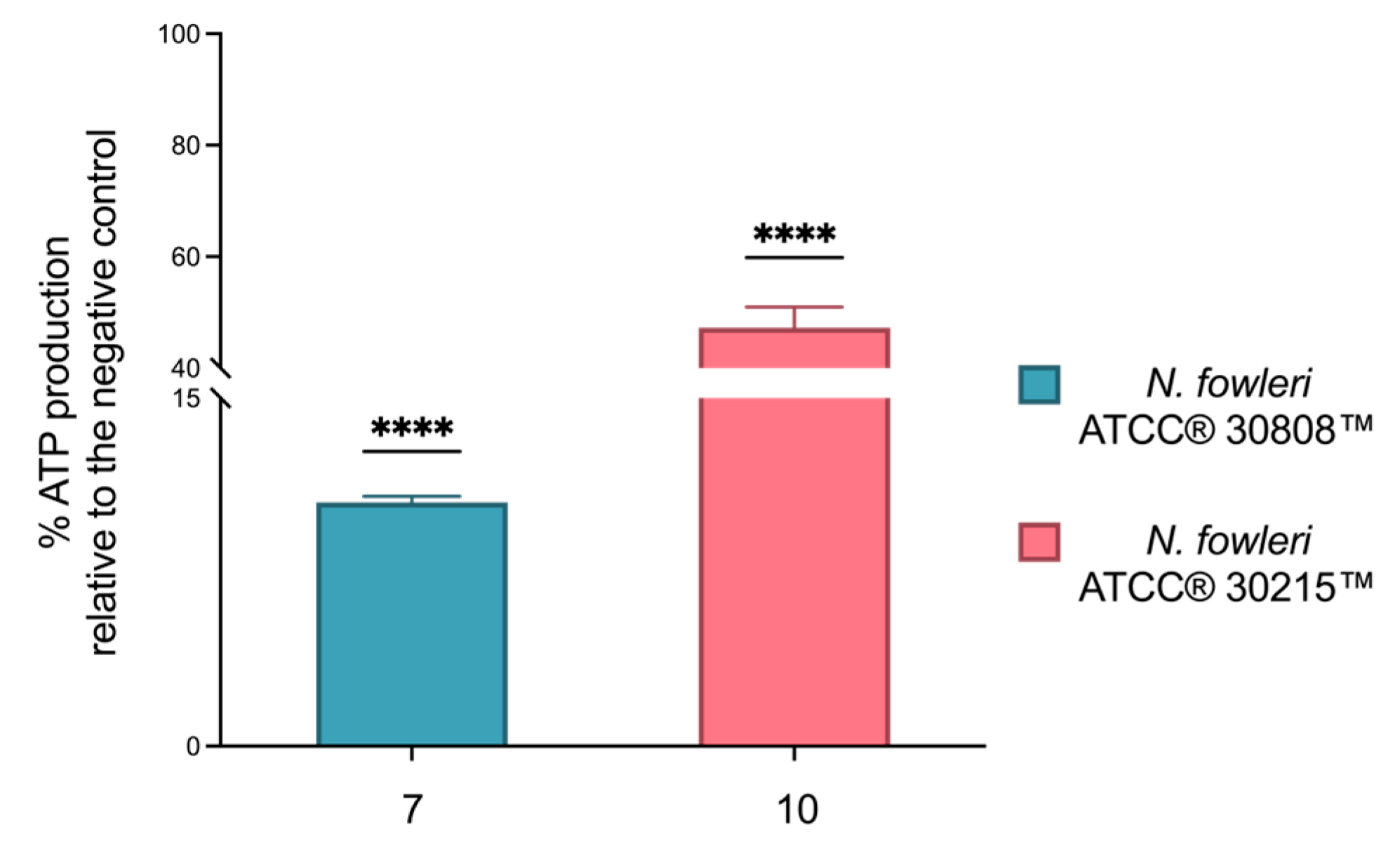
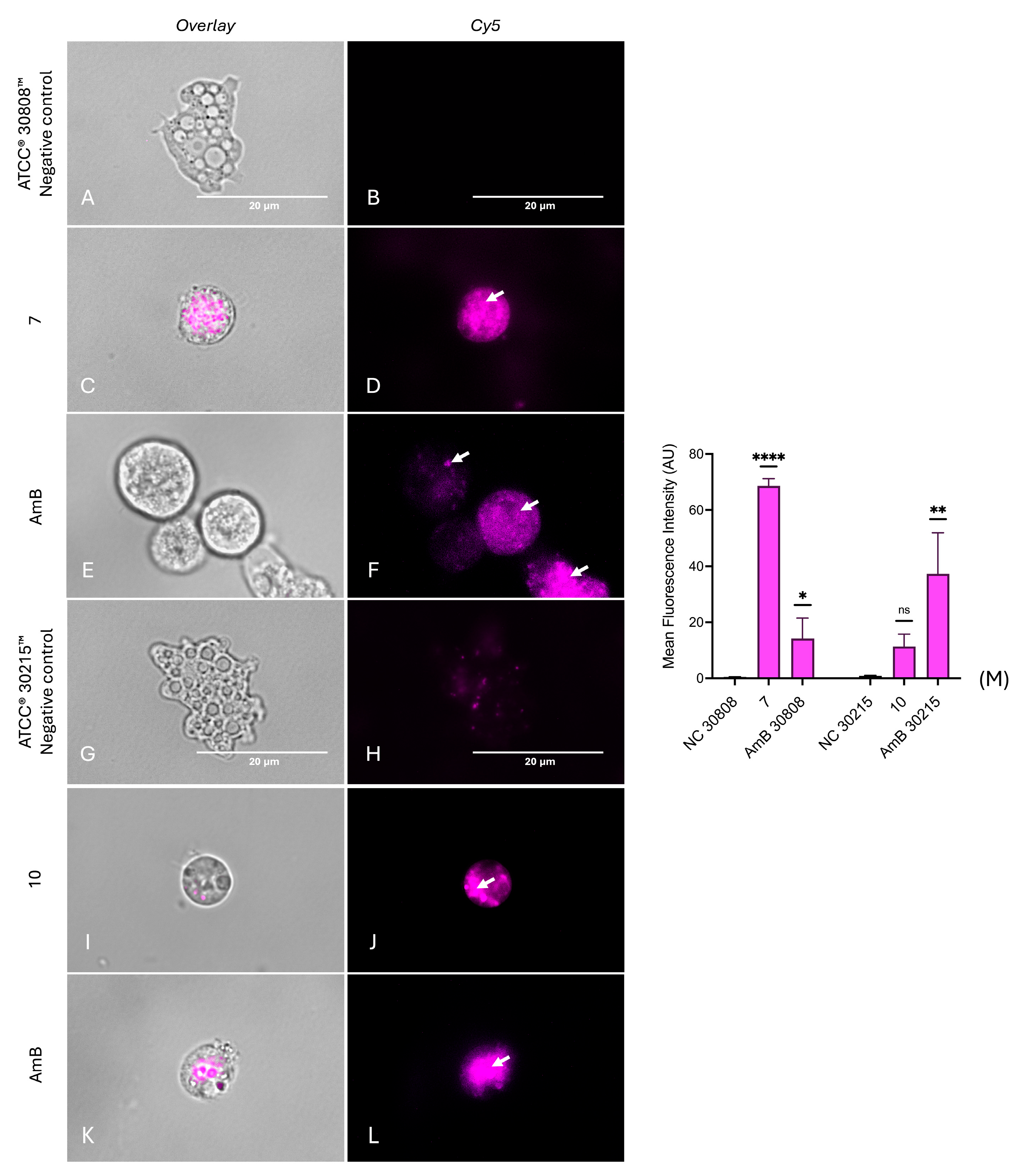
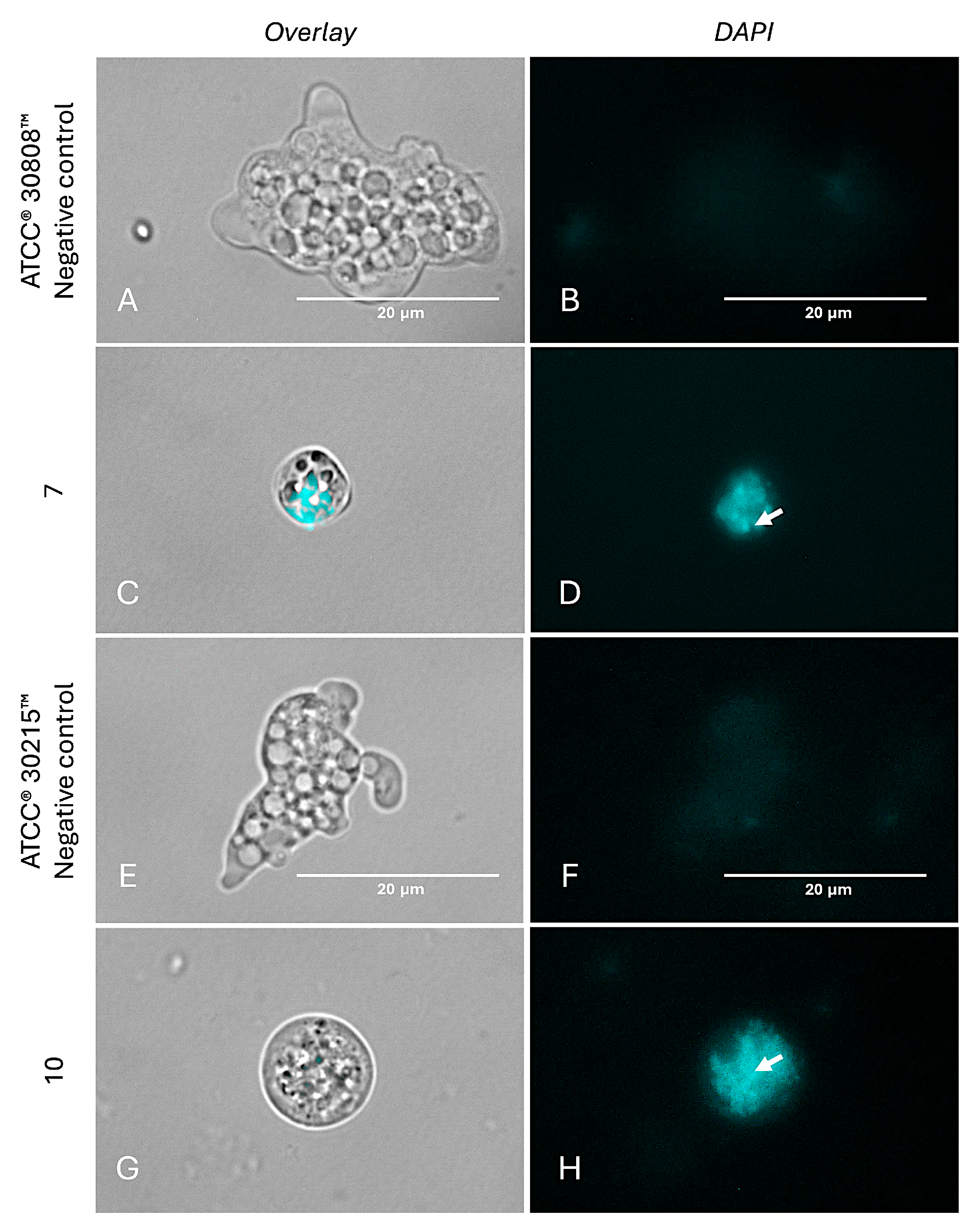
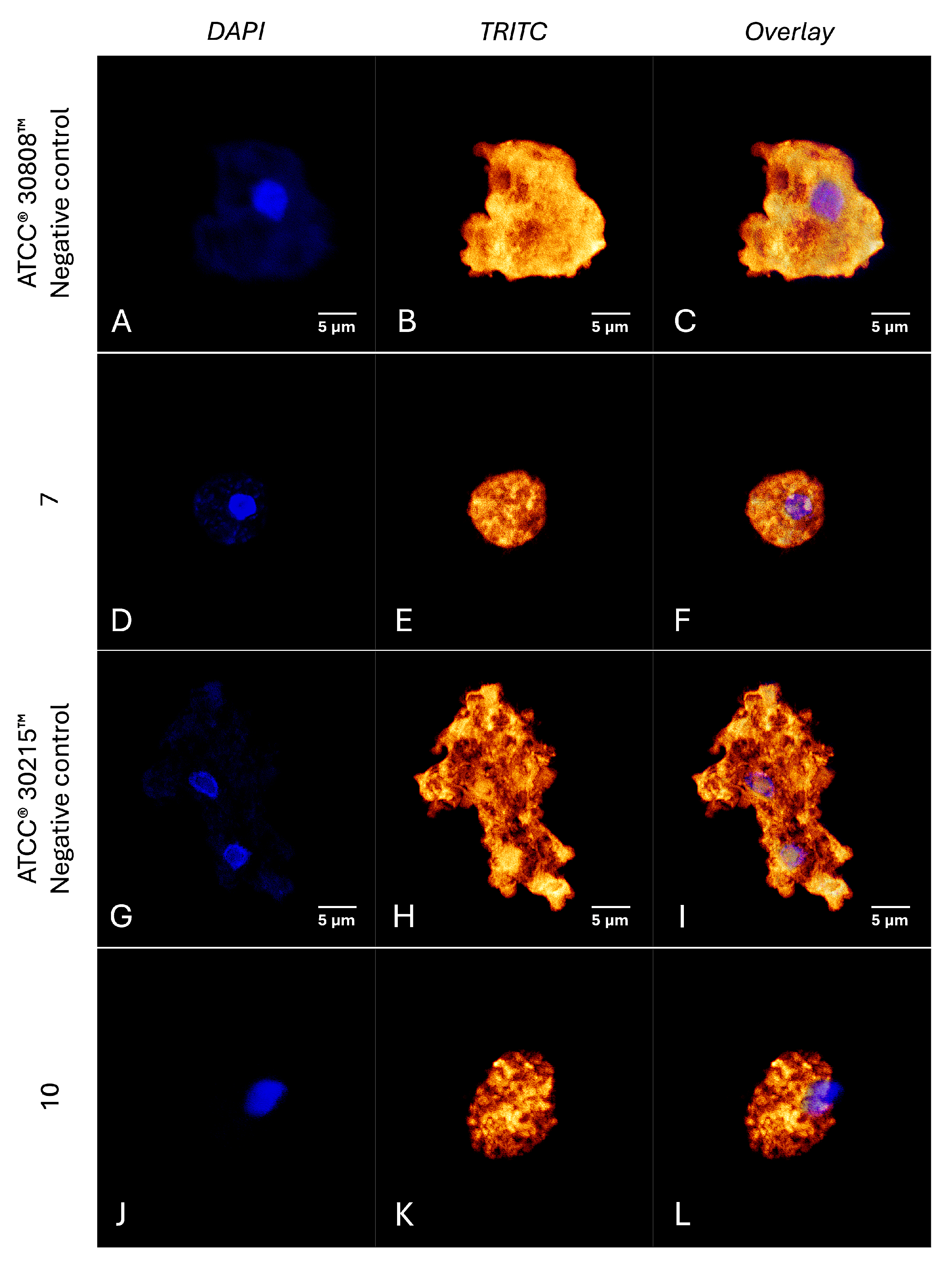
2.6. Investigation into the Molecular Mechanisms of Compounds 7 and 10
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Plant Material
4.3. Dried of Vegetal Material
4.4. Extracts Preparation
4.5. Bioactivity-Guided Chromatographic Fractionation and Isolation
4.6. Structural Chemical Modification of Ursolic Acid
4.6.1. Preparation of Derivative 2
4.6.2. General Procedure for Preparation of Derivatives 3–8
Preparation of Derivative 3
Preparation of Derivative 4
Preparation of Derivative 5
Preparation of Derivative 6
Preparation of Derivative 7
Preparation of Derivative 8
4.6.3. General Procedure for Preparation of Derivatives 9–11
Preparation of Derivative 9
Preparation of Derivative 10
Preparation of Derivative 11
4.7. Biological Activity
4.7.1. Amoebic Strains and Cell Maintenance
4.7.2. In Vitro Amoebicidal Activity Against N. fowleri Trophozoites
4.7.3. In Vitro Amoebicidal Activity Against N. fowleri Cyst
4.7.4. In Vitro Cytotoxicity on Murine Macrophage
4.8. Mechanism of Action on N. fowleri Trophozoites for Compounds 7 and 10
4.8.1. Analysis of Programmed Cell Death
4.8.2. Analysis of Chromatin Condensation
4.8.3. Analysis of Plasma Membrane Permeability
4.8.4. Analysis of Mitochondrial Membrane Potential
4.8.5. Measurement of Intracellular Adenosine Triphosphate Levels
4.8.6. Measurement of Intracellular Reactive Oxygen Species Levels
4.8.7. Detection of Autophagic Vacuole Formation
4.8.8. Analysis of Cytoskeletal Integrity
4.8.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alanazi, A.; Younas, S.; Ejaz, H.; Alruwaili, M.; Alruwaili, Y.; Mazhari, B.B.Z.; Atif, M.; Junaid, K. Advancing the understanding of Naegleria fowleri: Global epidemiology, phylogenetic analysis, and strategies to combat a deadly pathogen. J. Infect. Public Health 2025, 18, 102690. [Google Scholar] [CrossRef]
- De Jonckheere, J.F. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect. Genet. Evol. 2011, 11, 1520–1528. [Google Scholar] [CrossRef]
- Martinez-Castillo, M.; Cardenas-Zuniga, R.; Coronado-Velazquez, D.; Debnath, A.; Serrano Luna, J.; Shibayama, M. Naegleria fowleri after 50 years: Is it a neglected pathogen? J. Med. Microbiol. 2016, 65, 885–896. [Google Scholar] [CrossRef]
- Moseman, E.A. Battling brain-eating amoeba: Enigmas surrounding immunity to Naegleria fowleri. PLoS Pathog. 2020, 16, e1008406. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Iqbal, J.; Khan, N.A. Immunotherapeutic approaches and vaccine development against Naegleria fowleri. Front. Immunol. 2023, 14, 1284621. [Google Scholar] [CrossRef]
- Kim, J.; Jung, S.; Lee, Y.; Song, K.; Kwon, D.; Kim, K.; Park, S.; Im, K.; Shin, H. Effect of therapeutic chemical agents in vitro and on experimental meningoencephalitis due to Naegleria fowleri. Antimicrob. Agents Chemother. 2008, 52, 4010–4016. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Hussain, K.; Khalid, M.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Azole and 5-nitroimidazole based nanoformulations are potential antiamoebic drug candidates against brain-eating amoebae. J. Appl. Microbiol. 2023, 134, lxad072. [Google Scholar] [CrossRef]
- Lê, H.G.; Kang, J.M.; Võ, T.C.; Na, B.K. Kaempferol induces programmed cell death in Naegleria fowleri. Phytomedicine 2023, 119, 154994. [Google Scholar] [CrossRef]
- Arberas-Jiménez, I.; Nocchi, N.; Chao-Pellicer, J.; Sifaoui, I.; Soares, A.R.; Díaz-Marrero, A.R.; Fernández, J.J.; Piñero, J.E.; Lorenzo-Morales, J. Chamigrane-type sesquiterpenes from Laurencia dendroidea as lead compounds against Naegleria fowleri. Mar. Drugs 2023, 21, 224. [Google Scholar] [CrossRef]
- Ben Youssef, M.; Omrani, A.; Sifaoui, I.; Hernández-Álvarez, E.; Chao-Pellicer, J.; Bazzocchi, I.L.; Sebai, H.; Piñero, J.E.; Jimenez, I.A.; Lorenzo-Morales, J. Amoebicidal thymol analogues against brain eating amoeba, Naegleria fowleri. Bioorg. Chem. 2025, 159, 108346. [Google Scholar] [CrossRef]
- Moore, B.S.; Newman, D.J. The extraordinary benefit of nature’s chemistry to health, society, and the economy. J. Nat. Prod. 2025, 88, 1541–1548. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.S.; Kumari, S.; Sahal, D.; Sharma, U. Innate functions of natural products: A promising path for the identification of novel therapeutics. Eur. J. Med. Chem. 2023, 260, 115748. [Google Scholar] [CrossRef]
- Bharate, S.B.; Lindsley, C.W. Natural products driven medicinal chemistry. J. Med. Chem. 2024, 67, 20723–20730. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Ahmad, M. Mentha: A review of its ethnobotanical and pharmacological applications. Pharmacogn. Rev. 2018, 12, 179–184. [Google Scholar] [CrossRef]
- Jaiswal, D.; Pande, V. Phytochemical and pharmacological review of Mentha species. Pharmacogn. Rev. 2018, 12, 120–128. [Google Scholar]
- Monzote, L.; Herrera, I.; Satyal, P.; Setzer, W.N. In-vitro evaluation of 52 commercially-available essential oils against Leishmania amazonensis. Molecules 2019, 24, 1248. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Lima, T.C.; Steverding, D. Evaluation of antiparasitic activity of Mentha crispa essential oil, its major constituent rotundifolone, and analogues against Trypanosoma brucei. Planta Med. 2016, 82, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Jebali, J.; Ghazghazi, H.; Aouadhi, C.; ELBini-Dhouib, I.; Ben Salem, R.; Srairi-Abid, N.; Marrakchi, N.; Rigane, G. Tunisian Native Mentha pulegium L. extracts: Phytochemical composition and biological activities. Molecules 2022, 27, 314. [Google Scholar] [CrossRef]
- Morteza, M.A. Morphological and essential oils diversities among twelve Tunisian Mentha spp. Accessions. Med. J. Clin. Trials Case Stud. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Boualam, K.; Bouhaddou, N.; Sobeh, M.; Tabyaoui, M.; Taghzouti, K. Mentha rotundifolia (L.) Huds. aqueous extract attenuates H2O2-induced oxidative stress and neurotoxicity. Front. Neurosci. 2023, 17, 1121029. [Google Scholar] [CrossRef]
- Riahi, L.; Chakroun, H.; Klay, I.; Masmoudi, A.S.; Cherif, A.; Zoghlami, N. Metabolomic fingerprint of Mentha rotundifolia L. leaf tissues promotes this species as a potential candidate for sustainable production of biologically active molecules. J. Complement. Integr. Med. 2018, 16, 20180048. [Google Scholar] [CrossRef]
- Guardo, N.I.; Sainz, P.; González-Coloma, A.; Burillo, J.; Martínez-Díaz, R.A. Trypanocidal effects of essential oils from selected medicinal plants: Synergy among the main components. Nat. Prod. Commun. 2017, 12, 653–656. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Siddiqui, R.; Boghossian, A.; Khatoon, B.; Kawish, M.; Alharbi, A.M.; Shah, M.R.; Alfahemi, H.; Khan, N.A. Antiamoebic properties of metabolites against Naegleria fowleri and Balamuthia mandrillaris. Antibiotics 2022, 11, 539. [Google Scholar] [CrossRef]
- Liu, L.-F.; Li, W.-H.; Li, M.-Y.; Wu, X.-Z.; Yang, F.; Xu, J.-N.; Yuan, C.-S. Chemical constituents from common vetch (Vicia sativa L.) and their antioxidant and cytotoxic activities. Nat. Prod. Res. 2020, 34, 3205–3211. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.H.R.; Holness, N.J. Triterpenoids. V. Some relative configurations in rings C, D, and E of the β-amyrin and the lupeol group of triterpenoids. J. Chem. Soc. 1952, 78–85. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, H.; Gao, Y.; Hu, J.; Li, M.-H. Fabrication of chiral polydiacetylene nanotubes via supramolecular gelation of a triterpenoid-derived amphiphile. Mater. Adv. 2021, 2, 1909–1915. [Google Scholar] [CrossRef]
- Chao-Pellicer, J.; Arberas-Jiménez, I.; Sifaoui, I.; Piñero, J.E.; Lorenzo-Morales, J. Exploring therapeutic approaches against Naegleria fowleri infections through the COVID box. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100545. [Google Scholar] [CrossRef]
- Chao-Pellicer, J.; Delgado-Hernández, S.; Arberas-Jiménez, I.; Sifaoui, I.; Tejedor, D.; García-Tellado, F.; Piñero, J.E.; Lorenzo-Morales, J. Synthesis and biological evaluation of cyanoacrylamides and 5-iminopyrrol-2-ones against Naegleria fowleri. ACS Infect. Dis. 2024, 10, 3332–3345. [Google Scholar] [CrossRef]
- Cárdenas-Zúñiga, R.; Silva Olivares, A.; Villalba-Magdaleno, J.A.; Sánchez Monroy, V.; Serrano Luna, J.; Shibayama, M. Amphotericin B induces apoptosis-like programmed cell death in Naegleria fowleri and Naegleria gruberi. Microbiology 2017, 163, 940–949. [Google Scholar] [CrossRef]
- Chao-Pellicer, J.; Arberas-Jiménez, I.; Delgado-Hernández, S.; Sifaoui, I.; Tejedor, D.; García-Tellado, F.; Piñero, J.E.; Lorenzo-Morales, J. Cyanomethyl vinyl ethers against Naegleria fowleri. ACS Chem. Neurosci. 2023, 14, 2961–2970. [Google Scholar] [CrossRef]
- Ranasinghe, S.; Armson, A.; Lymbery, A.J.; Zahedi, A.; Ash, A. Medicinal plants as a source of antiparasitics: An overview of experimental studies. Pathog. Glob. Health. 2023, 117, 535–553. [Google Scholar] [CrossRef]
- Riahi, L.; Ben Mbarek, W.; Ayari, B.; Elferchichi, M.; Ghazghazi, H.; Jebali, J.; Zoghlami, N.; Ferchichi, A. Chemical composition and antioxidant activity of essential oil of Mentha rotundifolia. Ind. Crops Prod. 2013, 47, 21–27. [Google Scholar] [CrossRef]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Evdokiou, A.; Marciano-Cabral, F.; Jamerson, M. Studies on the cyst stage of Naegleria fowleri in vivo and in vitro. J. Eukaryot. Microbiol. 2022, 69, e12881. [Google Scholar] [CrossRef]
- Chen, C.F.; Yang, J.S.; Chen, W.K.; Lu, C.C.; Chiang, J.H.; Chiu, H.Y.; Tsai, S.C.; Juan, Y.N.; Huang, H.J.; Way, T.D. Ursolic acid elicits intrinsic apoptotic machinery by downregulating the phosphorylation of AKT/BAD signaling in human cisplatin-resistant oral cancer CAR cells. Oncol. Rep. 2018, 40, 1752–1760. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Q.; Liu, C.; Tang, Y.; Sun, C.; Zhuang, J. Nanoformulations of ursolic acid: A modern natural anticancer molecule. Front. Pharmacol. 2021, 12, 706121. [Google Scholar] [CrossRef]
- Jesus, J.A.; Fragoso da Silva, T.N.; Yamamoto, E.S.; Lago, J.H.G.; Laurenti, M.D.; Passero, L.F.D. Ursolic acid potentializes conventional therapy in experimental Leishmaniasis. Pathogens 2020, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Vanrell, M.C.; Martinez, S.J.; Muñoz, L.I.; Salassa, B.N.; Gambarte Tudela, J.; Romano, P.S. Induction of autophagy by ursolic acid promotes the elimination of Trypanosoma cruzi amastigotes from macrophages and cardiac cells. Front. Cell. Infect. Microbiol. 2022, 12, 919096. [Google Scholar] [CrossRef] [PubMed]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Ticona, J.C.; Reyes-Batlle, M.; Mejri, M.; Jiménez, A.I.; Lopez-Bazzocchi, I.; Valladares, B.; Lorenzo-Morales, J.; et al. In vitro effects of triterpenic acids from olive leaf extracts on the mitochondrial membrane potential of promastigote stage of Leishmania spp. Phytomedicine 2014, 21, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Gnoatto, S.C.B.; Vechia, L.D.; Lencina, C.L.; Dassonville-Klimpt, A.; Da Nascimento, S.; Mossalayi, D.; Sonnet, P. Synthesis and preliminary evaluation of new ursolic and oleanolic acids derivatives as antileishmanial agents. J. Enzyme Inhib. Med. Chem. 2008, 23, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Martín-Navarro, C.M.; González-Moreno, O.; Martínez-Carrasco, C.; López-Arencibia, A.; Arberas-Jiménez, I.; Sifaoui, I.; Reyes-Batlle, M.; Piñero, J.E.; Lorenzo-Morales, J. PrestoBlue® and AlamarBlue® are equally useful as viability assays for Acanthamoeba trophozoites and cysts. Exp. Parasitol. 2014, 141, 1–6. [Google Scholar] [CrossRef]
- Rodríguez-Expósito, R.L.; Sifaoui, I.; Reyes Batlle, M.; Maciver, S.K.; Piñero, J.E.; Lorenzo Morales, J. Statins induce actin cytoskeleton disassembly and an apoptosis-like process in Acanthamoeba spp. Antibiotics 2022, 11, 280. [Google Scholar] [CrossRef]




| Samples | N. fowleri ATCC® 30808TM IC50 a µg/mL |
|---|---|
| Hx b | 134.59 ± 14.57 |
| Hx c | 129.38 ± 7.18 |
| M4 | 76.28 ± 2.39 |
| M4A | 122.5 ± 6.51 |
| M4B1 | 54.75 ± 8.56 |
| M4B2 | 65.11 ± 8.08 |
| M4C1 | 96.91 ± 8.46 |
| M4C2 | 74.11 ± 10.18 |
| M4D | 94.35 ± 10.39 |
| M4E | 77.6 ± 0.85 |
| UA (1) d | 65.91 ± 3.73 |
| M e | 12.73 ± 1.49 |
| A e | 0.162 ± 0.002 |
| Cpds a | N. fowleri ATCC® 30808™ | N. fowleri ATCC® 30215™ | Murine Macrophages | N. fowleri ATCC® 30808™ | N. fowleri ATCC® 30215™ |
|---|---|---|---|---|---|
| IC50 b (µM) | IC50 b (µM) | CC50 c (µM) | SI d | SI d | |
| 1 | 141.53 ± 8.17 | 82.53 ± 8.43 | 35.93 ± 9.28 | 0.22 | 0.43 |
| 2 | 88.70 ± 3.02 | 84.47 ± 6.31 | 49.65 ± 5.65 | 1.12 | 1.06 |
| 7 | 28.66 ± 1.80 | 29.69 ± 1.59 | >175.19 | >6.11 | >5.90 |
| 8 | 66.58 ± 9.25 | 38.71 ± 2.15 | 84.96 ± 5.00 | 1.24 | 2.20 |
| 10 | 73.89 ± 3.22 | 7.61 ± 1.44 | 57.80 ± 2.32 | 0.87 | 7.60 |
| 11 | 78.55 ± 5.82 | 34.60 ± 8.86 | 40.75 ± 4.99 | 0.84 | 1.92 |
| M e | 38.74 ± 4.23 | 81.57 ± 7.23 | 127.89 ± 8.85 | 3.30 | 1.57 |
| A e | 0.12 ± 0.03 | 0.16 ± 0.01 | >200 | >1650.89 | >1250 |
| Cpds a | N. fowleri ATCC® 30808TM | N. fowleri ATCC® 30215TM | Murine Macrophages | N. fowleri ATCC® 30808TM | N. fowleri ATCC® 30215TM |
|---|---|---|---|---|---|
| IC50 b (µM) | IC50 b (µM) | CC50 c (µM) | SI d | SI d | |
| 7 | 36.65 ± 1.49 | 28.03 ± 1.59 | >175.19 | 4.78 | 6.25 |
| 10 | 66.47 ± 20.64 | 17.14 ± 2.59 | 57.80 ± 2.32 | 0.87 | 3.37 |
| M e | 21.52 ± 2.62 | 37.80 ± 5.60 | 127.89 ± 8.85 | 5.94 | 3.38 |
| A f | 0.53 ± 0.03 | 0.62 ± 0.04 | >200 | 377.36 | 322.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Youssef, M.; Chao-Pellicer, J.; Hernández-Álvarez, E.; Omrani, A.; Sifaoui, I.; Sebai, H.; Bazzocchi, I.L.; Piñero, J.E.; Jiménez, I.A.; Lorenzo-Morales, J. Mentha rotundifolia, a Source of Amoebicidal Agents Against Naegleria fowleri. Int. J. Mol. Sci. 2025, 26, 9048. https://doi.org/10.3390/ijms26189048
Ben Youssef M, Chao-Pellicer J, Hernández-Álvarez E, Omrani A, Sifaoui I, Sebai H, Bazzocchi IL, Piñero JE, Jiménez IA, Lorenzo-Morales J. Mentha rotundifolia, a Source of Amoebicidal Agents Against Naegleria fowleri. International Journal of Molecular Sciences. 2025; 26(18):9048. https://doi.org/10.3390/ijms26189048
Chicago/Turabian StyleBen Youssef, Meriam, Javier Chao-Pellicer, Eduardo Hernández-Álvarez, Amani Omrani, Ines Sifaoui, Hichem Sebai, Isabel L. Bazzocchi, José E. Piñero, Ignacio A. Jiménez, and Jacob Lorenzo-Morales. 2025. "Mentha rotundifolia, a Source of Amoebicidal Agents Against Naegleria fowleri" International Journal of Molecular Sciences 26, no. 18: 9048. https://doi.org/10.3390/ijms26189048
APA StyleBen Youssef, M., Chao-Pellicer, J., Hernández-Álvarez, E., Omrani, A., Sifaoui, I., Sebai, H., Bazzocchi, I. L., Piñero, J. E., Jiménez, I. A., & Lorenzo-Morales, J. (2025). Mentha rotundifolia, a Source of Amoebicidal Agents Against Naegleria fowleri. International Journal of Molecular Sciences, 26(18), 9048. https://doi.org/10.3390/ijms26189048











