Functional Complexity of Thermogenic Adipose Tissue: From Thermogenesis to Metabolic and Fibroinflammatory Crosstalk
Abstract
1. Adipose Tissue: Origins and Structural Characteristics
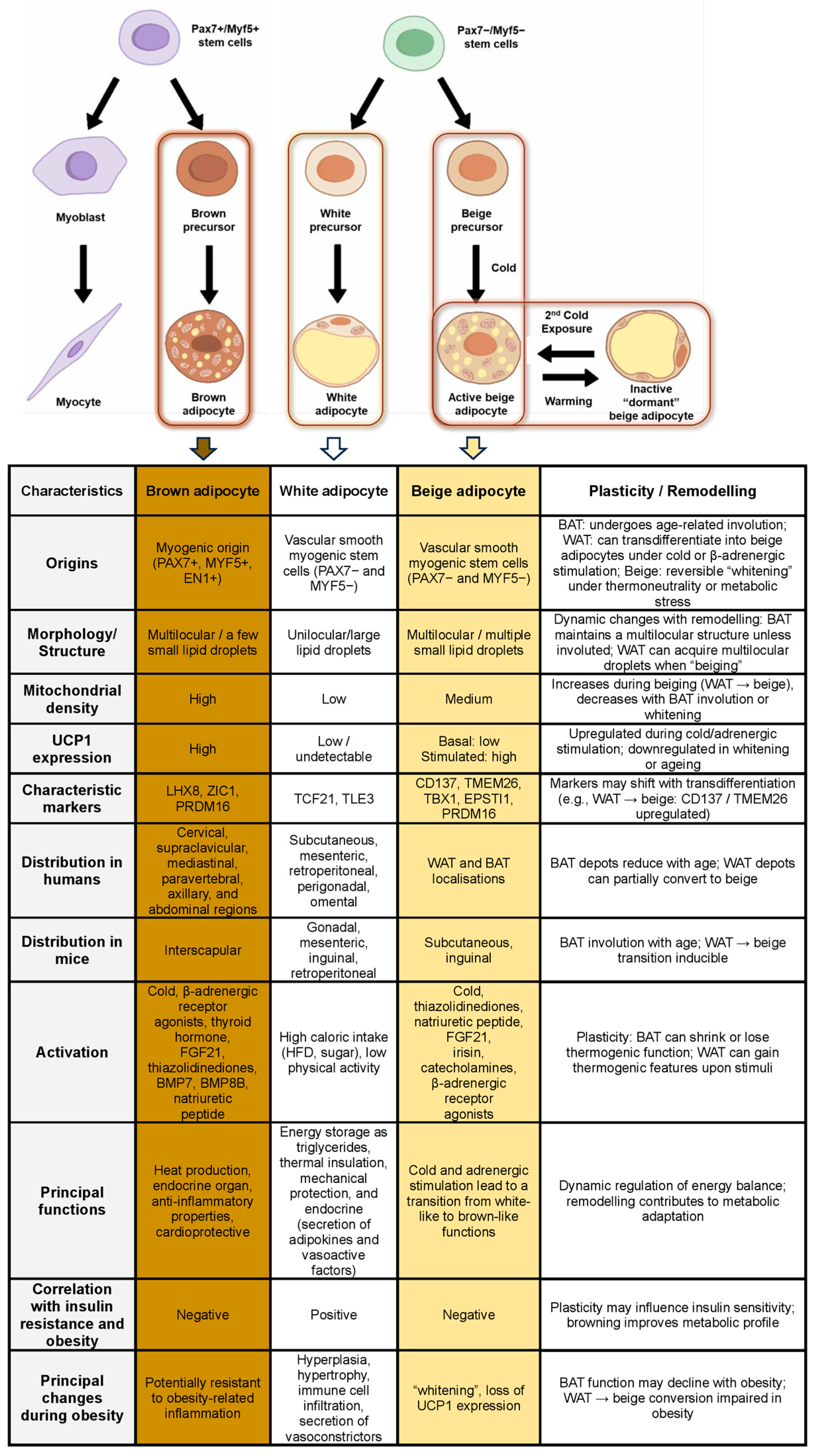
1.1. White Adipose Tissue
1.2. Beige Adipose Tissue
1.3. Brown Adipose Tissue
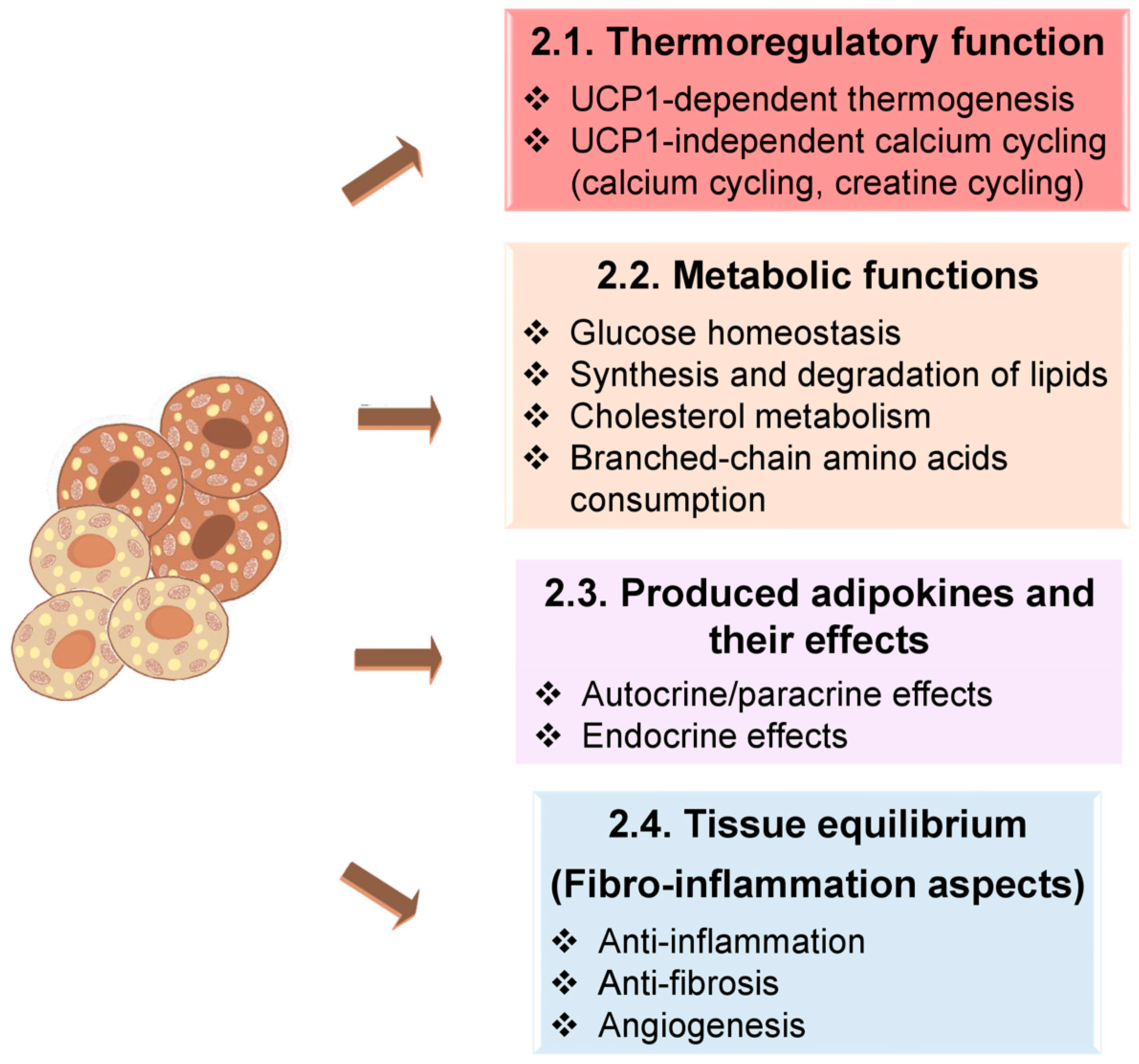
2. How Does BAT Work?
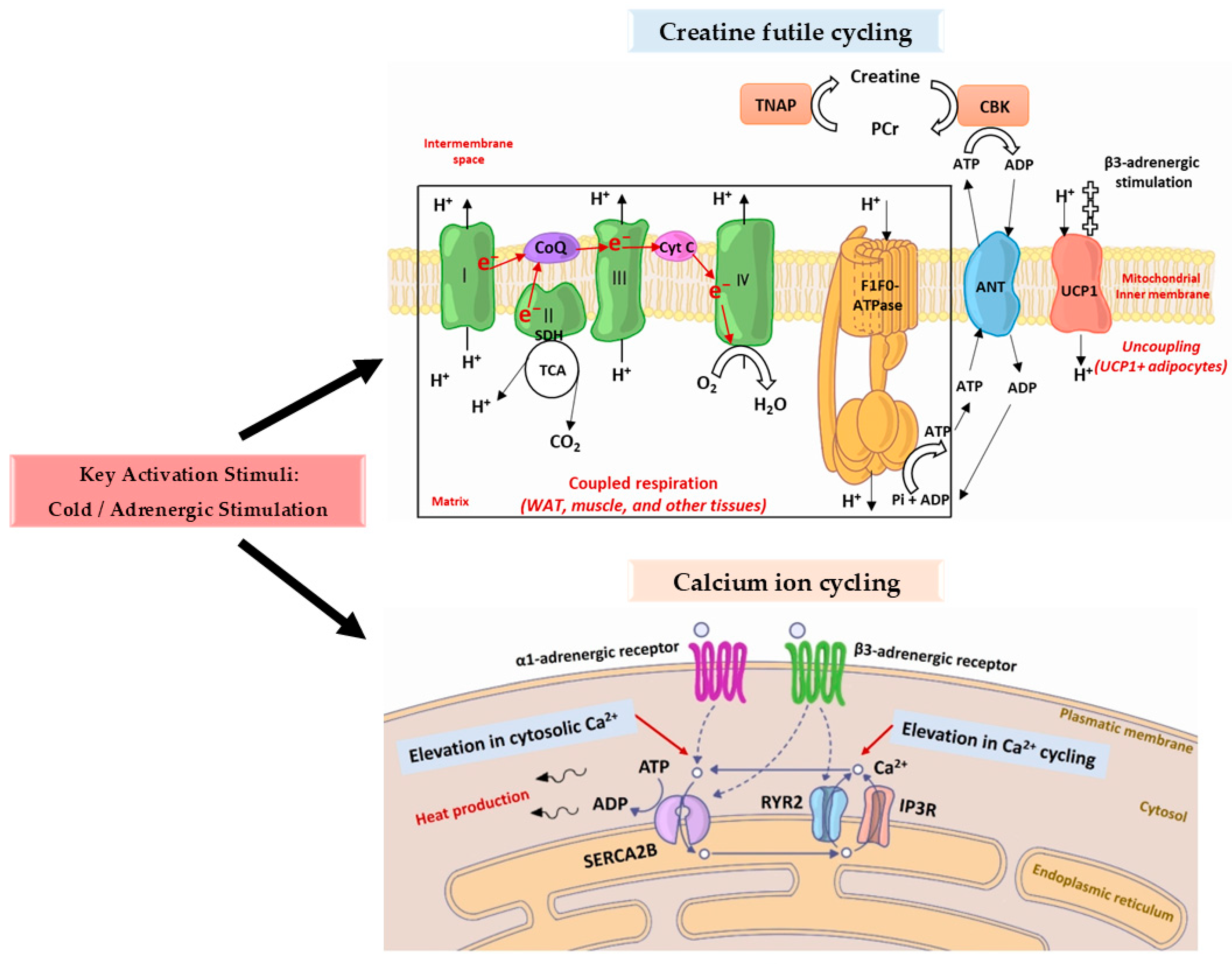
2.1. Thermoregulatory Function
- ❖
- The import of calcium ions into the intracellular space is stimulated by activated α1-adrenergic receptors.
- ❖
- Type 2 RYR (RYR2) is stimulated by activated β3-adrenergic receptors, resulting in an increase in calcium ion discharge from the endoplasmic reticulum.
2.2. Principal Metabolic Functions
2.2.1. Glucose Homeostasis
- ❖
- Genetic deletion of UCP1 in murine models leads to hyperglycaemia, hyperinsulinaemia, and increased adiposity [86].
- ❖
- ❖
- ❖
- In models with PRDM16 overexpression but lacking UCP1, glucose uptake remained elevated, suggesting that BeigeAT retains insulin sensitivity independently of UCP1-mediated thermogenesis [69].
- ❖
- Studies have identified a non-thermogenic function of BAT in the regulation of systemic insulin sensitivity. Specifically, impaired mitochondrial BCAA uptake via the transporter Solute Carrier Family 25 Member 44 (SLC25A44) in BAT leads to intracellular accumulation of BCAAs and their ketoacid derivatives (BCKAs), resulting in elevated oxidative stress, disrupted hepatic insulin signalling, and widespread insulin resistance. Notably, these effects occur independently of changes in energy expenditure or overall adiposity [90,91].
- ❖
- ❖
- In obese individuals, reduced BAT thermogenesis and insulin sensitivity are commonly observed. Nonetheless, multiple studies suggest that activation of thermogenic pathways, regardless of the stimulus, can restore glycaemic control and insulin responsiveness in this population [97]. Indeed, human imaging studies suggest that declines in BAT glucose uptake often precede measurable changes in thermogenic activity and may serve as sensitive early indicators of metabolic dysfunction [31,32,98].
2.2.2. Synthesis and Degradation of Lipids
- ❖
- Cold stimulation of the body initiates hepatic conversion of cholesterol into bile acids, followed by their excretion.
- ❖
- These bile acids play a key role in maintaining intestinal microbiota homeostasis.
- ❖
- In turn, the bile acids stimulate gut microbial activity and metabolite production.
- ❖
- The combination of microbiota-derived signalling molecules and bile acids in the circulation activates brown adipocytes.
- ❖
- Activated BAT secretes phospholipid transfer protein (PLTP).
- ❖
- PLTP supports HDL biogenesis, particularly in structural stabilisation.
- ❖
- The resulting HDL particles reduce circulating ceramide and phospholipid levels and enhance lipid excretion via faeces.
2.2.3. Branched-Chain Amino Acid Consumption
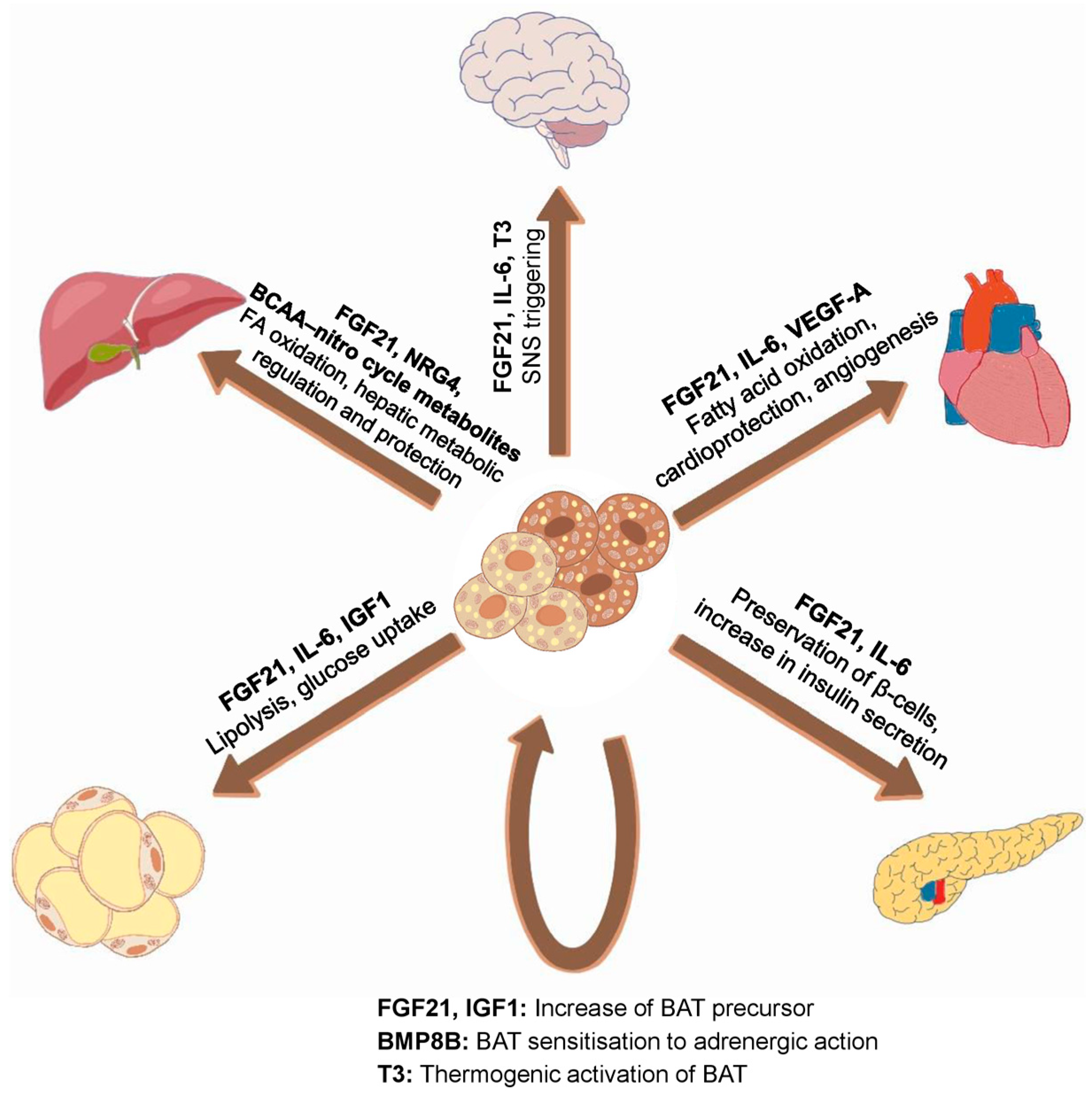
2.3. Produced Adipokines and Their Effects
2.4. Fibro-Inflammatory Aspects
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 12,13-diHOME | 12,13-dihydroxy-9z-octadecenoic acid |
| AMPK | AMP-activated protein kinase |
| ANP | atrial natriuretic peptide |
| ANT | adenine nucleotide translocator |
| APOA5 | apolipoprotein A-V |
| ATGL | adipose triglyceride lipase |
| ATP | adenosine triphosphate |
| BAT | brown adipose tissue |
| BCAAs | branched-chain amino acids |
| BCAT1/2 | branched-chain amino acid aminotransferase 1 or 2 |
| BCAT2 | branched-chain amino acid transaminase 2 |
| BCKAs | branched-chain keto acids |
| BCKDHA | branched-chain keto acid dehydrogenase e1 α subunit |
| BCKDHB | branched-chain keto acid dehydrogenase e1 β subunit |
| BeigeAT | beige adipose tissue |
| BHB | β-hydroxybutyrate |
| BMP | bone morphogenetic protein |
| BMP7 | bone morphogenetic protein 7 |
| BMP8B | bone morphogenetic protein 8b |
| C4orf3 | chromosome 4 open reading frame 3 |
| CACT | carnitine-acylcarnitine translocase |
| Ca2+ | calcium |
| CD36 | cluster of differentiation 36 |
| CFH | complement factor H |
| CGI-58 | coactivator comparative gene identification 58 |
| cGMP–p38 MAPK | cyclic guanosine monophosphate–p38 mitogen-activated protein kinase |
| CIDEA | Cell death–inducing DNA fragmentation factor-like effector A |
| CKB | creatine kinase B |
| COL6A3 | collagen type VI α 3 |
| CoQ | coenzyme Q |
| CPT1 | carnitine palmitoyltransferase I |
| CPT2 | carnitine palmitoyltransferase II |
| CTGF | connective tissue growth factor |
| CXCL14 | C-X-C motif chemokine ligand 14 |
| Cyt C | cytochrome C |
| ECM | extracellular matrix |
| EN1 | engrailed 1 |
| EPSTI1 | epithelial stromal interaction 1 |
| ETC | electron transport chain |
| ETP | endotrophin |
| FAs | fatty acids |
| FGF21 | fibroblast growth factor 21 |
| GDF15 | growth differentiation factor 15 |
| GLUT1 | glucose transporter type 1 |
| GLUT4 | glucose transporter type 4 |
| GTF2IRD1 | general transcription factor 2 I repeat domain-containing 1 |
| HDL-C | high-density lipoprotein cholesterol |
| IL-1β | interleukin-1β |
| IL-6 | Interleukin-6 |
| Ile | isoleucine |
| IP3R | inositol trisphosphate receptors |
| LAMA4 | laminin α 4 |
| Leu | leucine |
| LHX8 | lim homeobox protein 8 |
| LPL | lipoprotein lipase |
| miR-99b | microRNA-99b |
| mmBCFAs | monomethyl branched-chain fatty acids |
| MMP | metalloproteinase |
| mTORC2 | mechanistic target of rapamycin complex 2 |
| MYF5 | myogenic factor 5 |
| NGF | nerve growth factor |
| NRG4 | neuregulin 4 |
| PAX7 | paired box 7 |
| PCr | phosphocreatine |
| PD-L1 | programmed death ligand 1 |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1-α |
| Pi | inorganic phosphate |
| PLTP | phospholipid transfer protein |
| PPARγ | peroxisome proliferator activated receptor |
| PRDM16 | pr domain containing 16 |
| ROS | reactive oxygen species |
| RYR2 | type 2 RYR |
| RYRs | ryanodine receptors |
| S100B | s100 calcium-binding protein B |
| SDH | succinate dehydrogenase |
| SERCA | sarcoplasmic/endoplasmic reticulum calcium ATPase |
| SERCA1 | type 1 SERCA |
| SERCA2B | type 2b SERCA |
| SIRT5 | desuccinylase sirtuin 5 |
| SLC25A44 | solute carrier family 25 member 44 |
| SLIT2 | slit guidance ligand 2 |
| Sln | sarcolipin |
| SNS | sympathetic nervous system |
| T2D | type 2 diabetes |
| T3 | triiodothyronine |
| TBX1 | t-box 1 |
| TCA | tricarboxylic acid |
| TCF21 | transcription factor 21 |
| TGF-β | transforming growth factor-β |
| TLE3 | transducin-like enhancer of split 3 |
| TMEM26 | transmembrane protein 26 |
| TNAP | tissue-nonspecific alkaline phosphatase |
| TNF-α | tumour necrosis factor-α |
| TRLs | triglyceride-rich lipoproteins |
| UCP1 | uncoupling protein 1 |
| Val | valine |
| VEGF-A | vascular endothelial growth factor A |
| VLDL-TG | very-low-density lipoprotein triglycerides |
| WAT | white adipose tissue |
| ZIC1 | zinc finger protein of the cerebellum 1 |
References
- Cypess, A.M. Reassessing Human Adipose Tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef]
- Mo, Y.-Y.; Han, Y.-X.; Xu, S.-N.; Jiang, H.-L.; Wu, H.-X.; Cai, J.-M.; Li, L.; Bu, Y.-H.; Xiao, F.; Liang, H.-D.; et al. Adipose Tissue Plasticity: A Comprehensive Definition and Multidimensional Insight. Biomolecules 2024, 14, 1223. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Trivisonno, A.; Trivisonno, G.; Toietta, G. Dissecting Human Adipose Tissue Heterogeneity Using Single-cell Omics Technologies. Stem Cell Res. Ther. 2024, 15, 322. [Google Scholar] [CrossRef]
- Sun, K.; Li, X.; Scherer, P.E. Extracellular Matrix (ECM) and Fibrosis in Adipose Tissue: Overview and Perspectives. Compr. Physiol. 2023, 13, 4387–4407. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Valentić, S.; Šestan, M.; Turk Wensveen, T.; Polić, B. The “Big Bang” in Obese Fat: Events Initiating Obesity-Induced Adipose Tissue Inflammation. Eur. J. Immunol. 2015, 45, 2446–2456. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of Fat Cell Turnover in Humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Sender, R.; Milo, R. The Distribution of Cellular Turnover in the Human Body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.C.; Sampath, S.C.; Bredella, M.A.; Cypess, A.M.; Torriani, M. Imaging of Brown Adipose Tissue: State of the Art. Radiology 2016, 280, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Stenesen, D.; Zeve, D.; Graff, J.M. The Developmental Origins of Adipose Tissue. Development 2013, 140, 3939–3949. [Google Scholar] [CrossRef]
- Berry, R.; Rodeheffer, M.S. Characterization of the Adipocyte Cellular Lineage in Vivo. Nat. Cell Biol. 2013, 15, 302–308. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy Percentage Body Fat Ranges: An Approach for Developing Guidelines Based on Body Mass Index123. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef]
- Levitt, D.G.; Heymsfield, S.B.; Pierson, R.N.; Shapses, S.A.; Kral, J.G. Physiological Models of Body Composition and Human Obesity. Nutr. Metab. 2007, 4, 19, Erratum in Nutr. Metab. 2009, 6, 197. [Google Scholar] [CrossRef]
- Emont, M.P.; Jacobs, C.; Essene, A.L.; Pant, D.; Tenen, D.; Colleluori, G.; Di Vincenzo, A.; Jørgensen, A.M.; Dashti, H.; Stefek, A.; et al. A Single-Cell Atlas of Human and Mouse White Adipose Tissue. Nature 2022, 603, 926–933, Erratum in Nature 2023, 620, E14. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What We Talk about When We Talk about Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and Adipose Tissue Dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Arner, P.; Andersson, D.P.; Bäckdahl, J.; Dahlman, I.; Rydén, M. Weight Gain and Impaired Glucose Metabolism in Women Are Predicted by Inefficient Subcutaneous Fat Cell Lipolysis. Cell Metab. 2018, 28, 45–54.e3. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, V.G.; Aroor, A.R.; Sowers, J.R. The Pathophysiology of Hypertension in Patients with Obesity. Nat. Rev. Endocrinol. 2014, 10, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; McKarns, S.; Demarco, V.G.; Jia, G.; Sowers, J.R. Maladaptive Immune and Inflammatory Pathways Lead to Cardiovascular Insulin Resistance. Metabolism 2013, 62, 1543–1552. [Google Scholar] [CrossRef]
- Long, J.Z.; Svensson, K.J.; Tsai, L.; Zeng, X.; Roh, H.C.; Kong, X.; Rao, R.R.; Lou, J.; Lokurkar, I.; Baur, W.; et al. A Smooth Muscle-like Origin for Beige Adipocytes. Cell Metab. 2014, 19, 810–820. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 Controls a Brown Fat/Skeletal Muscle Switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the White Adipose Tissue Regulation: New Insights into Nutritional and Metabolic Relevance in Health and Diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef]
- Lizcano, F. The Beige Adipocyte as a Therapy for Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 5058. [Google Scholar] [CrossRef]
- Schulz, T.J.; Tseng, Y.-H. Brown Adipose Tissue: Development, Metabolism and Beyond. Biochem. J. 2013, 453, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Weng, Y.; Shi, F.; Jin, W. The Engrailed-1 Gene Stimulates Brown Adipogenesis. Stem Cells Int. 2016, 2016, 7369491. [Google Scholar] [CrossRef] [PubMed]
- Lidell, M.E.; Betz, M.J.; Dahlqvist Leinhard, O.; Heglind, M.; Elander, L.; Slawik, M.; Mussack, T.; Nilsson, D.; Romu, T.; Nuutila, P.; et al. Evidence for Two Types of Brown Adipose Tissue in Humans. Nat. Med. 2013, 19, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Leitner, B.P.; Huang, S.; Brychta, R.J.; Duckworth, C.J.; Baskin, A.S.; McGehee, S.; Tal, I.; Dieckmann, W.; Gupta, G.; Kolodny, G.M.; et al. Mapping of Human Brown Adipose Tissue in Lean and Obese Young Men. Proc. Natl. Acad. Sci. USA 2017, 114, 8649–8654. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, G.; Shao, M.; Nham, K.; An, Y.; Wang, Q.; Zhu, Y.; Kusminski, C.M.; Hassan, G.; Gupta, R.K.; et al. An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab. 2018, 27, 252–262.e3. [Google Scholar] [CrossRef]
- Jalloul, W.; Moscalu, M.; Moscalu, R.; Jalloul, D.; Grierosu, I.C.; Gutu, M.; Haba, D.; Mocanu, V.; Gutu, M.M.; Stefanescu, C. Are MTV and TLG Accurate for Quantifying the Intensity of Brown Adipose Tissue Activation? Biomedicines 2024, 12, 151. [Google Scholar] [CrossRef]
- Jalloul, W.; Moscalu, M.; Moscalu, R.; Jalloul, D.; Grierosu, I.C.; Ionescu, T.; Stolniceanu, C.R.; Ghizdovat, V.; Mocanu, V.; Iliescu, R.; et al. Off the Beaten Path in Oncology: Active Brown Adipose Tissue by Virtue of Molecular Imaging. Curr. Issues Mol. Biol. 2023, 45, 7891–7914. [Google Scholar] [CrossRef]
- Jalloul, W.; Moscalu, M.; Grierosu, I.; Ionescu, T.; Stolniceanu, C.R.; Gutu, M.; Ghizdovat, V.; Mocanu, V.; Azoicai, D.; Iliescu, R.; et al. Brown Adipose Tissue Biodistribution and Correlations Particularities in Parathyroid Pathology Personalized Diagnosis. Diagnostics 2022, 12, 3182. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.C.; Pauli, J.R.; Shulman, G.I.; Muñoz, V.R. An Update on Brown Adipose Tissue Biology: A Discussion of Recent Findings. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E488–E495. [Google Scholar] [CrossRef]
- Enerbäck, S. The Origins of Brown Adipose Tissue. N. Engl. J. Med. 2009, 360, 2021–2023. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Cannon, B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Poekes, L.; Lanthier, N.; Leclercq, I.A. Brown Adipose Tissue: A Potential Target in the Fight against Obesity and the Metabolic Syndrome. Clin. Sci. 2015, 129, 933–949. [Google Scholar] [CrossRef]
- Shao, M.; Wang, Q.A.; Song, A.; Vishvanath, L.; Busbuso, N.C.; Scherer, P.E.; Gupta, R.K. Cellular Origins of Beige Fat Cells Revisited. Diabetes 2019, 68, 1874–1885. [Google Scholar] [CrossRef]
- Bordicchia, M.; Liu, D.; Amri, E.-Z.; Ailhaud, G.; Dessì-Fulgheri, P.; Zhang, C.; Takahashi, N.; Sarzani, R.; Collins, S. Cardiac Natriuretic Peptides Act via P38 MAPK to Induce the Brown Fat Thermogenic Program in Mouse and Human Adipocytes. J. Clin. Investig. 2012, 122, 1022–1036. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Takahashi, N.; Yasubuchi, M.; Kim, Y.-I.; Hashizaki, H.; Kim, M.-J.; Sakamoto, T.; Goto, T.; Kawada, T. Triiodothyronine Induces UCP-1 Expression and Mitochondrial Biogenesis in Human Adipocytes. Am. J. Physiol. Cell Physiol. 2012, 302, C463–C472. [Google Scholar] [CrossRef]
- De Jesus, L.A.; Carvalho, S.D.; Ribeiro, M.O.; Schneider, M.; Kim, S.W.; Harney, J.W.; Larsen, P.R.; Bianco, A.C. The Type 2 Iodothyronine Deiodinase Is Essential for Adaptive Thermogenesis in Brown Adipose Tissue. J. Clin. Investig. 2001, 108, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New Role of Bone Morphogenetic Protein 7 in Brown Adipogenesis and Energy Expenditure. Nature 2008, 454, 1000–1004, Erratum in Nature 2009, 459, 122.. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1α-Dependent Myokine That Drives Browning of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Xue, S.; Lee, D.; Berry, D.C. Thermogenic Adipose Tissue in Energy Regulation and Metabolic Health. Front. Endocrinol. 2023, 14, 1150059. [Google Scholar] [CrossRef]
- Abdelhafez, Y.G.; Wang, G.; Li, S.; Pellegrinelli, V.; Chaudhari, A.J.; Ramirez, A.; Sen, F.; Vidal-Puig, A.; Sidossis, L.S.; Klein, S.; et al. The Role of Brown Adipose Tissue in Branched-Chain Amino Acid Clearance in People. iScience 2024, 27, 110559. [Google Scholar] [CrossRef]
- Ying, Z.; Tramper, N.; Zhou, E.; Boon, M.R.; Rensen, P.C.N.; Kooijman, S. Role of Thermogenic Adipose Tissue in Lipid Metabolism and Atherosclerotic Cardiovascular Disease: Lessons from Studies in Mice and Humans. Cardiovasc. Res. 2023, 119, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Berbée, J.F.P.; Boon, M.R.; Khedoe, P.P.S.J.; Bartelt, A.; Schlein, C.; Worthmann, A.; Kooijman, S.; Hoeke, G.; Mol, I.M.; John, C.; et al. Brown Fat Activation Reduces Hypercholesterolaemia and Protects from Atherosclerosis Development. Nat. Commun. 2015, 6, 6356. [Google Scholar] [CrossRef] [PubMed]
- Ziqubu, K.; Dludla, P.V.; Mabhida, S.E.; Jack, B.U.; Keipert, S.; Jastroch, M.; Mazibuko-Mbeje, S.E. Brown Adipose Tissue-Derived Metabolites and Their Role in Regulating Metabolism. Metabolism 2024, 150, 155709. [Google Scholar] [CrossRef]
- Markina, N.O.; Matveev, G.A.; Zasypkin, G.G.; Golikova, T.I.; Ryzhkova, D.V.; Kononova, Y.A.; Danilov, S.D.; Babenko, A.Y. Role of Brown Adipose Tissue in Metabolic Health and Efficacy of Drug Treatment for Obesity. J. Clin. Med. 2024, 13, 4151. [Google Scholar] [CrossRef]
- Cohen, P.; Kajimura, S. The Cellular and Functional Complexity of Thermogenic Fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- Chen, K.Y.; Brychta, R.J.; Abdul Sater, Z.; Cassimatis, T.M.; Cero, C.; Fletcher, L.A.; Israni, N.S.; Johnson, J.W.; Lea, H.J.; Linderman, J.D.; et al. Opportunities and Challenges in the Therapeutic Activation of Human Energy Expenditure and Thermogenesis to Manage Obesity. J. Biol. Chem. 2020, 295, 1926–1942. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M. UCP1—A Sophisticated Energy Valve. Biochimie 2017, 134, 19–27. [Google Scholar] [CrossRef]
- Symonds, M.E. Brown Adipose Tissue Growth and Development. Scientifica 2013, 2013, 305763. [Google Scholar] [CrossRef]
- Schreiber, R.; Diwoky, C.; Schoiswohl, G.; Feiler, U.; Wongsiriroj, N.; Abdellatif, M.; Kolb, D.; Hoeks, J.; Kershaw, E.E.; Sedej, S.; et al. Cold-Induced Thermogenesis Depends on ATGL-Mediated Lipolysis in Cardiac Muscle, but Not Brown Adipose Tissue. Cell Metab. 2017, 26, 753–763.e7. [Google Scholar] [CrossRef]
- Shin, H.; Ma, Y.; Chanturiya, T.; Cao, Q.; Wang, Y.; Kadegowda, A.K.G.; Jackson, R.; Rumore, D.; Xue, B.; Shi, H.; et al. Lipolysis in Brown Adipocytes Is Not Essential for Cold-Induced Thermogenesis in Mice. Cell Metab. 2017, 26, 764–777.e5. [Google Scholar] [CrossRef] [PubMed]
- Mouisel, E.; Bodon, A.; Noll, C.; Cassant-Sourdy, S.; Marques, M.-A.; Flores-Flores, R.; Riant, E.; Bergoglio, C.; Vezin, P.; Caspar-Bauguil, S.; et al. Cold-Induced Thermogenesis Requires Neutral-Lipase-Mediated Intracellular Lipolysis in Brown Adipocytes. Cell Metab. 2025, 37, 429–440.e5. [Google Scholar] [CrossRef]
- Anderson, C.M.; Kazantzis, M.; Wang, J.; Venkatraman, S.; Goncalves, R.L.S.; Quinlan, C.L.; Ng, R.; Jastroch, M.; Benjamin, D.I.; Nie, B.; et al. Dependence of Brown Adipose Tissue Function on CD36-Mediated Coenzyme Q Uptake. Cell Rep. 2015, 10, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Putri, M.; Syamsunarno, M.R.A.A.; Iso, T.; Yamaguchi, A.; Hanaoka, H.; Sunaga, H.; Koitabashi, N.; Matsui, H.; Yamazaki, C.; Kameo, S.; et al. CD36 Is Indispensable for Thermogenesis under Conditions of Fasting and Cold Stress. Biochem. Biophys. Res. Commun. 2015, 457, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.; Geoghegan, G.; Maschek, J.A.; Bensard, C.L.; Pasquali, M.; Miao, R.; Lee, S.; Jiang, L.; Huck, I.; Kershaw, E.E.; et al. Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell Metab. 2017, 26, 509–522.e6. [Google Scholar] [CrossRef]
- McGarry, J.D.; Brown, N.F. The Mitochondrial Carnitine Palmitoyltransferase System. From Concept to Molecular Analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef]
- Wade, G.; McGahee, A.; Ntambi, J.M.; Simcox, J. Lipid Transport in Brown Adipocyte Thermogenesis. Front. Physiol. 2021, 12, 787535. [Google Scholar] [CrossRef] [PubMed]
- Rahbani, J.F.; Roesler, A.; Hussain, M.F.; Samborska, B.; Dykstra, C.B.; Tsai, L.; Jedrychowski, M.P.; Vergnes, L.; Reue, K.; Spiegelman, B.M.; et al. Creatine Kinase B Controls Futile Creatine Cycling in Thermogenic Fat. Nature 2021, 590, 480–485. [Google Scholar] [CrossRef]
- Bunk, J.; Hussain, M.F.; Delgado-Martin, M.; Samborska, B.; Ersin, M.; Shaw, A.; Rahbani, J.F.; Kazak, L. The Futile Creatine Cycle Powers UCP1-Independent Thermogenesis in Classical BAT. Nat. Commun. 2025, 16, 3221. [Google Scholar] [CrossRef]
- Vargas-Castillo, A.; Sun, Y.; Smythers, A.L.; Grauvogel, L.; Dumesic, P.A.; Emont, M.P.; Tsai, L.T.; Rosen, E.D.; Zammit, N.W.; Shaffer, S.M.; et al. Development of a Functional Beige Fat Cell Line Uncovers Independent Subclasses of Cells Expressing UCP1 and the Futile Creatine Cycle. Cell Metab. 2024, 36, 2146–2155.e5. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Jedrychowski, M.P.; Lu, G.Z.; Erickson, B.K.; Szpyt, J.; Pierce, K.A.; Laznik-Bogoslavski, D.; Vetrivelan, R.; Clish, C.B.; et al. Mitochondrial ROS Regulate Thermogenic Energy Expenditure and Sulfenylation of UCP1. Nature 2016, 532, 112–116, Erratum in Nature 2016, 536, 360. [Google Scholar] [CrossRef]
- Wang, G.; Meyer, J.G.; Cai, W.; Softic, S.; Li, M.E.; Verdin, E.; Newgard, C.; Schilling, B.; Kahn, C.R. Regulation of UCP1 and Mitochondrial Metabolism in Brown Adipose Tissue by Reversible Succinylation. Mol. Cell 2019, 74, 844–857.e7. [Google Scholar] [CrossRef]
- de Meis, L. Uncoupled ATPase Activity and Heat Production by the Sarcoplasmic Reticulum Ca2+-ATPase. Regulation by ADP. J. Biol. Chem. 2001, 276, 25078–25087. [Google Scholar] [CrossRef]
- Bal, N.C.; Maurya, S.K.; Sopariwala, D.H.; Sahoo, S.K.; Gupta, S.C.; Shaikh, S.A.; Pant, M.; Rowland, L.A.; Bombardier, E.; Goonasekera, S.A.; et al. Sarcolipin Is a Newly Identified Regulator of Muscle-Based Thermogenesis in Mammals. Nat. Med. 2012, 18, 1575–1579, Erratum in Nat. Med. 2012, 18, 1857. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-Independent Signaling Involving SERCA2b-Mediated Calcium Cycling Regulates Beige Fat Thermogenesis and Systemic Glucose Homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Ikeda, K.; Tanabe, Y.; Thomson, E.A.; Yoneshiro, T.; Oguri, Y.; Ferro, M.D.; Poon, A.S.Y.; Kajimura, S. Wireless Optogenetics Protects against Obesity via Stimulation of Non-Canonical Fat Thermogenesis. Nat. Commun. 2020, 11, 1730. [Google Scholar] [CrossRef]
- Aquilano, K.; Sciarretta, F.; Turchi, R.; Li, B.-H.; Rosina, M.; Ceci, V.; Guidobaldi, G.; Arena, S.; D’Ambrosio, C.; Audano, M.; et al. Low-Protein/High-Carbohydrate Diet Induces AMPK-Dependent Canonical and Non-Canonical Thermogenesis in Subcutaneous Adipose Tissue. Redox Biol. 2020, 36, 101633. [Google Scholar] [CrossRef]
- Jones, S.A.; Sowton, A.P.; Lacabanne, D.; King, M.S.; Palmer, S.M.; Zögg, T.; Pardon, E.; Steyaert, J.; Ruprecht, J.J.; Kunji, E.R.S. Proton Conductance by Human Uncoupling Protein 1 Is Inhibited by Purine and Pyrimidine Nucleotides. EMBO J. 2025, 44, 2353–2365. [Google Scholar] [CrossRef]
- Bast-Habersbrunner, A.; Fromme, T. Purine Nucleotides in the Regulation of Brown Adipose Tissue Activity. Front. Endocrinol. 2020, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Divakaruni, A.S.; Humphrey, D.M.; Brand, M.D. Fatty Acids Change the Conformation of Uncoupling Protein 1 (UCP1). J. Biol. Chem. 2012, 287, 36845–36853. [Google Scholar] [CrossRef]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A Creatine-Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef]
- Guarnieri, A.R.; Benson, T.W.; Tranter, M. Calcium Cycling as a Mediator of Thermogenic Metabolism in Adipose Tissue. Mol. Pharmacol. 2022, 102, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Khandelwal, R.; Wolfrum, C. Futile Lipid Cycling: From Biochemistry to Physiology. Nat. Metab. 2024, 6, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Oeckl, J.; Janovska, P.; Adamcova, K.; Bardova, K.; Brunner, S.; Dieckmann, S.; Ecker, J.; Fromme, T.; Funda, J.; Gantert, T.; et al. Loss of UCP1 Function Augments Recruitment of Futile Lipid Cycling for Thermogenesis in Murine Brown Fat. Mol. Metab. 2022, 61, 101499. [Google Scholar] [CrossRef]
- Auger, C.; Li, M.; Fujimoto, M.; Ikeda, K.; Yook, J.-S.; O’Leary, T.R.; Caycedo, M.P.H.; Xiaohan, C.; Oikawa, S.; Verkerke, A.R.P.; et al. Identification of a Molecular Resistor That Controls UCP1-Independent Ca2+ Cycling Thermogenesis in Adipose Tissue. Cell Metab. 2025, 37, 1311–1325.e9. [Google Scholar] [CrossRef]
- Tabei, S.; Chamorro, R.; Meyhöfer, S.M.; Wilms, B. Metabolic Effects of Brown Adipose Tissue Activity Due to Cold Exposure in Humans: A Systematic Review and Meta-Analysis of RCTs and Non-RCTs. Biomedicines 2024, 12, 537. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Z.; Moazzami, Z.; Heck, R.; Hu, P.; Nanda, H.; Ren, K.; Sun, Z.; Bartolomucci, A.; Gao, Y.; et al. Brown Adipose Tissue Involution Associated with Progressive Restriction in Progenitor Competence. Cell Rep. 2022, 39, 110575. [Google Scholar] [CrossRef]
- Yu, X.; Benitez, G.; Wei, P.T.; Krylova, S.V.; Song, Z.; Liu, L.; Zhang, M.; Xiaoli, A.M.; Wei, H.; Chen, F.; et al. Involution of Brown Adipose Tissue through a Syntaxin 4 Dependent Pyroptosis Pathway. Nat. Commun. 2024, 15, 2856. [Google Scholar] [CrossRef]
- Guerra, C.; Navarro, P.; Valverde, A.M.; Arribas, M.; Brüning, J.; Kozak, L.P.; Kahn, C.R.; Benito, M. Brown Adipose Tissue-Specific Insulin Receptor Knockout Shows Diabetic Phenotype without Insulin Resistance. J. Clin. Investig. 2001, 108, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Dallner, O.S.; Chernogubova, E.; Brolinson, K.A.; Bengtsson, T. Beta3-Adrenergic Receptors Stimulate Glucose Uptake in Brown Adipocytes by Two Mechanisms Independently of Glucose Transporter 4 Translocation. Endocrinology 2006, 147, 5730–5739. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.M.; Sato, M.; Dallner, O.S.; Sandström, A.L.; Pisani, D.F.; Chambard, J.-C.; Amri, E.-Z.; Hutchinson, D.S.; Bengtsson, T. Glucose Uptake in Brown Fat Cells Is Dependent on mTOR Complex 2–Promoted GLUT1 Translocation. J. Cell Biol. 2014, 207, 365–374. [Google Scholar] [CrossRef]
- Lowell, B.B.; S-Susulic, V.; Hamann, A.; Lawitts, J.A.; Himms-Hagen, J.; Boyer, B.B.; Kozak, L.P.; Flier, J.S. Development of Obesity in Transgenic Mice after Genetic Ablation of Brown Adipose Tissue. Nature 1993, 366, 740–742. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.W.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.-H.; et al. Brown Adipose Tissue Regulates Glucose Homeostasis and Insulin Sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef]
- de Souza, C.J.; Hirshman, M.F.; Horton, E.S. CL-316,243, a Beta3-Specific Adrenoceptor Agonist, Enhances Insulin-Stimulated Glucose Disposal in Nonobese Rats. Diabetes 1997, 46, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Lo, J.C.; Zeng, X.; Ye, L.; Khandekar, M.J.; et al. Ablation of PRDM16 and Beige Adipose Causes Metabolic Dysfunction and a Subcutaneous to Visceral Fat Switch. Cell 2014, 156, 304–316. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. BCAA Catabolism in Brown Fat Controls Energy Homeostasis through SLC25A44. Nature 2019, 572, 614–619. [Google Scholar] [CrossRef]
- Verkerke, A.R.P.; Wang, D.; Yoshida, N.; Taxin, Z.H.; Shi, X.; Zheng, S.; Li, Y.; Auger, C.; Oikawa, S.; Yook, J.-S.; et al. BCAA-Nitrogen Flux in Brown Fat Controls Metabolic Health Independent of Thermogenesis. Cell 2024, 187, 2359–2374.e18. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, S.-W.; Tang, Y.; Tang, Q.-Q. The Secretory Function of Adipose Tissues in Metabolic Regulation. Life Metab. 2024, 3, loae003. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.D.L.; Gao, J.; Yang, Q.; Wu, Z.; Gromada, J. Fibroblast Growth Factor 21 Regulates Energy Metabolism by Activating the AMPK-SIRT1-PGC-1alpha Pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12553–12558. [Google Scholar] [CrossRef]
- Lewis, J.E.; Monnier, C.; Marshall, H.; Fowler, M.; Green, R.; Cooper, S.; Chiotellis, A.; Luckett, J.; Perkins, A.C.; Coskun, T.; et al. Whole-Body and Adipose Tissue-Specific Mechanisms Underlying the Metabolic Effects of Fibroblast Growth Factor 21 in the Siberian Hamster. Mol. Metab. 2020, 31, 45–54. [Google Scholar] [CrossRef]
- Hanssen, M.J.W.; Broeders, E.; Samms, R.J.; Vosselman, M.J.; van der Lans, A.A.J.J.; Cheng, C.C.; Adams, A.C.; van Marken Lichtenbelt, W.D.; Schrauwen, P. Serum FGF21 Levels Are Associated with Brown Adipose Tissue Activity in Humans. Sci. Rep. 2015, 5, 10275. [Google Scholar] [CrossRef]
- Justesen, S.; Haugegaard, K.V.; Hansen, J.B.; Hansen, H.S.; Andersen, B. The Autocrine Role of FGF21 in Cultured Adipocytes. Biochem. J. 2020, 477, 2477–2487. [Google Scholar] [CrossRef]
- Roberts-Toler, C.; O’Neill, B.T.; Cypess, A.M. Diet-Induced Obesity Causes Insulin Resistance in Mouse Brown Adipose Tissue. Obesity 2015, 23, 1765–1770. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P. Human Brown Adipose Tissue Is Not Enough to Combat Cardiometabolic Diseases. J. Clin. Investig. 2023, 133, 1150059. [Google Scholar] [CrossRef]
- Seki, T.; Yang, Y.; Sun, X.; Lim, S.; Xie, S.; Guo, Z.; Xiong, W.; Kuroda, M.; Sakaue, H.; Hosaka, K.; et al. Brown-Fat-Mediated Tumour Suppression by Cold-Altered Global Metabolism. Nature 2022, 608, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; An, K.; Ito, Y.; Kharbikar, B.N.; Sheng, R.; Paredes, B.; Murray, E.; Pham, K.; Bruck, M.; Zhou, X.; et al. Implantation of Engineered Adipocytes Suppresses Tumor Progression in Cancer Models. Nat. Biotechnol. 2025, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown Adipose Tissue Activity Controls Triglyceride Clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; John, C.; Schaltenberg, N.; Berbée, J.F.P.; Worthmann, A.; Cherradi, M.L.; Schlein, C.; Piepenburg, J.; Boon, M.R.; Rinninger, F.; et al. Thermogenic Adipocytes Promote HDL Turnover and Reverse Cholesterol Transport. Nat. Commun. 2017, 8, 15010. [Google Scholar] [CrossRef] [PubMed]
- Chondronikola, M.; Yoshino, J.; Ramaswamy, R.; Giardina, J.D.; Laforest, R.; Wahl, R.L.; Patterson, B.W.; Mittendorfer, B.; Klein, S. Very-Low-Density Lipoprotein Triglyceride and Free Fatty Acid Plasma Kinetics in Women with High or Low Brown Adipose Tissue Volume and Overweight/Obesity. Cell Rep. Med. 2024, 5, 101370. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, R.; Zhang, X.; Gao, Q.; Guo, X.; Gu, L.; Zhang, H.; Zhang, J.; Zheng, J.; Jiang, M. Associations between Brown Adipose Tissue Activity, Serum Lipid Profiles, and Cardiovascular Events: Insights from 18F-Fluorodeoxyglucose PET/Computed Tomography Analysis. Nucl. Med. Commun. 2025, 46, 855–861. [Google Scholar] [CrossRef]
- Cho, Y.K.; Lee, S.; Lee, J.; Doh, J.; Park, J.-H.; Jung, Y.-S.; Lee, Y.-H. Lipid Remodeling of Adipose Tissue in Metabolic Health and Disease. Exp. Mol. Med. 2023, 55, 1955–1973. [Google Scholar] [CrossRef]
- Magro, B.S.; Dias, D.P.M. Brown and Beige Adipose Tissue: New Therapeutic Targets for Metabolic Disorders. Health Sci. Rev. 2024, 10, 100148. [Google Scholar] [CrossRef]
- Sponton, C.H.; Hosono, T.; Taura, J.; Jedrychowski, M.P.; Yoneshiro, T.; Wang, Q.; Takahashi, M.; Matsui, Y.; Ikeda, K.; Oguri, Y.; et al. The Regulation of Glucose and Lipid Homeostasis via PLTP as a Mediator of BAT-Liver Communication. EMBO Rep. 2020, 21, e49828. [Google Scholar] [CrossRef]
- Worthmann, A.; John, C.; Rühlemann, M.C.; Baguhl, M.; Heinsen, F.-A.; Schaltenberg, N.; Heine, M.; Schlein, C.; Evangelakos, I.; Mineo, C.; et al. Cold-Induced Conversion of Cholesterol to Bile Acids in Mice Shapes the Gut Microbiome and Promotes Adaptive Thermogenesis. Nat. Med. 2017, 23, 839–849. [Google Scholar] [CrossRef]
- Balaz, M.; Becker, A.S.; Balazova, L.; Straub, L.; Müller, J.; Gashi, G.; Maushart, C.I.; Sun, W.; Dong, H.; Moser, C.; et al. Inhibition of Mevalonate Pathway Prevents Adipocyte Browning in Mice and Men by Affecting Protein Prenylation. Cell Metab. 2019, 29, 901–916.e8. [Google Scholar] [CrossRef] [PubMed]
- de Campos-Ferraz, P.L.; Ribeiro, S.M.L.; Luz, S.D.S.; Lancha, A.H.; Tirapegui, J. Exercise x BCAA Supplementation in Young Trained Rats: What Are Their Effects on Body Growth? J. Sports Sci. Med. 2011, 10, 483–490. [Google Scholar]
- Fernstrom, J.D. Branched-Chain Amino Acids and Brain Function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef]
- Neinast, M.D.; Jang, C.; Hui, S.; Murashige, D.S.; Chu, Q.; Morscher, R.J.; Li, X.; Zhan, L.; White, E.; Anthony, T.G.; et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched Chain Amino Acids. Cell Metab. 2019, 29, 417–429.e4. [Google Scholar] [CrossRef]
- Choi, B.H.; Hyun, S.; Koo, S.-H. The Role of BCAA Metabolism in Metabolic Health and Disease. Exp. Mol. Med. 2024, 56, 1552–1559. [Google Scholar] [CrossRef]
- Wallace, M.; Green, C.R.; Roberts, L.S.; Lee, Y.M.; McCarville, J.L.; Sanchez-Gurmaches, J.; Meurs, N.; Gengatharan, J.M.; Hover, J.D.; Phillips, S.A.; et al. Enzyme Promiscuity Drives Branched-Chain Fatty Acid Synthesis in Adipose Tissues. Nat. Chem. Biol. 2018, 14, 1021–1031. [Google Scholar] [CrossRef]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; Phillips, S.A.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-Chain Amino Acid Catabolism Fuels Adipocyte Differentiation and Lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef]
- Su, X.; Magkos, F.; Zhou, D.; Eagon, J.C.; Fabbrini, E.; Okunade, A.L.; Klein, S. Adipose Tissue Monomethyl Branched-Chain Fatty Acids and Insulin Sensitivity: Effects of Obesity and Weight Loss. Obesity 2015, 23, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, S.C.; Piston, D.W. Reversal of Type 1 Diabetes in Mice by Brown Adipose Tissue Transplant. Diabetes 2012, 61, 674–682. [Google Scholar] [CrossRef]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.E.; Kozak, L.P. Mice Lacking Mitochondrial Uncoupling Protein Are Cold-Sensitive but Not Obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Ali Khan, A.; Hansson, J.; Weber, P.; Foehr, S.; Krijgsveld, J.; Herzig, S.; Scheideler, M. Comparative Secretome Analyses of Primary Murine White and Brown Adipocytes Reveal Novel Adipokines. Mol. Cell Proteom. 2018, 17, 2358–2370. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Peijs, L.; Beaudry, J.L.; Jespersen, N.Z.; Nielsen, C.H.; Ma, T.; Brunner, A.D.; Larsen, T.J.; Bayarri-Olmos, R.; Prabhakar, B.S.; et al. Proteomics-Based Comparative Mapping of the Secretomes of Human Brown and White Adipocytes Reveals EPDR1 as a Novel Batokine. Cell Metab. 2019, 30, 963–975.e7. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, J.; Cereijo, R.; Giralt, M.; Villarroya, F. Secretory Proteome of Brown Adipocytes in Response to cAMP-Mediated Thermogenic Activation. Front. Physiol. 2019, 10, 67. [Google Scholar] [CrossRef]
- Yuko, O.-O.; Saito, M. Brown Fat as a Regulator of Systemic Metabolism beyond Thermogenesis. Diabetes Metab. J. 2021, 45, 840–852. [Google Scholar] [CrossRef]
- Dan, X.; Li, K.; Xu, J.; Yan, P. The Potential of Neuregulin 4 as a Novel Biomarker and Therapeutic Agent for Vascular Complications in Type 2 Diabetes Mellitus. J. Inflamm. Res. 2024, 17, 8543–8554. [Google Scholar] [CrossRef]
- Yan, C.; Burley, G.; Gao, H.; Shi, Y.-C. Emerging Insights into Brown Adipose Tissue Crosstalk With Pancreatic β-Cells in Metabolic Regulation. Endocrinology 2025, 166, bqaf118. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.J.; Long, J.Z.; Jedrychowski, M.P.; Cohen, P.; Lo, J.C.; Serag, S.; Kir, S.; Shinoda, K.; Tartaglia, J.A.; Rao, R.R.; et al. A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. Cell Metab. 2016, 23, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Campderrós, L.; Moure, R.; Cairó, M.; Gavaldà-Navarro, A.; Quesada-López, T.; Cereijo, R.; Giralt, M.; Villarroya, J.; Villarroya, F. Brown Adipocytes Secrete GDF15 in Response to Thermogenic Activation. Obesity 2019, 27, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Gutierrez, A.; Aguilera, C.M.; Cereijo, R.; Osuna-Prieto, F.J.; Martinez-Tellez, B.; Rico, M.C.; Sanchez-Infantes, D.; Villarroya, F.; Ruiz, J.R.; Sanchez-Delgado, G. Cold Exposure Modulates Potential Brown Adipokines in Humans, but Only FGF21 Is Associated with Brown Adipose Tissue Volume. Obesity 2024, 32, 560–570. [Google Scholar] [CrossRef]
- Jena, J.; García-Peña, L.M.; Weatherford, E.T.; Marti, A.; Bjorkman, S.H.; Kato, K.; Koneru, J.; Chen, J.H.; Seeley, R.J.; Abel, E.D.; et al. GDF15 Is Required for Cold-Induced Thermogenesis and Contributes to Improved Systemic Metabolic Health Following Loss of OPA1 in Brown Adipocytes. eLife 2023, 12, e86452. [Google Scholar] [CrossRef]
- Chung, H.K.; Ryu, D.; Kim, K.S.; Chang, J.Y.; Kim, Y.K.; Yi, H.-S.; Kang, S.G.; Choi, M.J.; Lee, S.E.; Jung, S.-B.; et al. Growth Differentiation Factor 15 Is a Myomitokine Governing Systemic Energy Homeostasis. J. Cell Biol. 2017, 216, 149–165. [Google Scholar] [CrossRef]
- Cereijo, R.; Gavaldà-Navarro, A.; Cairó, M.; Quesada-López, T.; Villarroya, J.; Morón-Ros, S.; Sánchez-Infantes, D.; Peyrou, M.; Iglesias, R.; Mampel, T.; et al. CXCL14, a Brown Adipokine That Mediates Brown-Fat-to-Macrophage Communication in Thermogenic Adaptation. Cell Metab. 2018, 28, 750–763.e6. [Google Scholar] [CrossRef]
- Stanford, K.I.; Goodyear, L.J. Muscle-Adipose Tissue Cross Talk. Cold Spring Harb. Perspect. Med. 2018, 8, a029801. [Google Scholar] [CrossRef]
- Chen, K.Y.; Cypess, A.M.; Laughlin, M.R.; Haft, C.R.; Hu, H.H.; Bredella, M.A.; Enerbäck, S.; Kinahan, P.E.; Lichtenbelt, W.V.M.; Lin, F.I.; et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): Recommendations for Standardized FDG-PET/CT Experiments in Humans. Cell Metab. 2016, 24, 210–222. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-Derived Circulating miRNAs Regulate Gene Expression in Other Tissues. Nature 2017, 542, 450–455, Erratum in Nature 2017, 545, 252. [Google Scholar] [CrossRef]
- Martinez-Sanchez, N.; Imbernon, M. Editorial: Adipokines, Batokines & Cardiokines: Crosstalk with Metabolic Organs. Front. Endocrinol. 2024, 15, 1481180. [Google Scholar] [CrossRef]
- Divoux, A.; Clément, K. Architecture and the Extracellular Matrix: The Still Unappreciated Components of the Adipose Tissue. Obes. Rev. 2011, 12, e494–e503. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Kitchener, R.L.; Grunden, A.M. Prolidase Function in Proline Metabolism and Its Medical and Biotechnological Applications. J. Appl. Microbiol. 2012, 113, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, V.; Figueroa-Juárez, E.; Samuelson, I.; U-Din, M.; Rodriguez-Fdez, S.; Virtue, S.; Leggat, J.; Çubuk, C.; Peirce, V.J.; Niemi, T.; et al. Defective Extracellular Matrix Remodeling in Brown Adipose Tissue Is Associated with Fibro-Inflammation and Reduced Diet-Induced Thermogenesis. Cell Rep. 2023, 42, 112640. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of Fibrosis: Therapeutic Translation for Fibrotic Disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- DeBari, M.K.; Abbott, R.D. Adipose Tissue Fibrosis: Mechanisms, Models, and Importance. Int. J. Mol. Sci. 2020, 21, 6030. [Google Scholar] [CrossRef]
- Lackey, D.E.; Burk, D.H.; Ali, M.R.; Mostaedi, R.; Smith, W.H.; Park, J.; Scherer, P.E.; Seay, S.A.; McCoin, C.S.; Bonaldo, P.; et al. Contributions of Adipose Tissue Architectural and Tensile Properties toward Defining Healthy and Unhealthy Obesity. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E233–E246. [Google Scholar] [CrossRef]
- Muir, L.A.; Neeley, C.K.; Meyer, K.A.; Baker, N.A.; Brosius, A.M.; Washabaugh, A.R.; Varban, O.A.; Finks, J.F.; Zamarron, B.F.; Flesher, C.G.; et al. Adipose Tissue Fibrosis, Hypertrophy, and Hyperplasia: Correlations with Diabetes in Human Obesity. Obesity 2016, 24, 597–605. [Google Scholar] [CrossRef]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol. Cell Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ikeda, K.; Chen, Y.; Alba, D.L.; Stifler, D.; Shinoda, K.; Hosono, T.; Maretich, P.; Yang, Y.; Ishigaki, Y.; et al. Repression of Adipose Tissue Fibrosis through a PRDM16-GTF2IRD1 Complex Improves Systemic Glucose Homeostasis. Cell Metab. 2018, 27, 180–194.e6. [Google Scholar] [CrossRef]
- Wang, W.; Ishibashi, J.; Trefely, S.; Shao, M.; Cowan, A.J.; Sakers, A.; Lim, H.-W.; O’Connor, S.; Doan, M.T.; Cohen, P.; et al. A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate. Cell Metab. 2019, 30, 174–189.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Wang, F.; You, L.; Xu, P.; Cao, Y.; Chen, L.; Wen, J.; Guo, X.; Cui, X.; et al. Identification of Intracellular Peptides Associated with Thermogenesis in Human Brown Adipocytes. J. Cell Physiol. 2019, 234, 7104–7114. [Google Scholar] [CrossRef]
- Grandoch, M.; Flögel, U.; Virtue, S.; Maier, J.K.; Jelenik, T.; Kohlmorgen, C.; Feldmann, K.; Ostendorf, Y.; Castañeda, T.R.; Zhou, Z.; et al. 4-Methylumbelliferone Improves the Thermogenic Capacity of Brown Adipose Tissue. Nat. Metab. 2019, 1, 546–559. [Google Scholar] [CrossRef]
- Gonzalez Porras, M.A.; Stojkova, K.; Vaicik, M.K.; Pelowe, A.; Goddi, A.; Carmona, A.; Long, B.; Qutub, A.A.; Gonzalez, A.; Cohen, R.N.; et al. Integrins and Extracellular Matrix Proteins Modulate Adipocyte Thermogenic Capacity. Sci. Rep. 2021, 11, 5442. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Park, J.; Gupta, O.T.; Holland, W.L.; Auerbach, P.; Zhang, N.; Goncalves Marangoni, R.; Nicoloro, S.M.; Czech, M.P.; Varga, J.; et al. Endotrophin Triggers Adipose Tissue Fibrosis and Metabolic Dysfunction. Nat. Commun. 2014, 5, 3485. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, M.; Kim, L.A.; Boucher, J.; Walshe, T.E.; Kahn, C.R.; D’Amore, P.A. Vascular Endothelial Growth Factor Is Important for Brown Adipose Tissue Development and Maintenance. FASEB J. 2013, 27, 3257–3271. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Sabiu, S. Adipose Tissue Inflammation Linked to Obesity: A Review of Current Understanding, Therapies and Relevance of Phyto-Therapeutics. Heliyon 2024, 10, e23114. [Google Scholar] [CrossRef]
- Kiran, S.; Kumar, V.; Murphy, E.A.; Enos, R.T.; Singh, U.P. High Fat Diet-Induced CD8+ T Cells in Adipose Tissue Mediate Macrophages to Sustain Low-Grade Chronic Inflammation. Front. Immunol. 2021, 12, 680944. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Xu, D. The Roles of T Cells in Obese Adipose Tissue Inflammation. Adipocyte 2021, 10, 435–445. [Google Scholar] [CrossRef]
- Perry, R.J.; Camporez, J.-P.G.; Kursawe, R.; Titchenell, P.M.; Zhang, D.; Perry, C.J.; Jurczak, M.J.; Abudukadier, A.; Han, M.S.; Zhang, X.-M.; et al. Hepatic Acetyl CoA Links Adipose Tissue Inflammation to Hepatic Insulin Resistance and Type 2 Diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef]
- Ingram, J.R.; Dougan, M.; Rashidian, M.; Knoll, M.; Keliher, E.J.; Garrett, S.; Garforth, S.; Blomberg, O.S.; Espinosa, C.; Bhan, A.; et al. PD-L1 Is an Activation-Independent Marker of Brown Adipocytes. Nat. Commun. 2017, 8, 647. [Google Scholar] [CrossRef] [PubMed]
- Eljaafari, A.; Pestel, J.; Le Magueresse-Battistoni, B.; Chanon, S.; Watson, J.; Robert, M.; Disse, E.; Vidal, H. Adipose-Tissue-Derived Mesenchymal Stem Cells Mediate PD-L1 Overexpression in the White Adipose Tissue of Obese Individuals, Resulting in T Cell Dysfunction. Cells 2021, 10, 2645. [Google Scholar] [CrossRef] [PubMed]

| UCP1-Dependent Thermogenesis | UCP1-Independent Thermogenesis | |
|---|---|---|
| Main Protein |
|
|
| Location |
|
|
| Activation Stimuli |
|
|
| Energy Source |
|
|
| Inhibitory Mechanisms |
|
|
| Post-translational Modifications |
|
|
| Role of ROS |
|
|
| Heat Production Efficiency |
|
|
| Fatty Acid Requirement |
|
|
| Metabolic Benefits and Non-thermogenic Roles |
|
|
| Additional Notes |
|
|
| Key References |
|
|
| Polypeptides | Effects/Organs | |
|---|---|---|
| Autocrine/Paracrine actions |
|
|
|
| |
|
| |
|
| |
| Endocrine actions |
|
|
|
| |
|
| |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalloul, W.; Grierosu, I.C.; Jalloul, D.; Stefanescu, C.; Ghizdovat, V. Functional Complexity of Thermogenic Adipose Tissue: From Thermogenesis to Metabolic and Fibroinflammatory Crosstalk. Int. J. Mol. Sci. 2025, 26, 9045. https://doi.org/10.3390/ijms26189045
Jalloul W, Grierosu IC, Jalloul D, Stefanescu C, Ghizdovat V. Functional Complexity of Thermogenic Adipose Tissue: From Thermogenesis to Metabolic and Fibroinflammatory Crosstalk. International Journal of Molecular Sciences. 2025; 26(18):9045. https://doi.org/10.3390/ijms26189045
Chicago/Turabian StyleJalloul, Wael, Irena Cristina Grierosu, Despina Jalloul, Cipriana Stefanescu, and Vlad Ghizdovat. 2025. "Functional Complexity of Thermogenic Adipose Tissue: From Thermogenesis to Metabolic and Fibroinflammatory Crosstalk" International Journal of Molecular Sciences 26, no. 18: 9045. https://doi.org/10.3390/ijms26189045
APA StyleJalloul, W., Grierosu, I. C., Jalloul, D., Stefanescu, C., & Ghizdovat, V. (2025). Functional Complexity of Thermogenic Adipose Tissue: From Thermogenesis to Metabolic and Fibroinflammatory Crosstalk. International Journal of Molecular Sciences, 26(18), 9045. https://doi.org/10.3390/ijms26189045