Chlorophylls and Polyphenols: Non-Enzymatic Regulation of the Production and Removal of Reactive Oxygen Species, as a Way of Regulating Abiotic Stress in Plants
Abstract
1. Introduction
2. Observations Regarding the Applied Research Methodology
3. Photosynthesis, Chlorophylls, and Other Photosynthetic Pigments and Their Interaction with Oxygen Metabolism
4. Polyphenols, Their Synthesis, Measurements, and Antioxidative Activity
- By handing over H-atoms, which directly bind (“trapping” and/or “quenching”) free oxygen or nitrogen radical species;
- By chelation of prooxidative metal ions (Fe2+, Cu2+, Zn2+ and Mn2+);
- By activating antioxidant enzymes;
5. Further Research Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Milivojević, D.B.; Nikolić, B.R. Effects of diquat on pigment-protein complexes of thylakoid membranes in soybean and maize plants. Biol. Plant. 1998, 41, 597–600. [Google Scholar] [CrossRef]
- Pavlović, D. Determination of Weed Resistance to Herbicides—Photosynthesis Inhibitors. Master’s Thesis, University of Belgrade, Faculty of Agriculture, Belgrade, Serbia, 2005. without identification number of work. (In Serbian). [Google Scholar]
- Pavlović, D. Sensitivity of Plants to Glyphosate: Morpho-Anatomical, Physiological and Biological Aspects. Ph.D. Thesis, University of Belgrade, Faculty of Agriculture, Belgrade, Serbia, 2010. without identification number of work. (In Serbian). [Google Scholar]
- Torres Netto, A.; Campostrini, E.; Jurandi Gonçalves de Oliveira, J.; Kiyoshi Yamanishi, O. Portable chlorophyll meter for the quantification of photosynthetic pigments, nitrogen and the possible use for assessment of the photochemical process in Carica papaya L. Braz. J. Plant Physiol. 2002, 14, 203–210. [Google Scholar] [CrossRef]
- Parry, C.; Blonquist, M.J., Jr.; Bugbee, B. In situ measurement of leaf chlorophyll concentration: Analysis of the optical/absolute relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, R.K. Quick Determination of Leaf Photosynthetic Pigments Using SPAD Readings. J. Cell Biol. Genet. 2020, 3, 145–151. [Google Scholar]
- Chowdhury, R.I.; Wahid, K.A.; Nugent, K.; Baulch, H. Design and Development of Low-Cost, Portable, and Smart Chlorophyll-A Sensor. IEEE Sens. J. 2020, 20, 7362–7371. [Google Scholar] [CrossRef]
- Ghani, M.N.O.; Hashim, H.; Mustaffer, N.; Rahman, F.S.A.; Razali, N.I.A. Calibration of Relative Chlorophyll Content Meter with Extracted Photosynthetic Pigment Concentration of Grain Corn Hybrid at Different Growth Stages. Trans. Malays. Soc. Plant Physiol. 2022, 29, 35–40. [Google Scholar]
- Dash, J.; Curran, P.J.; Tallis, M.J.; Llewellyn, G.M.; Taylor, G.; Snoeij, P. Validating the MERIS Terrestrial Chlorophyll Index (MTCI) with ground chlorophyll content data at MERIS spatial resolution. Int. J. Remote. Sens. 2010, 31, 5513–5532. [Google Scholar] [CrossRef]
- Zhao, Y.-R.; Li, X.; Yu, K.-Q.; Cheng, F.; He, Y. Hyperspectral Imaging for Determining Pigment Contents in Cucumber Leaves in Response to Angular Leaf Spot Disease. Sci. Rep. 2016, 6, 27790. [Google Scholar] [CrossRef] [PubMed]
- Badgley, G.; Field, C.B.; Berry, J.A. Canopy near-infrared reflectance and terrestrial photosynthesis. Sci. Adv. 2017, 3, e1602244. [Google Scholar] [CrossRef]
- Sonobe, R.; Sano, T.; Horie, H. Using spectral reflectance to estimate leaf chlorophyll content of tea with shading treatments. Biosyst. Eng. 2018, 175, 168–182. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Jiang, J.; Liu, J. Spectroscopic determination of leaf chlorophyll content and color for genetic selection on Sassafras tzumu. Plant Methods 2019, 15, 73. [Google Scholar] [CrossRef]
- Mulero, G.; Bacher, H.; Kleiner, U.; Peleg, Z.; Herrmann, I. Spectral Estimation of In Vivo Wheat Chlorophyll a/b Ratio under Contrasting Water Availabilities. Remote Sens. 2022, 14, 2585. [Google Scholar] [CrossRef]
- Červená, L.; Pinlová, G.; Lhotáková, Z.; Neuwirthová, E.; Kupková, L.; Potůčková, M.; Lysák, J.; Campbell, P.; Albrechtová, J. Determination of Chlorophyll Content in Selected Grass Communities of Krkonoše Mts. Tundra Based on Laboratory Spectroscopy and Aerial Hyperspectral Data. In The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Proceedings of the XXIV ISPRS Congress (2022 Edition), Nice, France, 6–11 June 2022; International Society for Photogrammetry and Remote Sensing (ISPRS): Paris, France, 2022; Volume XLIII-B3, pp. 381–388. [Google Scholar] [CrossRef]
- Brown, L.A.; Williams, O.; Dash, J. Calibration and characterisation of four chlorophyll meters and transmittance spectroscopy for non-destructive estimation of forest leaf chlorophyll concentration. Agric. For. Meteorol. 2022, 323, 109059. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F.; Langsdorf, G. Chlorophyll fluorescence imaging of photosynthetic activity in sun and shade leaves of trees. Photosynth. Res. 2007, 93, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Nikolić, B.; Janjić, V.; Ignjatović, S.; Jovanović, V. Source-Sink Manipulation in Herbicide Sulfosate Stressed Maize (Zea mays L.) Plants. In Proceedings of the XXXIV Annual ESNA Meeting, Novi Sad, Serbia and Montenegro, 29 August–2 September 2004; pp. 478–483. [Google Scholar]
- Nikolić, B.; Dodig, D.; Jovanović, V.; Oro, V.; Marković, A. The effect of temperature and light (PAR) on the induction of Chla fluorescence in situ. 2. Diurnal changes in stinging nettle (Urtica dioica) and red currant (Ribes spp.). Bot. Serbica 2013, 37, 161–166. [Google Scholar]
- Nikolić, B.; Dragičević, V.; Waisi, H.; Đurović, S.; Milićević, Z.; Spasojević, I.; Brankov, M. Iimpact of Root Manipulation and Brassinosteroids on Growth, Photosynthesis and Thermodinamics of Maize at Lower Temperatures. In Physical Chemistry 2014, Proceedings of the 12th International Conference on Fundamental and Applied Aspects of Physical Chemistry, Belgrade, Serbia, 22–26 September 2014; Čupić, Ž., Anić, S., Eds.; Society of Physical Chemists of Serbia: Belgrade, Serbia, 2014; pp. 477–481. ISBN 978-86-82475-30-9. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Vegetation Stress: An Introduction to the Stress Concept in Plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygen and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1419–1431. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35 (Suppl. S4), 1011–1019. [Google Scholar] [CrossRef]
- Waisi, H. The Influence of Brassinosteroid 24-Epibrassinolide on Germination and Early Stages of Growth and Development of Different Maize Hybrids (Zea mays L.). Ph.D. Thesis, University of Belgrade, Faculty of Biology, Belgrade, Serbia, 2016. on serbian with summary on English. Available online: https://nardus.mpn.gov.rs/handle/123456789/6772 (accessed on 30 June 2025).
- Božilović, B.; Nikolić, B.; Waisi, H.; Trifković, J.; Dodevski, V.; Janković, B.; Krstić, S.; Mojović, M. Influence of 24-Epibrassinolide on the Energetic Parameters and Early Stages of Growth and Development in Seedlings of Two Maize (Zea mays L.) Genotypes. Agronomy 2023, 13, 1673. [Google Scholar] [CrossRef]
- Nikolić, B.; Jovanović, V.; Knežević, B.; Nikolić, Z.; Babović-Đorđević, M. Mode of Action of Brassinosteroids: Seed Germination and Seedling Growth and Development—One Hypothesis. Int. J. Mol. Sci. 2025, 26, 2559. [Google Scholar] [CrossRef]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N. Structure and biogenesis of the cell walls of grasses. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 445–476. [Google Scholar] [CrossRef]
- Waisi, H.; Kosović, A.; Krstić, Đ.; Milojković-Opsenica, D.; Nikolić, B.; Dragičević, V.; Trifković, J. Polyphenolic Profile of Maize Seedlings Treated with 24-Epibrassinolide. J. Chem. 2015, 2015, 976971. [Google Scholar] [CrossRef]
- Đurović, S. The Influence of Different Extraction Procedures on the Content and Biological Properties of Polyphenols and Proteins in Yellow Soybean Seeds of Different Origin. Ph.D. Thesis, University of Belgrade, Faculty of Technology and Metallurgy, Belgrade, Serbia, 2019. on Serbian with Summary on English. Available online: https://nardus.mpn.gov.rs/handle/123456789/12113?locale-attribute=sr_RS (accessed on 30 June 2025).
- Đurović, S.; Nikolić, B.; Luković, N.; Jovanović, J.; Stefanović, A.; Šekuljica, N.; Mijin, D.; Knežević-Jugović, Z. The impact of high-power ultrasound and microwave on the phenolic acid profile and antioxidant activity of the extract from yellow soybean seeds. Ind. Crop. Prod. 2018, 122, 223–231. [Google Scholar] [CrossRef]
- Đurović, S.; Nikolić, B.; Pisinov, B.; Mijin, D.; Knežević-Jugović, Z. Microwave Irradiation as a Powerful Tool for Isolating Isoflavones from Soybean Flour. Molecules 2024, 29, 4685. [Google Scholar] [CrossRef]
- Geiger, D.R.; Servaites, J.C. Diurnal regulation of photosynthetic carbon metabolism in C3 plants. Annu. Rev. Plant Biol. 1994, 45, 235–256. [Google Scholar] [CrossRef]
- Heber, U. Irrungen, Wirrungen? The Mehler reaction in relation to cyclic electron transport in C3 plants. Photosynth. Res. 2002, 73, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Roberty, S.; Bailleul, B.; Berne, N.; Franck, F.; Cardol, P. PSI Mehler reaction is the main alternative photosynthetic electron pathway in Symbiodinium sp., symbiotic dinoflagellates of cnidarians. New Phytol. 2014, 204, 81–91. [Google Scholar] [CrossRef]
- Yamori, W.; Makino, A.; Shikanai, T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef]
- Ermakova, M.; Fitzpatrick, D.; Larkum, A.W.D. Cyclic electron flow and Photosystem II-less photosynthesis. Funct. Plant Biol. 2024, 51, FP24185. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M.; Roell, M.; Weber, A.P.M. Mechanistic understanding of photorespiration paves the way to a new green revolution. New Phytol. 2019, 223, 1762–1769. [Google Scholar] [CrossRef]
- Shi, X.; Bloom, A. Photorespiration: The Futile Cycle? Plants 2021, 10, 908. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.V.; Walters, R.G. Regulation of Light Harvesting in Green Plants. Indication by Nonphotochemical Quenching of Chlorophyll Fluorescence. Plant Physiol. 1994, 106, 415–420. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.V.; Walters, R.G. Regulation of Light Harvesting in Green Plants. Annu. Rev. Plant Biol. 1996, 47, 655–684. [Google Scholar] [CrossRef]
- Bjorkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypiński, P.; Samborska, I.A.; et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Bąba, W.; Kompała-Bąba, A.; Zabochnicka-Świątek, M.; Luźniak, J.; Hanczaruk, R.; Adamski, A.; Kalaji, H.M. Discovering trends in photosynthesis using modern analytical tools: More than 100 reasons to use chlorophyll fluorescence. Photosynthetica 2019, 57, 668–679. [Google Scholar] [CrossRef]
- Padhi, B.; Chauhan, G.; Kandoi, D.; Stirbet, A.; Tripathy, B.C.; Govindjee. A comparison of chlorophyll fluorescence transient measurements, using Handy PEA and FluorPen fluorometers. Photosynthetica 2021, 59, 39–48. [Google Scholar] [CrossRef]
- Nikolić, B.; Đurović, S.; Pisinov, B.; Jovanović, V.; Dudić, D.; Dugalić, M. Paraquat and other dessicants and bleaching herbicides—Their influence on weeds, crops and human and animal health. Acta Herbol. 2024, 33, 5–14. [Google Scholar] [CrossRef]
- Averina, N.G.; Shaligo, N.V.; Linnik, N.N. Study of chlorophyll pheophotonization under the action of pyridine metal chelators. Russ. J. Plant Physiol. 1991, 38, 1059–1065. (in Russian). [Google Scholar]
- Liu, X.; Zhu, L.; Song, Q.; Chang, J.; Ye, J.; Zhang, W.; Liao, Y.; Xu, F. Effects of 5-aminolevulinic Acid on the Photosynthesis, Antioxidant System, and α-Bisabolol Content of Matricaria recutita. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 418–425. [Google Scholar] [CrossRef]
- Silla, F.; González-Gil, A.; González-Molina, M.E.; Mediavilla, S.; Escudero, A. Estimation of chlorophyll in Quercus leaves using a portable chlorophyll meter: Effects of species and leaf age. Ann. For. Sci. 2010, 67, 108. [Google Scholar] [CrossRef]
- Yu, Z.-C.; Lin, W.; He, W.; Yan, G.-Z.; Zheng, X.-T.; Luo, Y.-N.; Zhu, H.; Peng, C.-L. Dynamic changes of the contents of photoprotective substances and photosynthetic maturation during leaf development of evergreen tree species in subtropical forests. Tree Physiol. 2023, 43, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Rühle, T.; Reiter, B.; Leister, D. Chlorophyll Fluorescence Video Imaging: A Versatile Tool for Identifying Factors Related to Photosynthesis. Front. Plant Sci. 2018, 9, 55. [Google Scholar] [CrossRef]
- Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Araniti, F. Imaging of Chlorophyll a Fluorescence in Natural Compound-Induced Stress Detection. Front. Plant Sci. 2020, 11, 583590. [Google Scholar] [CrossRef]
- Cruz, J.A.; Savage, L.J.; Zegarac, R.; Hall, C.C.; Satoh-Cruz, M.; Davis, G.A.; Kovac, W.K.; Chen, J.; Kramer, D.M. Dynamic Environmental Photosynthetic Imaging Reveals Emergent Phenotype. Cell Syst. 2016, 2, 365–377. [Google Scholar] [CrossRef]
- Hida, E.Z.; Çako, V.; Babani, F.; Karaja, T. Activity Imaging Photosynthetic of Populus × Canadensis Moench Plants in Air Pollution. Int. J. Eng. Invent. 2014, 3, 35–40. [Google Scholar]
- Pfündel, E.; Neubohn, B. Assessing photosystem I and II distribution in leaves from C4 plants using confocal laser scanning microscopy. Plant Cell Environ. 1999, 22, 1569–1577. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L. Chlorophyll Fluorescence Effects on Vegetation Apparent Reflectance: I. Leaf-Level Measurements and Model Simulation. Remote Sens. Environ. 2000, 74, 582–595. [Google Scholar] [CrossRef]
- Sayed, O.H. Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 2003, 41, 321–330. [Google Scholar] [CrossRef]
- Mereu, S.; Gerosa, G.; Marzuoli, R.; Fusaro, L.; Salvatori, E.; Finco, A.; Spano, D.; Manes, F. Gas exchange and JIP-test parameters of two Mediterranean maquis species are affected by sea spray and ozone interaction. Environ. Exp. Bot. 2011, 73, 80–88. [Google Scholar] [CrossRef]
- Schreiber, U.; Klughammer, C.; Kolbowski, J. Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer. Photosynth. Res. 2012, 113, 127–144. [Google Scholar] [CrossRef]
- Malapascua, J.R.F.; Jerez, C.G.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Photosynthesis monitoring to optimize growth of microalgal mass cultures: Application of chlorophyll fluorescence techniques. Aquat. Biol. 2014, 22, 123–140. [Google Scholar] [CrossRef]
- Rahimzadeh-Bajgiran, P.; Tubuxin, B.; Omasa, K. Estimating Chlorophyll Fluorescence Parameters Using the Joint Fraunhofer Line Depth and Laser-Induced Saturation Pulse (FLD-LISP) Method in Different Plant Species. Remote Sens. 2017, 9, 599. [Google Scholar] [CrossRef]
- Magney, T.S.; Frankenberg, C.; Köhler, P.; North, G.; Davis, T.S.; Dold, C.; Dutta, D.; Fisher, J.B.; Grossmann, K.; Harrington, A.; et al. Porcar-Castell8 Disentangling changes in the spectral shape of chlorophyll fluorescence: Implications for remote sensing of photosynthesis. J. Geophys. Res. Biogeosci. 2019, 124, 1491–1507. [Google Scholar] [CrossRef]
- Møller, I.M. Plant Mitochondria and Oxidative Stress: Electron Transport, NADPH Turnover, and Metabolism of Reactive Oxygen Species. Annu. Rev. Plant Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef]
- Brosa, C. Biological Effects of Brassinosteroids. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 339–358. [Google Scholar] [CrossRef]
- Wolf, S.; Mravec, J.; Greiner, S.; Mouille, G.; Höfte, H. Plant Cell Wall Homeostasis Is Mediated by Brassinosteroid Feedback Signaling. Curr. Biol. 2012, 22, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Spalding, E.P.; Gray, W.M. Rapid Auxin-Mediated Cell Expansion. Annu. Rev. Plant Biol. 2020, 71, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Xie, Z.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Hayat, S.; Fariduddin, Q.; Ahmad, A. Enhanced Tolerance to Heavy Metals. In Brassinosteroids: Practical Applications in Agriculture and Human Health; Pereira-Netto, A.B., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 44–56. ISBN 9781608056545. [Google Scholar] [CrossRef][Green Version]
- Radchenko, M.P.; Sychuk, A.M.; Morderer, Y.Y. Decrease of the herbicide fenoxaprop phytotoxicity in drought conditions: The role of the antioxidant enzymatic system. J. Plant Prot. Res. 2014, 54, 390–394. [Google Scholar] [CrossRef]
- Jiang, B.; Gao, G.; Ruan, M.; Bian, Y.; Geng, F.; Yan, W.; Xu, X.; Shen, M.; Wang, J.; Chang, R.; et al. Quantitative Assessment of Abiotic Stress on the Main Functional Phytochemicals and Antioxidant Capacity of Wheatgrass at Different Seedling Age. Front. Nutr. 2021, 8, 731555. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Puzanskiy, R.K.; Bogdanova, E.M.; Vanisov, S.A.; Kirpichnikova, A.A.; Biktasheva, M.O.; Mukhina, Z.M.; Shavarda, A.L.; Shishova, M.F. Alterations in the Rice Coleoptile Metabolome During Elongation Under Submergence Stres. Int. J. Mol. Sci. 2024, 25, 13256. [Google Scholar] [CrossRef]
- Negi, P.; Pandey, M.; Paladi, R.K.; Majumdar, A.; Pandey, S.P.; Barvkar, V.T.; Devarumath, R.; Srivastava, A.K. Stomata-Photosynthesis Synergy Mediates Combined Heat and Salt Stress Tolerance in Sugarcane Mutant M4209. Plant Cell Environ. 2025, 48, 4668–4684. [Google Scholar] [CrossRef]
- Wang, P.; Duan, W.; Takabayashi, A.; Endo, T.; Shikanai, T.; Ye, J.-Y.; Mi, H. Chloroplastic NAD(P)H Dehydrogenase in Tobacco Leaves Functions in Alleviation of Oxidative Damage Caused by Temperature Stress. Plant Physiol. 2006, 141, 465–474. [Google Scholar] [CrossRef]
- Zorina, A.A.; Mironov, K.S.; Stepanchenko, N.S.; Sinetova, M.A.; Koroban, N.V.; Zinchenko, V.V.; Kupriyanova, E.V.; Allakhverdiev, S.I.; Los, D.A. Regulation Systems for Stress Responses in Cyanobacteria. J. Plant Physiol. 2011, 58, 749–767. [Google Scholar] [CrossRef]
- Zafar, S.A.; Zaidi, S.S.-E.A.; Gaba, Y.; Singla-Pareek, S.L.; Dhankher, O.P.; Li, X.; Mansoor, S.; Pareek, A. Engineering abiotic stress tolerance via CRISPR/Cas-mediated genome editing. J. Exp. Bot. 2020, 71, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhu, J.; Li, Z.; Wang, W.; Bao, M.; Qiu, X.; Yao, P.; Bi, Z.; Sun, C.; Li, Y.; et al. Comprehensive Analysis of the OASTL Gene Family in Potato (Solanum tuberosum L.) and Its Expression Under Abiotic Stress. Int. J. Mol. Sci. 2024, 25, 13170. [Google Scholar] [CrossRef]
- Waisi, H.K.; Petković, A.Z.; Nikolić, B.R.; Janković, B.Ž.; Raičević, V.B.; Lalević, B.T.; Giba, Z.S. Influence of 24-epibrassinolide on seedling growth and distribution of mineral elements in two maize hybrids. Hem. Ind. 2017, 71, 201–209. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Schmitzer, V.; Mikulic-Petkovsek, M.; Stampar, F.; Cunja, V. Phenolic Accumulation in Hybrid Primrose and Pigment Distribution in Different Flower Segments. J. Am. Soc. Hortic. Sci. 2017, 142, 192–199. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Handa, N.; Sharma, R.; Kaur, H.; Kohli, S.; Kumar, V.; Kaur, P. Lignins and Abiotic Stress: An Overview. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment: Volume 1; Ahmad, P., Wani, M.R., Eds.; Springer Science + Business Media: New York, NY, USA, 2014; pp. 267–296. ISBN 978-1-4614-8590-2. [Google Scholar] [CrossRef]
- Mane, A.V.; Karadge, B.A.; Samant, J.S. Salt Stress Induced Alteration in Photosynthetic Pigments and Polyphenols of Pennisetum alopecuroides (L.). J. Ecophysiol. Occup. Health 2010, 10, 177–182. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An Overview of Plant Phenolics and Their Involvement in Abiotic Stress Tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Herrmann, K.M. The shikimate pathway: Early steps in the biosynthesis of aromatic compounds. Plant Cell 1995, 7, 907–919. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-lnduced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Hatamnia, A.A.; Rostamzad, A.; Malekzadeh, P.; Darvishzadeh, R.; Abbaspour, N.; Hosseini, M.; Nourollahi, K.; Mehr, R.S.A. Antioxidant activity of different parts of Pistacia khinjuk Stocks fruit and its correlation to phenolic composition. Nat. Prod. Res. 2015, 30, 1445–1450. [Google Scholar] [CrossRef]
- Gong, F.; Meng, J.; Xu, H.; Zhou, X. The Molecular Mechanism Regulating Flavonoid Production in Rhododendron chrysanthum Pall. Against UV-B Damage Is Mediated by RcTRP5. Int. J. Mol. Sci. 2024, 25, 13383. [Google Scholar] [CrossRef]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Martínez, E.; Bovy, A.; Plazas, M.; Tikunov, Y.; Prohens, J.; Pereira-Dias, L. Genetics and breeding of phenolic content in tomato, eggplant and pepper fruits. Front. Plant Sci. 2023, 14, 1135237. [Google Scholar] [CrossRef]
- Djurovic, S.; Dragicevic, V.; Waisi, H.; Pagnacco, M.; Lukovic, N.; Knezevic-Jugovic, Z.; Nikolic, B. Enhancement of antioxidant activity and bioactive compound contents in yellow soybean by plant-extract-based products. Arch. Biol. Sci. 2019, 71, 425–434. [Google Scholar] [CrossRef]
- Gururani, M.A.; Mohanta, T.K.; Bae, H. Current Understanding of the Interplay between Phytohormones and Photosynthesis under Environmental Stress. Int. J. Mol. Sci. 2015, 16, 19055–19085. [Google Scholar] [CrossRef]
- Paul, M.J.; Foyer, C.H. Sink regulation of photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Larcher, W. Physiological Plant Ecology. In Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2003; (on English); ISBN 978-3540435167. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant. 1994, 92, 696–717. [Google Scholar] [CrossRef]
- MülLer, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Z.; Zhao, L.; Zhao, H.; Ren, S. Diurnal Response of Sun-Induced Fluorescence and PRI to Water Stress in Maize Using a Near-Surface Remote Sensing Platform. Remote. Sens. 2018, 10, 1510. [Google Scholar] [CrossRef]
- Fichman, Y.; Miller, G.; Mittler, R. Whole-Plant Live Imaging of Reactive Oxygen Species. Mol. Plant 2019, 12, 1203–1210. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Perez-Perez, J.M. Optimization of ROS Measurement and Localization in Plant Tissues: Challenges and Solutions; Protocols: Berkeley, CA, USA, 2021; Available online: https://www.protocols.io/view/optimization-of-ros-measurement-and-localization-i-ewov146o2vr2/v1 (accessed on 30 June 2025).
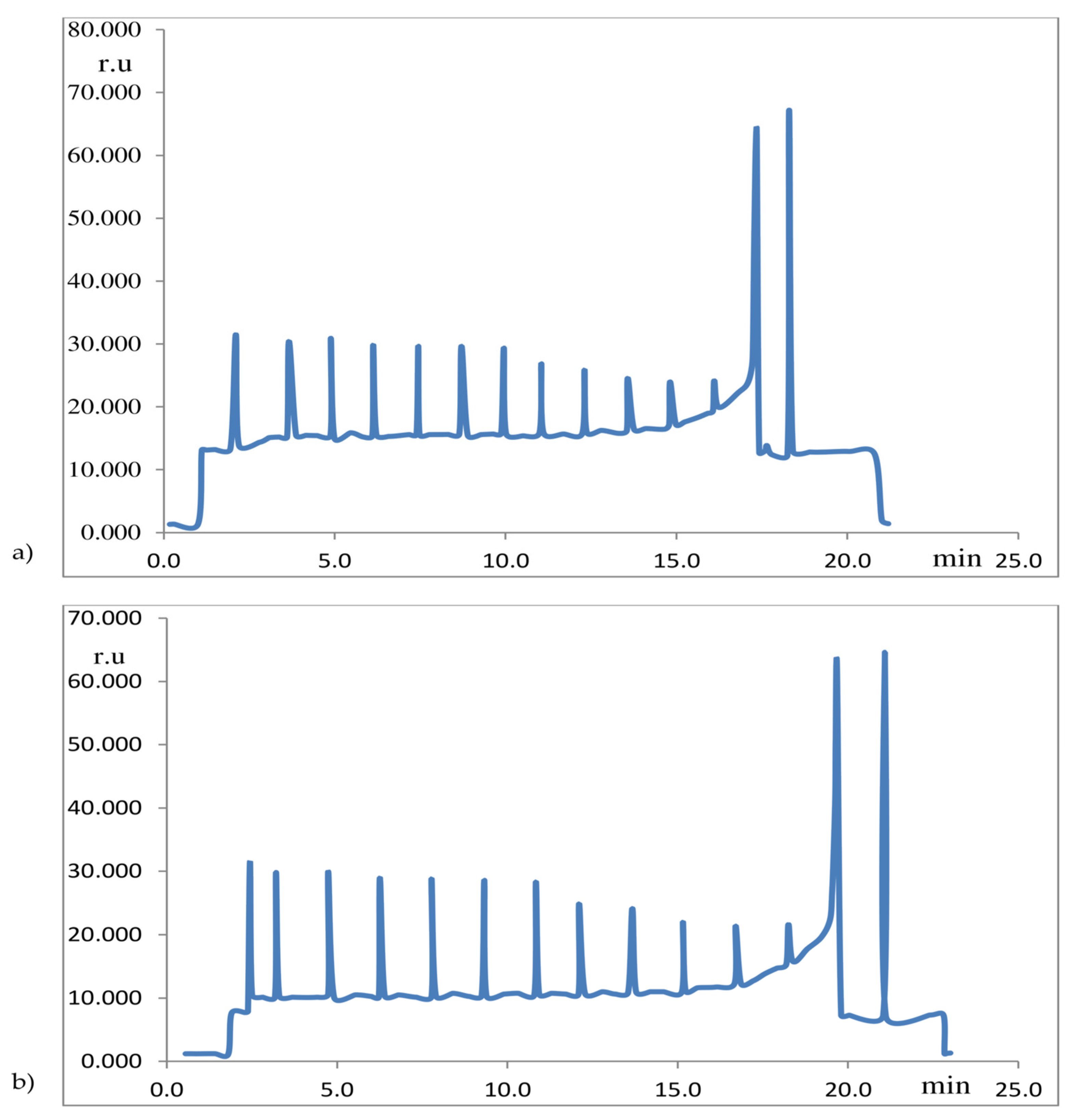
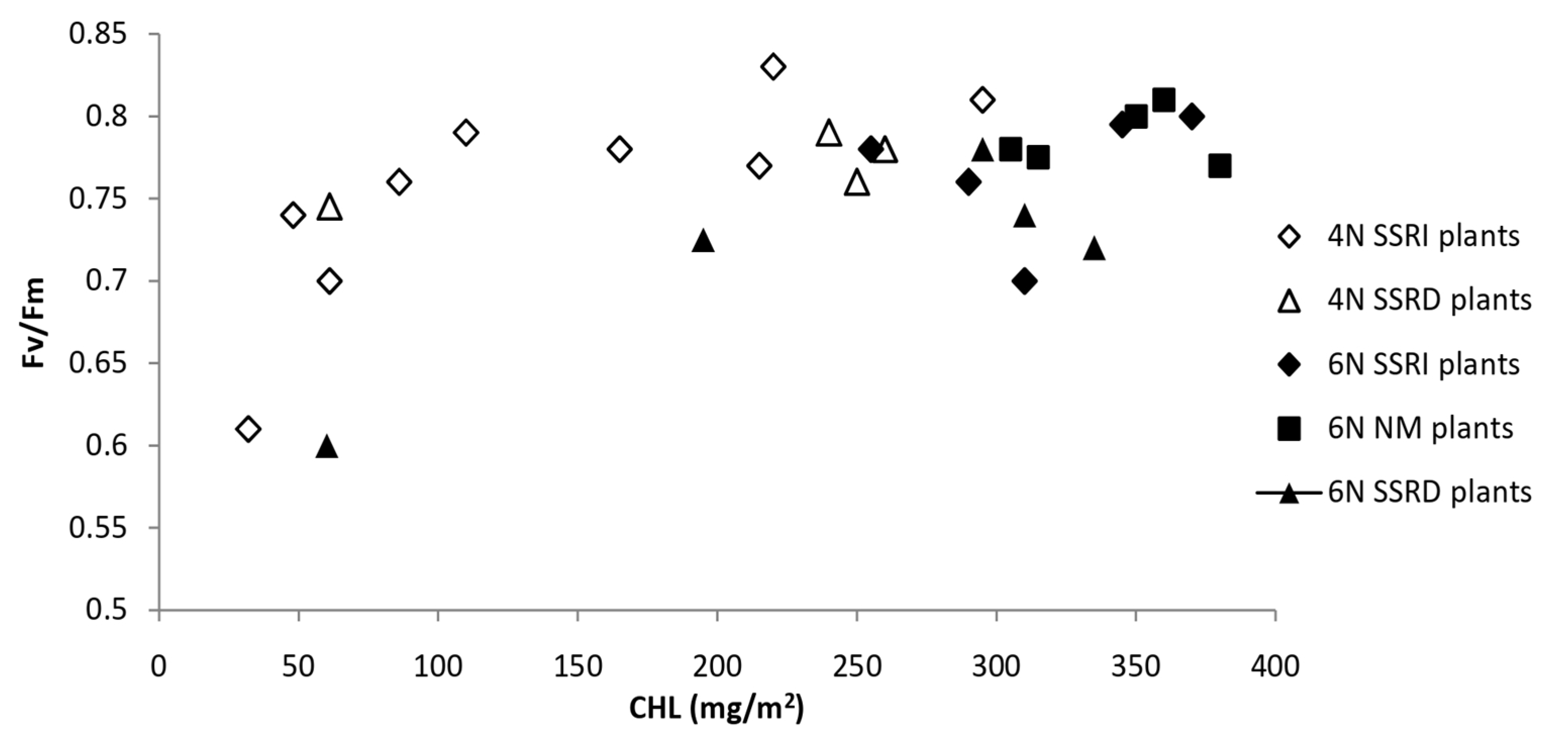
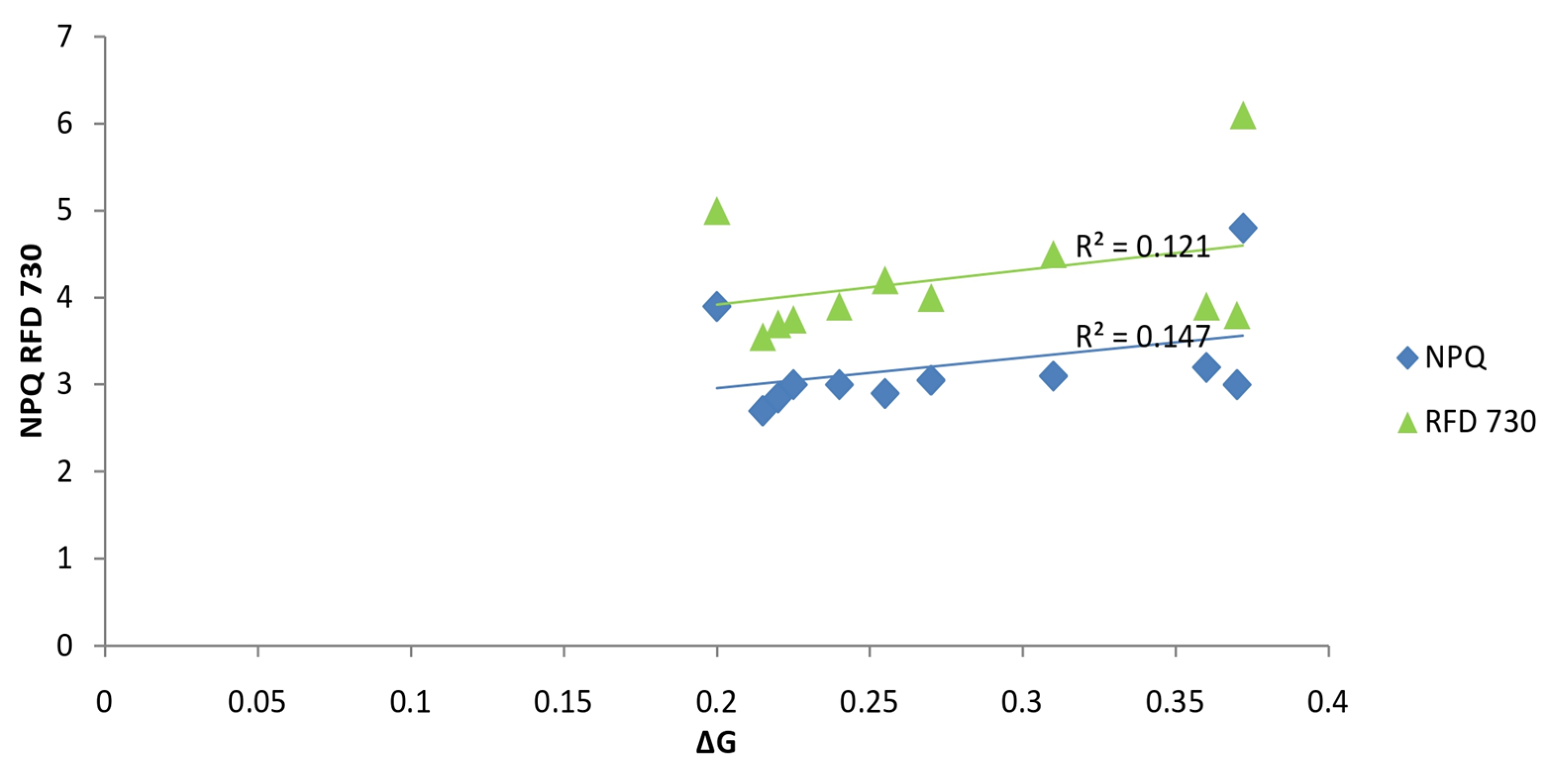
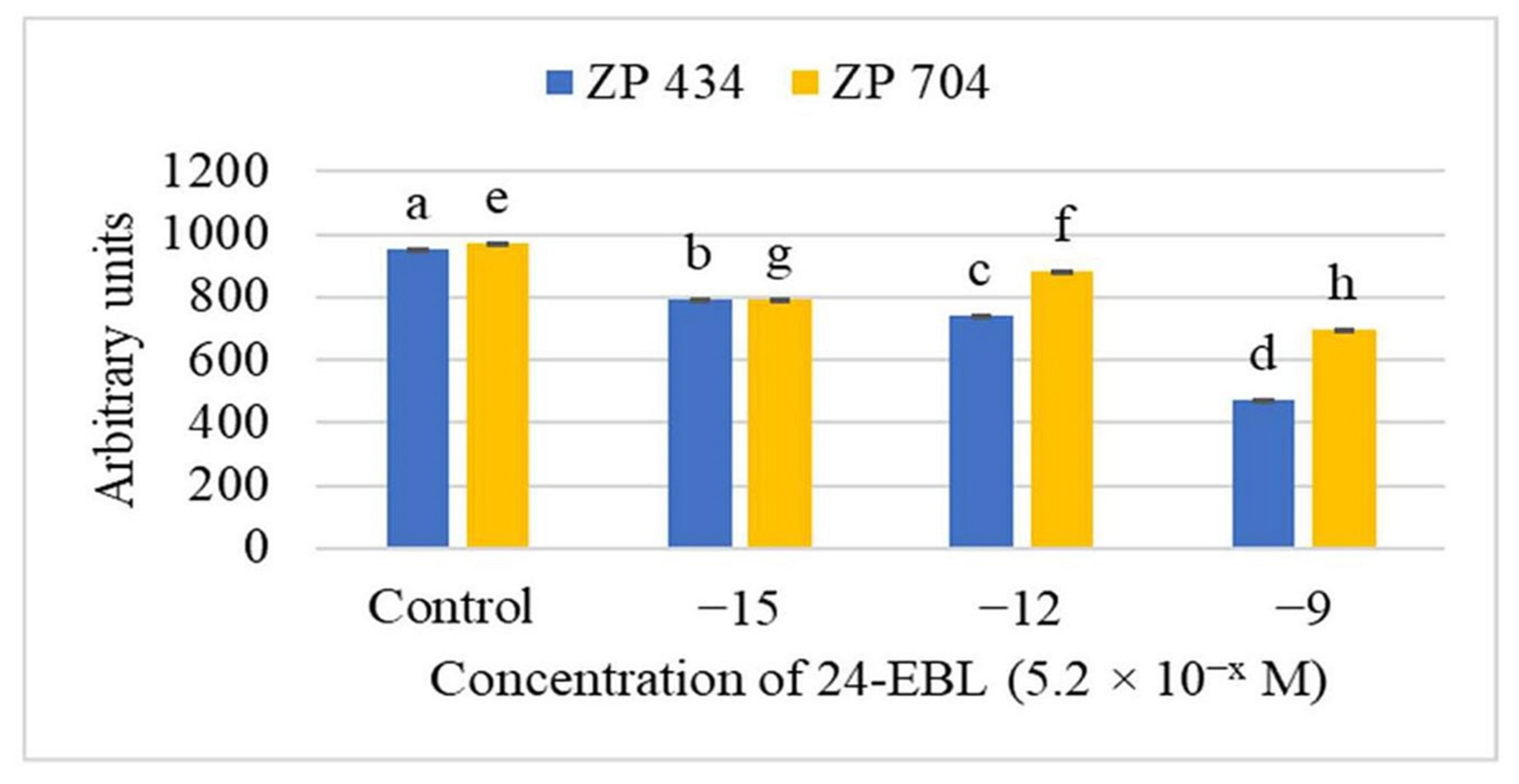
| Crop | Soybean | Maize | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Chl a/b | Chl a/Carr | Chl a/b | Chl a/Carr | ||||||||||||
| Treatment/treatment time (h) | 0 | 24 | 48 | 0 | 24 | 48 | 5 | 12 | 18 | 36 | 48 | 5 | 12 | 18 | 36 | 48 |
| WL | 2.1 | 2.3 | 2.4 | 5.3 | 5.6 | 4.6 | 2.6 | 2.5 | 2.3 | 2.5 | n.m | 4.9 | 4.8 | 4.9 | 5.5 | 15.3 |
| WL + diquat | 2.5 | 2.0 | 1.5 | 4.4 | 6.6 | 8.9 | 2.6 | 2.6 | 2.3 | 1.5 | 0.8 | 4.7 | 5.4 | 6.1 | 9.8 | 35.8 |
| FR | 2.2 | 2.1 | 1.7 | 3.4 | 3.4 | 3.9 | 2.6 | 2.4 | 2.4 | 2.1 | 2.1 | 4.9 | 4.6 | 4.9 | 4.6 | 4.9 |
| FR + diquat | 2.1 | 2.1 | 1.5 | 3.5 | 4.4 | 6.9 | 2.6 | 2.4 | 1.5 | 1.9 | 0.9 | 5.2 | 4.6 | 15.8 | 6.8 | 11.6 |
| Dark | 2.1 | 2.3 | 2.6 | 5.3 | 4.1 | 4.2 | 2.8 | 2.7 | 2.5 | 1.6 | 1.2 | 5.0 | 4.7 | 5.2 | 5.7 | 6.0 |
| Dark + diquat | 2.3 | 2.4 | 2.1 | 4.1 | 4.1 | 5.1 | 2.7 | 2.6 | 2.4 | 1.4 | 1.0 | 4.9 | 3.6 | 5.7 | 13.8 | 10.7 |
| LSD0.05 | 0.07 | 0.09 | 0.24 | 2.13 | ||||||||||||
| Crop | Soybean | Maize | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | PS1 | PS2 | LHC | PS2/PS1 | LHC/PS2 | PS1 | PS2 | LHC | PS2/PS1 | LHC/PS2 | ||||||||||
| Treatment/time (h) | 0 h→24 h | 5 h→24 h | 5 h→24 h | 5 h→24 h | 5 h→24 h | 0 h→24 h | 5 h→24 h | 5 h→24 h | 5 h→24 h | 5 h→24 h | ||||||||||
| WL | 7.1 | 3.0 | 23.2 | 38.6 | 35.4 | 34.3 | 3.3 | 1.8 | 1.7 | 1.0 | 6.9 | 5.2 | 22.2 | 33.8 | 36.7 | 29.8 | 3.2 | 6.5 | 1.7 | 0.8 |
| WL + diquat | 4.0 | 2.6 | 19.1 | 25.4 | 40.7 | 43.7 | 4.8 | 9.8 | 2.1 | 1.7 | 1.9 | 4.0 | 24.7 | 33.6 | 42.6 | 25.6 | 13.0 | 8.4 | 1.7 | 0.8 |
| FR | 5.3 | 2.6 | 24.6 | 23.8 | 23.9 | 43.7 | 4.6 | 9.2 | 1.0 | 1.8 | 2.9 | 5.8 | 28.4 | 25.7 | 37.1 | 27.9 | 9.8 | 4.4 | 1.3 | 1.1 |
| FR + diquat | 3.5 | 2.0 | 12.9 | 15.0 | 50.7 | 34.3 | 3.7 | 7.5 | 3.9 | 1.4 | 1.2 | 3.6 | 21.2 | 23.4 | 45.9 | 23.4 | 17.7 | 6.5 | 2.2 | 1.0 |
| Dark | 3.5 | 3.1 | 19.9 | 22.0 | 39.0 | 46.3 | 5.5 | 8.7 | 2.0 | 1.7 | 1.5 | 4.0 | 25.0 | 26.3 | 35.8 | 30.9 | 16.7 | 10.0 | 1.4 | 1.2 |
| Dark + diquat | 4.9 | 1.8 | 34.9 | 30.0 | 34.9 | 37.0 | 4.7 | 16.7 | 1.5 | 1.2 | n.m. | 2.6 | 45.0 | 32.0 | 36.3 | 25.1 | 45.0 | 12.3 | 0.8 | 0.8 |
| LSD0.05 | 0.8 | 10.5 | 9.3 | 1.5 | 1.2 | 1.1 | 5.1 | 8.5 | 7.6 | 0.4 | ||||||||||
| Weed Population | Control Plants | 5 Days After Treatment (8 kg ha−1 Atrazine) | ||||
|---|---|---|---|---|---|---|
| Average Amounts | Average Amounts | LSD Test | ||||
| R | S | R | S | C:R | C:S | |
| Abutilon teophrasti | 24.58 | 25.27 | 22.42 | 23.45 | ns | ** |
| Amaranthus retroflexus | 18.45 | 22.17 | 22.80 | 21.86 | ns | ** |
| Chenopodium album | 36.04 | 36.38 | 34.89 | 30.40 | ** | ** |
| Weed Population | Control Plants | 6 Days After Treatment (1 kg a.i.ha−1 Glyphosate) | ||||
|---|---|---|---|---|---|---|
| Average Amounts | Average Amounts | LSD Test | ||||
| R | S | R | S | C:R | C:S | |
| Lolium rigidum | 33.43 | 38.33 | 37.06 | 34.69 | ns | ** |
| Conyza canadensis | - | 48.46 | - | 50.81 | - | Ns |
| Conyza bonariensis | - | 39.17 | - | 43.06 | - | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, B.R.; Đurović, S.; Pisinov, B.; Jovanović, V.; Šikuljak, D. Chlorophylls and Polyphenols: Non-Enzymatic Regulation of the Production and Removal of Reactive Oxygen Species, as a Way of Regulating Abiotic Stress in Plants. Int. J. Mol. Sci. 2025, 26, 9039. https://doi.org/10.3390/ijms26189039
Nikolić BR, Đurović S, Pisinov B, Jovanović V, Šikuljak D. Chlorophylls and Polyphenols: Non-Enzymatic Regulation of the Production and Removal of Reactive Oxygen Species, as a Way of Regulating Abiotic Stress in Plants. International Journal of Molecular Sciences. 2025; 26(18):9039. https://doi.org/10.3390/ijms26189039
Chicago/Turabian StyleNikolić, Bogdan Radomir, Sanja Đurović, Boris Pisinov, Vladan Jovanović, and Danijela Šikuljak. 2025. "Chlorophylls and Polyphenols: Non-Enzymatic Regulation of the Production and Removal of Reactive Oxygen Species, as a Way of Regulating Abiotic Stress in Plants" International Journal of Molecular Sciences 26, no. 18: 9039. https://doi.org/10.3390/ijms26189039
APA StyleNikolić, B. R., Đurović, S., Pisinov, B., Jovanović, V., & Šikuljak, D. (2025). Chlorophylls and Polyphenols: Non-Enzymatic Regulation of the Production and Removal of Reactive Oxygen Species, as a Way of Regulating Abiotic Stress in Plants. International Journal of Molecular Sciences, 26(18), 9039. https://doi.org/10.3390/ijms26189039






