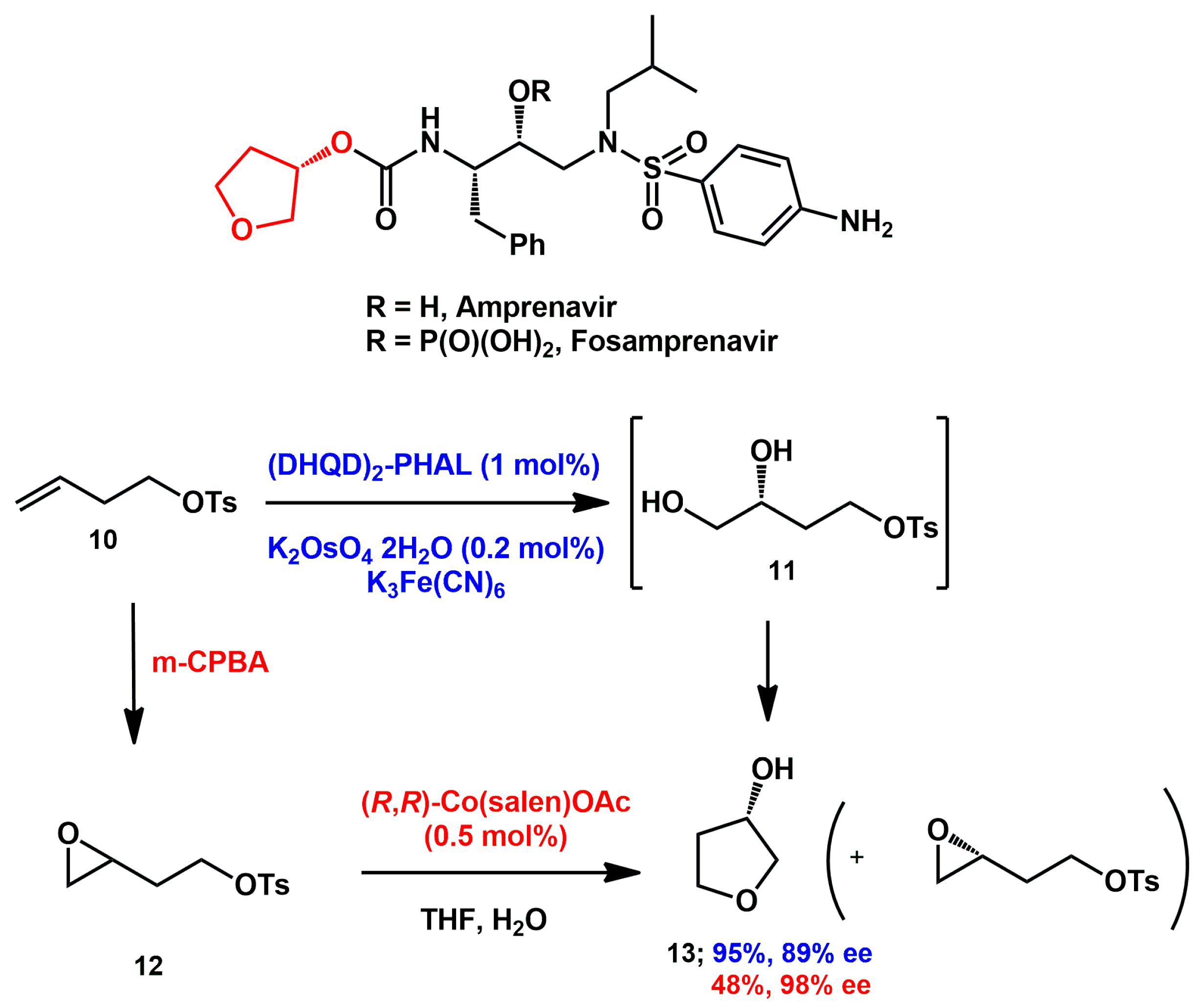
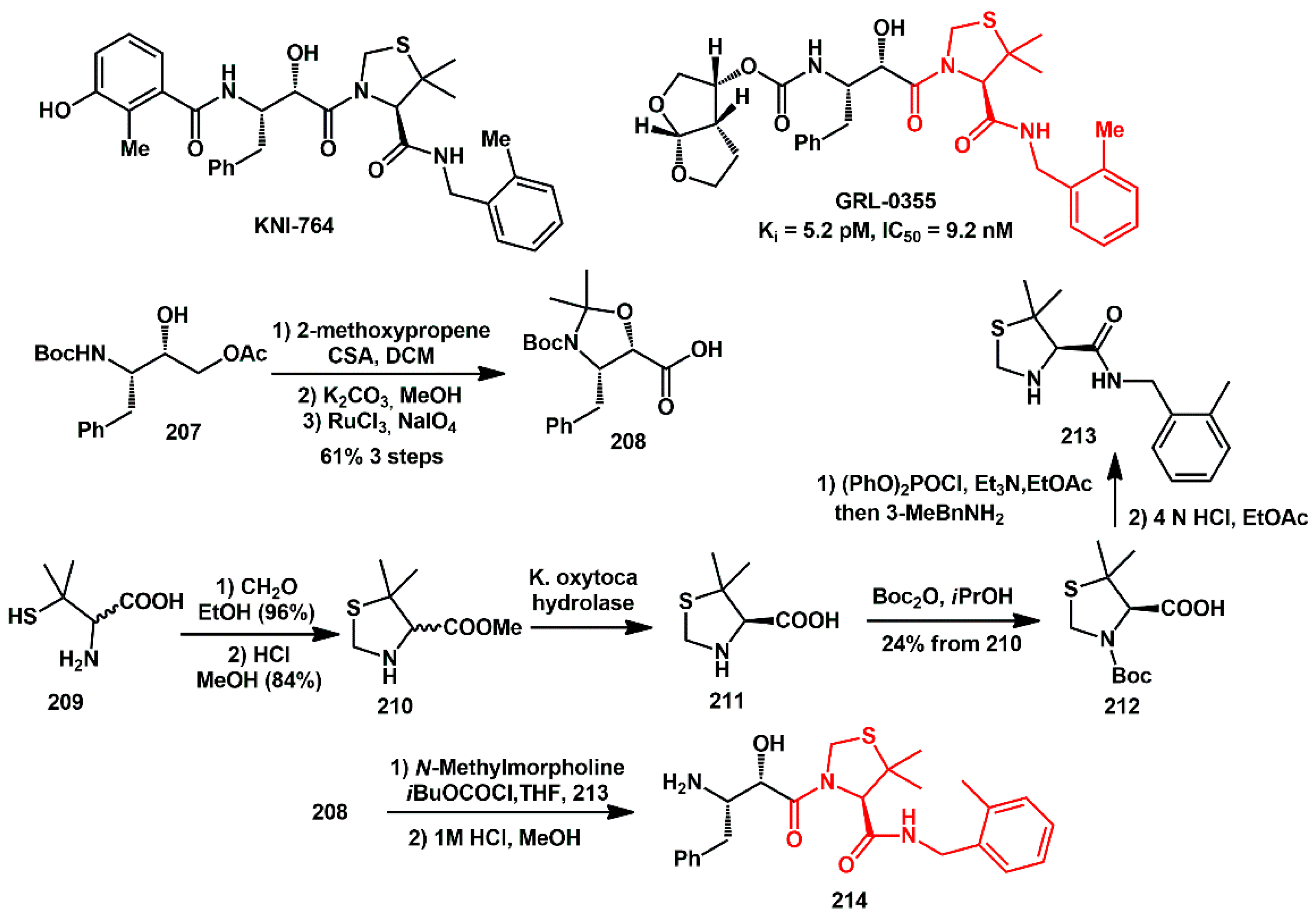
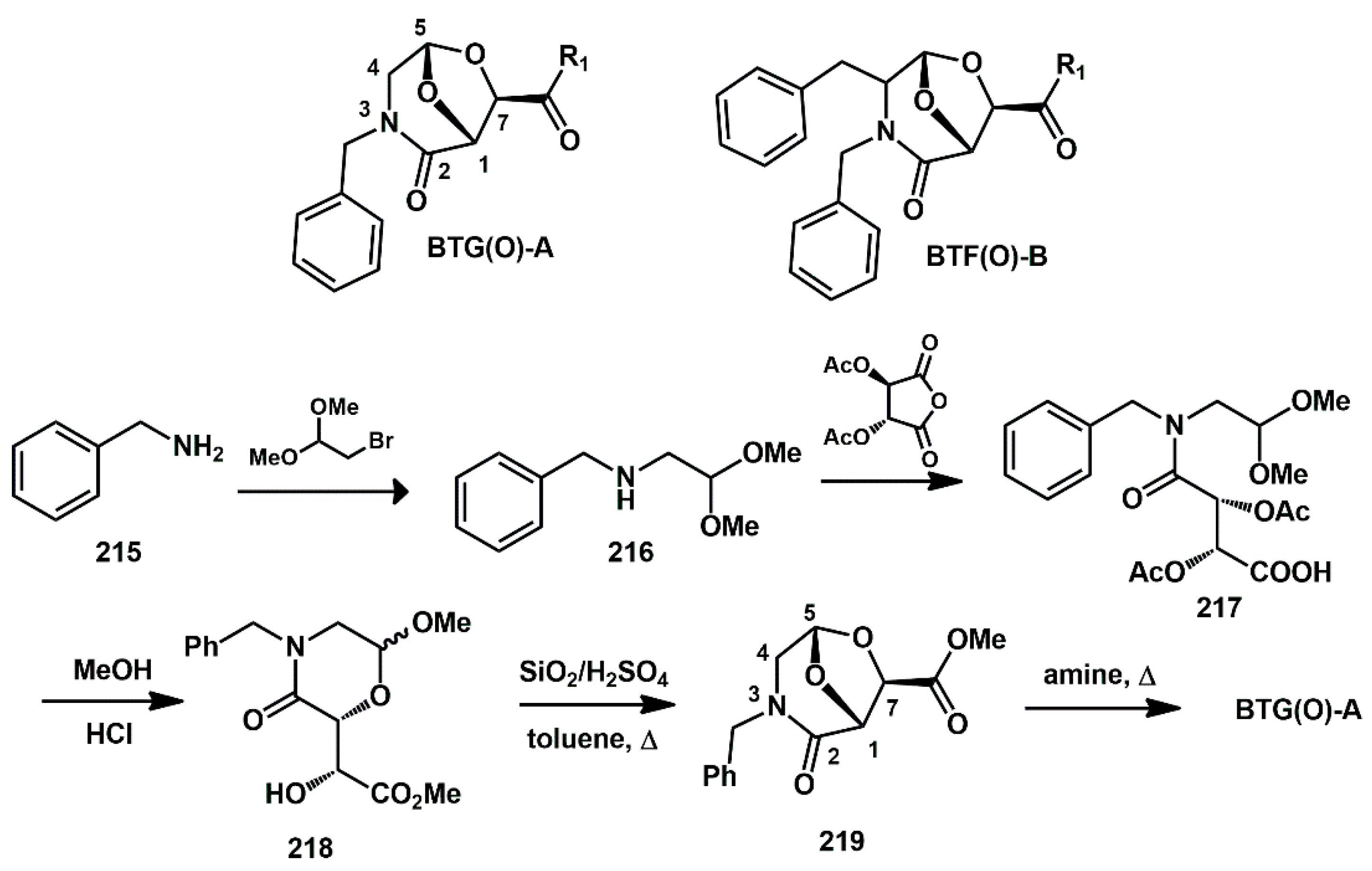
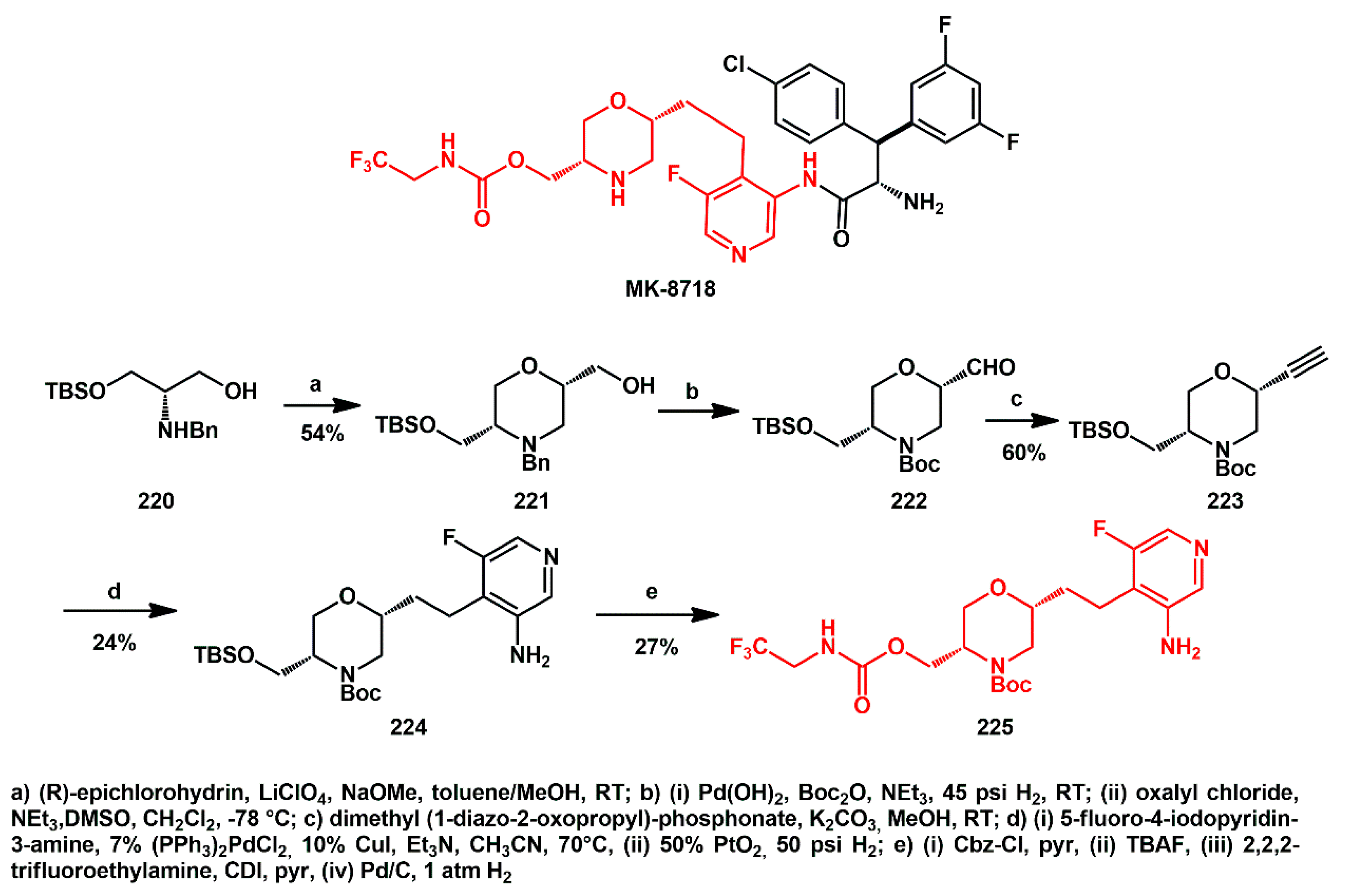
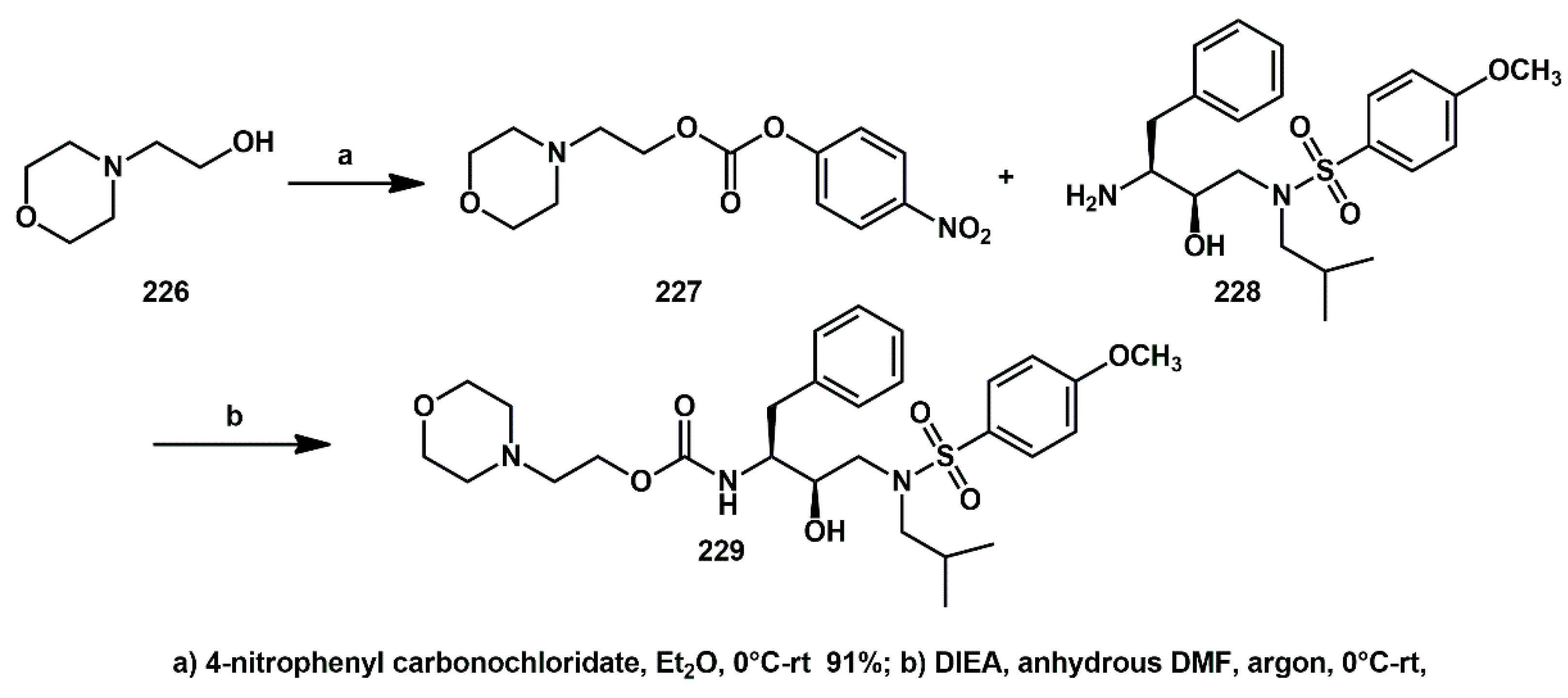
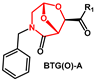
2.1. Non-Aromatic O-Heterocycles
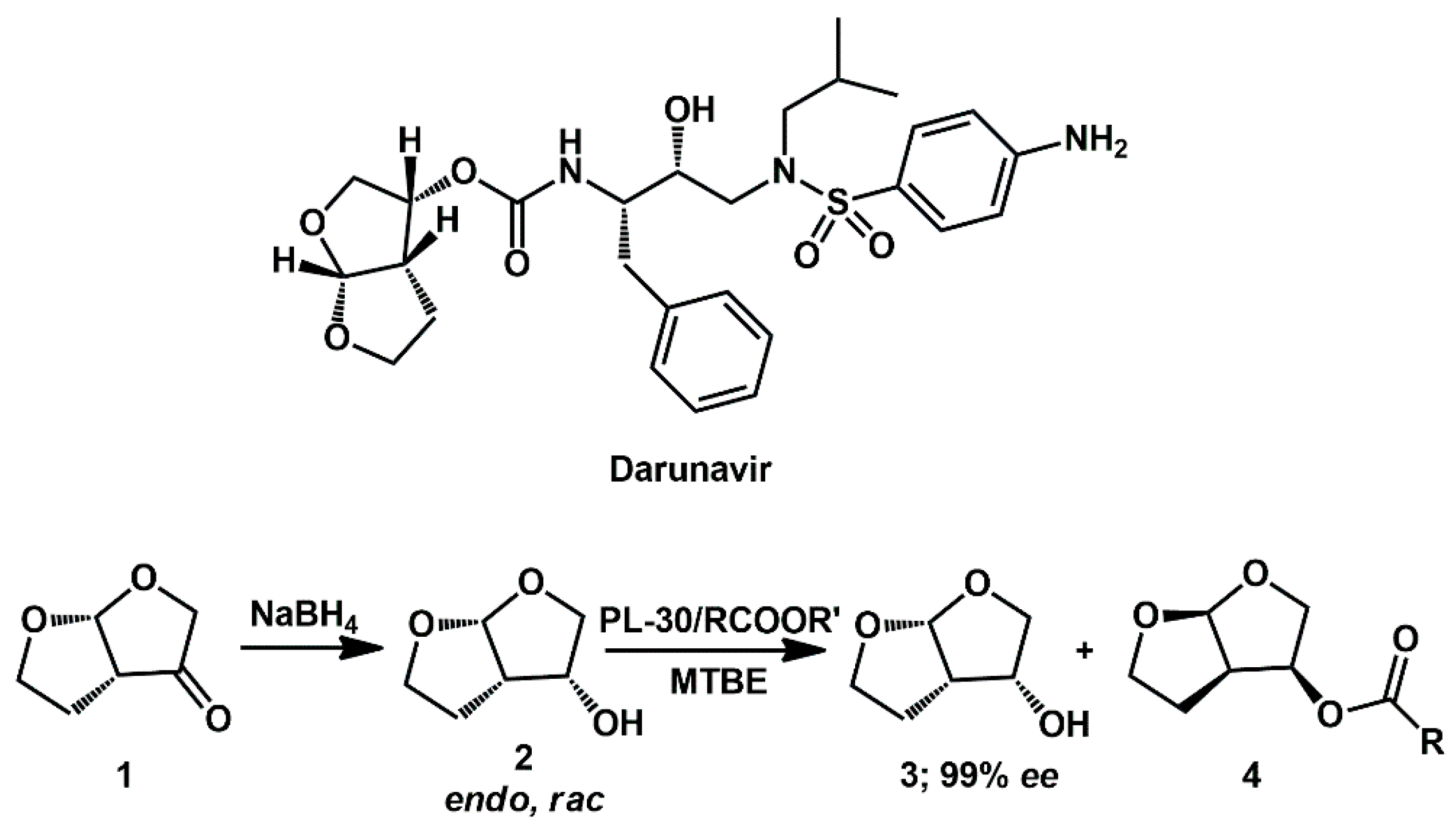
Fused bicyclic
bis-tetrahydrofuran (
bis-THF) is critical for darunavir’s durable drug resistance profile. Due to its challenging double-ring structure with three chiral centers, many different synthetic approaches have been developed according to the source of the chiral carbons in
bis-THF alcohol
2 (
Scheme 1) [
15]. In this respect, enzyme-catalyzed resolution has been exploited by Khmelnitsky [
16], who described the simple and efficient kinetic resolution of the racemic alcohol
2 using immobilized lipase to afford the desired optically pure (
R)-
bis-tetrahydrofuran (
bis-THF) alcohol
3. The reaction solvent, acyl donor, and immobilized biocatalyst proved to be critical factors.
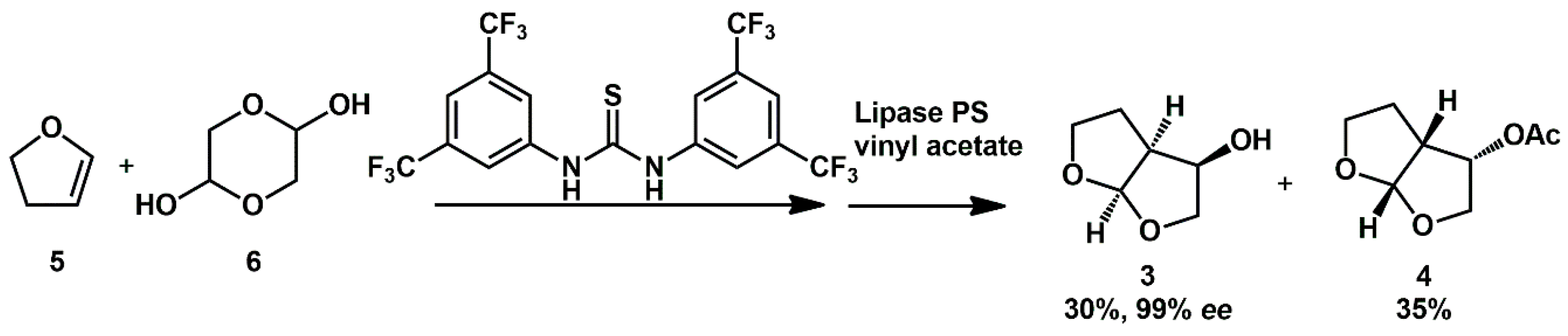
The organocatalytic condensation of 1,2-dihydrofuran
5 (
Scheme 2) with glycolaldehyde catalyzed by Schreiner’s thiourea, coupled with enzymatic (Lipase PS) resolution, was described as a one-pot procedure by Itoh for the preparation of enantiopure
3 and applied to the environmentally friendly synthesis of darunavir [
17].
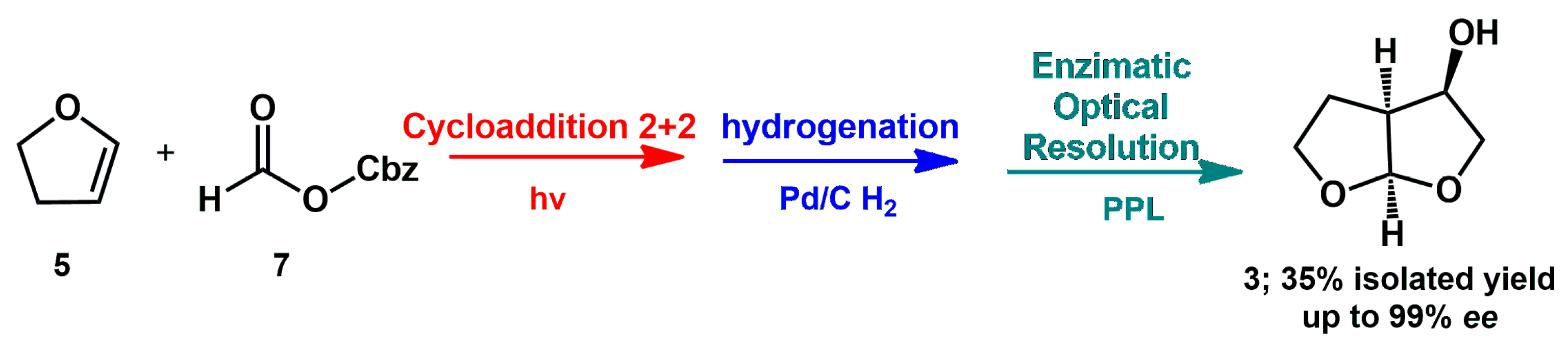
A one-pot procedure using 1,2-dihydrofuran
5 and Cbz-protected glycol aldehyde as the starting materials was developed by Opatz through [2+2]-photocycloaddition between both reactants, followed by hydrogenation and lipase-catalyzed kinetic resolution, affording the target compound with a high yield and up to 99%
ee (
Scheme 3) [
18].
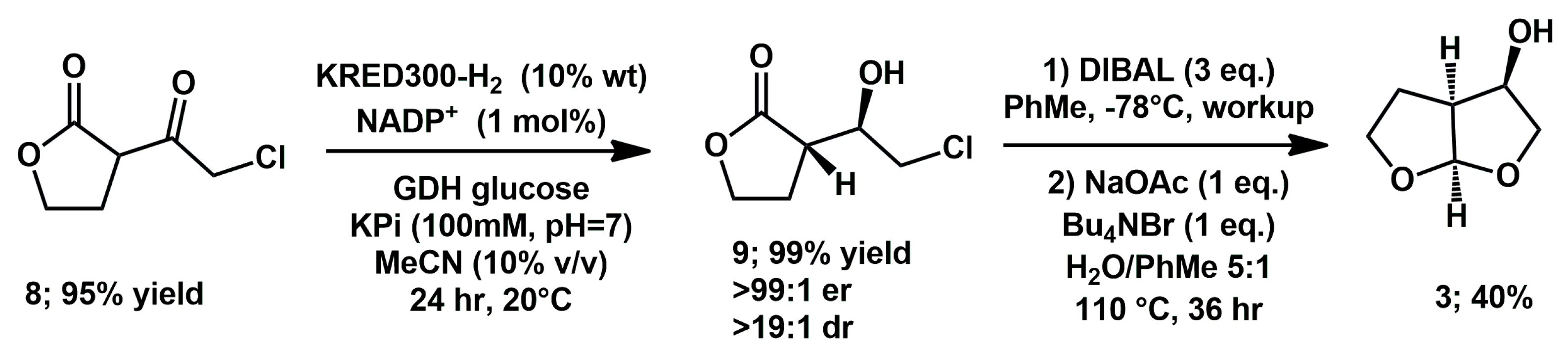
In 2020, Ghosh used the kinetic resolution of
bis-THF alcohol by lipase (PS-30), in a late stage of the study as an alternative stereoselective approach to
cis and
trans 2,3-disubstituted tetrahydrofuran derivatives [
19]. More recently, Hyster proposed a different chemoenzymatic approach to the use of
bis-THF alcohol [
20]. β-Ketolactone
8 was easily prepared and subjected to enantio- and diastereoselective dynamic kinetic resolution using suitable ketoreductase (KRED) and glutamate dehydrogenase (GDH) in phosphate buffer (KPi) from metagenomic mining (
Scheme 4). Subsequent lactone reduction with diisobutylaluminum hydride and phase-transfer cyclization afforded the desired fragment
3 in an acceptable yield.
Asymmetric catalysis was also exploited in the last period. Sudalai [
21] proposed an efficient synthesis of (
S)-tetrahydrofuran-3-ol
13, which is a critical fragment in amprenavir and fosamprenavir.
Olefinic tosylate
10 was dihydroxylated under Sharpless asymmetric dihydroxylation conditions, furnishing, in a single step, the desired alcohol in good yield and moderate enantiomeric purity (
Scheme 5). Alternatively, epoxidation with mCPBA yielded the racemic epoxide
12, which was subjected to co-catalyzed hydrolytic kinetic resolution, affording the alcohol along with the chiral epoxide in a lower chemical yield but with higher enantiomeric excess.
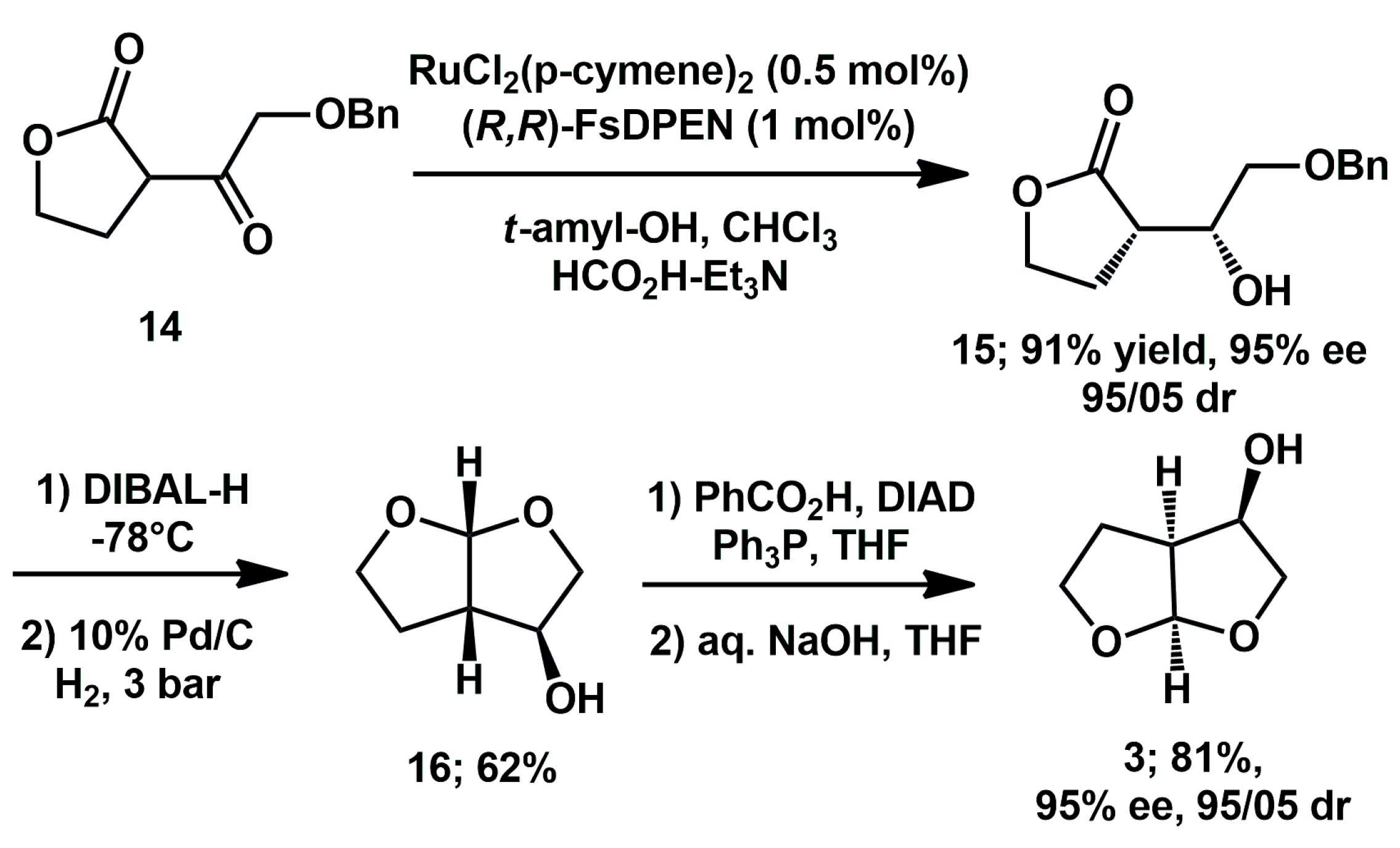
More and Ramana [
22] described a Ru-catalyzed enantio- and diastereoselective dynamic kinetic resolution of benzyloxy/benzoyloxy-α-acyl-γ-butyrolactones
via transfer hydrogenation, achieved through the in situ prepared (
R,
R)-Ru-FsDPEN catalyst. Starting from benzyloxy butyrolactone
14, (3R,3aS,6aR)-hexahydrofuro[2,3–b]furan-3-ol
3 was prepared in good yield, with high
de and
ee through elaboration of the key intermediate (
Scheme 6).
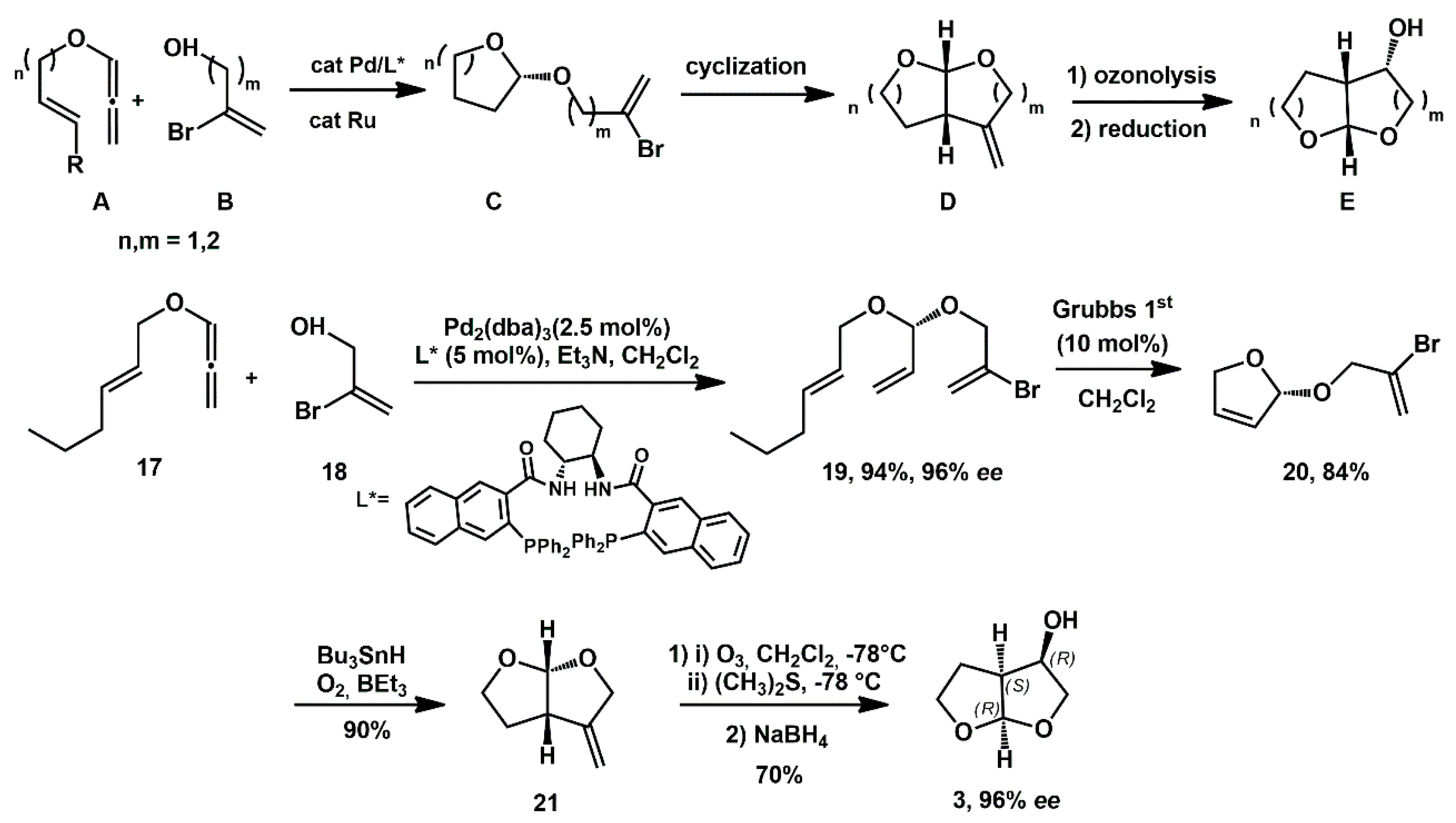
Pd-catalyzed asymmetric hydroalkoxylation of ene-alkoxyallene, followed by ring-closing metathesis (RCM), was successfully employed by Rhee [
23] for the preparation of hexahydrofurofuran-3-ol of type
E, and was generalized to provide access to pyranofuranol and furopyranol derivatives in good yields and
ee (
Scheme 7). For the synthesis of
3, readily available allene
17 was coupled with commercial 2-bromo allylic alcohol
18 in the presence of Pd
2(dba)
3 as the palladium source and suitable ligand, affording the chiral adduct
19 in high yield and enantiomeric excess. Subsequent RCM provided the cyclic acetal
20, which underwent a radical, mediated 5-
exo cyclization to intermediate
21, promoted by Bu
3SnH in the presence of Et
3B. Ozonolysis of this compound, followed by final reduction with NaBH
4, furnished the target alcohol
3 in high yield with complete diastereoselectivity and no erosion of enantiopurity.
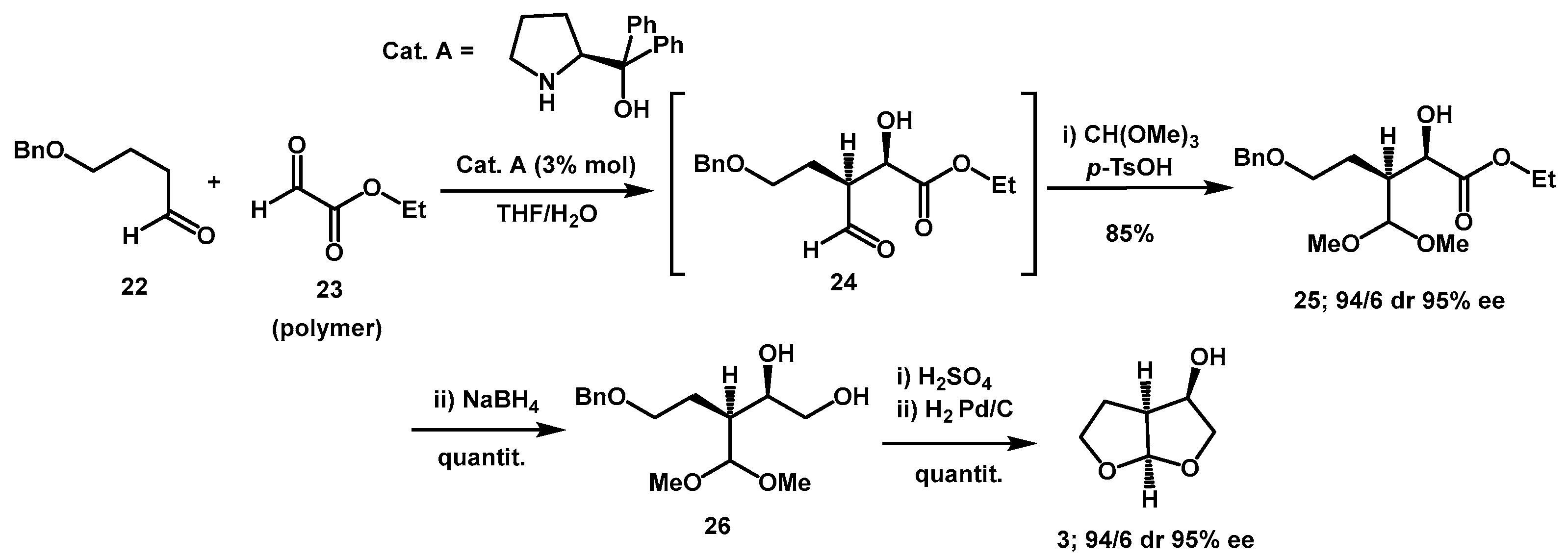
Organocatalytic methods were also successfully used. Ikemoto described an efficient synthesis of
bis-THF alcohol
3 and its carbonyloxy-pyrrolidine-2,5-dione derivative taking advantage of diphenylprolinol-catalyzed enantio- and diastereoselective cross aldol reaction of polymeric ethyl glyoxylate
23 with the aldehyde
22 as the key step (
Scheme 8) [
24]. Optimization of the reaction involved stirring polymeric ethyl glyoxylate in toluene solution with water prior to aldol reaction for an appropriate period. This pre-treatment was highly effective in accelerating the reaction when using 3 mol % of catalyst
A. It also ensured excellent reproducibility, even when polymeric ethyl glyoxylate from different manufacturers was utilized. The aldehyde of intermediate
24 was then protected as dimethyl acetal and the ester was reduced, yielding diol
26 in an overall yield of 85%. An acetal exchange reaction catalyzed by H
2SO
4 followed by hydrogenation with Pd/C catalyst afforded
3 in quantitative yield over two steps. Shortly thereafter, the same group described a series of amino perfluoroalkanesulfonamide derivatives of diarylprolinols, which proved to be effective organocatalysts [
25].
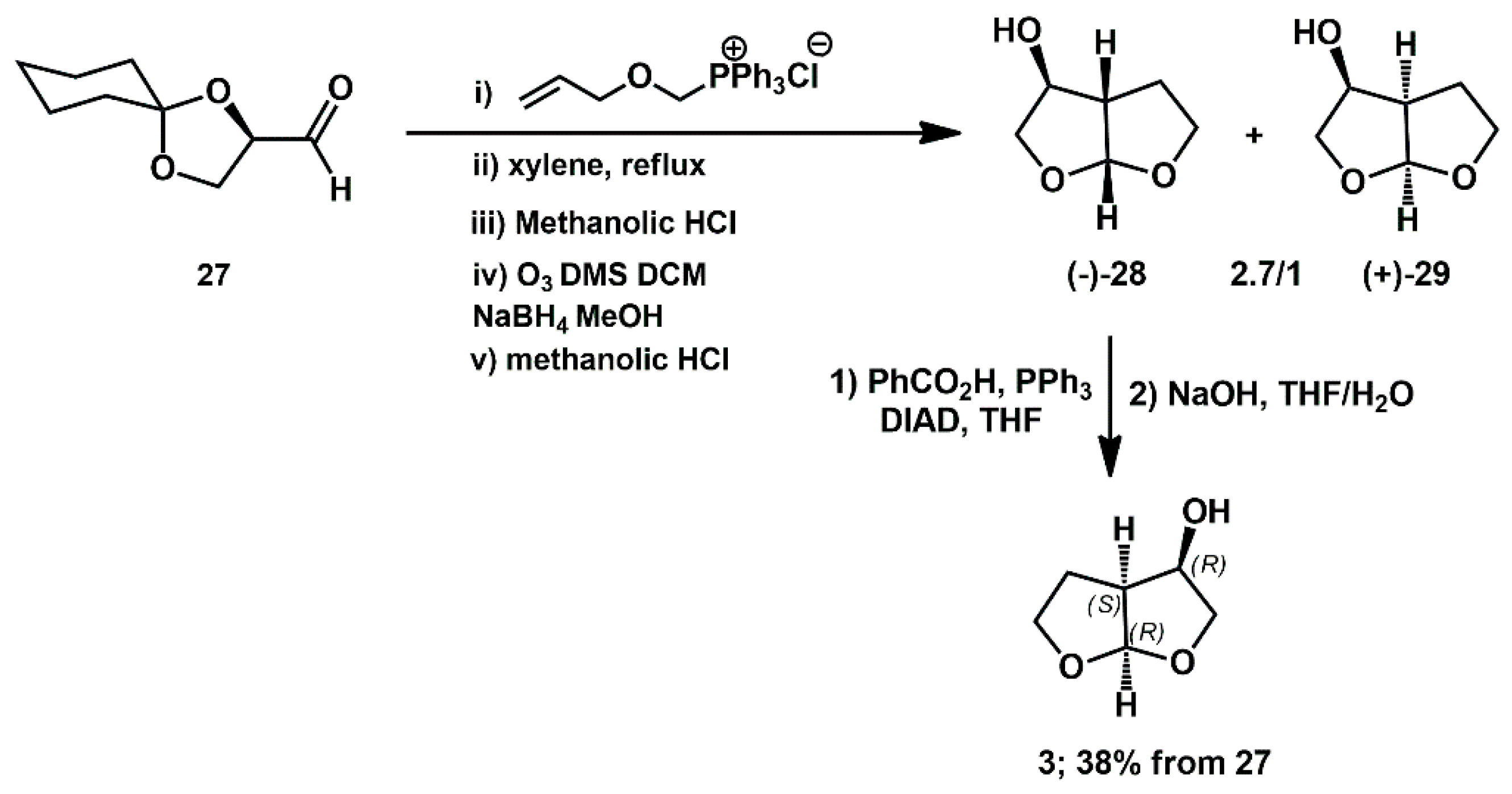
Traditional chiral-substrate approaches have also been employed over the past fifteen years. A spirocyclic dioxolane derivative from
D-glyceraldehyde
27 was used by Kulkarni as starting chiral substrate for the preparation of all four isomers of bis-tetrahydrofuran alcohol in good overall yield (38%) of the active isomer
3 (
Scheme 9) [
26].
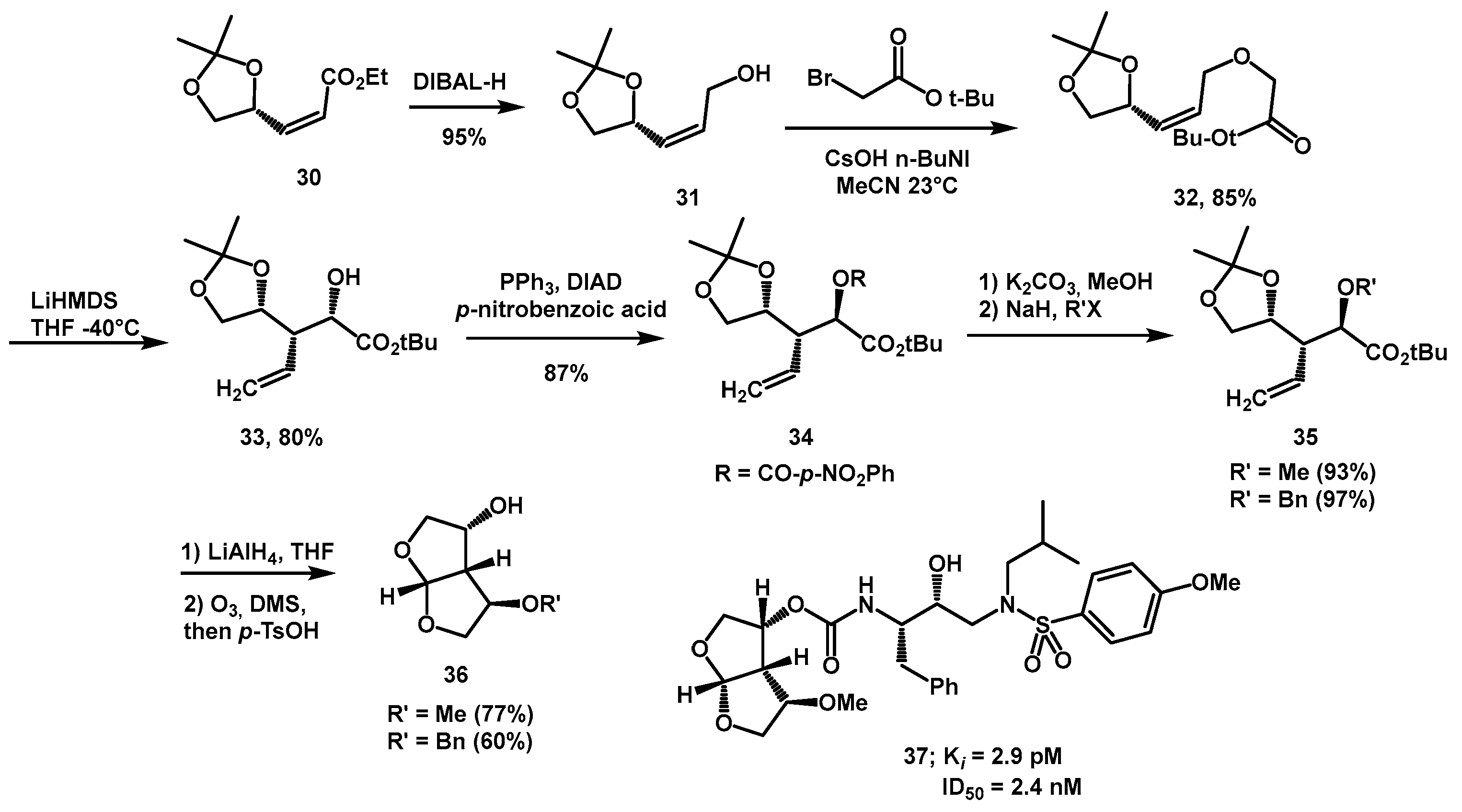
(S)-Glyceraldehyde was used as a chiral source by Ghosh in the convergent synthesis of various substituted bis-THF derivatives, to find new HIV-protease inhibitors with enhanced binding capacity [
27]. The synthesis of the
bis-THF ligand began with known compound
30 (
Scheme 10) prepared in multigram quantities by Wittig olefination of (S)-glyceraldehyde acetonide with (ethoxycarbonylmethylene) triphenylphosphorane. DIBAL-H reduction yielded the corresponding alcohol
31 in nearly quantitative yield. Subsequently,
O-alkylation and subsequent stereoselective [
3,
4]-sigmatropic rearrangement afforded the alcohol with three chiral centers
33, which was transformed alternatively to
bis-THF alcohol
3 or to different functionalized derivatives. The synthesis of alkyl substituted bis-THF ligands
36 required a Mitsunobu reaction on alcohol
33 with PPh
3/DIAD and
p-nitrobenzoic acid, to give the corresponding ester
34. This was first hydrolyzed, then alkylation with BnBr or MeI was performed, alternatively. Final reduction, ozonolysis, and cyclization sequence furnished the ligands
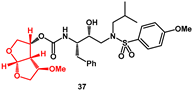
36 in good overall yield. Among the inhibitors bearing new
bis-THF fragments, compound
37 resulted in being the most potent.
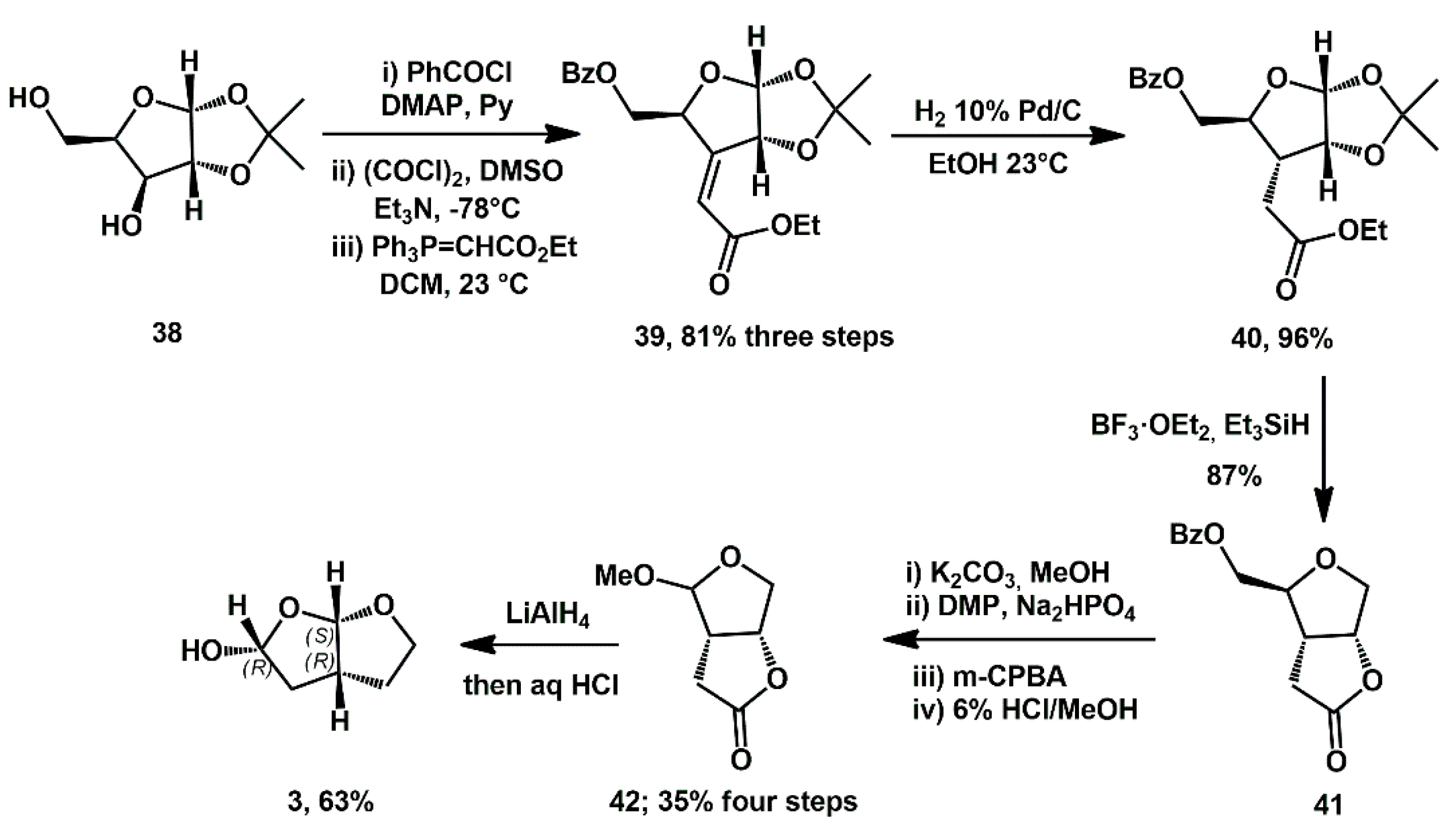
More recently, Ghosh described a new approach to bis-THF alcohol
3, starting from commercially available sugar derivatives [
28]. As an example, commercial 1,2-
O-isopropylidene-α-D-xylofuranose
38 was converted to α,β-unsaturated ester
39, by sequential selective protection of primary alcohol, Swern oxidation, and Wittig olefination (
Scheme 11). Ester
39 was then submitted to highly stereoselective substrate-controlled catalytic hydrogenation, which represented one of the key steps.
Saturated ester 40 was converted to γ-lactone derivative 41 by exposure to BF3·OEt2 followed by Et3SiH. Hydrolysis of the benzoate furnished the corresponding bicyclic alcohol, which was converted to methyl acetal 42 by a three-step sequence involving Dess–Martin oxidation of the primary alcohol to the corresponding aldehyde, Baeyer–Villiger oxidation promoted by m-CPBA, and exposure of the resulting formate to 6% HCl in MeOH. Acetal 42 was obtained in 35% yield over four steps. Final reduction of the lactone and acidification yielded the target bis-THF alcohol.
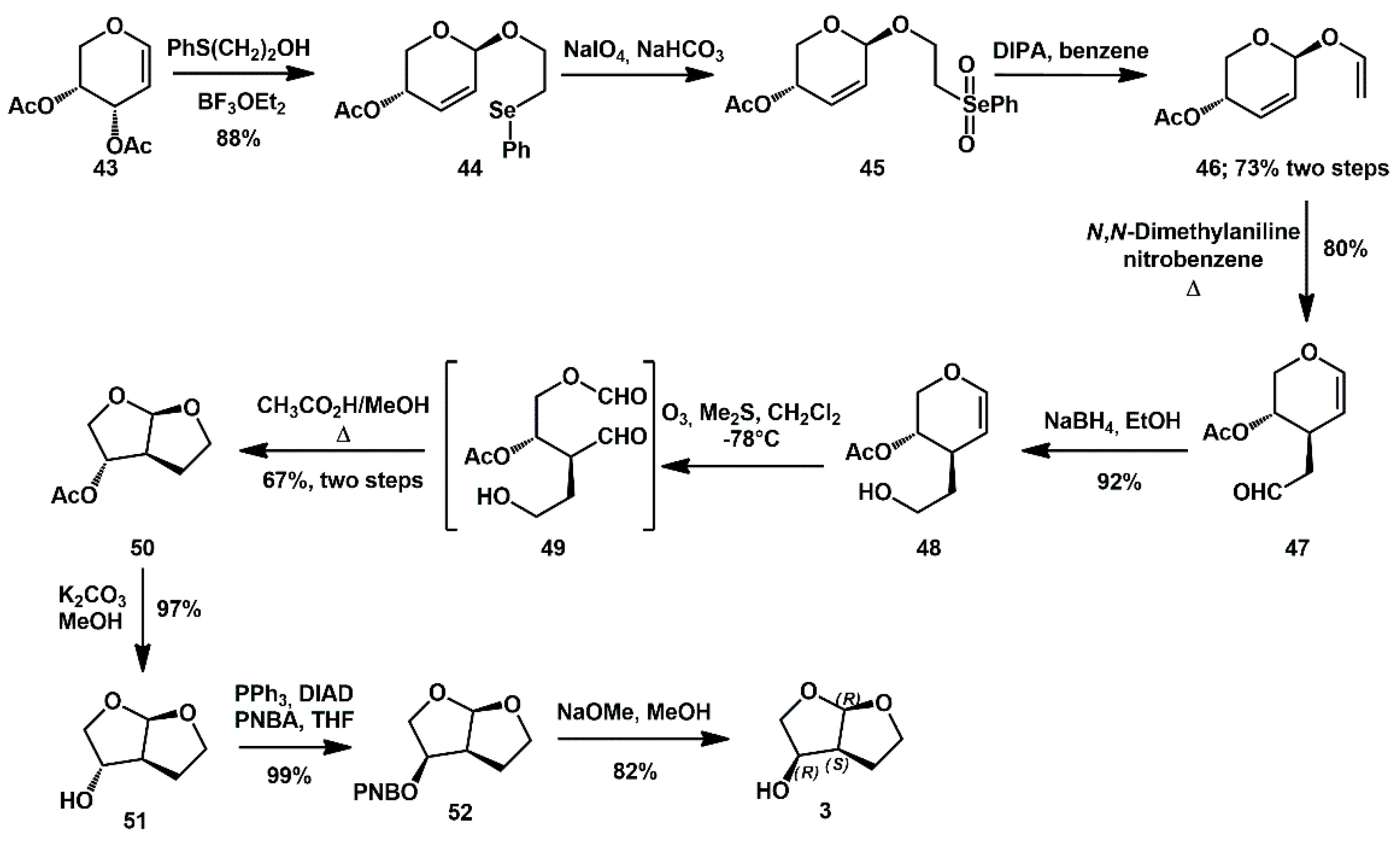
Regarding sugars as chiral pool materials, Sridhar developed a stereoselective synthesis of carbohydrate-derived perhydrofuro[2,3-
b]furan derivatives, starting from sugar-derived allyl vinyl ethers [
29]. In particular,
bis-THF alcohol
3 was prepared using 3,4-di-
O-acetyl-D-arabinal
43 as the chiral starting material (
Scheme 12). It was first subjected to Ferrier rearrangement with 2-(phenylselenyl)ethanol, which provided 2,3-unsaturated glycoside
44. After oxidation to selenone
45, a base-mediated thermal fragmentation furnished the allyl vinyl ether
46, which underwent a Claisen [
4]-sigmatropic rearrangement, affording the expected 3-C branched derivative
47. Reduction of the aldehyde, followed by ozonolysis of the olefin and acid-mediated acetalization, provided perhydrofuro[2,3-
b]furan
50 in good yield. The synthesis of
bis-THF alcohol
3 required deprotection of the ester, Mitsunobu inversion with
p-nitrobenzoic acid in PPh
3/DIAD system, and final hydrolysis.
The potassium isocitrate, obtained from high-yielding fermentation fed by sunflower oil, was the starting material for the preparation of bis-THF alcohol
3 in the work of Yue (
Scheme 13) [
30]. After several steps, it was transformed into a tertiary amide, which was reduced along with the ester functionalities to a transient aminal-triol. This latter was converted in situ to the desired
bis-THF alcohol. Each step was optimized, resulting in the key alcohol (in the form of activated carbonate) with an overall yield of 43%. Considering the low cost of the starting material and the efficiency of the overall synthesis, the method appeared to be effective in reducing the cost of darunavir.
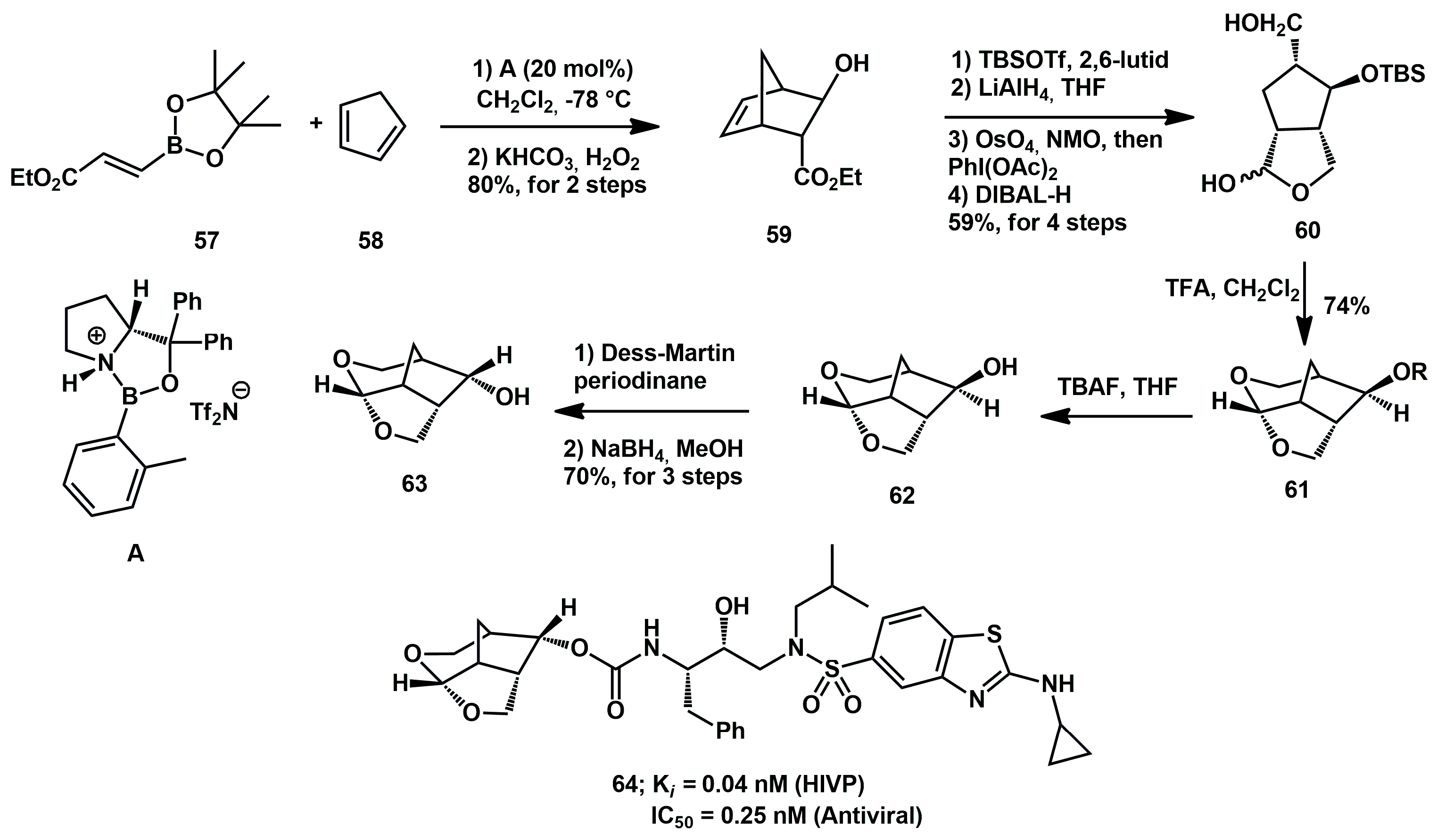
With the aim of creating a new class of inhibitors with improved pharmacological and drug-resistance profiles, Ghosh described the synthesis of a 6–5–5 ring-fused crown-like tetrahydropyranofurans and their incorporation into new potent inhibitors [
31,
32]. The synthesis started with an asymmetric Diels–Alder reaction between
57 and cyclopentadiene, using a chiral oxazaborolidinium cation
A, as a key step (
Scheme 14). The cycloadduct was oxidized with H
2O
2 to afford alcohol
59 in 80% yield over two steps, with 98%
ee. After protection as the TBS ether, the intermediate was converted to bicyclic acetal
60 in three-steps, involving reduction with LiAlH
4, one-pot oxidative cleavage of the olefin, and reduction of the resulting aldehyde with DIBAL-H. The lactol
60 (a 2:1 mixture) was treated with trifluoroacetic acid (TFA) to produce the bridged tricyclic derivative
61, and the TBS group was removed with tetrabutylammonium fluoride (TBAF), yielding the desired
exo-alcohol. This compound was alternatively epimerized by Dess–Martin oxidation followed by reduction of the resulting ketone with NaBH
4. The inhibitor
64 displayed superior antiviral activity and drug-resistance profiles compared to darunavir.
Shortly afterwards, this fragment was incorporated into new arylboronic derivatives, and they were tested against highly drug-resistant HIV-1 variants [
33].
Alternatively, the Diels–Alder reaction of cyclopentadiene with chiral 3-(acyloxy)acryloyloxazolidinone derivatives was later used for the synthesis of 6-5-5 fused crown-like THF ligands (
Scheme 15). In particular, 3-(4-methoxybenzoyl)acryloyl oxazolidinone derivative
65 and cyclopentadiene afforded the endo-diastereoselective derivative
66 in 98% yield. Reaction of the cycloadduct in MeOH provided the corresponding methyl ester
67 in excellent yield, which was a key intermediate in the synthesis of the bicyclic alcohol
63 [
34].
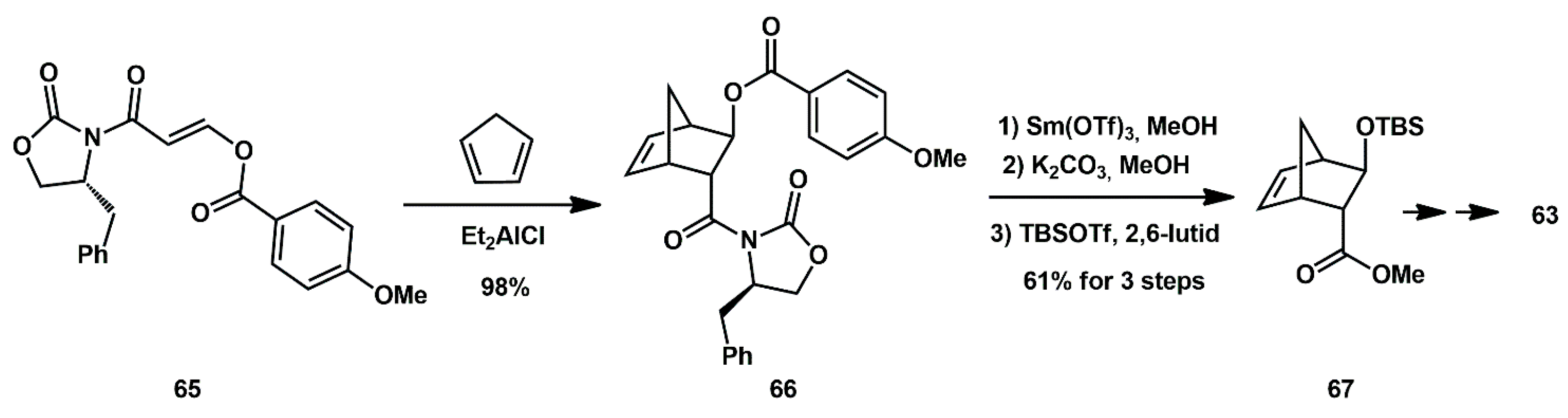
An optically active (1
R, 3aS, 5
R, 6
S, 7
aR)-octahydro-1,6-epoxy-isobenzo-furan-5-ol derivative was recently described as a high affinity ligand for HIV-1 protease inhibitors [
35]. The stereoselective synthesis was based on enantioselective enzymatic desymmetrization of
meso-1,2(dihydroxy methyl)cyclohex-4-ene
69 using porcine pancreatic lipase (PPL) to produce optically active alcohol
70 (
Scheme 16) [
28]. It was then converted to a functionalized cyclohexene derivative
71, which underwent Sharpless asymmetric dihydroxylation to yield a 1:1 mixture of diastereomeric diols
72 and
73. The diols were separated and only diastereomer
73 was utilized for the synthesis of ligand alcohol
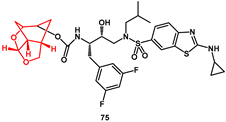
74. In general, inhibitors bearing such ligands showed very potent activity. Moreover, compound
75, with a difluorophenylmethyl P1 ligand and an aminobenzothiazole as the P2′ ligand, maintained high potency against a panel of highly multidrug-resistant HIV-1 variants.
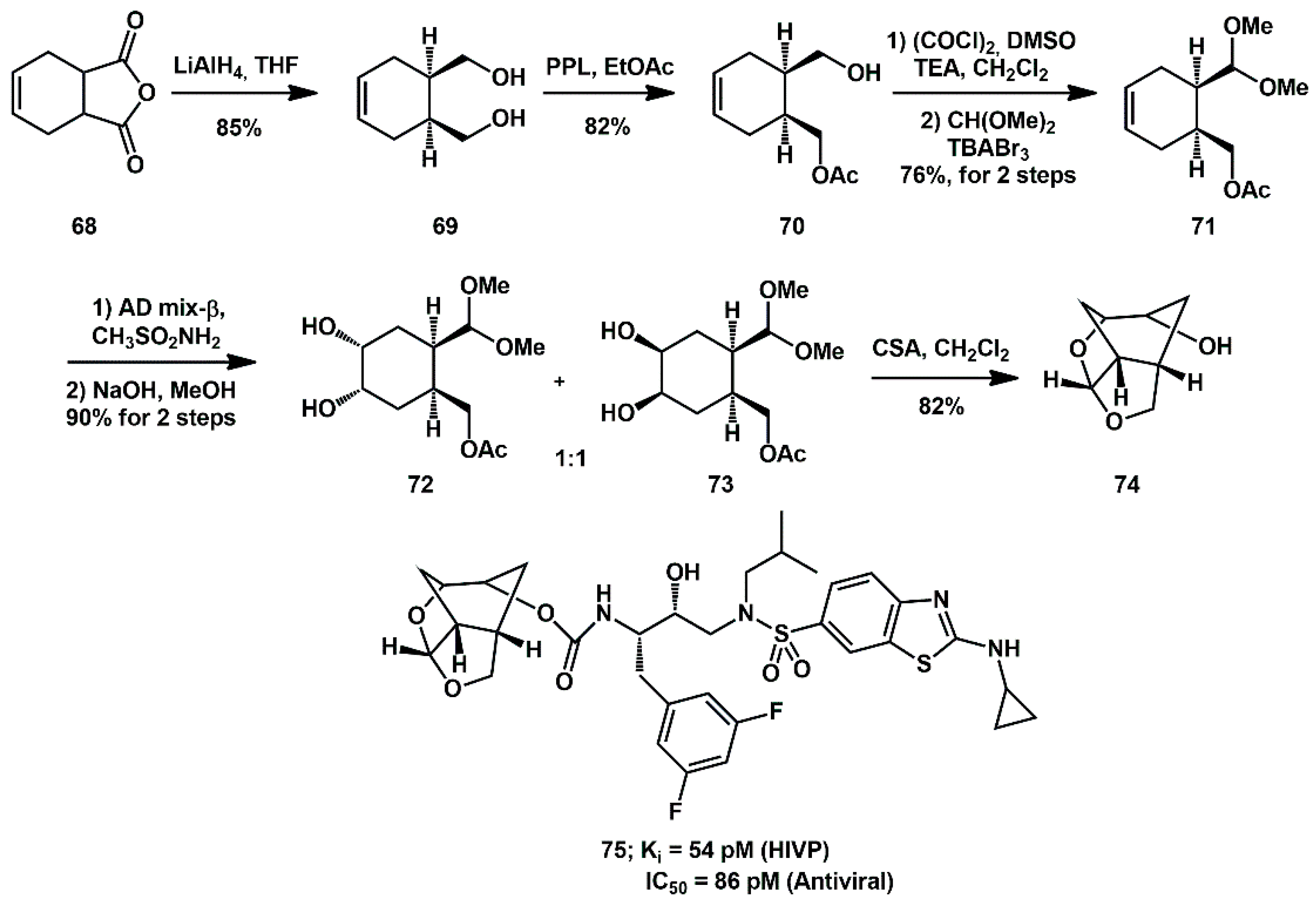
More recently, a modified procedure was described [
36]. Optically active alcohol
70 was converted to methyl acetal
76 (in 75% yield over 3 steps) as the major isomer in a sequence involving, (1) Swern oxidation of alcohol
70 to the aldehyde, (2) treatment of the resulting aldehyde with CH(OMe)
3 in the presence of tetrabutylammonium tribromide (TBABr
3), followed by treatment with 1 N NaOH, and (3) reaction of the resulting methyl acetal with a catalytic amount of CSA (
Scheme 17).
Stereoselective syn-1,2-diol functionality was introduced on methyl acetal 76 by substrate-controlled dihydroxylation, with K2OsO4/K3Fe(CN)6 system, affording syn diol 77, as 95/5 diastereomeric mixture. Swern oxidation provided 1,2-diketone derivative 78, which was reduced with L-Selectride to give the corresponding inverted syn-1,2-diol, stereoselectively. Acid-catalyzed cyclization then furnished tricyclic ligand alcohol 74 with high enantiomeric purity.
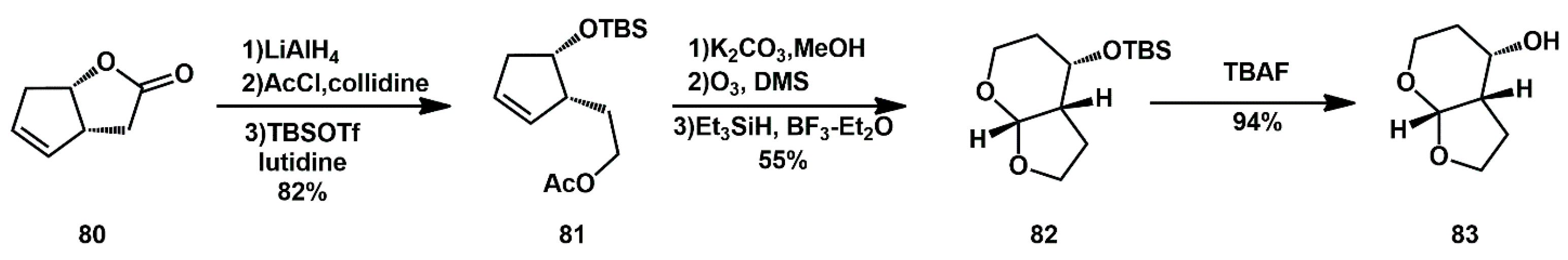
The synthesis of enantiomerically pure (3aS,4S,7aR)-hexahydro-2H-furo[2,3-b]pyran-4-ol
83 was originally described by Ghosh [
37] starting from known enantiomerically pure lactone
80 (
Scheme 18). Subsequent steps involved reduction, selective protection/deprotection, ozonolysis, and reduction of the hemiacetal moiety. Final removal of the silyl group with TBAF furnished the desired ligand.
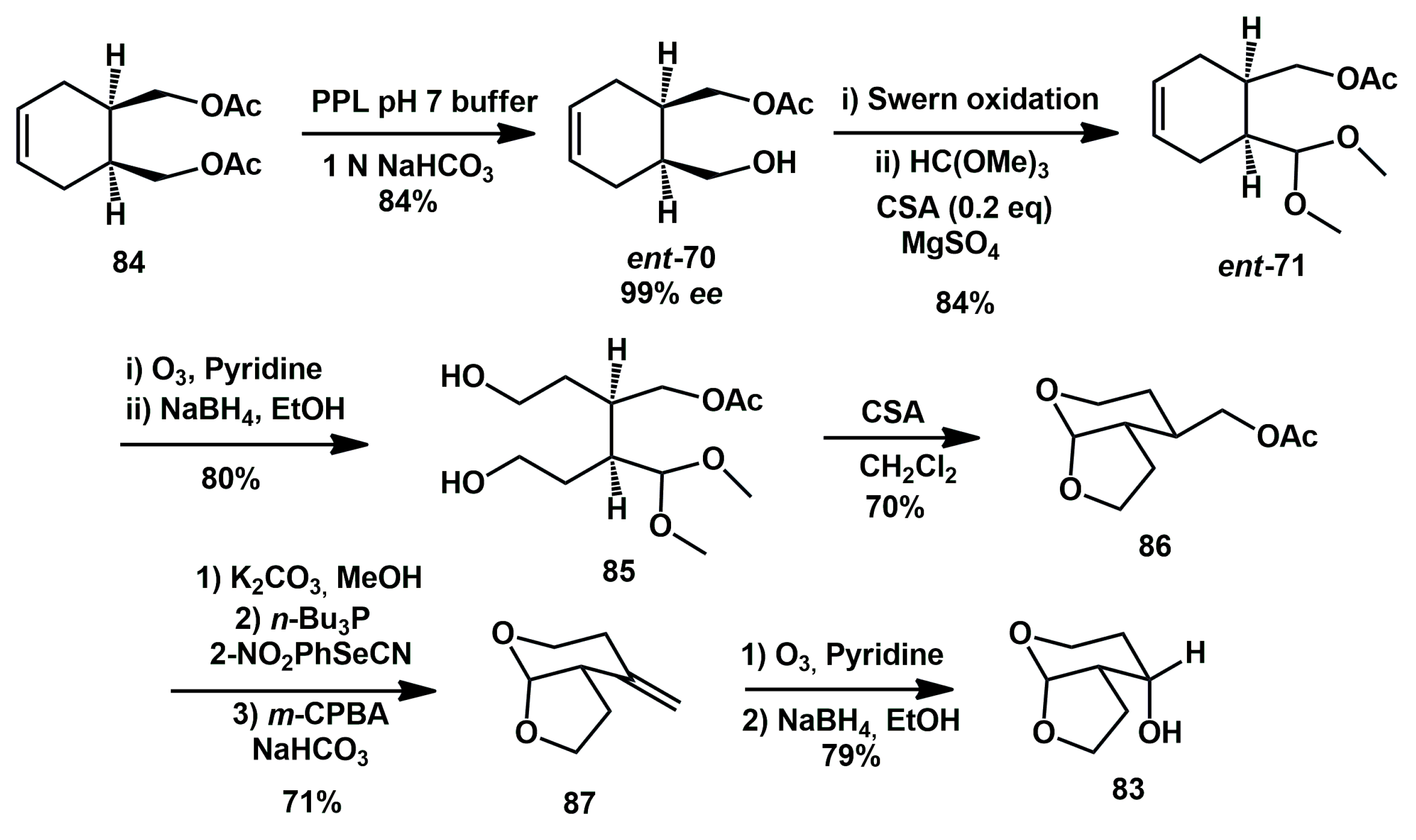
A more recent alternative route has been proposed, starting from inexpensive materials and taking advantage of highly enantioselective enzymatic desymmetrization of a
meso-diacetate, already developed by the same group [
38]. The enzymatic desymmetrization of
84 was optimized by using aqueous 1 N NaHCO
3 for neutralization of acetic acid formed during the reaction (
Scheme 19). Swern oxidation followed by protection of the aldehyde furnished the corresponding acetal
ent-71. Oxidative cleavage of the olefin by ozonolysis in the presence of pyridine as the organocatalyst, followed by reduction of the resulting dialdehyde with sodium borohydride afforded diol
85. Treatment of the diol with a catalytic amount of CSA furnished bicyclic acetal
86 in 56% yield over 3 steps. After hydrolysis of acetate, the corresponding alcohol was treated with
o-nitrophenylselenonitrile and
n-tributylphosphine to give the corresponding selenide derivative. Oxidation of the resulting selenide with
m-CPBA resulted in elimination of the corresponding selenoxide to afford olefin
87 in 71% yield over 3 steps. Ozonolysis of
exo-olefin followed by reduction of the corresponding ketone yielded alcohol
83 in good yield.
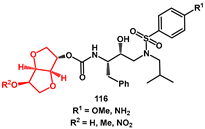
Various C3-functionalized cyclopentanyltetrahydrofurans (Cp-THF) were prepared and combined with hydroxyethylsulfonamide isosteres to obtain new inhibitors with high antiviral activity, including against a panel of multidrug-resistant HIV-1 variants [
39]. As an example, 3-(
R)-acetoxy and 3-(
R)-methoxy ligands
92 and
93 were prepared from the known alcohol
89, which was first alkylated to provide methyl ester
90 (
Scheme 20). DIBAL-H reduction followed by radical cyclization provided 3-(
R)-hydroxy derivative
91 in 10:1 diastereomeric ratio. The major isomer was transformed into the desired ligand
92 by acetylation, followed by removal of the silyl group, in 73% yield over two steps. Alternatively, the methoxy derivative
93 was prepared by methylation followed by removal of silyl group, in 71% yield.
Novel oxatricyclic ligands were designed and synthesized by Ghosh to enhance interactions with the protease backbone [
9]. In particular, the inhibitor
101, bearing the tris-THF
syn-
anti-
syn configuration, showed very high activity against a variety of multidrug resistant HIV-1 variants and blocked HIV-protease dimerization about 10-fold better than darunavir (
Scheme 21) [
40]. Starting from the known acetate
94, the bicyclic enol-ether
95 was easily obtained. Subsequently, after epoxidation with dimethyldioxirane (DMDO) and regio- and stereoselective oxiranyl ring opening with sodium methoxide, the desired
endo-alcohol
96 was obtained by Dess–Martin oxidation followed by L-selectride reduction of the resulting ketone. Acylation of
96, followed by treatment with propargyl alcohol, afforded acetals
97 and
98 in a 1:4 ratio. After removal of the acetate, the major diastereomeric alcohol deriving from
97 was converted into the corresponding tricyclic olefin
99. Ozonolysis followed by reduction furnished
syn-
anti-
syn type oxatricyclic tris-THF
100 as a single isomer.
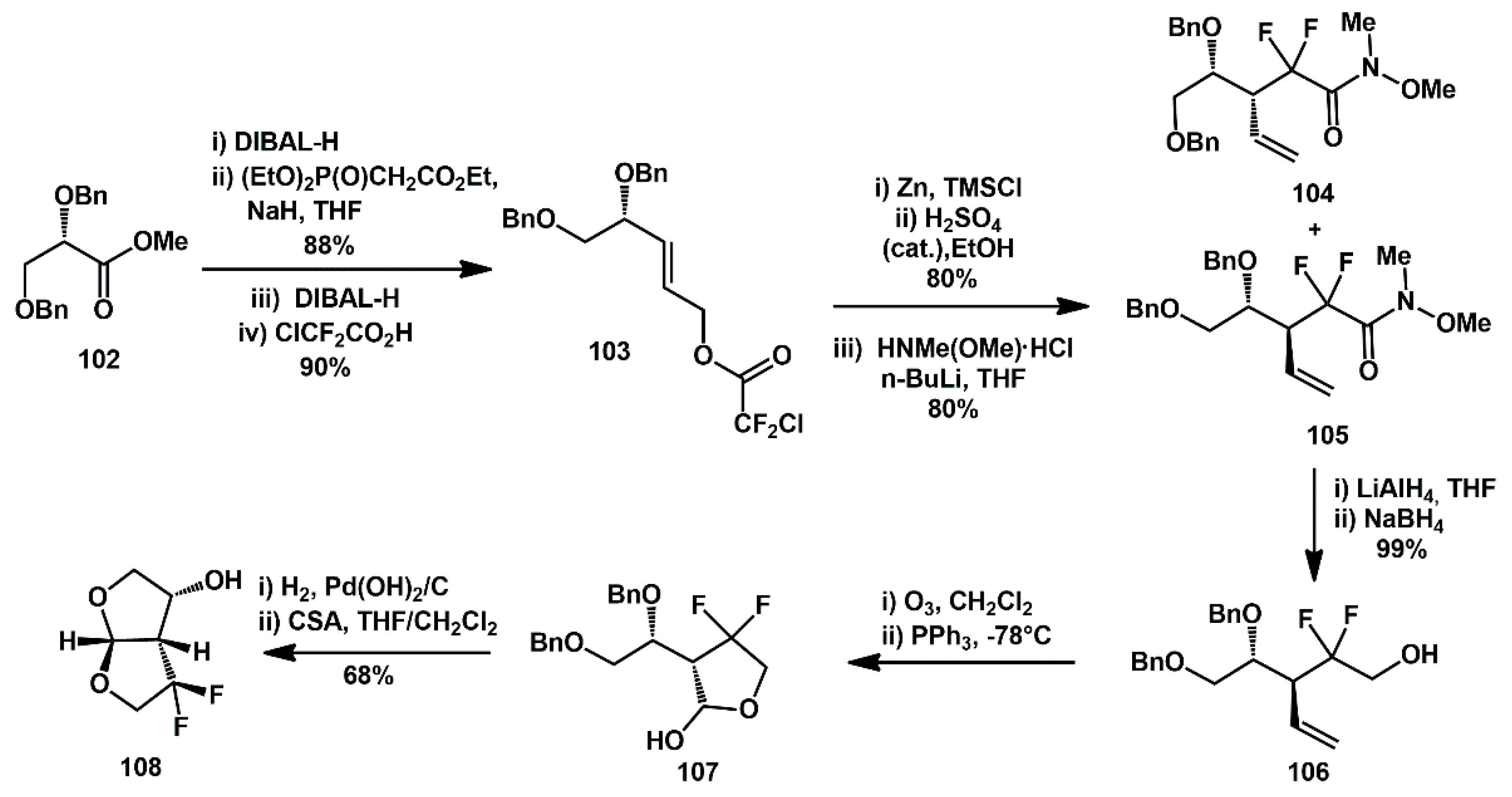
Gem-difluoro-bis-THF ligands were prepared and converted into suitable inhibitors, which exhibited very high activity and better lipophilicity profiles than darunavir, along with significantly improved blood–brain barrier permeability in an in vitro model [
41]. The synthesis began with DIBAL-H reduction of optically active methyl ester
102 (
Scheme 22), producing dibenzyl-L-glyceraldehyde. This intermediate underwent the Horner–Emmons reaction with sodium hydride and triethyl phosphonoacetate, affording the corresponding α,β-unsaturated ester in 88% yield over two steps. Reduction with DIBAL-H provided the corresponding allylic alcohol, which was treated with chlorodifluoroacetic acid in chloroform at reflux, yielding difluoroacetate derivative
103 in 90% yield over two steps. Subsequently, it underwent a Reformatskii–Claisen reaction by treatment with trimethylsilyl chloride and activated zinc dust. The subsequent acid-catalyzed esterification furnished a 2:1 mixture of diastereomers in 80% yield over two steps. The mixture could be separated after its conversion into the Weinreb amides, by treatment with HN(Me)OMe·HCl and
n-BuLi. These amide diastereomers were separated by silica gel chromatography, providing the syn diastereomer
104 as the major product and the anti diastereomer
105 as the minor product, in a 2:1 ratio and with an overall yield of 80%.
Reduction of the Weinreb amide 105 was carried out with lithium aluminum hydride, and the resulting crude aldehyde was reduced with sodium borohydride in a one-pot operation to provide difluoro alcohol 106 in near quantitative yield. Ozonolysis, followed by reductive cleavage with PPh3, provided cyclic acetal 107 upon cyclization. Final catalytic hydrogenation and treatment with CSA of the triol afforded difluoro-bis-THF 108.
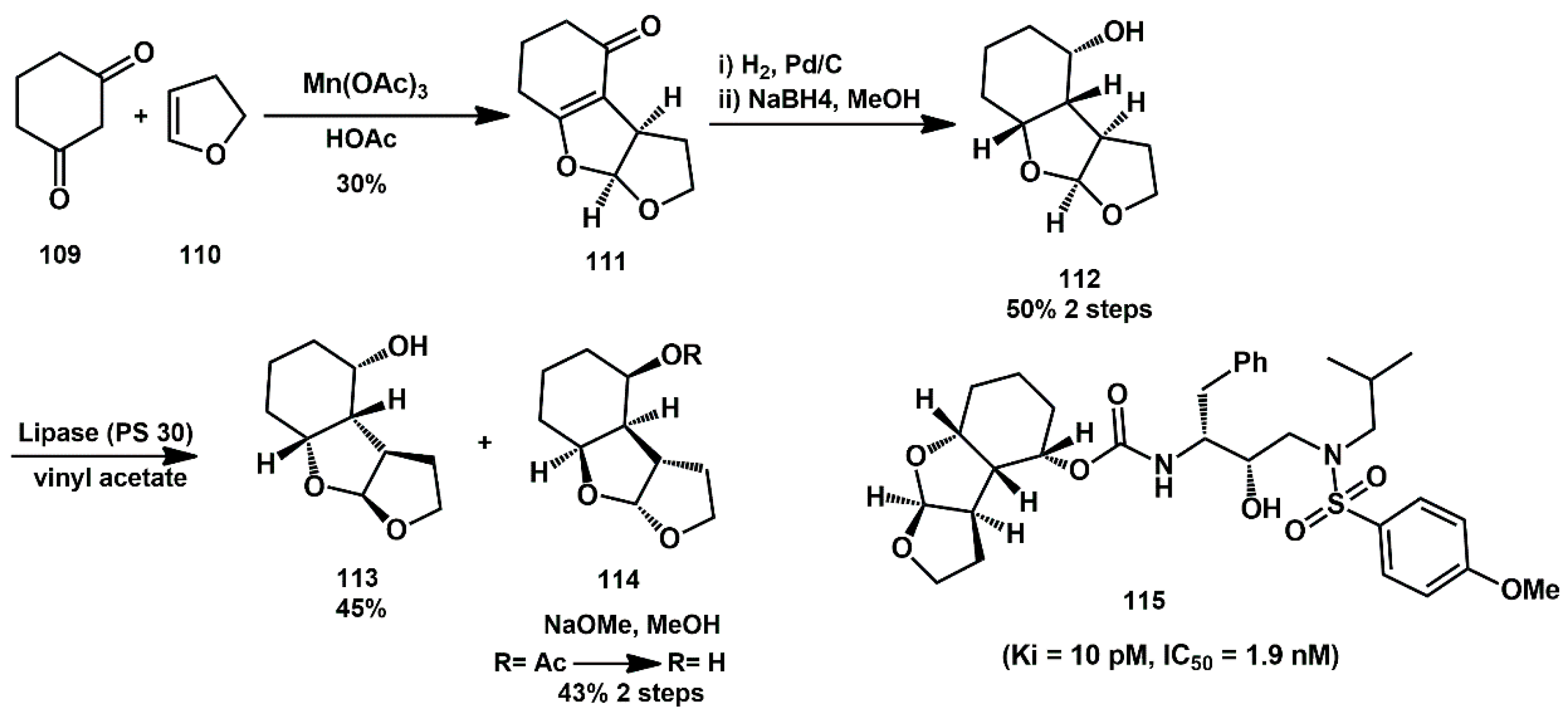
To maximize the ligand-binding site interactions in the protease active site, Ghosh described cyclohexyl-derived ligands within a 6-5-5 fused ring system [
42].
Starting from 1,3-diketone
109, reaction with dihydrofuran
110 in the presence of Mn(OAc)
3·2H
2O afforded the corresponding tricyclic derivative
111 (
Scheme 23). Enone
111 was first hydrogenated, and the resulting ketone was reduced with NaBH
4, to give racemic
endo alcohol
112. This racemic alcohol was subjected to enzymatic resolution using lipase PS-30, which provided the optically active acetate derivative
114 (45%yield) and alcohol
113 (45% yield). Acetate
114 was converted back to the alcohol
113 in 89% yield by transesterificaton using NaOMe in MeOH. A series of ligands was prepared and incorporated into new inhibitors. Notably, compound
115 exhibited very impressive enzyme and antiviral potency (Ki = 10 pM, antiviral IC
50 = 1.9 nM).
Novel isosorbide scaffolds were proposed by Liu as P2 ligands of HIV-1 protease inhibitors bearing the hydroxyethylamine core
116 (
Scheme 24) [
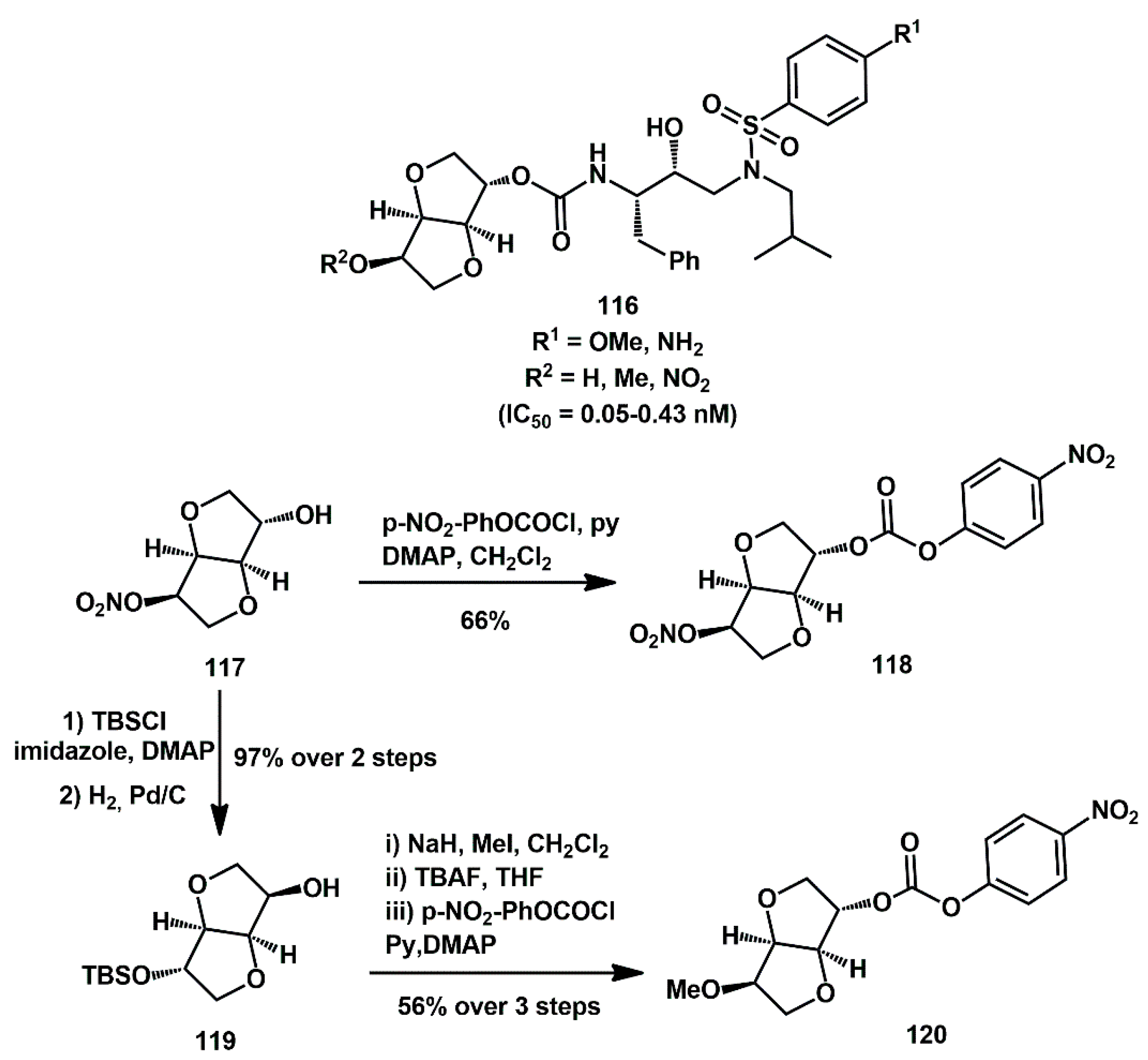
43]. They showed very high inhibition activity with IC50 in the nanomolar or picomolar range. From a preparative perspective, they leveraged the commercial availability of starting isosorbide mononitrate
117, which was easily elaborated and activated as a mixed carbonate. Specifically, it was transformed into mixed carbonate
118 with
p-NO
2PhOCOCl in good yield or converted into TBS-protected alcohol
119 via TBS ether formation, followed by cleavage of nitrate under reductive conditions. The alcohol was then methylated; the TBS group was cleaved, and final carbonate formation afforded the key intermediate
120.
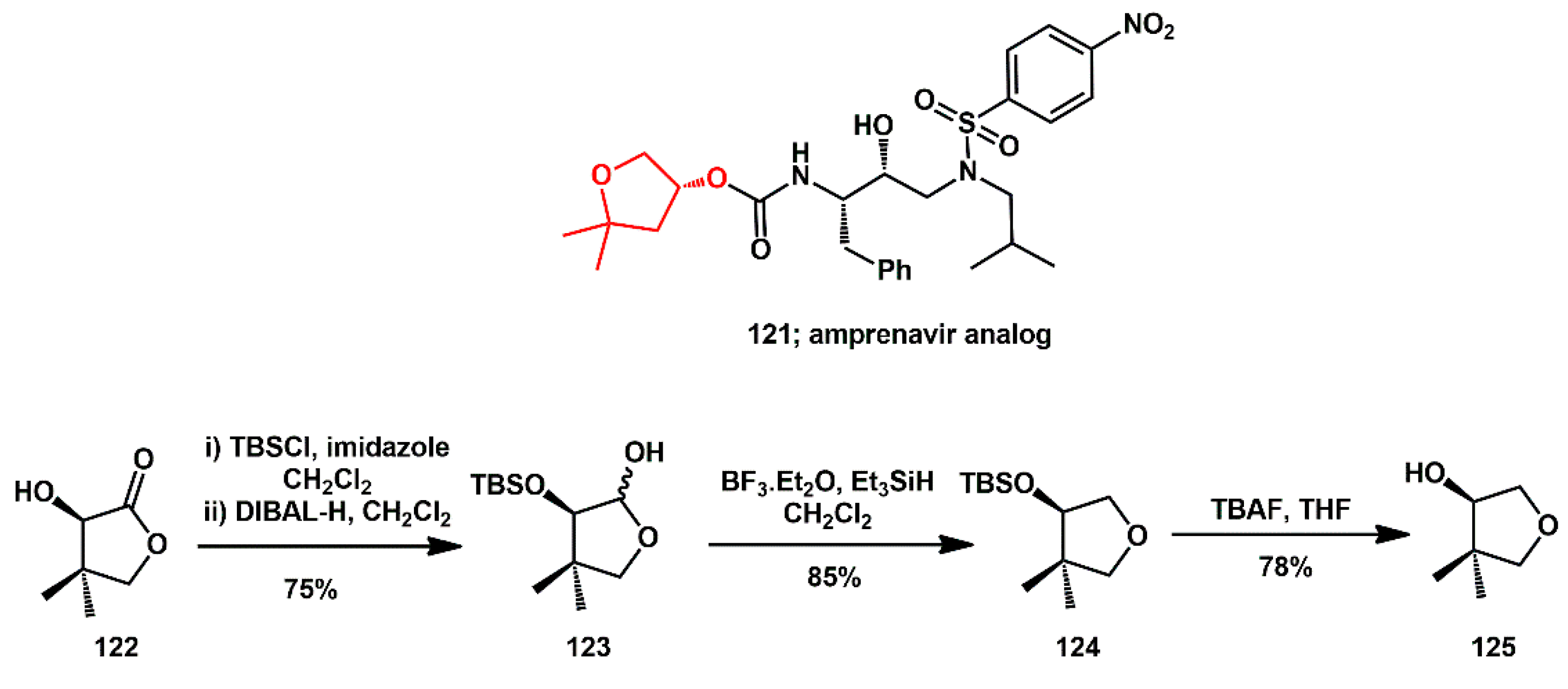
Chiral 4,4-dimethyltetrahydrofuran-3-ol was prepared by Srinivasa Reddy and used for the synthesis of new amprenavir analogs [
44]. Starting from pantolactone
122 (
Scheme 25), which is available in both enantiomeric forms, lactol
123 was obtained by protection and reduction. Subsequent reduction with BF
3Et
2O and triethylsilane, followed by final deprotection with TBAF, afforded the desired THF-alcohol.
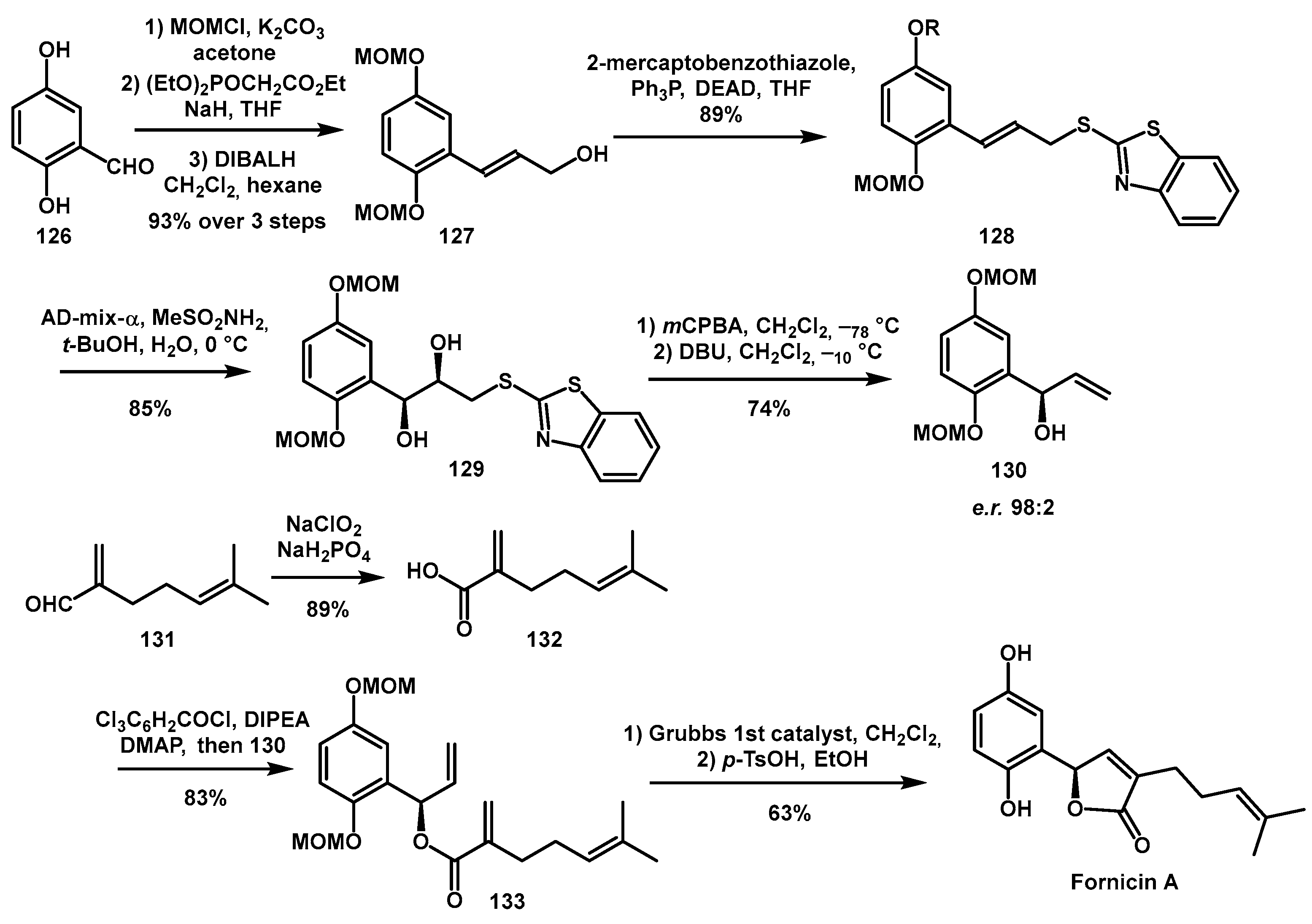
Different
O-heterocycles were also proposed by Yajima as HIV-1 protease inhibitors [
45]. Fornicin A, a meroterpenoid with a γ-butylolactone moiety in its side chains showed weak anti-HIV-1 protease activity without cytotoxicity against E-PR293 cells. The synthesis involved the preparation of optically active alcohol
130 from the starting material 2,5-dihydroxybenzaldehyde
126 (
Scheme 26), using Sharpless asymmetric dihydroxylation on the suitable benzothiazolsulfide
129 as the key step. Oxidation with
mCPBA and a final Smiles rearrangement of β-hydroxysulfone yielded the desired alcohol
130, which was coupled with the suitable acid segment prepared from the known aldehyde
131. Ring-closing metathesis using Grubbs 1st catalyst and a final deprotection step furnished Fornicin A.
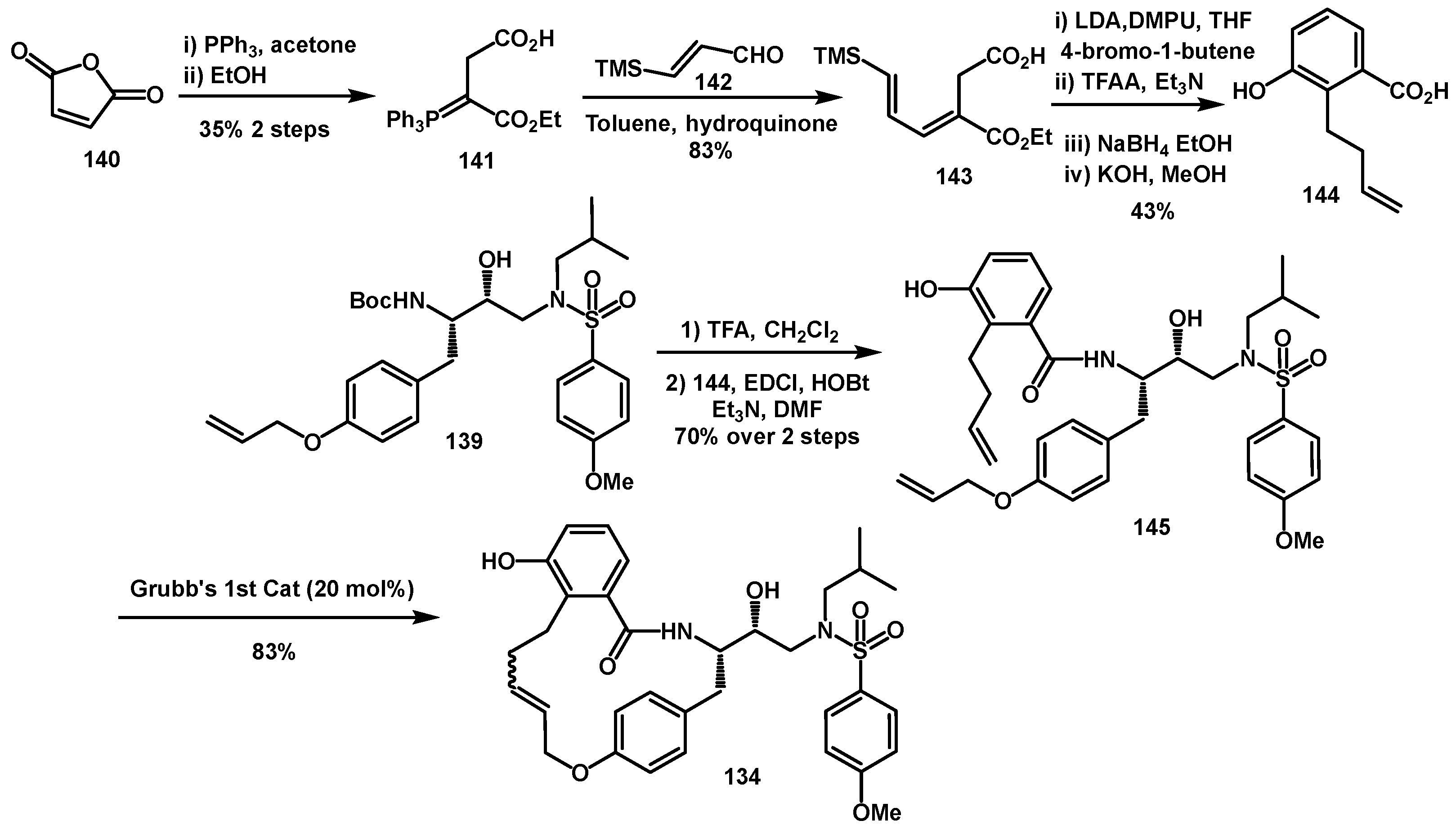
Flexible macrocycles between the P1′-side chain and a suitable P2′-ligand were proposed by Ghosh to broaden the activity of the inhibitor by achieving a better fit in the S2 hydrophobic pocket, which increases in size upon certain mutations [
46]. New inhibitors bearing 16- to 19-membered macrocyclic rings, connecting a nelfinavir-like P2 ligand and a tyrosine side chain containing a hydroxyethylamine sulfonamide isostere, were synthesized and found to be more potent than their corresponding acyclic counterparts. In particular, compound
134 showed the best enzyme inhibitory and antiviral activity (K
i = 0.2 nM, IC
50 = 0.21 mM) (
Scheme 27). The synthesis of the desired tyrosine-derived hydroxyethylamine sulfonamide isostere
138 began with butadiene monoxide
135, which was transformed into the corresponding allylic alcohol. This was subjected to Sharpless asymmetric epoxidation, and the resulting epoxide
136 was regioselectively opened by TMSN
3/Ti(O-
iPr)
4 system to afford the corresponding azido diol, which was subsequently converted to epoxide
137. After introducing the sulfonamide moiety, a Boc-protected amine and a free phenol were formed in
one pot process via catalytic hydrogenation in the presence of Boc
2O. Finally, the introduction of an allyl chain furnished the desired intermediate
138.
The P2 fragment was synthesized as 3-hydroxy-2-alkenylbenzoic acid from maleic anhydride
140 via formation of phosphorane
141, followed by Wittig reaction with known aldehyde
142. The resulting dienoic acid was then alkylated and subjected to 1,6-electrocyclization by exposure to TFAA/Et
3N, followed by reduction with NaBH
4 (
Scheme 28). Final saponification yielded the desired benzoic acid
144. After amine deprotection and coupling with the benzoic acid, the resulting acyclic diene was subjected to ring-closing metathesis (RCM) with Grubbs’ 1st generation catalyst, furnishing the desired macrocycles.
2.2. Non-Aromatic N-Heterocycles
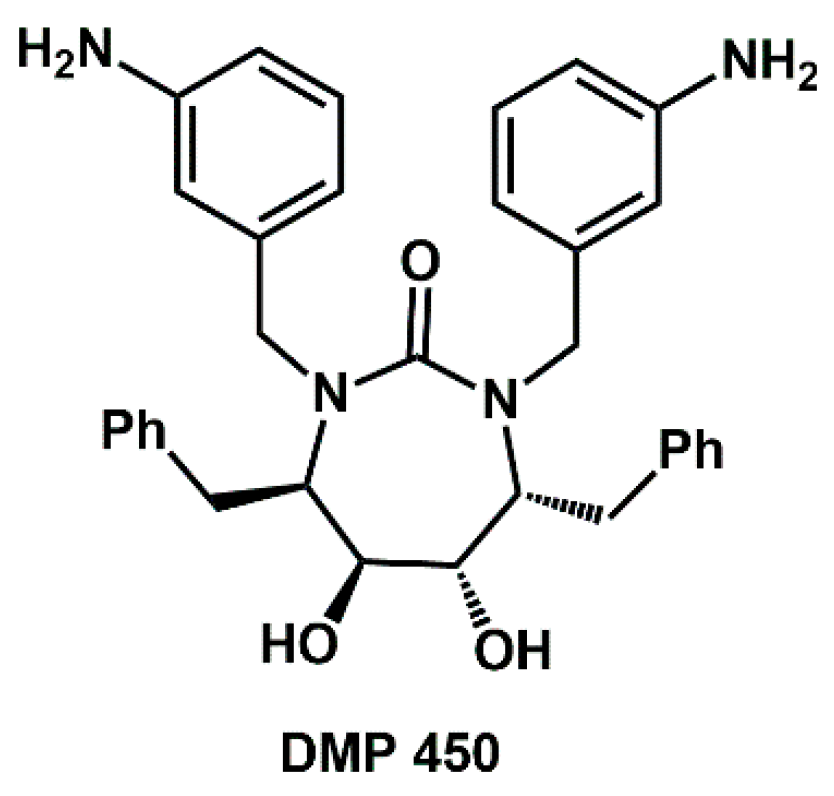
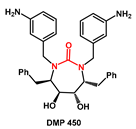
Among non-aromatic
N-heterocycles, the structure of 1,3-diazacycloalkan-2-ones is of interest due to its presence as the core of HIV-protease inhibitor DMP 450 (
Figure 1).
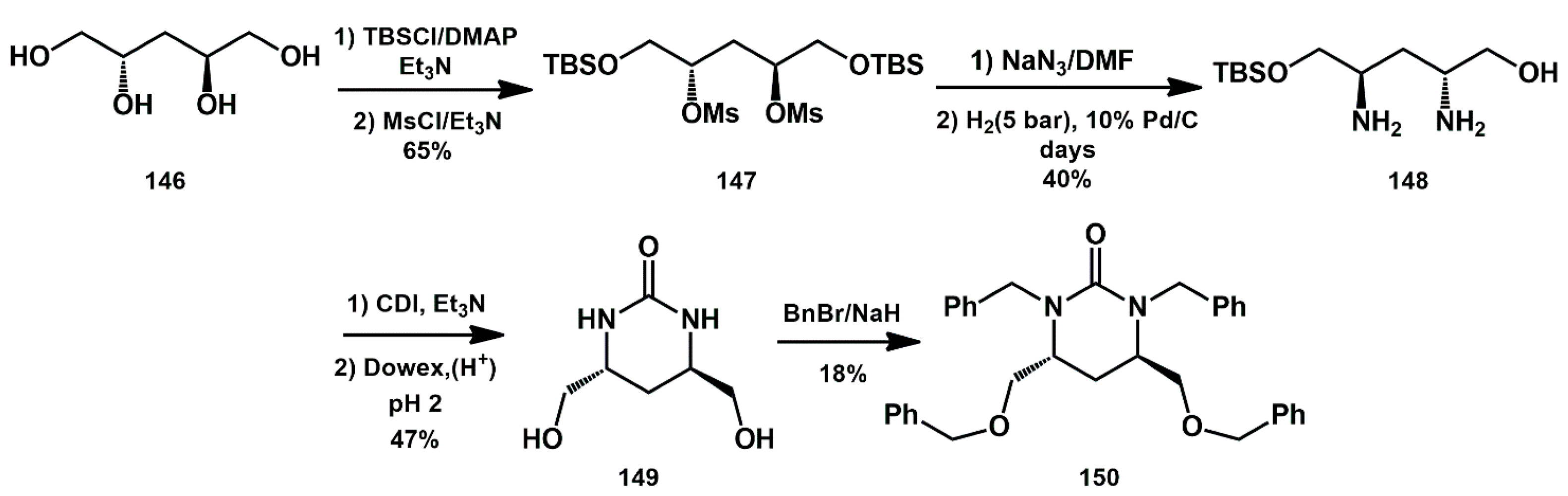
Frain described the synthesis of novel tetrahydropyrimidinones, starting from tetraols, that are easily prepared from suitable alditols [
47]. Tetraol
146, from D-(+)-arabitol, was first protected in primary hydroxyl groups as TBS ethers (
Scheme 29). The introduction of the amines was performed by mesylate activation of alcohols, followed by displacement with azide and reduction by hydrogenation. Extended hydrogenation periods led to the isolation of the mono TBS-protected diaminodialcohol
148. Subsequent reaction with carbonyl diimidazole in the presence of base provided the crude tetrahydropyrimidinone, which was treated with acid to remove the TBS group. Final perbenzylation furnished the functionalized tetrahydropyrimidinone
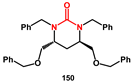
150, which exhibited modest HIV-protease inhibition activity.
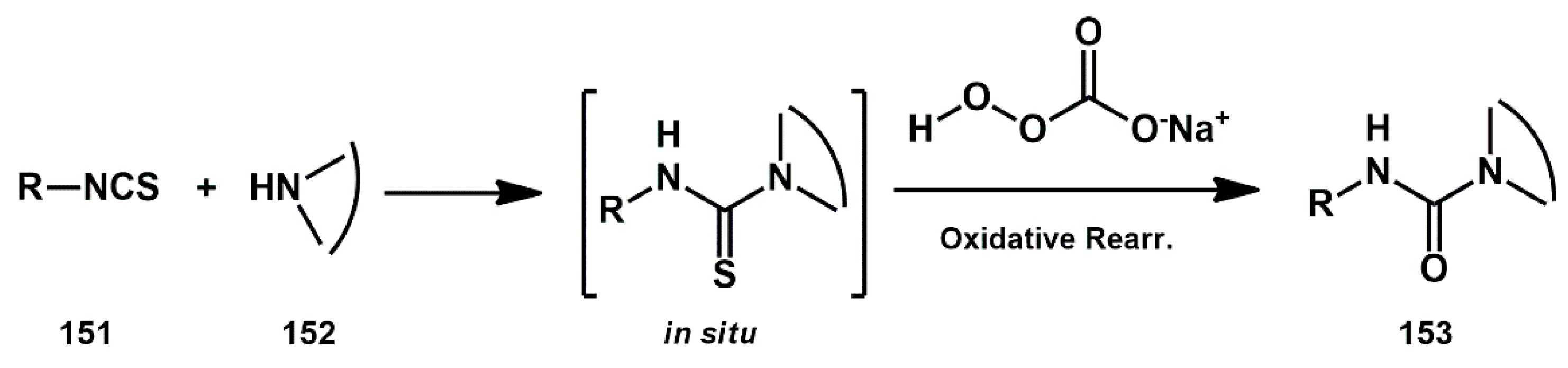
Unsymmetrical 1,3-diazacycloalkan-2-ones were prepared by Jamir [
48] from thiocarbamate salts using sodium percarbonate as an oxidant. These compounds were evaluated as HIV-1 protease inhibitors via in silico approach. Secondary amines
152, such as morpholine, piperidine, 4-hydroxy piperidine, diethylamine, and di-isopropylamine, were reacted with phenyl isothiocyanate and other substituted phenyl isothiocyanates
151 to afford the corresponding unsymmetrical 1,3-diazacycloalkan-2-one in high yields (
Scheme 30). Computational assessment of IC
50 values using known references satisfactorily confirmed the inhibitory activity of the selected compounds against HIV-1 protease.
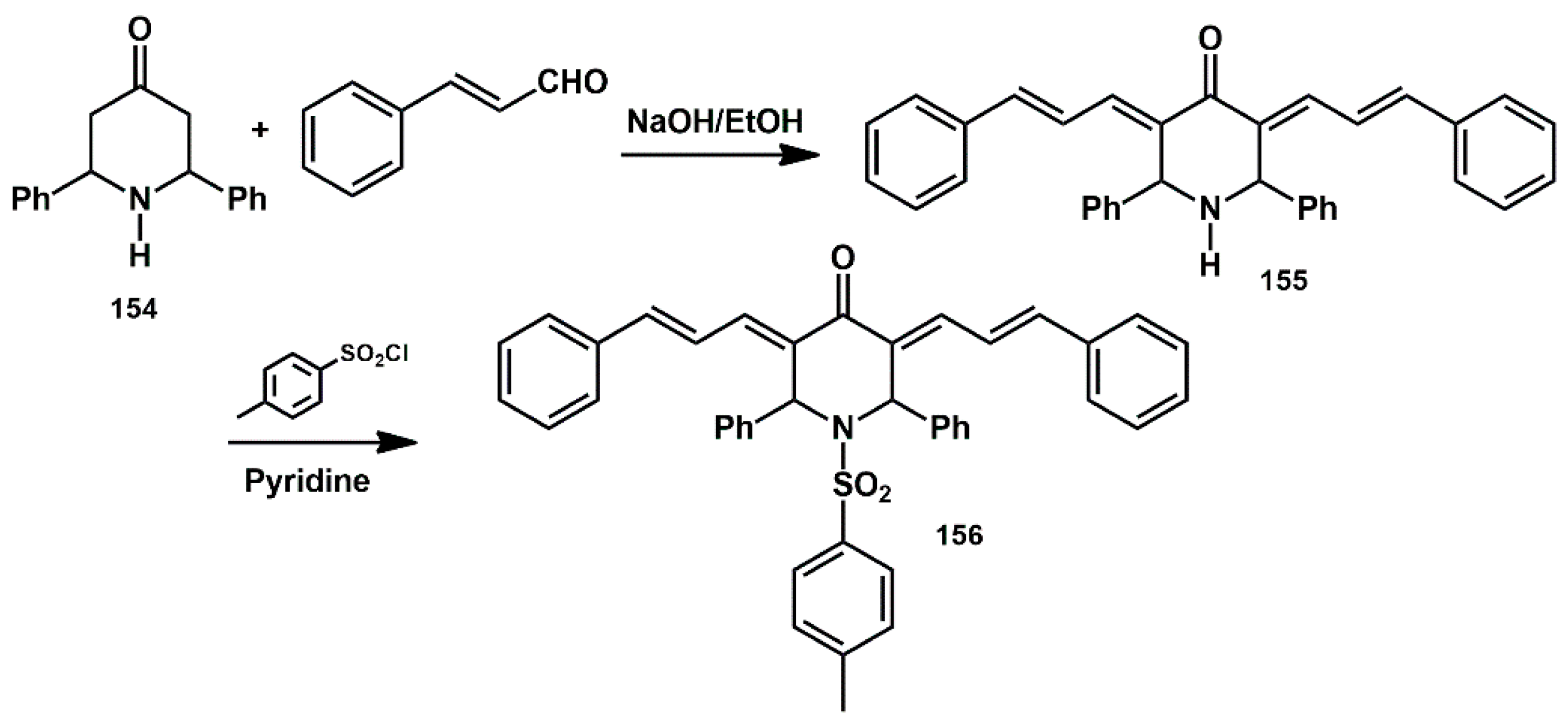
In the work of Jadhav [
49],
bis-allylidene-4-piperidones analogues were prepared by Claisen–Schmidt condensation between 2,6-substituted piperidin-4-ones and cinnamaldehyde in a basic medium (
Scheme 31). In particular, 3,5-
bis (3-phenyl-allylidene)-2,6-diphenyl-piperidine-4-one
155 was obtained from the parent compound
154. The
N-tosyl derivative
156 was prepared by reacting
155 with tosyl chloride. Compound
156 showed moderate HIV-1 protease inhibitor activity compared to the standard, pepstatin-A.
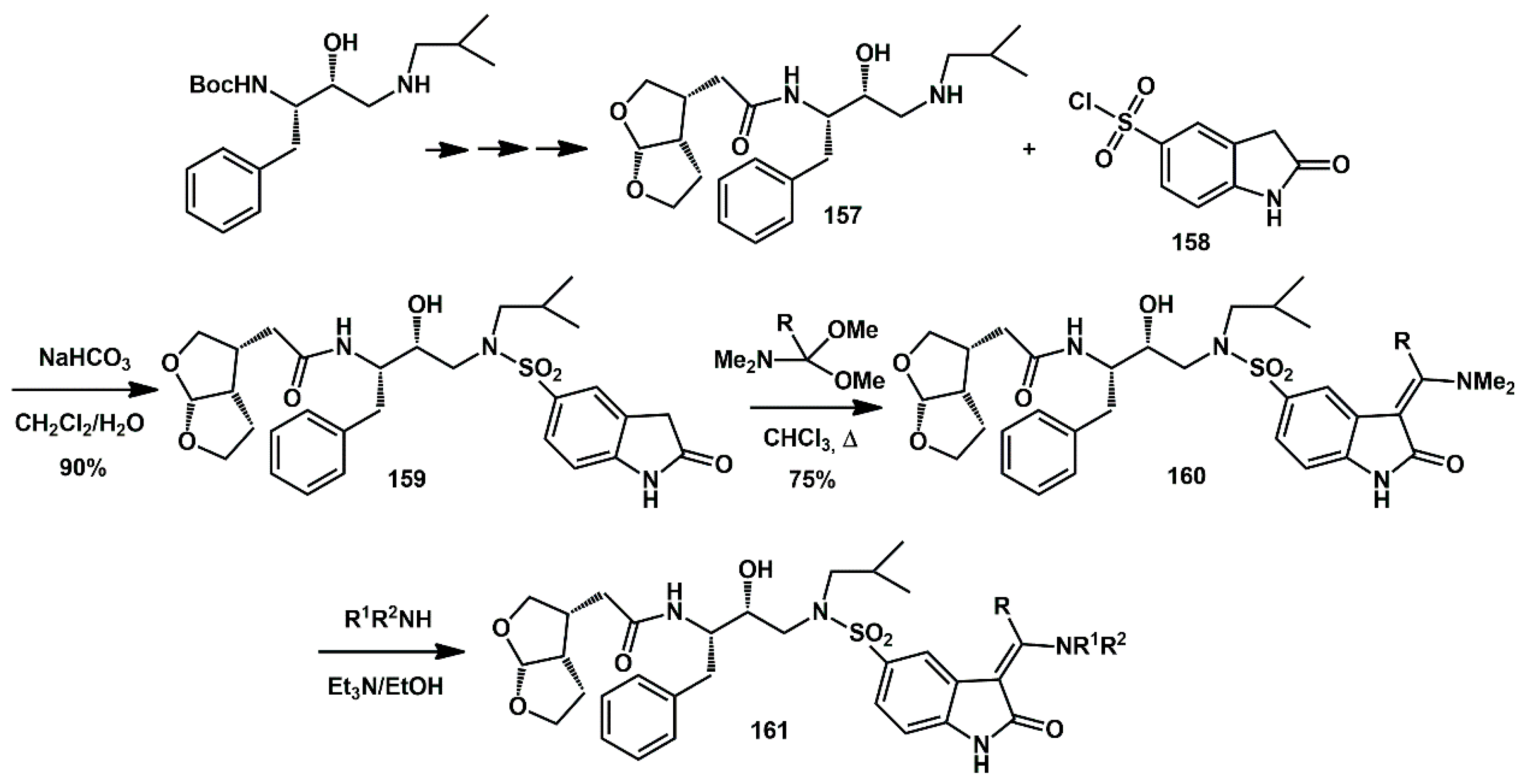
Indolin-2-one moieties were proposed by Eissenstat to interact with the S20 subsite of the HIV protease binding pocket [
50]. Several of these inhibitors were synthesized and exhibited sub-nanomolar Ki values and antiviral IC50s in the low nM range against wild-type (WT) HIV and a panel of multi-drug resistant (MDR) strains. The darunavir sulfonamide was replaced by an indolin-2-one moiety, reacting
bis-THF amino analog
157 with indolin-2-one sulfonyl chloride
158, affording compound
159. Subsequent heating in the presence of DMF dimethylacetal or related acetals led to the formation of indolin-2-one
160 (
Scheme 32). The amine portion of the enamines could be readily varied through an exchange reaction of dimethamino analog
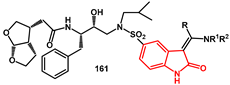
161 with excess amine.
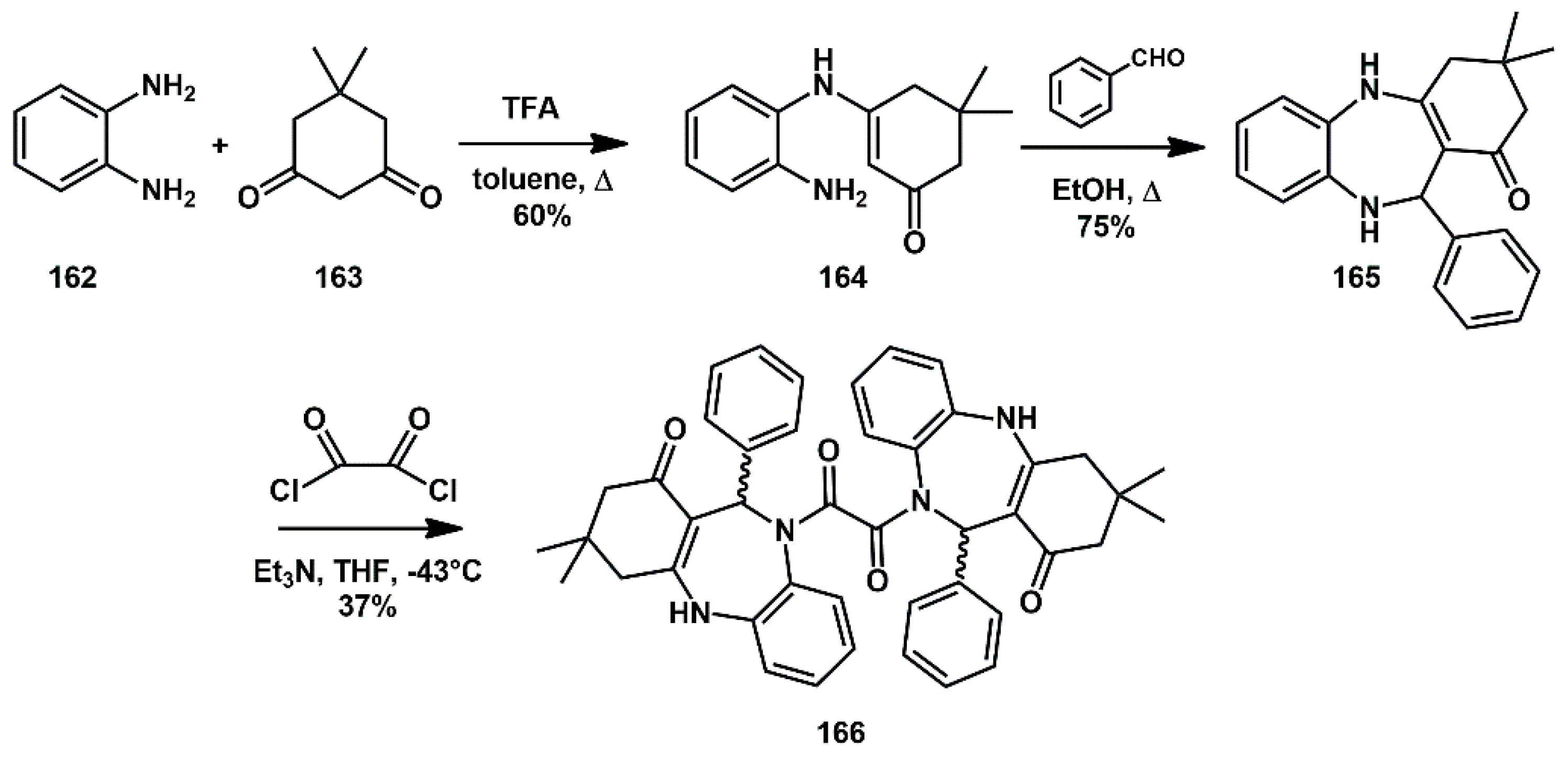
1,4-benzodiazepine derivatives have been recognized as mimicking the β-hairpin flap of HIV-1 protease, thereby interfering with the flap–flap protein–protein interaction which controls access to the active site. Recently, the Konvalinka group screened a variety of chemical structures for inhibition of HIV replication [
51]. This led to the identification of several new compounds that inhibited HIV PR in the low micromolar range. In particular, compound
166 resulted in being the most potent and selective for HIV PR. The monomer was prepared by reacting
o-phenylenediamine
162 with dimedone in the presence of catalytic amount of TFA, affording the corresponding enamine
164 which was then reacted with benzaldehyde to obtain monomer
165. Treatment with oxalyl chloride at low temperature, furnished the desired dimer
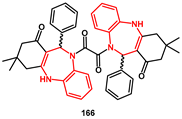
166 in modest yield as a diastereoisomeric mixture (
Scheme 33), whose racemate (11
R, 11′
R + 11
S, 11′
S) showed inhibition activity in the nanomolar range (IC
50 = 30 nM).
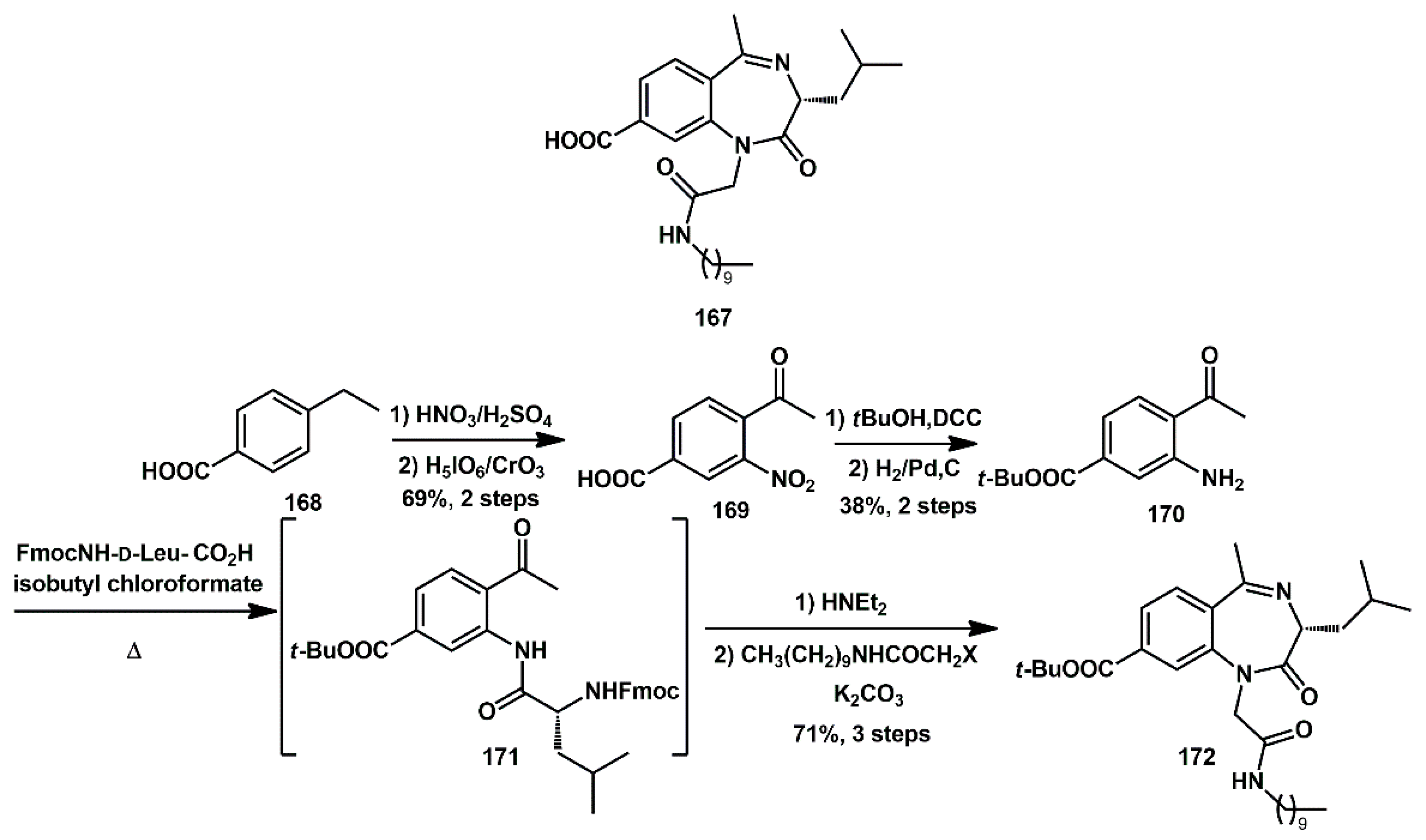
New bioactive compounds containing 1,4-benzodiazepine scaffolds were proposed by Fletcher, with the most potent compound,
167, inhibiting the protease with a modest Ki of 11 µM (
Scheme 34) [
52]. It was prepared starting with regioselective nitration of benzoic acid
168, followed by benzylic oxidation, which afforded ketone
169. After esterification, catalytic hydrogenation yielded aniline
170. Amide
171 was prepared coupling aniline
170 with Fmoc-protected pre-activated D-Leu, and then subjected to base-mediated cyclization, furnishing the key benzodiazepine nucleus. After deprotection and
N-alkylation, the final compound
167 was obtained. Different compounds were obtained by deprotection and/or alkylation. Methyl imine could be replaced by a phenyl imine, resulting in the formation of different compounds.
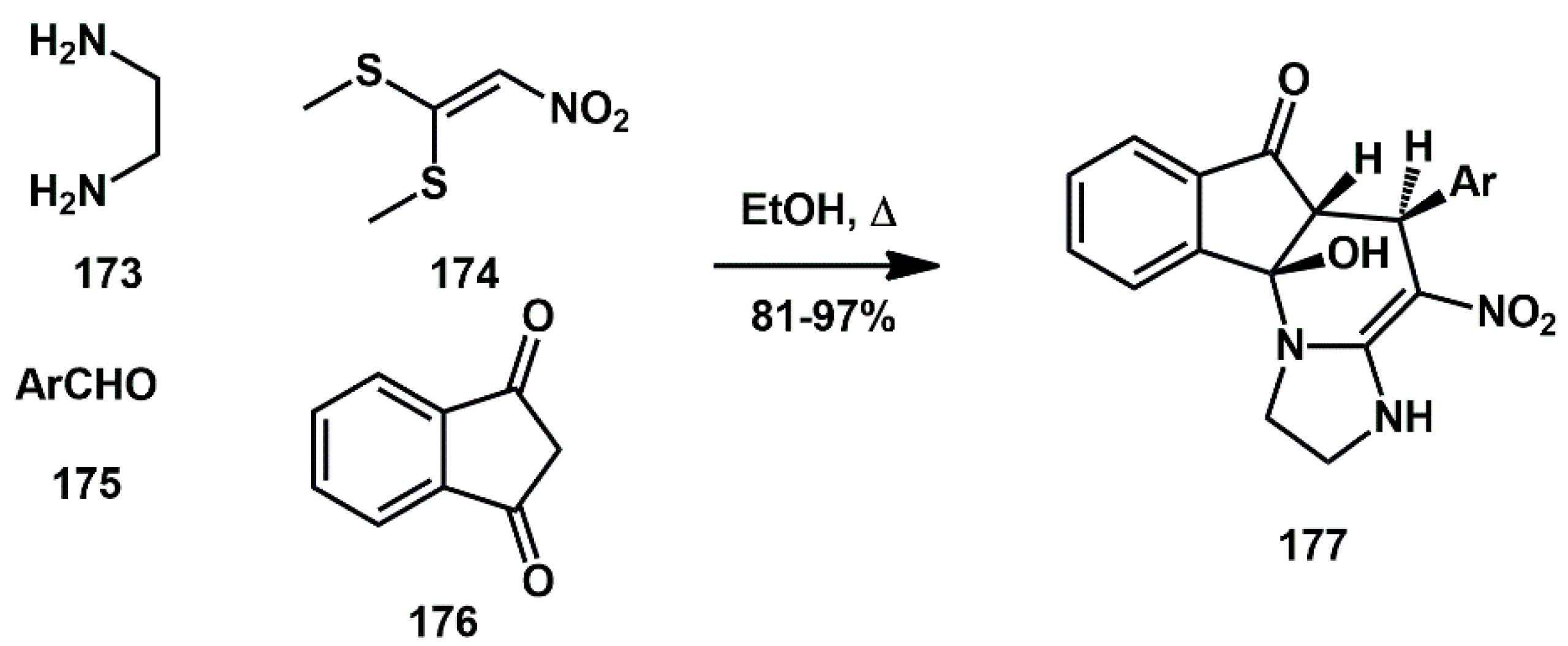
The tetrahydropyridine ring system is a widely distributed structural framework involved in numerous pharmaceuticals and natural products. Mohammadi [
53] recently described an efficient,
one-pot, catalyst-free, four-components procedure for the synthesis of novel 10β-hydroxy-4-nitro-5-phenyl-2,3,5,5a-tetrahydro-1
H-imidazo[1,2-a]indeno[2,1-e]pyridin-6(10b
H)-one derivatives
177 from the corresponding diamine
173, nitro ketene dithioacetal
174, aryl aldehydes
175, and 1,3-indandione
176 (
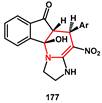
Scheme 35). The overall transformation consisted of a Knoevenagel condensation, Michael addition, tautomerism, and cyclisation sequence. All newly synthesized compounds were screened for molecular docking studies. Some of them showed minimum binding energy and good affinity toward the active pocket of HIV protease enzyme compared to Saquinavir.
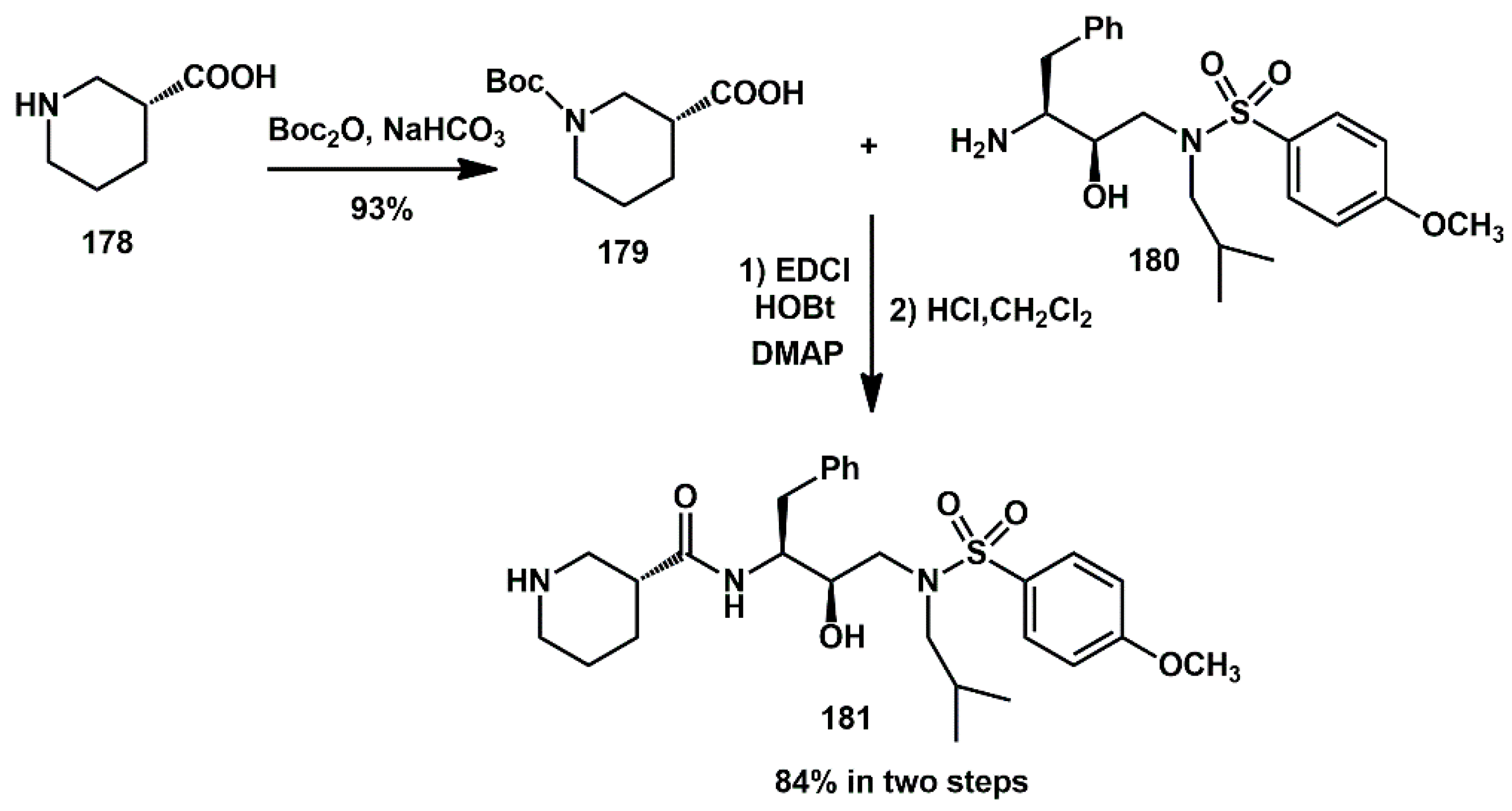
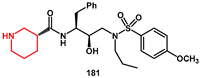
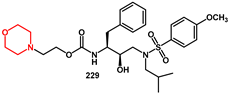
A flexible piperidine moiety was introduced into a novel class of HIV-1 protease inhibitors as the P2 ligand by Wang Y., Cen, and Wang J [
54]. In particular, inhibitor
181, which features (
R)-piperidine-3-carboxamide as the P2 ligand and 4-methoxybenzenesulfonamide as the P2′ ligand, showed more than a 6-fold enhancement of activity compared to darunavir (IC
50 0.13 ± 0.01 nM). Furthermore, there was no significant change in potency against darunavir-resistant mutations and HIV-1
NL4-3 variant. For the synthesis, easily prepared (
R)-piperidine-3-carboxylic acid
178 was reacted with (Boc)
2O to obtain the corresponding Boc-amino derivative
179 in excellent yield. Coupling of the acid with a suitable amine, under the catalysis of EDCI, HOBt, and DMAP, furnished the amide
181 (
Scheme 36). Removal of the Boc group provided the target compound.
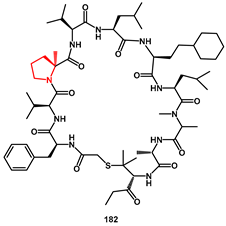
Macrocyclic peptides have emerged as a new class of drug discovery modalities because they are considered more likely to acquire strong interactions with target proteins, owing to their restricted molecular motion and rigidity compared to linear peptides. Recently, Mikamiyama [
55] discovered a novel HIV-1 protease inhibitor,
182 (
Figure 2), with potent antiviral activity (EC
50 37 nM) and oral bioavailability, using a structure-based drug design approach via X-ray crystal structure analysis. This approach started from hit macrocyclic peptides identified by mRNA display against HIV-1 protease. In particular, the improvement of the proteolytic stability of macrocyclic peptides by introducing a methyl group at the
α-position of certain amino acids, such as proline, is crucial for exhibiting strong antiviral activity.
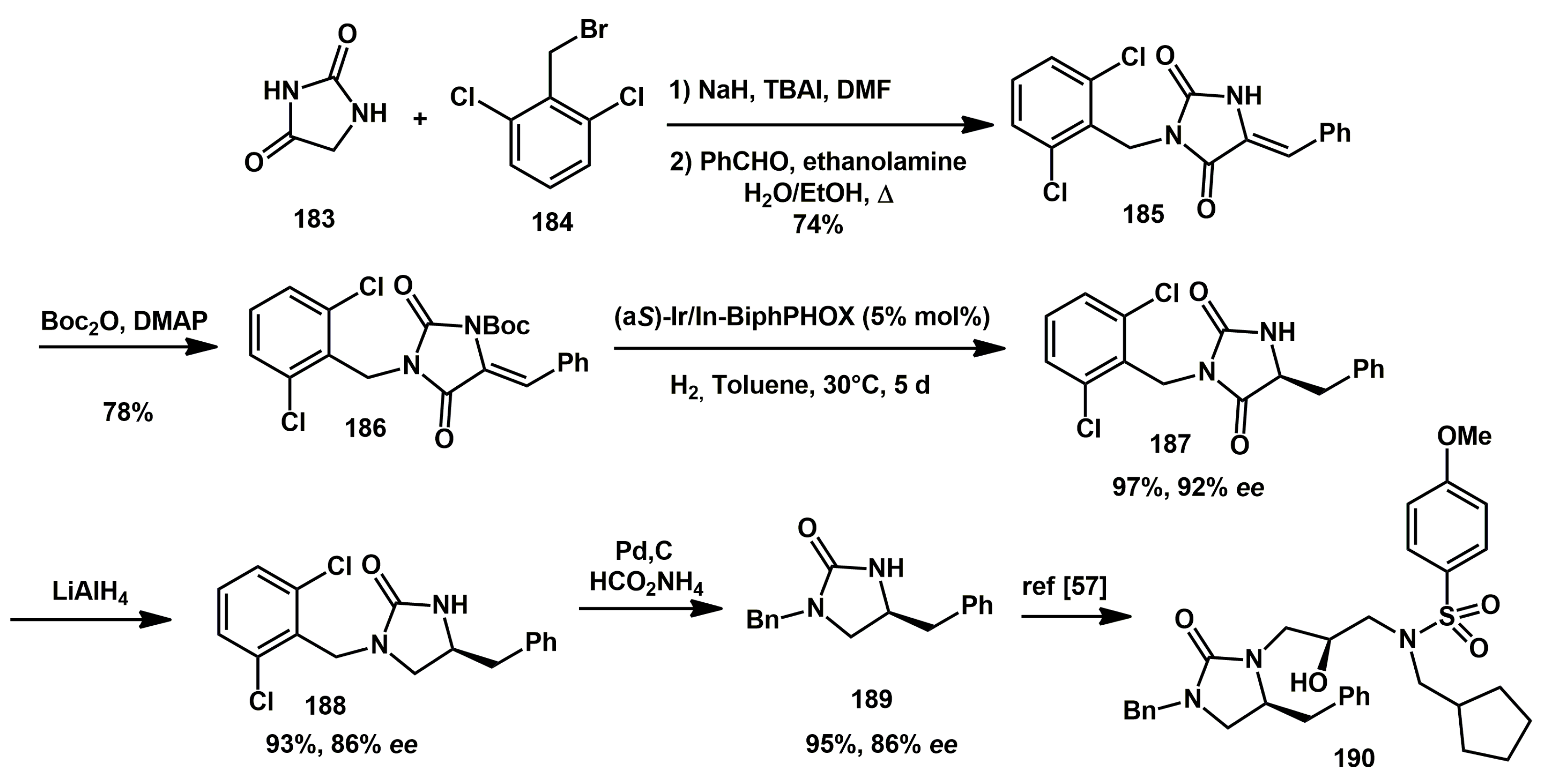
The five membered hydantoin scaffold was already recognized as a key fragment in HIV protease inhibitors. Recently, Zhang reported a new general iridium-catalyzed asymmetric hydrogenation of hydantoin and thiazolidinedione-derived exocyclic alkenes using BiphPHOX as a ligand [
56]. The transformation, which showed good functional group tolerance, high yields, and enantioselectivities in the hydrogenated products, was applied to the synthesis of
189, a key intermediate in the preparation of an HIV protease inhibitor
190 (
Scheme 37) [
57]. A gram-scale reaction was carried out starting from alkene
186, yielding the hydrogenated product
187 with 92%
ee and 97% yield. This product was then reduced with LiAlH
4, providing chiral 1,3-diazacycloalkan-2-one
188. The reduced product
188 was transformed into the key intermediate
189 + dechlorination in the presence of Pd/C.
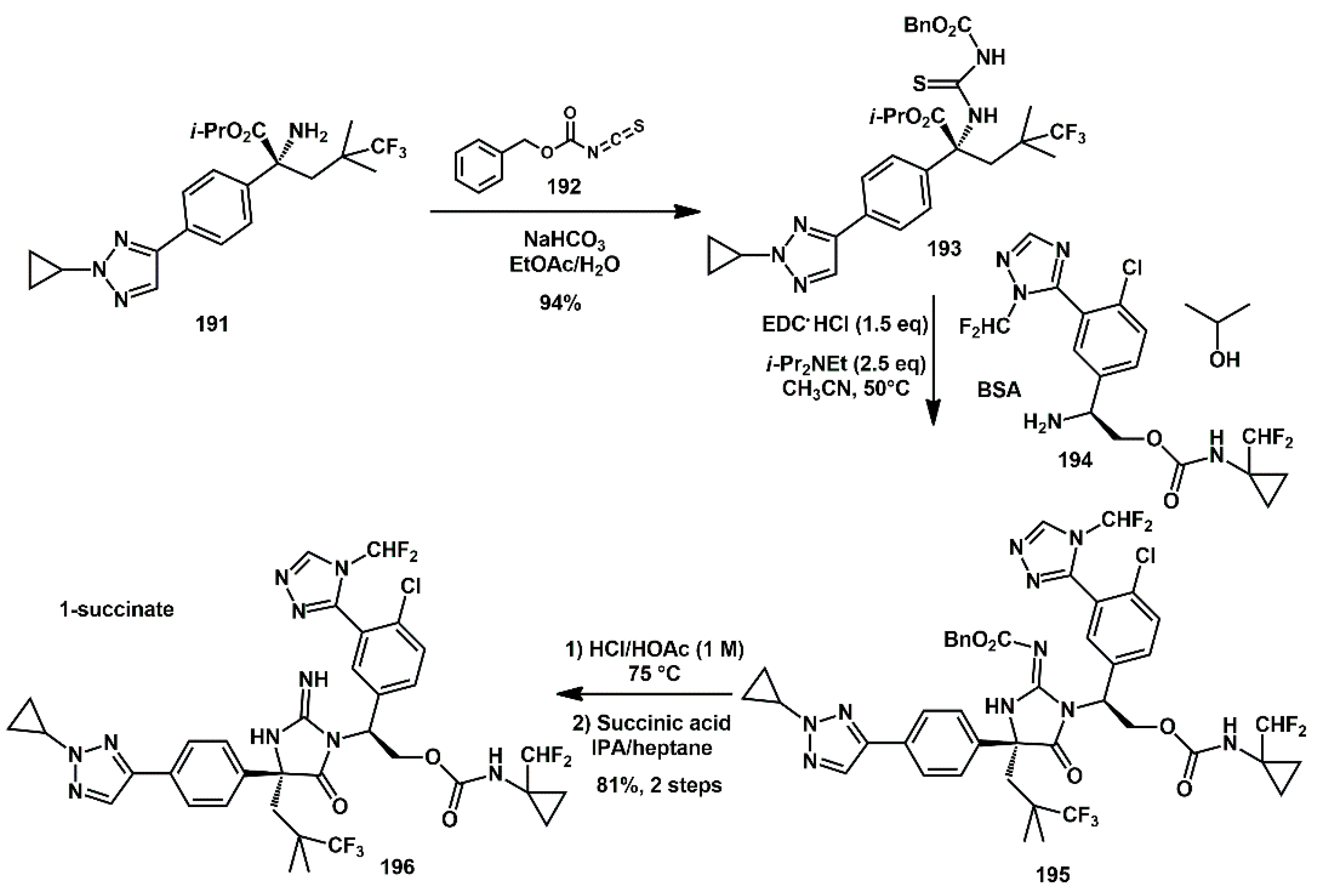
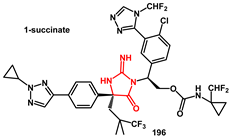
A densely functionalized iminohydantoin, bearing a quaternary stereocenter, was recently described and developed as an HIV protease inhibitor [
58,
59]. Ischay and Hoang described process development efforts that enabled the first scale-up of compound
196 (
Scheme 38). Several challenges were addressed, including reaction optimization, purification, stabilization of the intermediates, and deprotection procedures. For the reaction of iminohydantoin
195 formation, the addition order was evaluated. It was found that the presence of the amine coupling partner, in the form of BSA (benzensulphonic acid) salt, was required during the loading of the EDC·HCl reagent. Reagent stoichiometry was then investigated, showing that the loading of EDC·HCl could be reduced to 1.5 equivalents and 6·BSA to 1.1 equivalents without negatively affecting reaction performance. The use of 2–3.5 equiv.
i-Pr
2NEt proved optimal for scale-up to 687 g. A MIBK (methyl isobutylketone) solvate was identified as a crystalline form that provided an additional isolable intermediate, serving as a purity control point prior to Cbz-group removal and API isolation. The Cbz protecting group was removed from iminohydantoin
195 using HCl in acetic acid, that mitigated hydrolysis of the iminohydantoin. After work-up, crystallization afforded
196 1-succinate (
Scheme 38).
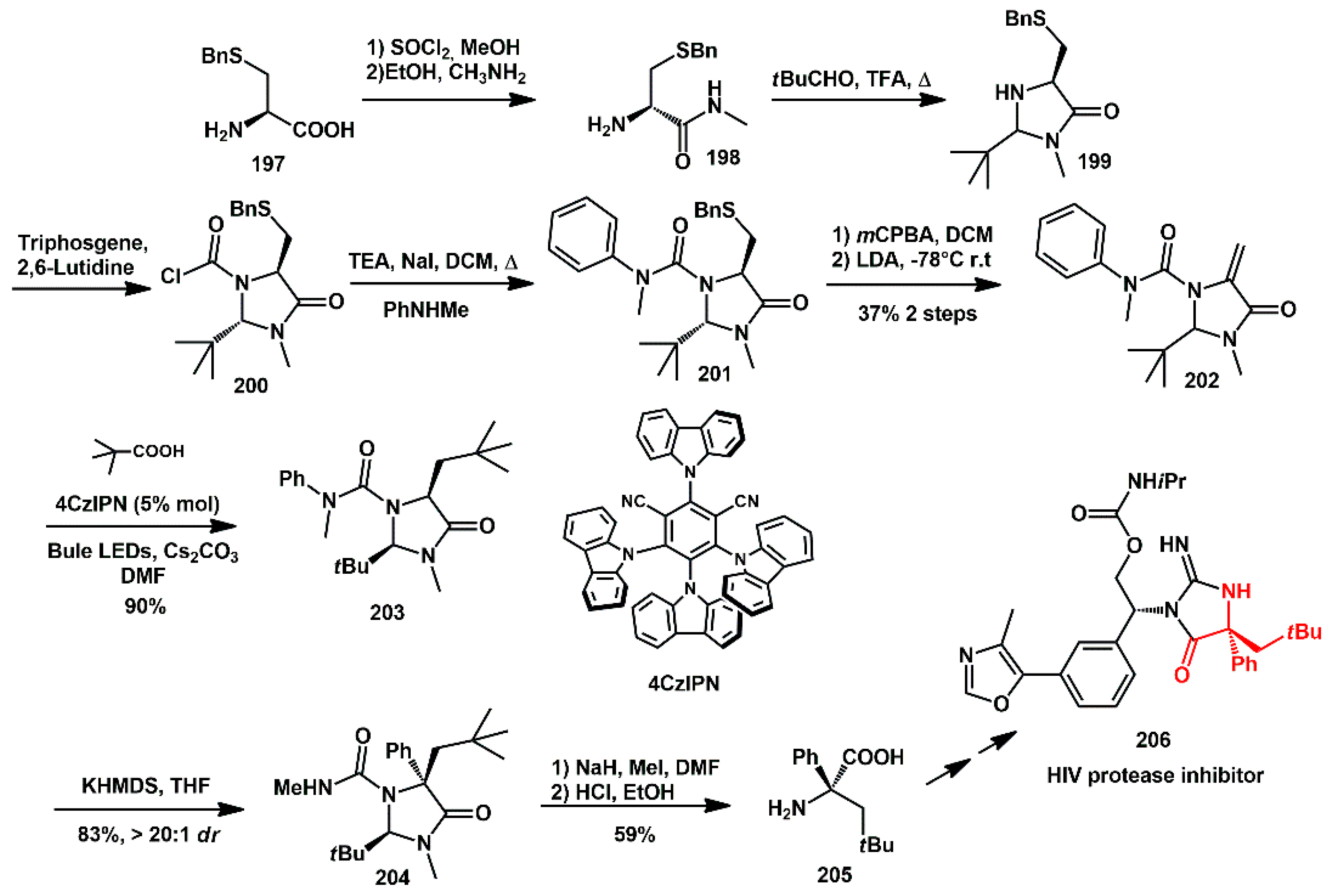
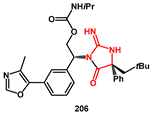
Wang and coll. recently reported a new strategy for the synthesis of an iminohydantoin derived from chiral quaternary
α-aryl amino acids [
60]. Such fragments are present in certain HIV protease inhibitors [
58]. They took advantage of newly developed chiral Karady–Beckwith dehydroalanines, which were prepared for use in a photoredox-mediated highly stereoselective Giese-type reaction with carboxylic acids and tertiary amines. A final stereoselective Clayden rearrangement afforded chiral quaternary
α-aryl amino acid derivatives. A new chiral dehydroalanine bearing an
N-methyl,
N-phenyl-urea moiety
202 was synthesized starting from
S-benzyl-L-cysteine
197, which was first transformed into amino amide
198 (
Scheme 39). Cyclization with pivalaldehyde was then performed, and the free NH group was converted into the corresponding urea moiety, affording intermediate
201. An exocyclic double bond was obtained by oxidation of sulfide and subsequent elimination of the sulfoxide. Compound
202 underwent a Giese-type reaction with pivalic acid under the irradiation with a blue light-emitting diode (LED), using 4CzIPN as the photocatalyst (PC) and Cs
2CO
3 as the base in DMF, affording the desired adduct
203 with a high diastereoisomeric ratio (>20:1). This intermediate smoothly underwent a Clayden rearrangement, giving rise to the quaternary amino acid derivative
204 in high yield and diastereoselectivity. Final deprotection afforded free amino acid
205, suitable for incorporation into the HIV protease inhibitor
206.
2.4. Heteroaromatics
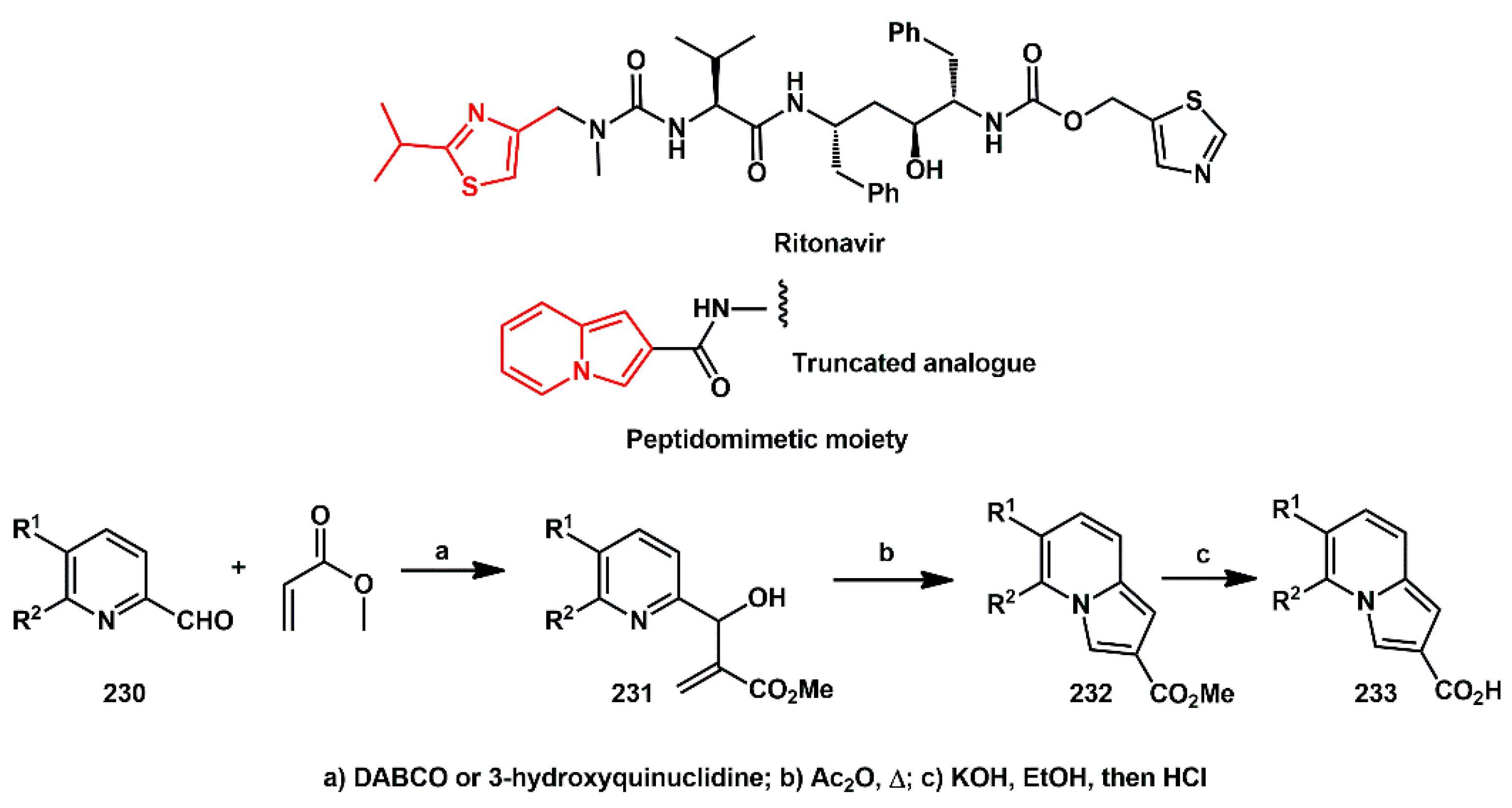
Based on Ritonavir, Kaye designed novel, structurally simplified, truncated analogs bearing heteroaryl groups linked to the peptidomimetic chain [
67]. Starting from pyridine-2-carbaldehydes
230, the indolizine-2-carboxylate esters
232 (
Scheme 44) were synthesized by Baylis–Hillman reaction followed by cyclization promoted by acetic anhydride at reflux, through acetylation of the alcohol, which facilitated the reaction. Saponification of the esters yielded the corresponding carboxylic acids
233. Different amides were prepared by coupling with suitable amines in presence of carbonyl diimidazole (CDI). According to the authors, inhibition activity was not measured; however, enzyme-binding, enzyme inhibition, and in silico docking studies of those compounds were planned.
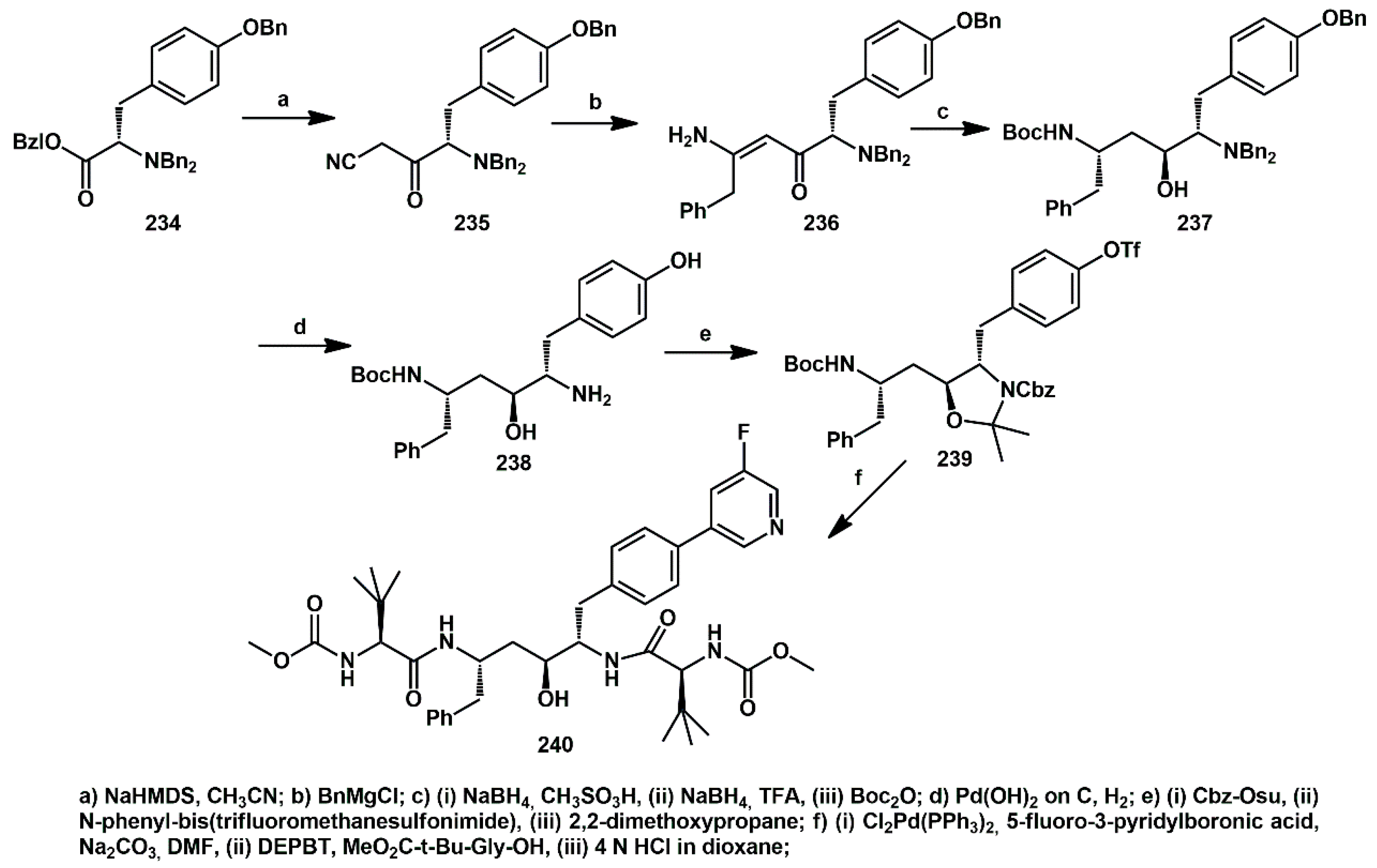
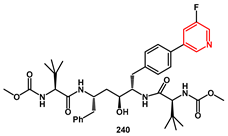
The incorporation of substituents with hydrogen bond donor and acceptor groups at the P1 position of a symmetry-based HIV protease inhibitor series was described by DeGoey and resulted in significant improvement in potency against the resistant mutants [
68]. Overall, compound
240 (
Scheme 45) demonstrated the best balance of potency against drug resistant strains of HIV and exhibited oral bioavailability in pharmacokinetic studies. The synthesis began with benzylated tyrosine
234 and reaction with sodio acetonitrile yielded nitrile
235, which was then treated with a benzyl Grignard reagent to give the enaminone
236. A high degree of stereocontrol was observed during the stepwise reduction of
236 with NaBH
4, followed by Boc protection of the resulting amine. Removal of the benzyl protecting groups through hydrogenolysis gave the amine
238. The corresponding triflate was generated using
N-phenyl-bis(trifluoromethanesulfonimide) affording
239. Palladium-mediated coupling with 5-fluoro-3-pyridylboronic acid furnished the bis aryl intermediate, which was finally coupled with a suitable
t-butyl-glycine derivative, yielded the desired inhibitor
240.
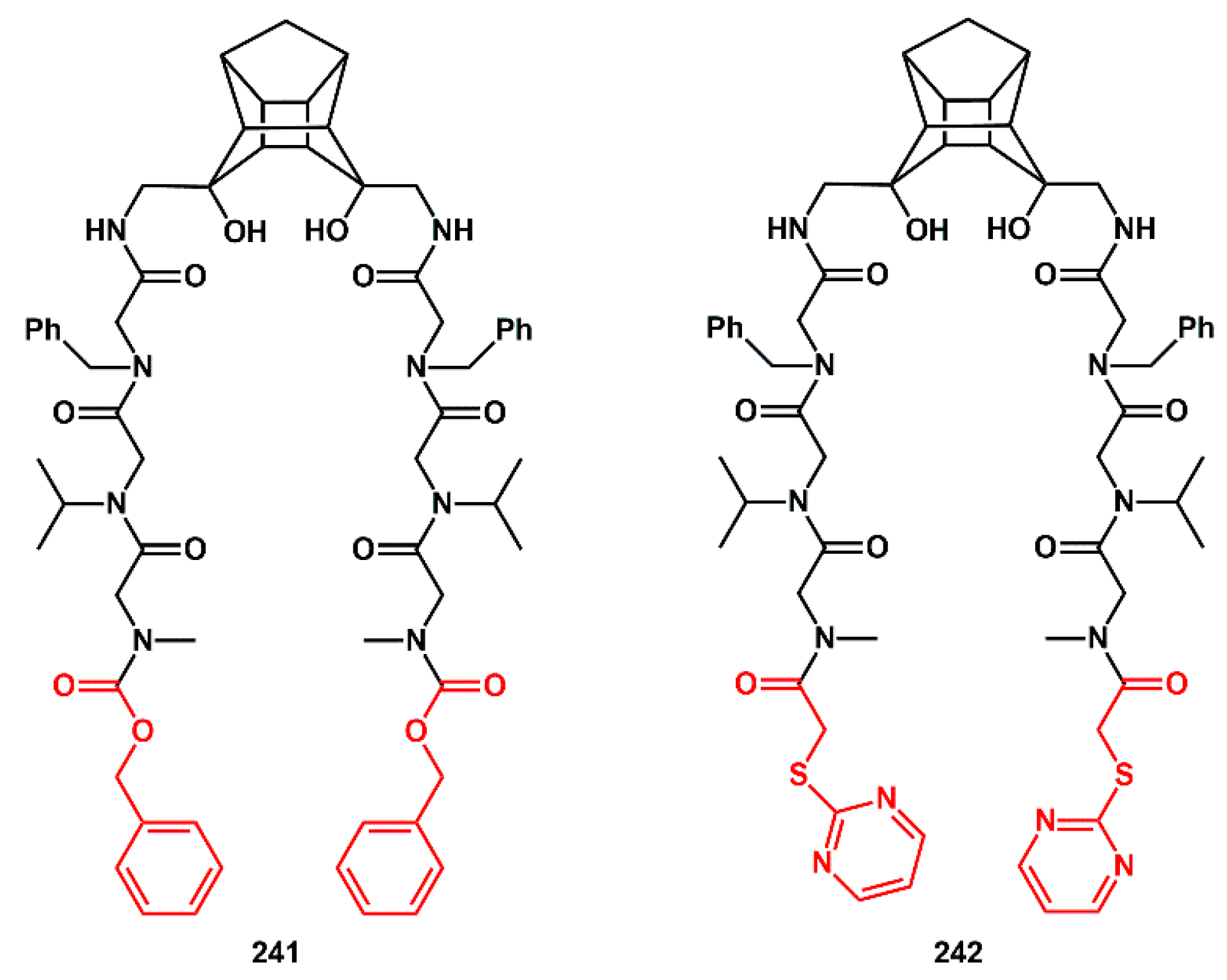
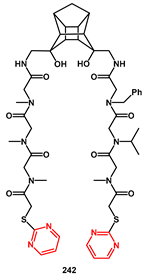
During the study of novel inhibitors against South African wild-type (C-SA) HIV protease, Makatini [
69] described the first pentacycloundecane (PCU) diol peptoid-derived inhibitors, with IC
50 values ranging from 6.5 to 0.075 µM. Starting from inhibitor
241, which showed very good activity (IC
50 0.075 µM), it was derivatized by substituting the carbobenzyloxy group with the (2-pyrimidinylthio)acetic acid group in order to produce compound
242 for improved solubility (
Scheme 46). Unfortunately, this molecule exhibited significantly less binding affinity to the enzyme (IC
50 1.0 µM).
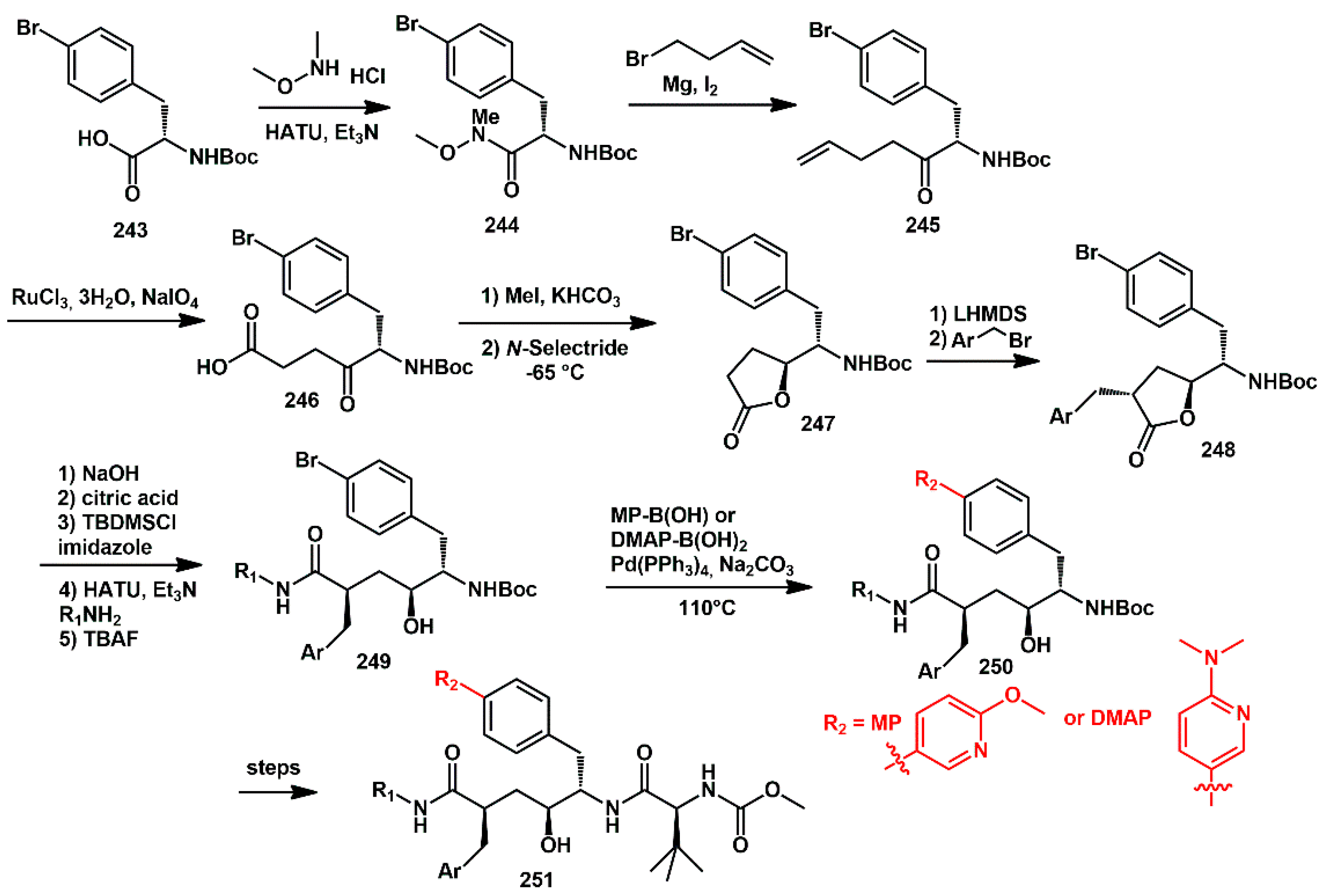
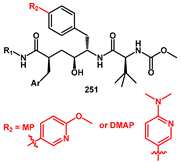
The discovery of HIV-1 protease inhibitors, which exhibit high potency against both HIV-1 wild-type and multi-PI-resistant HIV-mutants is always a key focus. The work of Kesteleyn [
70] was oriented towards discovering new PIs suitable for a long-acting injectable drug applications. In this regard, they described new compounds bearing a heterocyclic 6-methoxy-3-pyridinyl (MP) or a 6-(dimethylamino)-3-pyridinyl (DMAP) (R
3) group at the
para-position of the P1′ benzyl fragment, which showed antiviral activity in the low nanomolar range. The introduction of a heteroaryl moiety was performed via a Suzuki coupling reaction on polyfunctionalized bromoaryl intermediate
249. Bromo lactone
248 was recognized as a key intermediate. For the synthesis of
248, a new enantioselective methodology was developed. As shown in
Scheme 47, the first step involved converting
N-Boc-protected (S)-4-bromophenylalanine
243 into the Weinreb amide
244. Treatment with 3-butenylmagnesium bromide then furnished ketone
245, which was oxidized at the terminal double bond to afford the γ-ketocarboxylic acid
246. The desired bromo-lactone
247 was obtained by initially esterifying the carboxylic acid and then performing a reductive cyclization with
N-selectride. Final benzylation, using lithium hexamethyldisilazane as a base at low temperature, produced the alkylated lactone
248, stereoselectively. Hydrolysis of
248 provided the corresponding carboxylic acid derivative. To prevent re-lactonization, silylation of the alcohol group with TBDMSCl was performed. The introduction of the P2 amine was achieved via the HATU-mediated coupling. Subsequently, the heterocyclic aromatic group R
3 (MP or DMAP) was introduced through palladium-assisted Suzuki coupling of boronic acids, producing compounds
250. Finally,
N-Boc deprotection under acid conditions was followed by HATU-mediated coupling of
N-(methoxycarbonyl)-
L-
tert-leucine, resulting in the desired HIV-1 PIs
252 with high overall yields and enantioselective purity (
ee > 95%).
In the last period, studies on the synthesis of key heterocyclic fragments, particularly those oriented towards the pharmaceutical industry, were described. Kappe and his group developed multistep continuous flow reaction method for the synthesis of the biaryl-hydrazine unit of atazanavir [
71]. The synthesis involved Pd-catalyzed Suzuki–Miyaura cross-coupling, followed by hydrazone formation and a hydrogenation step. At the end, an additional liquid–liquid extraction step was performed. The method yielded the desired product with an overall yield of 74%, which exceeded the 53% overall batch yield previously described in the literature.
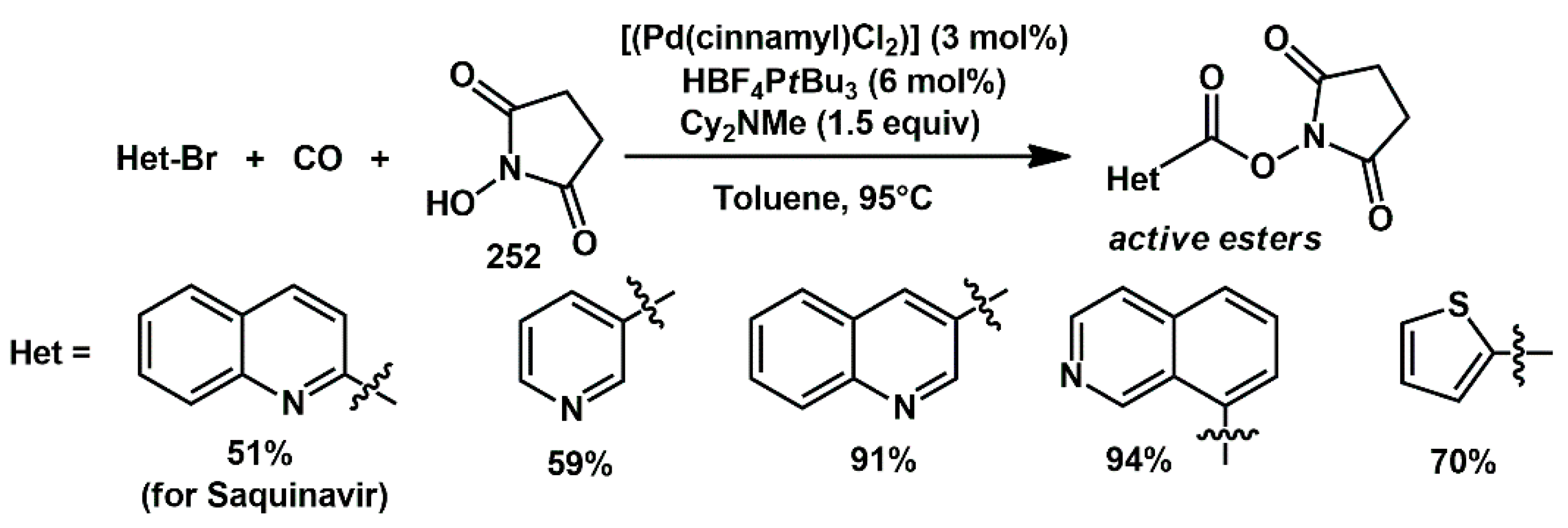
A useful method was developed by Lindhardt and Skrydstrup for the synthesis of active esters by palladium-catalyzed alkoxycarbonylation of (hetero)aromatic bromides (
Scheme 48) [
72]. The protocol was general for a range of oxygen nucleophiles including
N-hydroxysuccinimide (NHS)
252 and showed high functional group tolerance. The method enabled the synthesis of an important precursor to the HIV protease inhibitor saquinavir, through the formation of an NHS ester followed by acyl substitution.
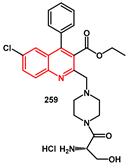
Quinoline and isoquinoline moieties have been extensively studied as scaffolds for HIV inhibition and have been recently reviewed [
73]. In the work of Sarveswari [
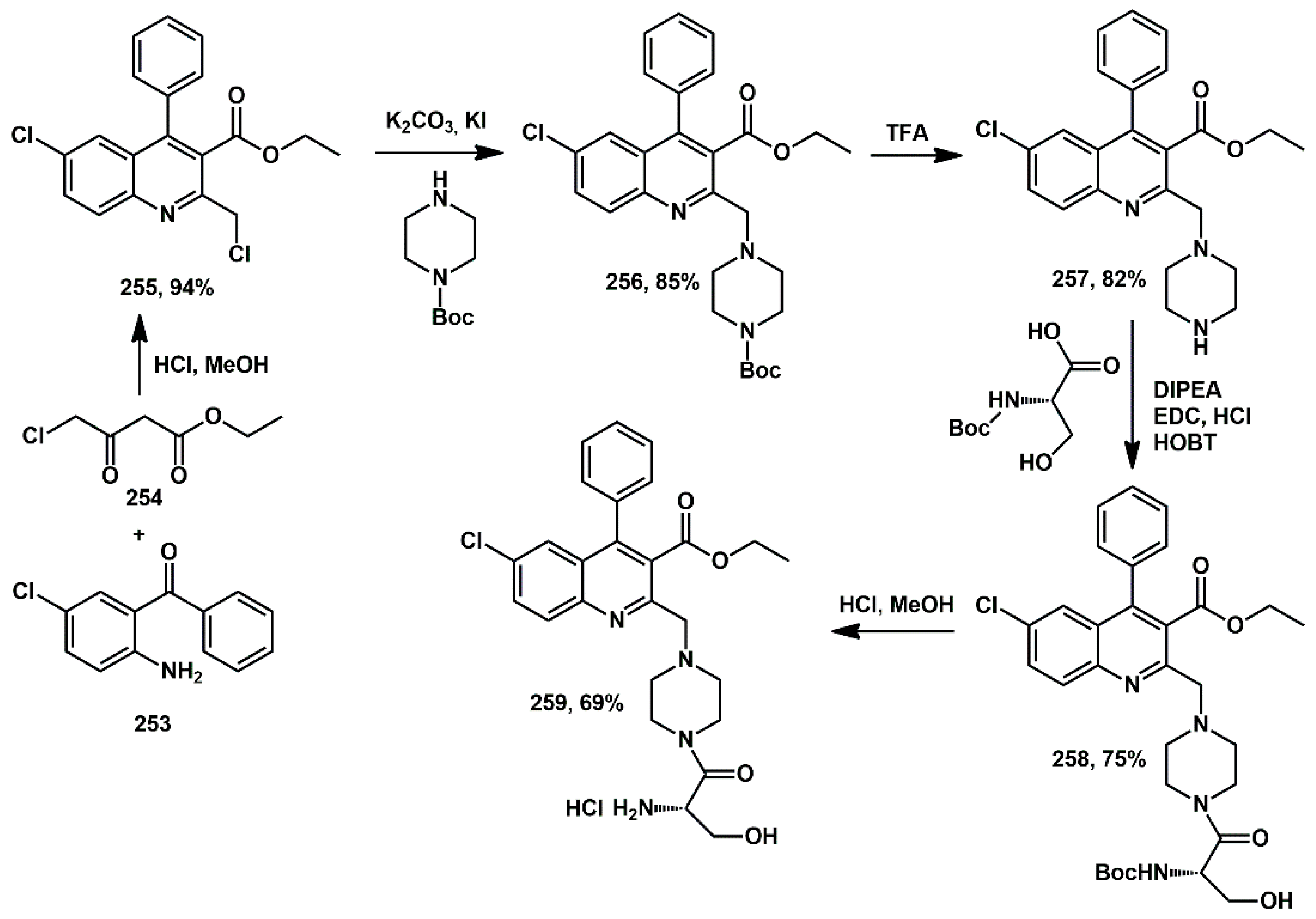
74], two diverging series of water-soluble, non-peptidic quinoline analogs were designed and synthesized through the sequential attachment of piperazine and various amino acids to the quinoline scaffold using peptide coupling procedures. All synthesized compounds were subjected to in silico screening against HIV protease-1 and the cytotoxicity of all compounds was examined on HCT116 cells. In particular, compound
259 (
Scheme 49), which bears serine and piperazine in sequence at C2 of quinoline, showed significant HIV protease inhibition properties and demonstrated cytotoxicity IC
50 value at 22.7 ± 0.59 nM. The synthesis began with the construction of quinoline core via HCl-promoted coupling between ethyl 4-chloroacetoacetate
254 and 2-amino-5-chlorobenzophenone
253 in methanol, yielding 94% of the desired compound
255. In the second step, Boc-piperazine was introduced by nucleophilic substitution, leading to compound
256. After removing the Boc protecting group, the piperazine amine was coupled with
L-serine using HOBt, EDC. Final deprotection of the amino acid in methanolic HCl afforded the target compound
259.
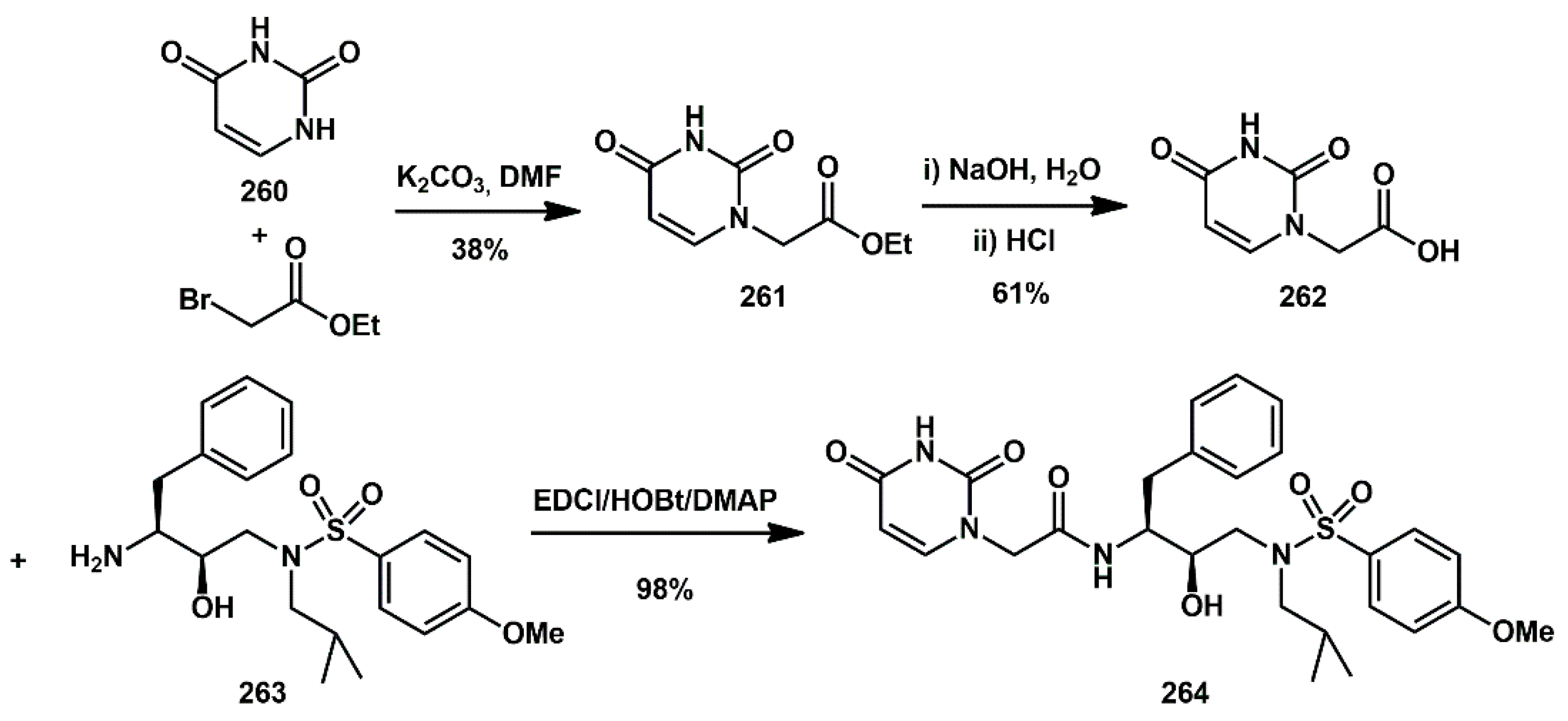
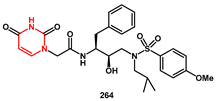
On the way to extending hydrogen bonding interactions between inhibitor and HIV-1 protease, pyrimidine bases were recognized as suitable as P2 ligands capable of enhancing the activity of the inhibitors. In Wang’s work [
75], inhibitor
264 (
Scheme 50), bearing
N-2-(2,4-Dioxo-3,4-dihydropyrimidin-1(2H)-yl) acetamide and a 4-methoxylphenylsulfonamide, showed high enzyme inhibitory activity, with an IC
50 of 2.53 nM in vitro and an inhibition ratio with 68% against wild-type HIV-1 in vivo, with low cytotoxicity. Its antiviral activity was also effective against DRV-resistant HIV-1 variants. The syntheses began with the preparation of substituted 2, 4-dioxopyrimidin-1(2H)-yl acetic acid
262 from uracil, via
N-alkylation with ethyl bromoacetate (38%), followed by saponification with sodium hydroxide (61%). Coupling with the suitable amine
263 under EDCl/HOBt/DMAP conditions yielded the final product in high yield (98%).
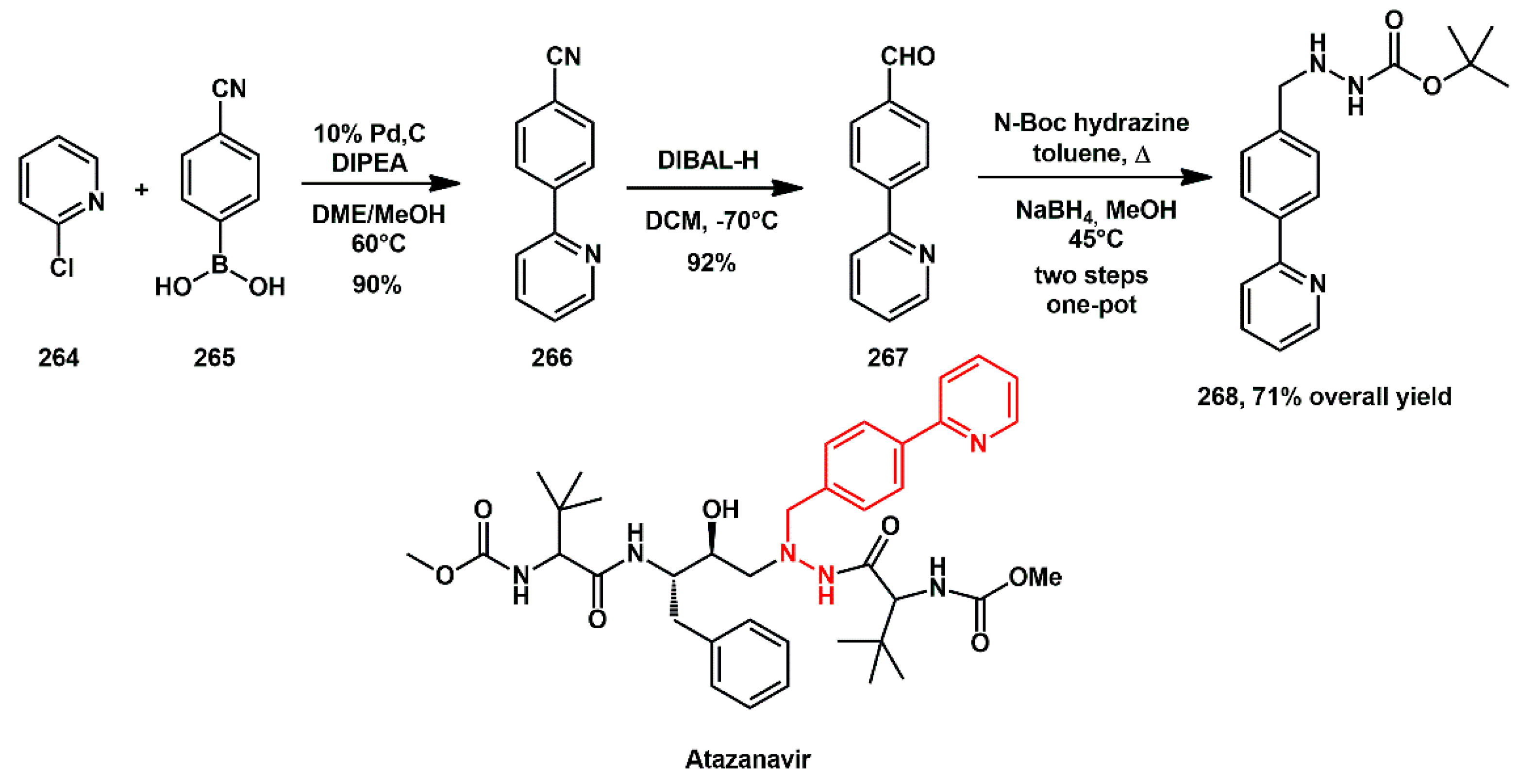
Atazanavir is one of the most prescribed HIV-1 protease inhibitors approved by the FDA. It was the first protease inhibitor approved for once-a-day dosing to treat AIDS, owing to its good oral bioavailability and favorable pharmacokinetic profile. Reddy’s work [
76] resulted in a new multistep synthesis for biaryl-hydrazine unit {
tert-butyl 2-[4-(2-pyridinyl)benzyl]hydrazinecarboxylate} of atazanavir
268 on a large scale (
Scheme 51). The synthesis began with a palladium catalyzed Suzuki–Miyaura coupling of readily available 2-chloropyridine
264 and (4-cyanophenyl)boronic acid
265, yielding the biaryl derivative
266. Next, the cyano group was reduced to the aldehyde
267 using DIBAL-H. Treatment with
tert-butyl carbazate, followed by in situ reduction with NaBH
4-furnished hydrazone
268. The entire process was scaled-up and completed in three steps with an overall yield of 71%.
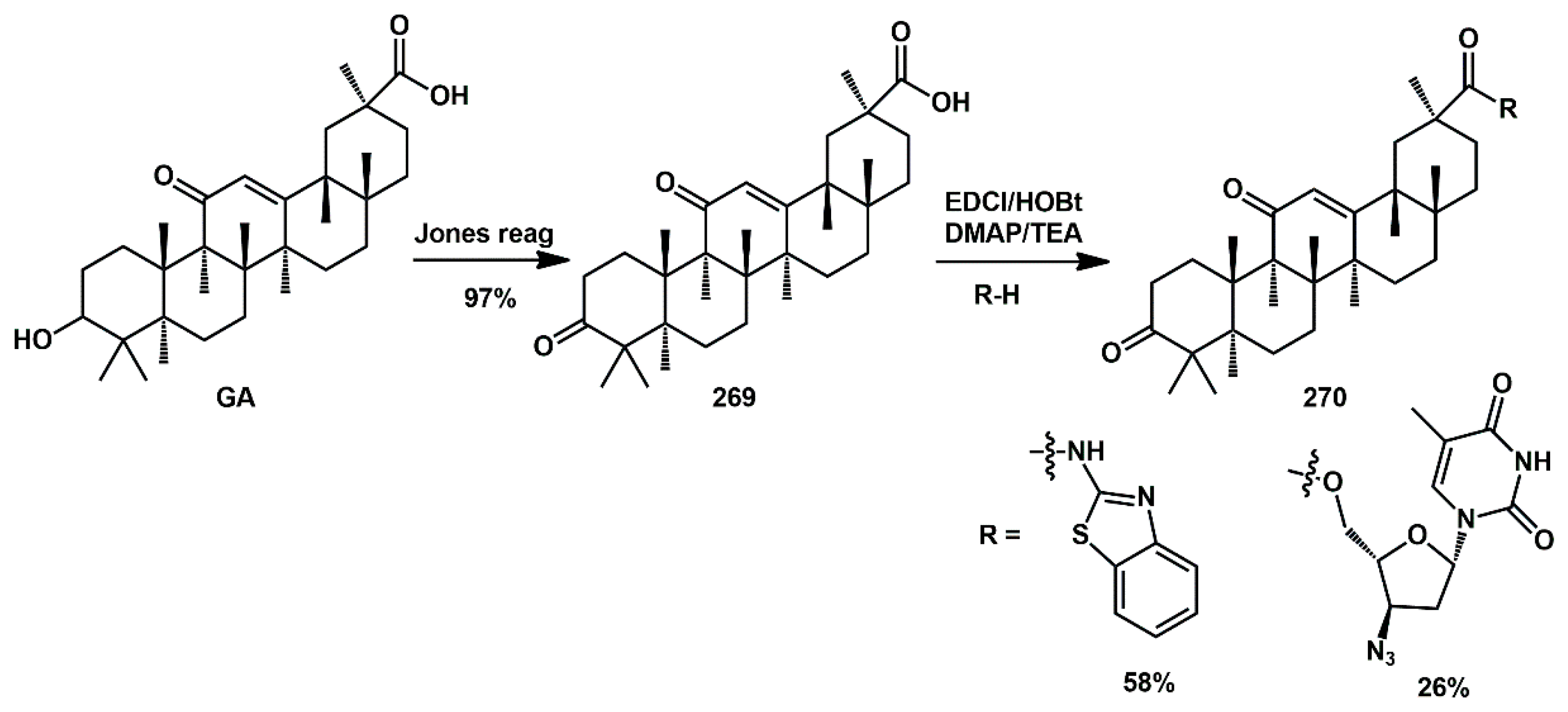
Several naturally occurring triterpenes and their semisynthetic analogs have demonstrated potent anti-HIV activities. Recently, Zheng [
77] synthesized thirteen nitrogen-containing derivatives of 3,11-dioxo-olean-12- en-30-oic acid by introducing various amino acids and nitrogen-containing heterocyclic groups at the 30-carboxyl group, starting from 18
β-glycyrrhetinic acid (
GA) (
Scheme 52). Among them, compound
270 displayed relatively moderate inhibitory activity, with IC
50 values below 0.24 mM. Molecular docking studies revealed favorable hydrophobic–hydrophobic and hydrogen bonding interactions in the active site of HIV-1PR. These findings underscore the potential of such derivatives as promising candidates for the development of HIV-1 PR inhibitors. The synthesis commenced with the oxidation of the 3-hydroxyl group of
GA to a carbonyl group (97%), yielding compound
269. The target compounds
270 were synthesized using an EDCI/HOBt/DMAP/TEA system in the presence of a suitable nucleophile.
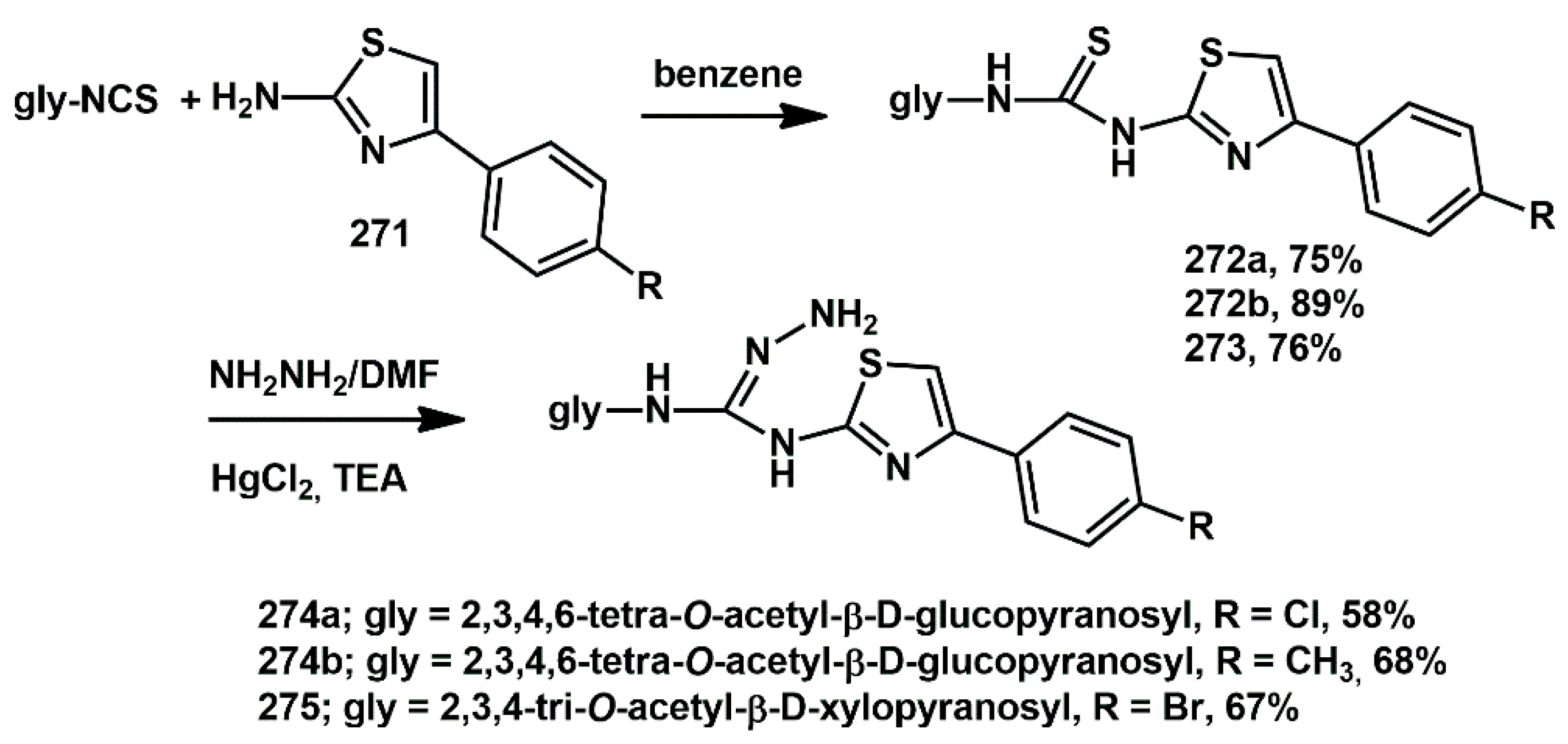
Certain thiazolyl and benzothiazolyl guanidines have been reported as exhibiting a wide range of pharmacological and antimicrobial activities. In the field of carbohydrates, glycosyl isothiocyanates are versatile synthetic intermediates for the synthesis of biologically active carbohydrate derivatives. Cao et al. synthesized and evaluated the bioactivity of some new
N-glucosyl-
N′-(4-arylthiazol-2-yl) aminoguanidines (
Scheme 53) [
78] The starting materials, 2-amino-4-arylthiazoles of type
271, were refluxed with substituted glycosyl isothiocyanates in dry benzene. Subsequent desulfurization of the resulting thioureas with HgCl
2 in DMF, in the presence of hydrazine hydrate and TEA, furnished the target compounds. In particular, compounds
274 and
275 showed moderate activity against HIV-1 protease (IC
50 between 22 and 107 mg/mL).
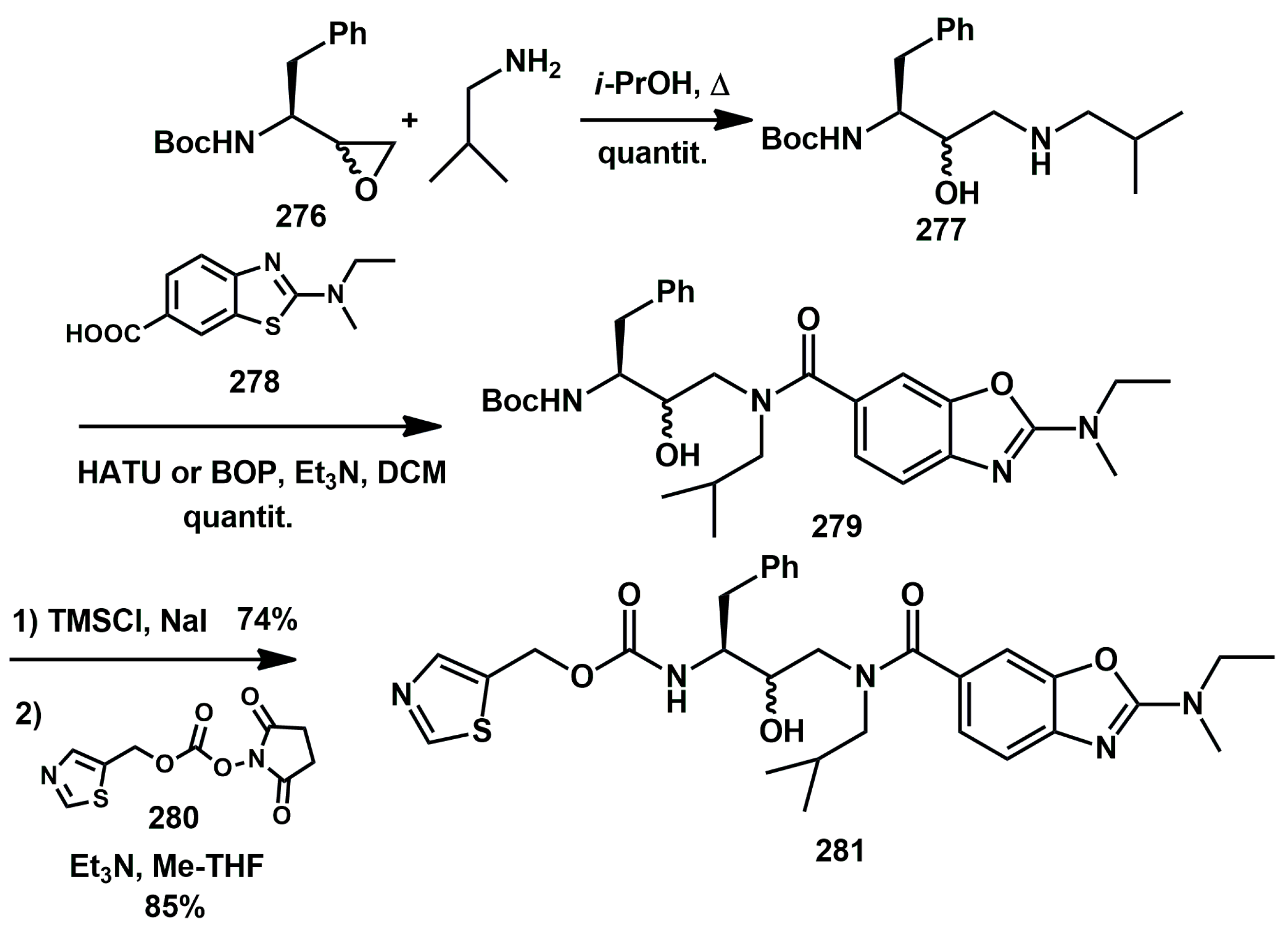
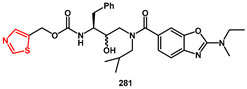
While ritonavir is approved as an HIV PI, it is hardly used as such, being more frequently used as a pharmacokinetic enhancer. Unfortunately, its use is often associated with various side effects. Therefore, novel derivatives have been described. Jonckers [
79] proposed thiazol-5-ylmethyl (2
S,3
R)-4-(2-(ethyl(methyl)amino)-
N-isobutylbenzo[d]oxazole-6-carboxamido)-3-hydroxy-1-phenylbutan-2-ylcarbamate
281 as a lead candidate for this class (
Scheme 54). This compound, together with structurally similar analogues, demonstrated excellent ‘boosting’ properties when tested in dogs. These findings made it attractive in the search for novel pharmacokinetic enhancers. The synthesis of the lead compound
279 began with commercially available epoxide
276, which was reacted with an excess of isobutyl amine, yielding monoprotected
bis-aminoalcohol
277. This was then coupled with acid derivative
278 using HATU or BOP as activating agent, yielding intermediate
279. After mild Boc-deprotection, the intermediate amine was coupled with suitable thiazolyl carbonate
280, affording target compound
281 in high yield. The in vitro antiviral activity against wild-type HIV-1 (EC
50 = 71 mM) was evaluated in an acutely infected lymphoblastic cell line (MT4-LTR-EGFP) and CYP 3A4 inhibition (IC
50 = 0.031 mM) was determined in vitro using a human liver microsome (HLM)-based assay.
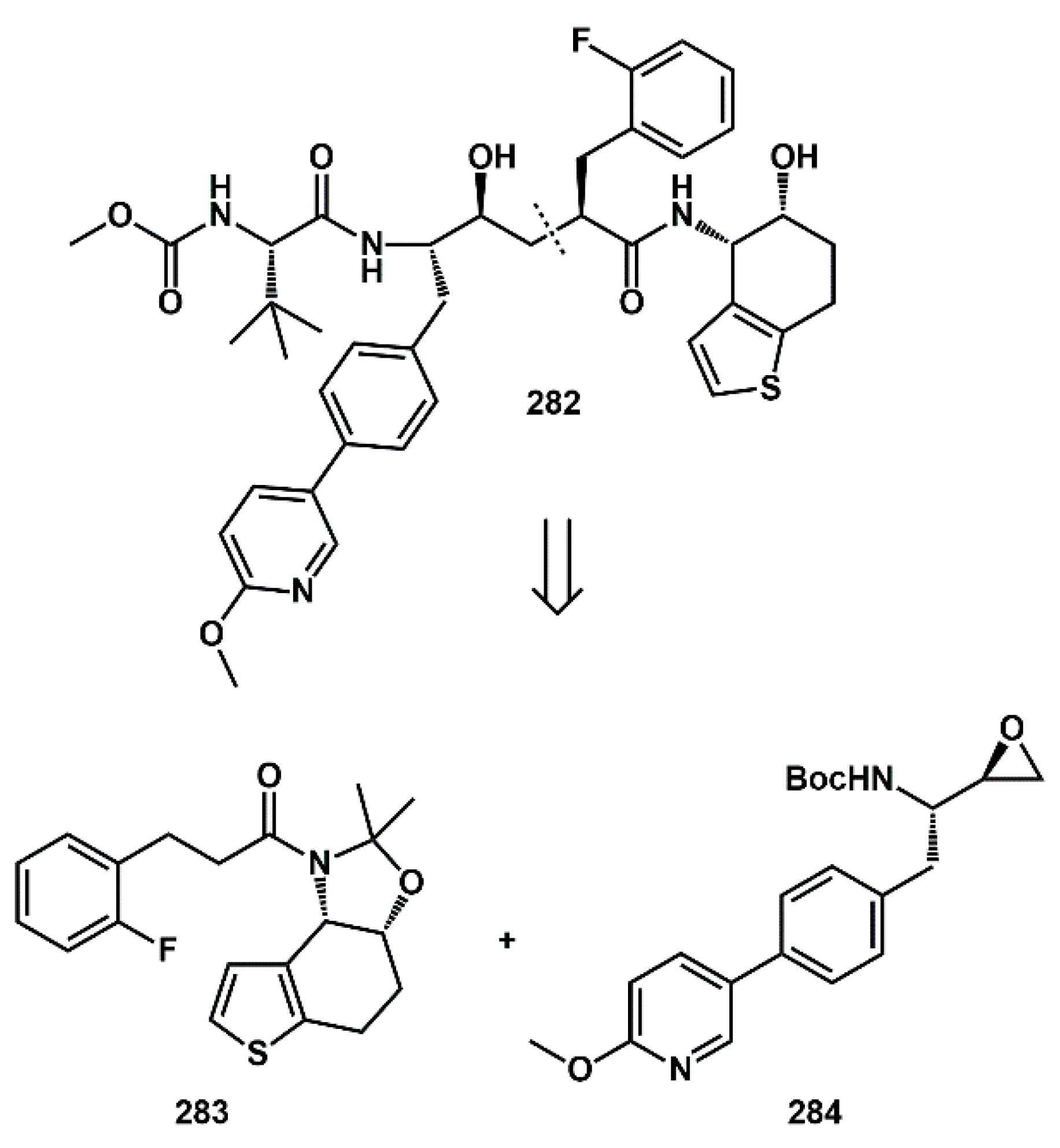
Multiple heteroaromatic fragments were introduced into novel inhibitors. In particular, Houpis [
80] described the convergent synthesis of clinical candidate
282 (
Scheme 55), a protease inhibitor specifically designed to allow for long acting-controlled release formulations. Central disconnection generated two synthons
283 and
284 bearing heteroaromatic moieties.
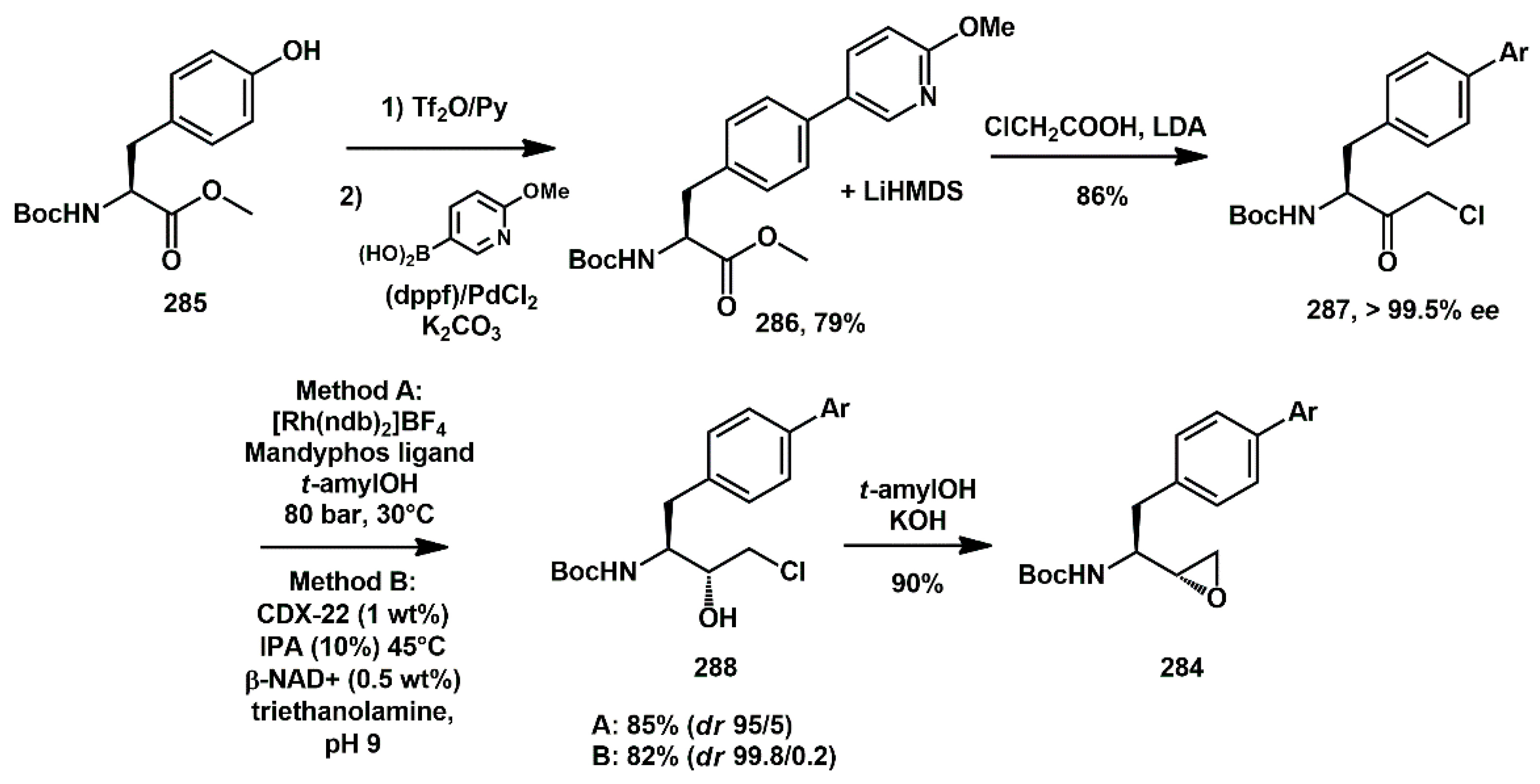
The preparation of epoxide
284 started from protected tyrosine
285 (
Scheme 56). The phenol functional group was activated as the triflate, using triflic anhydride in the presence of pyridine. The resulting product was then coupled with a suitable pyridyl boronic acid in the presence of commercially available ferrocenyl-based catalyst, Cl
2Pd (dppf)-DCM, yielding the
bis-aryl intermediate
286. The key intermediate, epoxide
284, could be obtained from either metal-catalyzed or enzymatic process in high yield by exposing
287 to aqueous potassium hydroxide in
tert-amyl alcohol.
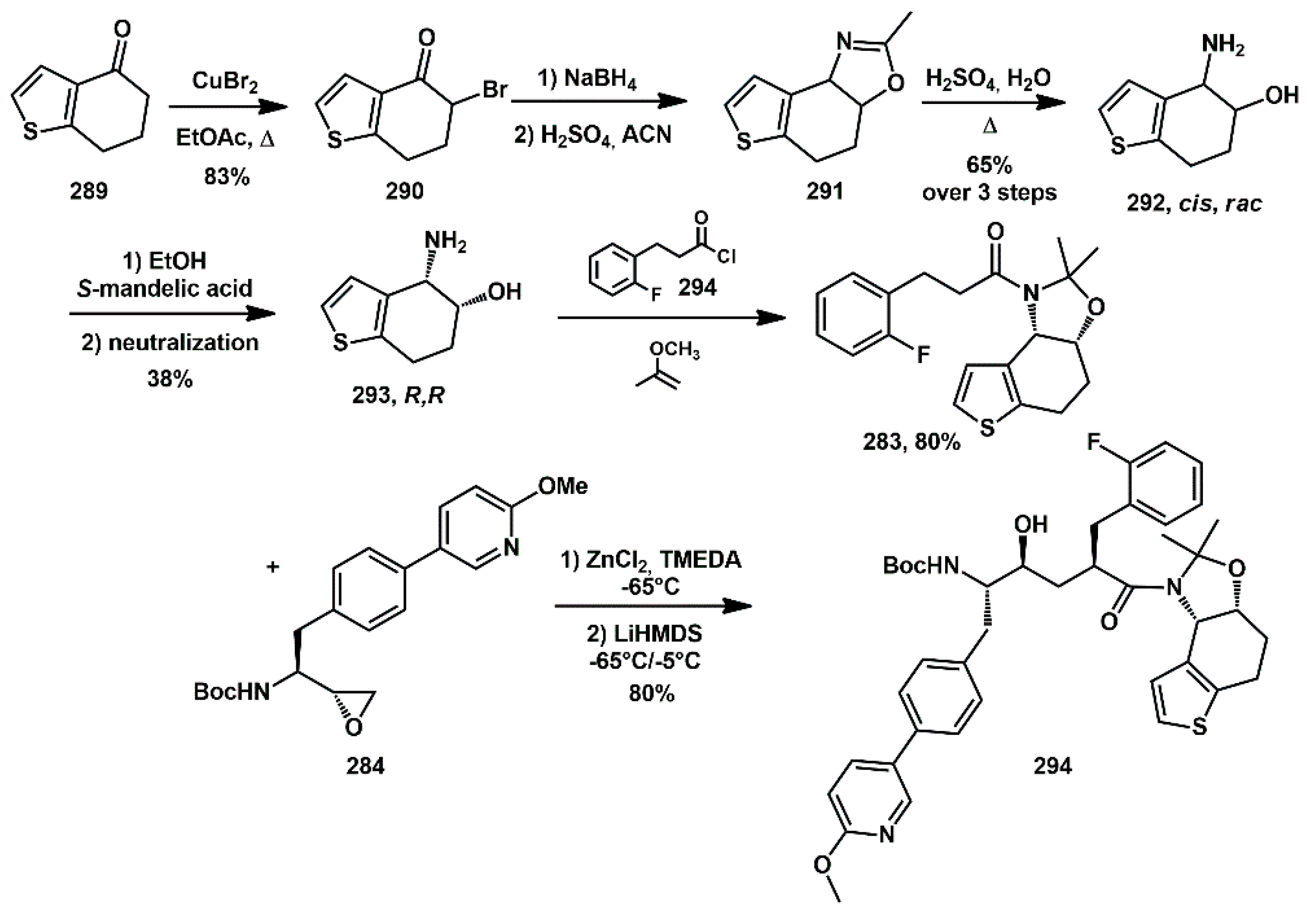
The optically pure amino alcohol (
R,
R-
293) was prepared starting from ketone
289 (
Scheme 57), which was brominated selectively at the α-aliphatic position using CuBr
2 in refluxing EtOAc to give
rac-
290. An NaBH
4 reduction followed by a Ritter reaction afforded the racemic
cis-amino alcohol
292. The optically active derivative (
R,
R-293) was obtained in good yield via a resolution with
S-mandelic acid. The optically active amide
283 was prepared using well established methods. Subsequent coupling with epoxide
284 was performed using ZnCl
2/TMEDA system, followed by the addition of LiHMDS. Deprotection of the BOC group and coupling with the methyl carbamate
tert-butylglycine furnished the desired inhibitor
282.
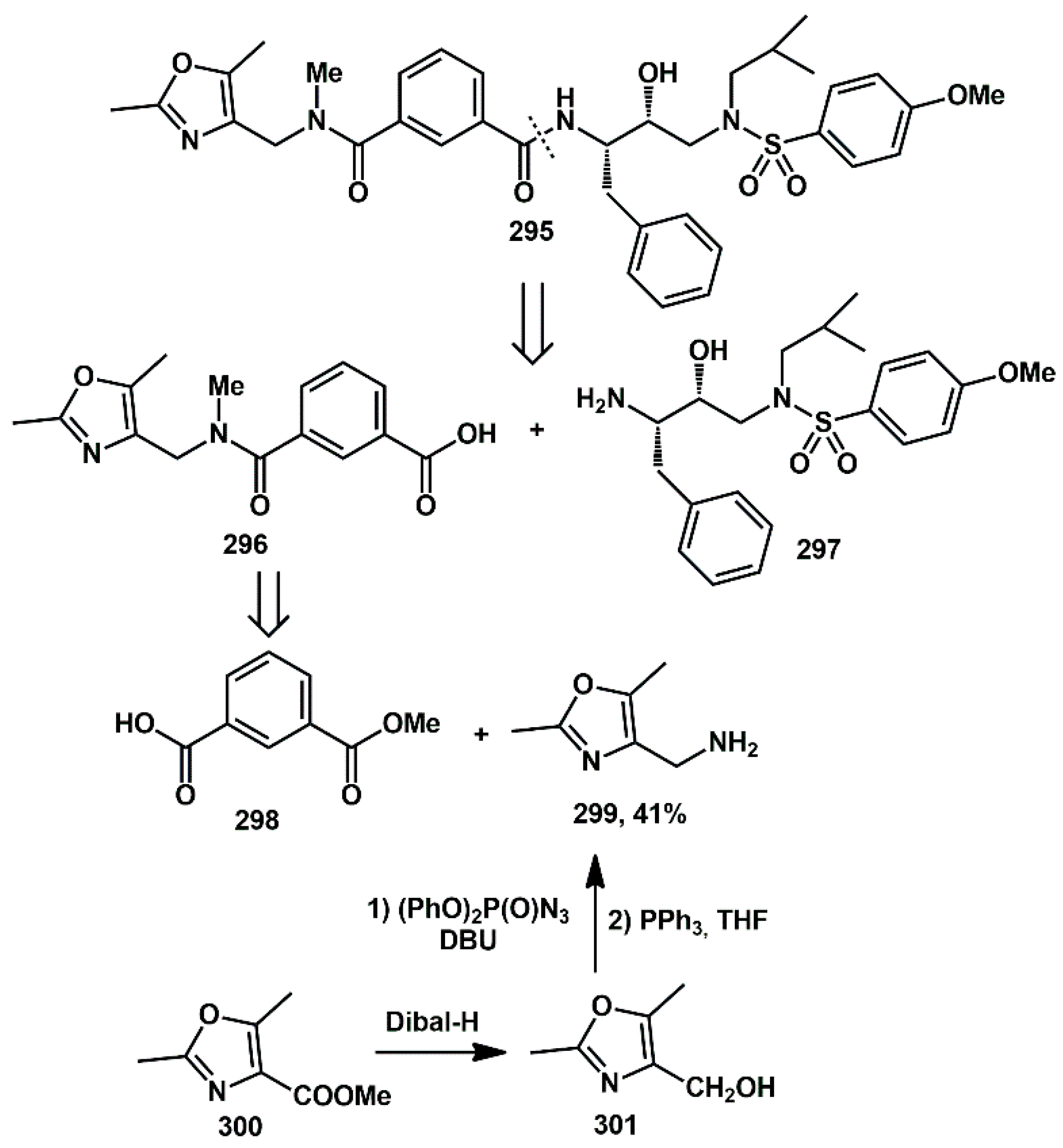
Ghosh [
81] described the design, synthesis, and biological evaluation of a series of novel HIV-1 protease inhibitors bearing isophthalamide derivatives as the P2–P3 ligands. In particular, compound
295 showed an enzyme Ki of 0.17 nM and antiviral IC
50 of 14 nM (
Scheme 58). The general synthetic strategy involved the coupling of isophthalic acid derivative
296 with the amine of the hydroxyethylamine sulfonamide isosteres
297 to produce HIV-1 protease inhibitors. Various isophthalic acid derivatives can be obtained by coupling readily available isophthalic monoacid
298 with various amines. In particular, amine
299 was prepared from oxazole ester
300, which was first reduced with excess of DIBAL-H to afford the corresponding alcohol
301. Subsequently, azidation of the alcohol with diphenylphosphoryl azide in the presence of DBU provided the corresponding azide. Finally, reduction of the azide with triphenyl phosphine in THF yielded amine
299.
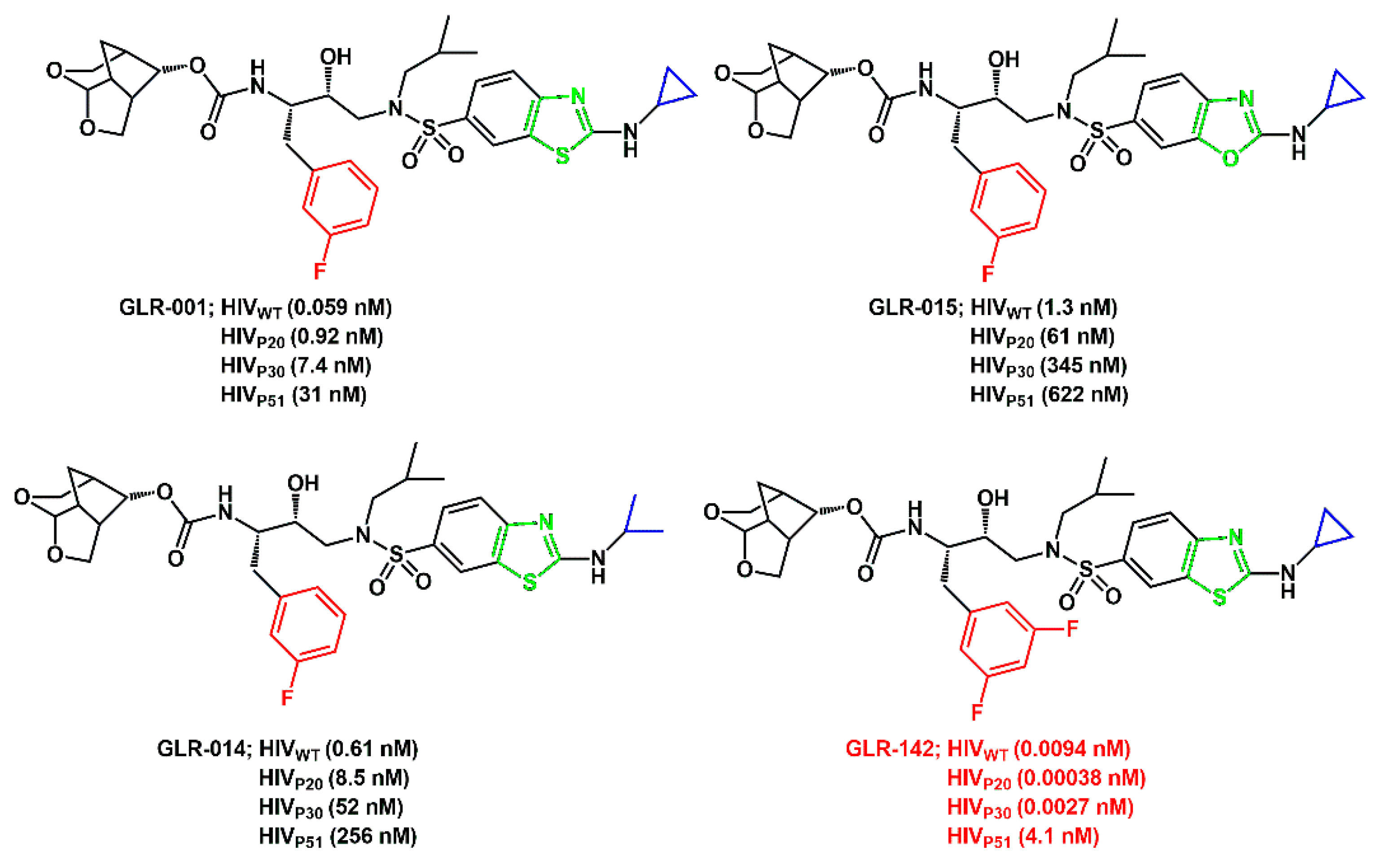
Ghosh and his group described the effect of a single atom change or a scission of a single bond on the activity of a panel of compounds structurally like darunavir. Seven novel PIs were synthesized, and the modifications involved an exchange of sulfur for oxygen, a scission of a single bond in P2′-cyclopropylaminobenzothiazole (or -oxazole), and/or introduction of P1-benzene ring with mono- or
bis-fluorine atoms (
Scheme 59) [
82]. X-ray structural analyses of the PIs complexed with wild-type protease (PR
WT) and highly multi-PI-resistant PR
DRV P51 revealed that the PIs adjusted themselves to the protease with resistance-associated amino acid substitutions. Inhibitors containing a benzothiazole moiety at the P2′ position showed greater anti-HIV-1 activity than those with a benzoxazole moiety. It was claimed that this greater potency is attributed to the capacity of sulfur atoms to form bidirectional σ-hole potentials with the carbonyl oxygen of G48 [
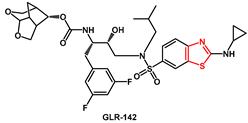
83]. On the other hand, substitution of cyclopropyl with isopropyl at the distal part of the inhibitor’s P2′ moiety resulted in a reduction in the antiviral activity. The membrane penetration data confirmed previous findings that the addition of two fluorine atoms greatly boosts the activity of the inhibitors.
More recently, Ghosh et al. [
84] described new inhibitors bearing various pyridyl-pyrimidine, aryl thiazole, or alkylthiazole moieties as P2 ligands in darunavir-like hydroxyethylamine sulfonamide isosteres, with the aim of promoting hydrogen bonding interactions with the backbone atoms in the S2 subsite of HIV-1 protease. The different ligands were introduced using suitable parent benzoic acids, whose synthesis was described. The new inhibitors showed sub-nanomolar levels of protease inhibitory activity and low nanomolar levels of antiviral activity. In particular, pyridyl-pyrimidine benzamide derivative
303a (
Scheme 60) displayed an enzyme inhibitory K
i of 28 pM and antiviral activity of 154 nM, whereas compound
303b displayed potent antiviral activity with an IC
50 value of 66 nM. Among the thiazole-derived inhibitors, compound
308, with a pyridyl thiazole as the P2 ligand, showed the best result, exhibiting an HIV-1 protease inhibitory
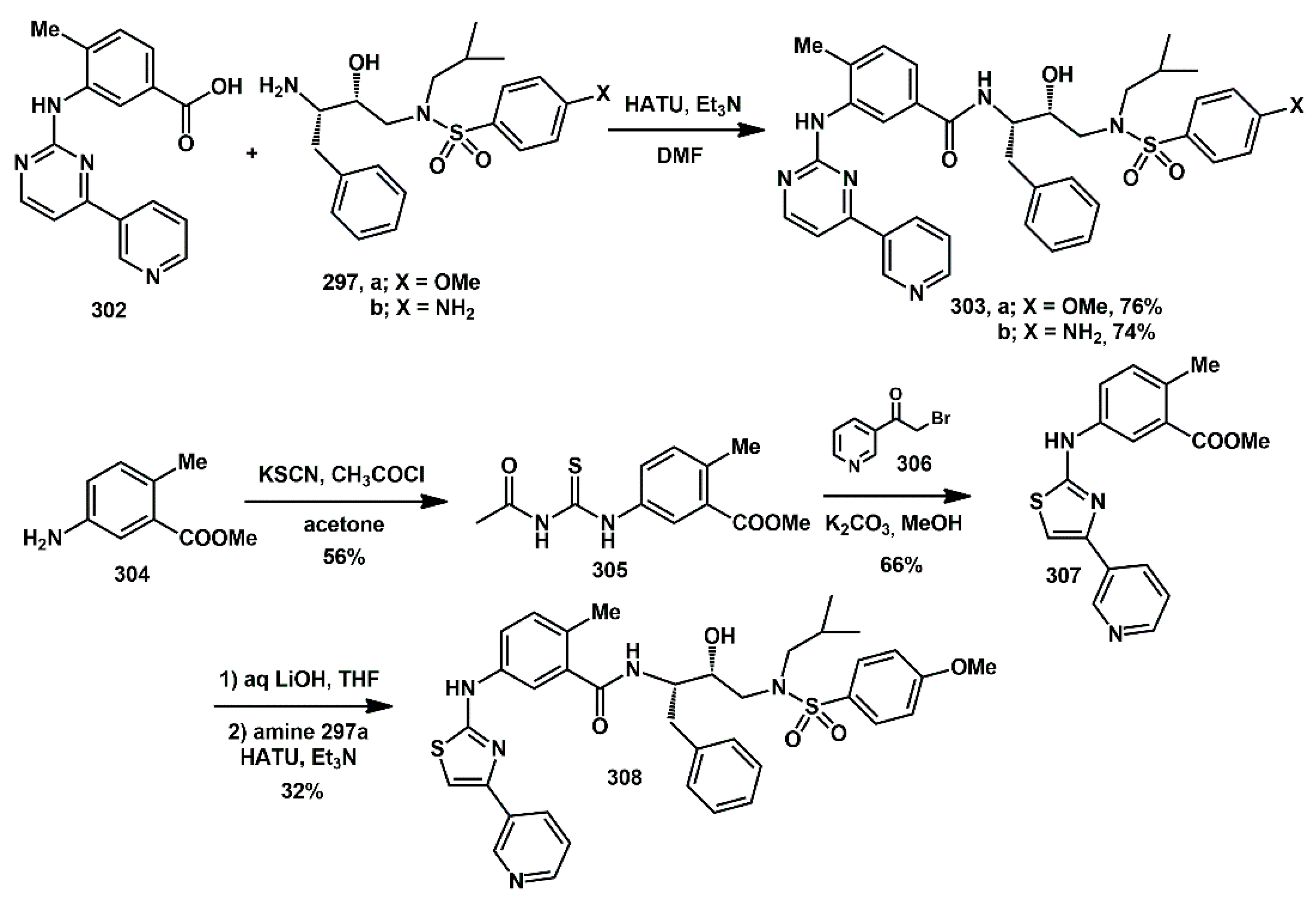
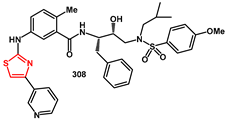
Ki of 8.7 nM and antiviral activity of 580 nM.
For the synthesis of 303, commercially available 4-methyl-3-[[4-(3- pyridinyl)-2-pyrimidinyl]amino]benzoic acid 302 was reacted with the previously reported hydroxyethylene isosteres 297 in the presence of HATU and Et3N in DMF. The synthesis of 308 started from 2-methyl 5-aminomethylbenzoate 304, which was reacted with potassium thiocyanate and acetyl chloride in acetone to yield the thiourea derivative 305. Exposure of 305 to potassium carbonate in methanol, followed by the addition of α-bromo ketone 306, furnished the pyridinylthiazole derivative 307. Saponification of methyl ester with aqueous LiOH in THF afforded the corresponding carboxylic acid. Final coupling with the hydroxyethylsulfonamide isosteric amine 297a produced inhibitor 308 in good yields.
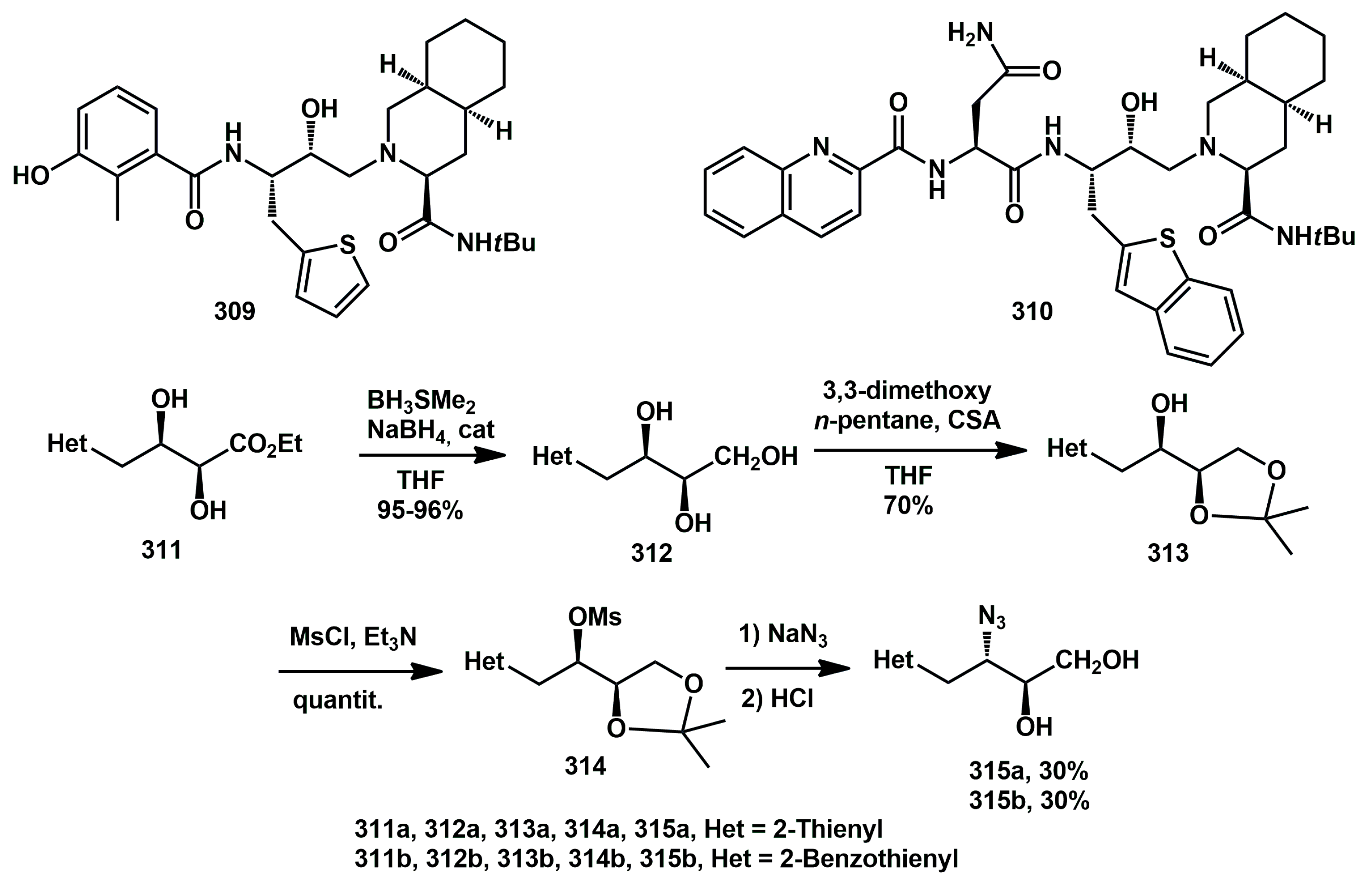
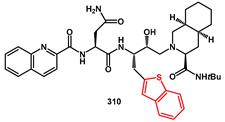
In 2010, we synthesized a series of new thienyl analogues of nelfinavir and saquinavir with different substitution patterns, derived from suitable enantiopure diols [
85]. Their inhibitory activity against wild-type recombinant HIV-1 protease was evaluated. In general, thienyl groups spaced from the core by a methylene group yielded products with IC
50 values in the nanomolar range, regardless of the heterocycle’s type or substitution pattern. Notably, compounds
309 and
310 (
Scheme 61) were the most active, and their activity was substantially maintained or even increased against two common mutants under drug pressure, such as V32I and V82A. The synthesis began with dihydroxybutylesters
311, which were reduced by BH
3SMe
2, producing triols
312 in nearly quantitative yield. Freshly prepared 3,3-dimethoxypentane was used for selective protection of the hydroxyl groups on carbons 1 and 2. The free hydroxyl was then transformed into the mesylate
313 and subsequently substituted with an azido group. Final hydrolysis yielded azidodiols
314 with modest to good overall yield.
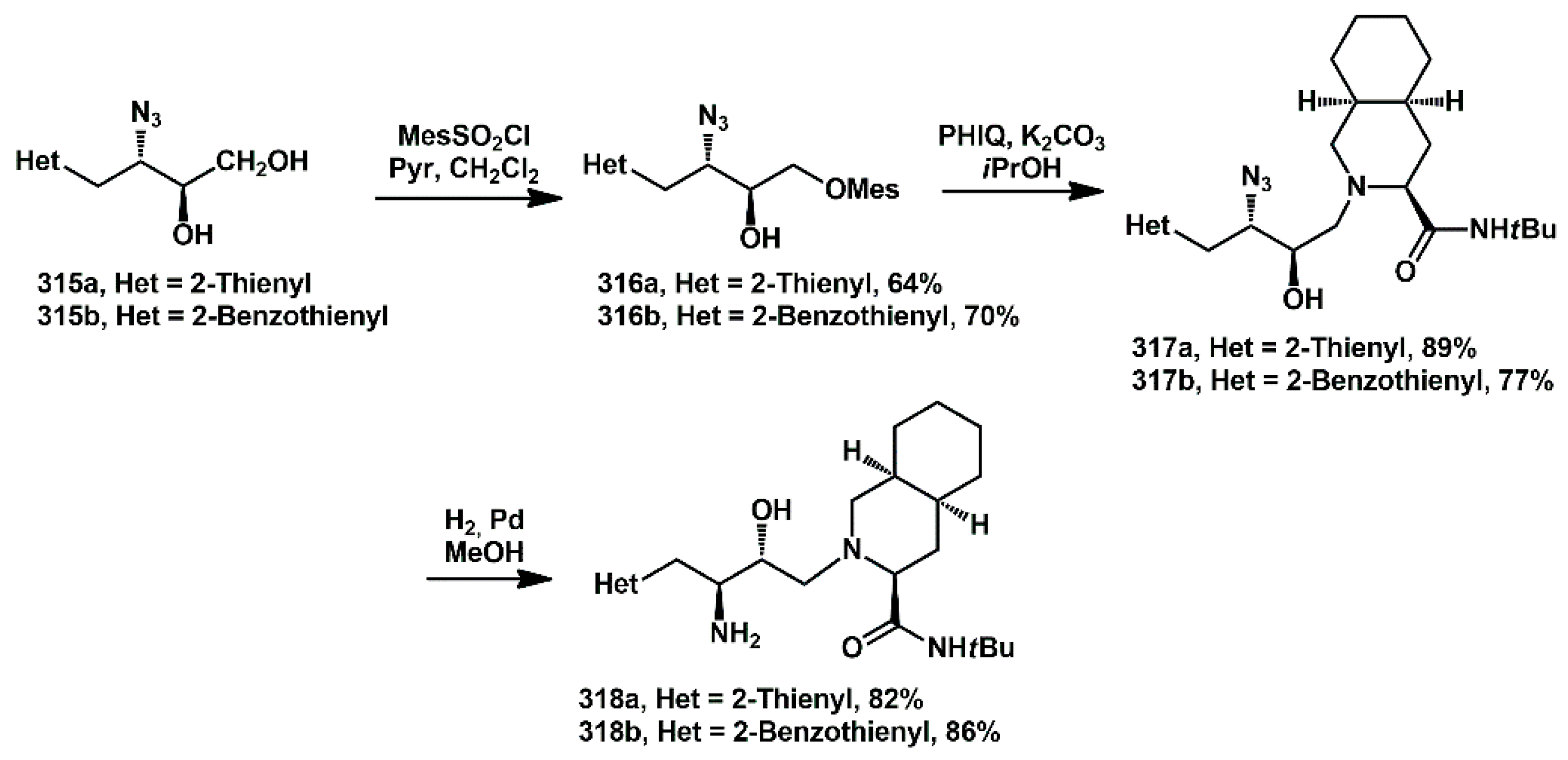
The perhydroisoquinolinic fragment was linked via selective activation of the hydroxyl group as a mesitylene sulfonyl derivative
316, followed by reaction with the commercially available substituted perhydroisoquinoline (
Scheme 62). Finally, the azidoalcohols were transformed into amino alcohols
318a and
318b by Pd-catalyzed hydrogenation in excellent yield.
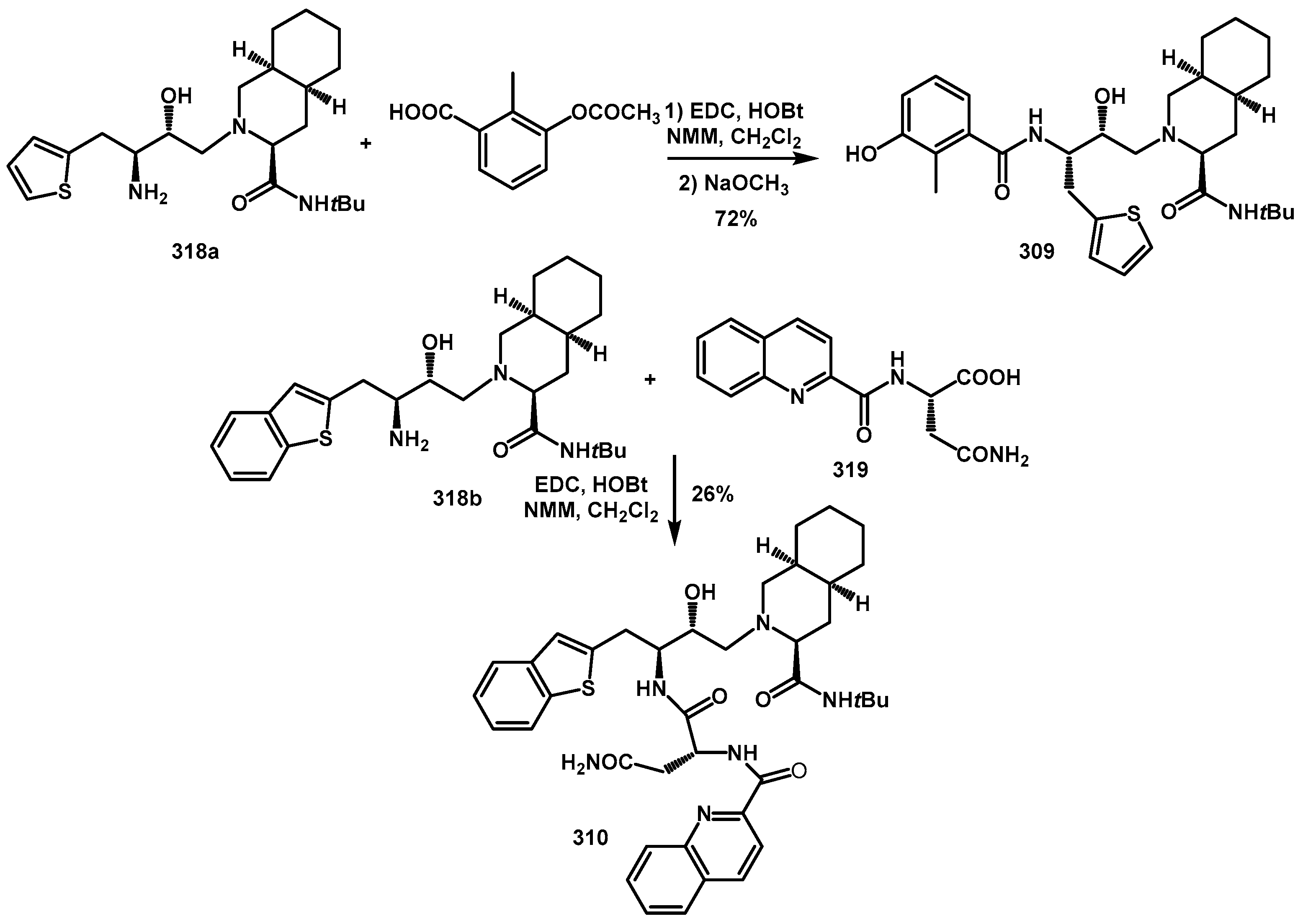
For the synthesis of the nelfinavir thienyl analog, amino alcohol
318a was reacted with 3-acetoxy-2-methylbenzoic acid. Final deacetylation of the phenolic group yielded the target compounds
309 in good yield (
Scheme 63). The characteristic dipeptide unit of saquinavir derivatives
319 was prepared by coupling asparagine tert-butyl ester with quinolinic acid. Hydrolysis with TFA afforded acid
319, which was subsequently coupled with amino alcohol
318b, resulting in the desired saquinavir derivatives
310.
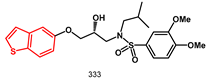
Investigating the synthesis and biological evaluation of new structurally simplified non-peptidic heteroaromatic molecules as PIs, we described the synthesis and biological evaluation of a new series of potential HIV-1 protease inhibitors of types
330–
333, incorporating different benzofused heterocycles (
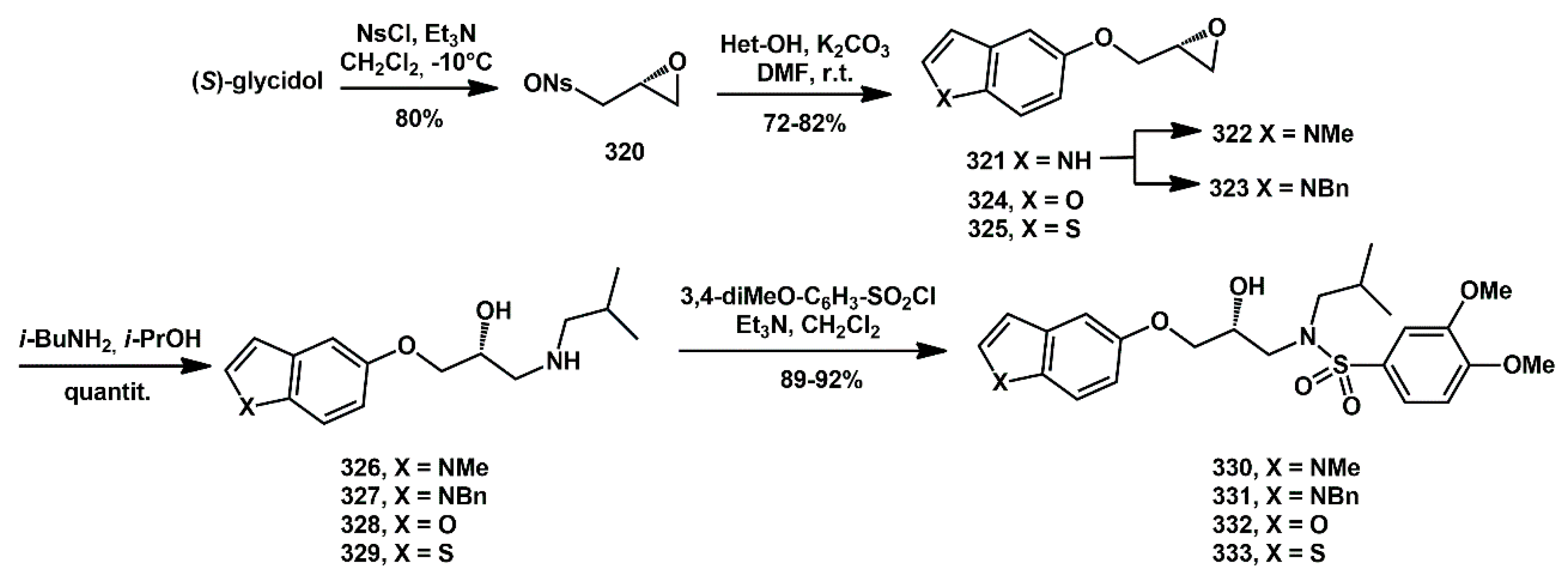
Scheme 64) [
86]. The variation in heteroatoms in such molecules affected biological activities and a benzothiophene containing inhibitor
333 exhibited high potency against wild-type HIV-1 protease with an IC
50 of 60 nM, thanks to the lower desolvation penalty paid by this hydrophobic moiety. The synthesis began with commercially available (
S)-glycidol (98%
ee), which was reacted with 3-nitrobenzenesulfonyl chloride (NsCl) and triethylamine (Et
3N) at −10 °C to afford
320 in 80% yield. Subsequently, it underwent regioselective displacement of the nosylate with the appropriate 5-hydroxyheteroarene and K
2CO
3 to yield the corresponding epoxides
321,
324, and
325 in 70%, 78%, and 82% yield, respectively. The indole derivative
321 was alkylated with methyl iodide and benzyl chloride to produce compounds
322 and
323 in 90% and 86% yield. The opening of the oxiranyl ring with
i-BuNH
2 in
i-PrOH provided aminoalcohols
326–
329 in quantitative yield. These compounds were then reacted with 3,4-dimethoxybenzenesulfonyl chloride and Et
3N in dry DCM to afford target compounds
330–
333 in high yield.
With the aim of facilitating access to new HIV-1 protease inhibitors bearing heteroaryl moieties as P1-ligands, we speculated on a convenient synthetic route to introduce diversity into the common hydroxyethylamino core present in several approved PIs [
87] In a straightforward retrosynthetic approach, variously functionalized aromatic groups could be incorporated via Suzuki coupling between an activated C(sp3) bromide (allylic electrophile) and an array of arylboronic acids, thereby furnishing methyl 4-arylcrotonates. An effective ligand-free Suzuki coupling protocol was described for coupling methyl (
E)-4-bromobut-2-enoate with several arylboronic acids. Given the strong interest in methodologies that produce polyarylated frameworks, we subsequently reported a nickel-catalyzed double phenylation of methyl 4-bromocrotonate, which furnished suitable doubly phenylated building blocks [
88].
Different aspects regarding the preparation of peptidomimetic and pseudopeptidic structures containing heterocycles were also reviewed in 2012 by us, with particular focus on novel tricyclic structures as potential drugs [
89]. Following the concept of targeting the protein backbone, we systematically investigated various substitution patterns on the common stereo-defined isopropanolamine core. In 2014, we described the synthesis of new, structurally simple indolic non-peptidic HIV-protease inhibitors from (S)-glycidol using regioselective methods [
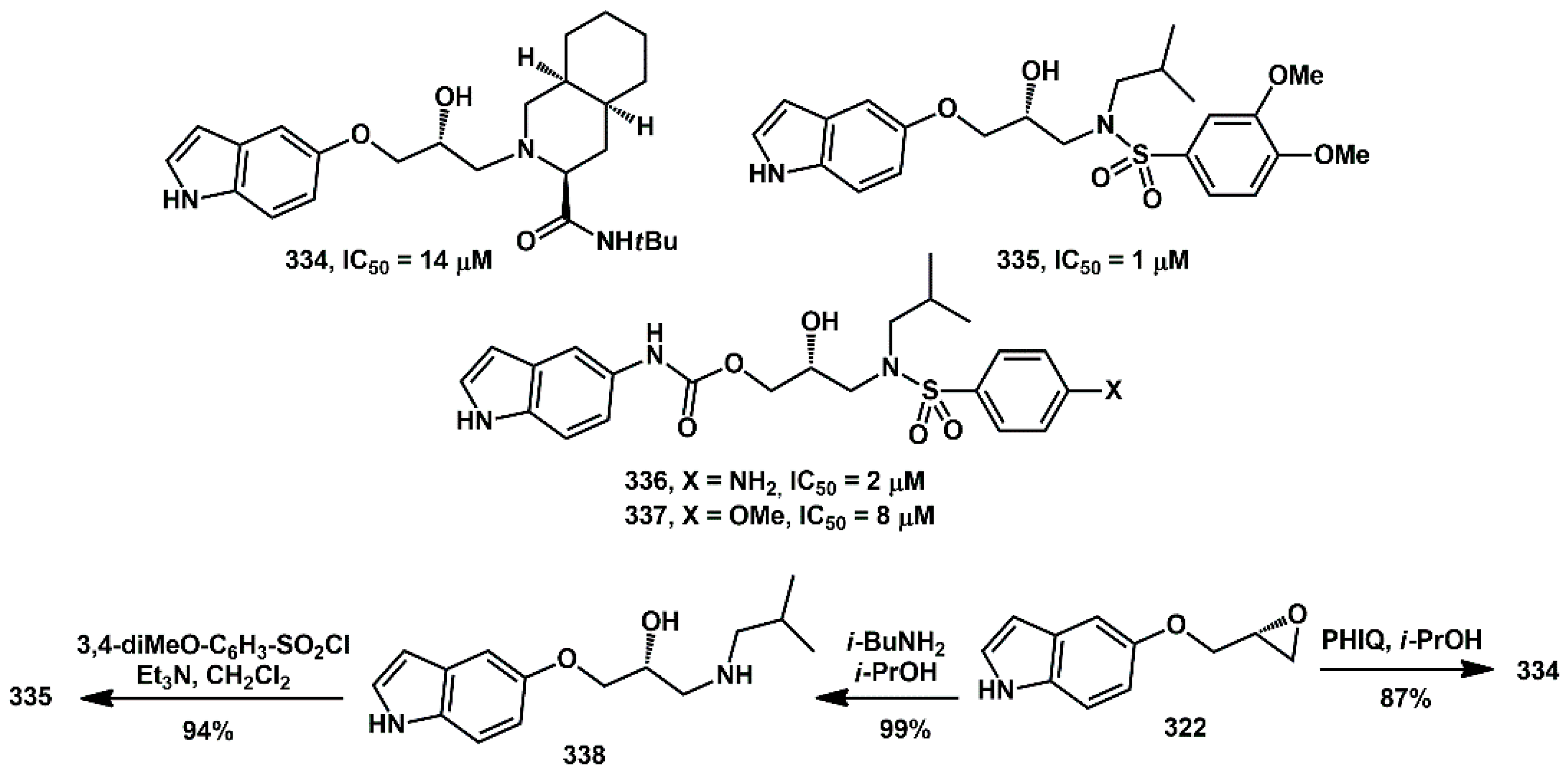
90], varying the type and/or the position of the functional group on the indole and the nature of the nitrogen containing group (sulfonamides or perhydroisoquinoline). The systematic study of in vitro inhibition activity of these compounds confirmed the general beneficial effect of the 5-indolyl substituents in the presence of arylsulfonamide moieties, which showed activities in the micromolar range. Oxyindoles and carbamoyl indoles showed general good activity, whereas simple aminoindoles were much less active (
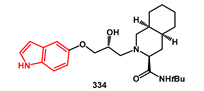
Scheme 65). For the synthesis of oxyindoles, epoxide
322 was opened with perhydroisoquinoline, yielding inhibitor
334, or alternatively with
i-BuNH
2, giving intermediate
338. This last amino alcohol was reacted with 3,4-diOMe-phenylsulfonyl chloride, affording compound
335 in excellent yield.
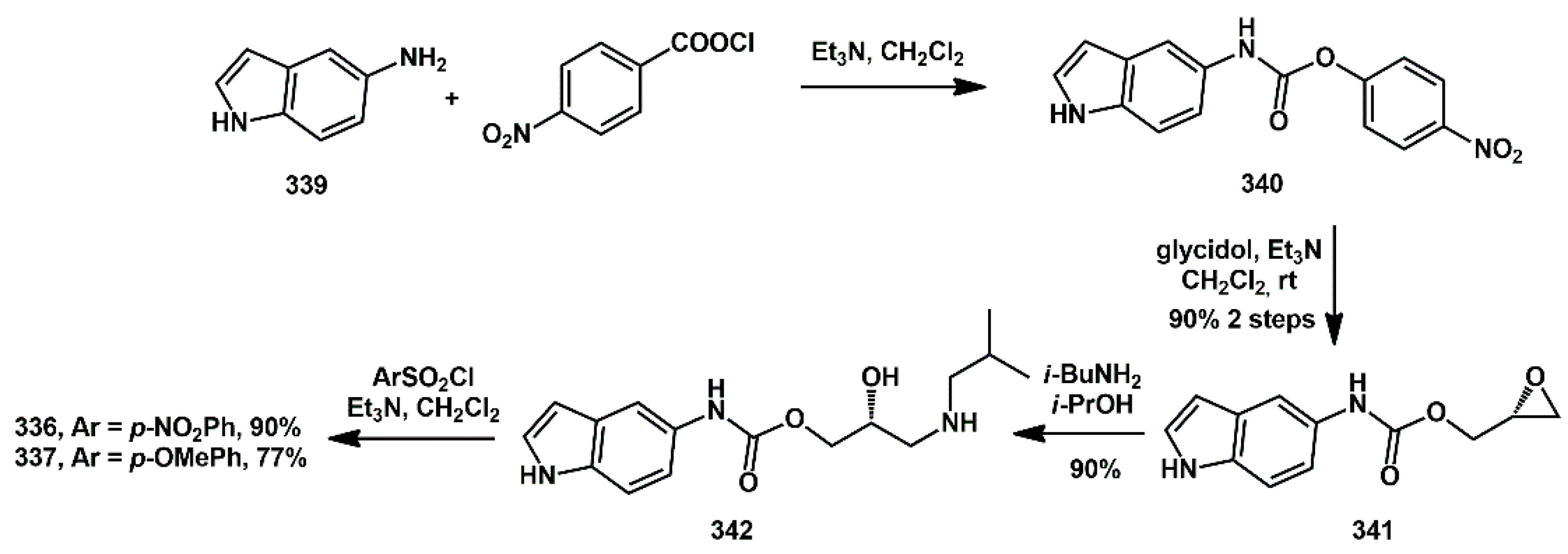
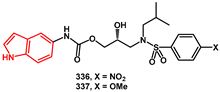
The preparation of the corresponding carbamoyl derivatives
336 and
337 was straightforward. 5-Aminoindole
339 was first reacted with
p-nitrophenylchlorocarbonate to afford the activated carbamate
340 (
Scheme 66). Glycidol was then introduced via a substitution reaction, yielding the oxiranyl carbamate
341 in good yield. The introduction of
i-BuNH
2, followed by sulfonylation with a suitable ArSO
2Cl, furnished the final compounds
336 and
337.
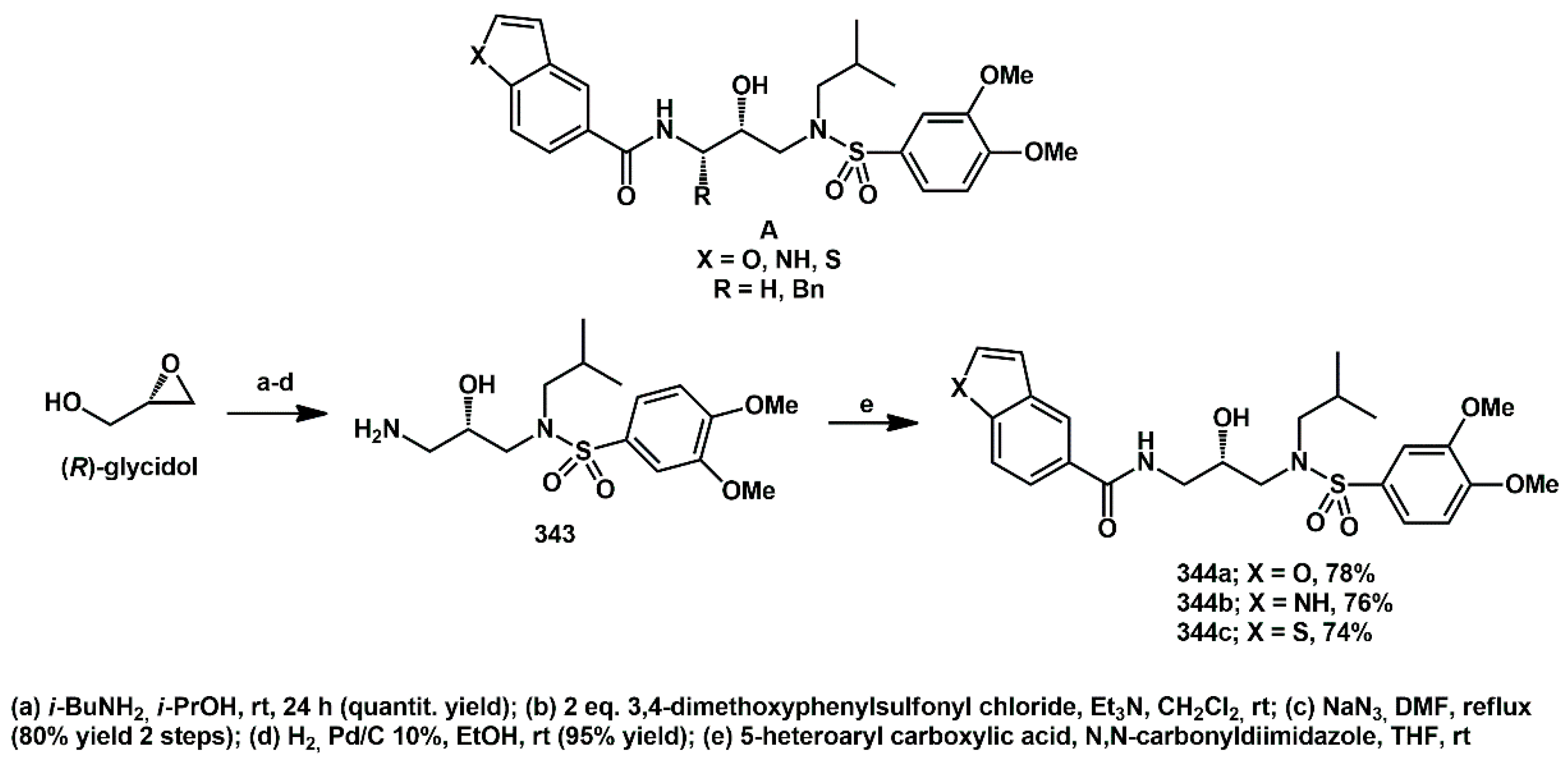
Different spacers were studied, connecting heteroaryl moieties to the hydroxyethylamine core. Thus, in 2017, new heteroaryl HIV protease inhibitors, bearing a carboxy–amide spacer, were synthesized in our lab in a few steps and with high yield, starting from commercially available homochiral epoxides [
91]. Onto a given hydroxyethyl-amino-isopropanoyl-sulfonamide core, we introduced different heteroarenes and modified the central core, with the presence of either H or a benzyl group (structure
A in
Scheme 67). For the synthesis of the simple, unsubstituted isopropanolamine core (R = H), we took advantage of an established route, starting from the commercially available bidentate electrophile (S)-glycidol. The epoxide was opened by
i-PrNH
2; the aryl sulfonyl moiety was introduced, and the primary OH group was substituted by NH
2, furnishing the key aminoalcohol
343 in good overall yield. Coupling with suitable 5-heteroaryl acids afforded the corresponding amides
344.
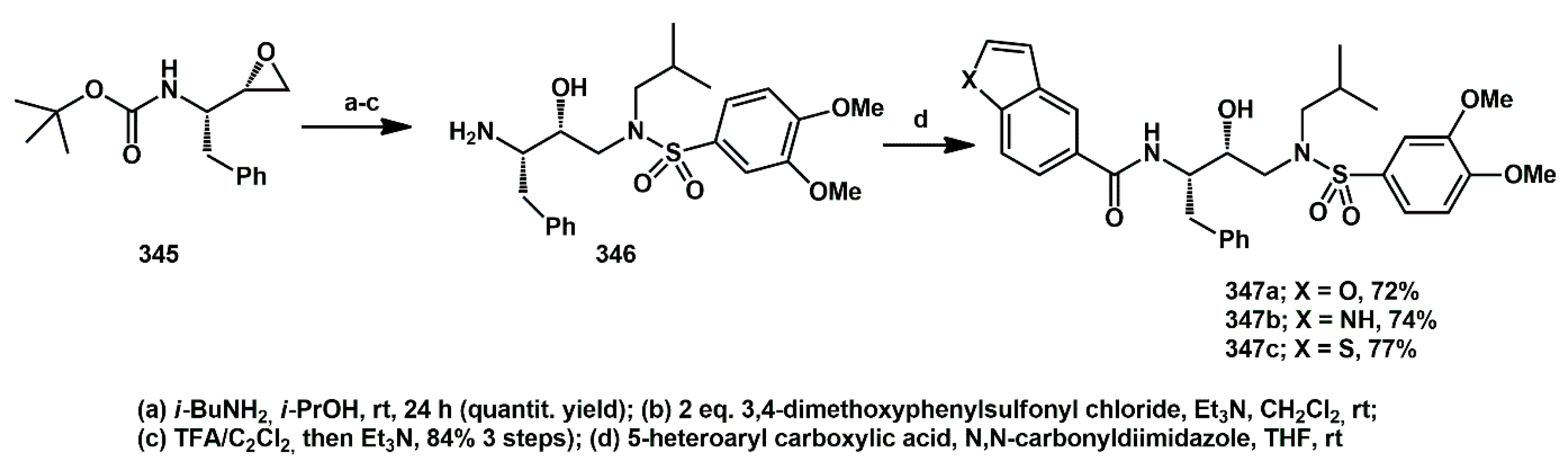
The synthesis of benzyl derivatives
347 was even shorter (
Scheme 68) and started from the commercially available homochiral
N-Boc-protected amino-epoxide
345. After opening with
iBuNH
2 and the subsequent introduction of the arylsulfonyl moiety, the
N-Boc group was displaced by treatment with trifluoroacetic acid in dichloromethane. The resulting ammonium trifluoroacetate was treated with NEt
3, affording amine
346, which was then reacted with suitable 5-heteroarylcarboxylic acids previously activated with
N,
N′-carbonyldiimidazole. Thus, the final products
347 were obtained in four steps and excellent overall yield.
In general, the presence of a carboxyamide moiety showed a positive effect on in vitro inhibition activity against recombinant protease. The IC50 values ranged between 1 and 15 nM. In particular, benzofuryl derivatives 344a and 347a displayed some of the best IC50 values among such structurally simple inhibitors. Docking analysis supported the experimental results regarding activity, demonstrating that these benzofuryl derivatives exhibited a favorable number of interactions within the active site. The inhibitory activity of these molecules was also evaluated in HEK293 cells.
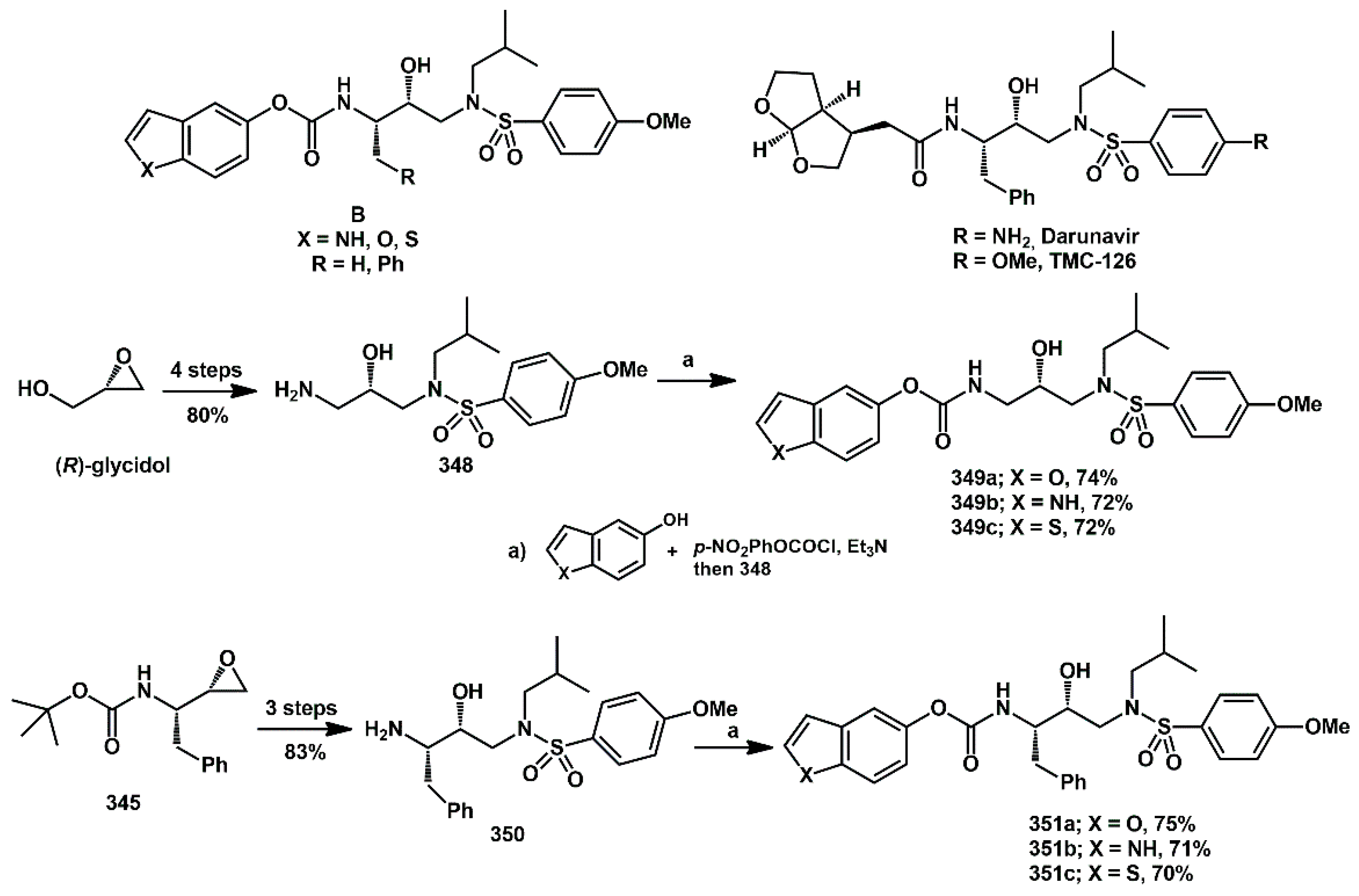
The study of new heteroaryl HIV protease inhibitors was extended to those bearing a carbamoyl spacer [
92]. In particular, we focused on new derivatives with the general structure
B (
Scheme 69), in which the heterocycle is spaced from the core by a carbamoyl function, like the arrangement in darunavir and TMC-126. Their synthesis was straightforward from commercially available homochiral epoxides. Different substitution patterns were introduced onto a given isopropanoyl-sulfonamide
core, which could have either H or benzyl group.
Both the carbamoyl moiety and benzyl group displayed a general beneficial effect on the in vitro inhibition activity against recombinant protease. The IC
50 values ranged from 11 to 0.6 nM. In particular, benzofuryl and indolyl derivatives
351a and
351c showed some of the best IC
50 values (IC
50 0.6 nM for both). Regarding the amide inhibitors, their activity was also confirmed in HEK293 mammalian cells and was maintained against protease mutants. Furthermore, the metabolic stability of all compounds was studied and found to be comparable to that of commercially available inhibitors. More recently, following the concept of repositioning HIV protease inhibitors as cancer therapeutics, compound
349b was also evaluated for its ability to induce cytotoxicity in hepatocellular carcinoma cell lines [
93].
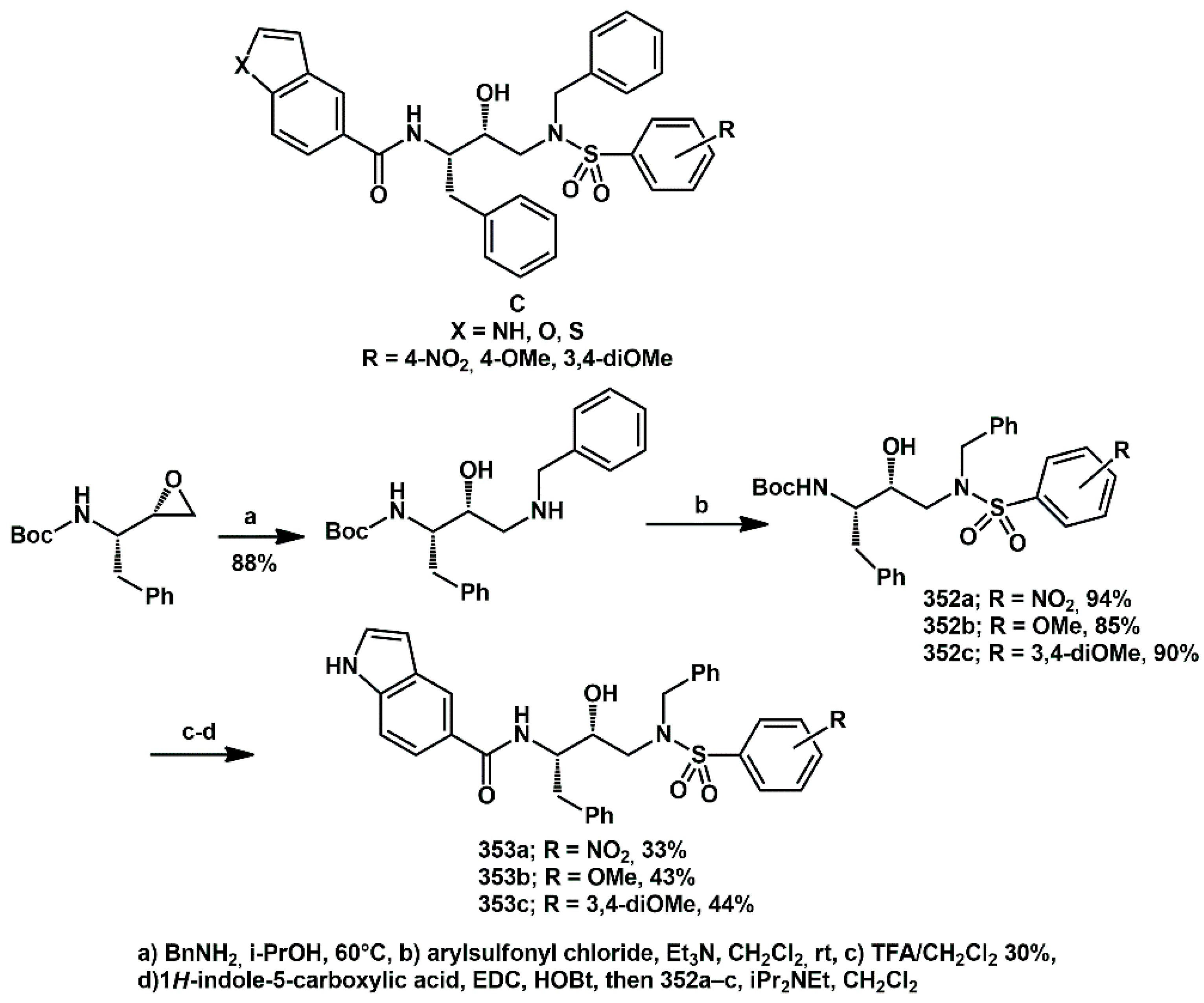
Considering the structure of HIV protease as a C2-symmetric homodimer in its active form, we recently reported on the synthesis, enzyme inhibition, and structure–activity relationship of a new class of HIV-1 protease inhibitors containing a pseudo-symmetric hydroxyethylamine core and heteroarylcarboxyamide moieties [
94]. To obtain a pseudo-symmetric hydroxyethylamine core, a benzyl group was placed on the sulfonamide nitrogen, resulting in the general structure
C (
Scheme 70). A straightforward synthetic pathway yielded nine compounds in a few steps with high yields. Potent inhibitory activity, with nanomolar IC50 values measured with a standard fluorimetric test, was achieved. In particular, compounds
353a–
c (whose synthesis is described in
Scheme 70), which contain the indole ring in P1, exhibited HIV-1 protease inhibitory activity that was more potent than darunavir in the same assay.
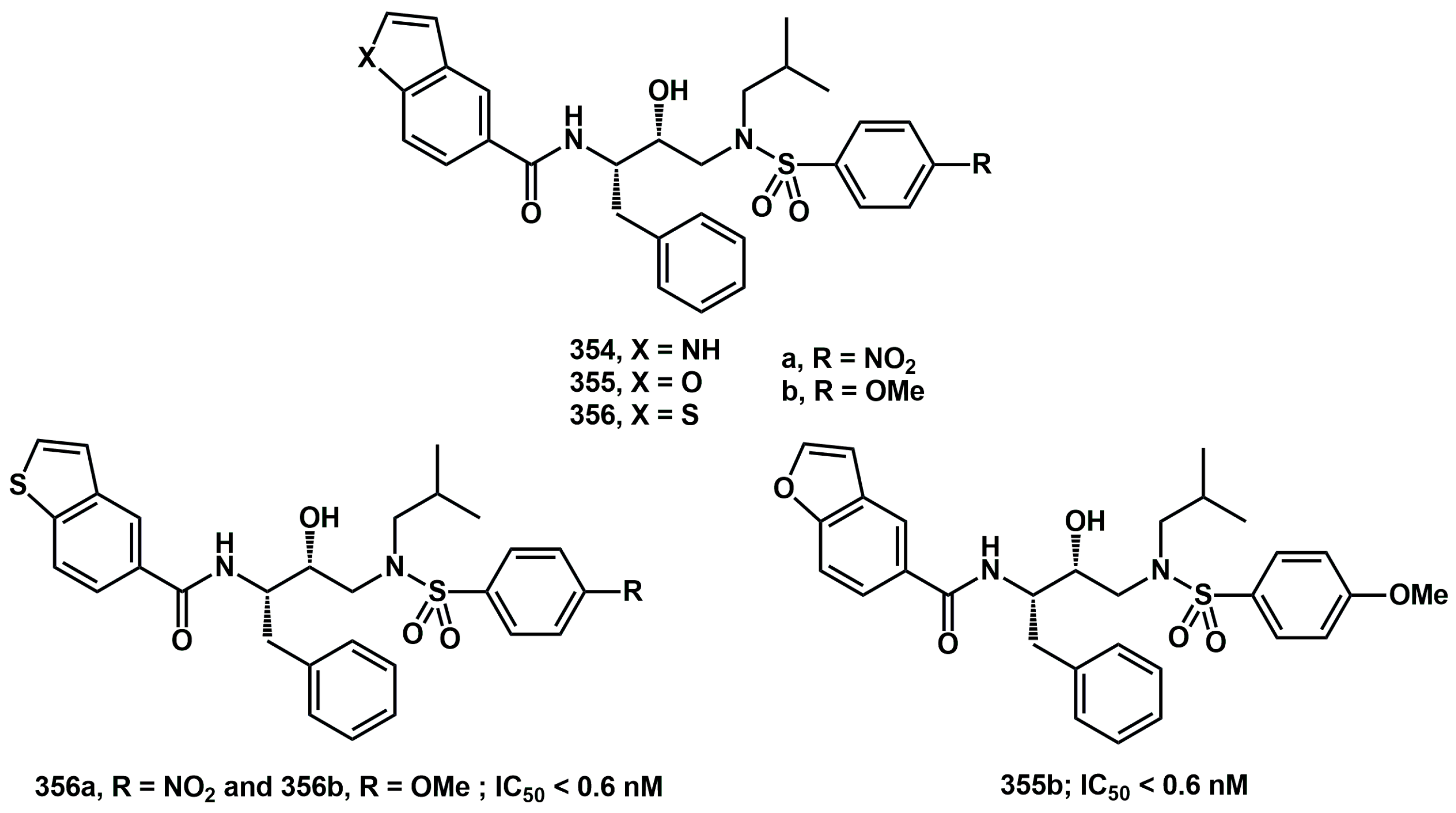
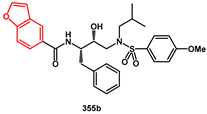
Recently, considering all our data on heteroaryl non-peptidic inhibitors, specifically their high HIV protease inhibitory activity and easy of synthetic approach, we reported novel simple heteroaryl carboxamides (
Scheme 71), bearing
p-NO
2 electron withdrawing group or
p-OMe electron releasing group on arylsulfonamide. We compared their in vitro activity in HEK293 cells with that of our previously described compounds [
95].
Benzofuryl-, benzothienyl-, and indolyl rings were introduced via efficient synthetic procedures. All compounds showed inhibitory activity comparable to the commercial drug darunavir, with particularly potent examples such as 356a, 356b, and 355b (IC50 < 0.6 nM). These compounds were effective against both wild-type HIV-1 protease and mutants containing V32I or V82A mutations. In silico evaluation of their absorption, distribution, metabolism, and excretion (ADME) properties was also conducted, comparing the results with the predicted properties of darunavir. As a result, 12 out 27 compounds (including 355b) performed better than or equal to darunavir across all ADME prediction models, demonstrating the potential of these compounds for further drug development.