Indole–Pyrazole Hybrids: Synthesis, Structure, and Assessment of Their Hemolytic and Cytoprotective Properties
Abstract
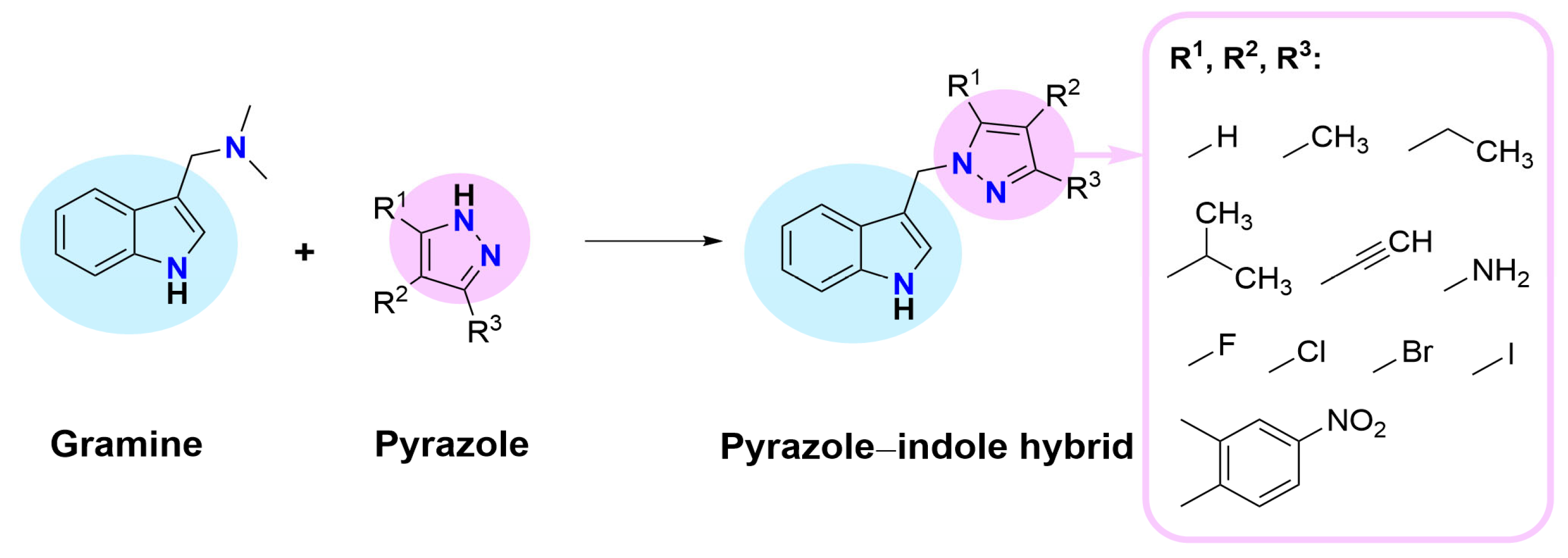
1. Introduction
2. Results and Discussion
2.1. Synthesis and Spectroscopic Characterization of Indole Hybrids
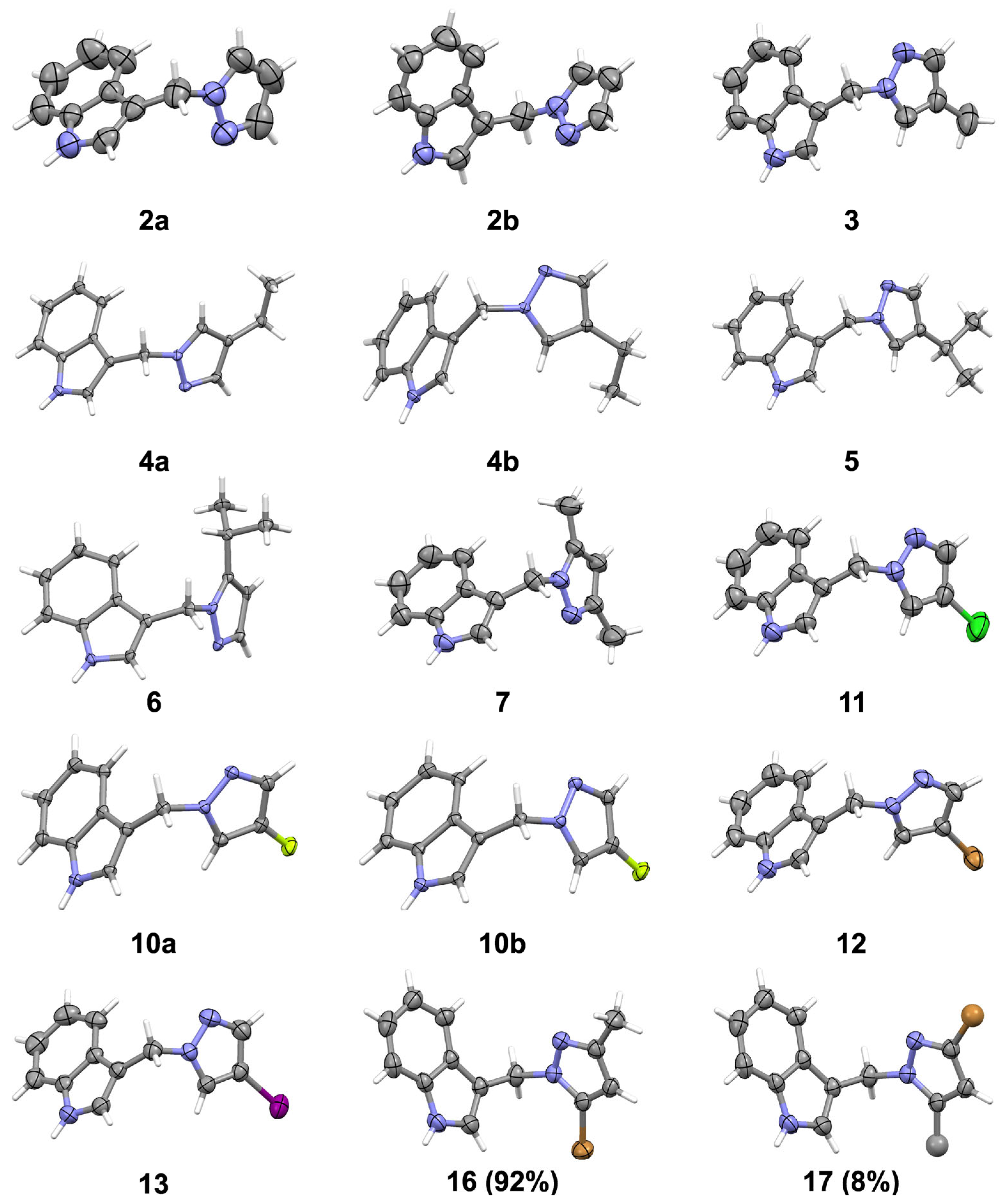
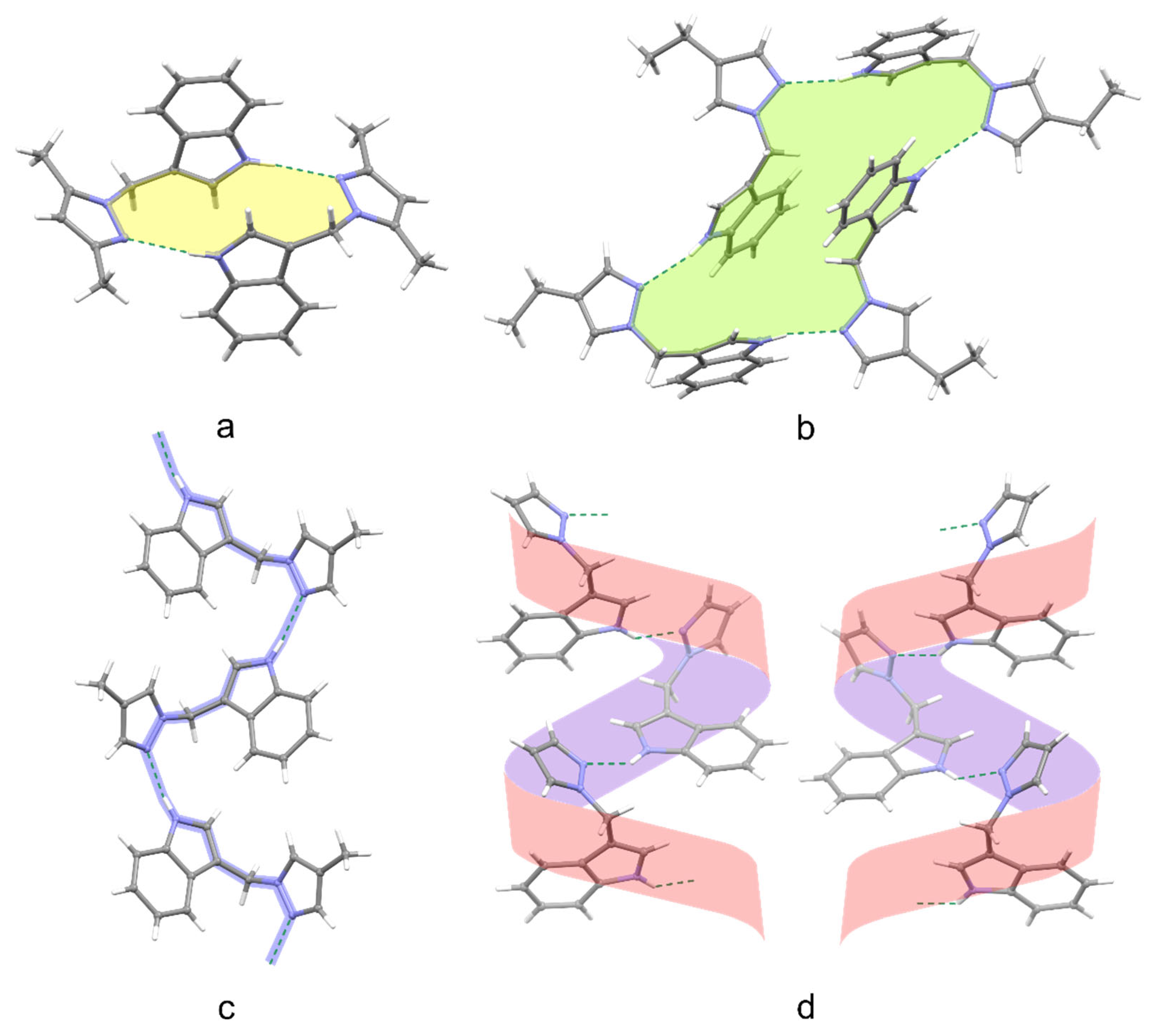
2.2. X-Ray Analysis
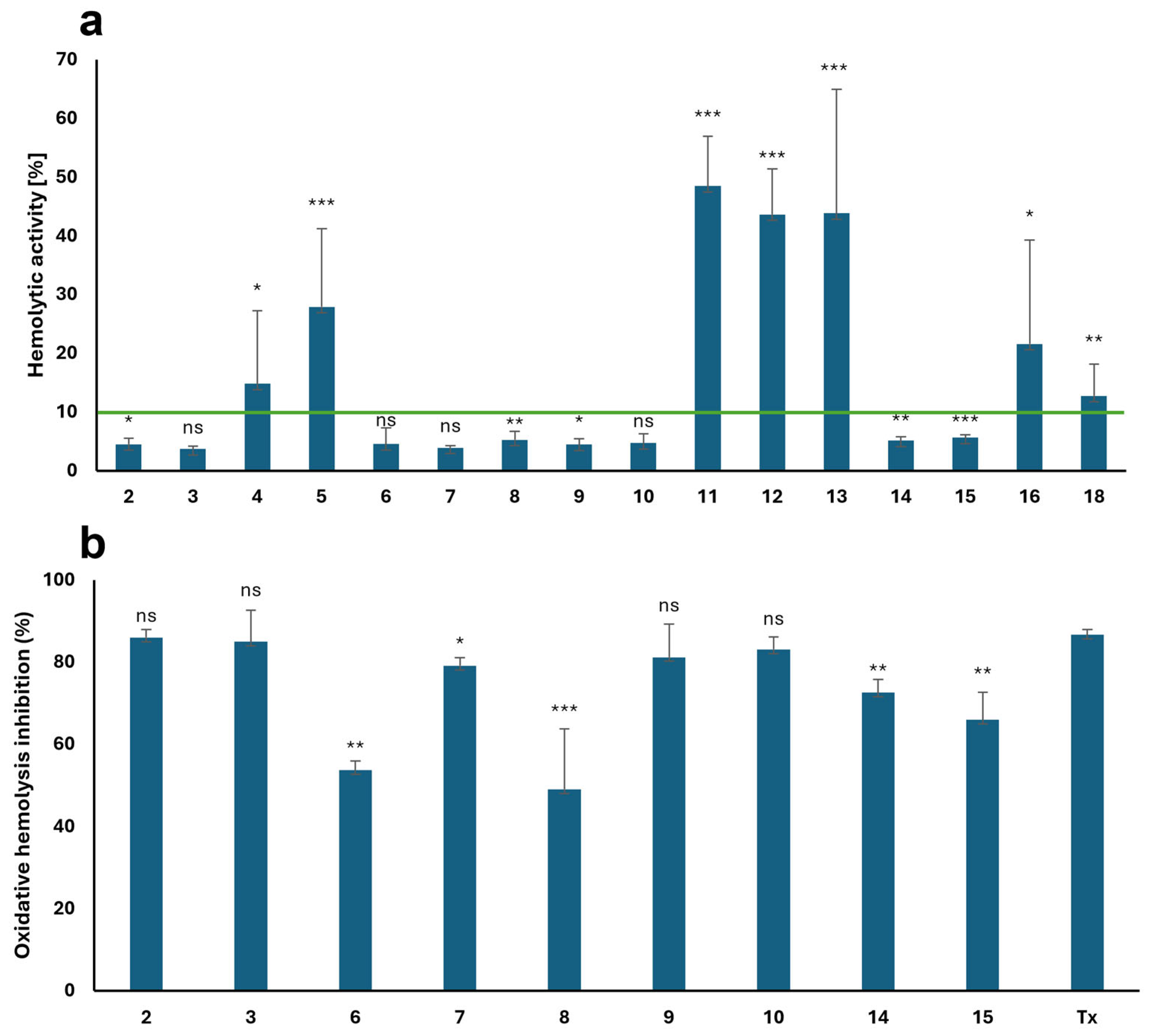
2.3. Hemolytic Properties and Cytoprotective Activity Against Free Radical-Induced Hemolysis
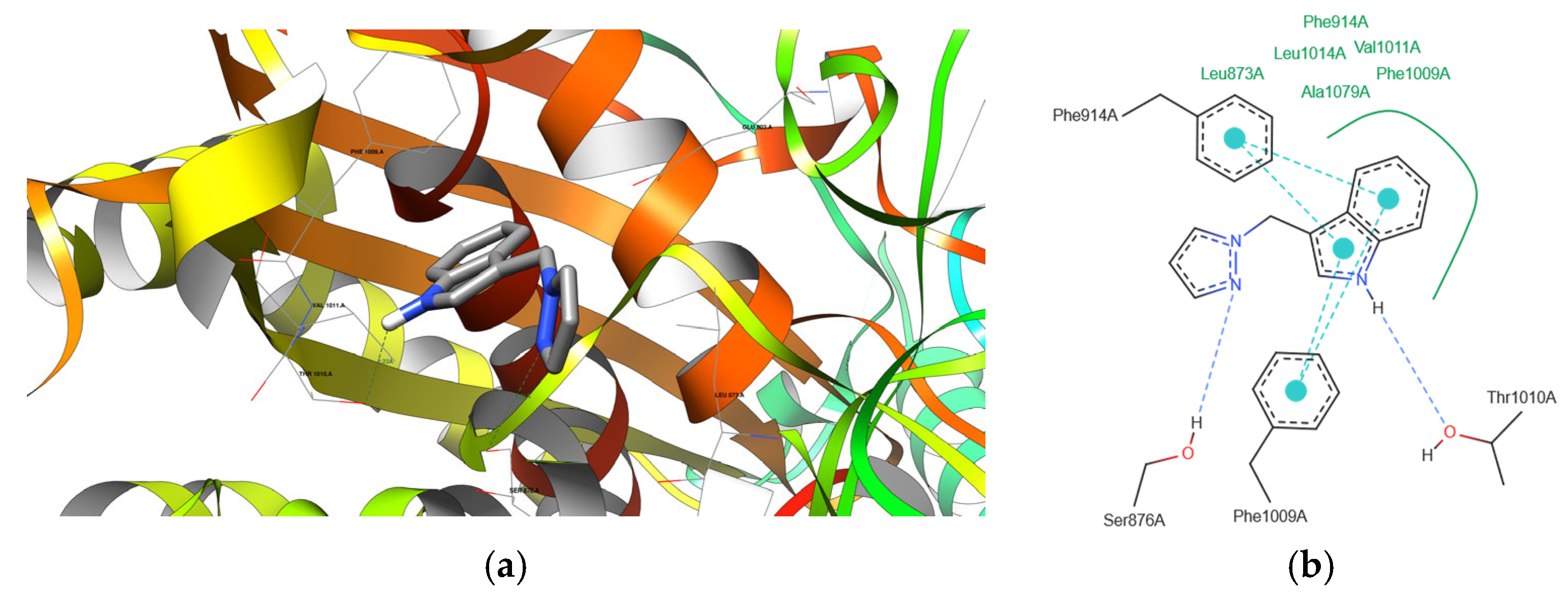
2.4. Molecular Docking
- Similarities and Differences between Novel and Endogenous Ligands
2.5. Physicochemical Properties of Indole Derivatives—In Silico Study
3. Materials and Methods
3.1. Instrumentation and Chemicals
3.2. Synthesis of Indole Derivatives
- The general synthesis procedure for 2-16
- 3-((1H-pyrazol-1-yl)methyl)-1H-indole (2)
- 3-((4-methyl-1H-pyrazol-1-yl)methyl)-1H-indole (3)
- 3-((4-ethyl-1H-pyrazol-1-yl)methyl)-1H-indole (4)
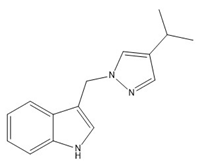
- 3-((4-isopropyl-1H-pyrazol-1-yl)methyl)-1H-indole (5)
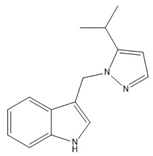
- 3-((5-isopropyl-1H-pyrazol-1-yl)methyl)-1H-indole (6)
- 3-((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-1H-indole (7)
- 3-((4-ethynyl-1H-pyrazol-1-yl)methyl)-1H-indole (8)
- 3-((5-methoxy-1H-pyrazol-1-yl)methyl)-1H-indole (9)
- 3-((4-fluoro-1H-pyrazol-1-yl)methyl)-1H-indole (10)
- 3-((4-chloro-1H-pyrazol-1-yl)methyl)-1H-indole (11)
- 3-((4-bromo-1H-pyrazol-1-yl)methyl)-1H-indole (12)
- 3-((4-iodo-1H-pyrazol-1-yl)methyl)-1H-indole (13)
- 3-((4-bromo-5-methyl-1H-pyrazol-1-yl)methyl)-1H-indole (14)
- 3-((4-bromo-3-methyl-1H-pyrazol-1-yl)methyl)-1H-indole (15)
- 3-((3-bromo-5-methyl-1H-pyrazol-1-yl)methyl)-1H-indole (16)
- Synthesis of 18:
- 1-((1H-indol-3-yl)methyl)-5-nitro-1H-indazole (18)
3.3. X-Ray Data Collection and Refinement of the Structures
3.4. Biological Study
3.4.1. Human Red Blood Cells Preparation
3.4.2. Hemolytic Assay
3.4.3. Inhibition of Oxidative Stress-Induced Hemolysis
3.4.4. Statistical Analysis
3.5. Molecular Docking—Experimental
3.6. In Silico Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Assress, H.A.; Selvarajan, R.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Antifungal azoles and azole resistance in the environment: Current status and future perspectives—A review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 1011–1041. [Google Scholar] [CrossRef]
- Devasia, J.; Nizam, A.; Vasantha, V.L. Azole-Based Antibacterial Agents: A Review on Multistep Synthesis Strategies and Biology. Polycycl. Aromat. Compd. 2021, 42, 5474–5495. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Zacconi, F.; Gupta, G.; Aljabali, A.A.; Mishra, V.; Tambuwala, M.M.; Kapoor, D.N.; Negi, P.; Pinto, T.D.J.A.; et al. Synthesis and Anticancer Properties of ‘Azole’ Based Chemotherapeutics as Emerging Chemical Moieties: A Comprehensive Review. Curr. Org. Chem. 2021, 25, 654–668. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M. ‘Azole’ as privileged heterocycle for targeting the inducible cyclooxygenase enzyme. Drug Dev. Res. 2021, 82, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Curutchet, C.; Poater, J.; Solà, M.; Elguero, J. Analysis of the Effects of N-Substituents on Some Aspects of the Aromaticity of Imidazoles and Pyrazoles. J. Phys. Chem. A 2011, 115, 8571–8577. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Portilla, J. Recent Advances in the Synthesis of New Pyrazole Derivatives. Targets Heterocycl. Syst. 2018, 22, 194–223. [Google Scholar] [CrossRef]
- Mortada, S.; Karrouchi, K.; Hamza, E.H.; Oulmidi, A.; Bhat, M.A.; Mamad, H.; Aalilou, Y.; Radi, S.; Ansar, M.; Masrar, A.; et al. Synthesis, structural characterizations, in vitro biological evaluation, and computational investigations of pyrazole derivatives as potential antidiabetic and antioxidant agents. Sci. Rep. 2024, 14, 1312. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Abbes Fauzi, M.E. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef]
- Costa, R.F.; Turones, L.C.; Cavalcante, K.V.N.; Rosa Júnior, I.A.; Xavier, C.H.; Rosseto, L.P.; Napolitano, H.B.; Castro, P.F.d.S.; Neto, M.L.F.; Galvão, G.M.; et al. Heterocyclic Compounds: Pharmacology of Pyrazole Analogs From Rational Structural Considerations. Front. Pharmacol. 2021, 12, 666725. [Google Scholar] [CrossRef]
- Suliphuldevara Mathada, B.; Gunavanthrao Yernale, N.; Basha, J.N. The Multi-Pharmacological Targeted Role of Indole and Its Derivatives: A Review. ChemistrySelect 2023, 8, e202204181. [Google Scholar] [CrossRef]
- Umer, S.M.; Solangi, M.; Khan, K.M.; Saleem, R.S.Z. Indole-Containing Natural Products 2019–2022: Isolations, Reappraisals, Syntheses, and Biological Activities. Molecules 2022, 27, 7586. [Google Scholar] [CrossRef]
- Nisha; Singh, S.; Sharma, N.; Chandra, R. The Indole Nucleus as a Selective COX-2 Inhibitor and Anti-Inflammatory Agent (2011–2022). Org. Chem. Front. 2022, 9, 3624–3639. [Google Scholar] [CrossRef]
- Lima, E.; Medeiros, J. Marine Organisms as Alkaloid Biosynthesizers of Potential Anti-Alzheimer Agents. Mar. Drugs 2022, 20, 75. [Google Scholar] [CrossRef]
- Chiu, Y.-J.; Lin, C.-H.; Lin, C.-Y.; Yang, P.-N.; Lo, Y.-S.; Chen, Y.-C.; Chen, C.-M.; Wu, Y.-R.; Yao, C.-F.; Chang, K.-H.; et al. Investigating Therapeutic Effects of Indole Derivatives Targeting Inflammation and Oxidative Stress in Neurotoxin-Induced Cell and Mouse Models of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 2642. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Luo, L.; Gao, Y.; Bao, H.; Li, P.; Zhang, H. Research Progress of Indole Compounds with Potential Antidiabetic Activity. Eur. J. Med. Chem. 2021, 223, 113665. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, C.; Zhu, J.; Li, E.; Xu, Z. Therapeutic Potential of Indole Derivatives as Anti-HIV Agents: A Mini-Review. Curr. Top. Med. Chem. 2022, 22, 993–1008. [Google Scholar] [CrossRef]
- Girgis, A.S.; Panda, S.S.; Kariuki, B.M.; Bekheit, M.S.; Barghash, R.F.; Aboshouk, D.R. Indole-Based Compounds as Potential Drug Candidates for SARS-CoV-2. Molecules 2023, 28, 6603. [Google Scholar] [CrossRef]
- Fabitha, K.; Chandrakanth, M.; Pramod, R.N.; Arya, C.G.; Li, Y.; Banothu, J. Recent Developments in the Synthesis of Indole-Pyrazole Hybrids. ChemistrySelect 2022, 7, e202201064. [Google Scholar] [CrossRef]
- Khatab, T.K.; Hassan, A.S. Computational molecular docking and in silico ADMET prediction studies of pyrazole derivatives as Covid-19 main protease (MPRO) and papain-like protease (PLPRO) inhibitors. Bull. Chem. Soc. Ethiop. 2023, 37, 449–461. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Ibrahim, N.S.; Saddiq, A.A.; Abdelhamid, I.A. Novel 3-(pyrazol-4-yl)-2-(1H-indole-3-carbonyl)acrylonitrile derivatives induce intrinsic and extrinsic apoptotic death mediated P53 in HCT116 colon carcinoma. Sci. Rep. 2023, 13, 22486. [Google Scholar] [CrossRef]
- Hassan, A.S.; Moustafa, G.O.; Awad, H.M.; Nossier, E.S.; Mady, M.F. Design, Synthesis, Anticancer Evaluation, Enzymatic Assays, and a Molecular Modeling Study of Novel Pyrazole–Indole Hybrids. ACS Omega 2021, 6, 12361–12374. [Google Scholar] [CrossRef]
- El-Mekabaty, A.; Etman, H.A.; Mosbah, A. Synthesis of Some New Fused Pyrazole Derivatives Bearing Indole Moiety as Antioxidant Agents. J. Heterocycl. Chem. 2016, 53, 894–900. [Google Scholar] [CrossRef]
- Kerzare, D.R.; Menghani, S.S.; Rarokar, N.R.; Khedekar, P.B. Development of novel indole-linked pyrazoles as anticonvulsant agents: A molecular hybridization approach. Arch. Der Pharm. 2021, 354, 2000100. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Babijczuk, K.; Warżajtis, B.; Rychlewska, U.; Starzyk, J.; Cofta, G.; Mrówczyńska, L. Indole Derivatives Bearing Imidazole, Benzothiazole-2-Thione or Benzoxazole-2-Thione Moieties—Synthesis, Structure and Evaluation of Their Cytoprotective, Antioxidant, Antibacterial and Fungicidal Activities. Molecules 2023, 28, 708. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Kozanecka-Okupnik, W.; Przygodzki, M.; Warżajtis, B.; Rychlewska, U.; Pospieszny, T.; Mrówczyńska, L. Synthesis, Antioxidant and Cytoprotective Activity Evaluation of C-3 Substituted Indole Derivatives. Sci. Rep. 2021, 11, 15425. [Google Scholar] [CrossRef] [PubMed]
- Babijczuk, K.; Berdzik, N.; Nowak, D.; Warżajtis, B.; Rychlewska, U.; Starzyk, J.; Mrówczyńska, L.; Jasiewicz, B. Novel C3-Methylene-Bridged Indole Derivatives with and without Substituents at N1: The Influence of Substituents on Their Hemolytic, Cytoprotective, and Antimicrobial Activity. Int. J. Mol. Sci. 2024, 25, 5364. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Kobayashi, S.; Warang, P.; Madkaikar, M.; Mukherjee, M.B. Erythrocytes as a Preferential Target of Oxidative Stress in Blood. Free Radic. Res. 2021, 55, 781–799. [Google Scholar] [CrossRef]
- Kwiatek, D.; Mrówczyńska, L.; Stopikowska, N.; Runowski, M.; Lesicki, A.; Lis, S. Surface Modification of Luminescent LnIII Fluoride Core-Shell Nanoparticles with Acetylsalicylic acid (Aspirin): Synthesis, Spectroscopic and in vitro Hemocompatibility Studies. ChemMedChem 2020, 15, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Malczewska-Jaskóła, K.; Jasiewicz, B.; Mrówczyńska, L. Nicotine alkaloids as antioxidant and potential protective agents against in vitro oxidative haemolysis. Chem.-Biol. Interact. 2016, 243, 62–71. [Google Scholar] [CrossRef]
- Kargapolova, Y.; Geissen, S.; Zheng, R.; Baldus, S.; Winkels, H.; Adam, M. The Enzymatic and Non-Enzymatic Function of Myeloperoxidase (MPO) in Inflammatory Communication. Antioxidants 2021, 10, 562. [Google Scholar] [CrossRef]
- Berry, C.E.; Hare, J.M. Xanthine Oxidoreductase and Cardiovascular Disease: Molecular Mechanisms and Pathophysiological Implications. J. Physiol. 2004, 555, 589–606. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Demartin, F.; Palmisano, G.; Penoni, A.; Radice, T.; Tollari, S. Novel cyclometallated Pd(II) and Pt(II) complexes with indole derivatives and their use as catalysts in Heck reaction. J. Organomet. Chem. 2005, 690, 2017–2026. [Google Scholar] [CrossRef]
- Ballesteros, P.; Claramunt, R.M.; López, M.C.; Elguero, J.; Gómez-Alarcón, G. Synthesis and antifungal properties of some N,N’-bis-azolylarylmethanes. Chem. Pharm. Bull. 1988, 36, 2036–2041. [Google Scholar] [CrossRef]
- Secrieru, A.; O’Neill, P.M.; Cristiano, M.L.S. Revisiting the Structure and Chemistry of 3(5)-Substituted Pyrazoles. Molecules 2020, 25, 42. [Google Scholar] [CrossRef] [PubMed]
- Palomba, M.; Dias, I.F.C.; Cocchioni, M.; Marini, F.; Santi, C.; Bagnoli, L. Vinylation of N-Heteroarenes through Addition/Elimination Reactions of Vinyl Selenones. Molecules 2023, 28, 6026. [Google Scholar] [CrossRef]
- Haydl, A.M.; Xu, K.; Breit, B. Regio- and Enantioselective Synthesis of N-Substituted Pyrazoles by Rhodium-Catalyzed Asymmetric Addition to Allenes. Angew. Chem. Int. Ed. 2015, 54, 7149–7153. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Foces-Foces, C.; Infantes, L. Classification of hydrogen-bond motives in crystals of NH-pyrazoles: A mixed empirical and theoretical approach. Arkivoc 2006, 2, 15–30. [Google Scholar] [CrossRef]
- Nath, N.K.; Nangia, A. Isomorphous Crystals by Chloro–Methyl Exchange in Polymorphic Fuchsones. Cryst. Growth Des. 2012, 12, 5411–5425. [Google Scholar] [CrossRef]
- Vo, Q.V.; Mechler, A. In Silico Study of the Radical Scavenging Activities of Natural Indole-3-Carbinols. J. Chem. Inf. Model. 2020, 60, 316–321. [Google Scholar] [CrossRef]
- Silveira, C.C.; Mendes, S.R.; Soares, J.S.; Victoria, F.N.; Martinez, D.M.; Savegnago, L. Synthesis and antioxidant activity of new C-3 sulfenyl indoles. Tetrahedron Lett. 2013, 54, 4926–4929. [Google Scholar] [CrossRef]
- Estevão, M.S.; Carvalho, L.C.; Ferreira, L.M.; Fernandes, E.; Marques, M.M.B. Analysis of the antioxidant activity of an indole library: Cyclic voltammetry versus ROS scavenging activity. Tetrahedron Lett. 2011, 52, 101–106. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragmental Methods: An Analysis of ALOGP and CLOGP Methods. J. Phys. Chem. A 1998, 102, 3762–3772. [Google Scholar] [CrossRef]
- Schöning-Stierand, K.; Diedrich, K.; Ehrt, C.h.; Flachsenberg, F.; Graef, J.; Sieg, J.; Penner, P.; Poppinga, M.; Ungethum, A.; Rarey, M. ProteinsPlus: A comprehensive collection of web-based molecular modeling tools. Nucleic Acids Res. 2022, 50, W611–W615. [Google Scholar] [CrossRef]
- Stierand, K.; Maaß, P.C.; Rarey, M. Molecular Complexes at a Glance: Automated Generation of Two-Dimensional Complex Diagrams. Bioinformatics 2006, 22, 1710–1716. [Google Scholar] [CrossRef]
- Diedrich, K.; Krause, B.; Berg, O.; Rarey, M. PoseEdit: Enhanced Ligand Binding Mode Communication by Interactive 2D Diagrams. J. Comput. Aided Mol. Des. 2023, 37, 491–503. [Google Scholar] [CrossRef]
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef] [PubMed]
- Herschlag, D.; Pinney, M.M. Hydrogen Bonds: Simple after All? Biochemistry 2018, 57, 3338–3352. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-Five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- USA SAINT, version 8.40B. Area Detector Control and Integration Software. Bruker AXS Inc.: Madison, WI, USA, 2021.
- Rigaku, O.D. CrysAlis PRO, version 1.171.43; Rigaku Oxford Diffraction: Yarnton, UK, 2024.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Mrówczyńska, L.; Hägerstrand, H. Platelet-activating factor interaction with the human erythrocyte membrane. J. Biochem. Mol. Toxicol. 2009, 23, 345–348. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Morley, C.; Hutchison, G.R. Pybel: A Python wrapper for the OpenBabel cheminformatics toolkit. Chem. Cent. J. 2008, 2, 5. [Google Scholar] [CrossRef]
- Raschka, S. BioPandas: Working with molecular structures in pandas DataFrames. J. Open Source Softw. 2017, 2, 279. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Blair-Johnson, M.; Fiedler, T.; Fenna, R. Human Myeloperoxidase: Structure of a Cyanide Complex and Its Interaction with Bromide and Thiocyanate Substrates at 1.9 Å Resolution. Biochemistry 2001, 40, 13990–13997. [Google Scholar] [CrossRef]
- Blair-Johnson, M.; Fiedler, T.; Fenna, R. Structural analyses of human myeloperoxidase-thiocyanate complex. Biochemistry 2001, 40, 13990–13997. [Google Scholar] [CrossRef]
- Okamoto, K.; Eger, B.T.; Nishino, T.; Kondo, S.; Pai, E.F.; Nishino, T. An Extremely Potent Inhibitor of Xanthine Oxidoreductase: Crystal structure of the enzyme-inhibitor complex and mechanism of inhibition. J. Biol. Chem. 2003, 278, 1848–1855. [Google Scholar] [CrossRef]
- Okamoto, K.; Eger, B.T.; Kondo, S.; Pai, E.F.; Nishino, T. Xanthine Dehydrogenase from Bovine Milk with Inhibitor TEI-6720 Bound. J. Biol. Chem. 2002, 278, 1848–1855. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Miyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1966, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Kurumbail, R.G.; Stallings, W.C. Cyclooxygenase-2 (prostaglandin synthase-2) complexed with a non-selective inhibitor, indomethacin. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- ProteinsPlus Development Team. ProteinsPlus. Available online: https://proteins.plus (accessed on 26 November 2024).


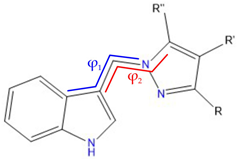 | |||
| Chemical formula | φ1 | φ2 | |
 2 | −53.5(4) 56.5(4) | 124.5(3) −124.0(3) |   |
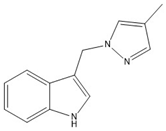 3 | −75.46(18) | −55.5(2) |  |
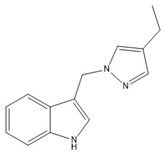 4 | −67.77(13) −77.64(13) | 121.04(11) −43.77(14) |  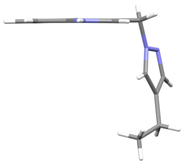 |
 5 | −74.5(3) | −60.3(3) | 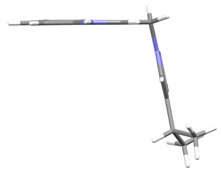 |
 6 | −68.4(6) | 112.6(5) |  |
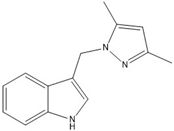 7 | −60.3(2) | 118.9(2) |  |
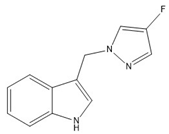 10 | −72.93(15) −70.32(14) | −41.00(16) −66.78(15) | 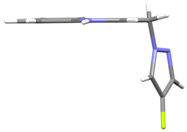  |
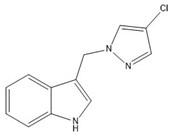 11 | −75.36(16) | −56.63(19) |  |
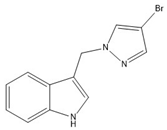 12 | −70.8(3) | −24.5(4) |  |
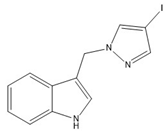 13 | −73.3(4) | −54.1(4) |  |
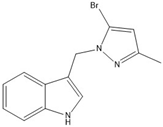 16 | 97.7(3) | 97.7(3) |  |
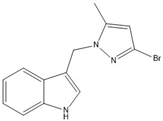 17 | 97.7(3) | 97.7(3) |  |
| Compound | MW [g/mol] | logP | HBD | HBA | RTB | TPSA [Å2] | GI Absorption | BBB Permeant | LogS | Solubility * |
|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin | 232.28 | 1.83 | 2 | 2 | 5 | 54.12 | High | Yes | −3.10 | Soluble |
| Febuxostan | 316.37 | 3.28 | 1 | 5 | 5 | 11.45 | High | No | −4.95 | Moderately |
| Indomethacin | 357.79 | 3.63 | 1 | 4 | 5 | 68.53 | High | Yes | −5.36 | Moderately |
| PDB ID | Compound | Average Binding Energy [kcal/mol] | Standard Deviation of Binding Energy [kcal/mol] | Ligand Efficiency [kcal/mol] |
|---|---|---|---|---|
| 1DNU | Melatonin | −5.5 | 0.09 | −0.32 |
| 2 | −5.6 | 0.15 | −0.37 | |
| 3 | −6.0 | 0.19 | −0.38 | |
| 10 | −5.8 | 0.18 | −0.36 | |
| 1N5X | Febuxostat | −7.7 | 0.55 | −0.35 |
| 2 | −8.6 | 0.76 | −0.57 | |
| 3 | −8.9 | 0.70 | −0.56 | |
| 10 | −8.8 | 0.70 | −0.55 | |
| 4COX | Indomethacin | −9.0 | 0.27 | −0.36 |
| 2 | −7.7 | 0.24 | −0.51 | |
| 3 | −8.4 | 0.19 | −0.53 | |
| 10 | −8.2 | 0.16 | −0.51 |
| Compound | Protein Domain | Amino Acid Residues | Distance [Å] | Is it a Compound Donor or Acceptor? |
|---|---|---|---|---|
| 2 | 1N5X | THR 1010 A SER 876 A | 2.23 3.21 | Donor Acceptor |
| 3 | 1N5X | THR 1010 A SER 876 A | 2.25 3.24 | Donor Acceptor |
| 10 | 1N5X | THR 1010 A SER 876 A | 2.18 3.30 | Donor Acceptor |
| 2 | 4COX | TYR 385 A | 2.53 | Donor |
| 10 | 4COX | TYR 385 A | 2.56 | Donor |
| Compound | MW [g/mol] | logP | HBD | HBA | RTB | TPSA [Å2] | GI Absorption | BBB Permeant | LogS | Solubility * |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 197.24 | 1.97 | 1 | 1 | 2 | 33.61 | High | Yes | −3.06 | Soluble |
| 3 | 211.26 | 2.34 | 1 | 1 | 2 | 33.61 | High | Yes | −3.47 | Soluble |
| 4 | 225.29 | 2.64 | 1 | 1 | 3 | 33.61 | High | Yes | −3.84 | Soluble |
| 5 | 239.32 | 2.95 | 1 | 1 | 3 | 33.61 | High | Yes | −4.05 | Moderately |
| 6 | 239.32 | 2.94 | 1 | 1 | 3 | 33.61 | High | Yes | −4.07 | Moderately |
| 7 | 225.29 | 2.67 | 1 | 1 | 2 | 33.61 | High | Yes | −3.85 | Soluble |
| 8 | 221.26 | 2.37 | 1 | 1 | 2 | 33.61 | High | Yes | −3.31 | Soluble |
| 9 | 227.26 | 2.14 | 1 | 1 | 3 | 42.84 | High | Yes | −3.41 | Soluble |
| 10 | 215.23 | 2.32 | 1 | 1 | 2 | 33.61 | High | Yes | −3.29 | Soluble |
| 11 | 231.68 | 2.57 | 1 | 1 | 2 | 33.61 | High | Yes | −3.73 | Soluble |
| 12 | 276.13 | 2.64 | 1 | 1 | 2 | 33.61 | High | Yes | −3.94 | Soluble |
| 13 | 323.13 | 2.67 | 1 | 1 | 2 | 33.61 | High | Yes | −4.03 | Moderately |
| 14 | 290.16 | 2.96 | 1 | 1 | 2 | 33.61 | High | Yes | −4.30 | Moderately |
| 15 | 290.16 | 2.99 | 1 | 1 | 2 | 33.61 | High | Yes | −4.30 | Moderately |
| 16 | 290.16 | 3.02 | 1 | 1 | 2 | 33.61 | High | Yes | −4.49 | Moderately |
| 17 | 290.16 | 3.02 | 1 | 1 | 2 | 33.61 | High | Yes | −4.49 | Moderately |
| 18 | 292.29 | 2.40 | 1 | 3 | 3 | 79.43 | High | No | −4.59 | Moderately |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babijczuk, K.; Wawrzyniak, K.; Warżajtis, B.; Rychlewska, U.; Nowak, D.; da Victoria Banda, Y.; Mrówczyńska, L.; Jasiewicz, B. Indole–Pyrazole Hybrids: Synthesis, Structure, and Assessment of Their Hemolytic and Cytoprotective Properties. Int. J. Mol. Sci. 2025, 26, 9018. https://doi.org/10.3390/ijms26189018
Babijczuk K, Wawrzyniak K, Warżajtis B, Rychlewska U, Nowak D, da Victoria Banda Y, Mrówczyńska L, Jasiewicz B. Indole–Pyrazole Hybrids: Synthesis, Structure, and Assessment of Their Hemolytic and Cytoprotective Properties. International Journal of Molecular Sciences. 2025; 26(18):9018. https://doi.org/10.3390/ijms26189018
Chicago/Turabian StyleBabijczuk, Karolina, Klaudia Wawrzyniak, Beata Warżajtis, Urszula Rychlewska, Damian Nowak, Yunna da Victoria Banda, Lucyna Mrówczyńska, and Beata Jasiewicz. 2025. "Indole–Pyrazole Hybrids: Synthesis, Structure, and Assessment of Their Hemolytic and Cytoprotective Properties" International Journal of Molecular Sciences 26, no. 18: 9018. https://doi.org/10.3390/ijms26189018
APA StyleBabijczuk, K., Wawrzyniak, K., Warżajtis, B., Rychlewska, U., Nowak, D., da Victoria Banda, Y., Mrówczyńska, L., & Jasiewicz, B. (2025). Indole–Pyrazole Hybrids: Synthesis, Structure, and Assessment of Their Hemolytic and Cytoprotective Properties. International Journal of Molecular Sciences, 26(18), 9018. https://doi.org/10.3390/ijms26189018








