Circulating Plasma Syncytin-1 mRNA in Preeclampsia—A Pilot Study
Abstract
1. Introduction
2. Results
2.1. Study Population
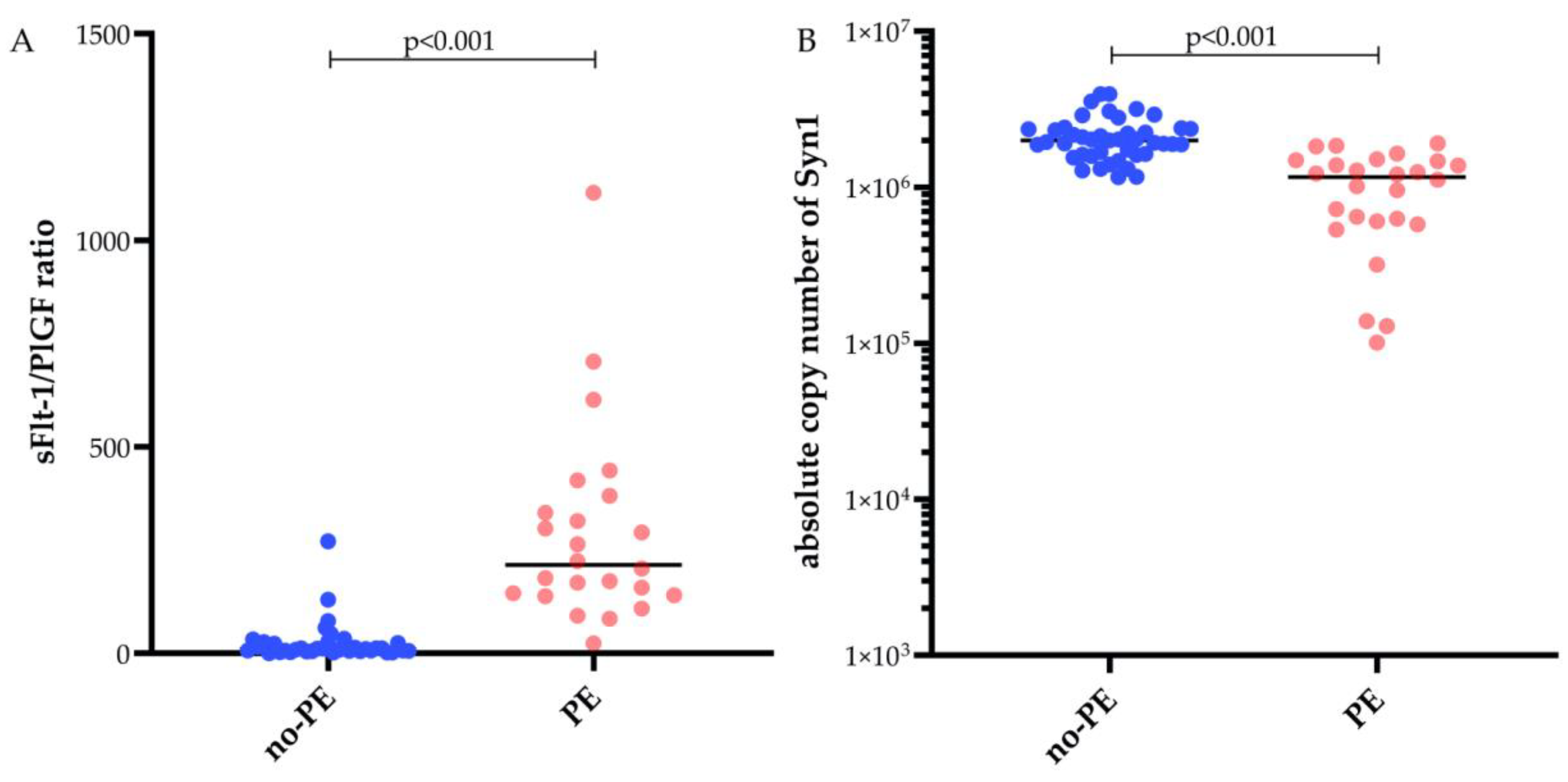
2.2. Fetal Syncytin-1 mRNA and sFlt-1/PIGF
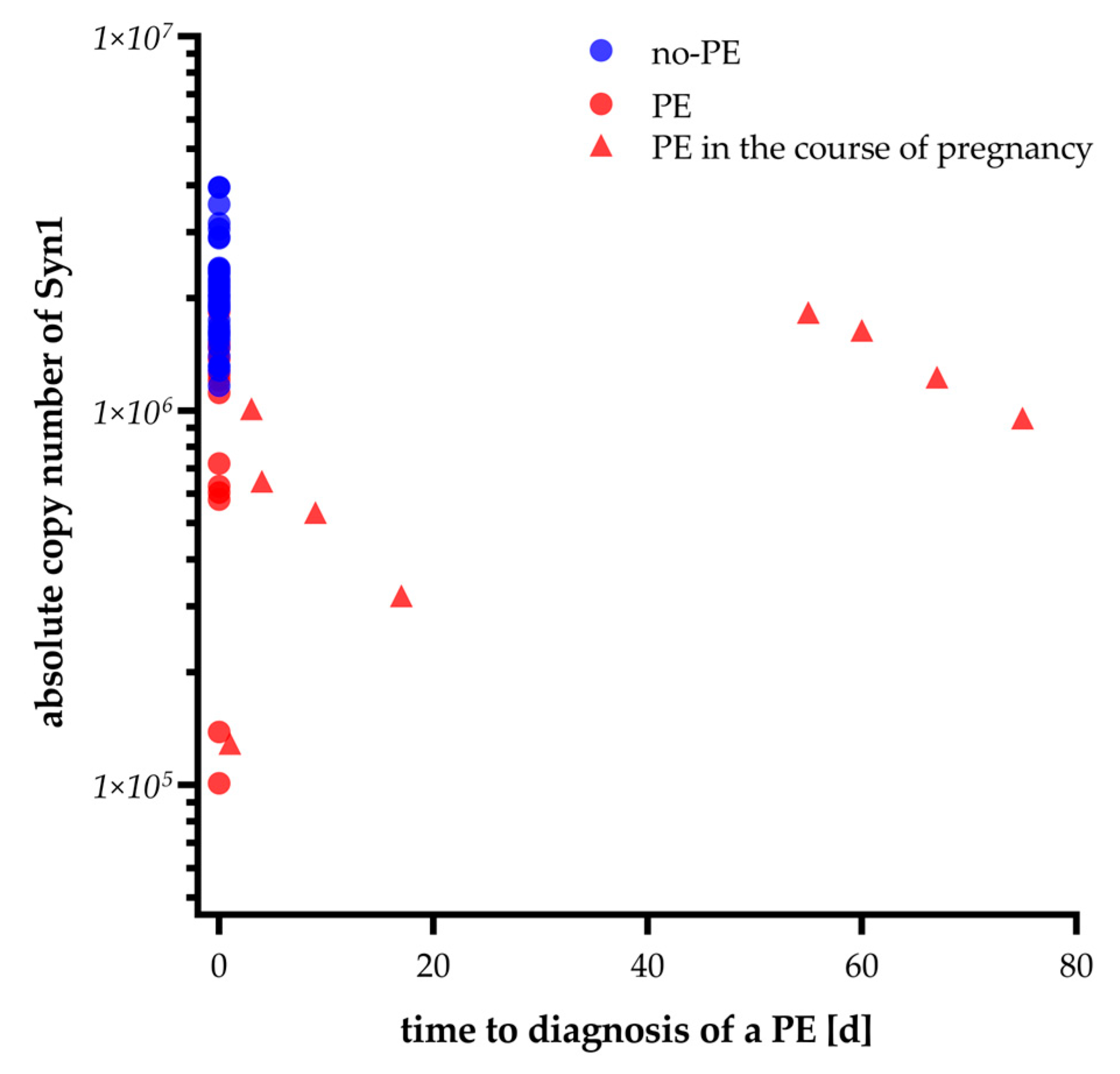
2.3. Syncytin-1 mRNA Expression over Time Prior to Preeclampsia Diagnosis
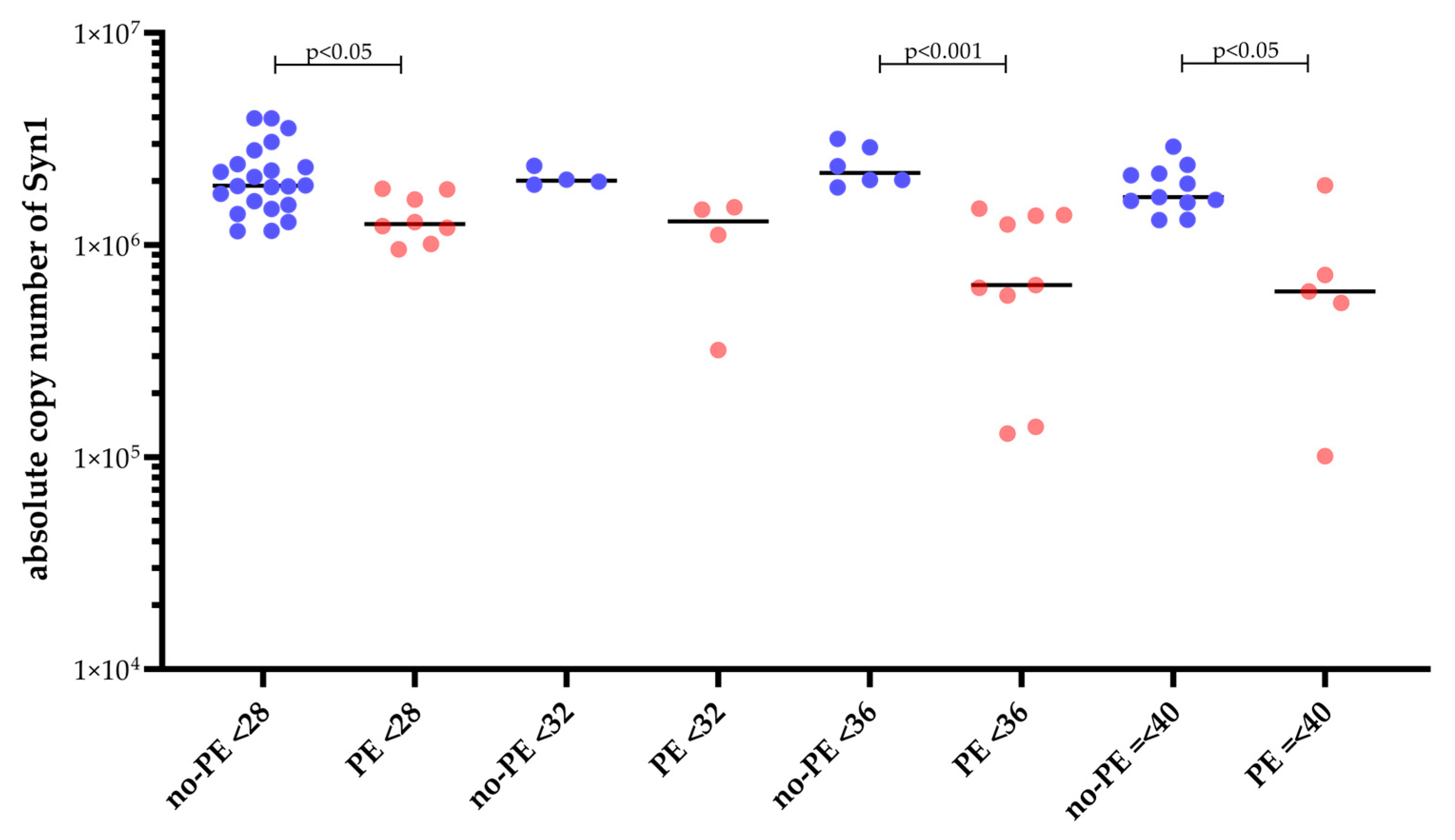
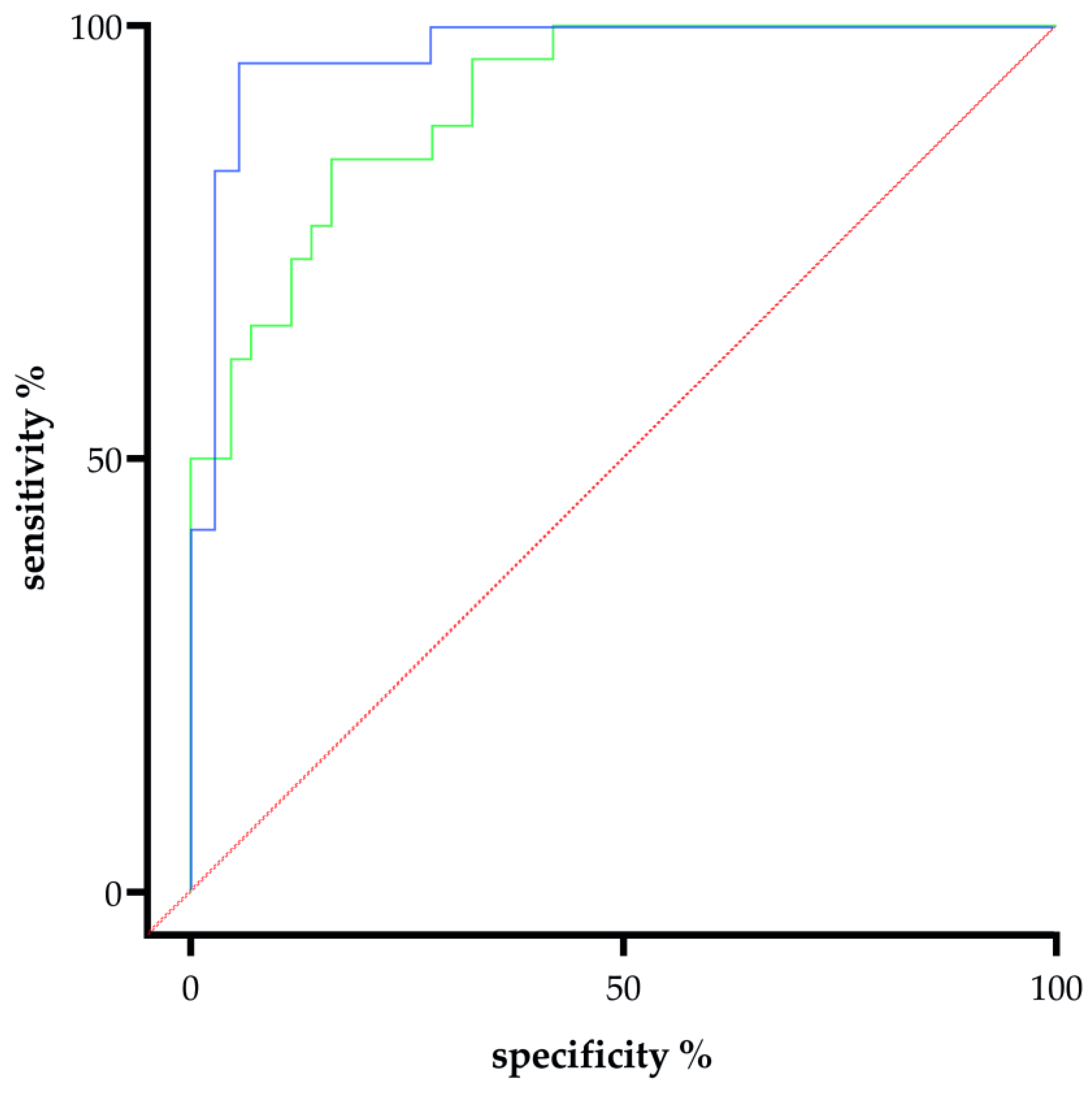
2.4. Reduced Maternal Syncytin-1 Expression Before and After Onset of PE and Its Diagnostic and Predictive Potential
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Design and Definition of Outcome Groups
4.3. Isolation of Fetal RNA from Maternal Whole Blood Samples
4.4. Reverse Transcription and Pre-Amplification
4.5. Quantitative PCR with Calibration Curve for Absolute Copy Number Determination
4.6. Quantification of the sFlt-1/PlGF Ratio in Maternal Serum
4.7. Doppler Ultrasonographic Evaluation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cresswell, J.A.; Alexander, M.; Chong, M.Y.C.; Link, H.M.; Pejchinovska, M.; Gazeley, U.; Ahmed, S.M.A.; Chou, D.; Moller, A.B.; Simpson, D.; et al. Global and regional causes of maternal deaths 2009-20: A WHO systematic analysis. Lancet Glob. Health 2025, 13, e626–e634. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1: PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- MacDonald, T.M.; Walker, S.P.; Hannan, N.J.; Tong, S.; Kaitu’u-Lino, T.J. Clinical tools and biomarkers to predict preeclampsia. EBioMedicine 2022, 75, 103780. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289, Correction in Nat. Rev. Nephrol. 2019, 15, 386. [Google Scholar] [CrossRef]
- Oike, A.; Shibata, S.; Arima, T.; Okae, H. Syncytin-1 Is Responsible for the Fusion Between Human Trophoblasts and Endometrial Stromal Cells. Dev. Growth Differ. 2025, 67, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Chen, X.L.; Yang, J.; Gong, X.X.; Zhang, H.F.; Zhang, Y.M.; Zeng, D.F.; Chen, P.S.; Chen, H.B. Reduced syncytin-1 regulates trophoblast invasion and apoptosis in preeclampsia. Placenta 2024, 155, 32–41. [Google Scholar] [CrossRef]
- Levine, L.; Habertheuer, A.; Ram, C.; Korutla, L.; Schwartz, N.; Hu, R.W.; Reddy, S.; Freas, A.; Zielinski, P.D.; Harmon, J.; et al. Syncytiotrophoblast extracellular microvesicle profiles in maternal circulation for noninvasive diagnosis of preeclampsia. Sci. Rep. 2020, 10, 6398. [Google Scholar] [CrossRef]
- Priščáková, P.; Svoboda, M.; Feketová, Z.; Hutník, J.; Repiská, V.; Gbelcová, H.; Gergely, L. Syncytin-1, syncytin-2 and suppressyn in human health and disease. J. Mol. Med. 2023, 101, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Krchlikova, V.; Braun, E.; Weiss, J.; Stafl, K.; Jech, L.; Badarinarayan, S.S.; Lotke, R.; Travnicek, M.; Baur, C.; Stark, P.; et al. Inhibition of placental trophoblast fusion by guanylate-binding protein 5. Sci. Adv. 2025, 11, eadt5388. [Google Scholar] [CrossRef]
- Zhuang, X.W.; Li, J.; Brost, B.C.; Xia, X.Y.; Chen, H.B.; Wang, C.X.; Jiang, S.W. Decreased expression and altered methylation of syncytin-1 gene in human placentas associated with preeclampsia. Curr. Pharm. Des. 2014, 20, 1796–1802. [Google Scholar] [CrossRef]
- Solt, I.; Cohen, S.M.; Admati, I.; Beharier, O.; Dominsky, O.; Yagel, S. Placenta at single-cell resolution in early and late preeclampsia: Insights and clinical implications. Am. J. Obstet. Gynecol. 2025, 232, S176–S189. [Google Scholar] [CrossRef]
- Roland, C.S.; Hu, J.; Ren, C.E.; Chen, H.; Li, J.; Varvoutis, M.S.; Leaphart, L.W.; Byck, D.B.; Zhu, X.; Jiang, S.W. Morphological changes of placental syncytium and their implications for the pathogenesis of preeclampsia. Cell. Mol. Life Sci. 2016, 73, 365–376. [Google Scholar] [CrossRef]
- Bolze, P.A.; Mommert, M.; Mallet, F. Contribution of Syncytins and Other Endogenous Retroviral Envelopes to Human Placenta Pathologies. Prog. Mol. Biol. Transl. Sci. 2017, 145, 111–162. [Google Scholar] [CrossRef] [PubMed]
- Bu, C.; Wang, Z.; Ren, Y.; Chen, D.; Jiang, S.W. Syncytin-1 nonfusogenic activities modulate inflammation and contribute to preeclampsia pathogenesis. Cell. Mol. Life Sci. 2022, 79, 290. [Google Scholar] [CrossRef] [PubMed]
- Ruebner, M.; Strissel, P.L.; Ekici, A.B.; Stiegler, E.; Dammer, U.; Goecke, T.W.; Faschingbauer, F.; Fahlbusch, F.B.; Beckmann, M.W.; Strick, R. Reduced syncytin-1 expression levels in placental syndromes correlates with epigenetic hypermethylation of the ERVW-1 promoter region. PLoS ONE 2013, 8, e56145. [Google Scholar] [CrossRef] [PubMed]
- Göhner, C.; Plösch, T.; Faas, M.M. Immune-modulatory effects of syncytiotrophoblast extracellular vesicles in pregnancy and preeclampsia. Placenta 2017, 60 (Suppl. S1), S41–S51. [Google Scholar] [CrossRef]
- Moufarrej, M.N.; Vorperian, S.K.; Wong, R.J.; Campos, A.A.; Quaintance, C.C.; Sit, R.V.; Tan, M.; Detweiler, A.M.; Mekonen, H.; Neff, N.F.; et al. Early prediction of preeclampsia in pregnancy with cell-free RNA. Nature 2022, 602, 689–694. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.; Yang, W.; Xue, P.; Yin, Y.; Wang, Y.; Tian, P.; Peng, H.; Jiang, H.; Xu, W.; et al. Noninvasive preeclampsia prediction using plasma cell-free RNA signatures. Am. J. Obstet. Gynecol. 2023, 229, 553.e1–553.e16. [Google Scholar] [CrossRef]
- Munchel, S.; Rohrback, S.; Randise-Hinchliff, C.; Kinnings, S.; Deshmukh, S.; Alla, N.; Tan, C.; Kia, A.; Greene, G.; Leety, L.; et al. Circulating transcripts in maternal blood reflect a molecular signature of early-onset preeclampsia. Sci. Transl. Med. 2020, 12, 550. [Google Scholar] [CrossRef]
- Raymond, D.; Peterson, E. A critical review of early-onset and late-onset preeclampsia. Obstet. Gynecol. Surv. 2011, 66, 497–506. [Google Scholar] [CrossRef]
- Han, L.; Holland, O.J.; Da Silva Costa, F.; Perkins, A.V. Potential biomarkers for late-onset and term preeclampsia: A scoping review. Front. Physiol. 2023, 14, 1143543. [Google Scholar] [CrossRef]
- Redman, C.W.G.; Staff, A.C.; Roberts, J.M. Syncytiotrophoblast stress in preeclampsia: The convergence point for multiple pathways. Am. J. Obstet. Gynecol. 2022, 226, S907–S927. [Google Scholar] [CrossRef]
- Vargas, A.; Toufaily, C.; LeBellego, F.; Rassart, É.; Lafond, J.; Barbeau, B. Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod. Sci. 2011, 18, 1085–1091. [Google Scholar] [CrossRef]
- Knerr, I.; Beinder, E.; Rascher, W. Syncytin, a novel human endogenous retroviral gene in human placenta: Evidence for its dysregulation in preeclampsia and HELLP syndrome. Am. J. Obstet. Gynecol. 2002, 186, 210–213. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Wiley, R.L.; Sarker, M.R.; Woelkers, D.A. Novel biomarkers for preeclampsia: Promises and pitfalls. Curr. Opin. Obstet. Gynecol. 2025, 37, 294–301. [Google Scholar] [CrossRef]
- Chen, C.P.; Wang, K.G.; Chen, C.Y.; Yu, C.; Chuang, H.C.; Chen, H. Altered placental syncytin and its receptor ASCT2 expression in placental development and pre-eclampsia. Bjog 2006, 113, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.T.; Seow, K.M.; Chen, K.H. The Pathophysiological, Genetic, and Hormonal Changes in Preeclampsia: A Systematic Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2024, 25, 4532. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, A.L.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.M.; Steyn, W.; Zeeman, G.G.; Brown, M.A. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014, 4, 97–104. [Google Scholar] [CrossRef]
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 2000, 183, S1–S22. [CrossRef]
- Kehl, S.; Dötsch, J.; Hecher, K.; Schlembach, D.; Schmitz, D.; Stepan, H.; Gembruch, U. Intrauterine Growth Restriction. Guideline of the German Society of Gynecology and Obstetrics (S2k-Level, AWMF Registry No. 015/080, October 2016). Geburtshilfe Frauenheilkd. 2017, 77, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Roemer, V.M.; Fusch, C.; Wenzlaff, P.; Hesse, V.; Krentz, H. Methodische Aspekte der Berechnung von Normwertkurven am Beispiel des Geburtsgewichtes. Geburtshilfe Frauenheilkd. 2005, 65, 279–283. [Google Scholar] [CrossRef]
- Bhide, A.; Acharya, G.; Bilardo, C.M.; Brezinka, C.; Cafici, D.; Hernandez-Andrade, E.; Kalache, K.; Kingdom, J.; Kiserud, T.; Lee, W.; et al. ISUOG practice guidelines: Use of Doppler ultrasonography in obstetrics. Ultrasound. Obstet. Gynecol. 2013, 41, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Signorell, A. DescTools: Tools for Descriptive Statistics, R Package Version 0.99.59; R Core Team: Vienna, Austria, 2025. Available online: https://andrisignorell.github.io/DescTools/ (accessed on 8 January 2025).
- Stevenson, M.S.E. epiR: Tools for the Analysis of Epidemiological Data, R Package Version 2.0.85; R Core Team: Vienna, Austria, 2025. Available online: https://cran.r-project.org/package=epiR (accessed on 8 January 2025).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
| No PE n = 43 | PE n = 26 | p-Value | |
|---|---|---|---|
| Maternal characteristics | |||
| maternal age, years | 33.0 (28.0–26.0) | 35.0 (29.5–36.0) | 0.471 |
| body mass index, kg/m2 | 26.3 (23.0–30.8) | 27.0 (23.3–33.7) | 0.665 |
| nulliparity | 23 (53.5%) | 19 (73.1%) | 0.131 |
| ethnicity | |||
| Caucasian | 40 (93%) | 23 (88.5%) | 0.665 |
| black | 3 (7%) | 1 (3.8%) | >0.999 |
| unknown | - | 2 (7.7%) | 0.139 |
| PE in previous pregnancy | - | 4 (15.4%) | 0.017 * |
| antiphospholipid syndrome | - | - | >0.999 |
| pre-existing kidney disease | - | - | >0.999 |
| maternal age > 40 years | 2 (4.7%) | 3 (11.5%) | 0.358 |
| familiar risk | 2 (4.7%) | - | 0.523 |
| pre-existing diabetes | - | - | <0.999 |
| BMI > 30 | 13 (30.2%) | 5 (19.2%) | 0.402 |
| conception mode | |||
| ICSI or IVF | 4 (9.3%) | 3 (11.5%) | <0.999 |
| gestational age at sampling, weeks + days | 28 + 5 (25 + 1–35 + 3) | 32 + 5 (26 + 6–35 + 2) | 0.093 |
| patients presenting −20 weeks | 7 (16.3%) | - | 0.040 |
| patients presenting 20–24 + 0 weeks | 5 (11.6%) | 3 (11.5%) | >0.999 |
| patients presenting 24 + 1–34 + 0 weeks | 20(46.5%) | 14 (53.8%) | 0.624 |
| patients presenting 34 + 1–37 + 0 weeks | 2 (4.7%) | 6 (23.1%) | 0.046 |
| patients presenting > 37 + 1 weeks | 9 (20.9%) | 3 (11.5%) | 0.514 |
| Maternal symptoms at sampling | |||
| systolic blood pressure, mmHg | 116.0 (110.0–127.0) | 150.0 (142.0–160.0) | <0.001 * |
| (n = 16 missing) | (n = 1 missing) | ||
| diastolic blood pressure, mmHg | 69.0 (59.0–73.0) | 96.0 (84.0–100.0) | <0.001 * |
| (n = 16 missing) | (n = 1 missing) | ||
| mean PI of uterine arteries | 0.84 (0.70–1.38) | 1.30 (1.11–1.69) | 0.010 * |
| (n = 22 missing) | (n = 8 missing) | ||
| urine dipstick | |||
| negative | 35 (81.4%) | 4 (15.4%) | <0.001 * |
| traces | 1(2.3%) | 2 (7.7%) | 0.552 |
| + | - | 7 (26.9%) | <0.001 * |
| ++ | - | 6 (23.1%) | <0.001 * |
| +++ | - | 1 (3.8%) | 0.379 |
| unknown | 7(16.3%) | 6 (23.1%) | 0.535 |
| new onset of hypertension | 3 (7.0%) | 15 (57.7%) | <0.001 * |
| new onset of proteinuria | 1 2.3%) | 9 (34.6%) | <0.001 * |
| headache | - | 7 (26.9%) | <0.001 * |
| visual disturbances | - | - | >0.999 |
| progressive edemas | - | 1 (3.8%) | 0.377 |
| weight gain ≥ 1 kg per week | - | - | >0.999 |
| low platelet count < 150/nL | 2 (4.7%) | - | 0.523 |
| elevated liver enzymes | 4 (9.3%) | 1 (3.8%) | 0.643 |
| upper abdominal pain | 1 (2.3%) | 3 (11.5%) | 0.147 |
| ascites or pleural effusion | - | - | |
| PI of the umbilical artery | 0.95 (0.78–1.13) | 1.0 (0.84–1.36) | 0.201 |
| (n = 6 missing) | (n = 4 missing) | ||
| PI of the MCA | 1.74 (1.22–2.13) | 1.39 (1.30–1.73) | 0.090 |
| (n = 21 missing) | (n = 9 missing) | ||
| suspected FGR | 5 (11.6%) | 9 (34.6%) | 0.031 * |
| Pregnancy outcome | |||
| gestational age at delivery, weeks + days | 36 + 5 (29 + 1–38 + 5) | 34 + 1 (32 + 2–36 + 6) | 0.396 |
| gestational age at delivery ≥ 37 + 0 weeks | 21 (48.8%) | 5 (19.2%) | 0.003 * |
| interval from sampling till delivery, days | 16.0 (3–57) | 8.5 (1.0–18.0) | 0.133 |
| interval from sampling till diagnosis, days | - | −1.0 (−5.8–5.3) | |
| early-onset PE ≤ 34 weeks | - | 17 (65.4%) | |
| no-PE nor FGR | 37 (86%) | - | |
| PE and FGR | - | 13 (50%) | |
| HELLP syndrome | - | 3 (11.5%) | |
| FGR without PE | 6 (14%) | - | |
| fetal birth weight, gram | 2560.0 (1160.0–3225) | 1807.5 (1368.8–2476.3) | 0.119 |
| percentile fetus | 40 (19–62) | 9.5 (3.8–26.3) | <0.001 |
| delivery mode | |||
| spontaneous | 18 (41.9%) | 5 (19.2%) | 0.068 |
| cesarean | 22 (51.2%) | 20 (76.9%) | 0.573 |
| vaginal-operative | 3 (7%) | 1(3.8%) | >0.999 |
| All Study Objectives | |||
|---|---|---|---|
| No PE (n = 43) | PE (n = 26) | ||
| gestational age at sampling (weeks + days) | 28 + 5 (25 + 1–35 + 3) | 32 + 5 (26 + 6–35 + 2) | 0.093 |
| sFlt-1 [pg/nL] | 2295.50 (1605.75–3661.75) | 8289.5 (6418.0–1 3496.8) | <0.001 * |
| PlGF [pg/nL] | 232 (116.50–348.50) | 43.7 (24.0–78.3) | <0.001 * |
| sFlt-1/PlGF | 10.5 (5.25–27.25) | 214.0 (142.0–371.5) | <0.001 * |
| (n = 9 missing) | (n = 3 missing) | ||
| syncytin-1 copies | 1 992 739 | 1 217 050 | <0.001 * |
| (1 616 811–3 126 225) | (610 900–1 482 069) | ||
| Patients with PE at time of sampling and GA-matched controls | |||
| No PE (n = 31) | PE at or before sampling (n = 17) | ||
| gestational age at | 32 + 2 (26 + 0–37 + 3) | 33 + 5 (30 + 6–36 + 1) | 0.382 |
| sampling (weeks + days) early-onset PE ≤ 34 weeks | min/max 24 + 4 weeks–40 + 2 weeks | min/max 24 + 4 weeks–40 + 1 weeks | |
| sFlt-1 [pg/nL] | 2 560.0 (1 647.75–3 873.00) | 8 215.00 (6 305.50–13 431.50) | <0.001 |
| PlGF [pg/nL] | 242.00 (125.50–393.25) | 41.30 (23.65–57.40) | <0.001 |
| sFlt-1/PlGF | 12.00 (5–28.00) | 264.00 (152.0–361.00) | <0.001 |
| syncytin-1 copies | 2 025 940 | 1 249 321 | <0.001 |
| (1 629 823–2 396 807) | (616 665–1 477 892) | ||
| Patients with PE later in pregnancy and GA-matched controls | |||
| No PE (n = 26) | development of PE after sampling (n = 9) | ||
| gestational age at sampling (weeks + days) time to diagnosis of PE (days) early-onset PE ≤ 34 weeks | 26 + 1 (24 + 6–32 + 1) min/max 20 + 5–36 + 2 weeks | 27 + 0 (22 + 4–33 + 3) min/max 20 + 4- 36 + 2 weeks 17 (3.5–63.5) 4 (44.4%) | 0.810 |
| sFlt-1 [pg/nL] | 1882.00 (1514.0–4251.0) | 8364.00 (6816.00–1,6107.00) | <0.001 |
| PlGF [pg/nL] | 248.00 (121.00–409.50) | 81.40 (23.10–94.30) | <0.001 |
| sFlt-1/PlGF | 9.00 (2–25.5) | 171.00 (91.00–442.00) | <0.001 |
| syncytin-1 copies | 2 031 055 | 954 986 | <0.001 |
| (1 888 707–281 675) | (427 006–1 434 322) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenz-Meyer, L.; Bührer, C.; Verlohren, S.; Endesfelder, S. Circulating Plasma Syncytin-1 mRNA in Preeclampsia—A Pilot Study. Int. J. Mol. Sci. 2025, 26, 9015. https://doi.org/10.3390/ijms26189015
Lorenz-Meyer L, Bührer C, Verlohren S, Endesfelder S. Circulating Plasma Syncytin-1 mRNA in Preeclampsia—A Pilot Study. International Journal of Molecular Sciences. 2025; 26(18):9015. https://doi.org/10.3390/ijms26189015
Chicago/Turabian StyleLorenz-Meyer, Lisa, Christoph Bührer, Stefan Verlohren, and Stefanie Endesfelder. 2025. "Circulating Plasma Syncytin-1 mRNA in Preeclampsia—A Pilot Study" International Journal of Molecular Sciences 26, no. 18: 9015. https://doi.org/10.3390/ijms26189015
APA StyleLorenz-Meyer, L., Bührer, C., Verlohren, S., & Endesfelder, S. (2025). Circulating Plasma Syncytin-1 mRNA in Preeclampsia—A Pilot Study. International Journal of Molecular Sciences, 26(18), 9015. https://doi.org/10.3390/ijms26189015







