From By-Product to Bioactive Molecular Ingredient: The Impact of Cranberry Pomace on Antioxidant Properties and Enzyme Modulation in Functional Biscuits
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterisation of American Cranberry Pomace
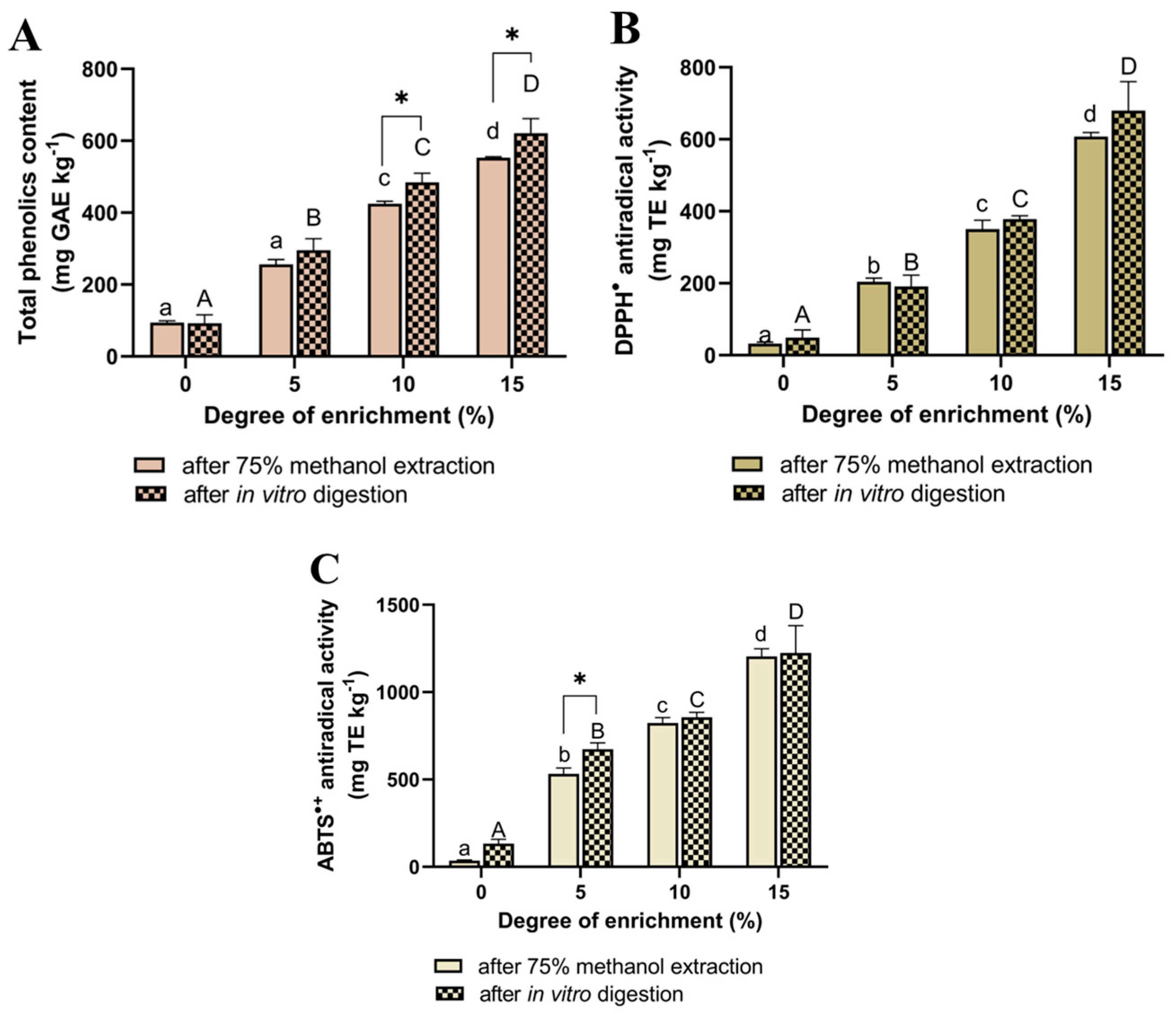
2.2. Phenolic Content and Antioxidant Activity of Enriched Biscuits
3. Materials and Methods
3.1. Preparation of Fruit Pomace
3.2. Biscuits Preparation
3.3. Analysis of Total Antioxidants Activity
3.4. Extraction Procedure and Determination of Individual Polyphenols
3.5. In Vitro Digestion of Biscuits
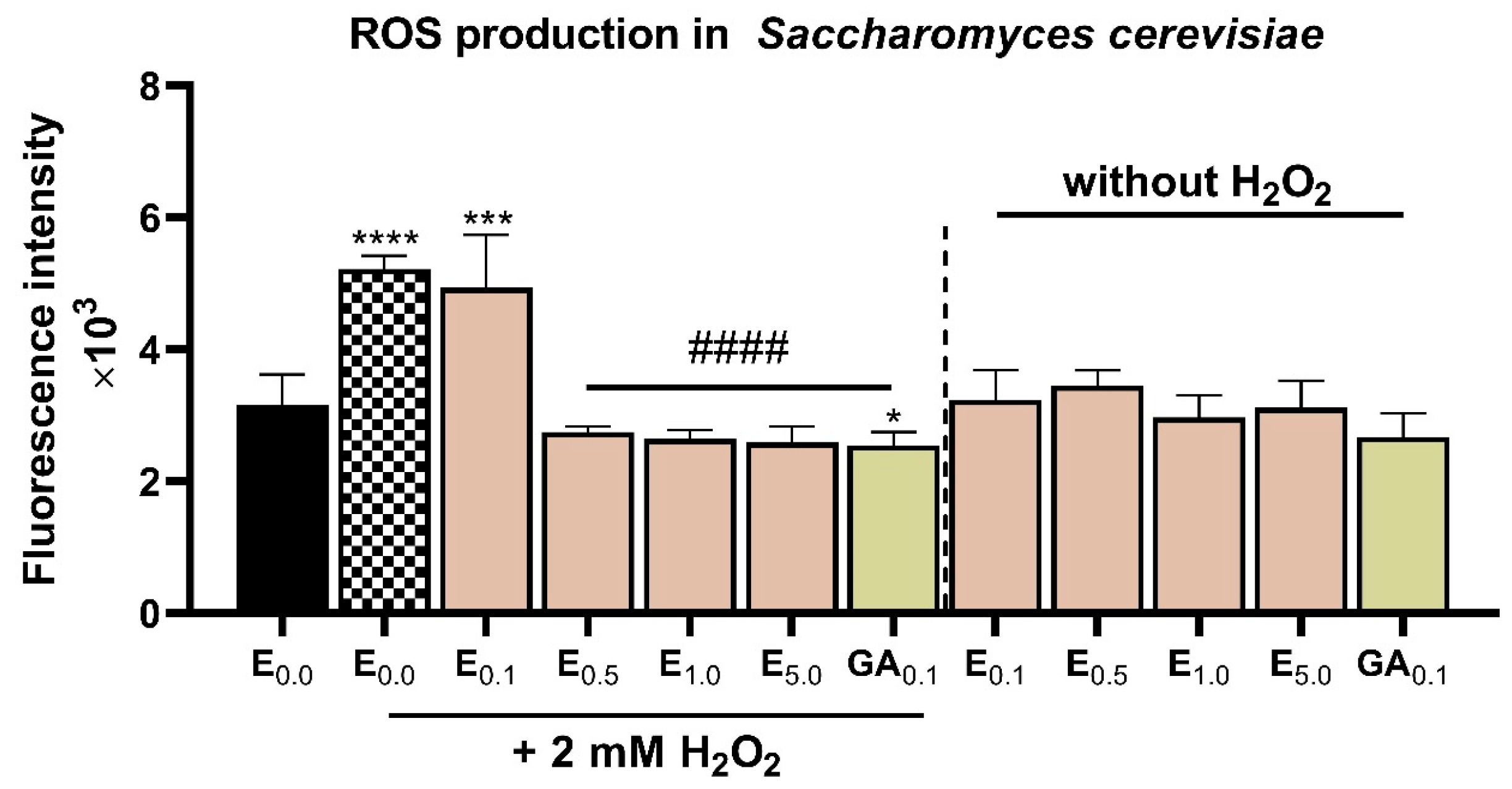
3.6. Assessment of Antioxidant Properties Using Saccharomyces Cerevisiae
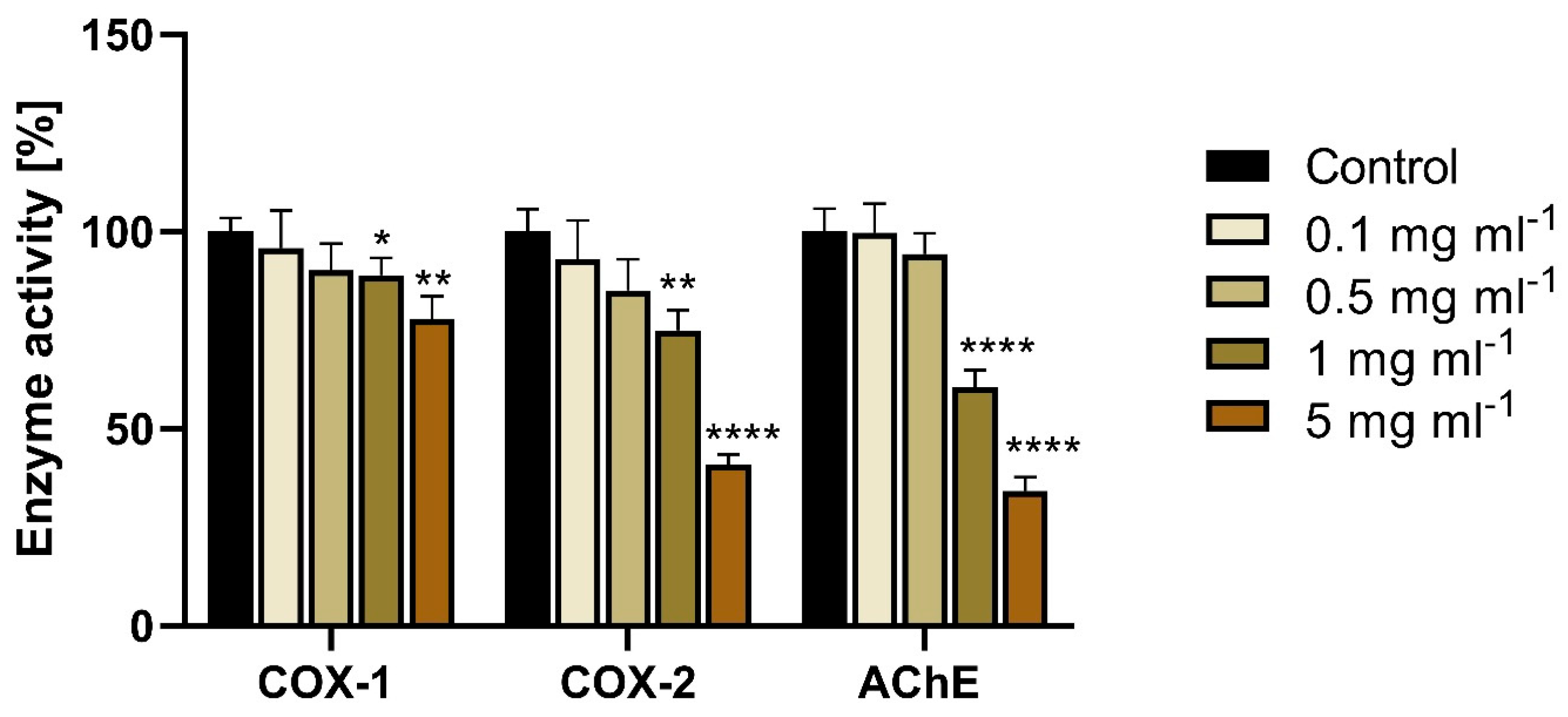
3.7. Inhibition of COX-1, COX-2, and AChE
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narwojsz, A.; Tańska, M.; Mazur, B.; Borowska, E.J. Fruit Physical Features, Phenolic Compounds Profile and Inhibition Activities of Cranberry Cultivars (Vaccinium macrocarpon) Compared to Wild-Grown Cranberry (Vaccinium oxycoccus). Plant Foods Hum. Nutr. 2019, 74, 300–306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Borowska, E.J.; Mazur, B.; Kopciuch, R.G.; Buszewski, B. Polyphenol, anthocyanin and resveratrol mass fractions and antioxidant properties of cranberry cultivars. Food Technol. Biotechnol. 2009, 47, 56–61. [Google Scholar]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Osorio, C.; Hurtado, N.; Dawid, C.; Hofmann, T.; Heredia-Mira, F.J.; Morales, A.L. Chemical characterisation of anthocyanins in tamarillo (Solanum betaceum Cav.) and Andes berry (Rubus glaucus Benth.) fruits. Food Chem. 2012, 132, 1915–1921. [Google Scholar] [CrossRef]
- Jayaprakash, S.; Paasi, J.; Pennanen, K.; Flores Ituarte, I.; Lille, M.; Partanen, J.; Sozer, N. Techno-Economic Prospects and Desirability of 3D Food Printing: Perspectives of Industrial Experts, Researchers and Consumers. Foods 2020, 9, 1725. [Google Scholar] [CrossRef]
- Liang, X.; Chang, L.; Hang, M.; Seeram, N.P.; Neto, C.C. Anti-inflammatory Activities of Cranberry Fruit Extracts in Human THP-1 Monocytes are Influenced by Their Phytochemical Composition. ACS Food Sci. Technol. 2022, 2, 75–83. [Google Scholar] [CrossRef]
- Zaa, C.A.; Marcelo, Á.J.; An, Z.; Medina-Franco, J.L.; Velasco-Velázquez, M.A. Anthocyanins: Molecular Aspects on Their Neuroprotective Activity. Biomolecules 2023, 13, 1598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roopchand, D.E.; Krueger, C.G.; Moskal, K.; Fridlender, B.; Lila, M.A.; Raskin, I. Food-compatible method for the efficient extraction and stabilization of cranberry pomace polyphenols. Food Chem. 2013, 141, 3664–3669. [Google Scholar] [CrossRef]
- Viskelis, P.; Rubinskienė, M.; Jasutienė, I.; Šarkinas, A.; Daubaras, R.; Česonienė, L. Anthocyanins, Antioxidative, and Antimicrobial Properties of American Cranberry (Vaccinium macrocarpon Ait.) and their Press Cakes. J. Food Sci. 2009, 74, C157–C161. [Google Scholar] [CrossRef]
- Debnath, S.C.; An, D. Antioxidant properties and structured biodiversity in a diverse set of wild cranberry clones. Heliyon 2019, 5, e01493. [Google Scholar] [CrossRef]
- Schendel, R.R.; Pandeya, P.R. Determination of (Total) Phenolics and Antioxidant Capacity in Food and Ingredients. In Nielsen’s Food Analysis; Ismail, B.P., Nielsen, S.S., Eds.; Food Science Text Series; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Balawejder, M.; Piechowiak, T.; Kapusta, I.; Chęciek, A.; Matłok, N. In Vitro Analysis of Selected Antioxidant and Biological Properties of the Extract from Large-Fruited Cranberry Fruits. Molecules 2023, 28, 7895. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Chapter 10—Non-Extractable Polyphenols in Plant Foods: Nature, Isolation, and Analysis. In Polyphenols in Plants; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 203–218. ISBN 9780123979346. [Google Scholar] [CrossRef]
- Ding, Y.; Morozova, K.; Scampicchio, M.; Ferrentino, G. Non-Extractable Polyphenols from Food By-Products: Current Knowledge on Recovery, Characterisation, and Potential Applications. Processes 2020, 8, 925. [Google Scholar] [CrossRef]
- Macagnan, F.T.; Bender, A.B.B.; Speroni, C.S. Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Saura-Calixto, F., Pérez-Jiménez, J., Eds.; The Royal Society of Chemistry: London, UK, 2018; pp. 17–36. [Google Scholar]
- Arcia, P.; Curutcheta, A.; Pérez-Pirotto, C.; Hernando, I. Upcycling fruit pomaces (orange, apple, and grape-wine): The impact of particle size on phenolic compounds’ bioaccessibility. Heliyon 2024, 10, e38737. [Google Scholar] [CrossRef] [PubMed]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. Potential bioaccessibility and functionality of polyphenols and cynaropicrin from breads enriched with artichoke stem. Food Chem. 2018, 245, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Alnsser, M.N.; Mellor, I.R.; Carter, W.G. A preliminary assessment of the nutraceutical potential of acai berry (Euterpe sp.) as a potential natural treatment for Alzheimer’s disease. Molecules 2022, 27, 4891. [Google Scholar] [CrossRef]
- Pervin, M.; Hasnat, M.A.; Lee, Y.M.; Kim, D.H.; Jo, J.E.; Lim, B.O. Antioxidant activity and acetylcholinesterase inhibition of grape skin anthocyanin (GSA). Molecules 2014, 19, 9403–9418. [Google Scholar] [CrossRef]
- Samani, S.; Costa, S. Cai. Neuroprotective effects of blueberries through inhibition on cholinesterase, tyrosinase, cyclooxygenase-2, and amyloidogenesis. Nutraceuticals 2023, 3, 39–57. [Google Scholar] [CrossRef]
- Borowiec, K.; Szwajgier, D.; Targoński, Z.; Demchuk, O.M.; Cybulska, J.; Czernecki, T.; Malik, A. Cholinesterase inhibitors isolated from bilberry fruit. J. Funct. Foods 2014, 11, 313–321. [Google Scholar] [CrossRef]
- Balawejder, M.; Matłok, N.; Piechowiak, T.; Szostek, M.; Kapusta, I.; Niemiec, M.; Komorowska, M.; Wróbel, M.; Mudryk, K.; Szeląg-Sikora, A.; et al. The Modification of Substrate in the Soilless Cultivation of Raspberries (Rubus Idaeus L.) as a Factor Stimulating the Biosynthesis of Selected Bioactive Compounds in Fruits. Molecules 2023, 28, 118. [Google Scholar] [CrossRef]
- Złotek, U.; Szychowski, K.A.; Świeca, M. Potential in vitro antioxidant, anti-inflammatory, antidiabetic, and anticancer effect of arachidonic acid-elicited basil leaves. J. Funct. Foods. 2017, 36, 290–299. [Google Scholar] [CrossRef]

| Compound | Rt (min) | λmax | MS | MS/MS | Content (mg kg−1) | |
|---|---|---|---|---|---|---|
| Anthocyanins | ||||||
| 1. | Cyanidin 3-O-glucoside | 2.54 | 279, 512 | 449+ | 287 | 598.08 ± 12.68 |
| 2. | Cyanidin 3-O-galactoside | 2.68 | 279, 517 | 449+ | 287 | 10.75 ± 0.13 |
| 3. | Cyanidin 3-O-arabinoside | 2.86 | 279, 512 | 419+ | 287 | 375.27 ± 3.65 |
| 4. | Peonidin 3-O-glucoside | 3.11 | 279, 512 | 463+ | 301 | 795.91 ± 72.01 |
| 5. | Peonidin 3-O-galactoside | 3.27 | 278, 519 | 463+ | 301 | 44.61 ± 0.10 |
| 6. | Peonidin 3-O-arabinoside | 3.45 | 279, 517 | 433+ | 301 | 266.68 ± 9.84 |
| Other phenolics | ||||||
| 7. | Caffeic acid O-glucoside | 2.40 | 329 | 341− | 179 | 5.17 ± 0.01 |
| 8. | 3-O-Caffeoylquinic acid | 2.79 | 324 | 353− | 191 | 20.03 ± 2.07 |
| 9. | Coumaric acid O-glucoside | 2.96 | 310 | 325− | 163 | 33.79 ± 0.98 |
| 10. | 3-O-Feruloylquinic acid | 3.32 | 329 | 355− | 193 | 19.36 ± 0.41 |
| 11. | Sinapic acid 3-O-glucoside | 3.39 | 329 | 385− | 223 | 33.30 ± 5.61 |
| 12. | Myricetin 3-O-glucoside | 3.78 | 257, 355 | 479− | 317 | 120.90 ± 12.51 |
| 13. | Myricetin 3-O-arabinofuranoside | 4.23 | 257, 352 | 449− | 317 | 26.96 ± 0.26 |
| 14. | Quercetin 3-O-glucoside | 4.40 | 255, 355 | 463− | 301 | 186.79 ± 16.90 |
| 15. | Laricitrin 3-O-glucoside | 4.51 | 271, 354 | 493− | 331 | 19.23 ± 0.04 |
| 16. | Quercetin 3-O-xylopyranoside | 4.72 | 255, 355 | 433− | 301 | 18.03 ± 0.67 |
| 17. | Coumaroyl-dihydromonotropein | 4.91 | 310 | 537− | 163, 119 | 17.53 ± 0.02 |
| 18. | Quercetin 3-O-arabinopyranoside | 4.96 | 255, 355 | 433− | 301 | 65.31 ± 6.73 |
| 19. | Quercetin 3-O-rhamnoside | 5.11 | 255, 352 | 447− | 301 | 31.83 ± 0.93 |
| 20. | Syringetin 3-O-glucoside | 5.17 | 271, 352 | 507− | 345 | 16.53 ± 0.35 |
| 21. | Kaempferol 3-O-glucoside | 5.64 | 267, 352 | 447− | 285 | 6.44 ± 1.08 |
| 22. | Kaempferol 3-O-galactoside | 5.76 | 265, 350 | 447− | 285 | 4.84 ± 0.50 |
| 23. | Syringetin 3-O-pentoside | 5.89 | 271, 350 | 477− | 345 | 4.54 ± 0.11 |
| Sum of polyphenols identified by LC-MS (mg kg−1) | 2721.89 ± 147.62 | |||||
| Total phenolics content (g GAE kg−1) | 5.12 ± 0.51 | |||||
| Antiradical activity against DPPH• (g TE kg−1) | 6.38 ± 1.96 | |||||
| Antiradical activity against ABTS•+ (g TE kg−1) | 12.29 ± 1.88 | |||||
| Compound | Content (mg kg−1) | ||
|---|---|---|---|
| Biscuits | Biscuits Subjected to In Vitro Digestion | ||
| Anthocyanins | |||
| 1. | Cyanidin 3-O-glucoside | 48.75 ± 1.03 * | 15.65 ± 1.55 * |
| 2. | Cyanidin 3-O-galactoside | 2.02 ± 0.03 * | 1.39 ± 0.03 * |
| 3. | Cyanidin 3-O-arabinoside | 25.20 ± 0.25 * | 6.51 ± 0.28 * |
| 4. | Peonidin 3-O-glucoside | 52.54 ± 4.75 * | 21.39 ± 0.41 * |
| 5. | Peonidin 3-O-galactoside | 3.57 ± 0.01 * | 1.28 ± 0.04 * |
| 6. | Peonidin 3-O-arabinoside | 16.59 ± 0.61 * | 5.32 ± 0.33 * |
| Other phenolics | |||
| 7. | Caffeic acid O-glucoside | 1.95 ± 0.00 | 1.05 ± 0.11 |
| 8. | 3-O-Caffeoylquinic acid | 7.48 ± 0.77 | 7.17 ± 0.35 |
| 9. | Coumaric acid O-glucoside | 8.46 ± 0.25 * | 5.12 ± 0.15 * |
| 10. | 3-O-Feruloylquinic acid | 5.91 ± 0.13 | 6.79 ± 0.12 |
| 11. | Sinapic acid 3-O-glucoside | 6.03 ± 1.02 * | 4.10 ± 0.01 * |
| 12. | Myricetin 3-O-glucoside | 36.94 ± 3.82 * | 4.22 ± 0.10 * |
| 13. | Myricetin 3-O-arabinofuranoside | 10.25 ± 1.02 * | 6.12 ± 0.13 * |
| 14. | Quercetin 3-O-glucoside | 64.71 ± 1.42 * | 2.20 ± 0.03 * |
| 15. | Laricitrin 3-O-glucoside | 11.31 ± 0.48 | 13.61 ± 0.13 |
| 16. | Quercetin 3-O-xylopyranoside | 6.82 ± 0.13 * | 4.46 ± 0.40 * |
| 17. | Coumaroyl-dihydromonotropein | 7.49 ± 0.26 * | 1.94 ± 0.00 * |
| 18. | Quercetin 3-O-arabinopyranoside | 20.30 ± 1.25 * | 0.45 ± 0.02 * |
| 19. | Quercetin 3-O-rhamnoside | 11.70 ± 1.24 * | 1.76 ± 0.00 * |
| 20. | Syringetin 3-O-glucoside | 6.47 ± 0.32 * | 1.91 ± 0.20 * |
| 21. | Kaempferol 3-O-glucoside | 1.88 ± 0.06 | <LOQ |
| 22. | Kaempferol 3-O-galactoside | 1.76 ± 0.03 | <LOQ |
| 23. | Syringetin 3-O-pentoside | 1.68 ± 0.01 | <LOQ |
| Sum of polyphenols identified by LC-MS (mg kg−1) | 359.81 ± 18.88 | 112.45 ± 4.41 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matłok, N.; Piechowiak, T.; Kapusta, I.; Balawejder, M. From By-Product to Bioactive Molecular Ingredient: The Impact of Cranberry Pomace on Antioxidant Properties and Enzyme Modulation in Functional Biscuits. Int. J. Mol. Sci. 2025, 26, 9002. https://doi.org/10.3390/ijms26189002
Matłok N, Piechowiak T, Kapusta I, Balawejder M. From By-Product to Bioactive Molecular Ingredient: The Impact of Cranberry Pomace on Antioxidant Properties and Enzyme Modulation in Functional Biscuits. International Journal of Molecular Sciences. 2025; 26(18):9002. https://doi.org/10.3390/ijms26189002
Chicago/Turabian StyleMatłok, Natalia, Tomasz Piechowiak, Ireneusz Kapusta, and Maciej Balawejder. 2025. "From By-Product to Bioactive Molecular Ingredient: The Impact of Cranberry Pomace on Antioxidant Properties and Enzyme Modulation in Functional Biscuits" International Journal of Molecular Sciences 26, no. 18: 9002. https://doi.org/10.3390/ijms26189002
APA StyleMatłok, N., Piechowiak, T., Kapusta, I., & Balawejder, M. (2025). From By-Product to Bioactive Molecular Ingredient: The Impact of Cranberry Pomace on Antioxidant Properties and Enzyme Modulation in Functional Biscuits. International Journal of Molecular Sciences, 26(18), 9002. https://doi.org/10.3390/ijms26189002









