Bio Meets Nano: Protein Exchange in Saline Biocoronae on Magnetic Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
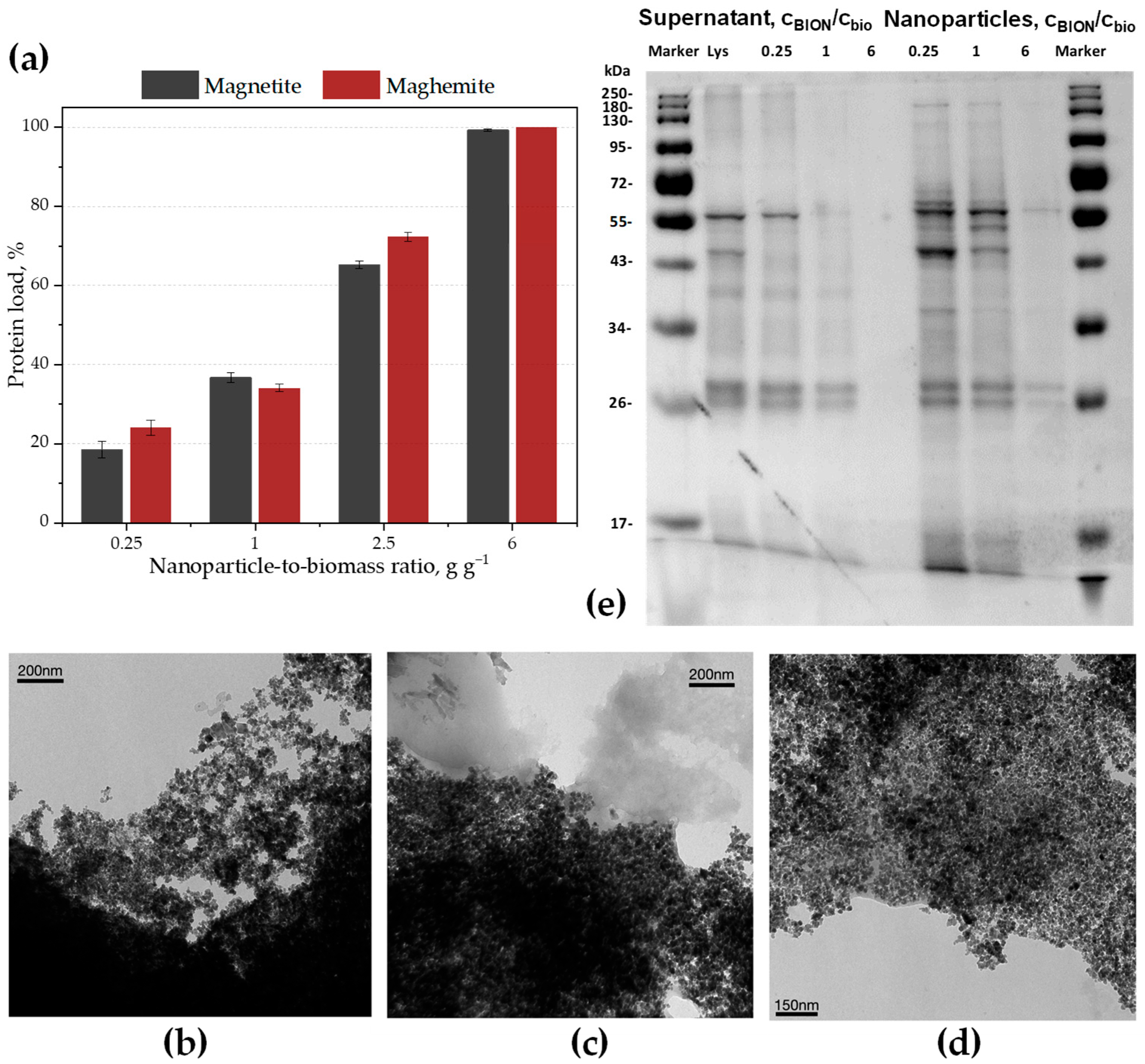
2.1. Quantitative Protein Separation
- -
- Magnetite nanoparticles (BIONs): size, 8.9 nm diameter measured via transmission electron microscopy (TEM) and 9.2 nm via X-ray diffraction (XRD); saturation magnetization, 70 emu g−1. See Figure S1 for characterization data.
- -
- Maghemite nanoparticles: size 9 and 7 nm from TEM and XRD data, respectively; saturation magnetization, 66 emu g−1 [48].
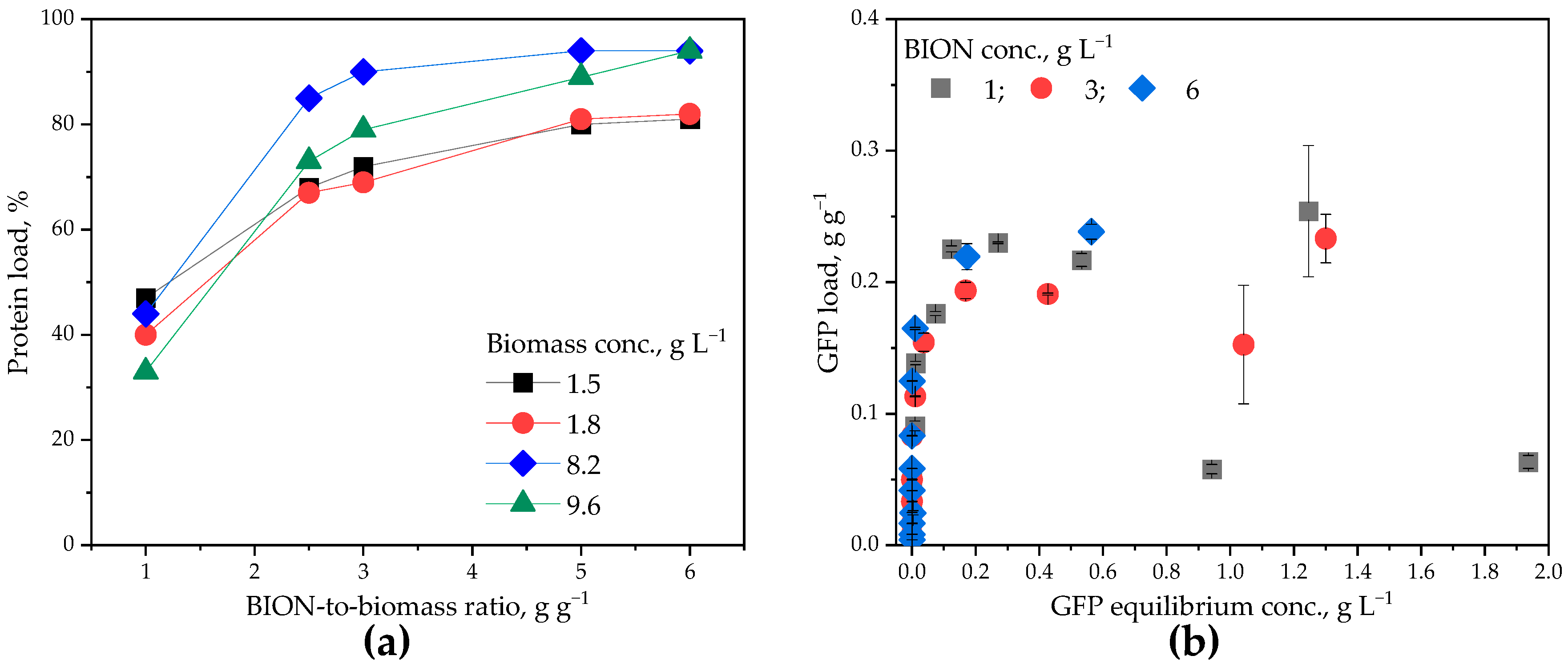
2.2. Potential to Find Predominating Interactions
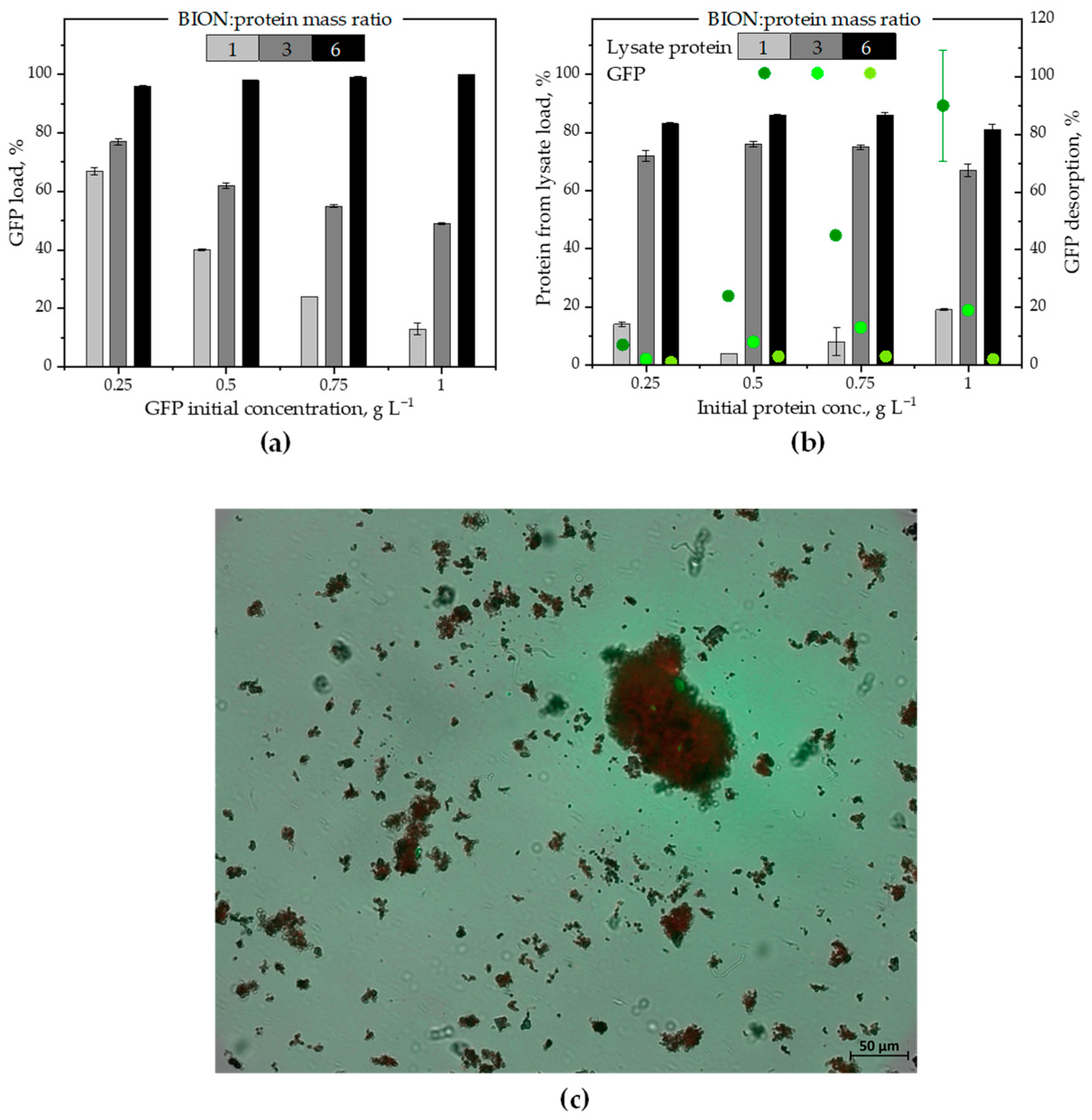
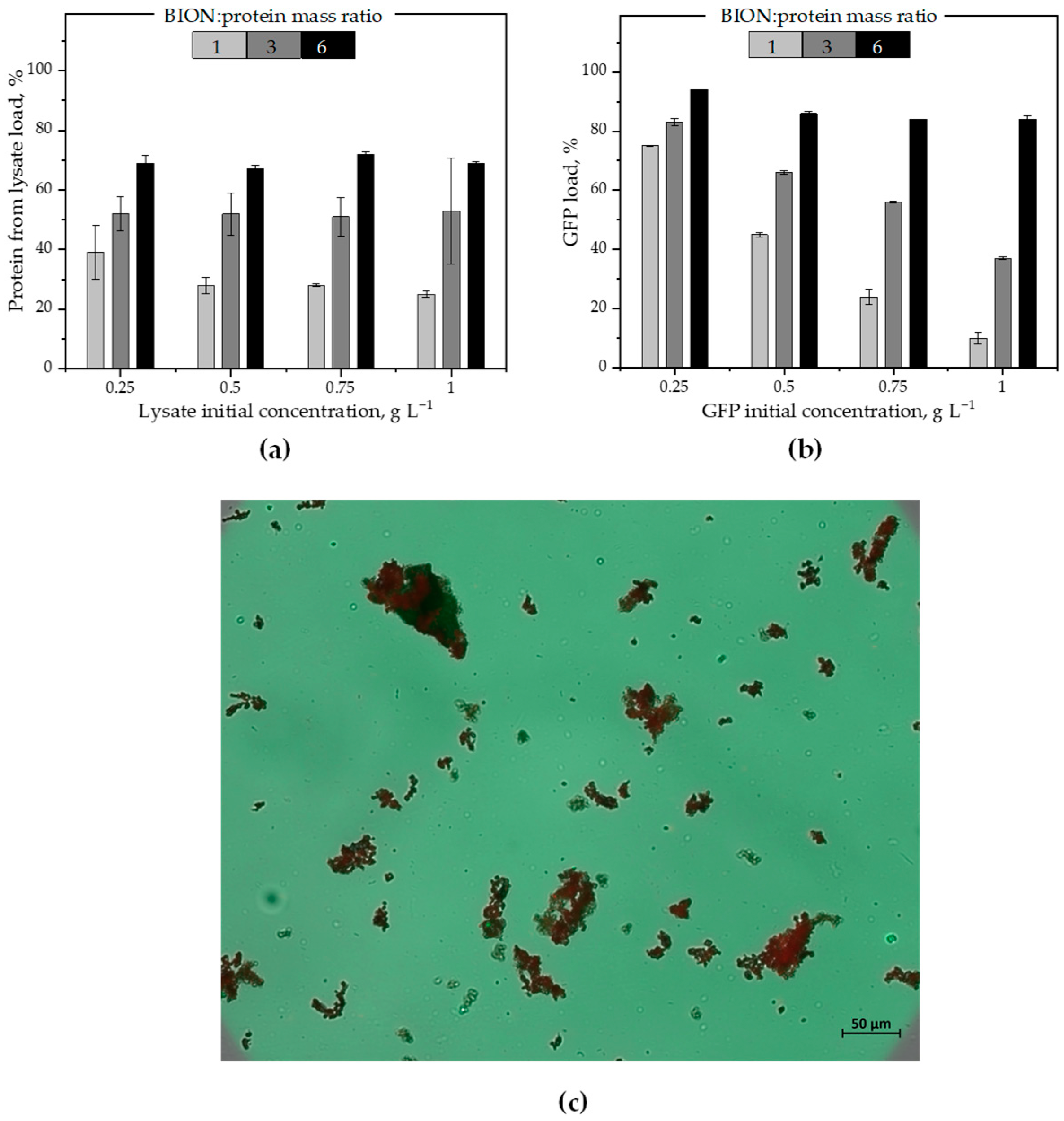
2.3. Protein Exchange Between the Corona and the Environment
3. Materials and Methods
- In Section 2.1, two iron oxides with different oxidation degrees and clearly different colors, black magnetite and brown maghemite, are used to compare their ability to separate proteins.
- In Section 2.2, the separated protein bands from the magnetite sample from Section 2.1 are compared to the protein bands remaining in solution through analysis of SDS-gels.
- Section 2.3 builds the core of the manuscript and presents data upon sequential adsorption of GFP and of lysate proteins onto the BIONs (i.e., the magnetite nanoparticles). This last section reinforces the impact of the nanoparticle-to-protein ratios on the nanoparticles’ adsorption capacity for proteins for different total molecule concentrations in the system and for a low nanoparticle-to-protein ratio (below the monolayer saturation value) compared to higher ratios.
3.1. Nanoparticle Synthesis and Characterization
3.2. Green Fluorescent Protein
3.3. Microchloropsis Salina
3.4. Microalgal Cell Lysis
3.5. Batch Adsorption of Microalgal Lysate onto Nanoparticles
3.6. Batch Adsorption of GFP onto BIONs
3.7. Sequential Batch Adsorption Experiments
3.8. Total Protein Quantification by BCA Assay
3.9. GFP Quantification via Fluorescence Measurement
3.10. Protein Visualization via SDS-PAGE
3.11. Optical Characterization of Solid Phases by Light Microscopy
3.12. Transmission Electron Microscopy Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpick, R.W.; Liu, G.-Y.; Hemminger, J.C. At the Cutting Edge of Surface Science: A Tribute to Miquel B. Salmeron. J. Phys. Chem. B 2018, 122, 399–400. [Google Scholar] [CrossRef]
- Guo, P.; Wang, Y.; Cui, H.; Yao, X.; Guan, G.; Han, M.-Y. Nano-bio-interface: Unleashing the Potential of Noble Nanometals. Small Sci. 2024, 4, 2300227. [Google Scholar] [CrossRef]
- Peng, M.; Li, C.; Wang, Z.; Wang, M.; Zhang, Q.; Xu, B.; Li, M.; Ma, D. Interfacial Catalysis at Atomic Level. Chem. Rev. 2025, 125, 2371–2439. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Fayad, M.A.; Abdul Wahhab, H.A.; Al-Azzawi, W.K.; Mohammed, J.K.; Majdi, H.S. Interfacial Engineering for Advanced Functional Materials: Surfaces, Interfaces, and Applications. Results Eng. 2024, 22, 102125. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef] [PubMed]
- Araújo, E.V.; Carneiro, S.V.; Neto, D.M.A.; Freire, T.M.; Costa, V.M.; Freire, R.M.; Fechine, L.M.U.D.; Clemente, C.S.; Denardin, J.C.; Dos Santos, J.C.S.; et al. Advances in surface design and biomedical applications of magnetic nanoparticles. Adv. Colloid Interface Sci. 2024, 328, 103166. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.-P.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, e2304848. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Jary, J.; Machnicka, B. Comprehensive Analysis of the Potential Toxicity of Magnetic Iron Oxide Nanoparticles for Medical Applications: Cellular Mechanisms and Systemic Effects. Int. J. Mol. Sci. 2024, 25, 12013. [Google Scholar] [CrossRef]
- Bushra, R.; Ahmad, M.; Alam, K.; Seidi, F.; Qurtulen; Shakeel, S.; Song, J.; Jin, Y.; Xiao, H. Recent advances in magnetic nanoparticles: Key applications, environmental insights, and future strategies. Sustain. Mater. Technol. 2024, 40, e00985. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Fraga-García, P.; Eigenfeld, M.; Becker, T.M.; Berensmeier, S. Magnetic Separation in Bioprocessing Beyond the Analytical Scale: From Biotechnology to the Food Industry. Front. Bioeng. Biotechnol. 2019, 7, 233. [Google Scholar] [CrossRef]
- Frost, R.; Langhammer, C.; Cedervall, T. Real-time in situ analysis of biocorona formation and evolution on silica nanoparticles in defined and complex biological environments. Nanoscale 2017, 9, 3620–3628. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef]
- Lundqvist, M.; Augustsson, C.; Lilja, M.; Lundkvist, K.; Dahlbäck, B.; Linse, S.; Cedervall, T. The nanoparticle protein corona formed in human blood or human blood fractions. PLoS ONE 2017, 12, e0175871. [Google Scholar] [CrossRef]
- Hellstrand, E.; Lynch, I.; Andersson, A.; Drakenberg, T.; Dahlbäck, B.; Dawson, K.A.; Linse, S.; Cedervall, T. Complete high-density lipoproteins in nanoparticle corona. FEBS J. 2009, 276, 3372–3381. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Nienhaus, K.; Nienhaus, G.U. Mechanistic Understanding of Protein Corona Formation around Nanoparticles: Old Puzzles and New Insights. Small 2023, 19, e2301663. [Google Scholar] [CrossRef]
- Li, S.; Cortez-Jugo, C.; Ju, Y.; Caruso, F. Approaching Two Decades: Biomolecular Coronas and Bio-Nano Interactions. ACS Nano 2024, 18, 33257–33263. [Google Scholar] [CrossRef]
- López-Estévez, A.M.; Lapuhs, P.; Pineiro-Alonso, L.; Alonso, M.J. Personalized Cancer Nanomedicine: Overcoming Biological Barriers for Intracellular Delivery of Biopharmaceuticals. Adv. Mater. 2024, 36, e2309355. [Google Scholar] [CrossRef]
- Hadjidemetriou, M.; Mahmoudi, M.; Kostarelos, K. In vivo biomolecule corona and the transformation of a foe into an ally for nanomedicine. Nat. Rev. Mater. 2024, 9, 219–222. [Google Scholar] [CrossRef]
- Wheeler, K.E.; Chetwynd, A.J.; Fahy, K.M.; Hong, B.S.; Tochihuitl, J.A.; Foster, L.A.; Lynch, I. Environmental dimensions of the protein corona. Nat. Nanotechnol. 2021, 16, 617–629. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Landry, M.P.; Moore, A.; Coreas, R. The protein corona from nanomedicine to environmental science. Nat. Rev. Mater. 2023, 8, 422–438. [Google Scholar] [CrossRef]
- Vu Thanh, C.; Gooding, J.J.; Kah, M. Learning lessons from nano-medicine to improve the design and performances of nano-agrochemicals. Nat. Commun. 2025, 16, 2306. [Google Scholar] [CrossRef]
- Abarca-Cabrera, L.; Milinovic, O.; Heitler, V.; Rühmann, B.; Kudermann, J.; Kube, M.; Dietz, H.; Sieber, V.; Berensmeier, S.; Fraga-García, P. Biocorona on Iron Oxide Nanoparticles in a Complex Biotechnological Environment: Analysis of Proteins, Lipids, and Carbohydrates. Small Sci. 2023, 3, 2300064. [Google Scholar] [CrossRef] [PubMed]
- Quagliarini, E.; Giulimondi, F.; Renzi, S.; Pirrottina, A.; Zingoni, A.; Carboni, N.; Pozzi, D.; Caracciolo, G. Protein-DNA Competition at the Bio-Nano Interface: Structural and Biological Insights From Graphene Oxide Coronas. Adv. Mater. Interfaces 2025, 12, 2400560. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Corbo, C.; Molinaro, R.; Lakey, J.R.T. Biohybrid Nanoparticles to Negotiate with Biological Barriers. Small 2019, 15, e1902333. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Weber, C.; Morsbach, S.; Landfester, K. Possibilities and Limitations of Different Separation Techniques for the Analysis of the Protein Corona. Angew. Chem. Int. Ed. Engl. 2019, 58, 12787–12794. [Google Scholar] [CrossRef]
- Gurtu, A.; Akhtar, N.; Verma, M.; Singh, K.; Moyle-Heyrman, G.; Bakshi, M.S. Functionalized Iron Oxide–Metal Hybrid Nanoparticles for Protein Extraction from Complex Fluids. Ind. Eng. Chem. Res. 2020, 59, 1045–1055. [Google Scholar] [CrossRef]
- Fraga-García, P.; Kubbutat, P.; Brammen, M.; Schwaminger, S.; Berensmeier, S. Bare Iron Oxide Nanoparticles for Magnetic Harvesting of Microalgae: From Interaction Behavior to Process Realization. Nanomaterials 2018, 8, 292. [Google Scholar] [CrossRef]
- Chapman, R.L. Algae: The world’s most important “plants”—An introduction. Mitig. Adapt. Strateg. Glob. Change 2013, 18, 5–12. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.; Zhang, Z.; Lan, C.Q. High cell density culture of microalgae in horizontal thin-layer algal reactor: Modeling of light attenuation and cell growth kinetics. Chem. Eng. J. 2024, 496, 154175. [Google Scholar] [CrossRef]
- Schädler, T.; Thurn, A.-L.; Brück, T.; Weuster-Botz, D. Continuous Production of Lipids with Microchloropsis salina in Open Thin-Layer Cascade Photobioreactors on a Pilot Scale. Energies 2021, 14, 500. [Google Scholar] [CrossRef]
- Koruyucu, A.; Peest, T.; Korzin, E.; Gröninger, L.; Patricia; Brück, T.; Weuster-Botz, D. Cell Disruption and Hydrolysis of Microchloropsis salina Biomass as a Feedstock for Fermentation. Appl. Sci. 2024, 14, 9667. [Google Scholar] [CrossRef]
- Apel, A.C.; Pfaffinger, C.E.; Basedahl, N.; Mittwollen, N.; Göbel, J.; Sauter, J.; Brück, T.; Weuster-Botz, D. Open thin-layer cascade reactors for saline microalgae production evaluated in a physically simulated Mediterranean summer climate. Algal Res. 2017, 25, 381–390. [Google Scholar] [CrossRef]
- Fernandes, T.; Cordeiro, N. Microalgae as Sustainable Biofactories to Produce High-Value Lipids: Biodiversity, Exploitation, and Biotechnological Applications. Mar. Drugs 2021, 19, 573. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Kang, N.K.; Choi, G.-G.; Kim, E.K.; Shin, S.-E.; Jeon, S.; Park, M.S.; Jeong, K.J.; Jeong, B.-R.; Chang, Y.K.; Yang, J.-W.; et al. Heterologous overexpression of sfCherry fluorescent protein in Nannochloropsis salina. Biotechnol. Rep. 2015, 8, 10–15. [Google Scholar] [CrossRef]
- Kang, N.K.; Kim, E.K.; Kim, Y.U.; Lee, B.; Jeong, W.-J.; Jeong, B.-R.; Chang, Y.K. Increased lipid production by heterologous expression of AtWRI1 transcription factor in Nannochloropsis salina. Biotechnol. Biofuels 2017, 10, 231. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Kiong, T.S.; Jathar, L.; Nik Ghazali, N.N.; Ramesh, S.; Awasarmol, U.; Ong, H.C. Perspectives on cultivation and harvesting technologies of microalgae, towards environmental sustainability and life cycle analysis. Chemosphere 2024, 353, 141540. [Google Scholar] [CrossRef]
- Schobesberger, M.; Helmhagen, S.; Mende, S.; Berensmeier, S.; Fraga-García, P. From Micro to Nano: Grinding Natural Magnetite Ore for Microalgae Harvesting. Magnetochemistry 2023, 9, 149. [Google Scholar] [CrossRef]
- Schobesberger, M.; Kopp Real, B.; Meijer, D.; Berensmeier, S.; Fraga-García, P. Natural magnetite ore as a harvesting agent for saline microalgae Microchloropsis salina. Bioresour. Technol. Rep. 2021, 15, 100798. [Google Scholar] [CrossRef]
- Schwaminger, S.; Syhr, C.; Berensmeier, S. Controlled Synthesis of Magnetic Iron Oxide Nanoparticles: Magnetite or Maghemite? Crystals 2020, 10, 214. [Google Scholar] [CrossRef]
- Roth, H.-C.; Schwaminger, S.P.; Schindler, M.; Wagner, F.E.; Berensmeier, S. Influencing factors in the CO-precipitation process of superparamagnetic iron oxide nano particles: A model based study. J. Magn. Magn. Mater. 2015, 377, 81–89. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Massart, R. Magnetic Fluids and Process for Obtaining Them. U.S. Patent No. 4,329,241, 11 May 1982. [Google Scholar]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. CrystEngComm 2017, 19, 246–255. [Google Scholar] [CrossRef]
- Strojan, K.; Leonardi, A.; Bregar, V.B.; Križaj, I.; Svete, J.; Pavlin, M. Dispersion of Nanoparticles in Different Media Importantly Determines the Composition of Their Protein Corona. PLoS ONE 2017, 12, e0169552. [Google Scholar] [CrossRef]
- Jedlovszky-Hajdú, A.; Bombelli, F.B.; Monopoli, M.P.; Tombácz, E.; Dawson, K.A. Surface coatings shape the protein corona of SPIONs with relevance to their application in vivo. Langmuir 2012, 28, 14983–14991. [Google Scholar] [CrossRef]
- De Castro, C.E.; Panico, K.; Stangherlin, L.M.; Albuquerque, L.J.C.; Ribeiro, C.A.S.; Da Silva, M.C.C.; Jäger, E.; Giacomelli, F.C. Evidence of protein coronas around soft nanoparticles regardless of the chemical nature of the outer surface: Structural features and biological consequences. J. Mater. Chem. B 2021, 9, 2073–2083. [Google Scholar] [CrossRef]
- Fomina, M.; Skorochod, I. Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications. Minerals 2020, 10, 861. [Google Scholar] [CrossRef]
- Weiss, A. Organic derivatives of clay minerals, zeolites and related minerals. In Organic Geochemistry; Springer: New York, NY, USA, 1969. [Google Scholar]
- Al-Ani, T.; Sarapää, O. Clay and Clay Mineralogy: Physical—Chemical Properties and Industrial Uses. 2008. Available online: https://www.researchgate.net/publication/292706105_Clay_and_clay_mineralogy (accessed on 9 September 2025).
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses, 2nd ed.; Completely Revised and Extended Edition; Wiley-VCH: Weinheim, Germany, 2003; ISBN 3527302743. [Google Scholar]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1993, 43, 30–59. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Overbeek, J. Theory of the stability of lyophobic colloids. J. Phys. Colloid Chem. 1947, 51, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Sapp, A.D.; Díaz-Cano, C.E.; Lengyel, J.; Abarca-Cabrera, L.; Fraga-García, P. Amino Acid Adsorption Onto Magnetic Nanoparticles Reveals Correlations with Physicochemical Parameters. ChemNanoMat 2024, 10, e202400280. [Google Scholar] [CrossRef]
- Abarca-Cabrera, L.; Xu, L.; Berensmeier, S.; Fraga-García, P. Competition at the Bio-nano Interface: A Protein, a Polysaccharide, and a Fatty Acid Adsorb onto Magnetic Nanoparticles. ACS Appl. Bio Mater. 2023, 6, 146–156. [Google Scholar] [CrossRef]
- Demirevska, K.; Zasheva, D.; Dimitrov, R.; Simova-Stoilova, L.; Stamenova, M.; Feller, U. Drought stress effects on Rubisco in wheat: Changes in the Rubisco large subunit. Acta Physiol. Plant 2009, 31, 1129–1138. [Google Scholar] [CrossRef]
- Postma, P.R.; Suarez-Garcia, E.; Safi, C.; Yonathan, K.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Energy efficient bead milling of microalgae: Effect of bead size on disintegration and release of proteins and carbohydrates. Bioresour. Technol. 2017, 224, 670–679. [Google Scholar] [CrossRef]
- Kargul, J.; Nield, J.; Barber, J. Three-dimensional reconstruction of a light-harvesting complex I-photosystem I (LHCI-PSI) supercomplex from the green alga Chlamydomonas reinhardtii. Insights into light harvesting for PSI. J. Biol. Chem. 2003, 278, 16135–16141. [Google Scholar] [CrossRef]
- Grewe, S.; Ballottari, M.; Alcocer, M.; D’Andrea, C.; Blifernez-Klassen, O.; Hankamer, B.; Mussgnug, J.H.; Bassi, R.; Kruse, O. Light-Harvesting Complex Protein LHCBM9 Is Critical for Photosystem II Activity and Hydrogen Production in Chlamydomonas reinhardtii. Plant Cell 2014, 26, 1598–1611. [Google Scholar] [CrossRef]
- Kosugi, M.; Ohtani, S.; Hara, K.; Toyoda, A.; Nishide, H.; Ozawa, S.-I.; Takahashi, Y.; Kashino, Y.; Kudoh, S.; Koike, H.; et al. Characterization of the far-red light absorbing light-harvesting chlorophyll a/b binding complex, a derivative of the distinctive Lhca gene family in green algae. Front. Plant Sci. 2024, 15, 1409116. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Fraga-García, P.; Blank-Shim, S.A.; Straub, T.; Haslbeck, M.; Muraca, F.; Dawson, K.A.; Berensmeier, S. Magnetic One-Step Purification of His-Tagged Protein by Bare Iron Oxide Nanoparticles. ACS Omega 2019, 4, 3790–3799. [Google Scholar] [CrossRef]
- Fraga García, P.; Brammen, M.; Wolf, M.; Reinlein, S.; Freiherr von Roman, M.; Berensmeier, S. High-gradient magnetic separation for technical scale protein recovery using low cost magnetic nanoparticles. Sep. Purif. Technol. 2015, 150, 29–36. [Google Scholar] [CrossRef]
- García, P.F.; Freiherr von Roman, M.; Reinlein, S.; Wolf, M.; Berensmeier, S. Impact of nanoparticle aggregation on protein recovery through a pentadentate chelate ligand on magnetic carriers. ACS Appl. Mater. Interfaces 2014, 6, 13607–13616. [Google Scholar] [CrossRef]
- Roth, H.-C.; Schwaminger, S.P.; Peng, F.; Berensmeier, S. Immobilization of Cellulase on Magnetic Nanocarriers. ChemistryOpen 2016, 5, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kaveh-Baghbaderani, Y.; Allgayer, R.; Schwaminger, S.P.; Fraga-García, P.; Berensmeier, S. Magnetic Separation of Antibodies with High Binding Capacity by Site-Directed Immobilization of Protein A-Domains to Bare Iron Oxide Nanoparticles. ACS Appl. Nano Mater. 2021, 4, 4956–4963. [Google Scholar] [CrossRef]
- Zimmermann, I.; Kaveh-Baghbaderani, Y.; Eilts, F.; Kohn, N.; Fraga-García, P.; Berensmeier, S. Direct Affinity Ligand Immobilization onto Bare Iron Oxide Nanoparticles Enables Efficient Magnetic Separation of Antibodies. ACS Appl. Bio Mater. 2024, 7, 3942–3952. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-W.; Holoman, T.R.; Kofinas, P.; Bentley, W.E. Towards oriented assembly of proteins onto magnetic nanoparticles. Biochem. Eng. J. 2008, 38, 164–170. [Google Scholar] [CrossRef]
- Ward, W.W. Biochemical and Physical Properties of Green Fluorescent Protein. In Green Fluorescent Protein; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 39–65. [Google Scholar]
- Chang, S.Y.; Zheng, N.-Y.; Chen, C.-S.; Chen, C.-D.; Chen, Y.-Y.; Wang, C.R.C. Analysis of peptides and proteins affinity-bound to iron oxide nanoparticles by MALDI MS. J. Am. Soc. Mass Spectrom. 2007, 18, 910–918. [Google Scholar] [CrossRef][Green Version]
- Huang, R.; Carney, R.P.; Stellacci, F.; Lau, B.L.T. Protein-nanoparticle interactions: The effects of surface compositional and structural heterogeneity are scale dependent. Nanoscale 2013, 5, 6928–6935. [Google Scholar] [CrossRef]
- Vilanova, O.; Mittag, J.J.; Kelly, P.M.; Milani, S.; Dawson, K.A.; Rädler, J.O.; Franzese, G. Understanding the Kinetics of Protein-Nanoparticle Corona Formation. ACS Nano 2016, 10, 10842–10850. [Google Scholar] [CrossRef]
- Yang, H.; Wang, M.; Zhang, Y.; Liu, X.; Yu, S.; Guo, Y.; Yang, S.; Yang, L. Detailed insight into the formation of protein corona: Conformational change, stability and aggregation. Int. J. Biol. Macromol. 2019, 135, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Schädler, T.; Caballero Cerbon, D.; de Oliveira, L.; Garbe, D.; Brück, T.; Weuster-Botz, D. Production of lipids with Microchloropsis salina in open thin-layer cascade photobioreactors. Bioresour. Technol. 2019, 289, 121682. [Google Scholar] [CrossRef] [PubMed]
- Schädler, T.; Neumann-Cip, A.-C.; Wieland, K.; Glöckler, D.; Haisch, C.; Brück, T.; Weuster-Botz, D. High-Density Microalgae Cultivation in Open Thin-Layer Cascade Photobioreactors with Water Recycling. Appl. Sci. 2020, 10, 3883. [Google Scholar] [CrossRef]
- Del Pino, P.; Pelaz, B.; Zhang, Q.; Maffre, P.; Nienhaus, G.U.; Parak, W.J. Protein corona formation around nanoparticles—From the past to the future. Mater. Horiz. 2014, 1, 301–313. [Google Scholar] [CrossRef]
- Han, G.S.; Domaille, D.W. Protein Corona, with a Twist. ACS Cent. Sci. 2020, 6, 14–15. [Google Scholar] [CrossRef]
- Ferdosi, S.; Stukalov, A.; Hasan, M.; Tangeysh, B.; Brown, T.R.; Wang, T.; Elgierari, E.M.; Zhao, X.; Huang, Y.; Alavi, A.; et al. Enhanced Competition at the Nano-Bio Interface Enables Comprehensive Characterization of Protein Corona Dynamics and Deep Coverage of Proteomes. Adv. Mater. 2022, 34, e2206008. [Google Scholar] [CrossRef]
- Davidson, A.M.; Brust, M.; Cooper, D.L.; Volk, M. Sensitive Analysis of Protein Adsorption to Colloidal Gold by Differential Centrifugal Sedimentation. Anal. Chem. 2017, 89, 6807–6814. [Google Scholar] [CrossRef]
- Danielsson, R.; Mile, I.; Eriksson, H. Adsorption and Desorption of Immune-Modulating Substances by Aluminium-Based Adjuvants: An Overlooked Feature of the Immune-Stimulating Mechanisms of Aluminium-Based Adjuvants. Int. J. Mol. Sci. 2024, 25, 12399. [Google Scholar] [CrossRef]
- Armengol-Badia, O.; Maggi, J.; Casal, C.; Cortés, R.; Abián, J.; Carrascal, M.; Closa, D. The Microenvironment in an Experimental Model of Acute Pancreatitis Can Modify the Formation of the Protein Corona of sEVs, with Implications on Their Biological Function. Int. J. Mol. Sci. 2024, 25, 12969. [Google Scholar] [CrossRef]
- Vianello, F.; Cecconello, A.; Magro, M. Toward the Specificity of Bare Nanomaterial Surfaces for Protein Corona Formation. Int. J. Mol. Sci. 2021, 22, 7625. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Wu, J.; Zhang, W.; Xia, Y. Illuminating the dark space of neutral glycosphingolipidome by selective enrichment and profiling at multi-structural levels. Nat. Commun. 2024, 15, 5627. [Google Scholar] [CrossRef]
- Juling, S.; Niedzwiecka, A.; Böhmert, L.; Lichtenstein, D.; Selve, S.; Braeuning, A.; Thünemann, A.F.; Krause, E.; Lampen, A. Protein Corona Analysis of Silver Nanoparticles Links to Their Cellular Effects. J. Proteome Res. 2017, 16, 4020–4034. [Google Scholar] [CrossRef]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef]
- Caputo, D.; Quagliarini, E.; Pozzi, D.; Caracciolo, G. Nanotechnology Meets Oncology: A Perspective on the Role of the Personalized Nanoparticle-Protein Corona in the Development of Technologies for Pancreatic Cancer Detection. Int. J. Mol. Sci. 2022, 23, 10591. [Google Scholar] [CrossRef] [PubMed]
- Khutsishvili, S.S.; Gagelidze, N.; Tsokolakyan, A.S.; Yeranosyan, M.A.; Tkesheliadze, E.; Sargsyan, V.A.; Dughashvili, D.; Dzebisashvili, N.; Aronia, K.; Benashvili, A.; et al. ι-Carrageenan Manganese Oxide Bionanocomposites as a Promising Solution to Agricultural Challenges. Materials 2025, 18, 495. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; La Cabrera-De Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and Nanomaterials as Plant Biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga-García, P.; Haßelt, S.; Díaz-Cano, C.E.; Abarca-Cabrera, L.; Kaveh-Baghbaderani, Y.; Schwaminger, S.P.; Kube, M.; Dietz, H. Bio Meets Nano: Protein Exchange in Saline Biocoronae on Magnetic Nanoparticles. Int. J. Mol. Sci. 2025, 26, 8995. https://doi.org/10.3390/ijms26188995
Fraga-García P, Haßelt S, Díaz-Cano CE, Abarca-Cabrera L, Kaveh-Baghbaderani Y, Schwaminger SP, Kube M, Dietz H. Bio Meets Nano: Protein Exchange in Saline Biocoronae on Magnetic Nanoparticles. International Journal of Molecular Sciences. 2025; 26(18):8995. https://doi.org/10.3390/ijms26188995
Chicago/Turabian StyleFraga-García, Paula, Sandra Haßelt, Carlos Eduardo Díaz-Cano, Lucía Abarca-Cabrera, Yasmin Kaveh-Baghbaderani, Sebastian P. Schwaminger, Massimo Kube, and Hendrik Dietz. 2025. "Bio Meets Nano: Protein Exchange in Saline Biocoronae on Magnetic Nanoparticles" International Journal of Molecular Sciences 26, no. 18: 8995. https://doi.org/10.3390/ijms26188995
APA StyleFraga-García, P., Haßelt, S., Díaz-Cano, C. E., Abarca-Cabrera, L., Kaveh-Baghbaderani, Y., Schwaminger, S. P., Kube, M., & Dietz, H. (2025). Bio Meets Nano: Protein Exchange in Saline Biocoronae on Magnetic Nanoparticles. International Journal of Molecular Sciences, 26(18), 8995. https://doi.org/10.3390/ijms26188995







