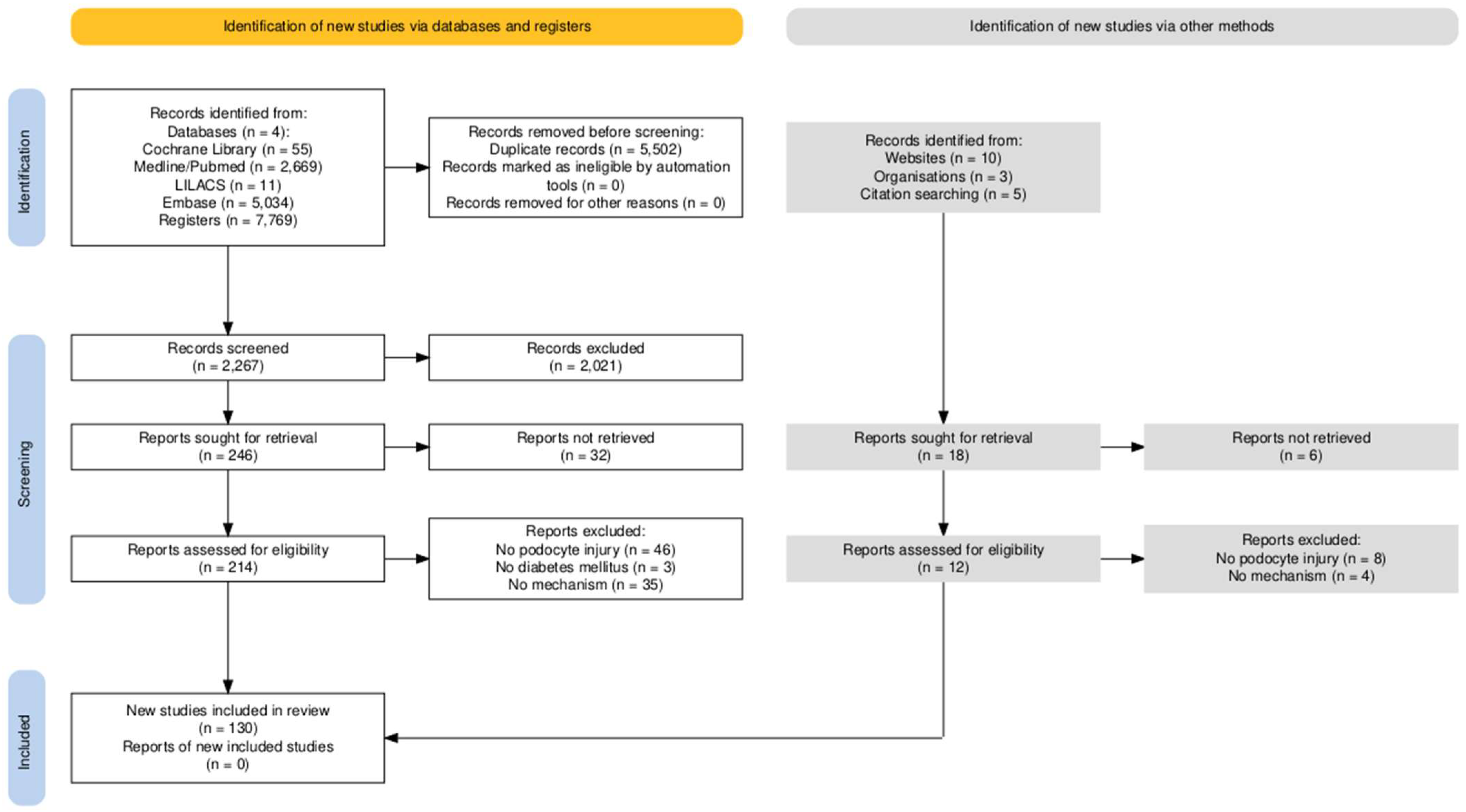
Approach to Studies on Podocyte Lesions Mediated by Hyperglycemia: A Systematic Review
Abstract
1. Introduction
2. Results
| Author | Year | Methodological Quality—% |
|---|---|---|
| Balint et al. [19] | 2023 | 87.50 |
| Zhou et al. [20] | 2017 | 62.50 |
| Yamashiro et al. [21] | 2024 | 68.75 |
| Ivanac-Janković et al. [22] | 2015 | 62.50 |
| Carson et al. [23] | 2014 | 81.25 |
| Arslan et al. [24] | 2025 | 81.25 |
| Shetty et al. [25] | 2021 | 68.75 |
| Ceol et al. [26] | 2012 | 62.50 |
| Canney et al. [27] | 2020 | 81.25 |
| Esselman et al. [28] | 2025 | 75.00 |
| Denhez et al. [29] | 2020 | 81.25 |
| Audzeyenka et al. [30] | 2020 | 87.50 |
| Hayashi et al. [31] | 2020 | 75.00 |
| Chen et al. [32] | 2020 | 87.50 |
| Angeletti et al. [33] | 2020 | 87.50 |
| Albrecht et al. [34] | 2023 | 81.25 |
| Endlich et al. [35] | 2018 | 62.50 |
| Fujimoto et al. [36] | 2020 | 81.25 |
| Hu et al. [37] | 2019 | 81.25 |
| Chen et al. [38] | 2024 | 50.00 |
| Fiorina et al. [39] | 2014 | 87.50 |
| Fang et al. [40] | 2021 | 81.25 |
| Hou et al. [41] | 2020 | 87.50 |
| Han et al. [42] | 2024 | 87.50 |
| Holderied et al. [43] | 2015 | 62.50 |
| Gujarati et al. [44] | 2024 | 87.50 |
| Cao et al. [45] | 2021 | 87.50 |
| Jiang et al. [46] | 2020 | 81.25 |
| Fu et al. [47] | 2020 | 50.00 |
| Hu et al. [48] | 2019 | 87.50 |
| Kimura et al. [49] | 2008 | 81.25 |
| Lei et al. [50] | 2024 | 87.50 |
| Hu et al. [51] | 2023 | 87.50 |
| Kondapi et al. [52] | 2021 | 87.50 |
| Hu et al. [53] | 2024 | 87.50 |
| Kondapi et al. [54] | 2021 | 87.50 |
| Shahzad et al. [55] | 2022 | 87.50 |
| Kawaguchi et al. [56] | 2021 | 87.50 |
| Jiang et al. [57] | 2022 | 87.50 |
| Inoki et al. [58] | 2011 | 87.50 |
| Gödel et al. [59] | 2011 | 87.50 |
| Langham et al. [60] | 2002 | 93.75 |
| Bai et al. [61] | 2018 | 87.50 |
| Wang et al. [62] | 2020 | 87.50 |
| Lai et al. [63] | 2020 | 87.50 |
| Hudkins et al. [64] | 2022 | 87.50 |
| Kostic et al. [65] | 2020 | 75.00 |
| Liebisch et al. [66] | 2020 | 75.00 |
| Liu et al. [67] | 2022 | 87.50 |
| Li et al. [68] | 2025 | 87.50 |
| Li et al. [69] | 2025 | 87.50 |
| Lu et al. [70] | 2021 | 87.50 |
| Liang et al. [71] | 2020 | 93.75 |
| Hu et al. [72] | 2025 | 87.50 |
| Lv et al. [73] | 2025 | 87.50 |
| Lu et al. [74] | 2021 | 87.50 |
| Miyauchi et al. [75] | 2009 | 87.50 |
| Martins et al. [76] | 2023 | 87.50 |
| Lizotte et al. [77] | 2023 | 87.50 |
| Lee et al. [78] | 2018 | 81.25 |
| Löwen et al. [79] | 2021 | 87.50 |
| Lu et al. [80] | 2023 | 87.50 |
| Li et al. [81] | 2024 | 87.50 |
| Nishad et al. [82] | 2021 | 87.50 |
| Petrica et al. [83] | 2021 | 100.00 |
| Matoba et al. [84] | 2021 | 87.50 |
| Palmer et al. [85] | 2021 | 100.00 |
| Naito et al. [86] | 2023 | 87.50 |
| Pan et al. [87] | 2024 | 75.00 |
| Morigi et al. [88] | 2020 | 87.50 |
| Khurana et al. [89] | 2023 | 100.00 |
| Mukhi et al. [90] | 2023 | 87.50 |
| Li et al. [91] | 2025 | 87.50 |
| Lv et al. [92] | 2024 | 62.50 |
| Shi et al. [93] | 2020 | 56.25 |
| Salvatore et al. [94] | 2014 | 81.25 |
| Liu et al. [95] | 2024 | 56.25 |
| Su et al. [96] | 2010 | 81.25 |
| Motrapu et al. [97] | 2020 | 68.75 |
| Rosenbloom et al. [98] | 2024 | 37.50 |
| Minakawa et al. [99] | 2019 | 68.75 |
| Sawada et al. [100] | 2023 | 81.25 |
| Sharma et al. [101] | 2016 | 56.25 |
| Sunilkumar et al. [102] | 2025 | 62.50 |
| Lu et al. [103] | 2024 | 62.50 |
| Petrica et al. [104] | 2023 | 100.00 |
| Boi et al. [105] | 2025 | 56.25 |
| Song et al. [13] | 2019 | 62.50 |
| Qin et al. [106] | 2020 | 56.25 |
| Vestra et al. [107] | 2003 | 81.25 |
| Tian et al. [108] | 2020 | 37.50 |
| Veron et al. [109] | 2021 | 62.50 |
| Su et al. [110] | 2022 | 62.50 |
| Suarez et al. [111] | 2024 | 56.25 |
| Song et al. [112] | 2022 | 62.50 |
| Sun et al. [113] | 2023 | 56.25 |
| Stefansson et al. [114] | 2022 | 87.50 |
| Woo et al. [115] | 2020 | 62.50 |
| Sun et al. [116] | 2025 | 62.50 |
| Tao et al. [117] | 2022 | 56.25 |
| Uil et al. [118] | 2021 | 87.50 |
| Yang et al. [119] | 2023 | 62.50 |
| Ward et al. [120] | 2025 | 31.25 |
| Yamaguchi et al. [121] | 2009 | 81.25 |
| Yao et al. [122] | 2020 | 56.25 |
| Li et al. [123] | 2020 | 62.50 |
| Zeng et al. [124] | 2023 | 100.00 |
| Xue et al. [125] | 2020 | 62.50 |
| Zeng et al. [126] | 2023 | 93.75 |
| Wang et al. [127] | 2021 | 62.50 |
| Zhang et al. [14] | 2016 | 56.25 |
| Wu et al. [128] | 2025 | 56.25 |
| Yu et al. [129] | 2022 | 62.50 |
| Sawada et al. [3] | 2016 | 81.25 |
| Li et al. [130] | 2024 | 93.75 |
| Wang et al. [131] | 2019 | 62.50 |
| Pan et al. [9] | 2018 | 56.25 |
| Xu et al. [132] | 2025 | 56.25 |
| Zhang et al. [133] | 2021 | 56.25 |
| Wang et al. [134] | 2024 | 62.50 |
| Sawai et al. [135] | 2006 | 81.25 |
| Zhou et al. [136] | 2019 | 62.50 |
| Zhao et al. [137] | 2023 | 87.50 |
| Zhang et al. [138] | 2024 | 62.50 |
| Zhang et al. [139] | 2024 | 62.50 |
| Zhu et al. [140] | 2025 | 62.50 |
| Zhang et al. [141] | 2025 | 62.50 |
| Zhou et al. [142] | 2024 | 87.50 |
| Zhu et al. [143] | 2021 | 62.50 |
| Zuo et al. [144] | 2024 | 62.50 |
| Mean | 81.25 | |
| SD | 18.84 | |
| CV—% | 23.19 |
| Autor (Year) | Mechanism of Lesion | Description |
|---|---|---|
| Langham et al., 2002 [60] | Reduced nephrin expression and glomerular permeability. | Nephrin downregulation increases glomerular permeability and is associated with proteinuria. ACE inhibition restores nephrin levels and mitigates albuminuria, indicating nephrin’s protective role in diabetic nephropathy. |
| Vestra et al., 2003 [107] | Mesangial expansion and podocyte injury | Podocyte density and structure are altered in type 2 diabetes, contributing to albuminuria. Podocyte density is inversely related to AER, and structural changes occur early in DN progression, indicating podocyte loss as a key pathogenic factor. |
| Sawai et al., 2006 [135] | Downregulation and heterogeneity of Cx43 in podocytes under hyperglycemia impairs intercellular communication and slit diaphragm integrity. | Loss of uniform Cx43 expression correlates with decreased renal function, implicating its role in diabetic nephropathy progression. |
| Kimura et al., 2008 [49] | Low alpha-actinin-4 expression damages podocytes and slit diaphragms, contributing to proteinuria. | Alpha-actinin-4 downregulation correlates with podocyte dysfunction and proteinuria severity in human diabetic kidneys. |
| Miyauchi et al., 2009 [75] | Podocyte loss is inversely correlated with proteinuria; hypertrophy compensates for podocyte loss due to glomerular pressure. | Human renal biopsy study shows reduced podocyte number correlates with increased proteinuria and hypertrophy. ACE-Is/ARBs included as treatment variables but their mechanistic role in podocyte hypertrophy is unclear. |
| Su et al., 2010 [96] | Reduced podocyte number and density in DN correlates inversely with proteinuria severity, involving WT1 changes and cytoplasmic structural alterations. | DN patients show decreased podocyte density and cytoplasmic coverage, with changes correlating with proteinuria levels. WT1 is used as a lesion marker. |
| Yamaguchi et al., 2009 [121] | Podocyte detachment from the glomerular basement membrane via EMT induced by FSP1, Snail1, ILK, and TGF-β1. | FSP1 expression in podocytes is associated with EMT markers and more severe clinical and pathological manifestations in diabetic nephropathy. |
| Inoki et al., 2011 [58] | mTORC1 hyperactivation. | Excessive mTORC1 activity in podocytes disrupts slit diaphragm protein localization, promotes epithelial–mesenchymal transition-like changes, induces ER stress, and leads to podocyte loss, mesangial expansion, and proteinuria in DN. |
| Gödel et al., 2011 [59] | mTOR dysregulation and podocyte stress. | Both overactivation and deletion of mTORC1 in podocytes lead to injury. mTOR signaling affects hypertrophy, foot process effacement, detachment, and autophagy suppression. Balanced mTOR activity is essential for podocyte homeostasis. |
| Ceol et al., 2012 [26] | ClC-5 overexpression in podocytes enhances albumin endocytosis, potentially compensating protein overload in nephropathies. | The study demonstrates ClC-5 is overexpressed in podocytes of proteinuric patients, supporting its role in albumin endocytosis. |
| Salvatore et al., 2014 [94] | Ischemic podocyte injury due to obliterative microvascular disease (arteriolosclerosis, hyalinosis), contributing to collapsing glomerulopathy (CG) in DN. | CG in DN is characterized by glomerular tuft collapse and epithelial proliferation, with loss of podocyte markers and VEGF overexpression. It is linked to poor prognosis and progression to ESRD. |
| Carson et al., 2014 [23] | Impaired lysosomal degradation in podocytes leads to albumin accumulation, cytokine production, and glomerulosclerosis. | Lysosomal dysfunction in podocytes impairs albumin processing, increases cytokine production and promotes glomerulosclerosis. |
| Fiorina et al., 2014 [39] | High glucose induces podocyte CD80/B7-1 via PI3Kα, leading to cytoskeleton disruption and apoptosis; reversed by CTLA4-Ig. | CD80/B7-1 upregulation mediates diabetic podocyte damage and albuminuria; blockade via CTLA4-Ig shows therapeutic potential. |
| Ivanac-Janković et al., 2015 [22] | Downregulation of BMP-7 in DN enhances TGF-β-driven fibrosis and reduces podocyte survival. | The study shows BMP-7 expression decreases in advanced DN stages, highlighting its protective role against inflammation and fibrosis. |
| Holderied et al., 2015 [43] | Diabetic-induced activation of PECs increases ECM secretion, thickening Bowman’s capsule under hyperglycemic and AGE conditions. | Diabetic PECs promote ECM expansion of Bowman’s capsule, worsening glomerular sclerosis in the absence of TGF-b1 autocrine feedback. |
| Sharma’s et al., 2016 [101] | MDM2 downregulation disrupts podocyte and tubular function, impairing metabolic pathways and reducing recovery capacity from injury. | MDM2 is reduced in DN and associated with metabolic dysfunction. Its loss leads to altered protein interactions and reduced renal resilience. |
| Zhang et al., 2016 [14] | Wnt/β-catenin signaling upregulates UCH-L1, altering podocyte morphology and increasing motility, contributing to DN. | High glucose increases UCH-L1 via Wnt/β-catenin signaling in podocytes; UCH-L1 is a potential therapeutic target in DN. |
| Sawada et al., 2016 [3] | Upregulation of α3β1-Integrin in podocytes contributes to foot process effacement and detachment via TGF-β1 signaling. | α3β1-Integrin is upregulated in early DN stages, facilitating detachment and cytoskeletal changes in podocytes. |
| Zhou et al., 2017 [20] | High glucose-induced miR-27a upregulation suppresses PPARγ, activates β-catenin, promotes mesenchymal transition and podocyte apoptosis. | miR-27a expression is stimulated by hyperglycemia, suppressing PPARγ and activating β-catenin, leading to podocyte injury and renal dysfunction in diabetic rats. |
| Bai et al., 2018 [61] | LINC01619/miR-27a/FOXO1 axis dysregulation. | Downregulation of LINC01619 removes its sponge effect on miR-27a, leading to suppression of FOXO1 and induction of ER stress. This enhances oxidative stress, apoptosis, and foot process effacement in podocytes. |
| Endlich et al., 2018 [35] | BDNF knockdown reduces nephrin/podocin, alters glomerular morphology, induces podocyte dedifferentiation and developmental defects. | Zebrafish and human data confirm BDNF’s role in podocyte function and as a biomarker for glomerular injury. |
| Lee et al., 2018 [78] | Palmitic acid induces mitochondrial ROS and reduces antioxidant enzymes, exacerbating oxidative stress in podocytes. | PA increases antioxidant proteins transiently but causes long-term suppression, correlating with oxidative damage in advanced DN. |
| Canney et al., 2020 [27] | Podocyte dedifferentiation and foot process effacement reversed by Roux-en-Y gastric bypass via improved glucose control. | Post-RYGB, patients showed reduced albuminuria and improved podocyte structure, associated with enhanced metabolic control. |
| Hu et al., 2020 [37] | Saxagliptin inhibits renal p38MAPK and enhances nephrin/podocin expression, independently of glucose-lowering effects. | Saxagliptin reduces renal injury by modulating nephrin/podocin expression and suppressing inflammation-related signaling. |
| Hu et al., 2023 [48] | LRH-1/GLS2 downregulation in podocytes impairs glutaminolysis, causing mitochondrial dysfunction and apoptosis. | LRH-1 loss impairs glutamine metabolism, driving podocyte apoptosis; restoring LRH-1 mitigates DKD injury. |
| Liang et al., 2020 [71] | GSK3β overactivity and ECM accumulation. | GSK3β is overexpressed in DKD, promoting ECM accumulation and podocyte injury. Its urinary activity predicts disease progression, serving as a potential biomarker. |
| Minakawa et al., 2019 [99] | Glomerular enlargement in early T2DM causes podocyte hypertrophic stress, detachment, and loss, leading to albuminuria. | In Zucker rats, glomerular volume increase precedes albuminuria, correlating with podocyte depletion. Detachment is driven by IGF1/2 and mTORC1 activation. |
| Song et al., 2019 [13] | TXNIP induction via high glucose. | Thioredoxin-interacting protein (TXNIP) is induced by high glucose and promotes oxidative stress by inhibiting thioredoxin. TXNIP knockdown disrupts EMT, reduces ROS, and inhibits mTOR pathway. In diabetic mice, TXNIP deficiency alleviates renal damage, and its expression correlates with mTOR activation in DN biopsies. |
| Qin et al., 2019 [106] | Mitochondrial dysfunction and impaired FAO. | Berberine improves insulin sensitivity and glucose tolerance, reduces albuminuria, and activates AMPK and PGC-1α pathways. These regulate mitochondrial energy homeostasis, fatty acid oxidation, and protect podocytes from oxidative stress in diabetic kidney disease. |
| Woo et al., 2020 [115] | Ceramide accumulation and mitochondrial injury. | Ceramide accumulation induces ROS-mediated mitochondrial damage in podocytes. Myriocin reduces ceramide synthesis and protects against DN progression. |
| Uil et al., 2021 [118] | miR-99a-5p regulation of mTOR and EMT. | miR-99a-5p inhibits mTOR and vimentin, protecting podocytes from EMT and injury in DN. Identified in extracellular vesicles from patients with micro/macroalbuminuria. |
| Wang et al., 2019 [131] | Downregulation of miR-27a/b increases FOXO1 expression, enhancing PEPCK and G6pase, leading to hepatic gluconeogenesis and hyperglycemia. | miR-27a/b regulates hepatic gluconeogenesis by targeting FOXO1. Overexpression reduces glucose output, suggesting a therapeutic role in type 2 diabetes. |
| Zhou et al., 2019 [136] | PGRN deficiency leads to mitochondrial dysfunction in podocytes by disrupting PGRN-Sirt1-PGC-1α/FoxO1 signaling, impairing mitophagy and biogenesis. | PGRN maintains mitochondrial homeostasis in podocytes. Its deficiency exacerbates injury, while rPGRN treatment restores function. |
| Shetty et al., 2021 [25] | Podocyte and tubular injury due to viral infection or systemic inflammation, with APOL1 genotypes increasing susceptibility. | Podocytopathy and protein overload tubulopathy were observed in COVID-19 patients, with APOL1 genotypes potentially influencing severity. |
| Denhez et al., 2020 [29] | Palmitate-induced FFA triggers IKKβ and mTORC1 activation, promoting insulin resistance in podocytes. | Palmitic acid induces podocyte insulin resistance via ceramide production and serine 307 phosphorylation of IRS1. |
| Audzeyenka et al., 2020 [30] | Cathepsin C overexpression in podocytes causes cytoskeletal disruption and insulin resistance under hyperglycemia. | Cathepsin C expression is increased in diabetic conditions, altering podocyte cytoskeleton and promoting albumin leakage. |
| Hayashi et al., 2020 [31] | Glomerular damage due to hyperglycemia-induced microvascular disorders, polyol pathway activation, cPKC activation, and podocyte loss; DGKα/67LR interactions maintain adhesion. | EGCg activates DGKα to maintain glomerular integrity and podocyte adhesion, mitigating diabetic nephropathy progression. |
| Chen et al., 2020 [32] | RARRES1 overexpression triggers podocyte apoptosis via RIOK1 interaction and p53 activation. | RARRES1 is upregulated in DN and induces podocyte injury via p53 signaling, supported by murine and human biopsy findings. |
| Fujimoto et al., 2020 [36] | High glucose suppresses podocyte ERAD pathway via mesangial crosstalk, leading to ER stress and nephrin phosphorylation suppression. | High-glucose-induced mesangial signals disrupt ERAD in podocytes, contributing to progressive DN and proteinuria. |
| Hou et al., 2020 [41] | HGF modulates PI3K/Akt-GSK3β-TFEB signaling to restore podocyte autophagy and lysosomal function. | HGF restores autophagy in diabetic podocytes, preserving function through PI3K/Akt-GSK3β-TFEB axis modulation. |
| Jiang et al., 2020 [46] | Smad3 activation under hyperglycemia disrupts cytoskeleton via transgelin and caspase-3, leading to podocyte damage. | Smad3 signaling under diabetic stress promotes actin remodeling and transgelin expression, compromising podocyte structure. |
| Fu et al., 2020 [47] | JAML activation impairs lipid metabolism in podocytes via SIRT1-SREBP1 and AMPK signaling. | JAML exacerbates podocyte lipid imbalance and injury through dysregulated SIRT1-mediated transcription and SREBP1 acetylation. |
| Wang et al., 2020 [62] | miR-770-5p-mediated TIMP3 suppression. | miR-770-5p upregulation promotes podocyte apoptosis and inflammation by targeting TIMP3, a protective factor. Its depletion reduces these effects, indicating a role in DN pathogenesis and a potential therapeutic target. |
| Lai et al., 2020 [63] | BAMBI deletion and TGF-β pathway overactivation. | Loss of BAMBI enhances TGF-β signaling. In podocytes, ALK5/Smad2/3 activation leads to apoptosis and loss. In endothelial cells, ALK1/Smad1/5 promotes proliferation and vascular dysfunction, contributing to DN progression. |
| Kostic et al., 2020 [65] | Tubulointerstitial fibrosis and mitochondrial damage. | CKD progression involves oxidative stress, glomerulosclerosis, and AIF-related pathways mediating apoptosis and cell survival. AIF is upregulated in glomeruli of diabetic rats, suggesting its role as a biomarker. |
| Liebisch et al., 2020 [66] | Epigenetic alterations due to AGEs. | AGEs reduce NIPP1 and EZH2 expression, decreasing H3K27me3 and promoting transcription of pro-disease genes in podocytes, contributing to DKD and metabolic memory. |
| Morigi et al., 2020 [88] | Complement activation via C3a causes podocyte mitochondrial damage and dysfunction, leading to proteinuria. | C3aR blockade restores mitochondrial function and podocyte density, offering therapeutic potential in DN. |
| Shi et al., 2020 [152] | Hyperglycemia upregulates HDAC4, which enhances calcineurin (CaN) signaling and promotes podocyte apoptosis. HDAC4 knockdown reduces CaN expression and apoptosis. | HDAC4 mediates CaN signaling in high-glucose conditions, leading to podocyte apoptosis. Its silencing attenuates this effect, suggesting a key role in DN pathophysiology. |
| Motrapu et al., 2020 [97] | Podocyte loss due to increased shear stress from RAS and SGLT2 pathways; MRE therapy increases podocyte density, further improved by BIO enhancing filtration slit density. | BIO combined with MRE attenuates CKD progression in diabetic mice by restoring podocyte numbers and filtration structure, improving GFR. |
| Tian et al., 2020 [108] | Loss of GAK and calpain activation. | Podocyte-specific GAK deficiency leads to albuminuria and glomerulosclerosis. Increased intracellular calcium activates calpain-1/2, causing NF-κB activation and GADD45B expression. Calpain inhibition mitigates these effects. |
| Yao et al., 2020 [122] | circ_0000285 sponges miR-654-3p, activating MAPK6 and promoting podocyte injury. | circ_0000285 promotes diabetic nephropathy progression via miR-654-3p suppression and MAPK6 activation. |
| Li et al., 2021 [123] | Activation of EGFR signaling increases rubicon expression and inhibits autophagy through mTOR-p70 S6K and RPS6 pathways, contributing to podocyte injury. | EGFR deletion in podocytes enhances autophagy and reduces albuminuria and inflammation, highlighting its role in DN progression. |
| Xue et al., 2020 [125] | Modulation of the PTEN-PDK1-Akt-mTOR pathway and reduction in Nox4-driven ROS production improves podocyte viability. | Xuesaitong treatment in diabetic rats reduces albuminuria and podocyte apoptosis via PTEN pathway regulation and oxidative stress mitigation. |
| Wang et al., 2021 [127] | Cdk5 upregulation under diabetic conditions leads to synaptopodin/nephrin downregulation, ROS increase, mitochondrial fission, and ATP depletion. | Cdk5-mediated mitochondrial dysfunction contributes to podocyte injury; inhibition of Cdk5 improves mitochondrial function and protects podocytes. |
| Cao et al., 2021 [45] | Diminished DACH1 reduces PTIP recruitment, increases H3K4Me3, and enhances gene transcription linked to podocyte vulnerability. | Loss of DACH1 epigenetically activates injury pathways; restoring DACH1 protects podocytes against DKD-related stress. |
| Kondapi et al., 2021 [52] | Hyperglycemia induces nephrin excretion, podocyte foot process retraction, cytoskeletal rearrangement, and glomerular/tubular thickening. | Urinary nephrin correlates with podocyte damage and is a sensitive early biomarker of diabetic nephropathy. |
| Kondapi et al., 2021 [54] | Podocyte structural damage and RAAS activation. | Podocyte injury in DKD involves foot process effacement, hypertrophy, apoptosis, and detachment. Disruption of slit diaphragm proteins (e.g., podocin) and RAAS activation with angiotensin II signaling further promote proteinuria and glomerulosclerosis. |
| Kawaguchi et al., 2021 [56] | Parietal epithelial cell (PEC) hypertrophy and injury. | High-glucose exposure leads to PEC hypertrophy, vacuolization, S-phase arrest, and mitotic catastrophe. These changes impair glomerular regeneration and may induce periglomerular inflammation, contributing to glomerular injury in DN. |
| Liu et al., 2022 [67] | Bcl-2-mediated regulation of apoptosis and autophagy. | Wogonin restores autophagy and inhibits apoptosis in podocytes via Bcl-2 interaction, reducing albuminuria and histological damage in diabetic mice. |
| Lu et al., 2021 [70] | METTL14-induced degradation of Sirt1. | METTL14 promotes m6A modification of Sirt1 mRNA, leading to podocyte injury. METTL14 deletion improves glomerular structure and reduces proteinuria. |
| Lu et al., 2021 [74] | GPR43-mediated insulin resistance. | GPR43 activation suppresses AMPKα via the PKC-PLC pathway, impairing podocyte insulin signaling. Its inhibition improves insulin sensitivity and reduces albuminuria. |
| Löwen et al., 2021 [79] | GBM component accumulation, endothelial proliferation, and podocyte death lead to glomerulosclerosis and interstitial fibrosis. | Glomerular vessel leakiness, tuft adhesions, and insudative damage propagate tubular degeneration and fibrosis in DN. |
| Nishad et al., 2021 [82] | Growth hormone induces podocyte cycle reentry via TGF-β1 and Notch, causing cytokinesis failure and cell death. | GH-driven TGF-β1/Notch signaling causes binucleation and mitotic catastrophe; inhibition prevents podocyte loss in DN. |
| Petrica et al., 2021 [83] | lncRNAs modulate podocyte injury by interacting with miRNAs and regulating oxidative stress and fibrogenesis. | lncRNAs like MALAT1 and NEAT1 worsen DKD by promoting inflammation, while MIAT and TUG1 offer protective effects. |
| Matoba et al., 2021 [84] | ROCK signaling mediates mesangial fibrosis, podocyte apoptosis, and inflammation, contributing to DKD. | Fasudil reduces proteinuria in diabetic patients by inhibiting ROCK without affecting blood pressure or eGFR. |
| Palmer et al., 2021 [85] | Glomerular epithelial hypertrophy and podocyte injury contribute to interstitial fibrosis and kidney function decline. | TRIDENT cohort shows eGFR correlates with interstitial fibrosis and glomerular epithelial changes in DKD patients. |
| Su et al., 2022 [110] | Risa overexpression and autophagy inhibition. | Risa, a long non-coding RNA, inhibits autophagy via Sirt1/GSK3Î2 axis, causing podocyte injury. Its suppression enhances autophagy and reduces injury, making it a potential therapeutic target. |
| Zhang et al., 2021 [133] | BASP1 acts as a cosuppressor of WT1, activating the p53 pathway and inducing podocyte apoptosis in diabetic nephropathy. | BASP1 is upregulated in diabetic nephropathy and promotes podocyte apoptosis via p53 activation, suggesting a role in disease progression. |
| Zhu et al., 2021 [150] | Caspase-1-mediated pyroptosis via GSDMD-N formation causes membrane rupture and cytokine release; carnosine inhibits this pathway. | Carnosine reduces inflammation and podocyte injury in DN by targeting caspase-1, suggesting its therapeutic potential. |
| Fang et al., 2021 [40] | β-hydroxybutyrate inhibits GSK3β, enhancing Nrf2 activity and reducing podocyte senescence and injury. | The ketone body β-hydroxybutyrate activates antioxidant pathways, reducing renal oxidative stress and podocyte aging. |
| Shahzad et al., 2022 [55] | NLRP3 inflammasome activation. | High glucose, AGEs, and ROS activate the NLRP3 inflammasome in podocytes, triggering canonical (caspase-1, IL-1β, IL-18) and non-canonical (autophagy regulation) pathways, contributing to sterile inflammation, podocyte dysfunction, and DKD progression. |
| Jiang et al., 2022 [57] | METTL3-mediated m6A modification and TIMP2 stabilization. | METTL3 enhances m6A modification of TIMP2 mRNA. IGF2BP2 binds to m6A sites, stabilizing TIMP2, which promotes Notch signaling, inflammation, and apoptosis in podocytes, leading to injury in DN. |
| Mukhi et al., 2023 [90] | GH induces TGF-β1 expression, activating SMAD signaling and increasing podocyte permeability. | GHR deletion or TGF-βR1 inhibition in podocytes prevents GH-induced SMAD activation and DN manifestations. |
| Sawada et al., 2023 [100] | PGNMID involves PV-1 overexpression in glomerular endothelial cells, triggering oxidative stress and inflammatory crosstalk to podocytes. | PV-1 expression correlates with podocyte injury in PGNMID. Complement activation and IgG deposition promote inflammation and podocyte damage. |
| Veron et al., 2021 [109] | VEGF-A knockdown and eNOS deficiency. | VEGF-A knockdown in eNOS-deficient mice induces diffuse glomerulosclerosis and proteinuria. S-nitrosylation of β3-integrin, laminin, and GSNOR contributes to renal damage. NO and thiol levels help protect renal function in diabetic mice. |
| Song et al., 2022 [112] | Sestrin2 and TSP-1/TGF-β1/Smad3 modulation. | Sestrin2 protects podocytes by modulating the TSP-1/TGF-β1/Smad3 pathway, reducing oxidative stress, phenotypic changes, and apoptosis in DKD. |
| Sun et al., 2023 [113] | Dynein-mediated nephrin degradation. | Hyperglycemia increases dynein expression, impairing nephrin trafficking and promoting degradation via DynII1 and DCTN1. This disrupts the kidney’s molecular sieve and contributes to DN. |
| Stefansson et al., 2022 [114] | Hyperfiltration and endothelial stress. | Hyperfiltration in early diabetes leads to podocyte depletion and GBM thickening. Endothelial stress response and mesangial cell crosstalk activate fibrosis-related pathways, contributing to DN. |
| Tao et al., 2022 [117] | Orai1-mediated SOCE and calpain activation. | Hyperglycemia induces SOCE via Orai1, activating calpain, which causes F-actin disorganization and nephrin loss, leading to podocyte injury. |
| Zeng et al., 2023 [126] | Podocyte damage and detachment from the GBM leads to urinary shedding of podocyte fragments and glomerulosclerosis. | Elevated urinary podocin and intrarenal podocalyxin levels predict DKD progression and correlate with kidney function decline. |
| Yu et al., 2022 [129] | TRPC6-mediated Ca2+ influx activates calpain-1, CDK5, and Drp1 phosphorylation, triggering mitochondrial fission in podocytes. | TRPC6 promotes podocyte mitochondrial dysfunction and apoptosis in diabetic conditions via the Ca2+/calpain-1/CDK5/Drp1 axis. |
| Balint et al., 2023 [19] | Endothelial dysfunction and BBB disruption due to gut-derived metabolites (e.g., indoxyl sulfate), oxidative stress, and loss of retinoic acid signaling. | This study used metabolomics of serum and urine in T2DM patients to identify early DKD biomarkers like indoxyl sulfate and all-trans retinoic acid, linked to podocyte and endothelial dysfunction. |
| Albrecht et al., 2023 [34] | HG and MGO disrupt GEC-podocyte crosstalk, impairing the filtration barrier and ECM structure; ID1/ID3 upregulation is insufficiently protective. | GEC-podocyte co-culture under diabetic stress reveals altered gene expression, confirming impaired intercellular signaling. |
| Chen et al., 2024 [38] | Renal inflammation and immune signaling cause podocyte injury; gene dysregulation (e.g., TGFBR3, PTGDS, FGF1/9) contributes to fibrosis and oxidative stress. | Bioinformatics revealed immune-related gene dysregulation and inflammatory mediators contributing to DKD pathology. |
| Hu et al., 2023 [51] | ENST00000436340 interacts with PTBP1 to degrade RAB3B mRNA, impairing cytoskeleton and GLUT4 translocation. | lncRNA ENST00000436340 disrupts cytoskeletal stability and glucose transport in podocytes, worsening DKD. |
| Martins et al., 2023 [76] | Hyperglycemia and angiotensin II lead to podocyte damage via actin destabilization, foot process effacement, and inflammation mediated by Mindin. | Mindin expression is elevated in DN and correlates with foot process effacement, suggesting its role as a biomarker of podocyte damage and chronic inflammation. |
| Lizotte et al., 2023 [77] | SHP-1 promotes podocyte injury by impairing slit diaphragm proteins and enhancing SUMO2 modification of podocin. | Podocyte-specific SHP-1 deletion in mice prevents albuminuria and structural damage in DKD, highlighting SHP-1 as a potential therapeutic target. |
| Lu et al., 2023 [80] | ACSS2 upregulation inhibits autophagy via mTORC1 activation, promoting podocyte injury and inflammation. | ACSS2 enhances raptor expression via histone acetylation, impairs autophagy, and contributes to DN progression. |
| Naito et al., 2023 [86] | Reduced GM3 in podocytes leads to albuminuria and glomerular lesions; VPA restores GM3 and reduces damage. | VPA-induced GM3 expression in podocytes mitigates podocyte loss and mesangial expansion in DN. |
| Khurana et al., 2023 [89] | Reduced DNA methylation at key sites affects gene expression related to insulin signaling and fibrosis. | Hypomethylation at CTCF/Pol2B sites in leukocytes from DN patients links to disease progression and renal decline. |
| Liu et al., 2024 [95] | Circ-0000953 modulates autophagy and inflammation by sponging Mir665-3p and regulating Atg4b in podocytes. | Circ-0000953 contributes to autophagy dysregulation in DN through the Mir665-3p-Atg4b axis, suggesting a regulatory role in podocyte injury. |
| Petrica et al., 2023 [104] | mtDNA damage and impaired OXPHOS lead to ROS overproduction, inflammation, and tissue injury at glomerular and tubular levels. | mtDNA alterations in blood/urine reflect inflammation in normoalbuminuric DKD. These changes are linked to podocyte and PT dysfunction. |
| Suarez et al., 2024 [111] | Impaired ENT2 activity and adenosine dysregulation. | In DN, insulin regulation of ENT2 is impaired, causing loss of adenosine homeostasis and glomerular alterations. Human podocyte and rat glomeruli models confirm ENT2 dysfunction in diabetes. |
| Yang et al., 2023 [119] | UCP2 deficiency impairs autophagy in podocytes by modulating mTORC1 phosphorylation and activating AMPK, which may inhibit the mTOR pathway. | UCP2 expression increases under diabetic conditions as a compensatory response. Its deficiency impairs autophagy, worsening podocyte injury and proteinuria, indicating its critical role in maintaining podocyte homeostasis. |
| Zeng et al., 2023 [124] | GSK3β overactivity induces dedifferentiation, ECM accumulation, and profibrotic cytokine expression, accelerating DKD. | Intrarenal and urinary GSK3β levels are elevated in DKD; the pY216-GSK3β/total GSK3β ratio correlates with disease progression. |
| Zhao et al., 2023 [137] | Alternative polyadenylation (APA) leads to 3′UTR lengthening, enhancing translation of inflammation-related proteins and activating ER stress and NF-κB signaling. | APA promotes diabetic nephropathy progression by increasing protein synthesis involved in inflammation and stress pathways. |
| Zhang et al., 2024 [139] | DHAP accumulation under hyperglycemia activates mTORC1/ROS/NLRP3 pathway, inducing podocyte pyroptosis. | This pathway links abnormal glucose metabolism to inflammatory podocyte death, identifying DHAP as a pathogenic factor in DKD. |
| Zuo et al., 2024 [144] | CCDC92 promotes podocyte lipotoxicity by dysregulating lipid homeostasis via ABCA1 signaling, leading to lipid accumulation and podocyte damage in diabetic kidney disease. | CCDC92 is upregulated in diabetic kidney disease and contributes to lipid deposition and podocyte injury by altering lipid metabolism. Its deletion reduces lipid accumulation and improves podocyte integrity, indicating its potential as a biomarker and therapeutic target. |
| Yamashiro et al., 2024 [21] | ERK activation in podocytes under high glucose conditions contributes to DN pathogenesis via VEGF and ribosomal biogenesis pathways. | ERK activation was confirmed in DN patient podocytes, suggesting involvement in DN via VEGF signaling and ribosomal regulation. |
| Esselman et al., 2025 [28] | Podocyte loss and mesangial expansion in glomeruli linked to specific lipid markers detected by MALDI IMS and MxIF. | Using MALDI IMS and MxIF, the study maps lipid markers linked to podocyte and mesangial changes in diabetic glomeruli. |
| Han et al., 2024 [42] | Podocyte hypoxia from severe microvascular injury promotes extracapillary hypercellularity and loss of podocyte phenotype. | Histological analysis links extracapillary hypercellularity with severe hypoxia-induced podocyte damage in DKD. |
| Gujarati et al., 2024 [44] | KLF6-induced ApoJ secretion from podocytes activates CaMK1D in proximal tubules, restoring mitochondrial function and protecting against injury. | KLF6 enhances podocyte-proximal tubule communication via ApoJ-CaMK1D axis, preserving renal mitochondrial function. |
| Lei et al., 2024 [50] | Mesangial expansion, podocyte depletion, and Kimmelstiel-Wilson lesions are key structural changes in DN. | AI-assisted pathology confirms mesangial and podocyte alterations as structural predictors of DN severity. |
| Hu et al., 2024 [53] | DOT1L/PLCL1 pathway dysregulation. | DOT1L expression is reduced in high-glucose conditions. Its overexpression protects against podocyte injury by upregulating PLCL1, which enhances fatty acid oxidation and reduces lipogenesis, mitigating podocyte damage in DKD. |
| Lv et al., 2025 [73] | TRAIL/DR5-induced PANoptosis. | TRAIL binds to DR5, triggering apoptosis, pyroptosis, and necroptosis (PANoptosis) in podocytes. Deletion of TRAIL/DR5 reduces kidney injury in DKD models. |
| Li et al., 2024 [81] | SGLT2 expression increases MAMs, impairing podocyte function; AMPK activation by SGLT2 inhibition restores balance. | SGLT2 inhibitors like empagliflozin reduce MAMs and podocyte injury in diabetic mice via AMPK activation. |
| Pan et al., 2024 [87] | BTG2 modulates autophagy via mTORC1 inhibition and suppresses EMT, reducing podocyte apoptosis. | BTG2 protects podocytes in DKD by linking autophagy regulation with inflammation pathways shared with periodontitis. |
| Lv et al., 2024 [92] | PVT1 promotes podocyte injury by modulating TRIM56-mediated AMPKα degradation, leading to mitochondrial dysfunction, mtDNA/mtROS release, and NF-κB-mediated inflammation. | PVT1 upregulation in DKD correlates with disease severity. Its deletion in mice reduces mitochondrial damage and inflammation, highlighting PVT1 as a potential therapeutic target. |
| Rosenbloom et al., 2024 [98] | Mechanism of vacuolar casts is unclear; hypothesized origin includes degenerated RTECs or podocyturia, with vesicles containing aqueous material. | Vacuolar casts are observed in advanced DN with proteinuria and kidney dysfunction, showing fluid-filled vesicles within a cast matrix on microscopy. |
| Sunilkumar et al., 2025 [102] | REDD1 reduces slit diaphragm proteins (podocin, nephrin), upregulates TRPC6 and Ca2+ influx, disrupting cytoskeleton via NF-κB. | REDD1 deletion preserves podocyte structure in diabetes, reduces albuminuria and glomerular damage, showing therapeutic promise for DN. |
| Lu et al., 2024 [103] | Rheb1 deficiency causes mitochondrial dysfunction and podocyte senescence through Atp5f1c acetylation, independent of mTORC1. | Rheb1 loss accelerates DKD progression via mitochondrial dysfunction and senescence, representing a novel therapeutic target. |
| Sun et al., 2025 [116] | AMPK/PGC-1α pathway and mitochondrial protection | Jinlida granules activate AMPK/PGC-1α, improving mitochondrial homeostasis and reducing podocyte apoptosis, offering renoprotection in diabetic mice. |
| Ward et al., 2025 [120] | Diabetic nephropathy involves vascular damage, mesangial expansion, glomerular scarring, podocyte loss, tubular atrophy, interstitial fibrosis, inflammatory infiltration, and maladaptive repair from fibroblast and macrophage activation. | The nPOD-K cohort includes kidneys from diabetic and non-diabetic donors, preserved for histological analysis to study DKD pathogenesis and progression. |
| Li et al., 2024 [130] | Podocyte-derived mRNA ratio (podocin:nephrin) reflects qualitative podocyte changes and correlates with fibrosis severity. | Urinary podocin:nephrin mRNA ratio is elevated in DKD and correlates with tubulointerstitial fibrosis, serving as a prognostic marker. |
| Wang et al., 2024 [134] | miR-193a suppresses WT1, triggering EZH2/β-catenin/NLRP3 pathway activation and inflammasome assembly, leading to inflammation. | Hyperglycemia induces miR-193a, which downregulates WT1 and activates inflammatory pathways, contributing to podocyte damage. |
| Zhang et al., 2024 [138] | lncRNA EVF-2 upregulation interacts with hnRNPU, promoting podocyte cell cycle re-entry and inflammation in diabetic nephropathy. | EVF-2 contributes to podocyte injury by modulating transcription and splicing, suggesting a novel target for DN therapy. |
| Zhou et al., 2024 [142] | Hyperglycemia and stress factors reduce α3β1 integrin and alter GBM, causing podocyte detachment and foot process widening. | Structural changes in podocytes correlate with proteinuria severity and DN classification, highlighting their diagnostic value. |
| Arslan et al., 2025 [24] | miR-342-3p targets SOX6, contributing to podocyte injury, fibrosis, and tubular loss via PI3K/Akt and TGF-β1 pathways. | The study links increased SOX6 expression and decreased miR-342-3p to renal dysfunction, implicating fibrosis-related pathways in DN. |
| Angeletti et al., 2020 [33] | DAF loss on podocytes leads to complement activation, C3a/C3aR and IL-1β/IL-1R1 signaling, cytoskeletal changes, and reduced nephrin. | DAF deficiency promotes FSGS-like glomerulosclerosis through complement activation and IL-1β-driven inflammation. |
| Hudkins et al., 2022 [64] | Podocyte loss, increased mesangial matrix, and mesangiolysis. | In a DN mouse model (BTBR ob/ob), podocyte loss and mesangiolysis were mitigated by atrasentan and losartan, which increased podocyte number and reduced mesangial matrix accumulation. |
| Li et al., 2025 [68] | Foot process effacement and nephrin autoantibodies. | Increased FPW and reduced nephrin expression are observed in DN + MCD. Autoantibodies against nephrin disrupt the slit diaphragm, and hyperglycemia impairs mitochondrial ATP, damaging cytoskeleton. |
| Li et al., 2025 [69] | RIPK3-mediated inflammation. | RIPK3 induces podocyte injury through NF-κB p65-mediated inflammatory signaling, independent of necroptosis. Its deletion reduces albuminuria and improves glomerular injury. |
| Hu et al., 2025 [72] | Lipotoxicity and impaired fatty acid oxidation. | DKD induces podocyte lipid accumulation. Dapagliflozin upregulates ERRα and ACOX1, enhancing fatty acid oxidation, reducing lipid toxicity, and restoring podocyte structure. |
| Li et al., 2025 [91] | Hyperglycemia-induced podocyte detachment, hypertrophy, and effacement compromising the glomerular filtration barrier. SGLT2 inhibitors mitigate these effects by reducing intraglomerular pressure and preserving actin cytoskeleton integrity. | SGLT2i treatment in DKD patients prevented increases in urinary levels of podocyte-specific molecules (podocin, podocalyxin, synaptopodin), indicating a protective effect on podocyte integrity. |
| Boi et al., 2025 [105] | CKAP4 deficiency disrupts podocyte cytoskeleton, leading to foot process effacement and detachment from basement membrane. | CKAP4 maintains actin and microtubule organization in podocytes. Its reduction in DKD contributes to cytoskeletal disarray and filtration barrier loss. |
| Wu et al., 2025 [128] | METTL3 induces m6A modification of MDM2, activating Notch signaling, leading to podocyte dedifferentiation and inflammation. | Targeting METTL3 may prevent MDM2-Notch1 mediated podocyte injury and glomerulosclerosis in DKD. |
| Pan et al., 2018 [9] | SRGAP2a inactivates RhoA/Cdc42 to suppress podocyte motility, maintaining structure and preventing injury under hyperglycemia or TGF-β stimulation. | SRGAP2a is downregulated in diabetic nephropathy. Its overexpression mitigates podocyte injury and proteinuria in diabetic mice. |
| Xu et al., 2025 [132] | GPR107 deficiency impairs endocytosis of collagen IV and AT1R, increasing membrane bound AT1R, activating AT1R/Ca2+ signaling, and promoting GBM thickening. | GPR107 regulates collagen IV balance in podocytes. Its deficiency leads to collagen accumulation and GBM thickening, suggesting therapeutic potential. |
| Zhu et al., 2025 [140] | CerS6-derived ceramide binds VDAC1, inducing mtDNA leakage and activating cGAS–STING pathway, promoting inflammation. | CerS6 knockout mitigates glomerular injury and inflammation, indicating its role in immune-mediated podocyte damage. |
| Zhang et al., 2025 [153] | QRXZYQF activates AMPK signaling, modulating ferroptosis by reducing iron overload, oxidative stress, and lipid peroxidation in podocytes. | This traditional Chinese formula protects against DKD by preventing ferroptosis, offering therapeutic benefits via AMPK activation. |
3. Discussion
4. Materials and Methods
4.1. Ethical Aspects
4.2. Type of Study and Protocol Record
4.3. Search Strategy and Eligibility Criteria
4.4. Evaluation of Study Selection and Methodological Quality
4.5. Data Analysis and Summarization
5. Conclusions
Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, H.; Liu, Q.; Liu, B. Research Progress on Mechanism of Podocyte Depletion in Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 2615286. [Google Scholar] [CrossRef]
- Zhang, C.; Hou, B.; Yu, S.; Chen, Q.; Zhang, N.; Li, H. HGF alleviates high glucose-induced injury in podocytes by GSK3β inhibition and autophagy restoration. Biochim. Biophys. Acta 2016, 1863, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Toyoda, M.; Kaneyama, N.; Shiraiwa, S.; Moriya, H.; Miyatake, H.; Tanaka, E.; Yamamoto, N.; Miyauchi, M.; Kimura, M.; et al. Upregulation of α3β1-Integrin in Podocytes in Early-Stage Diabetic Nephropathy. J. Diabetes Res. 2016, 2016, 9265074. [Google Scholar] [CrossRef] [PubMed]
- Parchwani, D.N.; Upadhyah, A.A. Diabetic nephropathy: Progression and pathophysiology. Int. J. Med. Sci. Public Health 2012, 1, 59–70. [Google Scholar] [CrossRef]
- Fried, L.F.; Folkerts, K.; Smela, B.; Deon Bowrin, K.; Mernagh, P.; Millier, A.; Kovesdy, C.P. Targeted literature review of the burden of illness in patients with chronic kidney disease and type 2 diabetes. Am. J. Manag. Care 2021, 27, S168–S177. [Google Scholar]
- Tereda, A. From pathophysiology to personalized care: A comprehensive review of diabetic kidney disease. J. Med. Sci. Res. 2024, 12, 246–252. [Google Scholar]
- Satirapoj, B.; Adler, S.G. Comprehensive approach to diabetic nephropathy. Kidney Res. Clin. Pract. 2014, 33, 121–131. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G. Renal progenitors in non-diabetic and diabetic nephropathies. Trends Endocrinol. Metab. 2013, 24, 13–20. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, S.; Hou, Q.; Qiu, D.; Shi, J.; Wang, L.; Chen, Z.; Zhang, M.; Duan, A.; Qin, W.; et al. Dissection of Glomerular Transcriptional Profile in Patients With Diabetic Nephropathy: SRGAP2a Protects Podocyte Structure and Function. Diabetes 2018, 67, 717–730. [Google Scholar] [CrossRef]
- Giunti, S.; Barit, D.; Cooper, M.E. Mechanisms of diabetic nephropathy: Role of hypertension. Hypertension 2006, 48, 519–526. [Google Scholar] [CrossRef]
- Shah, I.M.; Mackay, S.P.; McKay, G.A. Therapeutic strategies in the treatment of diabetic nephropathy—A translational medicine approach. Curr. Med. Chem. 2009, 16, 997–1016. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Nicholas, S.B. Pathomechanisms of diabetic kidney disease. J. Clin. Med. 2023, 12, 7349. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Qiu, D.; Shi, Y.; Wang, S.; Zhou, X.; Chen, N.; Wei, J.; Wu, M.; Wu, H.; Duan, H. Thioredoxin-interacting protein deficiency alleviates phenotypic alterations of podocytes via inhibition of mTOR activation in diabetic nephropathy. J. Cell. Physiol. 2019, 234, 16485–16502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, W.; Sun, Y.; Qiao, Y.; Zhang, L.; Zhao, Z.; Lv, S. Wnt/β-Catenin Signaling Mediated-UCH-L1 Expression in Podocytes of Diabetic Nephropathy. Int. J. Mol. Sci. 2016, 17, 1404. [Google Scholar] [CrossRef] [PubMed]
- Conserva, F.; Gesualdo, L.; Papale, M. A systems biology overview on human diabetic nephropathy: From genetic susceptibility to post-transcriptional and post-translational modifications. J. Diabetes Res. 2016, 2016, 7934504. [Google Scholar] [CrossRef]
- Mima, A. Renal protection by sodium-glucose cotransporter 2 inhibitors and its underlying mechanisms in diabetic kidney disease. J. Diabetes Its Complicat. 2018, 32, 720–725. [Google Scholar] [CrossRef]
- Jiang, H.; Shao, X.; Jia, S.; Qu, L.; Weng, C.; Shen, X.; Wang, Y.; Huang, H.; Wang, C.; Feng, S.; et al. The Mitochondria-Targeted Metabolic Tubular Injury in Diabetic Kidney Disease. Cell. Physiol. Biochem. 2019, 52, 156–171. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef]
- Balint, L.; Socaciu, C.; Socaciu, A.I.; Vlad, A.; Gadalean, F.; Bob, F.; Milas, O.; Cretu, O.M.; Suteanu-Simulescu, A.; Glavan, M.; et al. Metabolites Potentially Derived from Gut Microbiota Associated with Podocyte, Proximal Tubule, and Renal and Cerebrovascular Endothelial Damage in Early Diabetic Kidney Disease in T2DM Patients. Metabolites 2023, 13, 893. [Google Scholar] [CrossRef]
- Zhou, Z.; Wan, J.; Hou, X.; Geng, J.; Li, X.; Bai, X. MicroRNA-27a promotes podocyte injury via PPARγ-mediated β-catenin activation in diabetic nephropathy. Cell Death Dis. 2017, 8, e2658, Correction in Cell Death Dis. 2017, 9, 652. https://doi.org/10.1038/s41419-018-0637-3. [Google Scholar] [CrossRef]
- Yamashiro, A.; Satoh, Y.; Endo, S.; Oshima, N. Extracellular signal-regulated kinase is activated in podocytes from patients with diabetic nephropathy. Hum. Cell 2024, 37, 1553–1558. [Google Scholar] [CrossRef]
- Ivanac-Janković, R.; Ćorić, M.; Furić-Čunko, V.; Lovičić, V.; Bašić-Jukić, N.; Kes, P. BMP-7 protein expression is downregulated in human diabetic nephropathy. Acta Clin. Croat. 2015, 54, 164–168. [Google Scholar]
- Carson, J.M.; Okamura, K.; Wakashin, H.; McFann, K.; Dobrinskikh, E.; Kopp, J.B.; Blaine, J. Podocytes degrade endocytosed albumin primarily in lysosomes. PLoS ONE 2014, 9, e99771. [Google Scholar] [CrossRef] [PubMed]
- Arslan, G.; Karabulut, Y.Y.; Yeleser, İ.; Erdal, M.E.; Demir, S.; Özdemir, A.A. Correlation of hsa-mirna-342-3p and SOX 6 Expression with Diabetic Nephropathy Classification, Prognostic Histomorphological Parameters and Laboratory Findings in Diabetic Nephropathy. Ann. Diagn. Pathol. 2025, 76, 152461. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.A.; Tawhari, I.; Safar-Boueri, L.; Seif, N.; Alahmadi, A.; Gargiulo, R.; Aggarwal, V.; Usman, I.; Kisselev, S.; Gharavi, A.G.; et al. COVID-19-Associated Glomerular Disease. J. Am. Soc. Nephrol. 2021, 32, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ceol, M.; Tiralongo, E.; Baelde, H.J.; Vianello, D.; Betto, G.; Marangelli, A.; Bonfante, L.; Valente, M.; Della Barbera, M.; D’Angelo, A.; et al. Involvement of the tubular ClC-type exchanger ClC-5 in glomeruli of human proteinuric nephropathies. PLoS ONE 2012, 7, e45605. [Google Scholar] [CrossRef]
- Canney, A.L.; Cohen, R.V.; Elliott, J.A.; Aboud, C.M.; Martin, W.P.; Docherty, N.G.; le Roux, C.W. Improvements in diabetic albuminuria and podocyte differentiation following Roux-en-Y gastric bypass surgery. Diab Vasc. Dis. Res. 2020, 17, 1479164119879039. [Google Scholar] [CrossRef]
- Esselman, A.B.; Moser, F.A.; Tideman, L.E.M.; Migas, L.G.; Djambazova, K.V.; Colley, M.E.; Pingry, E.L.; Patterson, N.H.; Farrow, M.A.; Yang, H.; et al. In situ molecular profiles of glomerular cells by integrated imaging mass spectrometry and multiplexed immunofluorescence microscopy. Kidney Int. 2025, 107, 332–337. [Google Scholar] [CrossRef]
- Denhez, B.; Rousseau, M.; Spino, C.; Dancosst, D.A.; Dumas, M.; Guay, A.; Lizotte, F.; Geraldes, P. Saturated fatty acids induce insulin resistance in podocytes through inhibition of IRS1 via activation of both IKKβ and mTORC1. Sci. Rep. 2020, 10, 21628. [Google Scholar] [CrossRef]
- Audzeyenka, I.; Rachubik, P.; Rogacka, D.; Typiak, M.; Kulesza, T.; Angielski, S.; Rychłowski, M.; Wysocka, M.; Gruba, N.; Lesner, A.; et al. Cathepsin C is a novel mediator of podocyte and renal injury induced by hyperglycemia. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118723. [Google Scholar] [CrossRef]
- Hayashi, D.; Wang, L.; Ueda, S.; Yamanoue, M.; Ashida, H.; Shirai, Y. The mechanisms of ameliorating effect of a green tea polyphenol on diabetic nephropathy based on diacylglycerol kinase α. Sci. Rep. 2020, 10, 11790. [Google Scholar] [CrossRef]
- Chen, A.; Feng, Y.; Lai, H.; Ju, W.; Li, Z.; Li, Y.; Wang, A.; Hong, Q.; Zhong, F.; Wei, C.; et al. Soluble RARRES1 induces podocyte apoptosis to promote glomerular disease progression. J. Clin. Investig. 2020, 130, 5523–5535. [Google Scholar] [CrossRef]
- Angeletti, A.; Cantarelli, C.; Petrosyan, A.; Andrighetto, S.; Budge, K.; D’Agati, V.D.; Hartzell, S.; Malvi, D.; Donadei, C.; Thurman, J.M.; et al. Loss of decay-accelerating factor triggers podocyte injury and glomerulosclerosis. J. Exp. Med. 2020, 217, e20191699. [Google Scholar] [CrossRef]
- Albrecht, M.; Sticht, C.; Wagner, T.; Hettler, S.A.; De La Torre, C.; Qiu, J.; Gretz, N.; Albrecht, T.; Yard, B.; Sleeman, J.P.; et al. The crosstalk between glomerular endothelial cells and podocytes controls their responses to metabolic stimuli in diabetic nephropathy. Sci. Rep. 2023, 13, 17985. [Google Scholar] [CrossRef]
- Endlich, N.; Lange, T.; Kuhn, J.; Klemm, P.; Kotb, A.M.; Siegerist, F.; Kindt, F.; Lindenmeyer, M.T.; Cohen, C.D.; Kuss, A.W.; et al. BDNF: mRNA expression in urine cells of patients with chronic kidney disease and its role in kidney function. J. Cell. Mol. Med. 2018, 22, 5265–5277. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, D.; Kuwabara, T.; Hata, Y.; Umemoto, S.; Kanki, T.; Nishiguchi, Y.; Mizumoto, T.; Hayata, M.; Kakizoe, Y.; Izumi, Y.; et al. Suppressed ER-associated degradation by intraglomerular cross talk between mesangial cells and podocytes causes podocyte injury in diabetic kidney disease. FASEB J. 2020, 34, 15577–15590. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, S.; Xing, Y.; Lv, L.; Hu, W.; Zhou, W. Saxagliptin attenuates glomerular podocyte injury by increasing the expression of renal nephrin and podocin in type 2 diabetic rats. Acta Diabetol. 2020, 57, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liao, L.; Wang, B.; Wu, Z. Identification and validation of immune and cuproptosis-related genes for diabetic nephropathy by WGCNA and machine learning. Front. Immunol. 2024, 15, 1332279. [Google Scholar] [CrossRef]
- Fiorina, P.; Vergani, A.; Bassi, R.; Niewczas, M.A.; Altintas, M.M.; Pezzolesi, M.G.; D’Addio, F.; Chin, M.; Tezza, S.; Ben Nasr, M.; et al. Role of podocyte B7-1 in diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1415–1429. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, B.; Gong, A.Y.; Malhotra, D.K.; Gupta, R.; Dworkin, L.D.; Gong, R. The ketone body β-hydroxybutyrate mitigates the senescence response of glomerular podocytes to diabetic insults. Kidney Int. 2021, 100, 1037–1053, Correction in Kidney Int. 2022, 101, 1301–1302. https://doi.org/10.1016/j.kint.2022.04.002. [Google Scholar] [CrossRef]
- Hou, B.; Li, Y.; Li, X.; Zhang, C.; Zhao, Z.; Chen, Q.; Zhang, N.; Li, H. HGF protected against diabetic nephropathy via autophagy-lysosome pathway in podocyte by modulating PI3K/Akt-GSK3β-TFEB axis. Cell. Signal. 2020, 75, 109744. [Google Scholar] [CrossRef]
- Han, W.; Zheng, Q.; Zhang, Z.; Wang, X.; Gao, L.; Niu, D.; Li, R.; Wang, C. Association of the podocyte phenotype with extracapillary hypercellularity in patients with diabetic kidney disease. J. Nephrol. 2024, 37, 2209–2222. [Google Scholar] [CrossRef]
- Holderied, A.; Romoli, S.; Eberhard, J.; Konrad, L.A.; Devarapu, S.K.; Marschner, J.A.; Müller, S.; Anders, H.J. Glomerular parietal epithelial cell activation induces collagen secretion and thickening of Bowman’s capsule in diabetes. Lab. Investig. 2015, 95, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Gujarati, N.A.; Frimpong, B.O.; Zaidi, M.; Bronstein, R.; Revelo, M.P.; Haley, J.D.; Kravets, I.; Guo, Y.; Mallipattu, S.K. Podocyte-specific KLF6 primes proximal tubule CaMK1D signaling to attenuate diabetic kidney disease. Nat. Commun. 2024, 15, 8038. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Li, J.; Asadi, M.; Basgen, J.M.; Zhu, B.; Yi, Z.; Jiang, S.; Doke, T.; El Shamy, O.; Patel, N.; et al. DACH1 protects podocytes from experimental diabetic injury and modulates PTIP-H3K4Me3 activity. J. Clin. Investig. 2021, 131, 141279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Cui, H.; Ding, J. Smad3 signalling affects high glucose-induced podocyte injury via regulation of the cytoskeletal protein transgelin. Nephrology 2020, 25, 659–666. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, Y.; Wang, M.; Hou, Y.; Huang, W.; Zhou, D.; Wang, Z.; Yang, S.; Tang, W.; Zhen, J.; et al. Elevation of JAML Promotes Diabetic Kidney Disease by Modulating Podocyte Lipid Metabolism. Cell Metab. 2020, 32, 1052–1062.e1058. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Hu, H.; Yang, K.; Zhu, Z.; Yang, Q.; Liang, W. LRH-1 activation alleviates diabetes-induced podocyte injury by promoting GLS2-mediated glutaminolysis. Cell Prolif. 2023, 56, e13479. [Google Scholar] [CrossRef]
- Kimura, M.; Toyoda, M.; Kato, M.; Kobayashi, K.; Abe, M.; Kobayashi, T.; Miyauchi, M.; Yamamoto, N.; Umezono, T.; Suzuki, D. Expression of alpha-actinin-4 in human diabetic nephropathy. Intern. Med. 2008, 47, 1099–1106. [Google Scholar] [CrossRef]
- Lei, Q.; Hou, X.; Liu, X.; Liang, D.; Fan, Y.; Xu, F.; Liang, S.; Yang, J.; Xie, G.; Liu, Z.; et al. Artificial intelligence assists identification and pathologic classification of glomerular lesions in patients with diabetic nephropathy. J. Transl. Med. 2024, 22, 397. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Q.; Fan, X.; Zhen, J.; Wang, C.; Chen, H.; Liu, Y.; Zhou, P.; Zhang, T.; Huang, T.; et al. Long noncoding RNA ENST00000436340 promotes podocyte injury in diabetic kidney disease by facilitating the association of PTBP1 with RAB3B. Cell Death Dis. 2023, 14, 130. [Google Scholar] [CrossRef]
- Kondapi, K.; Kumar, N.L.; Moorthy, S.; Silambanan, S. A Study of Association of Urinary Nephrin with Albuminuria in Patients with Diabetic Nephropathy. Indian J. Nephrol. 2021, 31, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ye, S.; Kong, J.; Zhou, Q.; Wang, Z.; Zhang, Y.; Yan, H.; Wang, Y.; Li, T.; Xie, Y.; et al. DOT1L protects against podocyte injury in diabetic kidney disease through phospholipase C-like 1. Cell Commun. Signal. 2024, 22, 519. [Google Scholar] [CrossRef] [PubMed]
- Kondapi, K.; Silambanan, S.; Moorthy, S.; Kumar, N.L. A Study of the Risk Factors and Urinary Podocin as an Early Prognostic Indicator of Renal Injury in Diabetic Nephropathy. J. Assoc. Physicians India 2021, 69, 11–12. [Google Scholar] [PubMed]
- Shahzad, K.; Fatima, S.; Khawaja, H.; Elwakiel, A.; Gadi, I.; Ambreen, S.; Zimmermann, S.; Mertens, P.R.; Biemann, R.; Isermann, B. Podocyte-specific Nlrp3 inflammasome activation promotes diabetic kidney disease. Kidney Int. 2022, 102, 766–779. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Hasegawa, K.; Yasuda, I.; Muraoka, H.; Umino, H.; Tokuyama, H.; Hashiguchi, A.; Wakino, S.; Itoh, H. Diabetic condition induces hypertrophy and vacuolization in glomerular parietal epithelial cells. Sci. Rep. 2021, 11, 1515. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.; Hu, X.; Gao, L.; Zeng, H.; Wang, X.; Huang, Y.; Zhu, W.; Wang, J.; Wen, J.; et al. METTL3-mediated m(6)A modification of TIMP2 mRNA promotes podocyte injury in diabetic nephropathy. Mol. Ther. 2022, 30, 1721–1740. [Google Scholar] [CrossRef]
- Inoki, K.; Mori, H.; Wang, J.; Suzuki, T.; Hong, S.; Yoshida, S.; Blattner, S.M.; Ikenoue, T.; Rüegg, M.A.; Hall, M.N.; et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Investig. 2011, 121, 2181–2196. [Google Scholar] [CrossRef]
- Gödel, M.; Hartleben, B.; Herbach, N.; Liu, S.; Zschiedrich, S.; Lu, S.; Debreczeni-Mór, A.; Lindenmeyer, M.T.; Rastaldi, M.P.; Hartleben, G.; et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Investig. 2011, 121, 2197–2209. [Google Scholar] [CrossRef]
- Langham, R.G.; Kelly, D.J.; Cox, A.J.; Thomson, N.M.; Holthöfer, H.; Zaoui, P.; Pinel, N.; Cordonnier, D.J.; Gilbert, R.E. Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: Effects of angiotensin converting enzyme inhibition. Diabetologia 2002, 45, 1572–1576. [Google Scholar] [CrossRef]
- Bai, X.; Geng, J.; Li, X.; Wan, J.; Liu, J.; Zhou, Z.; Liu, X. Long Noncoding RNA LINC01619 Regulates MicroRNA-27a/Forkhead Box Protein O1 and Endoplasmic Reticulum Stress-Mediated Podocyte Injury in Diabetic Nephropathy. Antioxid. Redox Signal. 2018, 29, 355–376. [Google Scholar] [CrossRef]
- Wang, L.; Li, H. MiR-770-5p facilitates podocyte apoptosis and inflammation in diabetic nephropathy by targeting TIMP3. Biosci. Rep. 2020, 40, BSR20193653. [Google Scholar] [CrossRef]
- Lai, H.; Chen, A.; Cai, H.; Fu, J.; Salem, F.; Li, Y.; He, J.C.; Schlondorff, D.; Lee, K. Podocyte and endothelial-specific elimination of BAMBI identifies differential transforming growth factor-β pathways contributing to diabetic glomerulopathy. Kidney Int. 2020, 98, 601–614. [Google Scholar] [CrossRef]
- Hudkins, K.L.; Li, X.; Holland, A.L.; Swaminathan, S.; Alpers, C.E. Regression of diabetic nephropathy by treatment with empagliflozin in BTBR ob/ob mice. Nephrol. Dial. Transplant. 2022, 37, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Kostic, S.; Hauke, T.; Ghahramani, N.; Filipovic, N.; Vukojevic, K. Expression pattern of apoptosis-inducing factor in the kidneys of streptozotocin-induced diabetic rats. Acta Histochem. 2020, 122, 151655. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, M.; Wolf, G. AGE-Induced Suppression of EZH2 Mediates Injury of Podocytes by Reducing H3K27me3. Am. J. Nephrol. 2020, 51, 676–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Jiang, L.; Li, Y.Y.; Huang, Y.B.; Hu, X.R.; Zhu, W.; Wang, X.; Wu, Y.G.; Meng, X.M.; Qi, X.M. Wogonin protects glomerular podocytes by targeting Bcl-2-mediated autophagy and apoptosis in diabetic kidney disease. Acta Pharmacol. Sin. 2022, 43, 96–110. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Jiang, S.; Shang, S.; Zhang, J.; Liu, J.; Li, C.; Gao, Y.; Zhang, H.; Li, W. Utilizing Podocyte Foot Process Morphology for the Identification of Diabetic Nephropathy with or without Minimal Change Disease: Establishment of an Artificial Intelligence-Assisted Diagnostic Model. Diabetes Metab. Syndr. Obes. 2025, 18, 2141–2153. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Li, R.; Zhao, X.; Chen, Y.; Cai, Y.; Yang, Y.; Wang, W.; Zheng, S.; Zhang, L.; et al. Podocyte RIPK3 Deletion Improves Diabetic Kidney Disease by Attenuating NF-κB p65 Driven Inflammation. Adv. Sci. 2025, 12, e03325. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, H.; Song, N.; Liang, Y.; Zhu, J.; Chen, J.; Ning, Y.; Hu, J.; Fang, Y.; Teng, J.; et al. METTL14 aggravates podocyte injury and glomerulopathy progression through N(6)-methyladenosine-dependent downregulating of Sirt1. Cell Death Dis. 2021, 12, 881. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Chen, B.; Ge, Y.; Gong, A.Y.; Flickinger, B.; Malhotra, D.K.; Wang, L.J.; Dworkin, L.D.; Liu, Z.; et al. Glycogen synthase kinase 3β hyperactivity in urinary exfoliated cells predicts progression of diabetic kidney disease. Kidney Int. 2020, 97, 175–192. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Peng, Z.; Fan, Y.; Yang, Q.; Hu, J. Dapagliflozin attenuates diabetes-induced podocyte lipotoxicity via ERRα-Mediated lipid metabolism. Free Radic. Biol. Med. 2025, 234, 178–191. [Google Scholar] [CrossRef]
- Lv, Z.; Hu, J.; Su, H.; Yu, Q.; Lang, Y.; Yang, M.; Fan, X.; Liu, Y.; Liu, B.; Zhao, Y.; et al. TRAIL induces podocyte PANoptosis via death receptor 5 in diabetic kidney disease. Kidney Int. 2025, 107, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, P.P.; Zhang, J.X.; Li, X.Q.; Wang, G.H.; Yuan, B.Y.; Huang, S.J.; Liu, X.Q.; Jiang, T.T.; Wang, M.Y.; et al. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKα activity. Theranostics 2021, 11, 4728–4742. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Toyoda, M.; Kobayashi, K.; Abe, M.; Kobayashi, T.; Kato, M.; Yamamoto, N.; Kimura, M.; Umezono, T.; Suzuki, D. Hypertrophy and loss of podocytes in diabetic nephropathy. Intern. Med. 2009, 48, 1615–1620. [Google Scholar] [CrossRef][Green Version]
- Martins, A.; Bernardes, A.B.; Ferreira, V.A.; Wanderley, D.C.; Araújo, S.A.; do Carmo Neto, J.R.; da Silva, C.A.; Lira, R.C.P.; Araújo, L.S.; Dos Reis, M.A.; et al. In situ assessment of Mindin as a biomarker of podocyte lesions in diabetic nephropathy. PLoS ONE 2023, 18, e0284789. [Google Scholar] [CrossRef] [PubMed]
- Lizotte, F.; Rousseau, M.; Denhez, B.; Lévesque, D.; Guay, A.; Liu, H.; Moreau, J.; Higgins, S.; Sabbagh, R.; Susztak, K.; et al. Deletion of protein tyrosine phosphatase SHP-1 restores SUMOylation of podocin and reverses the progression of diabetic kidney disease. Kidney Int. 2023, 104, 787–802, Erratum in Kidney Int. 2023, 104, 1228. https://doi.org/10.1016/j.kint.2023.10.007. [Google Scholar] [CrossRef]
- Lee, E.; Lee, H.S. Peroxidase expression is decreased by palmitate in cultured podocytes but increased in podocytes of advanced diabetic nephropathy. J. Cell. Physiol. 2018, 233, 9060–9069. [Google Scholar] [CrossRef]
- Löwen, J.; Gröne, E.F.; Groß-Weißmann, M.L.; Bestvater, F.; Gröne, H.J.; Kriz, W. Pathomorphological sequence of nephron loss in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2021, 321, F600–F616, Correction in Am. J. Physiol. Ren. Physiol. 2022, 322, F245–F377. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.Q.; Chen, P.P.; Zhang, J.X.; Liu, L.; Wang, G.H.; Liu, X.Q.; Jiang, T.T.; Wang, M.Y.; Liu, W.T.; et al. Activation of acetyl-CoA synthetase 2 mediates kidney injury in diabetic nephropathy. JCI Insight 2023, 8, 165817. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Jiang, X.; Song, S.; Zou, W.; Yang, Q.; Liu, S.; Chen, S.; Wang, C. Inhibition of SGLT2 protects podocytes in diabetic kidney disease by rebalancing mitochondria-associated endoplasmic reticulum membranes. Cell Commun. Signal. 2024, 22, 534. [Google Scholar] [CrossRef]
- Nishad, R.; Mukhi, D.; Singh, A.K.; Motrapu, M.; Chintala, K.; Tammineni, P.; Pasupulati, A.K. Growth hormone induces mitotic catastrophe of glomerular podocytes and contributes to proteinuria. Cell Death Dis. 2021, 12, 342. [Google Scholar] [CrossRef]
- Petrica, L.; Hogea, E.; Gadalean, F.; Vlad, A.; Vlad, M.; Dumitrascu, V.; Velciov, S.; Gluhovschi, C.; Bob, F.; Ursoniu, S.; et al. Long noncoding RNAs may impact podocytes and proximal tubule function through modulating miRNAs expression in Early Diabetic Kidney Disease of Type 2 Diabetes Mellitus patients. Int. J. Med. Sci. 2021, 18, 2093–2101. [Google Scholar] [CrossRef]
- Matoba, K.; Sekiguchi, K.; Nagai, Y.; Takeda, Y.; Takahashi, H.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Renal ROCK Activation and Its Pharmacological Inhibition in Patients with Diabetes. Front. Pharmacol. 2021, 12, 738121. [Google Scholar] [CrossRef]
- Palmer, M.B.; Abedini, A.; Jackson, C.; Blady, S.; Chatterjee, S.; Sullivan, K.M.; Townsend, R.R.; Brodbeck, J.; Almaani, S.; Srivastava, A.; et al. The Role of Glomerular Epithelial Injury in Kidney Function Decline in Patients with Diabetic Kidney Disease in the TRIDENT Cohort. Kidney Int. Rep. 2021, 6, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; Nakayama, K.; Kawashima, N. Enhanced Levels of Glycosphingolipid GM3 Delay the Progression of Diabetic Nephropathy. Int. J. Mol. Sci. 2023, 24, 11355. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Teng, Y.; Wang, R.; Chen, D.; Chen, H. Deciphering the molecular nexus of BTG2 in periodontitis and diabetic kidney disease. BMC Med. Genom. 2024, 17, 152. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Corna, D.; Locatelli, M.; Cassis, P.; Carminati, C.E.; Bolognini, S.; Zoja, C.; Remuzzi, G.; Benigni, A.; et al. C3a receptor blockade protects podocytes from injury in diabetic nephropathy. JCI Insight 2020, 5, 131849. [Google Scholar] [CrossRef]
- Khurana, I.; Kaipananickal, H.; Maxwell, S.; Birkelund, S.; Syreeni, A.; Forsblom, C.; Okabe, J.; Ziemann, M.; Kaspi, A.; Rafehi, H.; et al. Reduced methylation correlates with diabetic nephropathy risk in type 1 diabetes. J. Clin. Investig. 2023, 133, 160959. [Google Scholar] [CrossRef]
- Mukhi, D.; Kolligundla, L.P.; Maruvada, S.; Nishad, R.; Pasupulati, A.K. Growth hormone induces transforming growth factor-β1 in podocytes: Implications in podocytopathy and proteinuria. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119391. [Google Scholar] [CrossRef]
- Li, C.; Ng, J.K.; Chan, G.C.; Fung, W.W.; Chow, K.M.; Szeto, C.C. Preservation of Urinary Podocyte Markers in Diabetic Kidney Disease by Sodium-Glucose Cotransporter 2 Inhibitor Therapy. Kidney Dis. 2025, 11, 218–225. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, Z.; Hu, J.; Su, H.; Liu, B.; Lang, Y.; Yu, Q.; Liu, Y.; Fan, X.; Yang, M.; et al. LncRNA PVT1 induces mitochondrial dysfunction of podocytes via TRIM56 in diabetic kidney disease. Cell Death Dis. 2024, 15, 697. [Google Scholar] [CrossRef]
- Shi, W.; Huang, Y.; Zhao, X.; Xie, Z.; Dong, W.; Li, R.; Chen, Y.; Li, Z.; Wang, W.; Ye, Z.; et al. Histone deacetylase 4 mediates high glucose-induced podocyte apoptosis via upregulation of calcineurin. Biochem. Biophys. Res. Commun. 2020, 533, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.P.; Reddi, A.S.; Chandran, C.B.; Chevalier, J.M.; Okechukwu, C.N.; Seshan, S.V. Collapsing glomerulopathy superimposed on diabetic nephropathy: Insights into etiology of an under-recognized, severe pattern of glomerular injury. Nephrol. Dial. Transplant. 2014, 29, 392–399. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, L.; Zeng, H.; Gao, L.; Guo, S.; Chen, C.; Zhang, M.; Ma, L.; Li, Y.; Qi, X.; et al. Circ-0000953 deficiency exacerbates podocyte injury and autophagy disorder by targeting Mir665-3p-Atg4b in diabetic nephropathy. Autophagy 2024, 20, 1072–1097. [Google Scholar] [CrossRef]
- Su, J.; Li, S.J.; Chen, Z.H.; Zeng, C.H.; Zhou, H.; Li, L.S.; Liu, Z.H. Evaluation of podocyte lesion in patients with diabetic nephropathy: Wilms’ tumor-1 protein used as a podocyte marker. Diabetes Res. Clin. Pract. 2010, 87, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Motrapu, M.; Świderska, M.K.; Mesas, I.; Marschner, J.A.; Lei, Y.; Martinez Valenzuela, L.; Fu, J.; Lee, K.; Angelotti, M.L.; Antonelli, G.; et al. Drug Testing for Residual Progression of Diabetic Kidney Disease in Mice Beyond Therapy with Metformin, Ramipril, and Empagliflozin. J. Am. Soc. Nephrol. 2020, 31, 1729–1745. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, S.; Ramanand, A.; Stark, A.; Varghese, V.; Chalmers, D.; Au-Yeung, N.; Kanduri, S.R.; Lukitsch, I.; Poloni, J.A.T.; Keitel, E.; et al. Urinary Vacuolar Casts Are a Unique Type of Casts in Advanced Proteinuric Glomerulopathies. Kidney360 2024, 5, 216–227. [Google Scholar] [CrossRef]
- Minakawa, A.; Fukuda, A.; Sato, Y.; Kikuchi, M.; Kitamura, K.; Wiggins, R.C.; Fujimoto, S. Podocyte hypertrophic stress and detachment precedes hyperglycemia or albuminuria in a rat model of obesity and type2 diabetes-associated nephropathy. Sci. Rep. 2019, 9, 18485. [Google Scholar] [CrossRef]
- Sawada, A.; Kawanishi, K.; Igarashi, Y.; Taneda, S.; Hattori, M.; Ishida, H.; Tanabe, K.; Koike, J.; Honda, K.; Nagashima, Y.; et al. Overexpression of Plasmalemmal Vesicle-Associated Protein-1 Reflects Glomerular Endothelial Injury in Cases of Proliferative Glomerulonephritis with Monoclonal IgG Deposits. Kidney Int. Rep. 2023, 8, 151–163. [Google Scholar] [CrossRef]
- Sharma, K.R.; Heckler, K.; Stoll, S.J.; Hillebrands, J.L.; Kynast, K.; Herpel, E.; Porubsky, S.; Elger, M.; Hadaschik, B.; Bieback, K.; et al. ELMO1 protects renal structure and ultrafiltration in kidney development and under diabetic conditions. Sci. Rep. 2016, 6, 37172. [Google Scholar] [CrossRef]
- Sunilkumar, S.; Yerlikaya, E.I.; VanCleave, A.; Subrahmanian, S.M.; Toro, A.L.; Kimball, S.R.; Dennis, M.D. REDD1-dependent GSK3β signaling in podocytes promotes canonical NF-κB activation in diabetic nephropathy. J. Biol. Chem. 2025, 301, 108244. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, X.; Hou, Q.; Yu, L.; Cao, K.; Ding, D.; Lu, Y.; Dai, C. Rheb1 deficiency elicits mitochondrial dysfunction and accelerates podocyte senescence through promoting Atp5f1c acetylation. Cell Signal. 2024, 124, 111451. [Google Scholar] [CrossRef]
- Petrica, L.; Vlad, A.; Gadalean, F.; Muntean, D.M.; Vlad, D.; Dumitrascu, V.; Bob, F.; Milas, O.; Suteanu-Simulescu, A.; Glavan, M.; et al. Mitochondrial DNA Changes in Blood and Urine Display a Specific Signature in Relation to Inflammation in Normoalbuminuric Diabetic Kidney Disease in Type 2 Diabetes Mellitus Patients. Int. J. Mol. Sci. 2023, 24, 9803. [Google Scholar] [CrossRef]
- Boi, R.; Lassén, E.; Johansson, A.; Liu, P.; Chaudhari, A.; Tati, R.; Müller-Deile, J.; Schiffer, M.; Ebefors, K.; Nyström, J. Cytoskeleton-associated protein 4 affects podocyte cytoskeleton dynamics in diabetic kidney disease. JCI Insight 2025, 10, 181298. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, M.; Zhao, Y.; Gong, J.; Su, H.; Yuan, F.; Fang, K.; Yuan, X.; Yu, X.; Dong, H.; et al. Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 2020, 177, 3646–3661. [Google Scholar] [CrossRef] [PubMed]
- Dalla Vestra, M.; Masiero, A.; Roiter, A.M.; Saller, A.; Crepaldi, G.; Fioretto, P. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 2003, 52, 1031–1035. [Google Scholar] [CrossRef]
- Tian, X.; Inoue, K.; Zhang, Y.; Wang, Y.; Sperati, C.J.; Pedigo, C.E.; Zhao, T.; Yan, M.; Groener, M.; Moledina, D.G.; et al. Inhibiting calpain 1 and 2 in cyclin G associated kinase-knockout mice mitigates podocyte injury. JCI Insight 2020, 5, 142740. [Google Scholar] [CrossRef]
- Veron, D.; Aggarwal, P.K.; Li, Q.; Moeckel, G.; Kashgarian, M.; Tufro, A. Podocyte VEGF-A Knockdown Induces Diffuse Glomerulosclerosis in Diabetic and in eNOS Knockout Mice. Front. Pharmacol. 2021, 12, 788886. [Google Scholar] [CrossRef] [PubMed]
- Su, P.P.; Liu, D.W.; Zhou, S.J.; Chen, H.; Wu, X.M.; Liu, Z.S. Down-regulation of Risa improves podocyte injury by enhancing autophagy in diabetic nephropathy. Mil. Med. Res. 2022, 9, 23. [Google Scholar] [CrossRef]
- Suarez, R.; Villarreal, C.; Nahuelpán, Y.; Jara, C.; Oyarzún, C.; Alarcón, S.; Díaz-Encarnación, M.M.; Guillén-Gómez, E.; Quezada, C.; San Martín, R. Defective insulin-stimulated equilibrative nucleoside transporter-2 activity and altered subcellular transporter distribution drive the loss of adenosine homeostasis in diabetic kidney disease progression. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166890. [Google Scholar] [CrossRef]
- Song, S.; Shi, C.; Bian, Y.; Yang, Z.; Mu, L.; Wu, H.; Duan, H.; Shi, Y. Sestrin2 remedies podocyte injury via orchestrating TSP-1/TGF-β1/Smad3 axis in diabetic kidney disease. Cell Death Dis. 2022, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Weidner, J.; Allamargot, C.; Piper, R.C.; Misurac, J.; Nester, C. Dynein-Mediated Trafficking: A New Mechanism of Diabetic Podocytopathy. Kidney360 2023, 4, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, V.T.N.; Nair, V.; Melsom, T.; Looker, H.C.; Mariani, L.H.; Fermin, D.; Eichinger, F.; Menon, R.; Subramanian, L.; Ladd, P.; et al. Molecular programs associated with glomerular hyperfiltration in early diabetic kidney disease. Kidney Int. 2022, 102, 1345–1358. [Google Scholar] [CrossRef]
- Woo, C.Y.; Baek, J.Y.; Kim, A.R.; Hong, C.H.; Yoon, J.E.; Kim, H.S.; Yoo, H.J.; Park, T.S.; Kc, R.; Lee, K.U.; et al. Inhibition of Ceramide Accumulation in Podocytes by Myriocin Prevents Diabetic Nephropathy. Diabetes Metab. J. 2020, 44, 581–591. [Google Scholar] [CrossRef]
- Sun, S.; Yang, S.; Cheng, Y.; Fang, T.; Qu, J.; Tian, L.; Zhang, M.; Wu, S.; Sun, B.; Chen, L. Jinlida granules alleviate podocyte apoptosis and mitochondrial dysfunction via the AMPK/PGC-1α pathway in diabetic nephropathy. Int. J. Mol. Med. 2025, 55, 26. [Google Scholar] [CrossRef]
- Tao, Y.; Chaudhari, S.; Shotorbani, P.Y.; Ding, Y.; Chen, Z.; Kasetti, R.; Zode, G.; Ma, R. Enhanced Orai1-mediated store-operated Ca2+ channel/calpain signaling contributes to high glucose-induced podocyte injury. J. Biol. Chem. 2022, 298, 101990. [Google Scholar] [CrossRef]
- Uil, M.; Hau, C.M.; Ahdi, M.; Mills, J.D.; Kers, J.; Saleem, M.A.; Florquin, S.; Gerdes, V.E.A.; Nieuwland, R.; Roelofs, J. Cellular origin and microRNA profiles of circulating extracellular vesicles in different stages of diabetic nephropathy. Clin. Kidney J. 2021, 14, 358–365. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, S.; Liang, Y.; Sun, Q.; Fang, Y.; Jiang, L.; Wen, P.; Yang, J. UCP2 deficiency impairs podocyte autophagy in diabetic nephropathy. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166705. [Google Scholar] [CrossRef]
- Ward, H.H.; Anquetil, F.; Das, V.; Gibson, C.B.; Dovmark, T.H.; Kusmartseva, I.; Yang, M.; Beery, M.; Atkinson, M.A.; Zeng, X.; et al. Network for Pancreatic Organ donors with Diabetes-Kidney: A Heterogenous Donor Cohort for the Investigation of Diabetic Kidney Disease Pathogenesis and Progression. Kidney360 2025, 6, 15–26. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Iwano, M.; Suzuki, D.; Nakatani, K.; Kimura, K.; Harada, K.; Kubo, A.; Akai, Y.; Toyoda, M.; Kanauchi, M.; et al. Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am. J. Kidney Dis. 2009, 54, 653–664. [Google Scholar] [CrossRef]
- Yao, T.; Zha, D.; Hu, C.; Wu, X. Circ_0000285 promotes podocyte injury through sponging miR-654-3p and activating MAPK6 in diabetic nephropathy. Gene 2020, 747, 144661. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, Y.; Cao, S.; Sasaki, K.; Wang, Y.; Niu, A.; Fan, X.; Wang, S.; Zhang, M.Z.; Harris, R.C. Podocyte EGFR Inhibits Autophagy Through Upregulation of Rubicon in Type 2 Diabetic Nephropathy. Diabetes 2021, 70, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Ng, J.K.; Fung, W.W.; Chan, G.C.; Chow, K.M.; Szeto, C.C. Intrarenal and Urinary Glycogen Synthase Kinase-3 Beta Levels in Diabetic and Nondiabetic Chronic Kidney Disease. Kidney Blood Press. Res. 2023, 48, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Zhai, R.; Xie, L.; Zheng, Z.; Jian, G.; Chen, T.; Su, J.; Gao, C.; Wang, N.; Yang, X.; et al. Xuesaitong Protects Podocytes from Apoptosis in Diabetic Rats through Modulating PTEN-PDK1-Akt-mTOR Pathway. J. Diabetes Res. 2020, 2020, 9309768. [Google Scholar] [CrossRef]
- Zeng, L.; Fung, W.W.; Chan, G.C.; Ng, J.K.; Chow, K.M.; Szeto, C.C. Urinary and Kidney Podocalyxin and Podocin Levels in Diabetic Kidney Disease: A Kidney Biopsy Study. Kidney Med. 2023, 5, 100569. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; He, X.; Yang, L.; Wang, J.; Xia, S.; Liu, D.; Liu, S.; Liu, W.; Duan, H. Cdk5-Mediated Phosphorylation of Sirt1 Contributes to Podocyte Mitochondrial Dysfunction in Diabetic Nephropathy. Antioxid. Redox Signal. 2021, 34, 171–190. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Z.; Yang, Y.; Han, Z.; Pan, Q.; Chen, Y.; Yu, H.; Shen, S.; Xu, L. The Impact of METTL3 on MDM2 Promotes Podocytes Injury During Diabetic Kidney Disease. J. Cell. Mol. Med. 2025, 29, e70627. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Ma, H.; Wang, Z.; Zhang, R.; Jiao, J. TRPC6 mediates high glucose-induced mitochondrial fission through activation of CDK5 in cultured human podocytes. Front. Physiol. 2022, 13, 984760. [Google Scholar] [CrossRef]
- Li, C.; Szeto, C.C. Urinary podocyte markers in diabetic kidney disease. Kidney Res. Clin. Pract. 2024, 43, 274–286. [Google Scholar] [CrossRef]
- Wang, S.; Ai, H.; Liu, L.; Zhang, X.; Gao, F.; Zheng, L.; Yi, J.; Sun, L.; Yu, C.; Zhao, H.; et al. Micro-RNA-27a/b negatively regulates hepatic gluconeogenesis by targeting FOXO1. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E911–E924. [Google Scholar] [CrossRef]
- Xu, D.; Tong, Z.; Yang, P.; Chen, Q.; Wang, S.; Zhao, W.; Han, L.; Yin, Y.; Xu, R.; Zhang, M.; et al. G protein-coupled receptor 107 deficiency promotes development of diabetic nephropathy. Mol. Biomed. 2025, 6, 10. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Ye, Q.; Tong, L.; Jiang, H.; Zhu, X.; Huang, L.; Lin, W.; Fu, H.; Wang, J.; et al. Podocyte apoptosis in diabetic nephropathy by BASP1 activation of the p53 pathway via WT1. Acta Physiol. 2021, 232, e13634. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, J.; Dai, S.; Gao, P.; Qi, Y.; Zhao, X.; Liu, J.; Wang, Y.; Gao, Y. miRNA-193a-mediated WT1 suppression triggers podocyte injury through activation of the EZH2/β-catenin/NLRP3 pathway in children with diabetic nephropathy. Exp. Cell Res. 2024, 442, 114238. [Google Scholar] [CrossRef] [PubMed]
- Sawai, K.; Mukoyama, M.; Mori, K.; Yokoi, H.; Koshikawa, M.; Yoshioka, T.; Takeda, R.; Sugawara, A.; Kuwahara, T.; Saleem, M.A.; et al. Redistribution of connexin43 expression in glomerular podocytes predicts poor renal prognosis in patients with type 2 diabetes and overt nephropathy. Nephrol. Dial. Transplant. 2006, 21, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, M.; Wang, Z.; Fu, Y.; Jia, M.; Wang, X.; Liu, M.; Zhang, Y.; Sun, Y.; Lu, Y.; et al. PGRN acts as a novel regulator of mitochondrial homeostasis by facilitating mitophagy and mitochondrial biogenesis to prevent podocyte injury in diabetic nephropathy. Cell Death Dis. 2019, 10, 524. [Google Scholar] [CrossRef]
- Zhao, T.; Zhan, D.; Qu, S.; Jiang, S.; Gan, W.; Qin, W.; Zheng, C.; Cheng, F.; Lu, Y.; Liu, M.; et al. Transcriptomics-proteomics Integration reveals alternative polyadenylation driving inflammation-related protein translation in patients with diabetic nephropathy. J. Transl. Med. 2023, 21, 86. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Yan, Y.; Li, Y.; Lei, M.; Liu, Y.; Yang, L.; Zhou, S.; Pan, S.; Liu, Z.; et al. LncRNA evf-2 Exacerbates Podocyte Injury in Diabetic Nephropathy by Inducing Cell Cycle Re-entry and Inflammation Through Distinct Mechanisms Triggered by hnRNPU. Adv. Sci. 2024, 11, e2406532. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, H.; Luo, Q.; Yang, K.; Zou, Z.; Shi, M.; Liang, W. Dihydroxyacetone phosphate accumulation leads to podocyte pyroptosis in diabetic kidney disease. J. Cell. Mol. Med. 2024, 28, e18073. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, Y.; Jian, Y.; Hu, H.; Yang, Q.; Hao, Y.; Jiang, H.; Luo, Z.; Yang, X.; Li, W.; et al. CerS6 links ceramide metabolism to innate immune responses in diabetic kidney disease. Nat. Commun. 2025, 16, 1528. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, W.; Lu, X.; Feng, L.; Li, J.; Zhu, B. The compound XueShuanTong promotes podocyte mitochondrial autophagy via the AMPK/mTOR pathway to alleviate diabetic nephropathy injury. Mitochondrion 2025, 83, 102024. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, S.; Huang, X.Y.; Chang, D.Y.; Wang, H.; Nie, L.; Xiong, Z.Y.; Chen, M.; Zhao, M.H.; Wang, S.X. Association of podocyte ultrastructural changes with proteinuria and pathological classification in type 2 diabetic nephropathy. Diabetes Metab. 2024, 50, 101547. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, S.; Sun, X.; Lou, Y.; Bao, J.; Yu, J. Hyperoside ameliorates diabetic nephropathy induced by STZ via targeting the miR-499-5p/APC axis. J. Pharmacol. Sci. 2021, 146, 10–20. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, Y.; Xu, X.; Ding, R.; Tang, W.; Sun, Y.; Wang, X.; Zhang, Y.; Wu, J.; Xie, Y.; et al. CCDC92 deficiency ameliorates podocyte lipotoxicity in diabetic kidney disease. Metabolism 2024, 150, 155724. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Wang, S.; Chen, D.; Shu, J.; Chong, N.; Wang, Q.; Xu, Y. Dapagliflozin improves podocytes injury in diabetic nephropathy via regulating cholesterol balance through KLF5 targeting the ABCA1 signalling pathway. Diabetol. Metab. Syndr. 2024, 16, 38. [Google Scholar] [CrossRef]
- Hu, J.; Yang, Q.; Chen, Z.; Liang, W.; Feng, J.; Ding, G. Small GTPase Arf6 regulates diabetes-induced cholesterol accumulation in podocytes. J. Cell. Physiol. 2019, 234, 23559–23570. [Google Scholar] [CrossRef]
- Qin, Z.; Hoh, C.K.; Olson, E.S.; Jahromi, A.H.; Hall, D.J.; Barback, C.V.; You, Y.H.; Yanagita, M.; Sharma, K.; Vera, D.R. Molecular Imaging of the Glomerulus via Mesangial Cell Uptake of Radiolabeled Tilmanocept. J. Nucl. Med. 2019, 60, 1325–1332. [Google Scholar] [CrossRef]
- Fang, R.; Cao, X.; Zhu, Y.; Chen, Q. Hsa_circ_0037128 aggravates high glucose-induced podocytes injury in diabetic nephropathy through mediating miR-31-5p/KLF9. Autoimmunity 2022, 55, 254–263. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.Y.; Zeng, H.X.; Liu, X.Q.; Sun, Y.T.; Jiang, L.; Xia, L.L.; Wu, Y.G. Carnosine alleviates podocyte injury in diabetic nephropathy by targeting caspase-1-mediated pyroptosis. Int. Immunopharmacol. 2021, 101, 108236. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Yang, J.; Liu, G. Melatonin Protects Against Diabetic Kidney Disease via the SIRT1/NLRP3 Signalling Pathway. Nephrology 2025, 30, e70073. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, Y.; Wang, T.; Wang, X.; He, J.; Xu, J.; Wu, B.; Li, Y. Ginsenoside Rg1 Alleviates Podocyte EMT Passage by Regulating AKT/GSK3 β/β-Catenin Pathway by Restoring Autophagic Activity. Evid. Based Complement. Altern. Med. 2020, 2020, 1903627. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Q.; Xia, C.; Zheng, H.; Jiang, W.; Wang, Y.; Sun, W. Qing-Re-Xiao-Zheng-(Yi-Qi) formula attenuates the renal podocyte ferroptosis in diabetic kidney disease through AMPK pathway. J. Ethnopharmacol. 2025, 351, 120157. [Google Scholar] [CrossRef]
- Kelly, D.J.; Aaltonen, P.; Cox, A.J.; Rumble, J.R.; Langham, R.; Panagiotopoulos, S.; Jerums, G.; Holthöfer, H.; Gilbert, R.E. Expression of the slit-diaphragm protein, nephrin, in experimental diabetic nephropathy: Differing effects of anti-proteinuric therapies. Nephrol. Dial. Transplant. 2002, 17, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guan, X.M.; Wang, R.Y.; Xie, Y.S.; Zhou, H.; Ni, W.J.; Tang, L.Q. Berberine mitigates high glucose-induced podocyte apoptosis by modulating autophagy via the mTOR/P70S6K/4EBP1 pathway. Life Sci. 2020, 243, 117277. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.Y.; Kc, R.; Kim, M.; Kim, H.S.; Baek, J.Y.; Koh, E.H. Autophagic flux defect in diabetic kidney disease results in megamitochondria formation in podocytes. Biochem. Biophys. Res. Commun. 2020, 521, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, H.; Yan, J.; Wang, X.; Xu, M.; Wang, M.; Fan, B.; Liu, J.; Lin, N.; Li, L.; et al. Loss of CLDN5 in podocytes deregulates WIF1 to activate WNT signaling and contributes to kidney disease. Nat. Commun. 2022, 13, 1600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Tu, W.; Long, Y.N.; Li, P.F.; He, K.Y.; Wu, J. Notch signaling in diabetic kidney disease: Recent progress. Front. Endocrinol. 2025, 16, 1537769. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, L.; Wei, X.; Chen, M.; Zhang, X.; Mo, R.; Huang, R.; Liang, T.; Xu, X. Puerarin alleviates diabetic nephropathy by inhibiting Caspase-1-mediated pyroptosis. J. Pharm. Pharmacol. 2024, 76, 213–223. [Google Scholar] [CrossRef]
- Bai, X.; Hou, X.; Tian, J.; Geng, J.; Li, X. CDK5 promotes renal tubulointerstitial fibrosis in diabetic nephropathy via ERK1/2/PPARγ pathway. Oncotarget 2016, 7, 36510–36528. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, Y.; Gong, J.; Huang, W.; Su, H.; Yuan, F.; Fang, K.; Wang, D.; Li, J.; Zou, X.; et al. Berberine Protects Glomerular Podocytes via Inhibiting Drp1-Mediated Mitochondrial Fission and Dysfunction. Theranostics 2019, 9, 1698–1713. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, N.; Li, J.; Li, X.; He, S. Upregulation of BRD7 protects podocytes against high glucose-induced apoptosis by enhancing Nrf2 in a GSK-3β-dependent manner. Tissue Cell 2022, 76, 101813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, S.; Yang, J.; Ma, J.; Wang, P.; Zhao, X.; Liu, J.; Xiao, A.; Song, Y.; Gao, L. MiR-33a Overexpression Exacerbates Diabetic Nephropathy Through Sirt6-dependent Notch Signaling. Iran. J. Kidney Dis. 2024, 18, 168–178. [Google Scholar] [PubMed]
- Li, Z.; Tan, D.; Lin, J.; Zhang, T.; Liu, B.; Zhao, B.; Liang, D.; Li, L.; Wei, X.; Lv, Z.; et al. Podocyte TLR4 deletion alleviates diabetic kidney disease through prohibiting PKCδ/SHP-1-dependent ER stress and relieving podocyte damage and inflammation. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef]
- Mallipattu, S.K.; Guo, Y.; Revelo, M.P.; Roa-Peña, L.; Miller, T.; Ling, J.; Shankland, S.J.; Bialkowska, A.B.; Ly, V.; Estrada, C.; et al. Krüppel-Like Factor 15 Mediates Glucocorticoid-Induced Restoration of Podocyte Differentiation Markers. J. Am. Soc. Nephrol. 2017, 28, 166–184. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, C.; Fan, Y.; Zeng, C.; Chen, Z.; Qin, W.; Zhang, C.; Zhang, W.; Wang, X.; Zhu, X.; et al. Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J. Am. Soc. Nephrol. 2014, 25, 92–104. [Google Scholar] [CrossRef]
- Agrawal, S.; Chanley, M.A.; Westbrook, D.; Nie, X.; Kitao, T.; Guess, A.J.; Benndorf, R.; Hidalgo, G.; Smoyer, W.E. Pioglitazone Enhances the Beneficial Effects of Glucocorticoids in Experimental Nephrotic Syndrome. Sci. Rep. 2016, 6, 24392. [Google Scholar] [CrossRef]
- Clement, L.C.; Avila-Casado, C.; Macé, C.; Soria, E.; Bakker, W.W.; Kersten, S.; Chugh, S.S. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med. 2011, 17, 117–122. [Google Scholar] [CrossRef]
- Chen, H.M.; Liu, Z.H.; Zeng, C.H.; Li, S.J.; Wang, Q.W.; Li, L.S. Podocyte lesions in patients with obesity-related glomerulopathy. Am. J. Kidney Dis. 2006, 48, 772–779. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Zhu, W.; Zhang, Y.; Liu, P.; Li, P. Morroniside attenuates podocytes lipid deposition in diabetic nephropathy: A network pharmacology, molecular docking and experimental validation study. Int. Immunopharmacol. 2024, 138, 112560. [Google Scholar] [CrossRef]
- Korbut, A.I.; Taskaeva, I.S.; Bgatova, N.P.; Muraleva, N.A.; Orlov, N.B.; Dashkin, M.V.; Khotskina, A.S.; Zavyalov, E.L.; Konenkov, V.I.; Klein, T.; et al. SGLT2 Inhibitor Empagliflozin and DPP4 Inhibitor Linagliptin Reactivate Glomerular Autophagy in db/db Mice, a Model of Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 2987. [Google Scholar] [CrossRef]
- Guo, R.; Wang, P.; Zheng, X.; Cui, W.; Shang, J.; Zhao, Z. SGLT2 inhibitors suppress epithelial-mesenchymal transition in podocytes under diabetic conditions via downregulating the IGF1R/PI3K pathway. Front. Pharmacol. 2022, 13, 897167, Correction in Front. Pharmacol. 2022, 13, 1074294. https://doi.org/10.3389/fphar.2022.1074294. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Chen, Y.; Cheng, Y.; Wang, Y.; Xu, N.; Wang, H.; Wang, L.; Chi, Y.; Ye, X.; et al. Canagliflozin Attenuates Podocyte Inflammatory Injury through Suppressing the TXNIP/NLRP3 Signaling Pathway in Diabetic Kidney Disease Mice. Inflammation 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ertracht, O.; Nakhoul, F. #993 The molecular effects of SGLT2i (Empagliflozin) on α-Klotho and Nephrin/Podocin proteins in type II diabetic mice model. Nephrol. Dial. Transplant. 2024, 39, 1057. [Google Scholar] [CrossRef]
- Cassis, P.; Locatelli, M.; Cerullo, D.; Corna, D.; Buelli, S.; Zanchi, C.; Villa, S.; Morigi, M.; Remuzzi, G.; Benigni, A.; et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 2018, 3, 98720. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Molina, J.; Kim, J.J.; Mallela, S.K.; Ahmad, A.; Varona Santos, J.; Al-Ali, H.; Mitrofanova, A.; Sharma, K.; Fontanesi, F.; et al. Empagliflozin reduces podocyte lipotoxicity in experimental Alport syndrome. elife 2023, 12, 83353. [Google Scholar] [CrossRef]
- Wang, X.X.; Levi, J.; Luo, Y.; Myakala, K.; Herman-Edelstein, M.; Qiu, L.; Wang, D.; Peng, Y.; Grenz, A.; Lucia, S.; et al. SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J. Biol. Chem. 2017, 292, 5335–5348. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Yang, Y.; Li, F.; Chen, Q.; Zhao, Z.; Zhang, N.; Li, H. Hepatocyte growth factor attenuates high glucose-disturbed mitochondrial dynamics in podocytes by decreasing ARF6-dependent DRP1 translocation. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119623. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Fan, Z.; Shen, Y.; Li, J.; Zhang, F. Identification of podocyte molecular markers in diabetic kidney disease via single-cell RNA sequencing and machine learning. PLoS ONE 2025, 20, e0328352. [Google Scholar] [CrossRef]
- Jiang, Z.; Qian, L.; Yang, R.; Wu, Y.; Guo, Y.; Chen, T. LncRNA TCF7 contributes to high glucose-induced damage in human podocytes by up-regulating SEMA3A via sponging miR-16-5p. J. Diabetes Investig. 2023, 14, 193–204. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2020, 134, 104–112. [Google Scholar]
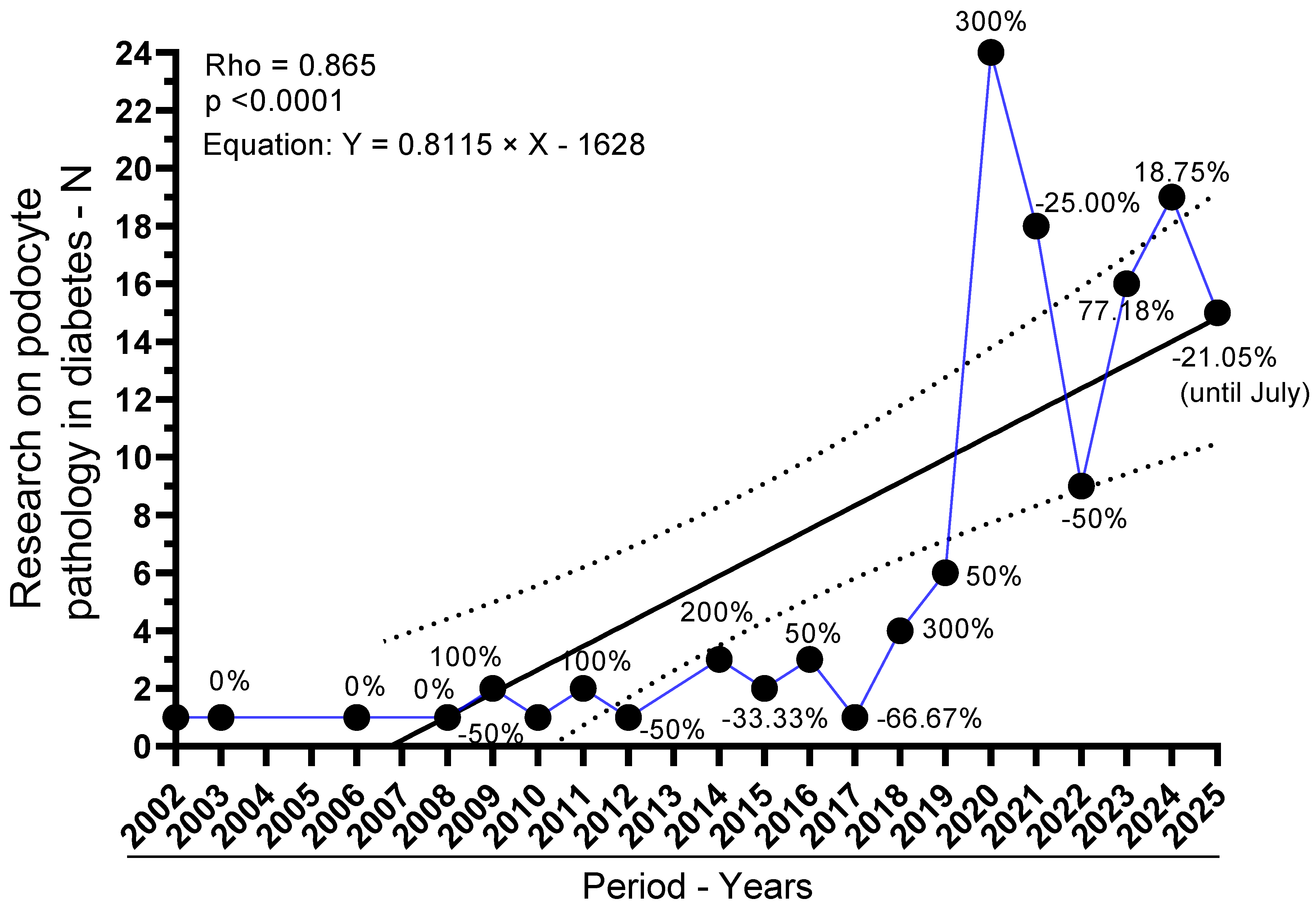
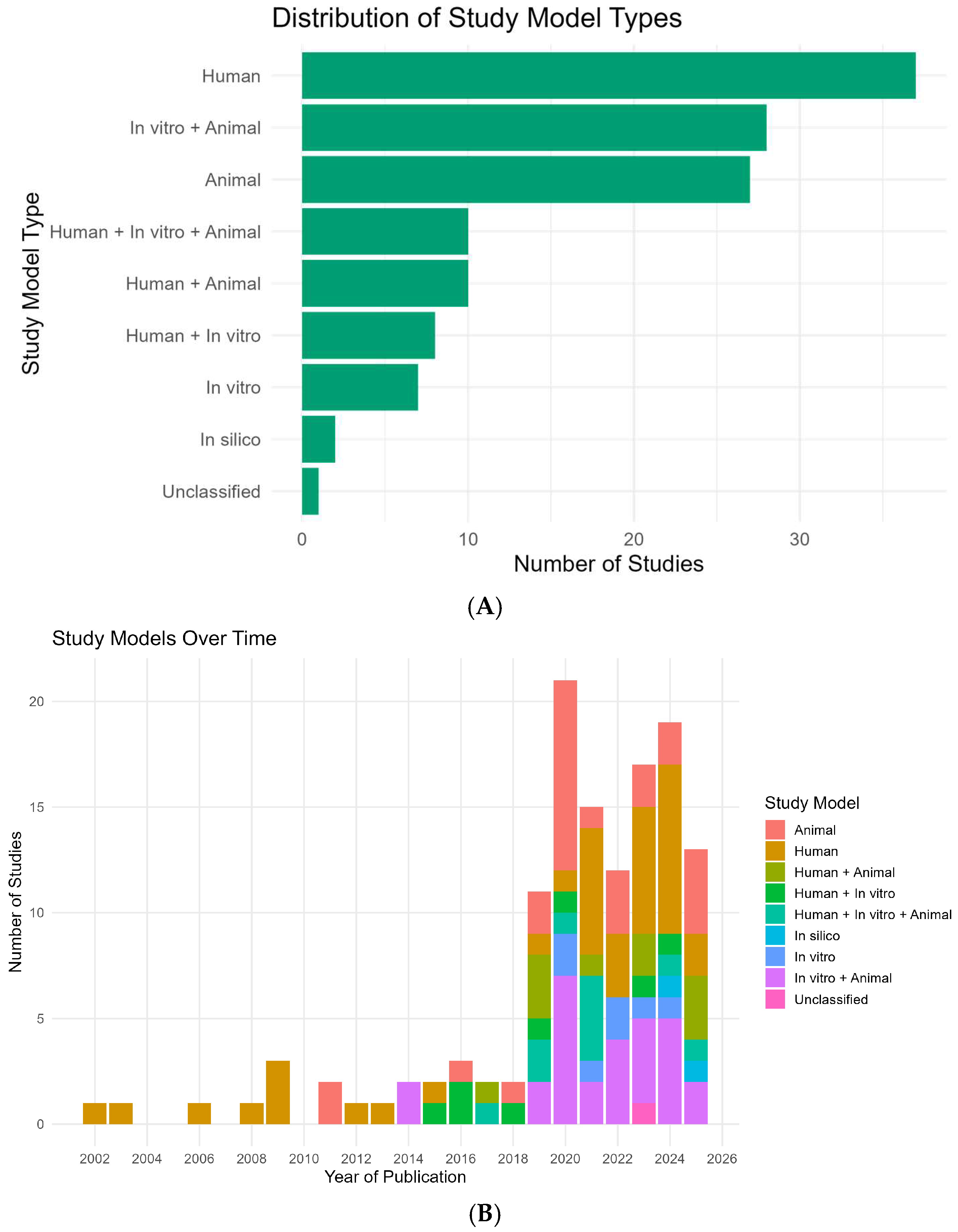
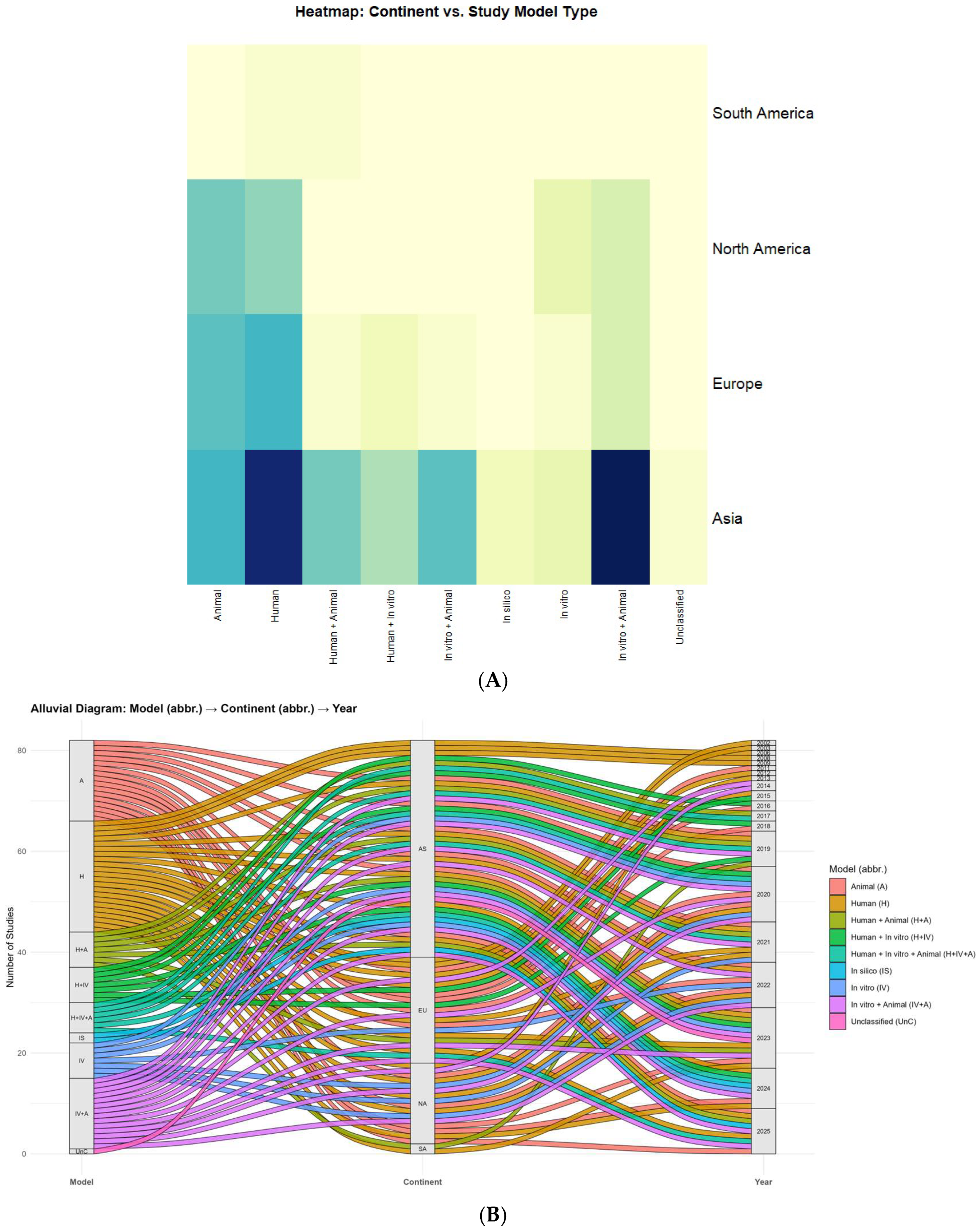


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.S.; Miguel, C.B.; Felipe, A.G.B.; Martins, A.L.M.d.S.; Miguel, R.B.; Carrijo, M.O.; Mazurek, L.; Araújo, L.S.; da Silva, C.A.; Góes-Neto, A.; et al. Approach to Studies on Podocyte Lesions Mediated by Hyperglycemia: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 8990. https://doi.org/10.3390/ijms26188990
Silva JS, Miguel CB, Felipe AGB, Martins ALMdS, Miguel RB, Carrijo MO, Mazurek L, Araújo LS, da Silva CA, Góes-Neto A, et al. Approach to Studies on Podocyte Lesions Mediated by Hyperglycemia: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(18):8990. https://doi.org/10.3390/ijms26188990
Chicago/Turabian StyleSilva, Jordana Souza, Camila Botelho Miguel, Alberto Gabriel Borges Felipe, Ana Luisa Monteiro dos Santos Martins, Renata Botelho Miguel, Maraiza Oliveira Carrijo, Laise Mazurek, Liliane Silvano Araújo, Crislaine Aparecida da Silva, Aristóteles Góes-Neto, and et al. 2025. "Approach to Studies on Podocyte Lesions Mediated by Hyperglycemia: A Systematic Review" International Journal of Molecular Sciences 26, no. 18: 8990. https://doi.org/10.3390/ijms26188990
APA StyleSilva, J. S., Miguel, C. B., Felipe, A. G. B., Martins, A. L. M. d. S., Miguel, R. B., Carrijo, M. O., Mazurek, L., Araújo, L. S., da Silva, C. A., Góes-Neto, A., Oliveira, C. J. F., Machado, J. R., Reis, M. A., & Rodrigues, W. F. (2025). Approach to Studies on Podocyte Lesions Mediated by Hyperglycemia: A Systematic Review. International Journal of Molecular Sciences, 26(18), 8990. https://doi.org/10.3390/ijms26188990






