The Interplay Between Therapeutic Self-Amplifying RNA and the Innate Immune System: Balancing Efficiency and Reactogenicity
Abstract
1. Introduction
2. Safety of saRNA-Based Therapeutics
3. Optimization of Non-Structural Proteins to Improve Safety of saRNA Products
3.1. saRNA Replication
3.2. The Influence of Non-Structural Proteins of Alphaviruses on the Cell and the Efficiency of saRNA Replication
3.2.1. Arthritogenic Alphavirus Genome-Based saRNA
3.2.2. saRNA Based on the Genomes of Encephalitic Alphaviruses
3.3. Modifications That Reduce Reactogenicity
3.3.1. Mutations in Non-Structural Proteins of Arthritogenic Alphaviruses Used for the saRNA Platform
3.3.2. Mutations in Non-Structural Proteins of Encephalitic Alphaviruses Used for the Development of the saRNA Platform
3.3.3. Other Modifications Used in the Development of the saRNA Platform
4. saRNA and Innate Immunity
4.1. The Role of Innate Immunity Receptors in saRNA Recognition
4.2. Innate Immunity Components Activated in Response to saRNA
4.3. Alleviating saRNA Immunogenicity by Regulating Intracellular Interactions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CD2AP | CD2-associated protein |
| CHIKV | Chikungunya virus |
| CPE | Cytopathic Effect |
| CSE | Conserved sequence elements |
| dsRNA | Double-stranded RNA |
| EEEV | Eastern Equine Encephalitis virus |
| FDA | Food and Drug Administration |
| FXR | Farnesoid X Receptor |
| GOI | Gene of Interest |
| HVD | Hypervariable Domain |
| IIP | Innate Inhibiting Proteins |
| IVT RNA | In vitro transcribed RNA |
| LNP | Lipid Nanoparticle |
| nsPs | Non-structural proteins |
| PAMP | Pathogen-Associated Molecular Pattern |
| PRR | Pattern recognition receptor |
| RdRp | RNA-dependent RNA polymerase |
| RNAPII | RNA polymerase II |
| RRV | Ross River virus |
| saRNA | Self-amplifying RNA |
| SFV | Semliki Forest virus |
| SIE | Superinfection exclusion |
| SINV | Sindbis virus |
| ssRNA | Single-stranded RNA |
| UTR | Untranslated region |
| VEEV | Venezuelan Equine Encephalitis virus |
| WEEV | Western Equine Encephalitis virus |
References
- Dolgin, E. The tangled history of mRNA vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct Gene Transfer into Mouse Muscle In Vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector with Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef]
- Vasileva, O.; Zaborova, O.; Shmykov, B.; Ivanov, R.; Reshetnikov, V. Composition of lipid nanoparticles for targeted delivery: Application to mRNA therapeutics. Front. Pharmacol. 2024, 15, 1466337. [Google Scholar] [CrossRef]
- Khlebnikova, A.; Kirshina, A.; Zakharova, N.; Ivanov, R.; Reshetnikov, V. Current Progress in the Development of mRNA Vaccines Against Bacterial Infections. Int. J. Mol. Sci. 2024, 25, 13139. [Google Scholar] [CrossRef]
- Żak, M.M.; Zangi, L. Clinical development of therapeutic mRNA applications. Mol. Ther. 2025, 33, 2583–2609. [Google Scholar] [CrossRef]
- Muslimov, A.; Tereshchenko, V.; Shevyrev, D.; Rogova, A.; Lepik, K.; Reshetnikov, V.; Ivanov, R. The Dual Role of the Innate Immune System in the Effectiveness of mRNA Therapeutics. Int. J. Mol. Sci. 2023, 24, 14820. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Simberg, D. Pro-inflammatory concerns with lipid nanoparticles. Mol. Ther. 2022, 30, 2109–2110. [Google Scholar] [CrossRef]
- Barmada, A.; Klein, J.; Ramaswamy, A.; Brodsky, N.N.; Jaycox, J.R.; Sheikha, H.; Jones, K.M.; Habet, V.; Campbell, M.; Sumida, T.S.; et al. Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine—Associated myocarditis. Sci. Immunol. 2023, 8, eadh3455. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Xu, Q. Current Developments and Challenges of mRNA Vaccines. Annu. Rev. Biomed. Eng. 2022, 24, 85–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jiang, L.; Liao, S.; Wu, F.; Yang, G.; Hou, L.; Liu, L.; Pan, X.; Jia, W.; Zhang, Y. Vaccines’ New Era-RNA Vaccine. Viruses 2023, 15, 1760. [Google Scholar] [CrossRef] [PubMed]
- Maruggi, G.; Mallett, C.P.; Westerbeck, J.W.; Chen, T.; Lofano, G.; Friedrich, K.; Qu, L.; Sun, J.T.; McAuliffe, J.; Kanitkar, A.; et al. A self-amplifying mRNA SARS-CoV-2 vaccine candidate induces safe and robust protective immunity in preclinical models. Mol. Ther. 2022, 30, 1897–1912. [Google Scholar] [CrossRef] [PubMed]
- Pateev, I.; Seregina, K.; Ivanov, R.; Reshetnikov, V. Biodistribution of RNA Vaccines and of Their Products: Evidence from Human and Animal Studies. Biomedicines 2023, 12, 59. [Google Scholar] [CrossRef]
- Gong, Y.; Yong, D.; Liu, G.; Xu, J.; Ding, J.; Jia, W. A Novel Self-Amplifying mRNA with Decreased Cytotoxicity and Enhanced Protein Expression by Macrodomain Mutations. Adv. Sci. 2024, 11, 2402936. [Google Scholar] [CrossRef]
- Arcturus Therapeutics and CSL Announce European Medicines Agency Validates Marketing Authorization Application for ARCT-154 Vaccine to Prevent COVID-19. Available online: https://ir.arcturusrx.com/news-releases/news-release-details/arcturus-therapeutics-and-csl-announce-european-medicines-agency (accessed on 15 June 2025).
- Vanluchene, H.; Gillon, O.; Peynshaert, K.; De Smedt, S.C.; Sanders, N.; Raemdonck, K.; Remaut, K. Less is more: Self-amplifying mRNA becomes self-killing upon dose escalation in immune-competent retinal cells. Eur. J. Pharm. Biopharm. 2024, 196, 114204. [Google Scholar] [CrossRef]
- Minnaert, A.-K.; Vanluchene, H.; Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Raemdonck, K.; Sanders, N.N.; Remaut, K. Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: Getting the message across. Adv. Drug Deliv. Rev. 2021, 176, 113900. [Google Scholar] [CrossRef]
- Casmil, I.C.; Jin, J.; Won, E.-J.; Huang, C.; Liao, S.; Cha-Molstad, H.; Blakney, A.K. The advent of clinical self-amplifying RNA vaccines. Mol. Ther. 2025, 33, 2565–2582. [Google Scholar] [CrossRef]
- Stokes, A.; Pion, J.; Binazon, O.; Laffont, B.; Bigras, M.; Dubois, G.; Blouin, K.; Young, J.K.; Ringenberg, M.A.; Ben Abdeljelil, N.; et al. Nonclinical safety assessment of repeated administration and biodistribution of a novel rabies self-amplifying mRNA vaccine in rats. Regul. Toxicol. Pharmacol. 2020, 113, 104648. [Google Scholar] [CrossRef]
- Donahue, D.A.; Ballesteros, C.; Maruggi, G.; Glover, C.; Ringenberg, M.A.; Marquis, M.; Ben Abdeljelil, N.; Ashraf, A.; Rodriguez, L.-A.; Stokes, A.H. Nonclinical Safety Assessment of Lipid Nanoparticle-and Emulsion-Based Self-Amplifying mRNA Vaccines in Rats. Int. J. Toxicol. 2023, 42, 37–49. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Wu, H.; Wang, M.; Mao, L.; Guo, X.; Zhu, J.; Ye, Z.; Luo, X.; Yang, X.; et al. Intravenous administration of IL-12 encoding self-replicating RNA-lipid nanoparticle complex leads to safe and effective antitumor responses. Sci. Rep. 2024, 14, 7366. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.A.; Fisher, M.A.; Simmons, C.A.; Farhood, A.; Jaeschke, H. Parenchymal cell apoptosis as a signal for sinusoidal sequestration and transendothelial migration of neutrophils in murine models of endotoxin and fas-antibody-induced liver injury. Hepatology 1998, 28, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Bajt, M.L. Protection against Fas Receptor-Mediated Apoptosis in Hepatocytes and Nonparenchymal Cells by a Caspase-8 Inhibitor in Vivo: Evidence for a Postmitochondrial Processing of Caspase-8. Toxicol. Sci. 2000, 58, 109–117. [Google Scholar] [CrossRef]
- Low, J.G.; De Alwis, R.; Chen, S.; Kalimuddin, S.; Leong, Y.S.; Mah, T.K.L.; Yuen, N.; Tan, H.C.; Zhang, S.L.; Sim, J.X.Y.; et al. A phase I/II randomized, double-blinded, placebo-controlled trial of a self-amplifying Covid-19 mRNA vaccine. Npj Vaccines 2022, 7, 161. [Google Scholar] [CrossRef]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef]
- Wojcechowskyj, J.A.; Jong, R.M.; Mäger, I.; Flach, B.; Munson, P.V.; Mukherjee, P.P.; Mertins, B.; Barcay, K.R.; Folliard, T. Controlling reactogenicity while preserving immunogenicity from a self-amplifying RNA vaccine by modulating nucleocytoplasmic transport. Npj Vaccines 2025, 10, 85. [Google Scholar] [CrossRef]
- Spikevax-Previously-COVID-19-Vaccine-Moderna-h-c-5791-ii-42-Epar-Assessment-Report-Variation_en. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax (accessed on 10 July 2025).
- Reshetnikov, V.; Shepelkova, G.; Rybakova, A.; Trashkov, A.; Yeremeev, V.; Ivanov, R. The candidate anti-tuberculosis mRNA vaccine immunogenicity and reactogenicity dependency on the animal’s sex and the vaccine dose. Bull. Russ. State Med. Univ. 2024, 25–31. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wu, J.; Wang, Y.; Huang, T.; Zhao, K.; Liu, J.; Wang, H.; Zhu, T.; Gou, J.; Huang, H.; et al. Safety and immunogenicity of the COVID-19 mRNA vaccine CS-2034: A randomized, double-blind, dose-exploration, placebo-controlled multicenter Phase I clinical trial in healthy Chinese adults. J. Infect. 2023, 87, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Pollock, K.M.; Cheeseman, H.M.; Szubert, A.J.; Libri, V.; Boffito, M.; Owen, D.; Bern, H.; McFarlane, L.R.; O’Hara, J.; Lemm, N.-M.; et al. Safety and immunogenicity of a self-amplifying RNA vaccine against COVID-19: COVAC1, a phase I, dose-ranging trial. eClinicalMedicine 2022, 44, 101262. [Google Scholar] [CrossRef] [PubMed]
- Szubert, A.J.; Pollock, K.M.; Cheeseman, H.M.; Alagaratnam, J.; Bern, H.; Bird, O.; Boffito, M.; Byrne, R.; Cole, T.; Cosgrove, C.A.; et al. COVAC1 phase 2a expanded safety and immunogenicity study of a self-amplifying RNA vaccine against SARS-CoV-2. eClinicalMedicine 2023, 56, 101823. [Google Scholar] [CrossRef]
- Hồ, N.T.; Hughes, S.G.; Ta, V.T.; Phan, L.T.; Đỗ, Q.; Nguyễn, T.V.; Phạm, A.T.V.; Thị Ngọc Đặng, M.; Nguyễn, L.V.; Trịnh, Q.V.; et al. Safety, immunogenicity and efficacy of the self-amplifying mRNA ARCT-154 COVID-19 vaccine: Pooled phase 1, 2, 3a and 3b randomized, controlled trials. Nat. Commun. 2024, 15, 4081. [Google Scholar] [CrossRef]
- Maine, C.J.; Miyake-Stoner, S.J.; Spasova, D.S.; Picarda, G.; Chou, A.C.; Brand, E.D.; Olesiuk, M.D.; Domingo, C.C.; Little, H.J.; Goodman, T.T.; et al. Safety and immunogenicity of an optimized self-replicating RNA platform for low dose or single dose vaccine applications: A randomized, open label Phase I study in healthy volunteers. Nat. Commun. 2025, 16, 456. [Google Scholar] [CrossRef]
- Kitonsa, J.; Serwanga, J.; Cheeseman, H.M.; Abaasa, A.; Lunkuse, J.F.; Ruzagira, E.; Kato, L.; Nambaziira, F.; Oluka, G.K.; Gombe, B.; et al. Safety and Immunogenicity of a Modified Self-Amplifying Ribonucleic Acid (saRNA) Vaccine Encoding SARS-CoV-2 Spike Glycoprotein in SARS-CoV-2 Seronegative and Seropositive Ugandan Individuals. Vaccines 2025, 13, 553. [Google Scholar] [CrossRef]
- Saraf, A.; Gurjar, R.; Kaviraj, S.; Kulkarni, A.; Kumar, D.; Kulkarni, R.; Virkar, R.; Krishnan, J.; Yadav, A.; Baranwal, E.; et al. An Omicron-specific, self-amplifying mRNA booster vaccine for COVID-19: A phase 2/3 randomized trial. Nat. Med. 2024, 30, 1363–1372. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Korzun, T.; Moses, A.S.; Jozic, A.; Grigoriev, V.; Newton, S.; Kim, J.; Diba, P.; Sattler, A.; Levasseur, P.R.; Le, N.; et al. Lipid Nanoparticles Elicit Reactogenicity and Sickness Behavior in Mice Via Toll-Like Receptor 4 and Myeloid Differentiation Protein 88 Axis. ACS Nano 2024, 18, 24842–24859. [Google Scholar] [CrossRef]
- Kang, D.D.; Hou, X.; Wang, L.; Xue, Y.; Li, H.; Zhong, Y.; Wang, S.; Deng, B.; McComb, D.W.; Dong, Y. Engineering LNPs with polysarcosine lipids for mRNA delivery. Bioact. Mater. 2024, 37, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef] [PubMed]
- Kulasegaran-Shylini, R.; Atasheva, S.; Gorenstein, D.G.; Frolov, I. Structural and Functional Elements of the Promoter Encoded by the 5′ Untranslated Region of the Venezuelan Equine Encephalitis Virus Genome. J. Virol. 2009, 83, 8327–8339. [Google Scholar] [CrossRef] [PubMed]
- Frolov, I.; Hardy, R.; Rice, C.M. Cis-acting RNA elements at the 5′ end of Sindbis virus genome RNA regulate minus- and plus-strand RNA synthesis. RNA 2001, 7, 1638–1651. [Google Scholar] [CrossRef]
- Michel, G.; Petrakova, O.; Atasheva, S.; Frolov, I. Adaptation of Venezuelan equine encephalitis virus lacking 51-nt conserved sequence element to replication in mammalian and mosquito cells. Virology 2007, 362, 475–487. [Google Scholar] [CrossRef]
- Shirako, Y.; Strauss, J.H. Regulation of Sindbis virus RNA replication: Uncleaved P123 and nsP4 function in minus-strand RNA synthesis, whereas cleaved products from P123 are required for efficient plus-strand RNA synthesis. J. Virol. 1994, 68, 1874–1885. [Google Scholar] [CrossRef]
- Lemm, J.A.; Rümenapf, T.; Strauss, E.G.; Strauss, J.H.; Rice, C.M. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: A model for the temporal regulation of minus- and plus-strand RNA synthesis. EMBO J. 1994, 13, 2925–2934. [Google Scholar] [CrossRef]
- Rupp, J.C.; Sokoloski, K.J.; Gebhart, N.N.; Hardy, R.W. Alphavirus RNA synthesis and non-structural protein functions. J. Gen. Virol. 2015, 96, 2483–2500. [Google Scholar] [CrossRef]
- Tan, Y.B.; Chmielewski, D.; Law, M.C.Y.; Zhang, K.; He, Y.; Chen, M.; Jin, J.; Luo, D. Molecular architecture of the Chikungunya virus replication complex. Sci. Adv. 2022, 8, eadd2536. [Google Scholar] [CrossRef]
- Laurent, T.; Kumar, P.; Liese, S.; Zare, F.; Jonasson, M.; Carlson, A.; Carlson, L.-A. Architecture of the chikungunya virus replication organelle. eLife 2022, 11, e83042. [Google Scholar] [CrossRef]
- Ahola, T.; Kääriäinen, L. Reaction in alphavirus mRNA capping: Formation of a covalent complex of nonstructural protein nsP1 with 7-methyl-GMP. Proc. Natl. Acad. Sci. USA 1995, 92, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Vasiljeva, L.; Merits, A.; Auvinen, P.; Kääriäinen, L. Identification of a Novel Function of the AlphavirusCapping Apparatus. J. Biol. Chem. 2000, 275, 17281–17287. [Google Scholar] [CrossRef] [PubMed]
- Rausalu, K.; Utt, A.; Quirin, T.; Varghese, F.S.; Žusinaite, E.; Das, P.K.; Ahola, T.; Merits, A. Chikungunya virus infectivity, RNA replication and non-structural polyprotein processing depend on the nsP2 protease’s active site cysteine residue. Sci. Rep. 2016, 6, 37124. [Google Scholar] [CrossRef] [PubMed]
- Rikkonen, M.; Peränen, J.; Kääriäinen, L. ATPase and GTPase activities associated with Semliki Forest virus nonstructural protein nsP2. J. Virol. 1994, 68, 5804–5810. [Google Scholar] [CrossRef]
- Karpe, Y.A.; Aher, P.P.; Lole, K.S. NTPase and 5′-RNA Triphosphatase Activities of Chikungunya Virus nsP2 Protein. PLoS ONE 2011, 6, e22336. [Google Scholar] [CrossRef]
- Das, P.K.; Merits, A.; Lulla, A. Functional Cross-talk between Distant Domains of Chikungunya Virus Non-structural Protein 2 Is Decisive for Its RNA-modulating Activity. J. Biol. Chem. 2014, 289, 5635–5653. [Google Scholar] [CrossRef]
- Akhrymuk, I.; Kulemzin, S.V.; Frolova, E.I. Evasion of the Innate Immune Response: The Old World Alphavirus nsP2 Protein Induces Rapid Degradation of Rpb1, a Catalytic Subunit of RNA Polymerase II. J. Virol. 2012, 86, 7180–7191. [Google Scholar] [CrossRef]
- Meshram, C.D.; Phillips, A.T.; Lukash, T.; Shiliaev, N.; Frolova, E.I.; Frolov, I. Mutations in Hypervariable Domain of Venezuelan Equine Encephalitis Virus nsP3 Protein Differentially Affect Viral Replication. J. Virol. 2020, 94, e01841-19. [Google Scholar] [CrossRef]
- Hick, T.A.H.; Zotler, T.; Bosveld, D.; Geertsema, C.; Van Oers, M.M.; Pijlman, G.P. Venezuelan equine encephalitis virus non-structural protein 3 dictates superinfection exclusion in mammalian cells. Npj Viruses 2024, 2, 43. [Google Scholar] [CrossRef]
- Rubach, J.K.; Wasik, B.R.; Rupp, J.C.; Kuhn, R.J.; Hardy, R.W.; Smith, J.L. Characterization of purified Sindbis virus nsP4 RNA-dependent RNA polymerase activity in vitro. Virology 2009, 384, 201–208. [Google Scholar] [CrossRef]
- Rupp, J.C.; Jundt, N.; Hardy, R.W. Requirement for the Amino-Terminal Domain of Sindbis Virus nsP4 during Virus Infection. J. Virol. 2011, 85, 3449–3460. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.; Hardy, R.W.; Smith, J.L.; Kuhn, R.J. Catalytic Core of Alphavirus Nonstructural Protein nsP4 Possesses Terminal Adenylyltransferase Activity. J. Virol. 2006, 80, 9962–9969. [Google Scholar] [CrossRef] [PubMed]
- Akhrymuk, I.; Lukash, T.; Frolov, I.; Frolova, E.I. Novel Mutations in nsP2 Abolish Chikungunya Virus-Induced Transcriptional Shutoff and Make the Virus Less Cytopathic without Affecting Its Replication Rates. J. Virol. 2019, 93, e02062-18. [Google Scholar] [CrossRef] [PubMed]
- Frolov, I.; Garmashova, N.; Atasheva, S.; Frolova, E.I. Random Insertion Mutagenesis of Sindbis Virus Nonstructural Protein 2 and Selection of Variants Incapable of Downregulating Cellular Transcription. J. Virol. 2009, 83, 9031–9044. [Google Scholar] [CrossRef]
- Garmashova, N.; Atasheva, S.; Kang, W.; Weaver, S.C.; Frolova, E.; Frolov, I. Analysis of Venezuelan Equine Encephalitis Virus Capsid Protein Function in the Inhibition of Cellular Transcription. J. Virol. 2007, 81, 13552–13565. [Google Scholar] [CrossRef]
- Garmashova, N.; Gorchakov, R.; Volkova, E.; Paessler, S.; Frolova, E.; Frolov, I. The Old World and New World Alphaviruses Use Different Virus-Specific Proteins for Induction of Transcriptional Shutoff. J. Virol. 2007, 81, 2472–2484. [Google Scholar] [CrossRef]
- Petrakova, O.; Volkova, E.; Gorchakov, R.; Paessler, S.; Kinney, R.M.; Frolov, I. Noncytopathic Replication of Venezuelan Equine Encephalitis Virus and Eastern Equine Encephalitis Virus Replicons in Mammalian Cells. J. Virol. 2005, 79, 7597–7608. [Google Scholar] [CrossRef]
- Li, Y.; Teague, B.; Zhang, Y.; Su, Z.; Porter, E.; Dobosh, B.; Wagner, T.; Irvine, D.J.; Weiss, R. In Vitro evolution of enhanced RNA replicons for immunotherapy. Sci. Rep. 2019, 9, 6932. [Google Scholar] [CrossRef]
- Maruggi, G.; Shaw, C.A.; Otten, G.R.; Mason, P.W.; Beard, C.W. Engineered alphavirus replicon vaccines based on known attenuated viral mutants show limited effects on immunogenicity. Virology 2013, 447, 254–264. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, Y. Mutations in the non-structural protein coding region regulate gene expression from replicon RNAs derived from Venezuelan equine encephalitis virus. Biotechnol. Lett. 2023, 45, 1029–1038. [Google Scholar] [CrossRef]
- Beitzel, B.F.; Bakken, R.R.; Smith, J.M.; Schmaljohn, C.S. High-Resolution Functional Mapping of the Venezuelan Equine Encephalitis Virus Genome by Insertional Mutagenesis and Massively Parallel Sequencing. PLoS Pathog. 2010, 6, e1001146. [Google Scholar] [CrossRef]
- Quintana, V.; Caillava, J.; Byk, L.A.; Mondotte, J.A.; Battini, L.; Tarte, P.; Samsa, M.M.; Filomatori, C.V.; Alvarez, D.E. Improvement of the potency of a N1-methylpseudouridine-modified self-amplifying RNA through mutations in the RNA-dependent-RNA-polymerase. J. Biol. Chem. 2025, 301, 110487. [Google Scholar] [CrossRef]
- Chamberlain, J.; Dowall, S.D.; Smith, J.; Pearson, G.; Graham, V.; Raynes, J.; Hewson, R. Attenuation of Chikungunya Virus by a Single Amino Acid Substitution in the nsP1 Component of a Non-Structural Polyprotein. Viruses 2025, 17, 281. [Google Scholar] [CrossRef] [PubMed]
- Utt, A.; Das, P.K.; Varjak, M.; Lulla, V.; Lulla, A.; Merits, A.; Perlman, S. Mutations Conferring a Noncytotoxic Phenotype on Chikungunya Virus Replicons Compromise Enzymatic Properties of Nonstructural Protein 2. J. Virol. 2015, 89, 3145–3162. [Google Scholar] [CrossRef] [PubMed]
- Garmashova, N.; Gorchakov, R.; Frolova, E.; Frolov, I. Sindbis Virus Nonstructural Protein nsP2 Is Cytotoxic and Inhibits Cellular Transcription. J. Virol. 2006, 80, 5686–5696. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, F.; Palchevska, O.; Frolova, E.I.; Frolov, I. Alphavirus-based replicons demonstrate different interactions with host cells and can be optimized to increase protein expression. J. Virol. 2023, 97, e01225-23. [Google Scholar] [CrossRef]
- Akhrymuk, I.; Frolov, I.; Frolova, E.I. Sindbis Virus Infection Causes Cell Death by nsP2-Induced Transcriptional Shutoff or by nsP3-Dependent Translational Shutoff. J. Virol. 2018, 92, e01388-18. [Google Scholar] [CrossRef]
- Kafai, N.M.; Diamond, M.S.; Fox, J.M. Distinct Cellular Tropism and Immune Responses to Alphavirus Infection. Annu. Rev. Immunol. 2022, 40, 615–649. [Google Scholar] [CrossRef]
- Guerrero-Arguero, I.; Tellez-Freitas, C.M.; Weber, K.S.; Berges, B.K.; Robison, R.A.; Pickett, B.E. Alphaviruses: Host pathogenesis, immune response, and vaccine & treatment updates. J. Gen. Virol. 2021, 102, 001644. [Google Scholar] [CrossRef]
- Leung, J.Y.-S.; Ng, M.M.-L.; Chu, J.J.H. Replication of Alphaviruses: A Review on the Entry Process of Alphaviruses into Cells. Adv. Virol. 2011, 2011, 249640. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Y.; Zhang, H.; Ge, T.; Pan, Y.; Liu, Y.; Wu, M.; Shan, T.; Zhu, G.; Wu, Q.; et al. Next-Generation saRNA Platforms: Systematic Screening and Engineering Enhances Superior Protein Expression and Organ-Specific Targeting for RNA Therapeutics. bioRxiv 2025. [Google Scholar] [CrossRef]
- Morais, P.; Adachi, H.; Yu, Y.-T. The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines. Front. Cell Dev. Biol. 2021, 9, 789427. [Google Scholar] [CrossRef]
- Martinez, N.M.; Su, A.; Burns, M.C.; Nussbacher, J.K.; Schaening, C.; Sathe, S.; Yeo, G.W.; Gilbert, W.V. Pseudouridine synthases modify human pre-mRNA co-transcriptionally and affect pre-mRNA processing. Mol. Cell 2022, 82, 645–659.e9. [Google Scholar] [CrossRef] [PubMed]
- Svitkin, Y.V.; Cheng, Y.M.; Chakraborty, T.; Presnyak, V.; John, M.; Sonenberg, N. N1-methyl-pseudouridine in mRNA enhances translation through eIF2α-dependent and independent mechanisms by increasing ribosome density. Nucleic Acids Res. 2017, 45, 6023–6036. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.; Eyler, D.E.; Mitchell, L.; Deb, I.; Bojanowski, A.; Srinivas, P.; Dunham, C.M.; Roy, B.; Frank, A.T.; Koutmou, K.S. N1-Methylpseudouridine and pseudouridine modifications modulate mRNA decoding during translation. Nat. Commun. 2024, 15, 8119. [Google Scholar] [CrossRef] [PubMed]
- Azizi, H.; Renner, T.M.; Agbayani, G.; Simard, B.; Dudani, R.; Harrison, B.A.; Iqbal, U.; Jia, Y.; McCluskie, M.J.; Akache, B. Self-amplifying RNAs generated with the modified nucleotides 5-methylcytidine and 5-methyluridine mediate strong expression and immunogenicity in vivo. NAR Mol. Med. 2024, 1, ugae004. [Google Scholar] [CrossRef]
- Miyazato, P.; Noguchi, T.; Ogawa, F.; Sugimoto, T.; Fauzyah, Y.; Sasaki, R.; Ebina, H. 1mΨ influences the performance of various positive-stranded RNA virus-based replicons. Sci. Rep. 2024, 14, 17634. [Google Scholar] [CrossRef]
- Komori, M.; Morey, A.L.; Quiñones-Molina, A.A.; Fofana, J.; Romero, L.; Peters, E.; Matsuda, K.; Gummuluru, S.; Smith, J.F.; Akahata, W.; et al. Incorporation of 5 methylcytidine alleviates innate immune response to self-amplifying RNA vaccine. bioRxiv 2023. [Google Scholar] [CrossRef]
- McGee, J.E.; Kirsch, J.R.; Kenney, D.; Cerbo, F.; Chavez, E.C.; Shih, T.-Y.; Douam, F.; Wong, W.W.; Grinstaff, M.W. Complete substitution with modified nucleotides in self-amplifying RNA suppresses the interferon response and increases potency. Nat. Biotechnol. 2025, 43, 720–726. [Google Scholar] [CrossRef]
- Aboshi, M.; Matsuda, K.; Kawakami, D.; Kono, K.; Kazami, Y.; Sekida, T.; Komori, M.; Morey, A.L.; Suga, S.; Smith, J.F.; et al. Safety and immunogenicity of VLPCOV-02, a SARS-CoV-2 self-amplifying RNA vaccine with a modified base, 5-methylcytosine. iScience 2024, 27, 108964. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K. Toll-like receptors in innate immunity. Int. Immunol. 2004, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Vercammen, E.; Staal, J.; Beyaert, R. Sensing of Viral Infection and Activation of Innate Immunity by Toll-Like Receptor 3. Clin. Microbiol. Rev. 2008, 21, 13–25. [Google Scholar] [CrossRef]
- Santoro, M.G. NEW EMBO MEMBER’S REVIEW: NF-kappaB and virus infection: Who controls whom. EMBO J. 2003, 22, 2552–2560. [Google Scholar] [CrossRef]
- Devoldere, J.; Dewitte, H.; De Smedt, S.C.; Remaut, K. Evading innate immunity in nonviral mRNA delivery: Don’t shoot the messenger. Drug Discov. Today 2016, 21, 11–25. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Recognition of viruses by innate immunity. Immunol. Rev. 2007, 220, 214–224. [Google Scholar] [CrossRef]
- Yoneyama, M.; Fujita, T. Structural Mechanism of RNA Recognition by the RIG-I-like Receptors. Immunity 2008, 29, 178–181. [Google Scholar] [CrossRef]
- Barral, P.M.; Sarkar, D.; Su, Z.; Barber, G.N.; DeSalle, R.; Racaniello, V.R.; Fisher, P.B. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: Key regulators of innate immunity. Pharmacol. Ther. 2009, 124, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Stirling, D.C.; Wang, Z.; Flight, K.E.; Brown, J.C.; Blakney, A.K.; McKay, P.F.; Cunliffe, R.F.; Murugaiah, V.; Fox, C.B.; et al. Formulation, inflammation, and RNA sensing impact the immunogenicity of self-amplifying RNA vaccines. Mol. Ther. Nucleic Acids 2023, 31, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Ueta, M.; Hamuro, J.; Kiyono, H.; Kinoshita, S. Triggering of TLR3 by polyI:C in human corneal epithelial cells to induce inflammatory cytokines. Biochem. Biophys. Res. Commun. 2005, 331, 285–294. [Google Scholar] [CrossRef]
- Nikonov, A.; Mölder, T.; Sikut, R.; Kiiver, K.; Männik, A.; Toots, U.; Lulla, A.; Lulla, V.; Utt, A.; Merits, A.; et al. RIG-I and MDA-5 Detection of Viral RNA-dependent RNA Polymerase Activity Restricts Positive-Strand RNA Virus Replication. PLoS Pathog. 2013, 9, e1003610. [Google Scholar] [CrossRef]
- Akhrymuk, I.; Frolov, I.; Frolova, E.I. Both RIG-I and MDA5 detect alphavirus replication in concentration-dependent mode. Virology 2016, 487, 230–241. [Google Scholar] [CrossRef]
- McDougal, M.B.; De Maria, A.M.; Ohlson, M.B.; Kumar, A.; Xing, C.; Schoggins, J.W. Interferon inhibits a model RNA virus via a limited set of inducible effector genes. EMBO Rep. 2023, 24, e56901. [Google Scholar] [CrossRef]
- Rutherford, M.N.; Hannigan, G.E.; Williams, B.R. Interferon-induced binding of nuclear factors to promoter elements of the 2-5A synthetase gene. EMBO J. 1988, 7, 751–759. [Google Scholar] [CrossRef]
- Dey, M.; Cao, C.; Dar, A.C.; Tamura, T.; Ozato, K.; Sicheri, F.; Dever, T.E. Mechanistic Link between PKR Dimerization, Autophosphorylation, and eIF2α Substrate Recognition. Cell 2005, 122, 901–913. [Google Scholar] [CrossRef]
- García, M.A.; Meurs, E.F.; Esteban, M. The dsRNA protein kinase PKR: Virus and cell control. Biochimie 2007, 89, 799–811. [Google Scholar] [CrossRef]
- Corbet, G.A.; Burke, J.M.; Bublitz, G.R.; Tay, J.W.; Parker, R. dsRNA-induced condensation of antiviral proteins modulates PKR activity. Proc. Natl. Acad. Sci. USA 2022, 119, e2204235119. [Google Scholar] [CrossRef]
- Yu, H.; Megawati, D.; Zheng, C.; Rothenburg, S. Protein Kinase R (PKR) as a Novel dsRNA Sensor in Antiviral Innate Immunity. In Antiviral Innate Immunity; Zheng, C., Ed.; Springer: New York, NY, USA, 2025; Volume 2854, pp. 265–282. [Google Scholar]
- Balachandran, S.; Kim, C.N.; Yeh, W.-C.; Mak, T.W.; Bhalla, K.; Barber, G.N. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 1998, 17, 6888–6902. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.J.; Nogusa, S.; Chen, P.; Maki, J.L.; Lerro, A.; Andrake, M.; Rall, G.F.; Degterev, A.; Balachandran, S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc. Natl. Acad. Sci. USA 2013, 110, E3109–E3118. [Google Scholar] [CrossRef] [PubMed]
- Dahal, B.; Lin, S.C.; Carey, B.D.; Jacobs, J.L.; Dinman, J.D.; van Hoek, M.L.; Adams, A.A.; Kehn-Hall, K. EGR1 upregulation following Venezuelan equine encephalitis virus infection is regulated by ERK and PERK pathways contributing to cell death. Virology 2020, 539, 121–128. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274, Erratum in Nature 1999, 398, 90. [Google Scholar] [CrossRef]
- Holm, L. DNA polymerase β belongs to an ancient nucleotidyltransferase superfamily. Trends Biochem. Sci. 1995, 20, 345–347. [Google Scholar] [CrossRef]
- Kristiansen, H.; Gad, H.H.; Eskildsen-Larsen, S.; Despres, P.; Hartmann, R. The Oligoadenylate Synthetase Family: An Ancient Protein Family with Multiple Antiviral Activities. J. Interferon Cytokine Res. 2011, 31, 41–47. [Google Scholar] [CrossRef]
- Ibsen, M.S.; Gad, H.H.; Andersen, L.L.; Hornung, V.; Julkunen, I.; Sarkar, S.N.; Hartmann, R. Structural and functional analysis reveals that human OASL binds dsRNA to enhance RIG-I signaling. Nucleic Acids Res. 2015, 43, 5236–5248. [Google Scholar] [CrossRef]
- Tanaka, N.; Nakanishi, M.; Kusakabe, Y.; Goto, Y.; Kitade, Y.; Nakamura, K.T. Structural basis for recognition of 2′,5′-linked oligoadenylates by human ribonuclease L. EMBO J. 2004, 23, 3929–3938. [Google Scholar] [CrossRef]
- Zhou, A. Expression cloning of 2-5A-dependent RNAase: A uniquely regulated mediator of interferon action. Cell 1993, 72, 753–765. [Google Scholar] [CrossRef]
- Bisbal, C.; Silverman, R.H. Diverse functions of RNase L and implications in pathology. Biochimie 2007, 89, 789–798. [Google Scholar] [CrossRef]
- Kubota, K.; Nakahara, K.; Ohtsuka, T.; Yoshida, S.; Kawaguchi, J.; Fujita, Y.; Ozeki, Y.; Hara, A.; Yoshimura, C.; Furukawa, H.; et al. Identification of 2′-Phosphodiesterase, Which Plays a Role in the 2-5A System Regulated by Interferon. J. Biol. Chem. 2004, 279, 37832–37841. [Google Scholar] [CrossRef]
- Schmidt, A.; Chernajovsky, Y.; Shulman, L.; Federman, P.; Berissi, H.; Revel, M. An interferon-induced phosphodiesterase degrading (2′–5′) oligoisoadenylate and the C-C-A terminus of tRNA. Proc. Natl. Acad. Sci. USA 1979, 76, 4788–4792. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H.; Gilbert, C.S.; Kerr, I.M.; Wreschner, D.H. Synthesis, Characterization and Properties of ppp(A2′p)nApCp and Related High-Specific-Activity 32P-Labelled Derivatives of ppp(A2′p)nA. Eur. J. Biochem. 2005, 115, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.A.; Gonçalves-Carneiro, D.; Zang, T.M.; Soll, S.J.; York, A.; Blanco-Melo, D.; Bieniasz, P.D. CG dinucleotide suppression enables antiviral defence targeting non-self RNA. Nature 2017, 550, 124–127. [Google Scholar] [CrossRef]
- Johnson, B.; VanBlargan, L.A.; Xu, W.; White, J.P.; Shan, C.; Shi, P.-Y.; Zhang, R.; Adhikari, J.; Gross, M.L.; Leung, D.W.; et al. Human IFIT3 Modulates IFIT1 RNA Binding Specificity and Protein Stability. Immunity 2018, 48, 487–499.e5. [Google Scholar] [CrossRef]
- Poleganov, M.A.; Eminli, S.; Beissert, T.; Herz, S.; Moon, J.-I.; Goldmann, J.; Beyer, A.; Heck, R.; Burkhart, I.; Barea Roldan, D.; et al. Efficient Reprogramming of Human Fibroblasts and Blood-Derived Endothelial Progenitor Cells Using Nonmodified RNA for Reprogramming and Immune Evasion. Hum. Gene Ther. 2015, 26, 751–766. [Google Scholar] [CrossRef]
- Ohto, T.; Konishi, M.; Tanaka, H.; Onomoto, K.; Yoneyama, M.; Nakai, Y.; Tange, K.; Yoshioka, H.; Akita, H. Inhibition of the Inflammatory Pathway Enhances Both the In Vitro and In Vivo Transfection Activity of Exogenous in Vitro-Transcribed mRNAs Delivered by Lipid Nanoparticles. Biol. Pharm. Bull. 2019, 42, 299–302. [Google Scholar] [CrossRef]
- Devasthanam, A.S. Mechanisms underlying the inhibition of interferon signaling by viruses. Virulence 2014, 5, 270–277. [Google Scholar] [CrossRef]
- Liu, Y.; Chin, J.M.; Choo, E.L.; Phua, K.K.L. Messenger RNA translation enhancement by immune evasion proteins: A comparative study between EKB (vaccinia virus) and NS1 (influenza A virus). Sci. Rep. 2019, 9, 11972. [Google Scholar] [CrossRef]
- Beissert, T.; Koste, L.; Perkovic, M.; Walzer, K.C.; Erbar, S.; Selmi, A.; Diken, M.; Kreiter, S.; Türeci, Ö.; Sahin, U. Improvement of In Vivo Expression of Genes Delivered by Self-Amplifying RNA Using Vaccinia Virus Immune Evasion Proteins. Hum. Gene Ther. 2017, 28, 1138–1146. [Google Scholar] [CrossRef]
- Perdiguero, B.; Esteban, M. The Interferon System and Vaccinia Virus Evasion Mechanisms. J. Interferon Cytokine Res. 2009, 29, 581–598. [Google Scholar] [CrossRef]
- Krug, R.M. Functions of the influenza A virus NS1 protein in antiviral defense. Curr. Opin. Virol. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Hale, B.G.; Randall, R.E.; Ortín, J.; Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008, 89, 2359–2376. [Google Scholar] [CrossRef]
- Blakney, A.K.; McKay, P.F.; Bouton, C.R.; Hu, K.; Samnuan, K.; Shattock, R.J. Innate Inhibiting Proteins Enhance Expression and Immunogenicity of Self-Amplifying RNA. Mol. Ther. 2021, 29, 1174–1185. [Google Scholar] [CrossRef]
- Blakney, A.K.; Zhu, Y.; McKay, P.F.; Bouton, C.R.; Yeow, J.; Tang, J.; Hu, K.; Samnuan, K.; Grigsby, C.L.; Shattock, R.J.; et al. Big Is Beautiful: Enhanced saRNA Delivery and Immunogenicity by a Higher Molecular Weight, Bioreducible, Cationic Polymer. ACS Nano 2020, 14, 5711–5727. [Google Scholar] [CrossRef] [PubMed]
- Borghese, F.; Sorgeloos, F.; Cesaro, T.; Michiels, T. The Leader Protein of Theiler’s Virus Prevents the Activation of PKR. J. Virol. 2019, 93, e01010-19. [Google Scholar] [CrossRef] [PubMed]
- Pepini, T.; Pulichino, A.-M.; Carsillo, T.; Carlson, A.L.; Sari-Sarraf, F.; Ramsauer, K.; Debasitis, J.C.; Maruggi, G.; Otten, G.R.; Geall, A.J.; et al. Induction of an IFN-Mediated Antiviral Response by a Self-Amplifying RNA Vaccine: Implications for Vaccine Design. J. Immunol. 2017, 198, 4012–4024. [Google Scholar] [CrossRef] [PubMed]
- De Beuckelaer, A.; Pollard, C.; Van Lint, S.; Roose, K.; Van Hoecke, L.; Naessens, T.; Udhayakumar, V.K.; Smet, M.; Sanders, N.; Lienenklaus, S.; et al. Type I Interferons Interfere with the Capacity of mRNA Lipoplex Vaccines to Elicit Cytolytic T Cell Responses. Mol. Ther. 2016, 24, 2012–2020. [Google Scholar] [CrossRef]
- Zhong, Z.; Portela Catani, J.P.; Mc Cafferty, S.; Couck, L.; van Den Broeck, W.; Gorlé, N.; Vandenbroucke, R.E.; Devriendt, B.; Ulbert, S.; Cnops, L.; et al. Immunogenicity and Protection Efficacy of a Naked Self-Replicating mRNA-Based Zika Virus Vaccine. Vaccines 2019, 7, 96. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Roose, K.; Ballegeer, M.; Zhong, Z.; Sanders, N.N.; De Koker, S.; Saelens, X.; Van Lint, S. The Opposing Effect of Type I IFN on the T Cell Response by Non-modified mRNA-Lipoplex Vaccines Is Determined by the Route of Administration. Mol. Ther. Nucleic Acids 2020, 22, 373–381. [Google Scholar] [CrossRef]
- McKay, P.F.; Hu, K.; Blakney, A.K.; Samnuan, K.; Brown, J.C.; Penn, R.; Zhou, J.; Bouton, C.R.; Rogers, P.; Polra, K.; et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020, 11, 3523. [Google Scholar] [CrossRef] [PubMed]
- Jureka, A.S.; Kleinpeter, A.B.; Tipper, J.L.; Harrod, K.S.; Petit, C.M. The influenza NS1 protein modulates RIG-I activation via a strain-specific direct interaction with the second CARD of RIG-I. J. Biol. Chem. 2020, 295, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, S.; Lin, R.; Mossman, K.L.; Zheng, C. Herpes Simplex Virus 1 Tegument Protein US11 Downmodulates the RLR Signaling Pathway via Direct Interaction with RIG-I and MDA-5. J. Virol. 2012, 86, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Childs, K.; Randall, R.; Goodbourn, S. Paramyxovirus V Proteins Interact with the RNA Helicase LGP2 To Inhibit RIG-I-Dependent Interferon Induction. J. Virol. 2012, 86, 3411–3421. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, F.; Zhu, N.; Wang, W.; Deng, Y.; Zhao, Z.; Tan, W. Middle East respiratory syndrome coronavirus ORF4b protein inhibits type I interferon production through both cytoplasmic and nuclear targets. Sci. Rep. 2015, 5, 17554. [Google Scholar] [CrossRef]
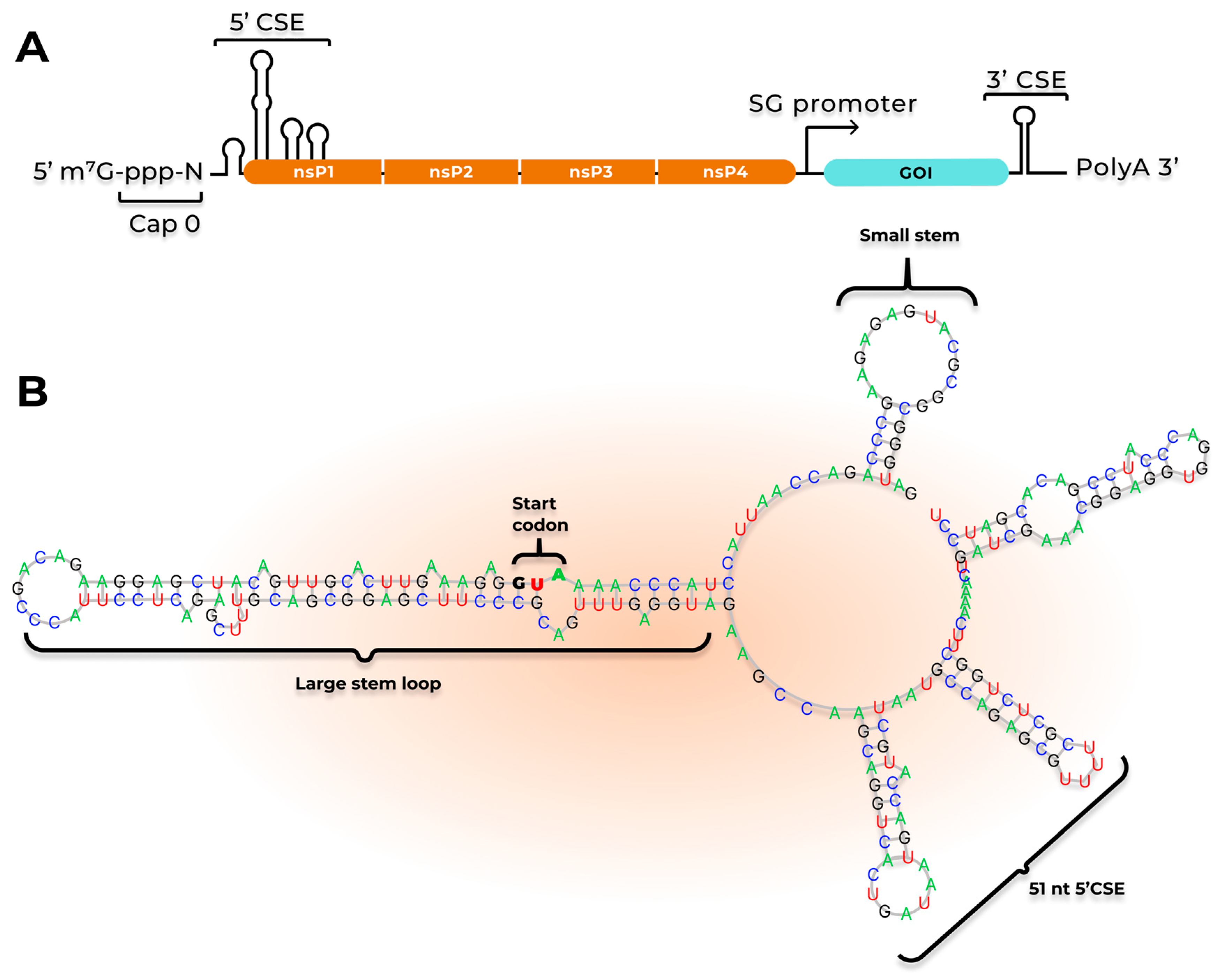
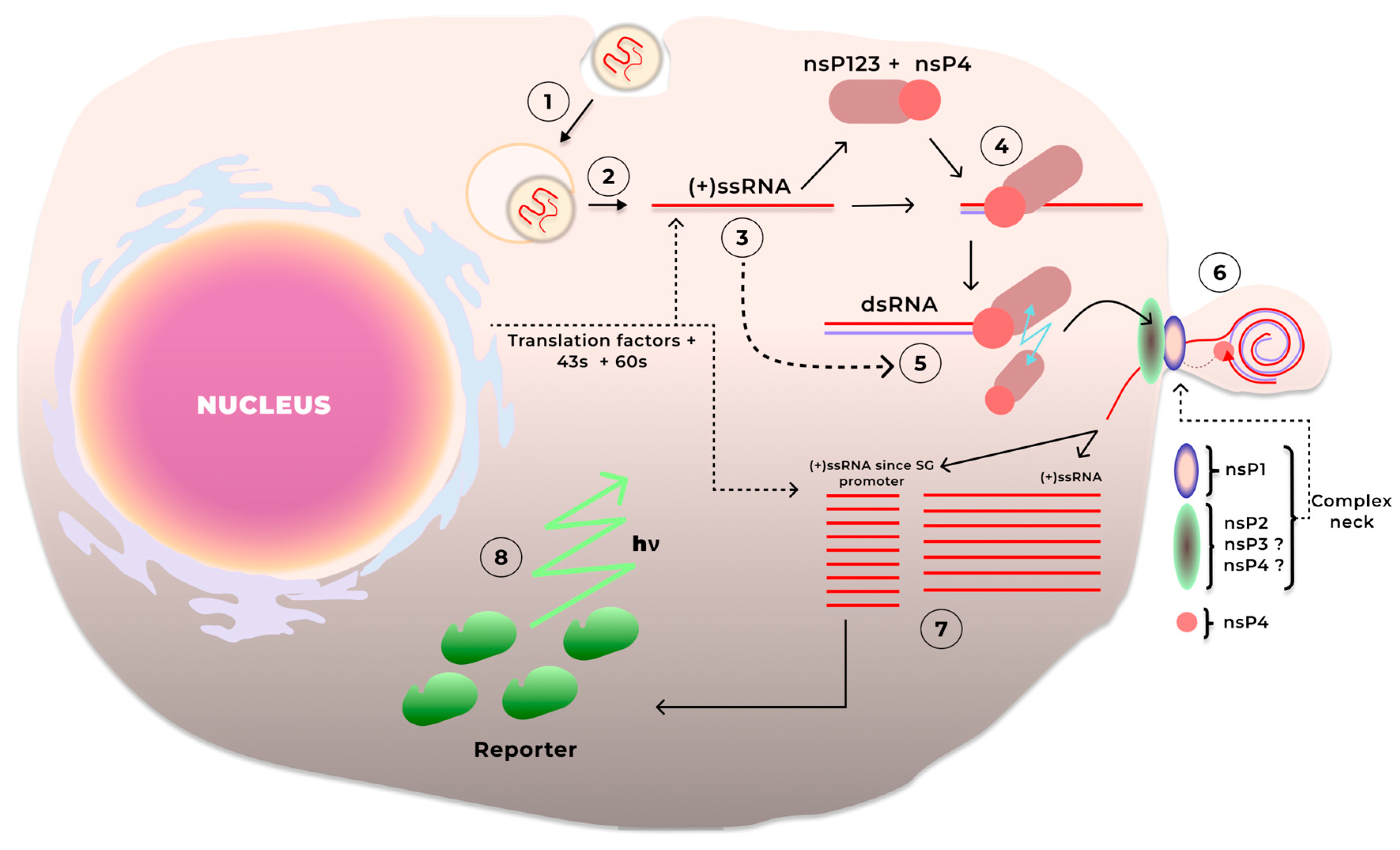

| Pathogen/Target | Replicon | Antigen | Dose/Regimen/Tissue | Delivery | Animals | Results and Safety | Reference |
|---|---|---|---|---|---|---|---|
| Rabies lyssavirus | VEEV | Rabies glycoprotein G | 15 μg; 1, 15, 29, 43 days; Blood collection day: 2, 8, 44, 50, 70 | Cationic nanoemulsion | Sprague Dawley rats (15f + 15m) | Local effects: mild erythema and/or edema 24 and 48 h after injections. Females: 2nd and 44th days—↑ ALT, ↑ AST, ↓ lymphocytes, ↑ neutrophils, ↑ fibrinogen; 44th day—↑ WBCs. Males: 2nd and 44th days—↓ lymphocytes, ↑ neutrophils, ↑ fibrinogen (+8th day); 44th day—↑ WBCs, ↑ monocytes | [22] |
| SARS-CoV-2 | VEEV | Glycoprotein S | 12 μg; 1, 15, 29 days; Blood collection day: 2, 8, 30, 36, 57 | LNP | Sprague Dawley rats (15f + 15m) | Local effects: mild erythema 72 h after vaccination. Swelling after 2nd and 3rd doses Females: 2nd day—↓ monocytes; 2nd and 30th days—↑ AST, ↓ lymphocytes, ↑ neutrophils, ↑ fibrinogen; Males: 2nd day—↓ monocytes, ↓ lymphocytes, ↑ neutrophils, ↑ fibrinogen; 30th day—↑WBCs, ↓ lymphocytes, ↑ neutrophils, ↑ fibrinogen | [15] |
| Rabies lyssavirus and SARS-CoV-2 | VEEV | Rabies glycoprotein G and glycoprotein S | 12 μg on the 1st day and 15 μg on the 15th day; Blood collection: 4 h, 1 day, 7 day | LNP | Sprague Dawley rats (12f + 12m) | ↑ TNF-α, ↑ IL-6—4 h and 24 h after vaccination; Mild erythema in all groups; Female: ↑ ALT, ↑ AST; No signs of toxicity were detected microscopically in the liver | [23] |
| Cancer | VEEV | Il-12 | 100 μg, 1, 8, 15 days; Blood collection day: –1, 2, 8, 9, 22 | LNP | Macaca fascicularis (1f + 1m) | In the male macaque group, ↑ fibrinogen, ↑ products of its breakdown in plasma, ↑ C-reactive protein were observed after the second injection. On day 9, the female showed a slight increase in ALT and AST levels. | [24] |
| Influenza | VEEV | Hemagglutinin | 0.5 µg 1st and 28th days; Blood collection day: 6 h, 28 days 6 h | LNP | C57BL/6 (n = 5) | ↑ IFNα2, ↑ IFNγ, ↑ TNF-α, ↑ KC, ↑ IL-10, ↑ IL-6, ↑ MCP-1 after the first dose; ↑ IFNα2, ↑ IFNγ, ↑ TNF-α, ↑ KC, ↑ IL-10, ↑ IL-6, ↑ MCP-1 after the second dose; | [30] |
| Name/Phase/Clinical Trial ID | Replicon/Modification/Antigen | Dose | Delivery | Results | Safety | Source |
|---|---|---|---|---|---|---|
| COVAC1 Phase I (ISRCTN17072692) | VEEV/G3A/Glycoprotein S | 0.1 μg, n = 39; 0.3 μg, n = 39; 1.0 μg, n = 42; 2.5 μg, n = 24; 5.0 μg, n = 24; 10,0 μg, n = 24; All: n = 192; 1st and 28th days | LNP | Mild ↑ IgG; 6 week—(Nab: 15–43%) 8 week—(SC: 8–57%; Nab: 8 week—11–52%) | Primer and booster 5 μg and 10 μg—100% systemic reaction; The frequency and severity of adverse reactions increased with the dose and also after the booster. Higher grades of headache were more common after the second dose. | [36] |
| COVAC1 Phase IIa (ISRCTN17072692) | VEEV/G3A/Glycoprotein S | 1st day—1 μg; 14th week—10 μg; n = 216 | LNP | After 2nd immunization ↑IgG; After 2nd immunization—SC had 80% participants and 56% had NAbs. | Primer 1 μg: Local reaction—53% (soreness/discomfort—49%; pain—20%, erythema—3%; swelling < 1%). No grade 3 (severe) reactions. Systemic reaction—58% (fatigue—29% and headache—29%). Booster 10 μg: Local reaction—94% (pain/discomfort—90%; pain—69%; swelling—2%; erythema—2%). 2 cases—grade 3 local reaction (pain, discomfort). Systemic reactions—88% (fatigue—74%; headache—67%; myalgia—63%; chills/rigors—60%; arthralgia—40%; nausea—27%; fever (≥38 °C)—13%). 24 cases—grade 3 systemic reaction. Neutrophil increase: 11%—not considered clinically significant. | [37] |
| ARCT-154 Approved (NCT05012943) | VEEV/G3A in 5′-UTR and Q739L in nsP2(US20230256083)/Glycoprotein S | 5 μg on the 1st and 28th days. 1, 2, 3a phases: n = 1001; 3b phase: n = 16,107 | LNP | 4 weeks after the 1st immunization SC—53.8%; 4 weeks after the 2nd immunization SC—94.1% | Prime and booster—myalgia, headache and chills, which resolved quicker than local reactions. | [38] |
| RBI-4000 Phase I (NCT06048770) | VEEV/G3A in 5′-UTR and Q739L in nsP2(WO2022226019A1)/Rabies glycoprotein G | 0.1 μg or 1 μg or 10 μg on the 1st or 57th day. n = 76 | LNP | NAb: 67–94% A dose-dependent increase in the titer from 0.1, 1, and 10 μg doses after a booster | Mild local reactions (pain/swelling at the injection site) were observed after single immunization. General reactions: headache and fatigue were the most common (in human studies). The booster dose (second immunization) was found to be more tolerable than the primer dose, with fewer participants reporting adverse effects. | [39] |
| LNP-nCOV saRNA-02 Phase I (NCT04934111) | VEEV/ORF4a/Glycoprotein S | from 0.1 µg to 10.0 µg on the 1st and 29th day. n = 42 | LNP | ↑IgG; NAb—91.6% participants at 14 days after stimulation. No placebo group, imbalance in groups | Primer: fatigue/malaise (47.6%), headache (42.9%), chills/rigors (40.1%); Booster: fatigue/malaise (63.4%), headache (61.0%), chills/rigors (58.5%); Local reactions—grade 1 and 2, pain (71.4%) and soreness (66.7%) | [40] |
| ARCT-021 Phase I/II (NCT04668339) | VEEV/-/Glycoprotein S | 1 µg, n = 5; 5 µg, n = 10; 7.5 µg, n = 5 (young); 7.5 µg, n = 5 (old); 10 µg, n = 5. | LNP | SC: 80–100%. 5.0 μg and 7.5 μg: best IgG | 10 µg dose—many local and systemic suspected AEs, including grade 3. 7.5 µg—mild and moderate reactions (pain, tenderness); The dose-related trend for ≥grade 2 lymphopenia with 0.0%, 25.0%, 26.5%, 30.0%, and 40.0% of participants immunized with 1.0, 5.0, 7.5, and 10 μg doses, respectively. ↑ ALT (5 participants) and ↑ AST (3 participants). | [28] |
| 3 µg, n = 12 (young); 5 µg, n = 12 (young); 3 µg, n = 12 (old); 5 µg, n = 12 (old); on the 1st and 28th day | ||||||
| GEMCOVAC-OM Phase III (CTRI/2022/10/046475) | VEEV/G3A/Glycoprotein S | 10 μg, n = 2980 | LNP | 90th day—Nab level: saRNA (754.0) > mRNA (383.1); The 1.76-fold increase in neutralizing antibody titers against Omicron BA.1; | The safety and tolerability of the saRNA vaccine were comparable to those of the mRNA vaccines. Most AEs (adverse effects) were mild to moderate and resolved spontaneously. No vaccine-related serious AEs or deaths were reported. Since mRNA vaccines have been associated with myocarditis, this AE has been included in the study as an AE of special interest; Adult women had a higher immune response than men. Women had significantly more severe local and systemic AEs | [41] |
| Protein | Origin | Activity | Functions | Characteristic Function |
|---|---|---|---|---|
| nsP1 | Arthritogenic viruses | Guanine-7N-methyltransferase; guanylyltransferase, scaffold | Catalytic capping [54,55]; membrane anchoring, neck complex assembly, and spherule formation [51,52] | — |
| Encephalitogenic viruses | — | |||
| nsP2 | Arthritogenic viruses | γ, β-ATPase, GTPase, Helicase, 7cysteine protease | Proteolytic cleavage of the nsP1234 polyprotein [49,56]; involvement in RNA capping [55]; helicase activity during replication [57,58,59] | Binding to DNA in the nucleus and RPB1 degradation. Cellular transcription termination [60] |
| Encephalitogenic viruses | — | |||
| nsP3 | Arthritogenic viruses | Homotypic interactions with cellular proteins, regulatory activity | The hypervariable domain (HVD) interacts with FXR cellular proteins, as well as proteins containing the SH3 domain: CD2AP and SH3KBP1, which facilitates replication [61]. The superinfection exclusion (SIE) effect—the limitation of excessive replication through the binding of cellular factors and their depletion in the cell [62] | — |
| Encephalitogenic viruses | — | |||
| nsP4 | Arthritogenic viruses | Rdrp; adenyltransferase activity | RNA synthesis based on an RNA template [63,64]. Polyadenylation of the RNA substrate at the 3′ end [65] | — |
| Encephalitogenic viruses | — |
| Genome | Region | SNV/Insertion | Cell Line | Effect | Origin |
|---|---|---|---|---|---|
| VEEV | 5′-UTR | (G3A) | BHK-V; L929 | Increased genome replication; suppressed subgenomic RNA replication; increased IFN-I levels | [72] |
| VEEV | 5′-UTR | (C24U) | BHK-V; L929 | Decreased IFN-I levels | [72] |
| VEEV | nsP1 and nsP2 | nsP1(G357C, G1569A, A1572C, C1575T) + nsP2 (A3821T, G3892C, T3922C) | HEK293T; RAW-Lucia ISG | Reduced innate immune response; increased target protein expression | [73] |
| VEEV | nsP2 | A533I | BHK-V; L929 | Reduced cytotoxicity; increased IFN-I levels | [72] |
| VEEV | nsP2 | Q739L, P773S | BHK-21 | Possible changes in helicase and protease activities; reduced cytotoxic effects; decreased replication levels | [70] |
| VEEV | nsP2 and nsP3 | G1298R (G3936C) + K1423E (A4311G) | Jurkat; Raw-Lucia ISG; B16F10 | Increased replication level; increased overall expression and specific expression of subgenomic RNA; increased IFN-I levels | [71] |
| VEEV | nsP3 | Q48P (A4174C) | BHK-21; Huh7.5.1; C2C12; RAW264.7 | Decreased saRNA replication; increased subgenomic RNA translation; elevated innate immune response to saRNA; significantly apoptosis inhibition; increased cell viability compared to wild-type saRNA | [17] |
| VEEV | nsP3 | I113F (A4368T) | BHK-21; Huh7.5.1; C2C12; RAW264.7 | Decreased saRNA replication; increased target protein expression; elevated innate immune response to saRNA; significant apoptosis inhibition; increased cell viability | [17] |
| VEEV | nsP3 | L121P | BHK-21 | Reduced cytotoxic effects | [70] |
| VEEV | nsP2 and nsP3 | G1298R (G3936C) + S1572G (A4758G) | Jurkat; Raw-Lucia ISG; B16F11 | Prolonged reporter expression | [71] |
| VEEV | nsP3 | S1572G (A4758G) + E1584D/ V1634 (G4796T/ G4946A) | Jurkat; Raw-Lucia ISG; B16F12 | Regulation of subgenomic (+)RNA translation; reduced subgenomic RNA expression; decreased IFN-I levels | [71] |
| VEEV | nsP3 | E1584/ V1634 (G4796T/ G4946A) | Jurkat; Raw-Lucia ISG; B16F13 | Decreased IFN-I levels | [71] |
| VEEV | nsP3 | Sequences 5628–5666 and 5684–5702 | BHK-21; Vero | Impaired nsP3-nsP4 proteolytic cleavage | [74] |
| VEEV | nsP3 | Sequence 5628-5666 | BHK-21; Vero | Disrupted degradation signaling and cellular localization of nsP3 | [74] |
| VEEV | nsP4 | C482Y and K290R | BHK21 | Increased accuracy of RdRp; possible use of uridine analogs; influence on replication initiation | [75] |
| CHIKV | nsP1 | A533V | Vero; L929 | Reduced cytotoxicity; increased IFN-I levels | [76] |
| CHIKV | nsP2 | A674R T675L L676E A730V | BHK-21; NIH 3T3 | Alleviated cytopathic effects due to the changes in the V-loop structure of the nsP2 C-terminus, which inhibits transcription | [66] |
| CHIKV | nsP2 | P718G + GEEGS insert between aa 647 and 648 | BHK-21 | Reduced cytopathic effects; impaired helicase and GTPase activities | [77] |
| CHIKV | nsP2 | (P718G + GEEGS insert between aa 647 and 648) + F391L + I175L | Huh7 | Reduced cytopathic effects; impaired helicase and GTPase activities; prolonged persistence in the cell | [77] |
| SINV | nsP2 | P726L and P726G | BHK-21 NIH 3T3 | Reduced cytopathic effects and efficiency of saRNA replication; absent nsP2 nuclear localization | [70,78,79] |
| SINV | nsP2 | H619Q and H643Q. P683Q | BHK-21 | Reduced cytopathic effects; absent nsP2 nuclear localization | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunyk, D.; Plotnikova, M.; Bespalov, M.; Shevyrev, D.; Klotchenko, S.; Ivanov, R.; Reshetnikov, V. The Interplay Between Therapeutic Self-Amplifying RNA and the Innate Immune System: Balancing Efficiency and Reactogenicity. Int. J. Mol. Sci. 2025, 26, 8986. https://doi.org/10.3390/ijms26188986
Kunyk D, Plotnikova M, Bespalov M, Shevyrev D, Klotchenko S, Ivanov R, Reshetnikov V. The Interplay Between Therapeutic Self-Amplifying RNA and the Innate Immune System: Balancing Efficiency and Reactogenicity. International Journal of Molecular Sciences. 2025; 26(18):8986. https://doi.org/10.3390/ijms26188986
Chicago/Turabian StyleKunyk, Dmitry, Marina Plotnikova, Mikhail Bespalov, Daniil Shevyrev, Sergey Klotchenko, Roman Ivanov, and Vasiliy Reshetnikov. 2025. "The Interplay Between Therapeutic Self-Amplifying RNA and the Innate Immune System: Balancing Efficiency and Reactogenicity" International Journal of Molecular Sciences 26, no. 18: 8986. https://doi.org/10.3390/ijms26188986
APA StyleKunyk, D., Plotnikova, M., Bespalov, M., Shevyrev, D., Klotchenko, S., Ivanov, R., & Reshetnikov, V. (2025). The Interplay Between Therapeutic Self-Amplifying RNA and the Innate Immune System: Balancing Efficiency and Reactogenicity. International Journal of Molecular Sciences, 26(18), 8986. https://doi.org/10.3390/ijms26188986








