Orexin and Lifestyle Habits: A Meaningful Connection Among Nutrition, Physical Activity, and Sleep Pattern in Health and Diseases
Abstract
1. Introduction
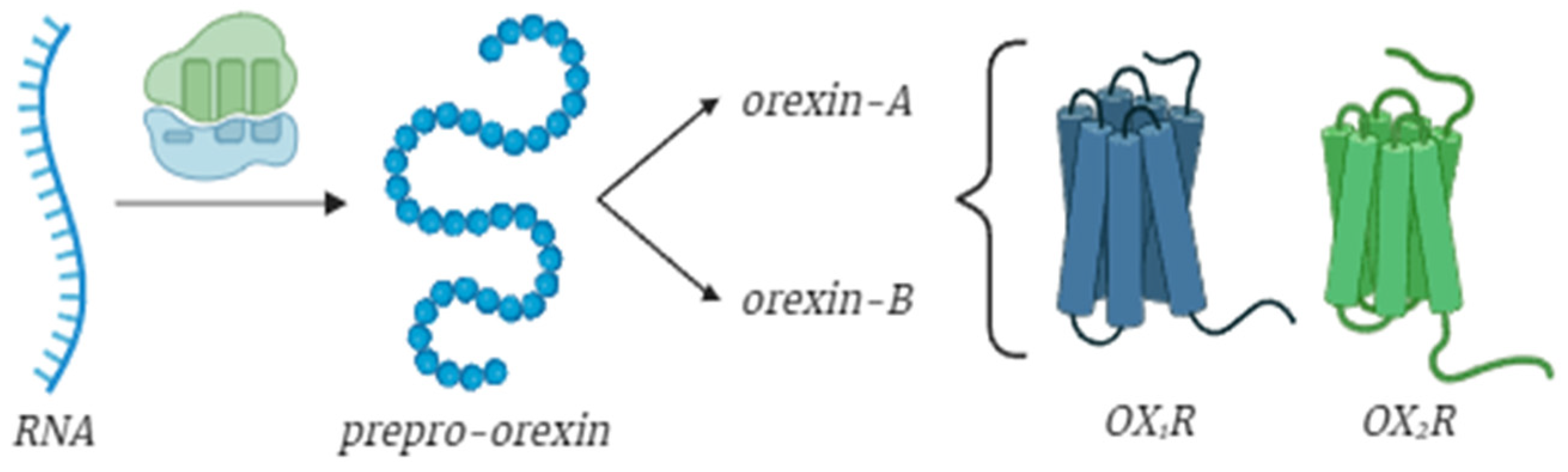
2. Brief Overview on Orexins: Structure and Functions
3. Molecular Mechanisms and Effects of Orexin Receptors
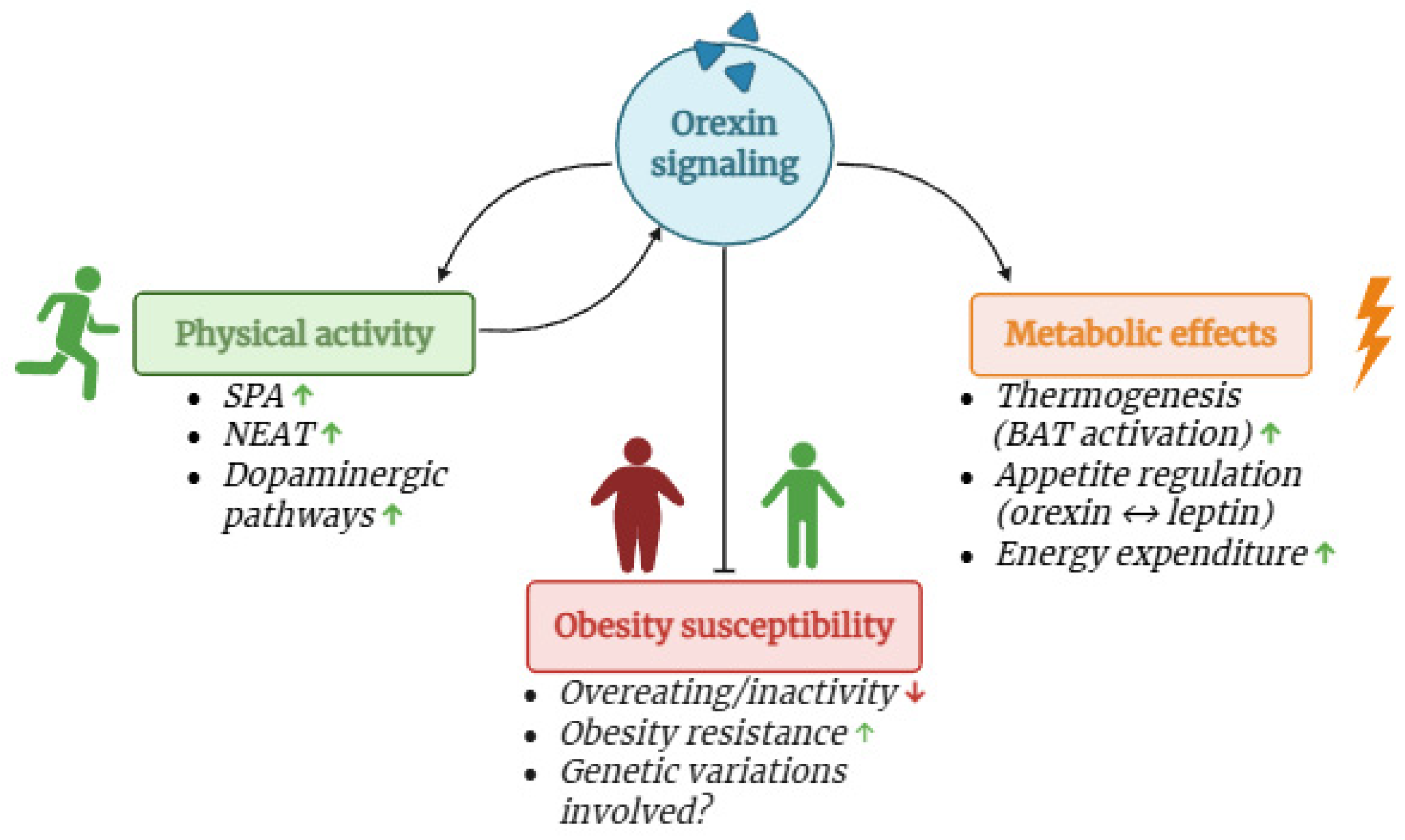
4. Orexin Increases Physical Activity Levels
4.1. Orexin and Motor Control
4.2. Correlation Between Orexin, Physical Activity, and Neurodegenerative Disorders
5. Orexin and Nutrition
Orexin in Metabolic Diseases: Obese vs. Normal Weight Subjects
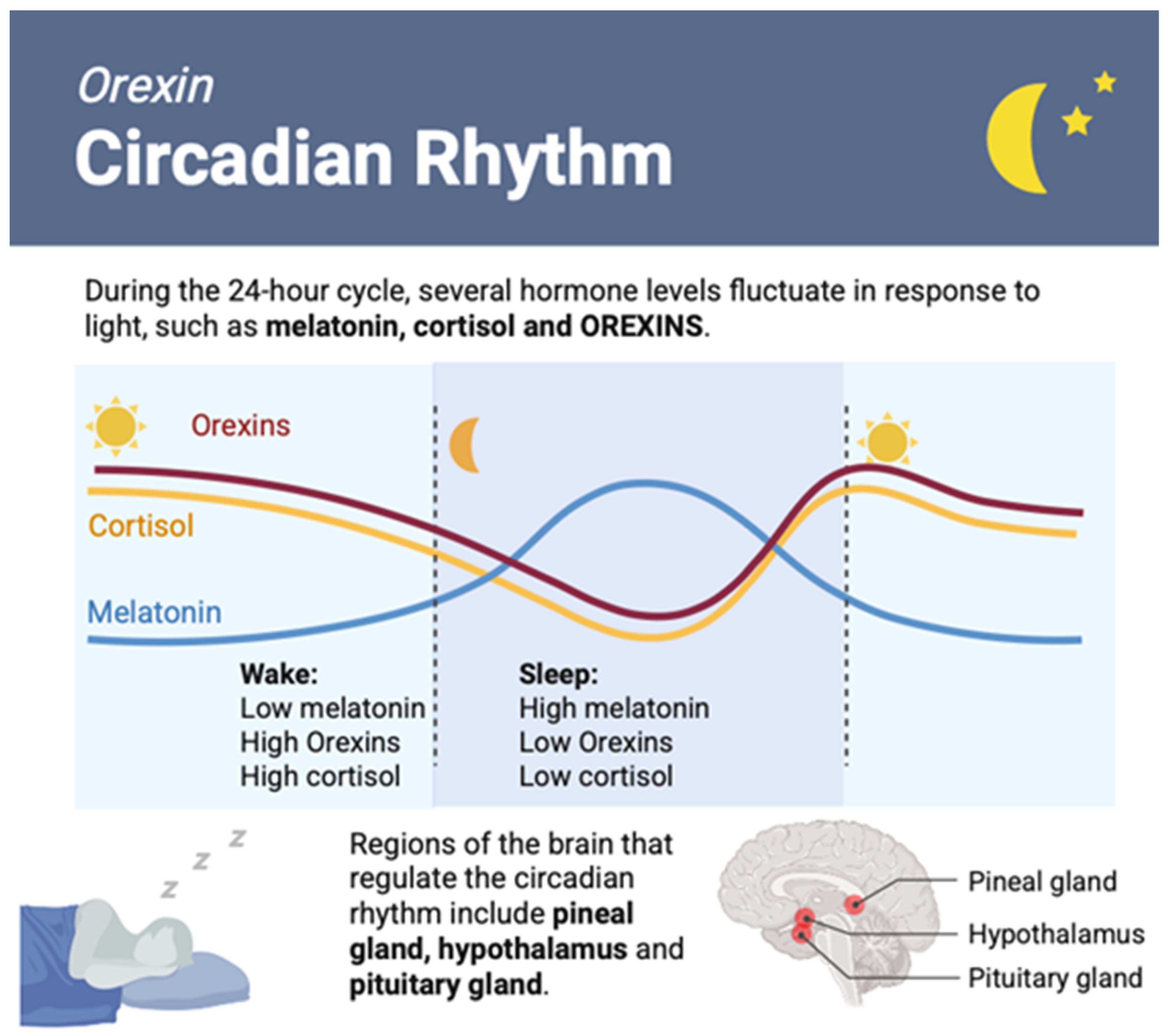
6. Orexin Role in Sleep Control and Disorders
7. Orexin in Clinical Trials
8. Conclusions
9. Methods
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OXR | Orexin receptor |
| HPA | Hypothalamic–pituitary–adrenal |
| PA | Physical activity |
| SPA | Spontaneous physical activity |
| NEAT | Non-exercise activity thermogenesis |
| BDNF | Brain-derived neurotrophic factor |
| IGF-1 | Insulin-like growth factor 1 |
| PLC | Phospholipase C |
| PLD | Phospholipase D |
| MAPK | Mitogen-activated protein kinase |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| CSF | Cerebrospinal fluid |
| PVN | Paraventricular nucleus |
| MS | Multiple sclerosis |
| Aβ | Amyloid-β |
| HFD | High-fat diet |
| DIO | Diet-induced obesity |
| DR | Diet-resistant |
| VTA | Ventral tegmental area |
| BAT | Brown adipose tissue |
| REM | Rapid eye movement |
| DORAs | Dual orexin receptor antagonists |
| FDA | Food and Drug Administration |
| SO1RAs | Selective orexin-1 receptor antagonists |
References
- Pizza, F.; Barateau, L.; Dauvilliers, Y.; Plazzi, G. The Orexin Story, Sleep and Sleep Disturbances. J. Sleep Res. 2022, 31, e13665. [Google Scholar] [CrossRef]
- Barson, J.R.; Leibowitz, S.F. Orexin/Hypocretin System: Role in Food and Drug Overconsumption. Int. Rev. Neurobiol. 2017, 136, 199–237. [Google Scholar] [CrossRef]
- Mahler, S.V.; Moorman, D.E.; Smith, R.J.; James, M.H.; Aston-Jones, G. Motivational Activation: A Unifying Hypothesis of Orexin/Hypocretin Function. Nat. Neurosci. 2014, 17, 1298–1303. [Google Scholar] [CrossRef]
- Mieda, M. The Roles of Orexins in Sleep/Wake Regulation. Neurosci. Res. 2017, 118, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Barson, J.R. Orexin/Hypocretin and Dysregulated Eating: Promotion of Foraging Behavior. Brain Res. 2020, 1731, 145915. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, S.; Carotenuto, M.; Monda, V.; Valenzano, A.; Villano, I.; Precenzano, F.; Tafuri, D.; Salerno, M.; Filippi, N.; Nuccio, F.; et al. Orexin System: The Key for a Healthy Life. Front. Physiol. 2017, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Zink, A.N.; Perez-Leighton, C.E.; Kotz, C.M. The Orexin Neuropeptide System: Physical Activity and Hypothalamic Function throughout the Aging Process. Front. Syst. Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef]
- Teske, J.A.; Mavanji, V. Chapter Six—Energy Expenditure: Role of Orexin. In Vitamins & Hormones; Litwack, G., Ed.; Sleep Hormones; Academic Press: Cambridge, MA, USA, 2012; Volume 89, pp. 91–109. [Google Scholar]
- Tsuneki, H.; Wada, T.; Sasaoka, T. Role of Orexin in the Central Regulation of Glucose and Energy Homeostasis [Review]. Endocr. J. 2012, 59, 365–374. [Google Scholar] [CrossRef]
- Sakurai, T. Orexins and Orexin Receptors: Implication in Feeding Behavior. Regul. Pept. 1999, 85, 25–30. [Google Scholar] [CrossRef]
- Muthmainah, M.; Gogos, A.; Sumithran, P.; Brown, R.M. Orexins (Hypocretins): The Intersection between Homeostatic and Hedonic Feeding. J. Neurochem. 2021, 157, 1473–1494. [Google Scholar] [CrossRef]
- España, R.A.; Plahn, S.; Berridge, C.W. Circadian-Dependent and Circadian-Independent Behavioral Actions of Hypocretin/Orexin. Brain Res. 2002, 943, 224–236. [Google Scholar] [CrossRef]
- Alexandre, C.; Andermann, M.L.; Scammell, T.E. Control of Arousal by the Orexin Neurons. Curr. Opin. Neurobiol. 2013, 23, 752–759. [Google Scholar] [CrossRef]
- Plaza-Zabala, A.; Maldonado, R.; Berrendero, F. The Hypocretin/Orexin System: Implications for Drug Reward and Relapse. Mol. Neurobiol. 2012, 45, 424–439. [Google Scholar] [CrossRef]
- Grafe, L.A.; Bhatnagar, S. Orexins and Stress. Front. Neuroendocrinol. 2018, 51, 132–145. [Google Scholar] [CrossRef]
- Silveyra, P.; Cataldi, N.I.; Lux-Lantos, V.A.; Libertun, C. Role of Orexins in the Hypothalamic-Pituitary-Ovarian Relationships. Acta Physiol. 2010, 198, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.H.; Small, C.J.; Kennedy, A.R.; Stanley, S.A.; Seth, A.; Murphy, K.G.; Taheri, S.; Ghatei, M.A.; Bloom, S.R. Orexin A Interactions in the Hypothalamo-Pituitary Gonadal Axis. Endocrinology 2001, 142, 5294–5302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-B.; Murata, T.; Narita, K.; Honda, K.; Higuchi, T. Variation in the Expression of Orexin and Orexin Receptors in the Rat Hypothalamus during the Estrous Cycle, Pregnancy, Parturition, and Lactation. Endocrine 2003, 22, 127–134. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T.; Kalinchuk, A.; Alanko, L.; Huhtaniemi, I.; Stenberg, D. Orexin A and B Levels in the Hypothalamus of Female Rats: The Effects of the Estrous Cycle and Age. Eur. J. Endocrinol. 2004, 150, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Silveyra, P.; Lux-Lantos, V.; Libertun, C. Both Orexin Receptors Are Expressed in Rat Ovaries and Fluctuate with the Estrous Cycle: Effects of Orexin Receptor Antagonists on Gonadotropins and Ovulation. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E977–E985. [Google Scholar] [CrossRef]
- Mavanji, V.; Pomonis, B.; Kotz, C.M. Orexin, Serotonin, and Energy Balance. WIREs Mech. Dis. 2022, 14, e1536. [Google Scholar] [CrossRef]
- Kotz, C.M.; Perez-Leighton, C.E.; Teske, J.A.; Billington, C.J. Spontaneous Physical Activity Defends Against Obesity. Curr. Obes. Rep. 2017, 6, 362–370. [Google Scholar] [CrossRef]
- Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 25 July 2024).
- Levine, J.A. Non-Exercise Activity Thermogenesis (NEAT). Best Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 679–702. [Google Scholar] [CrossRef]
- Kotz, C.; Nixon, J.; Butterick, T.; Perez-Leighton, C.; Teske, J.; Billington, C. Brain Orexin Promotes Obesity Resistance. Ann. N. Y. Acad. Sci. 2012, 1264, 72–86. [Google Scholar] [CrossRef]
- de Greeff, J.W.; Bosker, R.J.; Oosterlaan, J.; Visscher, C.; Hartman, E. Effects of Physical Activity on Executive Functions, Attention and Academic Performance in Preadolescent Children: A Meta-Analysis. J. Sci. Med. Sport 2018, 21, 501–507. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Tufik, S.; de Mello, M.T. Physical Exercise, Neuroplasticity, Spatial Learning and Memory. Cell. Mol. Life Sci. CMLS 2015, 73, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, B.; Maurya, N.; Lee, S.-D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, P.N.; Cotter, J.D.; George, K.P.; Lucas, S.; Murrell, C.; Shave, R.; Thomas, K.N.; Williams, M.J.A.; Atkinson, G. Elevation in Cerebral Blood Flow Velocity with Aerobic Fitness throughout Healthy Human Ageing. J. Physiol. 2008, 586, 4005–4010. [Google Scholar] [CrossRef]
- Messina, G.; Di Bernardo, G.; Viggiano, A.; De Luca, V.; Monda, V.; Messina, A.; Chieffi, S.; Galderisi, U.; Monda, M. Exercise Increases the Level of Plasma Orexin A in Humans. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 611–616. [Google Scholar] [CrossRef]
- Soya, S.; Sakurai, T. Evolution of Orexin Neuropeptide System: Structure and Function. Front. Neurosci. 2020, 14, 691. [Google Scholar] [CrossRef]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors That Regulate Feeding Behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Sun, Y.; Tisdale, R.K.; Kilduff, T.S. Hypocretin/Orexin Receptor Pharmacology and Sleep Phases. Front. Neurol. Neurosci. 2021, 45, 22–37. [Google Scholar] [CrossRef]
- Peyron, C.; Tighe, D.K.; van den Pol, A.N.; de Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar] [CrossRef] [PubMed]
- Zokaei, L.; Akbari, E.; Babapour, V.; Zendehdel, M. The Modulatory Role of Orexin 1 Receptor in Nucleus Accumbens (NAc) on Spatial Memory in Rats. Arch. Razi Inst. 2023, 78, 1285–1294. [Google Scholar] [CrossRef]
- Saito, Y.C.; Maejima, T.; Nishitani, M.; Hasegawa, E.; Yanagawa, Y.; Mieda, M.; Sakurai, T. Monoamines Inhibit GABAergic Neurons in Ventrolateral Preoptic Area That Make Direct Synaptic Connections to Hypothalamic Arousal Neurons. J. Neurosci. 2018, 38, 6366–6378. [Google Scholar] [CrossRef] [PubMed]
- Haghparast, A.; Fatahi, Z.; Arezoomandan, R.; Karimi, S.; Taslimi, Z.; Zarrabian, S. Functional Roles of Orexin/Hypocretin Receptors in Reward Circuit. Prog. Brain Res. 2017, 235, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Knez, R.; Stevanovic, D.; Fernell, E.; Gillberg, C. Orexin/Hypocretin System Dysfunction in ESSENCE (Early Symptomatic Syndromes Eliciting Neurodevelopmental Clinical Examinations). Neuropsychiatr. Dis. Treat. 2022, 18, 2683–2702. [Google Scholar] [CrossRef]
- Sears, R.M.; Fink, A.E.; Wigestrand, M.B.; Farb, C.R.; de Lecea, L.; LeDoux, J.E. Orexin/Hypocretin System Modulates Amygdala-Dependent Threat Learning through the Locus Coeruleus. Proc. Natl. Acad. Sci. USA 2013, 110, 20260–20265. [Google Scholar] [CrossRef]
- Kuwaki, T. Orexin Links Emotional Stress to Autonomic Functions. Auton. Neurosci. 2011, 161, 20–27. [Google Scholar] [CrossRef]
- Ammoun, S.; Holmqvist, T.; Shariatmadari, R.; Oonk, H.B.; Detheux, M.; Parmentier, M.; Kerman, K.E.O.; Kukkonen, J.P. Distinct Recognition of OX1 and OX2Receptors by Orexin Peptides. J. Pharmacol. Exp. Ther. 2003, 305, 507–514. [Google Scholar] [CrossRef]
- Kukkonen, J.P.; Turunen, P.M. Cellular Signaling Mechanisms of Hypocretin/Orexin. Front. Neurol. Neurosci. 2021, 45, 91–102. [Google Scholar] [CrossRef]
- Smart, D.; Jerman, J.C.; Brough, S.J.; Rushton, S.L.; Murdock, P.R.; Jewitt, F.; Elshourbagy, N.A.; Ellis, C.E.; Middlemiss, D.N.; Brown, F. Characterization of Recombinant Human Orexin Receptor Pharmacology in a Chinese Hamster Ovary Cell-Line Using FLIPR. Br. J. Pharmacol. 1999, 128, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.P. OX2 Orexin/Hypocretin Receptor Signal Transduction in Recombinant Chinese Hamster Ovary Cells. Cell. Signal. 2016, 28, 51–60. [Google Scholar] [CrossRef]
- Dale, N.C.; Hoyer, D.; Jacobson, L.H.; Pfleger, K.D.G.; Johnstone, E.K.M. Orexin Signaling: A Complex, Multifaceted Process. Front. Cell. Neurosci. 2022, 16, 812359. [Google Scholar] [CrossRef]
- Lund, P.E.; Shariatmadari, R.; Uustare, A.; Detheux, M.; Parmentier, M.; Kukkonen, J.P.; Akerman, K.E. The Orexin OX1 Receptor Activates a Novel Ca2+ Influx Pathway Necessary for Coupling to Phospholipase C. J. Biol. Chem. 2000, 275, 30806–30812. [Google Scholar] [CrossRef] [PubMed]
- Ammoun, S.; Johansson, L.; Ekholm, M.E.; Holmqvist, T.; Danis, A.S.; Korhonen, L.; Sergeeva, O.A.; Haas, H.L.; Åkerman, K.E.O.; Kukkonen, J.P. OX1 Orexin Receptors Activate Extracellular Signal-Regulated Kinase in Chinese Hamster Ovary Cells via Multiple Mechanisms: The Role of Ca2+ Influx in OX1 Receptor Signaling. Mol. Endocrinol. 2006, 20, 80–99. [Google Scholar] [CrossRef]
- Johansson, L.; Ekholm, M.E.; Kukkonen, J.P. Multiple Phospholipase Activation by OX1 Orexin/Hypocretin Receptors. Cell. Mol. Life Sci. CMLS 2008, 65, 1948–1956. [Google Scholar] [CrossRef]
- Tang, J.; Chen, J.; Ramanjaneya, M.; Punn, A.; Conner, A.C.; Randeva, H.S. The Signalling Profile of Recombinant Human Orexin-2 Receptor. Cell. Signal. 2008, 20, 1651–1661. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Conner, A.C.; Chen, J.; Kumar, P.; Brown, J.E.P.; Jöhren, O.; Lehnert, H.; Stanfield, P.R.; Randeva, H.S. Orexin-Stimulated MAP Kinase Cascades Are Activated through Multiple G-Protein Signalling Pathways in Human H295R Adrenocortical Cells: Diverse Roles for Orexins A and B. J. Endocrinol. 2009, 202, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Q.; Ji, B.; Pan, Y.; Xu, C.; Cheng, B.; Bai, B.; Chen, J. The Orexin/Receptor System: Molecular Mechanism and Therapeutic Potential for Neurological Diseases. Front. Mol. Neurosci. 2018, 11, 220. [Google Scholar] [CrossRef]
- Dalrymple, M.B.; Jaeger, W.C.; Eidne, K.A.; Pfleger, K.D.G. Temporal Profiling of Orexin Receptor-Arrestin-Ubiquitin Complexes Reveals Differences between Receptor Subtypes. J. Biol. Chem. 2011, 286, 16726–16733. [Google Scholar] [CrossRef]
- Kukkonen, J.P.; Leonard, C.S. Orexin/Hypocretin Receptor Signalling Cascades. Br. J. Pharmacol. 2014, 171, 314–331. [Google Scholar] [CrossRef]
- Cai, X.; Wang, H.; Wang, M.; Wang, D.; Zhang, Z.; Wei, R.; Gao, X.; Zhang, R.; Wang, C.; Chen, J. A Novel Phosphorylation Site on Orexin Receptor 1 Regulating orexinA-Induced GRK2-Biased Signaling. Cell. Signal. 2020, 75, 109743. [Google Scholar] [CrossRef]
- Xu, D.; Kong, T.; Zhang, S.; Cheng, B.; Chen, J.; Wang, C. Orexin-A Protects against Cerebral Ischemia-Reperfusion Injury by Inhibiting Excessive Autophagy through OX1R-Mediated MAPK/ERK/mTOR Pathway. Cell. Signal. 2021, 79, 109839. [Google Scholar] [CrossRef]
- Rouet-Benzineb, P.; Rouyer-Fessard, C.; Jarry, A.; Avondo, V.; Pouzet, C.; Yanagisawa, M.; Laboisse, C.; Laburthe, M.; Voisin, T. Orexins Acting at Native OX1 Receptor in Colon Cancer and Neuroblastoma Cells or at Recombinant OX1 Receptor Suppress Cell Growth by Inducing Apoptosis. J. Biol. Chem. 2004, 279, 45875–45886. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Tsuneki, H.; Honda, K.; Sekine, Y.; Yahata, K.; Yasue, M.; Fujishima, M.; Takeda, R.; Wada, T.; Sasaoka, T. C-Terminal Peptide of Preproorexin Enhances Brain-Derived Neurotrophic Factor Expression in Rat Cerebrocortical Cells and Recognition Memory in Mice. Eur. J. Pharmacol. 2024, 964, 176306. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease and Its Pharmaceutical Potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, P.; Cammarota, M.; Medina, J.H. BDNF and Memory Processing. Neuropharmacology 2014, 76 Pt C, 677–683. [Google Scholar] [CrossRef]
- Shahsavari, F.; Abbasnejad, M.; Esmaeili-Mahani, S.; Raoof, M. The Ability of Orexin-A to Modify Pain-Induced Cyclooxygenase-2 and Brain-Derived Neurotrophic Factor Expression Is Associated with Its Ability to Inhibit Capsaicin-Induced Pulpal Nociception in Rats. Korean J. Pain 2022, 35, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.; Atlas, R.; Lange, A.; Ginzburg, I. Brain-Derived Neurotrophic Factor Induces a Rapid Dephosphorylation of Tau Protein through a PI-3 Kinase Signalling Mechanism. Eur. J. Neurosci. 2005, 22, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; He, Z.; Xing, D. Low-Level Laser Therapy Rescues Dendrite Atrophy via Upregulating BDNF Expression: Implications for Alzheimer’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 13505–13517. [Google Scholar] [CrossRef]
- Weinstein, G.; Beiser, A.S.; Choi, S.H.; Preis, S.R.; Chen, T.C.; Vorgas, D.; Au, R.; Pikula, A.; Wolf, P.A.; DeStefano, A.L.; et al. Serum Brain-Derived Neurotrophic Factor and the Risk for Dementia: The Framingham Heart Study. JAMA Neurol. 2014, 71, 55–61. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson’s Disease: A Review. Front. Biosci. Sch. Ed. 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Toor, B.; Ray, L.B.; Pozzobon, A.; Fogel, S.M. Sleep, Orexin and Cognition. Front. Neurol. Neurosci. 2021, 45, 38–51. [Google Scholar]
- Kim, H.J.J.; Dickie, S.A.; Laprairie, R.B. Estradiol-Dependent Hypocretinergic/Orexinergic Behaviors throughout the Estrous Cycle. Psychopharmacology 2023, 240, 15–25. [Google Scholar] [CrossRef]
- Kiwaki, K.; Kotz, C.M.; Wang, C.; Lanningham-Foster, L.; Levine, J.A. Orexin A (Hypocretin 1) Injected into Hypothalamic Paraventricular Nucleus and Spontaneous Physical Activity in Rats. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E551–E559. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.M.; Kotz, C.M.; Levine, J.A. Central Orexin Sensitivity, Physical Activity, and Obesity in Diet-Induced Obese and Diet-Resistant Rats. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E396–E403. [Google Scholar] [CrossRef]
- Hagan, J.J.; Leslie, R.A.; Patel, S.; Evans, M.L.; Wattam, T.A.; Holmes, S.; Benham, C.D.; Taylor, S.G.; Routledge, C.; Hemmati, P.; et al. Orexin A Activates Locus Coeruleus Cell Firing and Increases Arousal in the Rat. Proc. Natl. Acad. Sci. USA 1999, 96, 10911–10916. [Google Scholar] [CrossRef]
- Lubkin, M.; Stricker-Krongrad, A. Independent Feeding and Metabolic Actions of Orexins in Mice. Biochem. Biophys. Res. Commun. 1998, 253, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, A.J.; Kotz, C.M. Orexin A in the Nucleus Accumbens Stimulates Feeding and Locomotor Activity. Brain Res. 2005, 1050, 156–162. [Google Scholar] [CrossRef]
- Kotz, C.M.; Wang, C.; Teske, J.A.; Thorpe, A.J.; Novak, C.M.; Kiwaki, K.; Levine, J.A. Orexin A Mediation of Time Spent Moving in Rats: Neural Mechanisms. Neuroscience 2006, 142, 29–36. [Google Scholar] [CrossRef]
- Novak, C.M.; Escande, C.; Burghardt, P.R.; Zhang, M.; Barbosa, M.T.; Chini, E.N.; Britton, S.L.; Koch, L.G.; Akil, H.; Levine, J.A. Spontaneous Activity, Economy of Activity, and Resistance to Diet-Induced Obesity in Rats Bred for High Intrinsic Aerobic Capacity. Horm. Behav. 2010, 58, 355–367. [Google Scholar] [CrossRef]
- Kosse, C.; Schöne, C.; Bracey, E.; Burdakov, D. Orexin-Driven GAD65 Network of the Lateral Hypothalamus Sets Physical Activity in Mice. Proc. Natl. Acad. Sci. USA 2017, 114, 4525–4530. [Google Scholar] [CrossRef]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Fliers, E. Daily regulation of hormone profiles. Handb. Exp. Pharmacol. 2013, 217, 185–226. [Google Scholar] [CrossRef]
- Hao, Y.Y.; Yuan, H.W.; Fang, P.H.; Zhang, Y.; Liao, Y.X.; Shen, C.; Wang, D.; Zhang, T.T.; Bo, P. Plasma orexin-A level associated with physical activity in obese people. Eat Weight Disord. 2017, 22, 69–77. [Google Scholar] [CrossRef]
- Martin, T.; Dauvilliers, Y.; Koumar, O.C.; Bouet, V.; Freret, T.; Besnard, S.; Dauphin, F.; Bessot, N. Dual orexin receptor antagonist induces changes in core body temperature in rats after exercise. Sci. Rep. 2019, 9, 18432. [Google Scholar] [CrossRef] [PubMed]
- Koumar, O.C.; Martin, T.; Bataille, A.; Bulla, J.; Crunel, V.; Bouet, V.; Freret, T.; Boumediene, K.; Bauge, C.; Moussay, S.; et al. Core body temperature varies according to the time of exercise without affecting orexin-A production in the dorsal hypothalamus in male rats. J. Therm. Biol. 2023, 114, 103522. [Google Scholar] [CrossRef]
- Hu, B.; Yang, N.; Qiao, Q.-C.; Hu, Z.-A.; Zhang, J. Roles of the Orexin System in Central Motor Control. Neurosci. Biobehav. Rev. 2015, 49, 43–54. [Google Scholar] [CrossRef]
- Cluderay, J.E.; Harrison, D.C.; Hervieu, G.J. Protein Distribution of the Orexin-2 Receptor in the Rat Central Nervous System. Regul. Pept. 2002, 104, 131–144. [Google Scholar] [CrossRef]
- Hervieu, G.J.; Cluderay, J.E.; Harrison, D.C.; Roberts, J.C.; Leslie, R.A. Gene Expression and Protein Distribution of the Orexin-1 Receptor in the Rat Brain and Spinal Cord. Neuroscience 2001, 103, 777–797. [Google Scholar] [CrossRef]
- Yamuy, J.; Fung, S.J.; Xi, M.; Chase, M.H. Hypocretinergic Control of Spinal Cord Motoneurons. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 5336–5345. [Google Scholar] [CrossRef] [PubMed]
- van den Pol, A.N. Hypothalamic Hypocretin (Orexin): Robust Innervation of the Spinal Cord. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 3171–3182. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Xue, Y.; Wang, Y.; Chen, A.-Q.; Liu, C.; Liu, Y.-H.; Chu, H.-Y.; Chen, L. The Subthalamic Neurons Are Activated by Both Orexin-A and Orexin-B. Neuroscience 2018, 369, 97–108. [Google Scholar] [CrossRef]
- Kotz, C.M.; Teske, J.A.; Billington, C.J. Neuroregulation of Nonexercise Activity Thermogenesis and Obesity Resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R699–R710. [Google Scholar] [CrossRef]
- Kiyashchenko, L.I.; Mileykovskiy, B.Y.; Lai, Y.Y.; Siegel, J.M. Increased and Decreased Muscle Tone with Orexin (Hypocretin) Microinjections in the Locus Coeruleus and Pontine Inhibitory Area. J. Neurophysiol. 2001, 85, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, B.; Yu, L.; He, Y.-C.; Li, H.-Z.; Zhu, J.-N.; Wang, J.-J. A Role for Orexin in Central Vestibular Motor Control. Neuron 2011, 69, 793–804. [Google Scholar] [CrossRef]
- Takakusaki, K.; Takahashi, K.; Saitoh, K.; Harada, H.; Okumura, T.; Kayama, Y.; Koyama, Y. Orexinergic Projections to the Cat Midbrain Mediate Alternation of Emotional Behavioural States from Locomotion to Cataplexy. J. Physiol. 2005, 568, 1003–1020. [Google Scholar] [CrossRef]
- Mochizuki, T.; Crocker, A.; McCormack, S.; Yanagisawa, M.; Sakurai, T.; Scammell, T.E. Behavioral State Instability in Orexin Knock-out Mice. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 6291–6300. [Google Scholar] [CrossRef]
- España, R.A.; Valentino, R.J.; Berridge, C.W. Fos Immunoreactivity in Hypocretin-Synthesizing and Hypocretin-1 Receptor-Expressing Neurons: Effects of Diurnal and Nocturnal Spontaneous Waking, Stress and Hypocretin-1 Administration. Neuroscience 2003, 121, 201–217. [Google Scholar] [CrossRef]
- Mileykovskiy, B.Y.; Kiyashchenko, L.I.; Siegel, J.M. Behavioral Correlates of Activity in Identified Hypocretin/Orexin Neurons. Neuron 2005, 46, 787–798. [Google Scholar] [CrossRef]
- Matsuki, T.; Sakurai, T. Orexins and Orexin Receptors: From Molecules to Integrative Physiology. In Orphan G Protein-Coupled Receptors and Novel Neuropeptides; Results and Problems in Cell Differentiation; Springer: Berlin/Heidelberg, Germany, 2008; Volume 46, pp. 27–55. [Google Scholar] [CrossRef]
- Phillips, C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; García-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; et al. Physical Exercise in the Prevention and Treatment of Alzheimer’s Disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Fan, R.; Xie, L.; Shi, X.; Dong, K.; Zhang, S.; Tao, J.; Xu, W.; Ma, D.; Chen, J.; et al. A Growing Link between Circadian Rhythms, Type 2 Diabetes Mellitus and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 504. [Google Scholar] [CrossRef]
- Blackman, J.; Swirski, M.; Clynes, J.; Harding, S.; Leng, Y.; Coulthard, E. Pharmacological and Non-pharmacological Interventions to Enhance Sleep in Mild Cognitive Impairment and Mild Alzheimer’s Disease: A Systematic Review. J. Sleep Res. 2021, 30, e13229. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Nuccetelli, M.; Izzi, F.; Sancesario, G.; Romigi, A.; Martorana, A.; Amoroso, C.; Bernardini, S.; Marciani, M.G.; Mercuri, N.B.; et al. Rapid Eye Movement Sleep Disruption and Sleep Fragmentation Are Associated with Increased Orexin-A Cerebrospinal-Fluid Levels in Mild Cognitive Impairment Due to Alzheimer’s Disease. Neurobiol. Aging 2016, 40, 120–126. [Google Scholar] [CrossRef]
- Fronczek, R.; van Geest, S.; Frölich, M.; Overeem, S.; Roelandse, F.W.C.; Lammers, G.J.; Swaab, D.F. Hypocretin (Orexin) Loss in Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 1642–1650. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Kratzsch, J.; Gertz, H.-J.; Tittmann, M.; Jahn, I.; Pietsch, U.-C.; Kaisers, U.X.; Thiery, J.; Hegerl, U.; Schönknecht, P. Cerebrospinal Fluid Melanin-Concentrating Hormone (MCH) and Hypocretin-1 (HCRT-1, Orexin-A) in Alzheimer’s Disease. PLoS ONE 2013, 8, e63136. [Google Scholar] [CrossRef]
- Slats, D.; Claassen, J.A.H.R.; Lammers, G.J.; Melis, R.J.; Verbeek, M.M.; Overeem, S. Association between Hypocretin-1 and Amyloid-Β42 Cerebrospinal Fluid Levels in Alzheimer’s Disease and Healthy Controls. Curr. Alzheimer Res. 2012, 9, 1119–1125. [Google Scholar] [CrossRef]
- An, H.; Cho, M.-H.; Kim, D.-H.; Chung, S.; Yoon, S.-Y. Orexin Impairs the Phagocytosis and Degradation of Amyloid-β Fibrils by Microglial Cells. J. Alzheimers Dis. JAD 2017, 58, 253–261. [Google Scholar] [CrossRef]
- Kang, J.-E.; Lim, M.M.; Bateman, R.J.; Lee, J.J.; Smyth, L.P.; Cirrito, J.R.; Fujiki, N.; Nishino, S.; Holtzman, D.M. Amyloid-Beta Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science 2009, 326, 1005–1007. [Google Scholar] [CrossRef]
- Alberts, J.L.; Rosenfeldt, A.B. The Universal Prescription for Parkinson’s Disease: Exercise. J. Park. Dis. 2020, 10, S21–S27. [Google Scholar] [CrossRef]
- Sakurai, T.; Saito, Y.C.; Yanagisawa, M. Interaction between Orexin Neurons and Monoaminergic Systems. Front. Neurol. Neurosci. 2021, 45, 11–21. [Google Scholar] [CrossRef]
- Guillaumin, M.C.C.; Burdakov, D. Neuropeptides as Primary Mediators of Brain Circuit Connectivity. Front. Neurosci. 2021, 15, 644313. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-F.; Xue, Y.; Liu, C.; Liu, Y.-H.; Diao, H.-L.; Wang, Y.; Pan, Y.-P.; Chen, L. Orexin-A Exerts Neuroprotective Effects via OX1R in Parkinson’s Disease. Front. Neurosci. 2018, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Manavis, J.; Yamanaka, A.; Ootsuka, Y.; Blumbergs, P.; Bobrovskaya, L. The Role of Orexin in Parkinson’s Disease. J. Neurosci. Res. 2024, 102, e25322. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Bardhan, M.; Ghosh, S.; Chandani, Y.; Satapathy, P.; Roy, P.; Shamim, M.A.; Gandhi, A.P.; Sandeep, M.; Rustagi, S.; et al. Exploring the Role of Orexin-A Neuropeptide in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Clin. Neurol. Neurosurg. 2024, 242, 108320. [Google Scholar] [CrossRef]
- Dauwan, M.; Begemann, M.J.H.; Slot, M.I.E.; Lee, E.H.M.; Scheltens, P.; Sommer, I.E.C. Physical Exercise Improves Quality of Life, Depressive Symptoms, and Cognition across Chronic Brain Disorders: A Transdiagnostic Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Neurol. 2021, 268, 1222–1246. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, F.; Wu, Y. Orexinergic System in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 713201. [Google Scholar] [CrossRef]
- Gencer, M.; Akbayır, E.; Şen, M.; Arsoy, E.; Yılmaz, V.; Bulut, N.; Tüzün, E.; Türkoğlu, R. Serum Orexin-A Levels Are Associated with Disease Progression and Motor Impairment in Multiple Sclerosis. Neurol. Sci. 2019, 40, 1067–1070. [Google Scholar] [CrossRef]
- Papuć, E.; Zbigniew, S.; Paweł, G.; Konrad, R. CSF Hypocretin-1 Concentrations Correlate with the Level of Fatigue in Multiple Sclerosis Patients. Neurosci. Lett. 2010, 474, 9–12. [Google Scholar] [CrossRef] [PubMed]
- de Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.; Gautvik, V.T.; Bartlett, F.S.; et al. The Hypocretins: Hypothalamus-Specific Peptides with Neuroexcitatory Activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.L.; Davis, J.F.; Fitzgerald, M.E.; Benoit, S.C. The Role of Orexin-A in Food Motivation, Reward-Based Feeding Behavior and Food-Induced Neuronal Activation in Rats. Neuroscience 2010, 167, 11–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, D.; Liu, R.; Gong, H.; Wang, C.; Zhao, M.-G.; Zhang, K. Transcutaneous Auricular Vagus Nerve Stimulation Inhibits Food Intake and Body Weight Gain through the Orexin Dependent Pathway in High Fat Diet Mice. Sci. Rep. 2025, 15, 19286. [Google Scholar] [CrossRef]
- Tsuneki, H.; Maeda, T.; Takatsuki, M.; Sekine, T.; Masui, S.; Onishi, K.; Takeda, R.; Sugiyama, M.; Sakurai, T.; Yanagisawa, M.; et al. Bromocriptine Improves Glucose Tolerance in Obese Mice via Central Dopamine D2 Receptor-Independent Mechanism. PLoS ONE 2025, 20, e0320157. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Xu, W.; Wang, X.; Tang, W.; Wang, X.; Jiao, Y.; Luan, X.; Li, P.; Guo, F. Melanin-Concentrating Hormone Attenuates the Hedonic Feeding Induced by Orexin-A in the Ventral Tegmental Area of High-Fat Diet Male Mice. Front. Nutr. 2024, 11, 1468874. [Google Scholar] [CrossRef]
- Steward, T.; Mestre-Bach, G.; Granero, R.; Sánchez, I.; Riesco, N.; Vintró-Alcaraz, C.; Sauchelli, S.; Jiménez-Murcia, S.; Agüera, Z.; Fernández-García, J.C.; et al. Reduced Plasma Orexin-A Concentrations Are Associated with Cognitive Deficits in Anorexia Nervosa. Sci. Rep. 2019, 9, 7910. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.J.; Marques, M.S.; Tufik, S.; D’Almeida, V. Orexin activation precedes increased NPY expression, hyperphagia, and metabolic changes in response to sleep deprivation. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E726–E734. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.; Bowes, S.C.; Semba, K.; Hirasawa, M. Sleep deprivation-induced pre- and postsynaptic modulation of orexin neurons. Neuropharmacology 2019, 154, 50–60. [Google Scholar] [CrossRef]
- Almeida Rojo, A.L.; Barnhardt, T.R.; Pham, T.Q.; Heim, B.; Cai, L.; Tseng, G.C.; Huang, Y.H. Sleep deprivation engages the orexin/hypocretin system to regulate food reward seeking. Int. J. Neuropsychopharmacol. 2025, 28, pyaf047. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.A.; Botticelli, L.; Bergamini, G.; Micioni Di Bonaventura, E.; Gatfield, J.; Williams, J.T.; Treiber, A.; Vaillant, C.; Cifani, C.; Micioni Di Bonaventura, M.V. Evaluating the Efficacy of the Selective Orexin 1 Receptor Antagonist Nivasorexant in an Animal Model of Binge-Eating Disorder. Int. J. Eat. Disord. 2024, 57, 1418–1432. [Google Scholar] [CrossRef]
- Mehr, J.B.; Mitchison, D.; Bowrey, H.E.; James, M.H. Sleep dysregulation in binge eating disorder and “food addiction”: The orexin (hypocretin) system as a potential neurobiological link. Neuropsychopharmacology 2021, 46, 2051–2061. [Google Scholar] [CrossRef]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Butterick, T.A.; Billington, C.J.; Kotz, C.M.; Nixon, J.P. Orexin: Pathways to Obesity Resistance? Rev. Endocr. Metab. Disord. 2013, 14, 357–364. [Google Scholar] [CrossRef]
- Zhang, S.; Zeitzer, J.M.; Sakurai, T.; Nishino, S.; Mignot, E. Sleep/Wake Fragmentation Disrupts Metabolism in a Mouse Model of Narcolepsy. J. Physiol. 2007, 581, 649–663. [Google Scholar] [CrossRef]
- Fujiki, N.; Yoshida, Y.; Zhang, S.; Sakurai, T.; Yanagisawa, M.; Nishino, S. Sex Difference in Body Weight Gain and Leptin Signaling in Hypocretin/Orexin Deficient Mouse Models. Peptides 2006, 27, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Hara, J.; Yanagisawa, M.; Sakurai, T. Difference in Obesity Phenotype between Orexin-Knockout Mice and Orexin Neuron-Deficient Mice with Same Genetic Background and Environmental Conditions. Neurosci. Lett. 2005, 380, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, B.; Wolińska-Witort, E.; Martyńska, M.; Chmielowska, M.; Baranowska-Bik, A. Plasma Orexin A, Orexin B, Leptin, Neuropeptide Y (NPY) and Insulin in Obese Women. Neuro Endocrinol. Lett. 2005, 26, 293–296. [Google Scholar]
- Teske, J.A.; Billington, C.J.; Kuskowski, M.A.; Kotz, C.M. Spontaneous Physical Activity Protects against Fat Mass Gain. Int. J. Obes. 2012, 36, 603–613. [Google Scholar] [CrossRef]
- Teske, J.A.; Levine, A.S.; Kuskowski, M.; Levine, J.A.; Kotz, C.M. Elevated Hypothalamic Orexin Signaling, Sensitivity to Orexin A, and Spontaneous Physical Activity in Obesity-Resistant Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R889–R899. [Google Scholar] [CrossRef]
- Tsujino, N.; Sakurai, T. Orexin/Hypocretin: A Neuropeptide at the Interface of Sleep, Energy Homeostasis, and Reward System. Pharmacol. Rev. 2009, 61, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Futatsuki, T.; Yamashita, A.; Ikbar, K.N.; Yamanaka, A.; Arita, K.; Kakihana, Y.; Kuwaki, T. Involvement of Orexin Neurons in Fasting- and Central Adenosine-Induced Hypothermia. Sci. Rep. 2018, 8, 2717. [Google Scholar] [CrossRef] [PubMed]
- Bouâouda, H.; Jha, P.K. Orexin and MCH Neurons: Regulators of Sleep and Metabolism. Front. Neurosci. 2023, 17, 1230428. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Arihara, Z.; Suzuki, T.; Sone, M.; Kikuchi, K.; Sasano, H.; Murakami, O.; Totsune, K. Expression of Orexin-A and Orexin Receptors in the Kidney and the Presence of Orexin-A-like Immunoreactivity in Human Urine. Peptides 2006, 27, 871–877. [Google Scholar] [CrossRef]
- Digby, J.E.; Chen, J.; Tang, J.Y.; Lehnert, H.; Matthews, R.N.; Randeva, H.S. Orexin Receptor Expression in Human Adipose Tissue: Effects of Orexin-A and Orexin-B. J. Endocrinol. 2006, 191, 129–136. [Google Scholar] [CrossRef]
- Shiuchi, T.; Haque, M.S.; Okamoto, S.; Inoue, T.; Kageyama, H.; Lee, S.; Toda, C.; Suzuki, A.; Bachman, E.S.; Kim, Y.-B.; et al. Hypothalamic Orexin Stimulates Feeding-Associated Glucose Utilization in Skeletal Muscle via Sympathetic Nervous System. Cell Metab. 2009, 10, 466–480. [Google Scholar] [CrossRef]
- Shin, S.-K.; Song, S.-E.; Oh, J.U.; Hwang, M.; Cho, H.-W.; Bae, J.-H.; Im, S.-S.; Kim, J.-I.; Song, D.-K. Orexin A-Induced Inhibition of Leptin Expression and Secretion in Adipocytes Reducing Plasma Leptin Levels and Hypothalamic Leptin Resistance. Pflügers Arch. 2019, 471, 1407–1418. [Google Scholar] [CrossRef]
- Mogavero, M.P.; Godos, J.; Grosso, G.; Caraci, F.; Ferri, R. Rethinking the Role of Orexin in the Regulation of REM Sleep and Appetite. Nutrients 2023, 15, 3679. [Google Scholar] [CrossRef]
- Thompson, J.L.; Borgland, S.L. A Role for Hypocretin/Orexin in Motivation. Behav. Brain Res. 2011, 217, 446–453. [Google Scholar] [CrossRef]
- Tunisi, L.; D’Angelo, L.; Fernández-Rilo, A.C.; Forte, N.; Piscitelli, F.; Imperatore, R.; de Girolamo, P.; Di Marzo, V.; Cristino, L. Orexin-A/Hypocretin-1 Controls the VTA-NAc Mesolimbic Pathway via Endocannabinoid-Mediated Disinhibition of Dopaminergic Neurons in Obese Mice. Front. Synaptic Neurosci. 2021, 13, 622405. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Sellayah, D.; Bharaj, P.; Sikder, D. Orexin Is Required for Brown Adipose Tissue Development, Differentiation, and Function. Cell Metab. 2011, 14, 478–490. [Google Scholar] [CrossRef]
- Sellayah, D.; Sikder, D. Orexin Receptor-1 Mediates Brown Fat Developmental Differentiation. Adipocyte 2012, 1, 58–63. [Google Scholar] [CrossRef]
- Monda, M.; Viggiano, A.; Viggiano, A.; Fuccio, F.; De Luca, V. Injection of Orexin A into the Diagonal Band of Broca Induces Symphatetic and Hyperthermic Reactions. Brain Res. 2004, 1018, 265–271. [Google Scholar] [CrossRef]
- Messina, G.; Dalia, C.; Tafuri, D.; Monda, V.; Palmieri, F.; Dato, A.; Russo, A.; De Blasio, S.; Messina, A.; De Luca, V.; et al. Orexin-A Controls Sympathetic Activity and Eating Behavior. Front. Psychol. 2014, 5, 997. [Google Scholar] [CrossRef]
- Monda, M.; Viggiano, A.; Viggiano, A.; Viggiano, E.; Messina, G.; Tafuri, D.; De Luca, V. Sympathetic and Hyperthermic Reactions by Orexin A: Role of Cerebral Catecholaminergic Neurons. Regul. Pept. 2007, 139, 39–44. [Google Scholar] [CrossRef]
- Shirasaka, T.; Kunitake, T.; Takasaki, M.; Kannan, H. Neuronal Effects of Orexins: Relevant to Sympathetic and Cardiovascular Functions. Regul. Pept. 2002, 104, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Perez-Leighton, C.E.; Boland, K.; Teske, J.A.; Billington, C.; Kotz, C.M. Behavioral Responses to Orexin, Orexin Receptor Gene Expression, and Spontaneous Physical Activity Contribute to Individual Sensitivity to Obesity. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E865–E874. [Google Scholar] [CrossRef]
- Kotz, C.M. Integration of Feeding and Spontaneous Physical Activity: Role for Orexin. Physiol. Behav. 2006, 88, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Kotz, C.M.; Teske, J.A.; Levine, J.A.; Wang, C. Feeding and Activity Induced by Orexin A in the Lateral Hypothalamus in Rats. Regul. Pept. 2002, 104, 27–32. [Google Scholar] [CrossRef]
- Ida, T.; Nakahara, K.; Katayama, T.; Murakami, N.; Nakazato, M. Effect of Lateral Cerebroventricular Injection of the Appetite-Stimulating Neuropeptide, Orexin and Neuropeptide Y, on the Various Behavioral Activities of Rats. Brain Res. 1999, 821, 526–529. [Google Scholar] [CrossRef]
- Chieffi, S.; Messina, G.; Villano, I.; Messina, A.; Esposito, M.; Monda, V.; Valenzano, A.; Moscatelli, F.; Esposito, T.; Carotenuto, M.; et al. Exercise Influence on Hippocampal Function: Possible Involvement of Orexin-A. Front. Physiol. 2017, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Smolko, N.A.; Valiev, R.I.; Kabdesh, I.M.; Fayzullina, R.A.; Mukhamedshina, Y.O. Eating disorder in children: Impact on quality of life, with a spotlight on autism spectrum disorder. Nutr. Res. 2024, 123, 38–52. [Google Scholar] [CrossRef]
- Colten, H.R.; Altevogt, B.M.; Institute of Medicine (US) Committee on Sleep Medicine and Research (Eds.) Extent and Health Consequences of Chronic Sleep Loss and Sleep Disorders. In Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; National Academies Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Chen, Q.; de Lecea, L.; Hu, Z.; Gao, D. The Hypocretin/Orexin System: An Increasingly Important Role in Neuropsychiatry. Med. Res. Rev. 2015, 35, 152–197. [Google Scholar] [CrossRef]
- Mieda, M.; Willie, J.; Hara, J.; Sinton, C.; Sakurai, T.; Yanagisawa, M. Orexin Peptides Prevent Cataplexy and Improve Wakefulness in an Orexin Neuron-Ablated Model of Narcolepsy in Mice. Proc. Natl. Acad. Sci. USA 2004, 101, 4649–4654. [Google Scholar] [CrossRef] [PubMed]
- Dhafar, H.O.; BaHammam, A.S. Body Weight and Metabolic Rate Changes in Narcolepsy: Current Knowledge and Future Directions. Metabolites 2022, 12, 1120. [Google Scholar] [CrossRef]
- Clark, J.W.; Brian, M.L.; Drummond, S.P.A.; Hoyer, D.; Jacobson, L.H. Effects of Orexin Receptor Antagonism on Human Sleep Architecture: A Systematic Review. Sleep Med. Rev. 2020, 53, 101332. [Google Scholar] [CrossRef]
- Zhang, W.; Lian, T.; He, M.; Guo, P.; Guan, H.; Li, J.; Qi, J.; Luo, D.; Li, J.; Zhang, Y.; et al. Sleep Disorders and Alzheimer’s Disease: Relationship and Mechanisms Involving Neuroinflammation, Orexin and Aβ. Fluids Barriers CNS 2025, 22, 62. [Google Scholar] [CrossRef]
- Liguori, C.; Romigi, A.; Nuccetelli, M.; Zannino, S.; Sancesario, G.; Martorana, A.; Albanese, M.; Mercuri, N.B.; Izzi, F.; Bernardini, S.; et al. Orexinergic System Dysregulation, Sleep Impairment, and Cognitive Decline in Alzheimer Disease. JAMA Neurol. 2014, 71, 1498–1505. [Google Scholar] [CrossRef]
- Gabelle, A.; Jaussent, I.; Hirtz, C.; Vialaret, J.; Navucet, S.; Grasselli, C.; Robert, P.; Lehmann, S.; Dauvilliers, Y. Cerebrospinal Fluid Levels of Orexin-A and Histamine, and Sleep Profile within the Alzheimer Process. Neurobiol. Aging 2017, 53, 59–66. [Google Scholar] [CrossRef]
- Asai, H.; Hirano, M.; Furiya, Y.; Udaka, F.; Morikawa, M.; Kanbayashi, T.; Shimizu, T.; Ueno, S. Cerebrospinal Fluid-Orexin Levels and Sleep Attacks in Four Patients with Parkinson’s Disease. Clin. Neurol. Neurosurg. 2009, 111, 341–344. [Google Scholar] [CrossRef]
- Nie, K.; Gao, Y.; Mei, M.; Guo, M.; Huang, Z.; Wang, L.; Zhao, J.; Zhang, Y.; Wang, L. The Clinical Characteristics and Cognitive Features of Mild Cognitive Impairment in Parkinson’s Disease and the Analysis of Relevant Factors. J. Clin. Neurosci. 2019, 63, 142–148. [Google Scholar] [CrossRef]
- Tracik, F.; Ebersbach, G. Sudden Daytime Sleep Onset in Parkinson’s Disease: Polysomnographic Recordings. Mov. Disord. 2001, 16, 500–506. [Google Scholar] [CrossRef]
- Brodsky, M.A.; Godbold, J.; Roth, T.; Olanow, C.W. Sleepiness in Parkinson’s Disease: A Controlled Study. Mov. Disord. 2003, 18, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Drouot, X.; Moutereau, S.; Nguyen, J.P.; Lefaucheur, J.P.; Créange, A.; Remy, P.; Goldenberg, F.; d’Ortho, M.P. Low Levels of Ventricular CSF Orexin/Hypocretin in Advanced PD. Neurology 2003, 61, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Inoue, Y.; Kanbayashi, T.; Nomura, T.; Kusumi, M.; Nakashima, K. CSF Orexin Levels of Parkinson’s Disease, Dementia with Lewy Bodies, Progressive Supranuclear Palsy and Corticobasal Degeneration. J. Neurol. Sci. 2006, 250, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Sahraian, M.A.; Rezaali, S.; Hosseiny, M.; Doosti, R.; Tajik, A.; Naser Moghadasi, A. Sleep Disorder as a Triggering Factor for Relapse in Multiple Sclerosis. Eur. Neurol. 2017, 77, 258–261. [Google Scholar] [CrossRef]
- Oka, Y.; Kanbayashi, T.; Mezaki, T.; Iseki, K.; Matsubayashi, J.; Murakami, G.; Matsui, M.; Shimizu, T.; Shibasaki, H. Low CSF Hypocretin-1/Orexin-A Associated with Hypersomnia Secondary to Hypothalamic Lesion in a Case of Multiple Sclerosis. J. Neurol. 2004, 251, 885–886. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Niepel, G.; Patterson, M.; Judd, A.; Braitch, M.; Fahey, A.J.; Harikrishnan, S.; Edwards, L.J.; Tench, C.R.; Bennett, G.W.; et al. Orexin A (Hypocretin-1) Levels Are Not Reduced While Cocaine/Amphetamine Regulated Transcript Levels Are Increased in the Cerebrospinal Fluid of Patients with Multiple Sclerosis: No Correlation with Fatigue and Sleepiness. J. Neurol. Sci. 2011, 307, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Burfeind, K.G.; Yadav, V.; Marks, D.L. Hypothalamic Dysfunction and Multiple Sclerosis: Implications for Fatigue and Weight Dysregulation. Curr. Neurol. Neurosci. Rep. 2016, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Boss, C.; Gatfield, J.; Brotschi, C.; Heidmann, B.; Sifferlen, T.; von Raumer, M.; Schmidt, G.; Williams, J.T.; Treiber, A.; Roch, C. The Quest for the Best Dual Orexin Receptor Antagonist (Daridorexant) for the Treatment of Insomnia Disorders. ChemMedChem 2020, 15, 2286–2305. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigro, E.; Argentino, F.; Musumeci, G.; Daniele, A. Orexin and Lifestyle Habits: A Meaningful Connection Among Nutrition, Physical Activity, and Sleep Pattern in Health and Diseases. Int. J. Mol. Sci. 2025, 26, 8980. https://doi.org/10.3390/ijms26188980
Nigro E, Argentino F, Musumeci G, Daniele A. Orexin and Lifestyle Habits: A Meaningful Connection Among Nutrition, Physical Activity, and Sleep Pattern in Health and Diseases. International Journal of Molecular Sciences. 2025; 26(18):8980. https://doi.org/10.3390/ijms26188980
Chicago/Turabian StyleNigro, Ersilia, Francesca Argentino, Giuseppe Musumeci, and Aurora Daniele. 2025. "Orexin and Lifestyle Habits: A Meaningful Connection Among Nutrition, Physical Activity, and Sleep Pattern in Health and Diseases" International Journal of Molecular Sciences 26, no. 18: 8980. https://doi.org/10.3390/ijms26188980
APA StyleNigro, E., Argentino, F., Musumeci, G., & Daniele, A. (2025). Orexin and Lifestyle Habits: A Meaningful Connection Among Nutrition, Physical Activity, and Sleep Pattern in Health and Diseases. International Journal of Molecular Sciences, 26(18), 8980. https://doi.org/10.3390/ijms26188980






