Effect of Tigecycline on the Homeostasis of Human Epidermal Melanocytes and Fibroblasts
Abstract
1. Introduction
2. Results
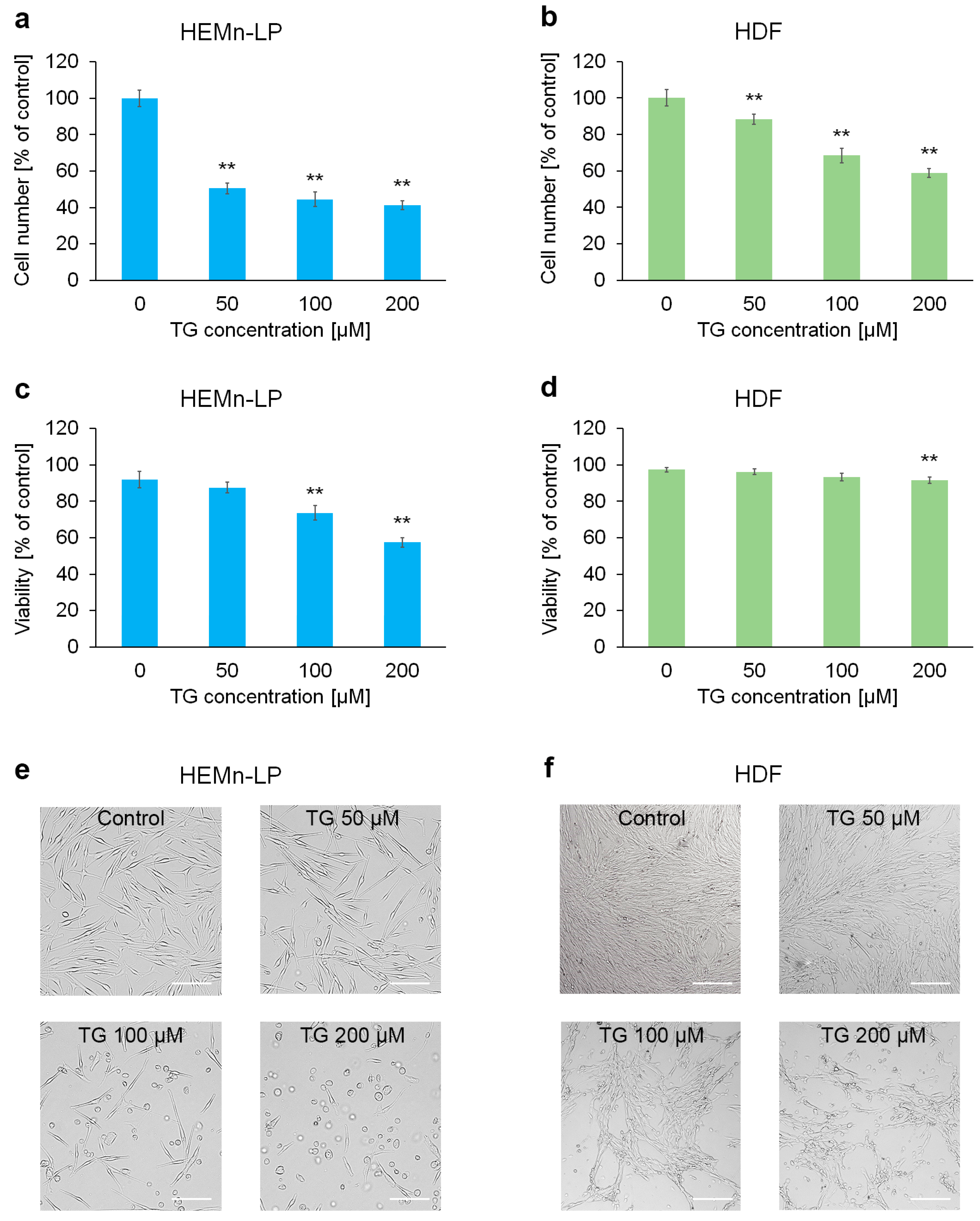
2.1. The Impact of Tigecycline on Proliferation and Viability of Human Skin Cells
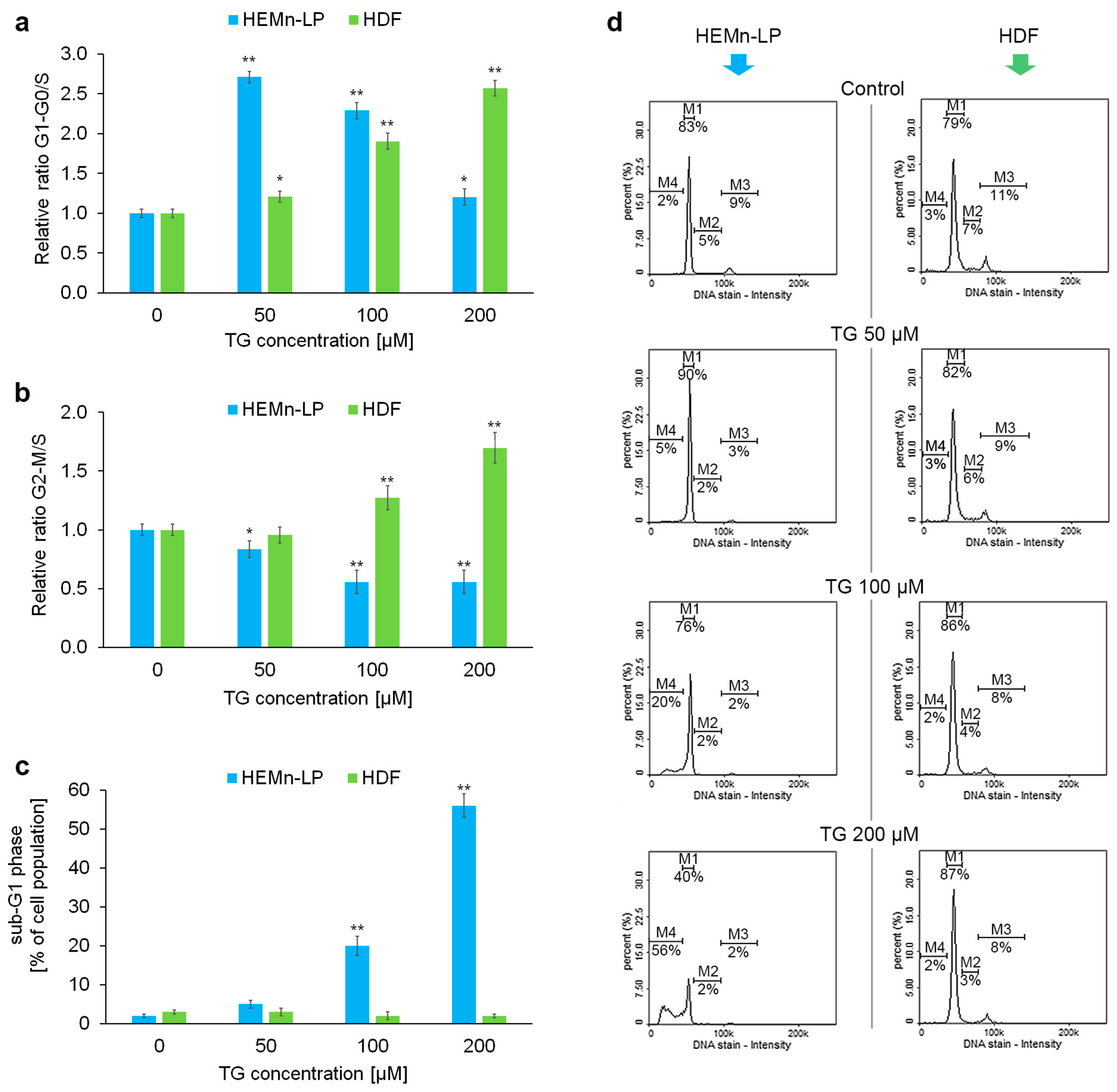
2.2. Effects of Tigecycline on the Cell Cycle of Melanocytes and Fibroblasts
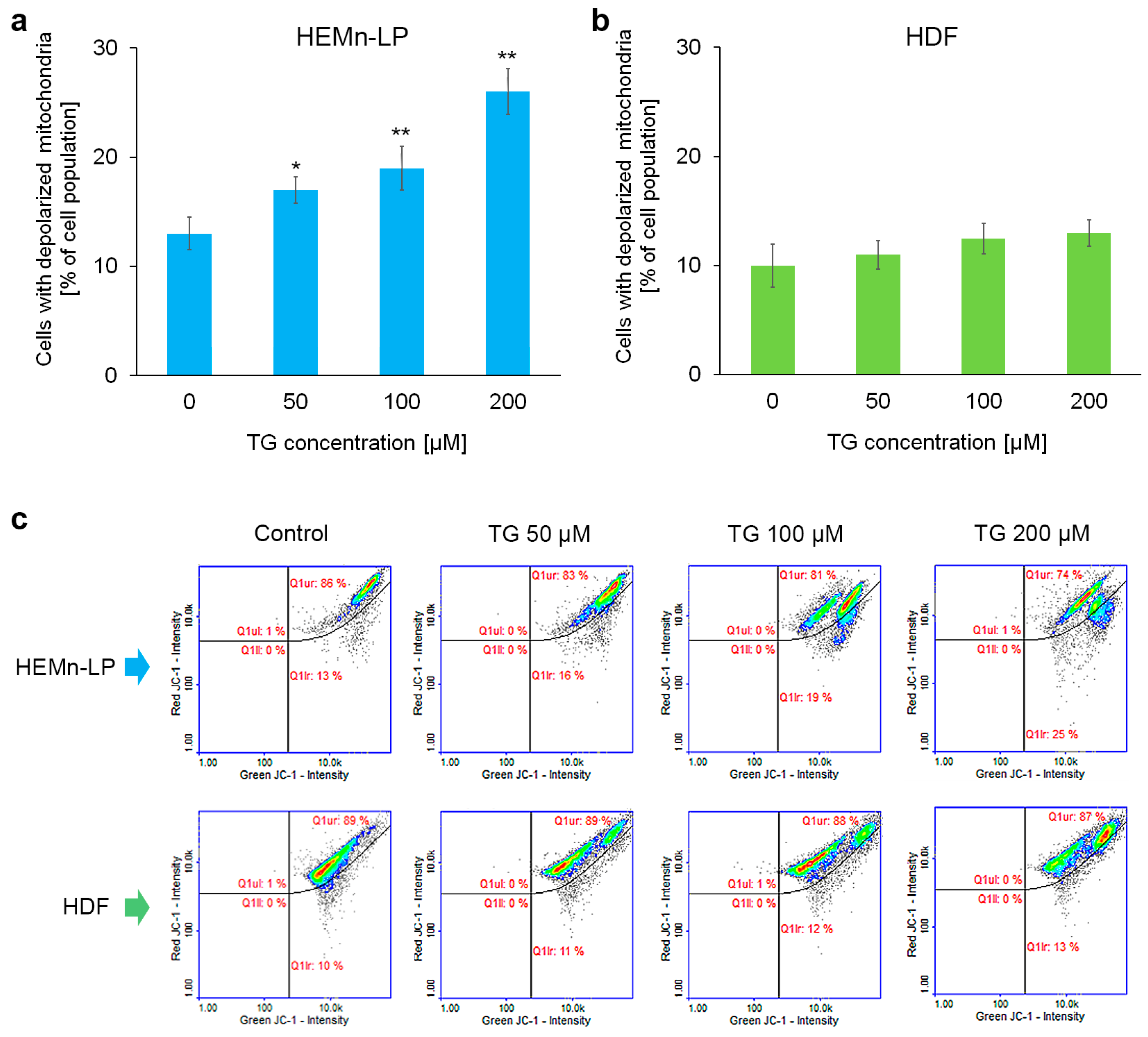
2.3. Mitochondrial Potential in Melanocytes and Fibroblasts Treated with Tigecycline
2.4. Reduced Thiols Status in Melanocytes and Fibroblasts Treated with Tigecycline
2.5. Results of EPR Examination of the Influence of Tigecycline on Free Radicals in Melanocytes
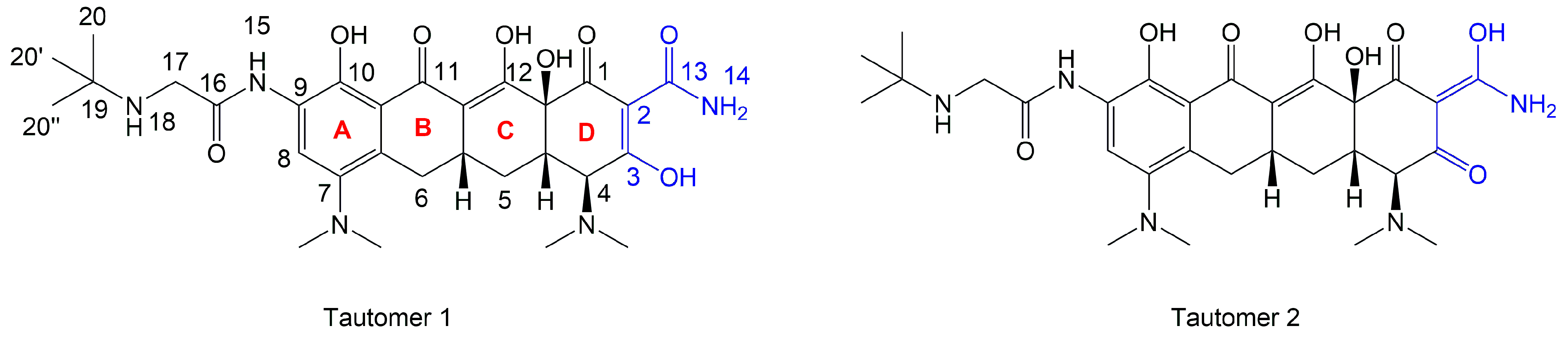
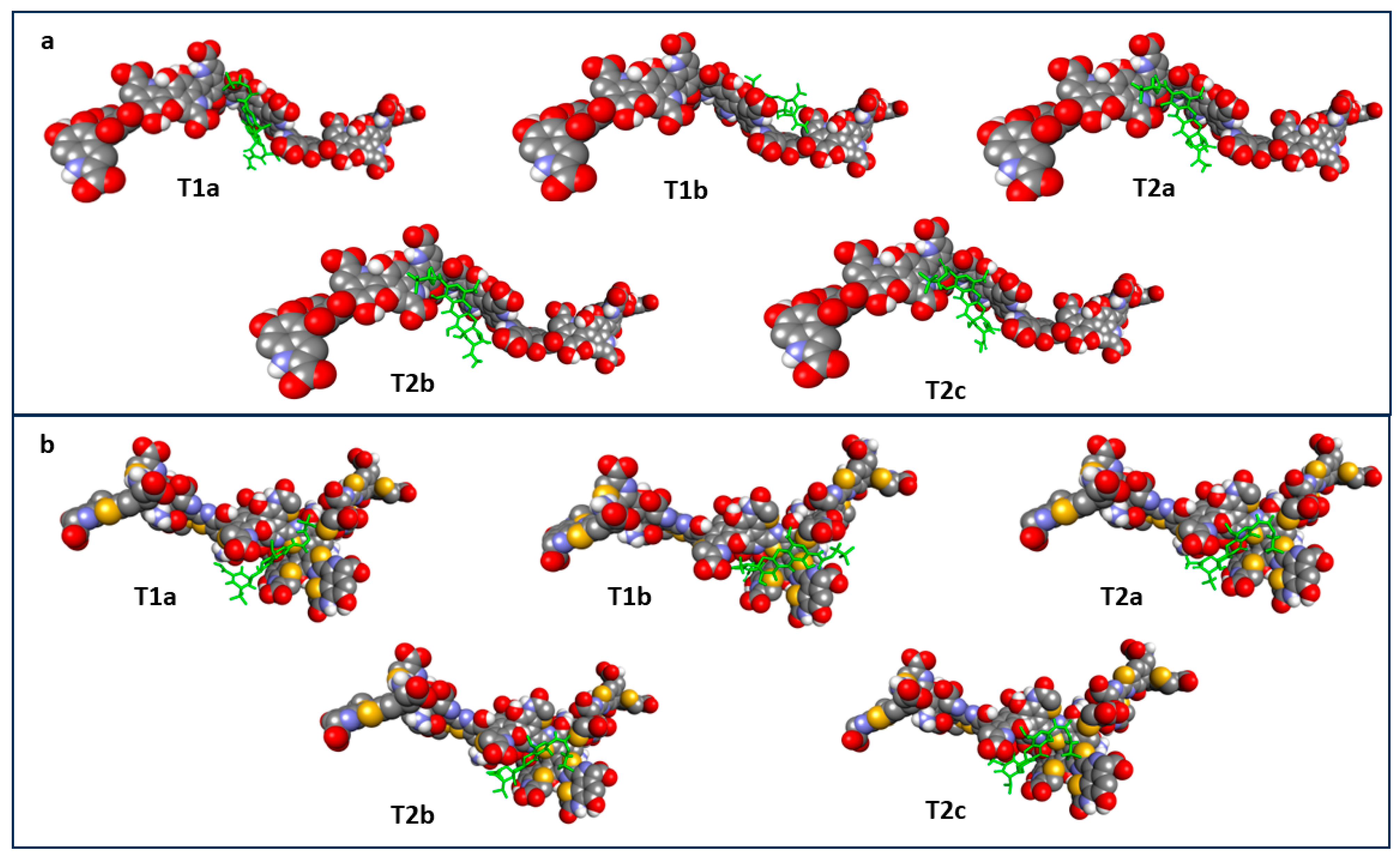
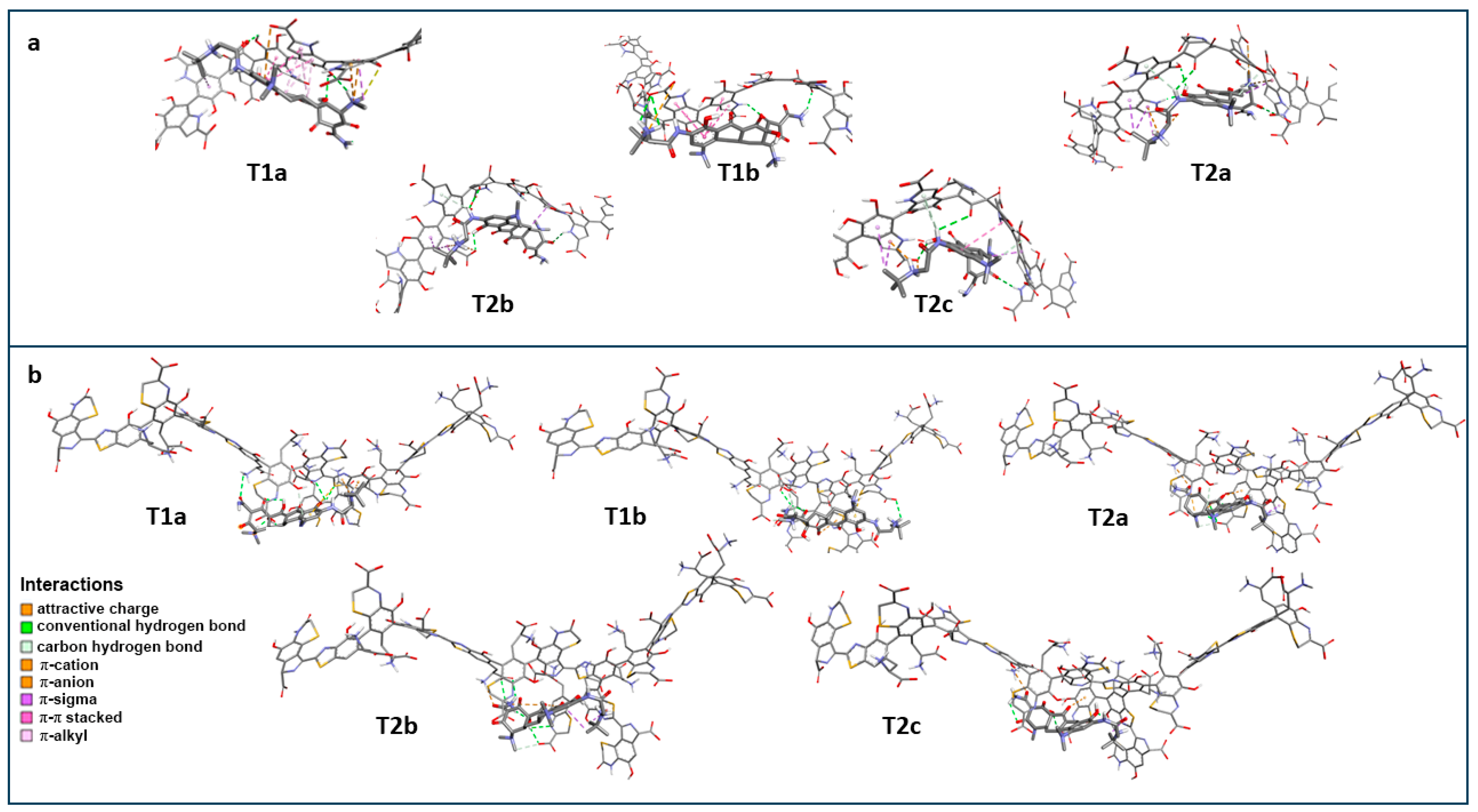
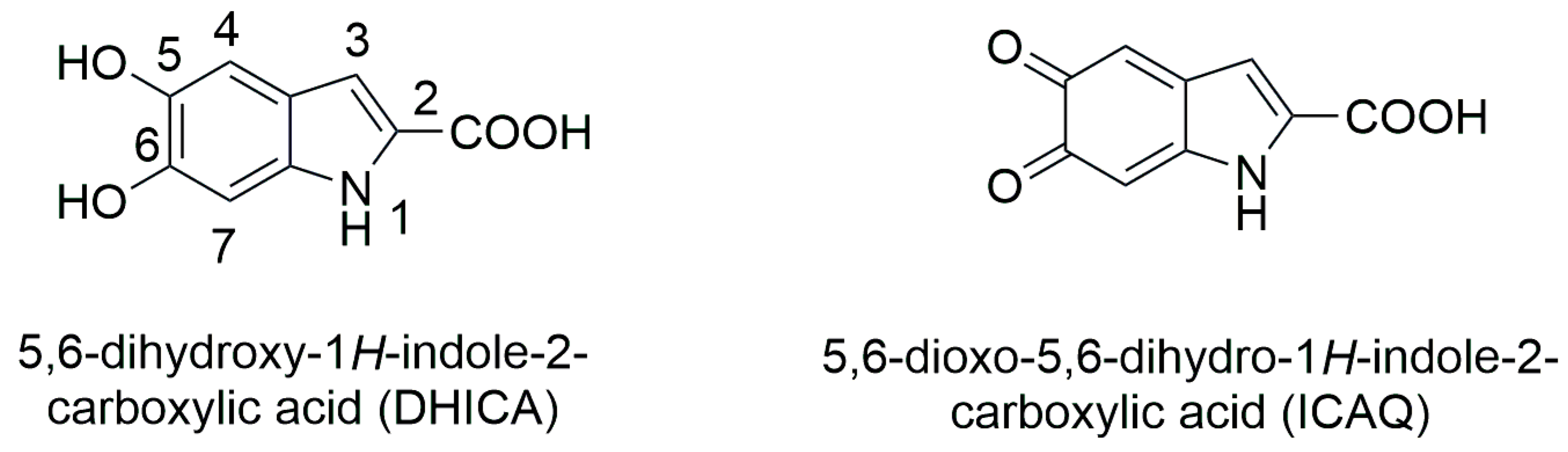
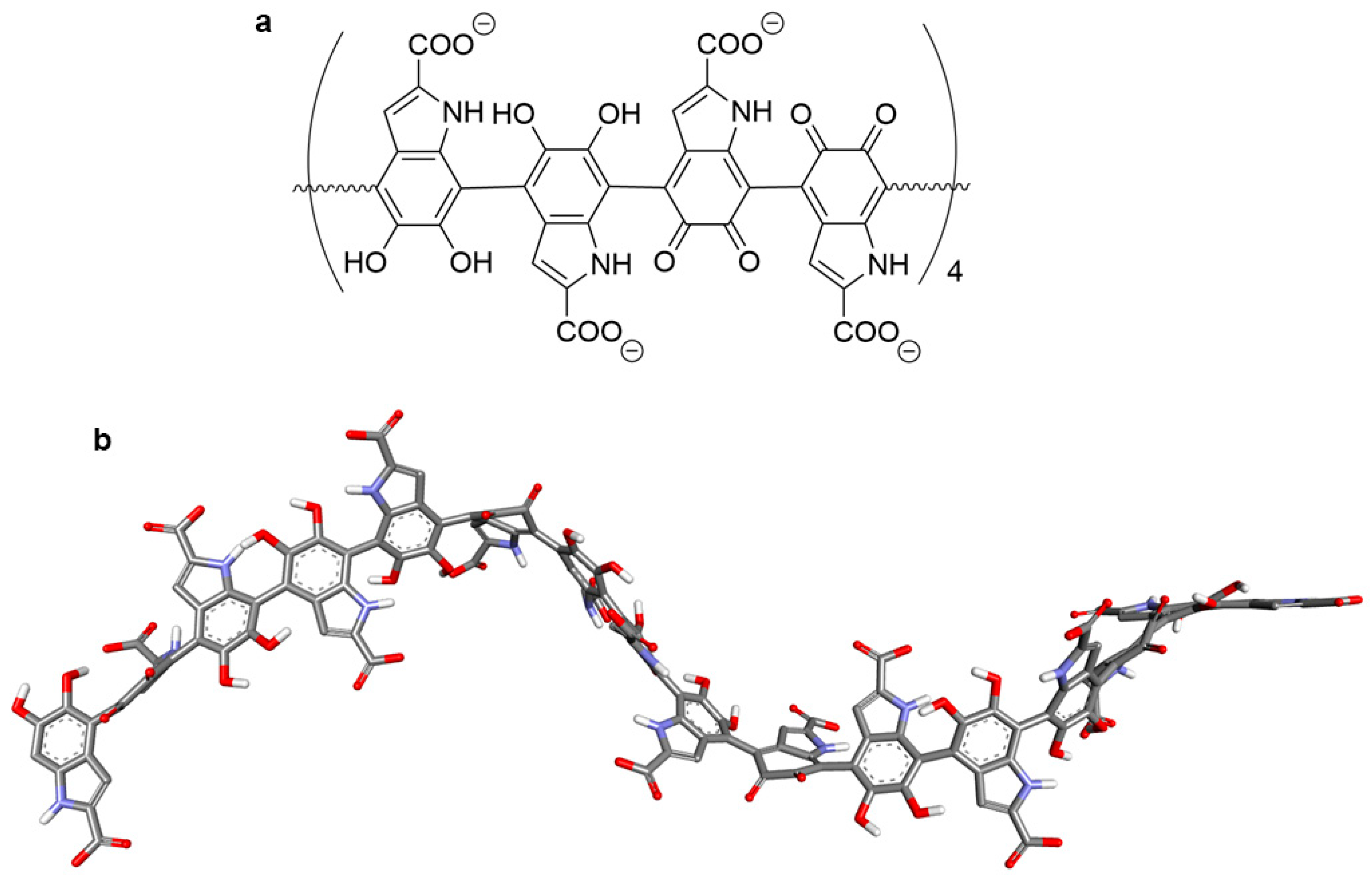
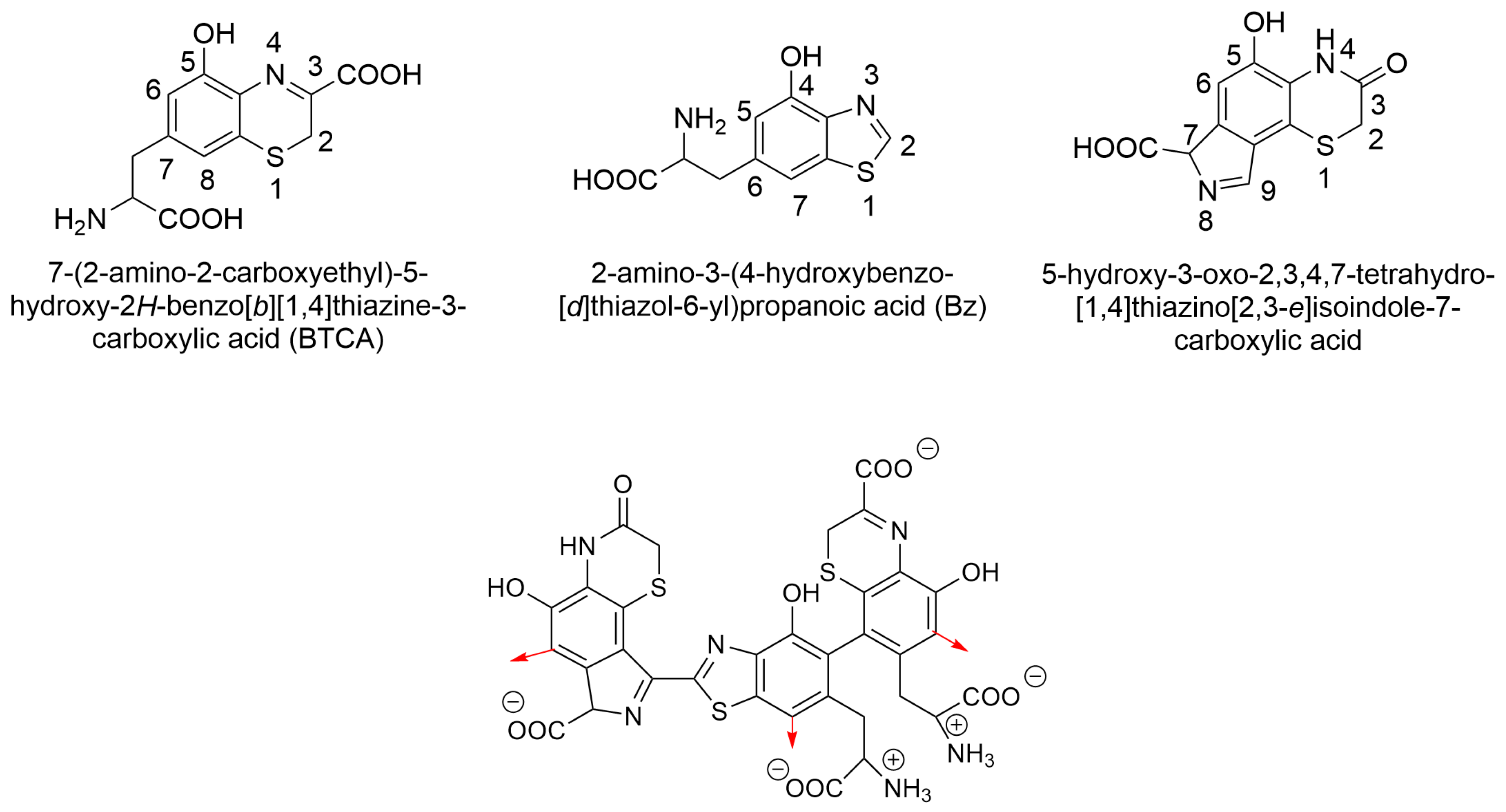
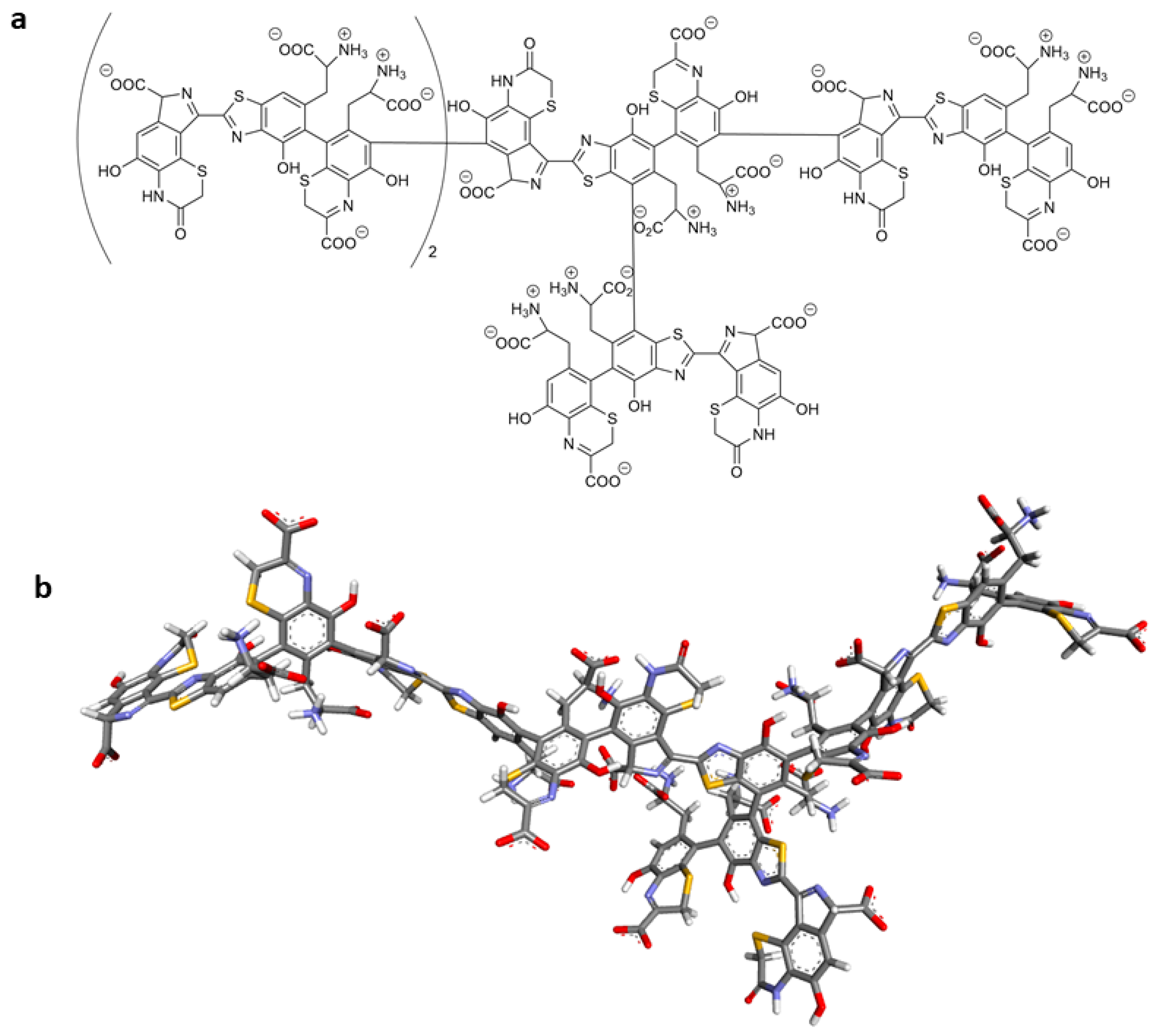
2.6. Molecular Docking of Tigecycline to Melanin
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. The Assessment of Cell Number and Viability
4.4. The Assessment of Cell Morphology
4.5. Cell Cycle Analysis
4.6. Transmembrane Mitochondrial Potential Assessment
4.7. Measurement of Intracellular Reduced Thiols Level
4.8. In Silico Study
4.9. EPR Examination of Free Radicals in Melanocytes
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffin, M.O.; Fricovsky, E.; Ceballos, G.; Villarreal, F. Tetracyclines: A pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am. J. Physiol. Cell Physiol. 2010, 299, C539–C548. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, F.; Morris, D.L.; Pourgholami, M.H. Tetracyclines: Drugs with huge therapeutic potential. Mini Rev. Med. Chem. 2012, 12, 44–52. [Google Scholar] [CrossRef]
- Perret, L.J.; Tait, C.P. Non-antibiotic properties of tetracyclines and their clinical application in dermatology. Australas. J. Dermatol. 2014, 55, 111–118. [Google Scholar] [CrossRef]
- Golub, L.M.; Elburki, M.S.; Walker, C.; Ryan, M.; Sorsa, T.; Tenenbaum, H.; Goldberg, M.; Wolff, M.; Gu, Y. Non-antibacterial tetracycline formulations: Host-modulators in the treatment of periodontitis and relevant systemic diseases. Int. Dent. J. 2016, 66, 127–135. [Google Scholar] [CrossRef]
- Rusu, A.; Buta, E.L. The Development of Third-Generation Tetracycline Antibiotics and New Perspectives. Pharmaceutics 2021, 13, 2085. [Google Scholar] [CrossRef]
- Sun, C.; Yu, Y.; Hua, X. Resistance mechanisms of tigecycline in Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2023, 13, 1141490. [Google Scholar] [CrossRef]
- Yaghoubi, S.; Zekiy, A.O.; Krutova, M.; Gholami, M.; Kouhsari, E.; Sholeh, M.; Ghafouri, Z.; Maleki, F. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: Narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1003–1022. [Google Scholar] [CrossRef]
- Kounatidis, D.; Dalamaga, M.; Grivakou, E.; Karampela, I.; Koufopoulos, P.; Dalopoulos, V.; Adamidis, N.; Mylona, E.; Kaziani, A.; Vallianou, N.G. Third-Generation Tetracyclines: Current Knowledge and Therapeutic Potential. Biomolecules 2024, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Odorici, G.; Monfrecola, G.; Bettoli, V. Tetracyclines and photosensitive skin reactions: A narrative review. Dermatol. Ther. 2021, 34, e14978. [Google Scholar] [CrossRef] [PubMed]
- Karkoszka, M.; Rok, J.; Wrześniok, D. Melanin Biopolymers in Pharmacology and Medicine-Skin Pigmentation Disorders, Implications for Drug Action, Adverse Effects and Therapy. Pharmaceuticals 2024, 17, 521. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; Ozeki, H. Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigment. Cell Res. 2000, 13, 103–109. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef]
- Bustamante, J.; Bredeston, L.; Malanga, G.; Mordoh, J. Role of melanin as a scavenger of active oxygen species. Pigment. Cell Res. 1993, 6, 348–353. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamashita, Y.; Umezawa, K.; Hirobe, T.; Ito, S.; Wakamatsu, K. The Pro-Oxidant Activity of Pheomelanin is Significantly Enhanced by UVA Irradiation: Benzothiazole Moieties Are More Reactive than Benzothiazine Moieties. Int. J. Mol. Sci. 2018, 19, 2889. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, S.; Wakamatsu, K.; Galván, I. Increase of the benzothiazole moiety content of pheomelanin pigment after endogenous free radical inducement. Dye. Pigment. 2020, 180, 108516. [Google Scholar] [CrossRef]
- Szewczyk, G.; Żądło, A.; Sarna, M.; Ito, S.; Wakamatsu, K.; Sarna, T. Aerobic photoreactivity of synthetic eumelanins and pheomelanins: Generation of singlet oxygen and superoxide anion. Pigment Cell Melanoma Res. 2016, 29, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef]
- Simon, J.D.; Hong, L.; Peles, D.N. Insights into melanosomes and melanin from some interesting spatial and temporal properties. J. Phys. Chem. B 2008, 112, 13201–13217. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Rok, J.; Rzepka, Z.; Wrześniok, D. Drug-Induced Photosensitivity—From Light and Chemistry to Biological Reactions and Clinical Symptoms. Pharmaceuticals 2021, 14, 723. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Jibreen, A.; Karaman, D.; Thawabteh, A.; Karaman, R. Skin Pigmentation Types, Causes and Treatment—A Review. Molecules 2023, 28, 4839. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- Rok, J.; Rzepka, Z.; Maszczyk, M.; Beberok, A.; Wrześniok, D. Minocycline Impact on Redox Homeostasis of Normal Human Melanocytes HEMn-LP Exposed to UVA Radiation and Hydrogen Peroxide. Int. J. Mol. Sci. 2021, 22, 1642. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Rzepka, Z.; Beberok, A.; Pawlik, J.; Wrześniok, D. Cellular and Molecular Aspects of Anti-Melanoma Effect of Minocycline—A Study of Cytotoxicity and Apoptosis on Human Melanotic Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 6917. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Kowalska, J.; Rzepka, Z.; Stencel, D.; Skorek, A.; Banach, K.; Wrześniok, D. The Assessment of Anti-Melanoma Potential of Tigecycline-Cellular and Molecular Studies of Cell Proliferation, Apoptosis and Autophagy on Amelanotic and Melanotic Melanoma Cells. Cells 2023, 12, 1564. [Google Scholar] [CrossRef]
- Rok, J.; Karkoszka, M.; Rzepka, Z.; Respondek, M.; Banach, K.; Beberok, A.; Wrześniok, D. Cytotoxic and proapoptotic effect of doxycycline—An in vitro study on the human skin melanoma cells. Toxicol. Vitro 2020, 65, 104790. [Google Scholar] [CrossRef]
- Maciag, M.C.; Ward, S.L.; O’Connell, A.E.; Broyles, A.D. Hypersensitivity to tetracyclines: Skin testing, graded challenge, and desensitization regimens. Ann. Allergy Asthma Immunol. 2020, 124, 589–593. [Google Scholar] [CrossRef]
- Yang, J.; Wu, F.; Luo, D.; Li, M.; Gou, X.; Xi, J.; Zhu, H. Toxic epidermal necrolysis syndrome induced by tigecycline: A case report. J. Int. Med. Res. 2020, 48, 300060520922416. [Google Scholar] [CrossRef]
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 2006, 54, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Alsemari, M.A.; Hakeam, H.A.; Alsalman, H.; Amin, T. Cutaneous Hyperpigmentation Secondary to High-Dose Tigecycline: A Case Report. Ther. Adv. Infect. Dis. 2020, 7, 2049936120952605. [Google Scholar] [CrossRef]
- Rok, J.; Rzepka, Z.; Respondek, M.; Beberok, A.; Wrześniok, D. Chlortetracycline and melanin biopolymer—The risk of accumulation and implications for phototoxicity: An in vitro study on normal human melanocytes. Chem.-Biol. Interact. 2019, 303, 27–34. [Google Scholar] [CrossRef]
- Vandecasteele, S.J.; De Ceulaer, J.; Wittouck, E. Tigecycline Induced Hyperpigmentation of the Skin. Open Forum Infect. Dis. 2016, 3, ofw033. [Google Scholar] [CrossRef] [PubMed]
- Atlasik, B.; Stepien, K.; Wilczok, T. Interaction of drugs with ocular melanin in vitro. Exp. Eye Res. 1980, 30, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Banning, T.P.; Heard, C.M. Binding of doxycycline to keratin, melanin and human epidermal tissue. Int. J. Pharm. 2002, 235, 219–227. [Google Scholar] [CrossRef]
- Rok, J.; Buszman, E.; Delijewski, M.; Otręba, M.; Beberok, A.; Wrześniok, D. Effect of tetracycline and UV radiation on melanization and antioxidant status of melanocytes. J. Photochem. Photobiol. B: Biol. 2015, 148, 168–173. [Google Scholar] [CrossRef]
- Rok, J.; Rzepka, Z.; Kowalska, J.; Banach, K.; Beberok, A.; Wrześniok, D. Molecular and Biochemical Basis of Minocycline-Induced Hyperpigmentation—The Study on Normal Human Melanocytes Exposed to UVA and UVB Radiation. Int. J. Mol. Sci. 2021, 22, 3755. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Wrześniok, D.; Beberok, A.; Otręba, M.; Delijewski, M.; Buszman, E. Phototoxic effect of oxytetracycline on normal human melanocytes. Toxicol. Vitro 2018, 48, 26–32. [Google Scholar] [CrossRef]
- Hu, H.; Dong, Z.; Tan, P.; Zhang, Y.; Liu, L.; Yang, L.; Liu, Y.; Cui, H. Antibiotic drug tigecycline inhibits melanoma progression and metastasis in a p21CIP1/Waf1-dependent manner. Oncotarget 2016, 7, 3171–3185. [Google Scholar] [CrossRef]
- Uitto, V.J.; Firth, J.D.; Nip, L.; Golub, L.M. Doxycycline and chemically modified tetracyclines inhibit gelatinase A (MMP-2) gene expression in human skin keratinocytes. Ann. N. Y. Acad. Sci. 1994, 732, 140–151. [Google Scholar] [CrossRef]
- Chatzispyrou, I.A.; Held, N.M.; Mouchiroud, L.; Auwerx, J.; Houtkooper, R.H. Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research. Cancer Res. 2015, 75, 4446–4449. [Google Scholar] [CrossRef]
- Sarna, T.; Plonka, P.M. Biophysical Studies of Melanin. In Biomedical EPR, Part A: Free Radicals, Metals, Medicine, and Physiology; Eaton, S.R., Eaton, G.R., Berliner, L.J., Eds.; Biological Magnetic Resonance; Springer: New York, NY, USA, 2005; Volume 23. [Google Scholar] [CrossRef]
- Chodurek, E.; Zdybel, M.; Pilawa, B.; Dzierzewicz, Z. Examination by EPR spectroscopy of free radicals in melanins isolated from A-375 cells exposed on valproic acid and cisplatin. Acta Pol. Pharm. 2012, 69, 1334–1341. [Google Scholar] [PubMed]
- Rzepka, Z.; Rok, J.; Zdybel, M.; Pilawa, B.; Beberok, A.; Wrześniok, D. Streptomycin generates oxidative stress in melanin-producing cells: In vitro study with EPR spectroscopy evidence. Toxicol. Vitro 2024, 98, 105844. [Google Scholar] [CrossRef] [PubMed]
- Wrześniok, D.; Rok, J.; Beberok, A.; Rzepka, Z.; Respondek, M.; Pilawa, B.; Zdybel, M.; Delijewski, M.; Buszman, E. Kanamycin induces free radicals formation in melanocytes: An important factor for aminoglycosides ototoxicity. J. Cell. Biochem. 2019, 120, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Ramachanderan, R.; Schaefer, B. Tetracycline Antibiotics. ChemTexts 2021, 7, 18. [Google Scholar] [CrossRef]
- Mojica, E.-R.E.; Autschbach, J.; Bright, F.V.; Aga, D.S. Tetracycline Speciation during Molecular Imprinting in Xerogels Results in Class-Selective Binding. Analyst 2011, 136, 749–755. [Google Scholar] [CrossRef]
- Blázquez-Castro, A.; Stockert, J. Biomedical Overview of Melanin. 1. Updating Melanin Biology and Chemistry, Physico-Chemical Properties, Melanoma Tumors, and Photothermal Therapy. BIOCELL 2021, 45, 849–862. [Google Scholar] [CrossRef]
- Panzella, L.; Ebato, A.; Napolitano, A.; Koike, K. The Late Stages of Melanogenesis: Exploring the Chemical Facets and the Application Opportunities. Int. J. Mol. Sci. 2018, 19, 1753. [Google Scholar] [CrossRef]
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; D’Ischia, M. “Fifty Shades” of Black and Red or How Carboxyl Groups Fine Tune Eumelanin and Pheomelanin Properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, L.; Wakamatsu, K.; Ito, S.; Adhyaru, B.; Cheng, C.-Y.; Bowers, C.R.; Simon, J.D. Comparison of Structural and Chemical Properties of Black and Red Human Hair Melanosomes. Photochem. Photobiol. 2005, 81, 135–144. [Google Scholar] [CrossRef]
- Watt, A.A.R.; Bothma, J.P.; Meredith, P. The Supramolecular Structure of Melanin. Soft Matter 2009, 5, 3754–3760. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A.; d’Ischia, M. Is DHICA the Key to Dopachrome Tautomerase and Melanocyte Functions? Pigment. Cell Melanoma Res. 2011, 24, 248–249. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.-M.; Dai, X.; Zhou, Q.; Lei, T.-C.; Beermann, F.; Wakamatsu, K.; Xu, S.-Z. Regulation of DHICA-Mediated Antioxidation by Dopachrome Tautomerase: Implica-tion for Skin Photoprotection against UVA Radiation. Free Radic. Biol. Med. 2010, 48, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, T.; Pezzella, A.; Di Mauro, E.; Cestola, S.; Ginsburg, D.; Luzi, M.; Rigucci, A.; Santato, C. On the Antioxidant Activity of Eumelanin Biopigments: A Quantitative Comparison between Free Radical Scavenging and Redox Properties. Nat. Prod. Res. 2020, 34, 2465–2473. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 98276. [Google Scholar] [CrossRef]
- Del Bino, S.; Ito, S.; Sok, J.; Nakanishi, Y.; Bastien, P.; Wakamatsu, K.; Bernerd, F. Chemical analysis of constitutive pigmentation of human epidermis reveals constant eumelanin to pheomelanin ratio. Pigment. Cell Melanoma Res. 2015, 28, 707–717. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ohtara, K.; Ito, S. Chemical analysis of late stages of pheomelanogenesis: Conversion of dihydrobenzothiazine to a benzothiazole structure. Pigment. Cell Melanoma Res. 2009, 22, 474–486. [Google Scholar] [CrossRef]
- ACD/Labs Releases ACD/Labs Percepta. Available online: https://www.acdlabs.com/resource/acd-labs-releases-acd-labs-percepta/ (accessed on 28 June 2024).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Systèmes, D. BIOVIA Discovery Studio. In Dassault Systmes BIOVIA, Discovery Studio Modeling Environment, Release 2017; Dassault Systmes: San Diego, CA, USA, 2016. [Google Scholar]


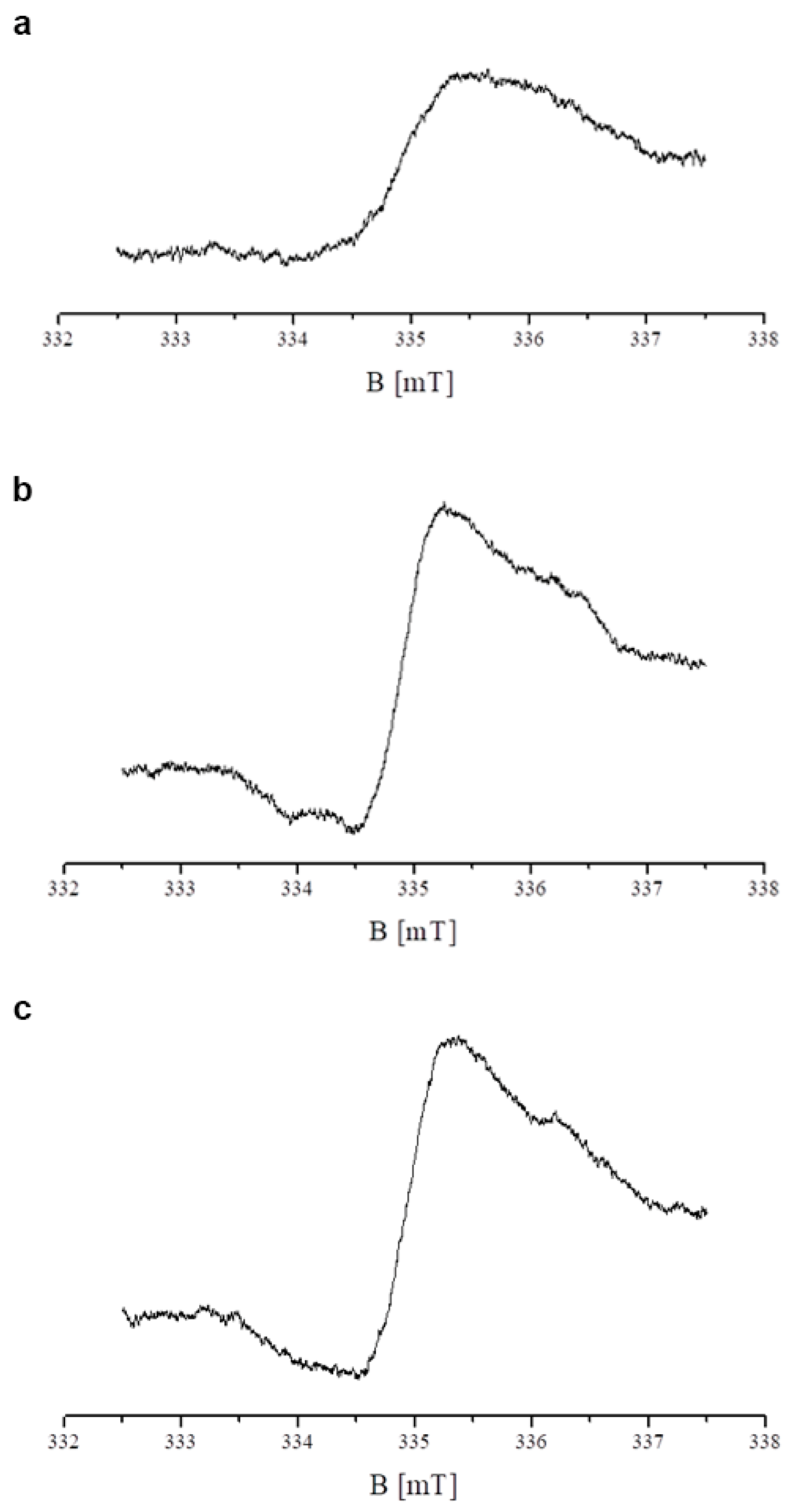
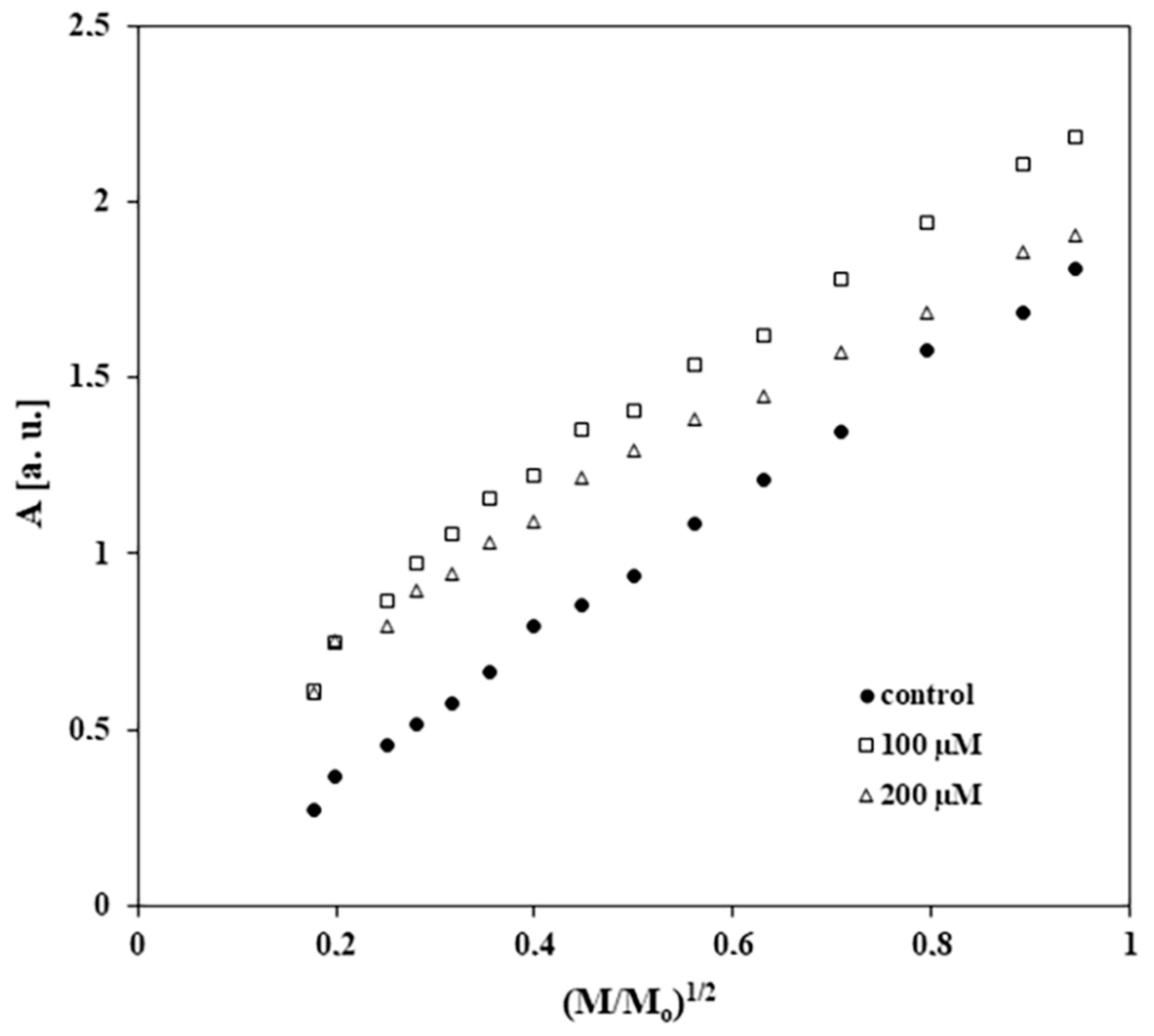
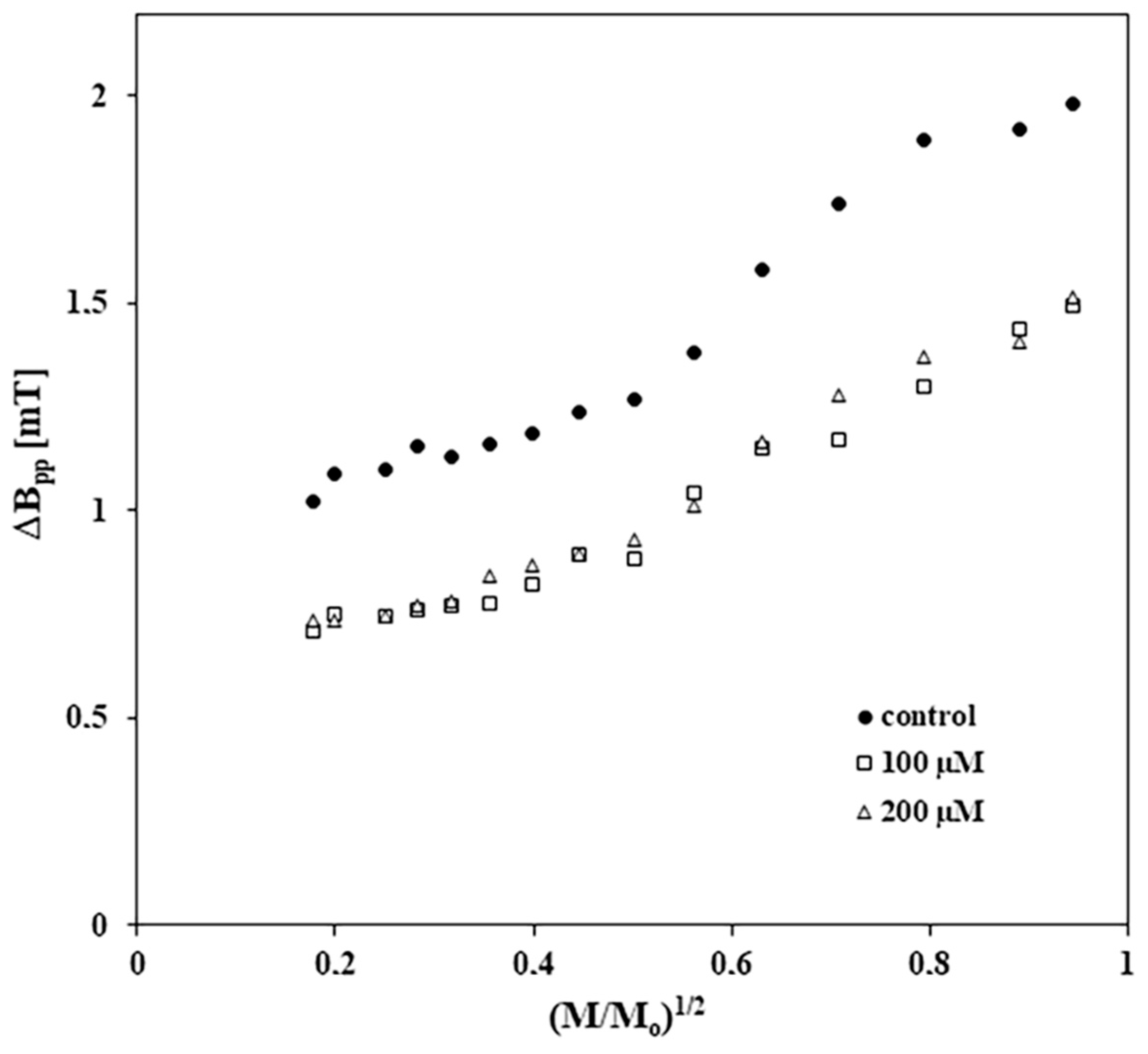

| Sample | I [a. u.] ± 0.2 | Bpp [mT] ± 0.02 |
|---|---|---|
| Control | 8.8 | 1.02 |
| TG 100 µM | 9.5 | 0.71 |
| TG 200 µM | 10.1 | 0.74 |
| Entry | Structure of Ligand | Percentage at pH = 7.4 |
|---|---|---|
| 1 | 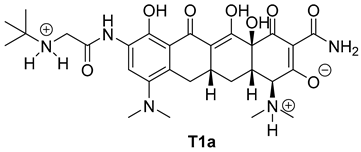 | 20 |
| 2 | 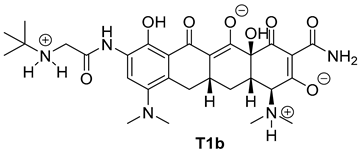 | 74 |
| 3 | 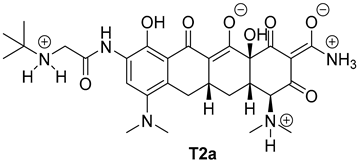 | 40 |
| 4 | 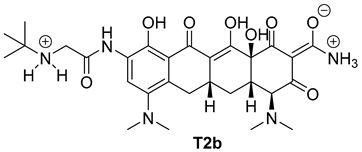 | 15 |
| 5 | 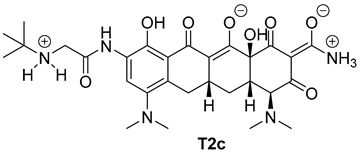 | 35 |
| Compound | Docking Score [kcal/mol] | |
|---|---|---|
| Eumelanin | Pheomelanin | |
| T1a | −7.2 | −6.3 |
| T1b | −7.0 | −6.5 |
| T2a | −7.4 | −6.2 |
| T2b | −7.1 | −6.8 |
| T2c | −7.5 | −6.5 |
| Ligand | Eumelanin | Interaction | ||
| Name | Residue | Residue | Type | Distance [Å] |
| T1a | C16-Carbonyl oxygen | C5-hydroxyl group of DHICA | conventional hydrogen bond | 2.37 |
| C12a hydroxyl group | C6-hydroxyl group of DHICA | conventional hydrogen bond | 2.45 | |
| N18 ammonium group | C5-Carbonyl oxygen of ICAQ | conventional hydrogen bond | 1.98 | |
| C4-ammonium group | C6-hydroxyl group of DHICA | conventional hydrogen bond | 3.07 | |
| C4-ammonium group | C5-hydroxyl group of DHICA | carbon hydrogen bond | 3.39 | |
| C4-ammonium group | benzene ring of DHICA | π-cation | 3.80 | |
| C4-ammonium group | pirole ring of DHICA | π-cation | 4.53 | |
| A-ring | carboxylate group of DHICA | π-anion | 4.86 | |
| C20 | benzene ring of DHICA | π-sigma | 3.90 | |
| Methyl of C4-ammonium group | benzene ring of DHICA | π-sigma | 3.94 | |
| A-ring | benzene ring of DHICA | π-π stacked | 5.04 | |
| A-ring | benzene ring of DHICA | π-π stacked | 5.08 | |
| A-ring | pirole ring of DHICA | π-π stacked | 4.33 | |
| Methyl of C7-amine group | pirole ring of DHICA | π-alkyl | 4.23 | |
| T1b | C13 amide group | C6-Carbonyl oxygen of ICAQ | conventional hydrogen bond | 2.22 |
| C1-Carbonyl oxygen | NH of pirole ring of DHICA | conventional hydrogen bond | 2.57 | |
| N18 ammonium group | carboxylate group of DHICA | conventional hydrogen bond | 2.63 | |
| N18 ammonium group | carboxylate group of DHICA | conventional hydrogen bond | 3.05 | |
| N18 ammonium group | carboxylate group of DHICA | attractive charge | 4.99 | |
| A-ring | benzene ring of DHICA | π-π stacked | 4.35 | |
| A-ring | pirole ring of DHICA | π-π stacked | 4.75 | |
| A-ring | benzene ring of DHICA | π-π stacked | 4.83 | |
| A-ring | pirole ring of DHICA | π-π stacked | 5.80 | |
| T2a | C4-ammonium group | carboxylate group of DHICA | attractive charge | 4.99 |
| N18 ammonium group | carboxylate group of DHICA | attractive charge | 5.16 | |
| C10 hydroxyl group | C5-hydroxyl group of DHICA | conventional hydrogen bond | 2.69 | |
| C10 hydroxyl group | NH of pirole ring of DHICA | conventional hydrogen bond | 2.01 | |
| N15 amide group | C5-Carbonyl oxygen of ICAQ | conventional hydrogen bond | 2.72 | |
| C3-carbonyl oxygen | NH of pirole ring of DHICA | conventional hydrogen bond | 2.28 | |
| Methyl of C7-amine group | C6-hydroxyl group of DHICA | carbon hydrogen bond | 3.79 | |
| Methyl of C4-ammonium group | carboxylate group of DHICA | carbon hydrogen bond | 3.70 | |
| C4-ammonium group | benzene ring of DHICA | π-cation | 4.73 | |
| N18 ammonium group | pirole ring of DHICA | π-cation | 4.89 | |
| N15 amide group | pirole ring of DHICA | π-donor hydrogen bond | 3.21 | |
| C20 | pirole ring of DHICA | π-sigma | 3.64 | |
| C20 | benzene ring of DHICA | π-sigma | 3.67 | |
| C20′ | pirole ring of DHICA | π-sigma | 3.97 | |
| C7 | benzene ring of DHICA | π-sigma | 3.89 | |
| T2b | N18 ammonium group | carboxylate group of DHICA | attractive charge | 4.97 |
| N15 amide group | C5-Carbonyl oxygen of ICAQ | conventional hydrogen bond | 2.83 | |
| C10 hydroxyl group | carboxylate group of DHICA | conventional hydrogen bond | 2.53 | |
| C3-carbonyl oxygen | NH of pirole ring of DHICA | conventional hydrogen bond | 2.28 | |
| Methyl of C7-amine group | C6-hydroxyl group of DHICA | carbon hydrogen bond | 3.70 | |
| N18 ammonium group | pirole ring of DHICA | π-cation | 4.78 | |
| N15 amide group | pirole ring of DHICA | π-donor hydrogen bond | 3.23 | |
| C20 | pirole ring of DHICA | π-sigma | 3.68 | |
| C20 | benzene ring of DHICA | π-sigma | 3.59 | |
| C20′ | pirole ring of DHICA | π-sigma | 3.96 | |
| C7 | benzene ring of DHICA | π-sigma | 3.73 | |
| T2c | N18 ammonium group | carboxylate group of DHICA | salt bridge;attractive charge | 2.64 |
| C3-carbonyl oxygen | NH of pirole ring of DHICA | conventional hydrogen bond | 2.70 | |
| C10 hydroxyl group | carboxylate group of DHICA | conventional hydrogen bond | 3.04 | |
| N15 amide group | C5-Carbonyl oxygen of ICAQ | conventional hydrogen bond | 2.45 | |
| Methyl of C7-amine group | C6-hydroxyl group of DHICA | carbon hydrogen bond | 3.79 | |
| N18 ammonium group | pirole ring of DHICA | π-cation | 2.82 | |
| N15 amide group | pirole ring of DHICA | π-donor hydrogen bond | 2.48 | |
| N15 amide group | benzene ring of DHICA | π-donor hydrogen bond | 3.16 | |
| C20 | pirole ring of DHICA | π-sigma | 3.55 | |
| C20 | benzene ring of DHICA | π-sigma | 3.84 | |
| C7 | benzene ring of DHICA | π-sigma | 3.67 | |
| A-ring | benzene ring of DHICA | π-π stacked | 5.41 | |
| Ligand | Pheomelanin | Interaction | ||
| Name | Residue | Residue | Type | Distance [Å] |
| T1a | C13-Carbonyl oxygen | tyrosine ammonium group at C6 of BZ | conventional hydrogen bond | 3.05 |
| C10 hydroxyl group | N3 of Bz | conventional hydrogen bond | 2.45 | |
| C10 hydroxyl group | N8 of isoindole-ODHBT | conventional hydrogen bond | 2.77 | |
| C12a hydroxyl group | carboxylate group of BTCA | conventional hydrogen bond | 2.62 | |
| C7-amine group | C5-hydroxyl group of BTCA | carbon hydrogen bond | 3.77 | |
| N18-ammonium group | thiazole ring of Bz | π-cation | 3.40 | |
| N18-ammonium group | benzene ring of Bz | π-cation | 3.08 | |
| A-ring | S1 of Bz | π-cation | 5.54 | |
| T1b | N18-ammonium group | carboxylate group of BTCA | conventional hydrogen bond | 2.86 |
| N14-amide group | Carboxylate group of isoindole-ODHBT | conventional hydrogen bond | 2.53 | |
| C4 | C5-hydroxyl group of BTCA | carbon hydrogen bond | 3.46 | |
| C4 ammonium group | C5-hydroxyl group of BTCA | carbon hydrogen bond | 3.51 | |
| C12 enolate anion | thiazole ring of BTCA | π-anion | 4.87 | |
| A-ring | S1 of BTCA | π-sulfur | 4.13 | |
| C6 | C2 of BTCA | alkyl | 5.37 | |
| T2a | C13 enolate anion | tyrosine ammonium group at C6 of BZ | attractive charge | 3.89 |
| C12 enolate anion | benzene ring of Bz | π-anion | 3.97 | |
| C20 | thiazole ring of Bz | π-sigma | 3.64 | |
| C12a hydroxyl group | Carboxylate group of BTCA | conventional hydrogen bond | 3.18 | |
| C12a hydroxyl group | S1 of BTCA | conventional hydrogen bond | 2.70 | |
| C4 ammonium group | carboxylate group of BTCA | conventional hydrogen bond | 2.71 | |
| Methyl of C7-amine group | C5-hydroxyl group of BTCA | carbon hydrogen bond | 3.58 | |
| Methyl of C4-ammonium group | carboxylate group of BTCA | carbon hydrogen bond | 2.79 | |
| T2b | C13 enolate anion | tyrosine ammonium group at C6 of BZ | salt bridge, attractive charge | 2.34 |
| C12a hydroxyl group | S1 of BTCA | conventional hydrogen bond | 3.04 | |
| C12a hydroxyl group | carboxylate group of BTCA | conventional hydrogen bond | 2.33 | |
| N14 ammonium group | carboxylate group of Bz | conventional hydrogen bond | 2.79 | |
| N14 ammonium group | carboxylate group of Bz | conventional hydrogen bond | 2.39 | |
| methyl of C4 amine group | carboxylate group of BTCA | carbon hydrogen bond | 3.19 | |
| N18 ammonium group | thiazole ring of Bz | π-cation | 4.86 | |
| C20 | thiazole ring of Bz | π-sigma | 3.73 | |
| T2c | C13 enolate anion | tyrosine ammonium group at C6 of BZ | attractive charge | 3.32 |
| C12a hydroxyl group | carboxylate group of BTCA | conventional hydrogen bond | 3.21 | |
| C20 | thiazole ring of Bz | π-sigma | 3.68 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzepka, Z.; Karkoszka-Stanowska, M.; Marciniec, K.; Zdybel, M.; Pilawa, B.; Wrześniok, D. Effect of Tigecycline on the Homeostasis of Human Epidermal Melanocytes and Fibroblasts. Int. J. Mol. Sci. 2025, 26, 8939. https://doi.org/10.3390/ijms26188939
Rzepka Z, Karkoszka-Stanowska M, Marciniec K, Zdybel M, Pilawa B, Wrześniok D. Effect of Tigecycline on the Homeostasis of Human Epidermal Melanocytes and Fibroblasts. International Journal of Molecular Sciences. 2025; 26(18):8939. https://doi.org/10.3390/ijms26188939
Chicago/Turabian StyleRzepka, Zuzanna, Marta Karkoszka-Stanowska, Krzysztof Marciniec, Magdalena Zdybel, Barbara Pilawa, and Dorota Wrześniok. 2025. "Effect of Tigecycline on the Homeostasis of Human Epidermal Melanocytes and Fibroblasts" International Journal of Molecular Sciences 26, no. 18: 8939. https://doi.org/10.3390/ijms26188939
APA StyleRzepka, Z., Karkoszka-Stanowska, M., Marciniec, K., Zdybel, M., Pilawa, B., & Wrześniok, D. (2025). Effect of Tigecycline on the Homeostasis of Human Epidermal Melanocytes and Fibroblasts. International Journal of Molecular Sciences, 26(18), 8939. https://doi.org/10.3390/ijms26188939







