The Protective Mechanism of Moderate Intensity Continuous Training on TMAO-Induced Myocardial Injury Based on NMR Metabolomics
Abstract
1. Introduction
2. Results
2.1. The Results of Body Weight, Heart Weight, and Ultrasound Examination
2.2. H&E Staining Results of Myocardial Tissue
2.3. NMR Metabolomics Analysis Results of Aqueous Metabolites in Mouse Myocardial Tissue
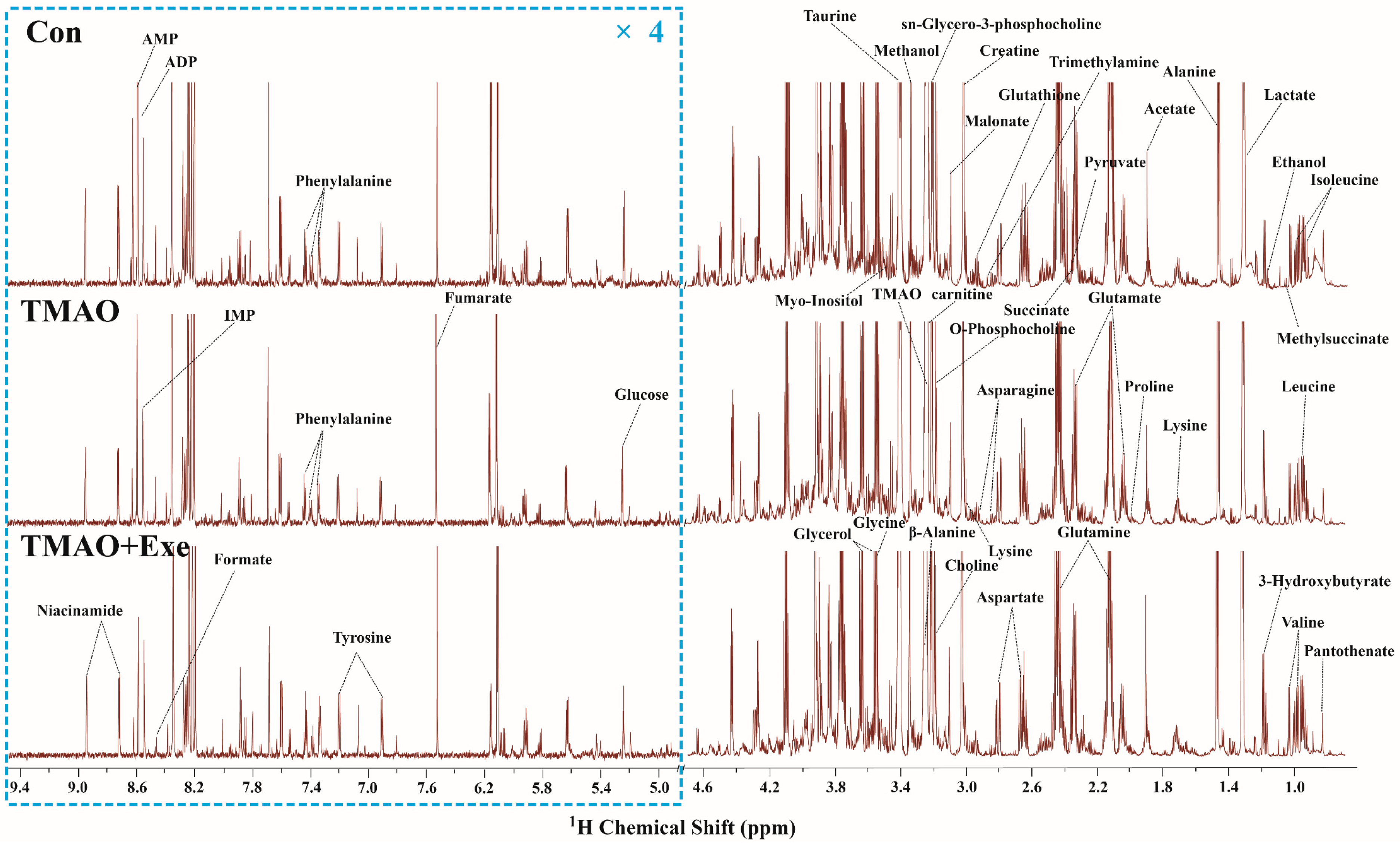
2.3.1. NMR Spectra and Metabolite Identification
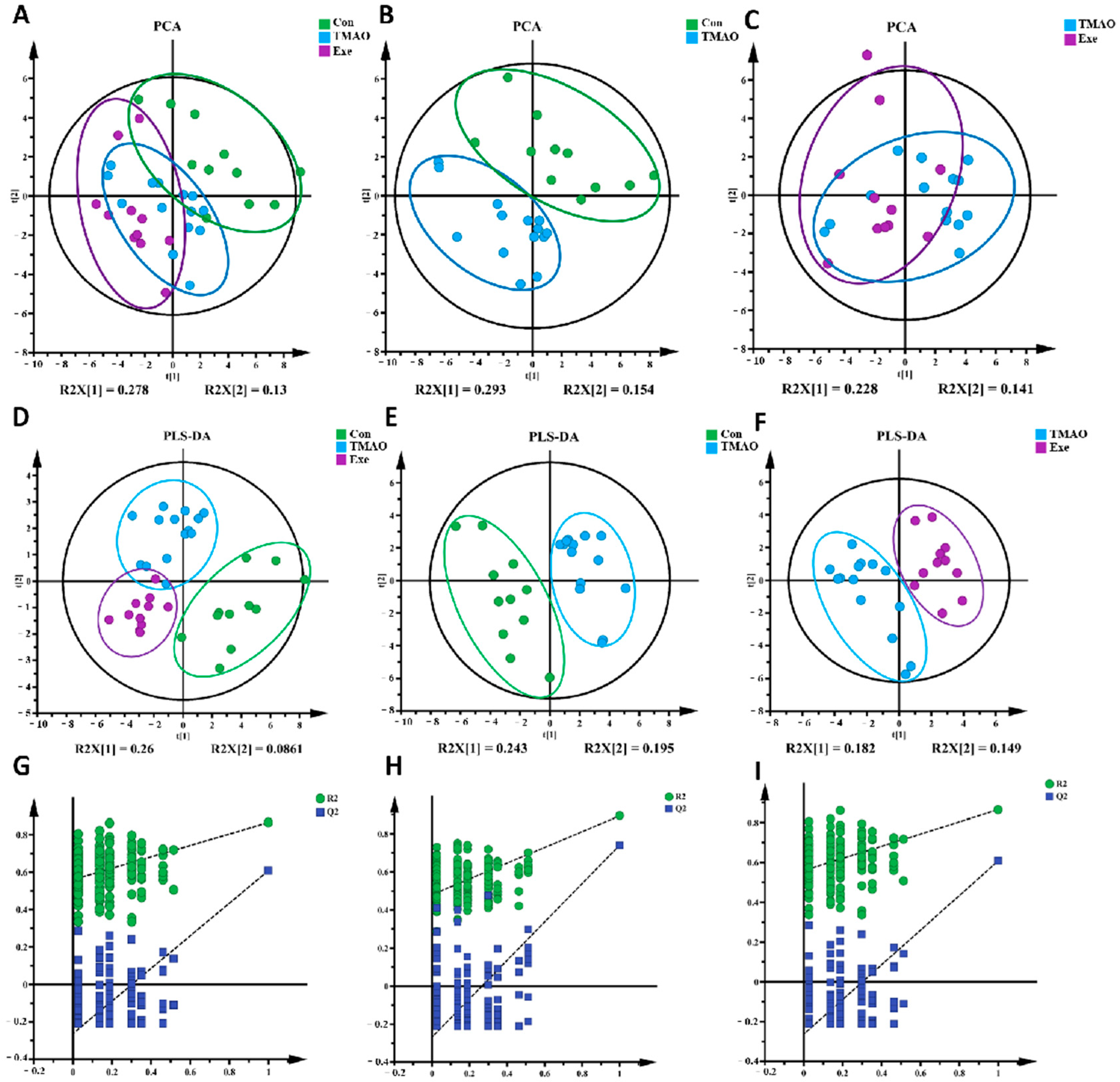
2.3.2. Multivariate Statistical Analysis for NMR Spectra of Metabolites Extracted from the Myocardial Tissue
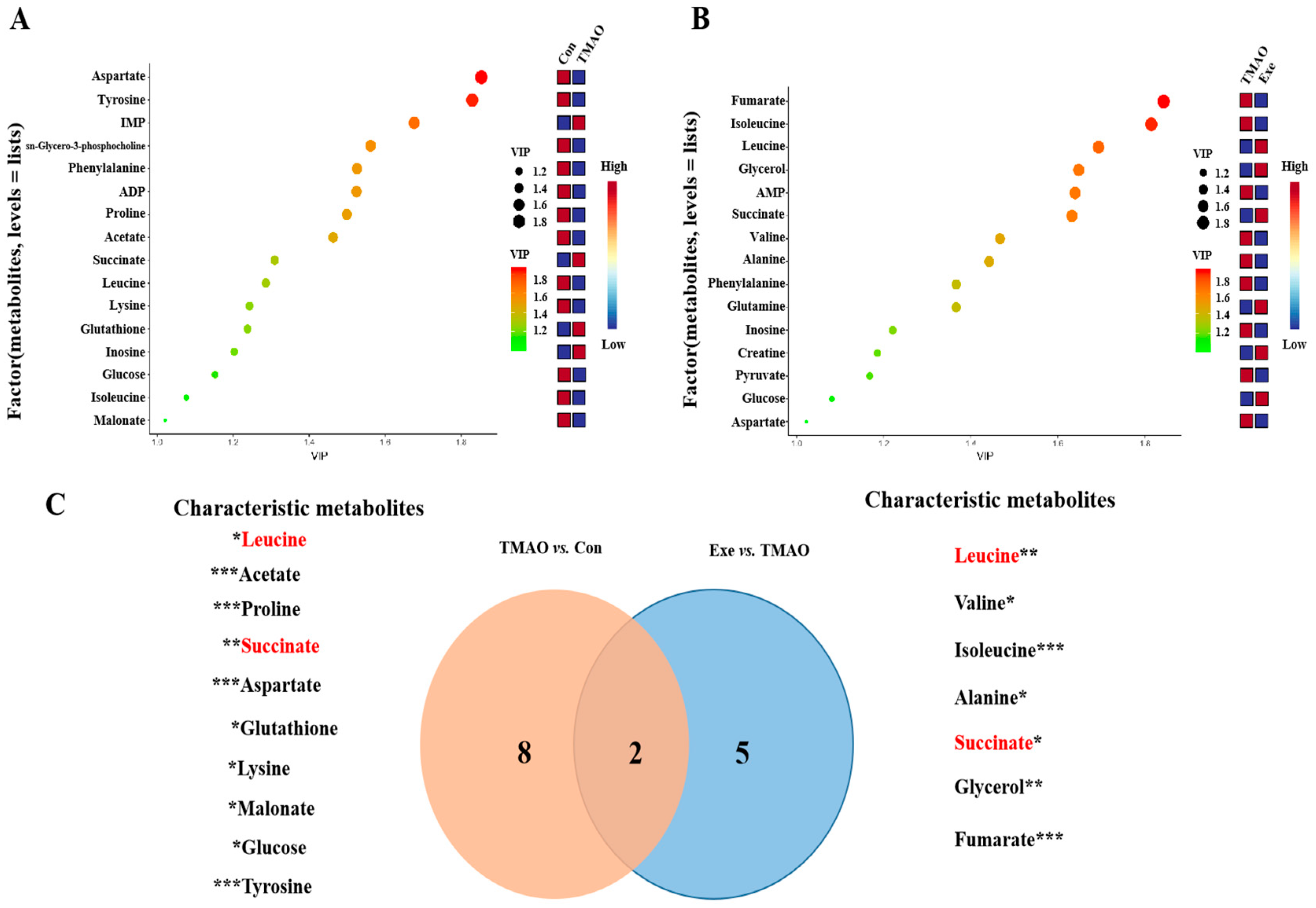
2.3.3. Identifications of Important, Differential, and Characteristic Metabolites of Aqueous Extracts Derived from the Myocardial Tissue
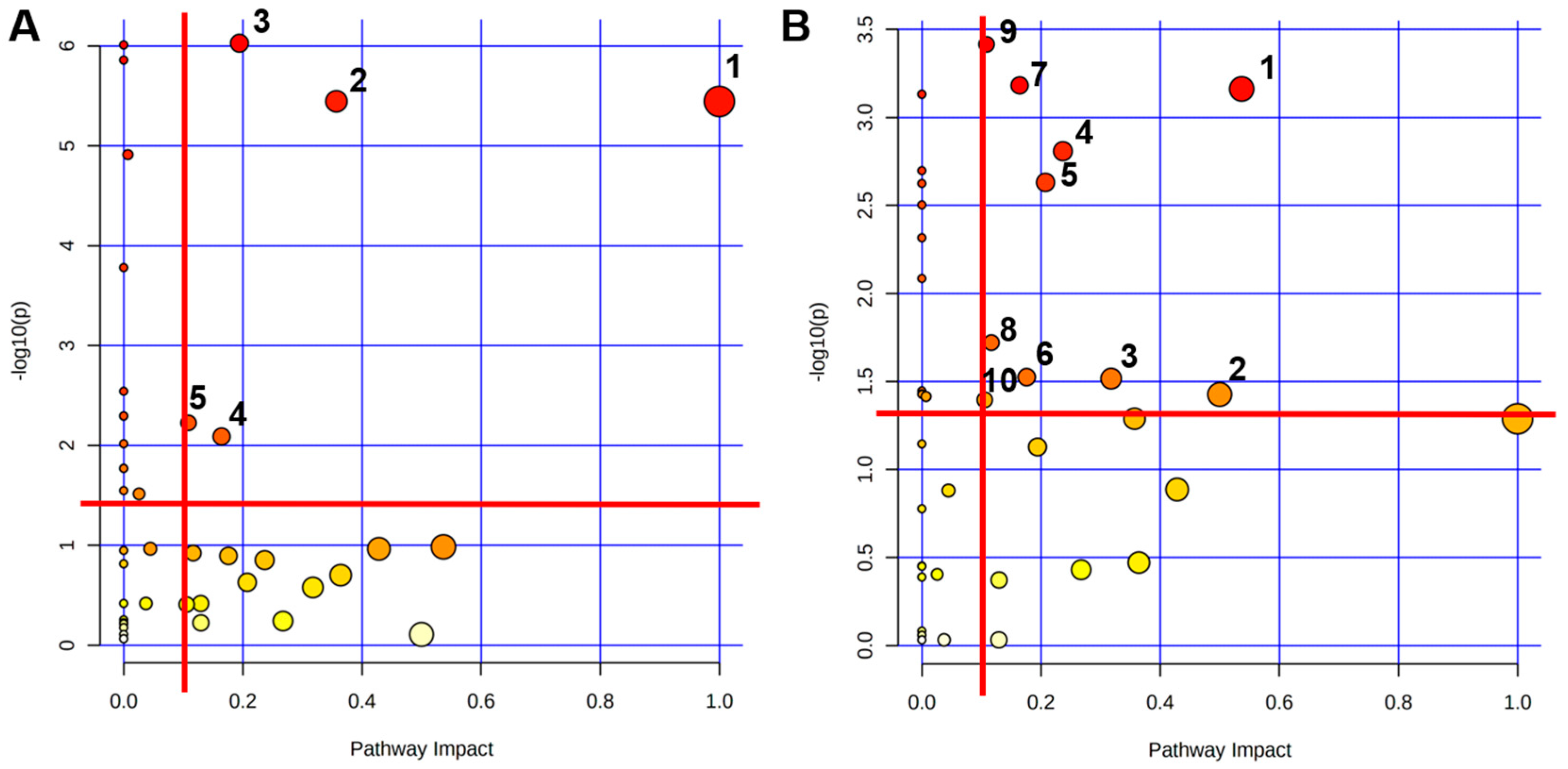
2.3.4. Identifications of Significantly Altered Metabolic Pathways of Aqueous Extracts Derived from the Myocardial Tissue of Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. Determination of the Maximal Running Capacity (MRC) on Treadmill
4.3. Training Protocols
4.4. TMAO Administration
4.5. Samples Collection
4.6. Cardiac Functional Assessment in Mice
4.6.1. Echocardiographic Examination
4.6.2. Myocardial Tissue H&E Staining Protocol
4.7. NMR Metabolomics Analysis of Mice Myocardial Tissue
4.7.1. Extraction of Mice Myocardial Tissue’s Aqueous Metabolites
4.7.2. Preparation for NMR Sample
4.7.3. Measurements of NMR Spectroscopy
4.7.4. Preprocess of NMR Spectra
4.7.5. Statistical Analyses of NMR Data
4.7.6. Metabolic Pathway Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs): Key Facts [Internet]; World Health Organization, 2021. Available online: https://knowledge-action-portal.com/en/content/cardiovascular-diseases-cvds (accessed on 9 September 2025).
- Manor, O.; Zubair, N.; Conomos, M.P.; Xu, X.; Rohwer, J.E.; Krafft, C.E.; Lovejoy, J.C.; Magis, A.T. A Multi-omic Association Study of Trimethylamine N-Oxide. Cell Rep. 2018, 24, 935–946. [Google Scholar] [CrossRef]
- Costabile, G.; Vetrani, C.; Bozzetto, L.; Giacco, R.; Bresciani, L.; Del Rio, D.; Vitale, M.; Della Pepa, G.; Brighenti, F.; Riccardi, G.; et al. Plasma TMAO increase after healthy diets: Results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. Am. J. Clin. Nutr. 2021, 114, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Koay, Y.C.; Chen, Y.-C.; A Wali, J.; Luk, A.W.S.; Li, M.; Doma, H.; Reimark, R.; Zaldivia, M.T.K.; Habtom, H.T.; E Franks, A.; et al. Plasma levels of trimethylamine-N-oxide can be increased with ‘healthy’ and ‘unhealthy’ diets and do not correlate with the extent of atherosclerosis but with plaque instability. Cardiovasc. Res. 2020, 117, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: Refining the gut hypothesis. J. Am. Coll. Cardiol. 2014, 64, 1908–1914. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Me-tabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.N.; Gregory, J.C.; Org, E.; Wu, Y.P.; Li, L.; Smith, J.D.; et al. γ-Butyrobetaine Is a Proatherogenic Intermediate in Gut Microbial Metabolism of L-Carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Long, J.; Chen, S.; Liao, G.; Wu, S.; Li, C.; Wang, L.; Ling, W.; Zhu, H. Trimethylamine N-Oxide Aggravates Liver Steatosis through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019, 63, e1900257. [Google Scholar] [CrossRef]
- Bjornestad, E.O.; Dhar, I.; Svingen, G.F.T.; Pedersen, E.R.; Orn, S.; Svenningsson, M.M.; Tell, G.S.; Ueland, P.M.; Sulo, G.; Laaksonen, R.; et al. Circulating trimethylamine N-oxide levels do not predict 10-year survival in patients with or without coronary heart disease. J. Intern. Med. 2022, 292, 915–924. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yang, P.; Liu, X.; Lu, L.; Chen, Y.; Zhong, X.; Li, Z.; Liu, H.; Ou, C.; et al. Trimethylamine-N-Oxide Promotes Vascular Calcification Through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-κB (Nuclear Factor κB) Signals. Arter. Thromb. Vasc. Biol. 2020, 40, 751–765. [Google Scholar] [CrossRef]
- Kong, Q.; Gu, J.; Lu, R.; Huang, C.; Chen, L.; Wu, W.; Lin, D. NMR-Based Metabolomic Analysis of Cardiac Tissues Clarifies Molecular Mechanisms of CVB3-Induced Viral Myocarditis and Dilated Cardiomyopathy. Molecules 2022, 27, 6115. [Google Scholar] [CrossRef] [PubMed]
- Engel, L.E.; de Souza, F.L.A.; Giometti, I.C.; Okoshi, K.; Mariano, T.B.; Ferreira, N.Z.; Pinheiro, D.G.; Floriano, R.S.; Aguiar, A.F.; Cicogna, A.C.; et al. The high-intensity interval training mitigates the cardiac remodeling in spontaneously hypertensive rats. Life Sci. 2022, 308, 120959. [Google Scholar] [CrossRef] [PubMed]
- Frasier, C.R.; Moore, R.L.; Brown, D.A. Exercise-induced cardiac preconditioning: How exercise protects your achy-breaky heart. J. Appl. Physiol. 2011, 111, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Quindry, J.C.; Kavazis, A.N. Exercise-induced cardioprotection against myocardial ischemia–reperfusion injury. Free. Radic. Biol. Med. 2008, 44, 193–201. [Google Scholar] [CrossRef]
- Padrao, A.I.; Moreira-Gonçalves, D.; Oliveira, P.A.; Teixeira, C.; Faustino-Rocha, A.I.; Helguero, L.; Vitorino, R.; Santos, L.L.; Amado, F.; Duarte, J.A.; et al. Endurance training prevents TWEAK but not myostatin-mediated cardiac remodelling in cancer cachexia. Arch. Biochem. Biophys. 2015, 567, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Pagan, L.U.; Gomes, M.J.; Damatto, R.L.; Lima, A.R.R.; Cezar, M.D.M.; Damatto, F.C.; Reyes, D.R.A.; Campos, D.H.S.; Caldonazo, T.M.M.; Polegato, B.F.; et al. Aerobic Exercise During Advance Stage of Uncontrolled Arterial Hypertension. Front. Physiol. 2021, 12, 675778. [Google Scholar] [CrossRef]
- Miyachi, M.; Yazawa, H.; Furukawa, M.; Tsuboi, K.; Ohtake, M.; Nishizawa, T.; Hashimoto, K.; Yokoi, T.; Kojima, T.; Murate, T.; et al. Exercise Training Alters Left Ventricular Geometry and Attenuates Heart Failure in Dahl Salt-Sensitive Hypertensive Rats. Hypertension 2009, 53, 701–707. [Google Scholar] [CrossRef][Green Version]
- Chacko, S.; Haseeb, Y.B.; Haseeb, S. Metabolomics Work Flow and Analytics in Systems Biology. Curr. Mol. Med. 2022, 22, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.A.N.; Raftery, D. NMR Metabolomics Methods for Investigating Disease. Anal. Chem. 2023, 95, 83–99. [Google Scholar] [CrossRef]
- Gowda, G.A.N.; Zhu, W.; Raftery, D. NMR-based metabolomics: Where are we now and where are we going? Prog. Nucl. Magn. Reson. Spectrosc. 2025, 150–151, 101564. [Google Scholar] [CrossRef]
- Emwas, A.-H.M.; Salek, R.M.; Griffin, J.L.; Merzaban, J. NMR-based metabolomics in human disease diagnosis: Applications, limitations, and recommendations. Metabolomics 2013, 9, 1048–1072. [Google Scholar] [CrossRef]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228–233. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Doenst, T.; Bugger, H.; Schwarzer, M.; Faerber, G.; Borger, M.A.; Mohr, F.W. Three good reasons for heart surgeons to understand cardiac metabolism. Eur. J. Cardio Thorac. Surg. 2008, 33, 862–871. [Google Scholar] [CrossRef]
- Li, X.; Wu, F.; Günther, S.; Looso, M.; Kuenne, C.; Zhang, T.; Wiesnet, M.; Klatt, S.; Zukunft, S.; Fleming, I.; et al. Inhibition of fatty acid oxidation enables heart regeneration in adult mice. Nature 2023, 622, 619–626, Correction in Nature 2023, 623, E7. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Zhu, Y.; Zhou, J.; Ai, S.; Yao, J.; Yin, M.; Hu, M.; Qi, W.; Spanos, M.; Li, L.; et al. Inhibition of Hmbox1 Promotes Cardiomyocyte Survival and Glucose Metabolism Through Gck Activation in Ischemia/Reperfusion Injury. Circulation 2024, 150, 848–866. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.; et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell 2020, 180, 862–877. [Google Scholar] [CrossRef]
- Hanley, W.B. Optimal serum phenylalanine for adult patients with phenylketonuria (PKU). Mol. Genet. Metab. 2013, 110, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.G.; Bermea, K.C.; Ayati, M.; Kim, H.B.; Yang, X.M.; Medina, A.; Fu, Z.M.; Heravi, A.; Zhang, X.Y.; Na, C.H.; et al. Alteration in tyrosine phosphorylation of cardiac proteome and EGFR pathway contribute to hypertrophic cardiomyopathy. Commun. Biol. 2022, 5, 1251. [Google Scholar] [CrossRef] [PubMed]
- Melita, V.; Reinis, V.; Stanislava, K.; Helena, C.; Eduards, S.; Maija, D.; Marina, M.-K. Microbiota-Derived Metabolite Trimethylamine N-Oxide Protects Mitochondrial Energy Metabolism and Cardiac Functionality in a Rat Model of Right Ventricle Heart Failure. Front. Cell Dev. Biol. 2021, 8, 622741. [Google Scholar]
- Tomasz, H.; Adrian, D.; Marta, G.; Marek, K.; Klaudia, B.; Ewelina, Z.; Emilia, S.; Aleksandra, W.-T.; Leszek, P.; Michal, D.; et al. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1805–H1820. [Google Scholar]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef]
- Wenlong, Y.; Shuning, Z.; Jianbing, Z.; Hao, J.; Daile, J.; Tiantong, O.; Zhiyong, Q.; Yunzeng, Z.; Juying, Q.; Aijun, S.; et al. Gut microbe-derived metabolite trimethylamine N-oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J. Mol. Cell. Cardiol. 2019, 134, 119–130. [Google Scholar]
- Zapata-Perez, R.; Wanders, R.J.A.; van Karnebeek, C.D.M.; Houtkooper, R.H. NAD(+) homeostasis in human health and disease. EMBO Mol. Med. 2021, 13, e13943. [Google Scholar] [CrossRef] [PubMed]
- Diguet, N.; Trammell, S.A.J.; Tannous, C.; Deloux, R.; Piquereau, J.; Mougenot, N.; Gouge, A.; Gressette, M.; Manoury, B.; Blanc, J.; et al. Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy. Circulation 2018, 137, 2256–2273, Correction in Circulation, 2018, 137, e690. [Google Scholar] [CrossRef]
- Hara, N.; Osago, H.; Hiyoshi, M.; Kobayashi-Miura, M.; Tsuchiya, M. Quantitative analysis of the effects of nicotinamide phosphoribosyltransferase induction on the rates of NAD+ synthesis and breakdown in mammalian cells using stable isotope-labeling combined with mass spectrometry. PLoS ONE 2019, 14, e0214000. [Google Scholar] [CrossRef]
- Lv, J.; Li, Y.; Shi, S.; Xu, X.; Wu, H.; Zhang, B.; Song, Q. Skeletal muscle mitochondrial remodeling in heart failure: An update on mechanisms and therapeutic opportunities. Biomed. Pharmacother. 2022, 155, 113833. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.L.B.; Mota, G.A.F.; da Silva, V.L.; Vileigas, D.F.; Sant’ANa, P.G.; Gregolin, C.S.; Figueira, R.L.; Batah, S.S.; Fabro, A.T.; Murata, G.M.; et al. Effects of early exercise on cardiac function and lipid metabolism pathway in heart failure. J. Cell. Mol. Med. 2023, 27, 2956–2969. [Google Scholar] [CrossRef]
- Zhou, Q.; Deng, J.; Pan, X.; Meng, D.; Zhu, Y.; Bai, Y.; Shi, C.; Duan, Y.; Wang, T.; Li, X. Gut microbiome mediates the protective effects of exercise after myocardial infarction. Microbiome 2022, 10, 82. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Ruilope, L.M.; Santos-Lozano, A.; Wilhelm, M.; Kraenkel, N.; Fiuza-Luces, C.; Lucia, A. Exercise benefits in cardiovascular diseases: From mechanisms to clinical implementation. Eur. Heart J. 2023, 44, 1874–1889. [Google Scholar] [CrossRef]
- Hou, S.L.; Liu, L.; Yao, J.; Zhao, Q.; Feng, W.; Liu, Q.Q.; Zou, M.; Zhang, R.X.; Yin, H.T.; Xian, H.M. Impact of exercise-based cardiac rehabilitation on cardiopulmonary function and prognosis in ST elevation myocardial infarction after PCI patients in extremely cold regions. BMC Cardiovasc. Disord. 2025, 25, 84. [Google Scholar] [CrossRef]
- Warnecke, G.; Schulze, B.; Steinkamp, T.; Haverich, A.; Klima, U. Glycine application and right heart function in a porcine heart transplantation model. Transpl. Int. 2006, 19, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, W.; Zhang, Q.; Xu, J.; Liu, H.; Luo, J.; Zhan, L.; Xia, Z.; Lei, S. Glycine Protects H9C2 Cardiomyocytes from High Glucose- and Hypoxia/Reoxygenation-Induced Injury via Inhibiting PKCbeta2 Activation and Improving Mitochondrial Quality. J. Diabetes Res. 2018, 2018, 9502895. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, Z.; Ren, S.; Zhu, J.; Morisawa, N.; Chua, G.L.; Zhang, X.; Wong, Y.K.; Su, L.; Wong, M.X.; et al. BCAA catabolism targeted therapy for heart failure with preserved ejection fraction. Theranostics 2025, 15, 6257–6273. [Google Scholar] [CrossRef]
- Farah, C.; Michel, L.Y.M.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef]
- Ferreira, R.; Nogueira-Ferreira, R.; Trindade, F.; Vitorino, R.; Powers, S.K.; Moreira-Goncalves, D. Sugar or fat: The metabolic choice of the trained heart. Metabolism 2018, 87, 98–104. [Google Scholar] [CrossRef]
- Saito, Y.; Sugiura, Y.; Sakaguchi, A.; Sada, T.; Nishiyama, C.; Maeda, R.; Kaneko, M.; Kiyonari, H.; Kimura, W. Redox-dependent purine degradation triggers postnatal loss of cardiac regeneration potential. Redox Biol. 2024, 79, 103442. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, A.G.; Passos, D.F.; Doleski, P.H.; Leitemperger, J.W.; Loro, V.L.; Leal, D.B.R. Purine Metabolism in Platelets and Heart Cells of Hyperlipidemic Rats. Cardiovasc. Drugs Ther. 2020, 34, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Alem, M.M.; Alshehri, A.M.; Cahusac, P.M.; Walters, M.R. Effect of Xanthine Oxidase Inhibition on Arterial Stiffness in Patients With Chronic Heart Failure. Clin. Med. Insights Cardiol. 2018, 12, 117954681877958. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.S.; Hawkey, C.J.; Ford, I.; Greenlaw, N.; Pigazzani, F.; Rogers, A.; Struthers, A.D.; Begg, A.G.; Wei, L.; Avery, A.J.; et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): A multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022, 400, 1195–1205. [Google Scholar] [CrossRef]
- Tanaka, A.; Node, K. Xanthine oxidase inhibition for cardiovascular disease prevention. Lancet 2022, 400, 1172–1173. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.F.; Ban, L.A.; Olaya-Agudo, L.F.; Maharjan, B.R.; Williams, P.F.; Tam, C.S.; Mclennan, S.V.; Twigg, S.M. Constant-Moderate and High-Intensity Interval Training Have Differential Benefits on Insulin Sensitive Tissues in High-Fat Fed Mice. Front. Physiol. 2019, 10, 459. [Google Scholar] [CrossRef]
- Baekkerud, F.H.; Salerno, S.; Ceriotti, P.; Morland, C.; Storm-Mathisen, J.; Bergersen, L.H.; Hoydal, M.A.; Catalucci, D.; Stolen, T.O. High Intensity Interval Training Ameliorates Mitochondrial Dysfunction in the Left Ventricle of Mice with Type 2 Diabetes. Cardiovasc. Toxicol. 2019, 19, 422–431. [Google Scholar] [CrossRef]
- Kemi, O.J.; Loennechen, J.P.; Wisloff, U.; Ellingsen, O. Intensity-controlled treadmill running in mice: Cardiac and skeletal muscle hypertrophy. J. Appl. Physiol. 2002, 93, 1301–1309. [Google Scholar] [CrossRef]
- Chavanelle, V.; Boisseau, N.; Otero, Y.F.; Combaret, L.; Dardevet, D.; Montaurier, C.; Delcros, G.; Peltier, S.L.; Sirvent, P. Effects of high-intensity interval training and moderate-intensity continuous training on glycaemic control and skeletal muscle mitochondrial function in db/db mice. Sci. Rep. 2017, 7, 204. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Gong, L.; Huang, C.; Lin, D.; Zhang, Y. The Protective Mechanism of Moderate Intensity Continuous Training on TMAO-Induced Myocardial Injury Based on NMR Metabolomics. Int. J. Mol. Sci. 2025, 26, 8902. https://doi.org/10.3390/ijms26188902
Zou H, Gong L, Huang C, Lin D, Zhang Y. The Protective Mechanism of Moderate Intensity Continuous Training on TMAO-Induced Myocardial Injury Based on NMR Metabolomics. International Journal of Molecular Sciences. 2025; 26(18):8902. https://doi.org/10.3390/ijms26188902
Chicago/Turabian StyleZou, Hong, Lijing Gong, Caihua Huang, Donghai Lin, and Yimin Zhang. 2025. "The Protective Mechanism of Moderate Intensity Continuous Training on TMAO-Induced Myocardial Injury Based on NMR Metabolomics" International Journal of Molecular Sciences 26, no. 18: 8902. https://doi.org/10.3390/ijms26188902
APA StyleZou, H., Gong, L., Huang, C., Lin, D., & Zhang, Y. (2025). The Protective Mechanism of Moderate Intensity Continuous Training on TMAO-Induced Myocardial Injury Based on NMR Metabolomics. International Journal of Molecular Sciences, 26(18), 8902. https://doi.org/10.3390/ijms26188902







